Abstract
Schizophrenia is a neurodevelopmental disorder characterized by abnormal processing of information and attentional deficits. Schizophrenia has a high genetic component but is precipitated by environmental factors, as proposed by the ‘two-hit’ theory of schizophrenia. Here we compared latent inhibition as a measure of learning and attention, in CHL1-deficient mice, an animal model of schizophrenia, and their wild-type littermates, under no-stress and chronic mild stress conditions. All unstressed mice as well as the stressed wild-type mice showed latent inhibition. In contrast, CHL1-deficient mice did not show latent inhibition after exposure to chronic stress. Differences in neuronal activation (c-Fos-positive cell counts) were noted in brain regions associated with latent inhibition: Neuronal activation in the prelimbic/infralimbic cortices and the nucleus accumbens shell was affected solely by stress. Neuronal activation in basolateral amygdala and ventral hippocampus was affected independently by stress and genotype. Most importantly, neural activation in nucleus accumbens core was affected by the interaction between stress and genotype. These results provide strong support for a ‘two-hit’ (genes x environment) effect on latent inhibition in CHL1-deficient mice, and identify CHL1-deficient mice as a model of schizophrenia-like learning and attention impairments.
Keywords: accumbens, c-Fos, chronic mild stress, close homolog to L1, latent inhibition, schizophrenia
1. Introduction
Schizophrenia (SZ) is a neurological disorder characterized by delusions, hallucinations, disorganized behavior and speech, and attentional control deficits that can lead to severe impairments in adaptive function and social integration. Schizophrenia is affecting 1% of the population and has a large economic impact on the affected individual as well as society as a whole, with an estimated economic cost of over $32 billion in the United States alone [1]. Genetic factors affect the individual’s susceptibility to schizophrenia, the heritability of SZ being estimated to be as high as 81% [2]; men show a higher incidence of SZ than women [3]. Additionally, individuals living in urban areas show a higher incidence of SZ than individuals in suburban or rural areas [4] and immigrant populations have a higher incidence rate than native populations [4]. This suggests that environmental factors also play a role in the onset of schizophrenia. In the absence of a singular genetic or environmental pathogenic agent for schizophrenia, disease models involving multiple factors were developed, starting from a ‘two-hit’ hypothesis [5] to a more recent ‘multi-hit’ model [6].
One of the human genes found to be associated with increased risks of developing schizophrenia is the Close Homolog of L1 (CHL1) [7–9], coding for a neuronal cell adhesion molecule. Three separate studies [8, 10, 11] have identified an association between a functional polymorphism Leu17Phe (rs2272522) in the signal peptide region of the CHL1 gene and SZ; although no experimental data exist to date, the CHL1 Leu17Phe polymorphism could alter protein trafficking and recruitment to the membrane. Another study has identified genomic copy number variations (CNVs) of the CHL1 gene in Scottish SZ patients [9]; CNVs involve mainly loss (e.g. deletions) or gain (e.g. duplications) of up to several million base pairs of DNA sequence [12] and thus can alter gene dosage and expression (e.g., a deletion of a gene or part of a gene would leave the individual with only one functional allele, i.e., heterozygous). CHL1 and other cell adhesion molecules of the immunoglobulin superfamily are highly expressed during the development of the nervous system [13], have multiple functions in the formation of appropriate neuronal connections during development [14] and in synaptic function and plasticity in the adult [15], processes which are thought to be disrupted in intellectual disabilities and schizophrenia [16]. CHL1-deficient mice [17] exhibit behavioral alterations suggestive of those found in schizophrenic patients [18–23], such as impaired sensorimotor gating (prepulse inhibition) [24, 25] and reduced exploratory behavior in novel environments [17, 26]. Timekeeping is disrupted in schizophrenic patients and individuals at risk for schizophrenia [22, 27–31], as well as CHL1-deficient mice [32]. Deficits in spatial-temporal integration were reported both in schizophrenics [18, 33], and in CHL1-deficient mice [32].
Latent Inhibition (LI) is a measure of attention and learning, defined as the loss of future associability by a stimulus that has been repeatedly presented without consequence [34]; the loss of associability results in slower learning of a new conditioned stimulus (CS) – unconditioned stimulus (US) relationship if the pre-exposed (PE) stimulus is presented with consequences in the future. LI is attenuated in schizophrenics [35, 36], which learn faster about stimuli previously presented with no consequence, and is thought to be related to the positive symptoms of SZ [37].
Here we explored a ‘two-hit’ hypothesis in LI expression, using CHL1-deficient mice (genetic component) under no-stress and chronic mild stress (CMS) conditions (environmental component). We also comparatively evaluated neuronal activation (c-Fos positive cell counts) during the LI paradigm in brain regions previously shown to be relevant to LI [38–43], in CHL1-deficient mice and their wild-type (WT) littermates.
2. Materials and Methods
2.1 Subjects
The subjects were 93 male CHL1 mice in a C57Bl/6J background, 6–8 weeks old at the beginning of the experiment, subdivided by genotype as follows: CHL1-deficient (KO, n=22), heterozygous (HET, n=36), and wild-type littermate controls (WT, n=35). CHL1 KO mice were originally developed by Montag-Sallaz et al. [26]. The subjects for this experiment were generated at Utah State University in a mouse colony maintained on C57Bl/6J background for more than 10 generations. The genotype of the subjects was confirmed by PCR genotyping from tail biopsy samples. The mice were housed in groups of three or four in a climate-controlled room under a 12-h light-dark cycle. The mice were further divided into Stress (S, n=52) and No-Stress (NS, n=41) groups. Water was provided ad libitum, while weight was maintained at 85% of the ad libitum weight by restricting food access (Teklad rodent diet 8604, Envigo, Denver, CO) during behavioral training and testing. Mice were tested during the light period of the cycle. All experimental procedures were conducted in accordance with the standards for the ethical treatment and approved by Utah State University IACUC Committee.
2.2 Chronic Mild Stress (CMS)
We have chosen a prolonged chronic mild stress paradigm to parallel exposure to mild stressors in daily life (being stuck in traffic, shift work, changes in living conditions etc). Stress mice received 6 weeks of CMS beginning at 6 weeks of age, while no-Stress mice were kept in standard laboratory conditions. The CMS regimen was modeled after [44] and consisted of three different stressors each day, with each stressor lasting a minimum of 2 hours. The different stressors were: 1) the water bottle removed from the home cage; 2) mice housed into a cage that had housed other mice; 3) mice housed into a clean home cage; 4) food removed from the home cage; 5) wet bedding in the home cage; 6) mice restrained into a small plastic container; 7) home cages placed at a 45-degree angle; 8) rat bedding spread evenly in the home cage; 9) light on during the dark cycle. Due to light-dark cycle manipulations, stress mice were housed in a different colony room from the no-stress mice, but using identical laboratory conditions except when the stress condition dictated otherwise. Behavioral testing for all mice occurred at 12–14 weeks of age, as described below.
2.3 Apparatus
The apparatus consisted of 8 standard mouse operant chambers housed inside sound-attenuating cubicles (Med Associates, St. Albans, VT) equipped with a house light, a fan, two nosepokes on the front wall and one nosepoke on the back wall, a programmable audio generator, a shocker/scrambler module, a lever, and a standard mouse 20-mg pellet feeder. The pre-exposed (PE) and non-pre-exposed (NPE) conditioned stimuli were a 80-dB tone and a 10-Hz click. The unconditioned stimulus was a 1-s 0.5mA footshock.
2.4 Latent inhibition (LI)
Latent inhibition was assessed using an “on baseline” conditioned emotional response (CER) procedure consisting of baseline, pre-exposure, conditioning, rebaseline and test phases (i.e. allowing the mouse to eat during the all stages of the LI paradigm). Mice were assigned to either a PE tone/NPE click (n=47) or PE click/NPE tone (n=46) in a counterbalanced manner. Mice were shaped to nosepoke for food pellets on an FR1 schedule throughout the LI task, which consisted of four daily sessions as follows: During the 60-min pre-exposure session mice received forty 30-s presentations of the PE stimulus separated by a 60-s inter-stimulus interval (ISI). During the 30-min conditioning session, the PE and NPE stimuli were presented for 30-sec three times, separated by a 240-s ISI. The last presentation of the PE and NPE stimuli was paired with a 1-s, 0.5-mA footshock. On the next day mice were given a 60-min rebaseline session during which mice were reinforced for nosepoking on an FR1 schedule. During a 30-min test session, mice were presented with 3-min PE and NPE stimuli with an 8-min ISI. Mouse behavior was video recorded and the duration of freezing behavior was estimated using FreezeScan software (CleverSys Inc., Reston, VA) [45].
2.5 c-Fos immunostaining
Minutes upon neuronal activation, a specific subset of immediate early genes is transcribed. Basal levels of brain expression for these genes (in home cage conditions) are very low; the expression of their protein products peaks at 90–120 min after neuronal activation in behavioral tasks. As previously shown in [46], we have assessed neuronal activation during the LI behavioral paradigm through analyses of expression (positive cell counts) of the immediate early gene c-Fos in brain regions known to be relevant to LI through lesion or pharmacological studies [38–43].
Two hours after the start of the test session 6–9 mice in each group were deeply anesthetized and transcardially perfused with a paraformaldehyde solution (4% in 0.1M phosphate buffer, pH 7.4). Brains were collected and sectioned on a vibrating microtome (VT1200S, Leica, Germany). Brain sections were prepared for immunohistochemistry using a Vectastain Elite ABC kit (Vector Labs, Burlingame, CA), according to manufacturer’s protocol. Briefly, 50um sections were treated with 0.3% hydrogen peroxide to inhibit endogenous enzymes, then permeabilized and blocked (5% goat serum, 0.5% triton x-100 in PBS solution) at room temperature for 2 hrs. Sections were incubated with a rabbit anti c-Fos antibody (pc-38, Calbiochem, San Diego, CA; diluted 1:20,000), then with a biotinylated goat anti-rabbit secondary antibody (Vector Labs, Burlingame, CA; diluted 1:200), and then with a avidin-biotinylated horseradish peroxidase complex (Elite ABC reagent). Staining was developed using 3,3′-Diaminobenzidine (DAB Substrate Kit SK4100, Vector Labs, Burlingame, CA), after which sections were washed in order to stop the reaction. Sections were then mounted on slides, left to dry, then dehydrated, and coverslipped with Permount (Fisher Scientific, Pittsburgh, PA).
2.6 Neural activation analysis
Image acquisition and neuronal activation analysis were performed on a Zeiss AxioImager M2 motorized research microscope with an imaging system. Analysis of neuronal activation was performed by counting c-Fos-positive nuclei, in corresponding areas in 2 sections/region of interest/mouse in the following areas of interest: prelimbic cortex (PrL: bregma 2.1 to 1.54), infralimbic cortex (IL: bregma 1.94 to 1.42), basolateral amygdala (BLA: Bregma −1.34 to −1.94), ventral hippocampus (vHipp: bregma −2.92 to −3.52), nucleus accumbens shell (Acb-shell: bregma 1.78 to 1.1), and nucleus accumbens core (Acb-core: bregma 1.78 – 1.10) [47], by two independent observers unaware of genotype. Neuronal activation in each region was averaged over observers and subjected to statistical analyses.
2.7 Statistical analyses
The estimated duration of freezing behavior in the first 30-s of the presentation of the PE and NPE stimuli, and the latency to freeze in the conditioning and test sessions were subjected to mixed ANOVAs with between-subjects variables stress (S, NS) and genotype (KO, HET, WT), and within-subjects variable pre-exposure (PE, NPE) followed by posthoc analyses. The number of rewards and nosepokes during the test session were subjected to mixed ANOVAs with between-subjects variables stress (S, NS) and genotype (KO, HET, WT), and within-subjects variable pre-exposure (PE, NPE) followed by posthoc analyses. Neuronal activation (counts of c-Fos+ cells in each brain region) was subjected to 2-way ANOVAs with factors stress (S, NS) and genotype (KO, HET, WT). All statistical analyses were conducted at an alpha level 0.05.
3. Results
3.1 Latent inhibition
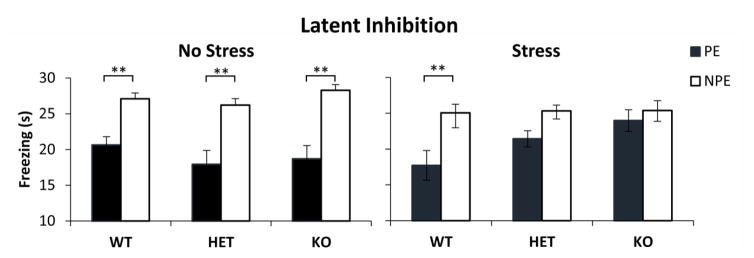
The average freezing duration during the PE and NPE stimuli in the test session is shown in Fig. 1. Analyses indicated a main effect of pre-exposure (F(1,87)=86.86, p<0.01), showing that mice froze longer during the NPE stimulus than during the PE stimulus (LI). However, LI was not expressed equally in all stress conditions: Analyses indicated a reliable pre-exposure x stress interaction (F(1,87)=8.62, p<0.01), showing that NS mice expressed more LI (larger difference in freezing to NPE and PE) than S mice. Furthermore, analyses indicated a reliable 3-way interaction pre-exposure x genotype x stress (F(2,87)=3.93, p<0.05). Post-hoc Tukey HSD and Bonferroni tests indicated that all unstressed mice as well as the stressed WT mice showed reliable LI (all ps<0.01), while neither HET nor KO stressed mice showed LI (all ps>0.05). These results provide support for a ‘two-hit’ under which environmental factors (stress) potentiate the effect of genetic background to reveal schizophrenia-like symptoms (lack of LI) only in stressed CHL1 HET and KO mice.
Fig. 1. Latent inhibition by stress and genotype.
Average duration of freezing (± SEM) to the pre-exposed (PE) and non-pre-exposed (NPE) stimulus in CHL1 knock-out (KO), heterozygotes (HET) and wild type littermate controls (WT) under no-stress (left) and chronic mild stress (right). A reliably latent inhibition (larger freezing to NPE than PE) was observed in all groups except in stressed CHL1 HET and KO mice. * = p<0.05; ** = p<0.01.
3.2 Unconditioned freezing
On the other hand, the difference in freezing to PE and NPE stimuli in Fig. 1 could be due not only to LI, but also to intrinsic (unconditioned) differences in freezing to the two stimuli. To evaluate this hypothesis we performed analyses of freezing behavior to the PE and NPE stimuli in the conditioning session, before these stimuli were paired with footshock. Analyses failed to indicate any main effects of stimulus (PE/NPE) (F(1,87)=0.03, p>0.05), genotype (F(2,87)=1.11, p>0.05), stress (F(1,87)=0.77, p>0.05), or any interactions (all Fs<2.21, p>0.05), suggesting no differences in unconditioned freezing to the PE and NPE stimuli, irrespective of genotype and stress condition. This result further suggests that the differences in freezing between genotypes/groups in Fig. 1 are not due to differences in unconditioned freezing, but rather due to differences in conditioned freezing (associability/learning), thus describing true differences in LI.
3.3 Reactivity to shock
Alternatively, the differences in LI between groups could be due to CHL1 HET and KO mice becoming more reactive to shock following stress. To evaluate this hypothesis we followed three lines of evidence: First, posthoc Tukey HSD and Bonferroni tests of the duration of freezing failed to indicate differences between genotypes in duration of freezing to the NPE stimulus (all ps>0.05) (see Fig. 1); same analyses also failed to indicate differences in duration of freezing to the NPE stimulus between unstressed and stressed mice for each genotype (all ps>0.05) (see Fig. 1). Taken together, these analyses suggest that all mice learned similarly about the NPE stimuli, thus making it unlikely that they had different reactivity to shock. Second, analyses of the number of rewards earned and number of nosepokes performed during the test session failed to indicate any effects of genotype (all Fs(2,87)<1.31, all ps>0.05), stress (all Fs(1,87)<2.99, all ps>0.05), or interactions (all Fs(2,87)<0.69, all ps>0.05), suggesting that mice poked and were rewarded similarly irrespective of stress and genotype, thus making it unlikely that stress and genotype affected reactivity to shock. Finally, analyses of the latency to freeze in the conditioning session (before stimuli being paired with shock) and in the test session (after stimuli were paired with shock), failed to indicate any effects of genotype (F(2,87)=0.43, p>0.05), stress (F(1,87)=1.81, p>0.05), session (F(1,87)=2.06, p>0.05) or any interactions (all Fs<1.96, all ps>0.05), suggesting that mice’s propensity to freeze in the given context did not change before and after stress, irrespective of genotype, thus making it unlikely that mice differed in their reactivity to shock. In summary, in spite of mice performing similarly in the FR1 task, learning similarly about the NPE stimulus, and also learning similarly about the context, they differed only in their freezing to the PE stimulus: Freezing to the PE stimulus was reliably smaller than freezing to the NPE stimulus in all unstressed mice and in the WT stressed mice, but increased (to levels not reliably different than freezing to the NPE stimulus) only in the stressed CHL1 HET and KO mice, suggestive of impaired LI.
3.4 Neuronal activation
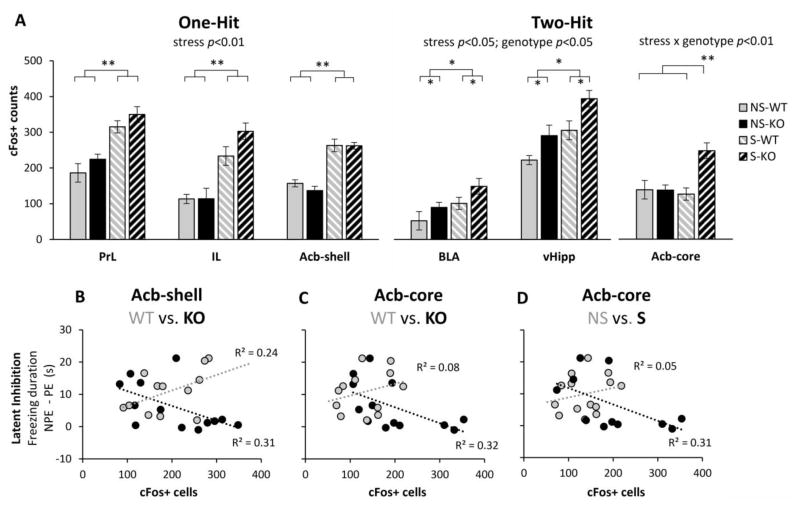
Neuronal activation during LI testing was evaluated in PrL, IL, Acb-core, Ach-shell, vHipp, and BLA, brain regions with relevant roles in latent inhibition [38–43, 46, 48, 49]. Fig. 2A indicates three different patterns of neuronal activation: First, neuronal activation in PrL, IL, and Acb-shell was affected only by stress (F(1,25)=33.11, p<0.01 for PrL; F(1,25)=47.60, p<0.01 for IL, and F(1,21)=25.72, p<0.01 for Acb-shell), but not by genotype (all Fs<2.71, p>0.05), or interactions (all Fs<2.34, p>0.05). Second, BLA and vHipp were independently affected by stress (F(1,23)=19.74, p<0.01 for BLA; F(1,24)=7.15, p<0.05 for vHipp), and genotype (F(1,23)=12.31, p<0.01 for BLA; F(1,24)=5.03, p<0.05 for vHipp), but not by stress x genotype interactions (all Fs<0.18, p>0.05). Third, Acb-core activation was reliably affected by genotype (F(1,21)=5.74, p<0.05), marginally affected by stress (F(1,21)=3.80, p=0.065), and reliably affected by a stress x genotype interaction (F(1,21)=5.93, p<0.05). These results indicate that various brain regions relevant to LI are differentially affected by stress, genotype, and their interaction, thus supporting a complex ‘two-hit’ stress x genotype model.
Fig. 2. Neural activation during latent inhibition testing.
A: Average c-Fos+ cell counts (± SEM) in prelimbic cortex (PrL), infralimbic cortex (IL), nucleus accumbens shell (Acb-shell), basolateral amygdala (BLA), ventral hippocampus (vHipp), and nucleus accumbens core (Acb-core) in the stress (S) and no-stress (NS) CHL1-deficient mice (KO) and wild-type littermate controls (WT). BCD: Correlations between latent inhibition (difference in freezing duration to the non-pre-exposed, NPE, and pre-exposed, PE, stimuli) and neural activation in Acb-shell (B) and Acb-core (CD). * = p<0.05.
To further understand the effect of stress and genotype on these brain regions, we evaluated the patterns of neuronal activation in the nuclei that control the behavioral output [48, 49], Acb-shell and Acb-core, as shown in Fig. 2BCD. Fig. 2A indicates that Acb-shell activation increases after exposure to stress. Consistent with previous studies [46], Fig. 2B indicates that LI (the difference in freezing duration to NPE and PE stimuli) correlates positively with Acb-shell activation (number of c-Fos+ cells) in WT mice (R2(13)=0.24, p<0.05); in contrast, in CHL1 KO mice LI correlates negatively with Acb-shell activation (R2(12)=0.31, p<0.05).
On the other hand, Fig. 2C indicates that in WT controls LI does not correlate with Acb-core activation (R2(13)=0.08, p>0.05). In contrast, in CHL1 KO mice LI correlates negatively with Acb-core activation (R2(12)=0.232, p<0.05). Indeed, stress determines an increase in Acb-core activation only in CHL1 KO mice (stress x genotype interaction) (see Fig. 2A), such that stressed CHL1 KO mice, but not stressed WT mice, show impaired LI (Fig. 1).
Finally, Fig. 2D shows that the correlation between LI and Acb-core activation differs drastically in no-stress (NS) and stress (S) mice: In NS mice LI does not correlate with Acb-core activation (R2(12)=0.05, p>0.05), while in S mice LI correlates negatively with Acb-core activation (R2(15)=0.31, p<0.05). Indeed, there is no reliable difference in Acb-core activation in S and NS WT mice (Fig. 2A), which show LI (Fig. 1), while stressed CHL1 KO mice show an increase in Acb-core activation (Fig. 2A), and fail to show LI (Fig. 1). In summary, all three patterns in Fig. 2BCD contribute to the reliable LI in stressed WT mice, and to the lack of LI in stressed CHL1 KO mice, as shown in Fig. 1.
4. Discussion
Using an “on baseline” within-subject CER LI procedure developed in our lab, the current study found that WT mice in a C57BL/6J background showed LI, consistent with previous findings [50]. Additionally, results indicate that both CHL1 HET and KO mice in C57BL/6J background showed LI under no-stress conditions. However, after exposure to a CMS regimen in adolescence/young adulthood, both CHL1 HET and KO mice failed to show LI, while WT littermates continued to show LI. These results are unlikely to be due to differences in unconditioned freezing to the two stimuli, to changes in auditory generalization for all cues as a result of stress, or to differences in reactivity to shock, as all mice froze similarly to the two stimuli (before they were paired with shock), learned similarly about the NPE stimulus and context, nosepoked similarly, and were rewarded similarly in the FR1 task.
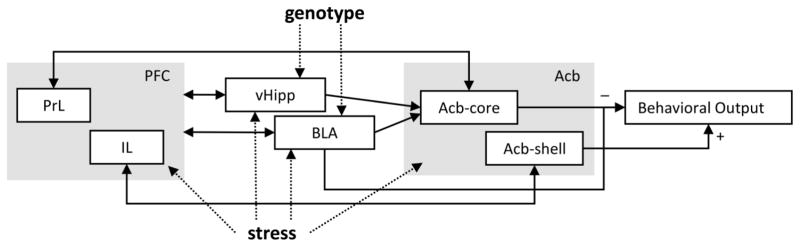
Neuronal activation analyses in brain regions proposed to be involved in LI suggested that in some brain regions activity was affected solely by stress (PrL, IL, Acb-shell), while in others it was affected by both stress and genotype (BLA, vHipp) or their interaction (Acb-core). Our results are in accord with current neurobiological [48] and neuro-computational models [49, 51, 52] of LI (Fig. 3, discussed below), which propose that, in regard to LI, Acb receives input from multiple brain regions (cortex, amygdala, hippocampus) and participates in the selection and integration of motivationally relevant cortico-limbic information that governs behavioral output. However, our data cannot differentiate whether the increased Acb-core activation is the underlying cause of decreased LI or a compensatory effect. Moreover, current results should be considered with caution since other brain regions not investigated in this study may contribute to the LI behavior, and to the current results.
Fig. 3. Modulation of a putative latent inhibition circuit by stress or the CHL1 genotype.
A putative circuit for latent inhibition (modified after [38–39]) indicating the brain regions where activity was affected by stress and/or CHL1 genotype. PFC = prefrontal cortex; PrL = prelimbic cortex; IL = infralimbic cortex; BLA = basolateral amygdala; vHipp = ventral hippocampus; Acb = nucleus accumbens; Acb-core = nucleus accumbens core; Acb-shell = nucleus accumbens shell.
4.1 Neural substrates of latent inhibition
Latent inhibition was introduced by Lubow and Moore [34] as the phenomenon by which repeated presentation of a neutral stimulus without consequences reduces its future associability relative to learning about novel stimuli; this phenomenon is similar in humans and other species [reviewed in 53]. LI promotes stimulus selectivity required for rapid, efficient learning [54], learning that is different from habituation [55] or conditioned inhibition [56]. Most theories explaining LI focus on attentional mechanisms, i.e. during pre-exposure of an inconsequential CS the animal or participant learns not to attend to it [49, 54, 57]. Weiner and Feldon [58] suggested a ‘switching’ theory; the CS-noUS association is learned during pre-exposure, the hippocampus is responsible for detecting mismatches, while switching from the dominant CS-noUS strategy to the new CS-US one is controlled by the core of the nucleus accumbens (with the shell having a modulatory role) [48, 59]. Since LI is disrupted by a change in context between pre-exposure and conditioning [60], Lubow and Gerwitz [54] proposed that the context serves as an occasion setter; since the context is also processed in the hippocampus, this theory does not contradict ‘switching’.
LI prominently depends on Acb, BLA, frontal cortex, and the hippocampus. The role of the hippocampus in LI is underlined by pharmacological and lesion studies showing maintenance of LI, but loss of context specificity of the CR and LI, in rats with specific hippocampal lesions [61–63]. LI is disrupted after ventral hippocampal (vHipp)/ventral subiculum (vSub) NMDA receptor activation [42, 64]. On the other hand, lesions to the BLA produce an abnormally persistent LI in rats, even with a low number of pre-exposures, but not with context shift [39, 65]. Lesions of the orbitofrontal cortex but not of the medial prefrontal cortex also produce an abnormally persistent latent inhibition in rats [39]. Acb is a central structure for the acquisition and expression of LI: lesions of the Acb-shell disrupt LI [66], while lesions of Acb-core or Acb-shell+core are associated with persistent LI [41, 66].
All these features, including the role of the hippocampus and nucleus accumbens are captured by a computational model of LI [49] which addresses not only the effect of hippocampal lesions [52], the complex LI pharmacology [51], and ‘switching’ [49], but also predicts that LI is may be altered by a ‘two-hit’ environment x neurobiology interaction [52]. For example, [52] predicts that hippocampal lesions (neurobiological component) may have vastly different (sometimes contradictory) effects depending on the setting of the LI paradigm (environmental component), ranging from facilitated LI to impaired LI. This model predicts that genetic manipulations which affect the hippocampus (e.g., CHL1 deficiency [26]) may have different effects depending on environmental factors, as in our study.
4.2 Latent inhibition and schizophrenia
On a neuroanatomical level, schizophrenia is characterized by many changes including: enlarged ventricles [67], localized decreases in the density of cortical gray matter [68], decreases in the size of the thalamus [69] and of the caudate nuclei [70]. Additionally, abnormalities are often reported in the medial temporal lobe [71], particularly in the entorhinal cortex [72, 73], the hippocampus and the amygdala [74–76]. Many of the neuroanatomical changes observed in SZ brains are thought to be related to neurodevelopmental processes [16, 77], although some may be the result of treatments with neuroleptics [78, 79]. Neuroanatomical changes observed in the cortex, thalamus, hippocampus and amygdala could be linked to the impairments in LI.
LI is absent or much reduced in patients with SZ [36, 80]. According to LI theories this may be explained by SZ patients not stopping attending the familiar inconsequential stimuli [53], having a hyperactive ‘switching’ mechanism [81], or not being able to use context as an occasion setter [54]. Most interestingly, our results are compatible with a computational model suggesting that LI is affected by the interaction between environmental stimuli and brain insults [52]. Under this model, LI is absent in SZ patients because some environmental conditions trigger their abnormally-developed hippocampus to transition from processing stimuli in an automatic mode, to processing stimuli in a controlled mode [49]. This is the only current LI model that incorporates at its core an interaction between biological and environmental factors, as detailed below [5].
In the Buhusi et al. [5] neuro-computational model, LI depends on the novelty of the PE and NPE stimuli relative to the environment, which depends on learned associations between stimuli, which in turn depend on normal hippocampal function. Under these assumptions, novelty depends both on the environment and on the state of the hippocampus, and the model is, at its core, a ‘two-hit’ LI model, while the hippocampus is a ‘vulnerability’ region. Indeed, simulations ([5], Fig. 21 and Fig. 22, pages 243–344) indicate that alterations in hippocampal function (e.g., due to lesions, drugs, genetic factors etc) are predicted to have variable effects on LI depending on environmental factors. Interestingly, CHL1-deficient mice show alterations of hippocampal circuitry and function, and behavioral anomalies such as impairment of novelty detection and altered exploratory behavior in novel environments [17, 26, 82]. Thus, according to the model in [5], current data could be explained by genetically-induced alterations in hippocampal function combined with environmental factors, which interact to alter novelty computation, and impair LI in stressed CHL1 KO mice.
4.3 CHL1 and schizophrenia
The Close Homolog to L1 cell adhesion molecule is highly expressed in the brain during development [13, 83] and regulates important processes such as neuronal migration, neurite outgrowth and axonal pathfinding, through interactions with other cell adhesion molecules, integrins [84, 85] and guidance receptors [86–88]. Recently, CHL1 was found to regulate neurite outgrowth through interactions with another protein linked to SZ – Disrupted in Schizophrenia (DISC1) [89]. CHL1 deficiency in mice is associated with neuroanatomical anomalies reminiscent of those found in SZ patients, such as enlarged ventricles [26], abnormal thalamocortical projections [86] and altered positioning and morphology of deep-layer cortical neurons [90]. CHL1 KO mice also exhibit many hippocampal anomalies, such as ectopic mossy fiber synapses in the lateral CA3 region, outside the trajectory of the infra-pyramidal mossy fiber bundle [26], and abnormal synapses with enhanced perisomatic inhibition and altered long-term potentiation in the CA1 region [91].
4.4 Stress and latent inhibition
Chronic stress affects gene expression, as well as neuronal morphology and function in many brain regions. For example, after stress, pyramidal neurons in the cortex and hippocampus exhibit altered dendritic and spine morphology, decreases in spine density [92–94], and changes in neurotransmission [95, 96]. Stress induces alterations in dopamine neurotransmission [95, 97, 98], which are particularly important for the acquisition and expression of LI [37, 99].
Stress may attenuate LI in humans [100] or rats [101], while, in some cases, it may potentiate it [102]. For example, a recent study revealed a disrupted LI in highly stress reactive mice [103], supporting our own observation that genetic factors are major contributors to the effects of stress: Only stressed CHL1 HET and KO mice, but not wild-type littermates, failed to show LI. Although some neuronal cell adhesion molecules of the immunoglobulin superfamily, such as NCAM and L1, are considered important mediators of the effects of stress on the brain [104, 105], to date nothing is known of the interaction between stress and CHL1 expression. It is possible that the effect is indirect, for example through effects on interneurons [106] or through effects on serotonergic circuits [107].
5 Conclusions
Our results show that a SZ-related behavioral deficit – impaired LI – may be uncovered in mice by the interaction of a genetic vulnerability to SZ (CHL1 deficiency) and environmental stressors (CMS). These results provide strong evidence for a ‘two-hit’ hypothesis of schizophrenia, and support CHL1-deficient mice as a valid model to study environment x genetic interactions on SZ-behavioral phenotypes. The neural dysfunction causing the loss of LI may be the result of interactions between the genetic background supporting changes in neuronal excitability and regional changes in neuronal activation due to stress.
Highlights.
Latent inhibition (LI) was evaluated in CHL1 KO mice under chronic mild stress
Stressed CHL1 KO and HET mice failed to show LI, while all other mice showed LI
Neuronal activation demonstrated regional differences relevant for LI
Some regions were affected by stress only, others by stress, genotype, and their interaction
Stressed CHL1-deficient mice are a two-hit model for schizophrenia-like phenotypes
Acknowledgments
This work was supported by a NARSAD Independent Investigator Award from the Brain and Behavior Research Foundation to CVB; MB was supported by grant NS090283 from the National Institutes of Health. DO and BG were supported by Utah State University URCO Fellowships. Parts of this work were included in an Honors Thesis by DO. We are grateful to Drs. Schachner and Montag for providing a pair of CHL1 breeder mice to start our CHL1 colony. We would like to thank Brooke Hansen for excellent assistance with colony management and genotyping, Chance Christensen for excellent assistance with the FreezeScan software, and Michael Wooley and Morgan Homan for excellent assistance with cell counting. The authors contributed to this work as follows: Experimental design: MB, CB. Behavior: CVB, DO, MJB. Immunostaining and imaging: MB, BG. Data analysis: MB, DO, BG, CVB. Wrote paper: MB, DO, CVB.
Abreviations
- Acb-core
nucleus accumbens core
- Acb-shell
nucleus accumbens shell
- ANOVA
analysis of variance
- BLA
basolateral amygdala
- CER
conditioned emotional response
- CHL1
Close Homolog of L1
- CMS
chronic mild stress
- CR
conditioned response
- CS
conditioned stimulus
- DISC1
Disrupted in Schizophrenia
- HET
heterozygous
- IL
infralimbic cortex
- ISI
inter-stimulus interval
- KO
knock-out
- LI
latent inhibition
- NPE
non-pre-exposed
- NS
no-stress group
- PE
pre-exposed
- PrL
prelimbic cortex
- S
stress group
- SZ
schizophrenia
- US
unconditioned stimulus
- vHipp
ventral hippocampus
- vSub
ventral subiculum
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Knapp M, Mangalore R, Simon J. The global costs of schizophrenia. Schizophr Bull. 2004;30:279–93. doi: 10.1093/oxfordjournals.schbul.a007078. [DOI] [PubMed] [Google Scholar]
- 2.Sullivan PF, Kendler KS, Neale MC. Schizophrenia as a complex trait: evidence from a meta-analysis of twin studies. Arch Gen Psychiatry. 2003;60:1187–92. doi: 10.1001/archpsyc.60.12.1187. [DOI] [PubMed] [Google Scholar]
- 3.Ochoa S, Usall J, Cobo J, Labad X, Kulkarni J. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophrenia research and treatment. 2012;2012:916198. doi: 10.1155/2012/916198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGrath J, Saha S, Welham J, El Saadi O, MacCauley C, Chant D. A systematic review of the incidence of schizophrenia: the distribution of rates and the influence of sex, urbanicity, migrant status and methodology. BMC Med. 2004;2:13. doi: 10.1186/1741-7015-2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bayer TA, Falkai P, Maier W. Genetic and non-genetic vulnerability factors in schizophrenia: the basis of the “two hit hypothesis”. Journal of psychiatric research. 1999;33:543–8. doi: 10.1016/s0022-3956(99)00039-4. [DOI] [PubMed] [Google Scholar]
- 6.Davis J, Eyre H, Jacka FN, Dodd S, Dean O, McEwen S, et al. A review of vulnerability and risks for schizophrenia: Beyond the two hit hypothesis. Neuroscience and biobehavioral reviews. 2016;65:185–94. doi: 10.1016/j.neubiorev.2016.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu TT, Liu Y. An integrated genomic analysis of gene-function correlation on schizophrenia susceptibility genes. J Hum Genet. 2010;55:285–92. doi: 10.1038/jhg.2010.24. [DOI] [PubMed] [Google Scholar]
- 8.Sakurai K, Migita O, Toru M, Arinami T. An association between a missense polymorphism in the close homologue of L1 (CHL1, CALL) gene and schizophrenia. Molecular psychiatry. 2002;7:412–5. doi: 10.1038/sj.mp.4000973. [DOI] [PubMed] [Google Scholar]
- 9.Tam GW, van de Lagemaat LN, Redon R, Strathdee KE, Croning MD, Malloy MP, et al. Confirmed rare copy number variants implicate novel genes in schizophrenia. Biochemical Society transactions. 2010;38:445–51. doi: 10.1042/BST0380445. [DOI] [PubMed] [Google Scholar]
- 10.Shaltout TE, Alali KA, Bushra S, Alkaseri AM, Jose ED, Al-Khainji M, et al. Significant association of close homologue of L1 gene polymorphism rs2272522 with schizophrenia in Qatar. Asia-Pacific psychiatry: official journal of the Pacific Rim College of Psychiatrists. 2013;5:17–23. doi: 10.1111/appy.12014. [DOI] [PubMed] [Google Scholar]
- 11.Chen QY, Chen Q, Feng GY, Lindpaintner K, Chen Y, Sun X, et al. Case-control association study of the close homologue of L1 (CHL1) gene and schizophrenia in the Chinese population. Schizophr Res. 2005;73:269–74. doi: 10.1016/j.schres.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 12.Bassett AS, Scherer SW, Brzustowicz LM. Copy number variations in schizophrenia: critical review and new perspectives on concepts of genetics and disease. The American journal of psychiatry. 2010;167:899–914. doi: 10.1176/appi.ajp.2009.09071016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillenbrand R, Molthagen M, Montag D, Schachner M. The close homologue of the neural adhesion molecule L1 (CHL1): patterns of expression and promotion of neurite outgrowth by heterophilic interactions. The European journal of neuroscience. 1999;11:813–26. doi: 10.1046/j.1460-9568.1999.00496.x. [DOI] [PubMed] [Google Scholar]
- 14.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10:19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 15.Sakurai T. The role of cell adhesion molecules in brain wiring and neuropsychiatric disorders. Molecular and cellular neurosciences. 2016 doi: 10.1016/j.mcn.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Rapoport JL, Addington AM, Frangou S, Psych MR. The neurodevelopmental model of schizophrenia: update 2005. Molecular psychiatry. 2005;10:434–49. doi: 10.1038/sj.mp.4001642. [DOI] [PubMed] [Google Scholar]
- 17.Montag-Sallaz M, Baarke A, Montag D. Aberrant neuronal connectivity in CHL1-deficient mice is associated with altered information processing-related immediate early gene expression. J Neurobiol. 2003;57:67–80. doi: 10.1002/neu.10254. [DOI] [PubMed] [Google Scholar]
- 18.Velasques B, Machado S, Paes F, Cunha M, Sanfim A, Budde H, et al. Sensorimotor integration and psychopathology: motor control abnormalities related to psychiatric disorders. The world journal of biological psychiatry: the official journal of the World Federation of Societies of Biological Psychiatry. 2011;12:560–73. doi: 10.3109/15622975.2010.551405. [DOI] [PubMed] [Google Scholar]
- 19.Wang ZR, Tan YL, Yang FD, Zhang WF, Zou YZ, Tan SP, et al. Impaired prepulse inhibition of acoustic startle in Chinese patients with first-episode, medication-naive schizophrenia. Chinese medical journal. 2013;126:526–31. [PubMed] [Google Scholar]
- 20.Hammer TB, Oranje B, Skimminge A, Aggernaes B, Ebdrup BH, Glenthoj B, et al. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. Journal of psychiatry & neuroscience: JPN. 2013;38:34–42. doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, et al. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47:662–9. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- 22.Penney TB, Meck WH, Roberts SA, Gibbon J, Erlenmeyer-Kimling L. Interval-timing deficits in individuals at high risk for schizophrenia. Brain Cogn. 2005;58:109–18. doi: 10.1016/j.bandc.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol Psychiatry. 2000;47:61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- 24.Irintchev A, Koch M, Needham LK, Maness P, Schachner M. Impairment of sensorimotor gating in mice deficient in the cell adhesion molecule L1 or its close homologue, CHL1. Brain Res. 2004;1029:131–4. doi: 10.1016/j.brainres.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 25.Morellini F, Lepsveridze E, Kahler B, Dityatev A, Schachner M. Reduced reactivity to novelty, impaired social behavior, and enhanced basal synaptic excitatory activity in perforant path projections to the dentate gyrus in young adult mice deficient in the neural cell adhesion molecule CHL1. Molecular and cellular neurosciences. 2007;34:121–36. doi: 10.1016/j.mcn.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 26.Montag-Sallaz M, Schachner M, Montag D. Misguided axonal projections, neural cell adhesion molecule 180 mRNA upregulation, and altered behavior in mice deficient for the close homolog of L1. Molecular and cellular biology. 2002;22:7967–81. doi: 10.1128/MCB.22.22.7967-7981.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green MF, Nuechterlein KH. Cortical oscillations and schizophrenia: timing is of the essence. Archives of general psychiatry. 1999;56:1007–8. doi: 10.1001/archpsyc.56.11.1007. [DOI] [PubMed] [Google Scholar]
- 28.Braus DF. Temporal perception and organisation, neuronal synchronisation and schizophrenia. Fortschr Neurol Psychiatr. 2002;70:591–600. doi: 10.1055/s-2002-35172. [DOI] [PubMed] [Google Scholar]
- 29.Kimura B. Disturbance of timing and selfhood in schizophrenia. Seishin shinkeigaku zasshi = Psychiatria et neurologia Japonica. 2003;105:729–32. [PubMed] [Google Scholar]
- 30.McDowell JE, Clementz BA, Wixted JT. Timing and amplitude of saccades during predictive saccadic tracking in schizophrenia. Psychophysiology. 1996;33:93–101. doi: 10.1111/j.1469-8986.1996.tb02112.x. [DOI] [PubMed] [Google Scholar]
- 31.Volz HP, Nenadic I, Gaser C, Rammsayer T, Hager F, Sauer H. Time estimation in schizophrenia: an fMRI study at adjusted levels of difficulty. Neuroreport. 2001;12:313–6. doi: 10.1097/00001756-200102120-00026. [DOI] [PubMed] [Google Scholar]
- 32.Buhusi M, Scripa I, Williams CL, Buhusi CV. Impaired Interval Timing and Spatial–Temporal Integration in Mice Deficient in CHL1, a Gene Associated with Schizophrenia. Timing & Time Perception. 2013;1:21–38. doi: 10.1163/22134468-00002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Herzog MH, Brand A. Pitting temporal against spatial integration in schizophrenic patients. Psychiatry research. 2009;168:1–10. doi: 10.1016/j.psychres.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 34.Lubow RE, Moore AU. Latent inhibition: the effect of nonreinforced pre-exposure to the conditional stimulus. J Comp Physiol Psychol. 1959;52:415–9. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- 35.Martins Serra A, Jones SH, Toone B, Gray JA. Impaired associative learning in chronic schizophrenics and their first-degree relatives: a study of latent inhibition and the Kamin blocking effect. Schizophr Res. 2001;48:273–89. doi: 10.1016/s0920-9964(00)00141-9. [DOI] [PubMed] [Google Scholar]
- 36.Baruch I, Hemsley DR, Gray JA. Differential performance of acute and chronic schizophrenics in a latent inhibition task. The Journal of nervous and mental disease. 1988;176:598–606. doi: 10.1097/00005053-198810000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Weiner I, Arad M. Using the pharmacology of latent inhibition to model domains of pathology in schizophrenia and their treatment. Behav Brain Res. 2009;204:369–86. doi: 10.1016/j.bbr.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 38.Yee BK, Feldon J, Rawlins JN. Latent inhibition in rats is abolished by NMDA-induced neuronal loss in the retrohippocampal region, but this lesion effect can be prevented by systemic haloperidol treatment. Behav Neurosci. 1995;109:227–40. doi: 10.1037//0735-7044.109.2.227. [DOI] [PubMed] [Google Scholar]
- 39.Schiller D, Weiner I. Lesions to the basolateral amygdala and the orbitofrontal cortex but not to the medial prefrontal cortex produce an abnormally persistent latent inhibition in rats. Neuroscience. 2004;128:15–25. doi: 10.1016/j.neuroscience.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Schiller D, Zuckerman L, Weiner I. Abnormally persistent latent inhibition induced by lesions to the nucleus accumbens core, basolateral amygdala and orbitofrontal cortex is reversed by clozapine but not by haloperidol. Journal of psychiatric research. 2006;40:167–77. doi: 10.1016/j.jpsychires.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 41.Gal G, Schiller D, Weiner I. Latent inhibition is disrupted by nucleus accumbens shell lesion but is abnormally persistent following entire nucleus accumbens lesion: The neural site controlling the expression and disruption of the stimulus preexposure effect. Behav Brain Res. 2005;162:246–55. doi: 10.1016/j.bbr.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Pouzet B, Zhang WN, Weiner I, Feldon J, Yee BK. Latent inhibition is spared by N-methyl-D-aspartate (NMDA)-induced ventral hippocampal lesions, but is attenuated following local activation of the ventral hippocampus by intracerebral NMDA infusion. Neuroscience. 2004;124:183–94. doi: 10.1016/j.neuroscience.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Ouhaz Z, Ba-M’hamed S, Bennis M. Haloperidol treatment at pre-exposure phase reduces the disturbance of latent inhibition in rats with neonatal ventral hippocampus lesions. Comptes rendus biologies. 2014;337:561–70. doi: 10.1016/j.crvi.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Willner P. Validity, reliability and utility of the chronic mild stress model of depression: a 10-year review and evaluation. Psychopharmacology (Berl) 1997;134:319–29. doi: 10.1007/s002130050456. [DOI] [PubMed] [Google Scholar]
- 45.Hsiao YH, Chen PS, Chen SH, Gean PW. The involvement of Cdk5 activator p35 in social isolation-triggered onset of early Alzheimer’s disease-related cognitive deficit in the transgenic mice. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2011;36:1848–58. doi: 10.1038/npp.2011.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sotty F, Sandner G, Gosselin O. Latent inhibition in conditioned emotional response: c-fos immunolabelling evidence for brain areas involved in the rat. Brain Res. 1996;737:243–54. doi: 10.1016/0006-8993(96)00737-8. [DOI] [PubMed] [Google Scholar]
- 47.Franklin KB, Paxinos G. The mouse brain in stereotaxic coordinates. New York: Elsevier, Academic Press; 2008. [Google Scholar]
- 48.Weiner I. The “two-headed” latent inhibition model of schizophrenia: modeling positive and negative symptoms and their treatment. Psychopharmacology (Berl) 2003;169:257–97. doi: 10.1007/s00213-002-1313-x. [DOI] [PubMed] [Google Scholar]
- 49.Schmajuk N, Buhusi CV, Gray JA. The Transition from Automatic to Controlled Processing. Neural networks: the official journal of the International Neural Network Society. 1997;10:1257–68. doi: 10.1016/s0893-6080(97)00058-0. [DOI] [PubMed] [Google Scholar]
- 50.Gould TJ, Wehner JM. Genetic influences on latent inhibition. Behav Neurosci. 1999;113:1291–6. doi: 10.1037//0735-7044.113.6.1291. [DOI] [PubMed] [Google Scholar]
- 51.Schmajuk NA, Buhusi CV, Gray JA. Psychopharmacology of latent inhibition: a neural network approach. Behavioural pharmacology. 1998;9:711–30. doi: 10.1097/00008877-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 52.Buhusi CV, Gray JA, Schmajuk NA. Perplexing effects of hippocampal lesions on latent inhibition: a neural network solution. Behav Neurosci. 1998;112:316–51. doi: 10.1037//0735-7044.112.2.316. [DOI] [PubMed] [Google Scholar]
- 53.Lubow RE. Latent Inhibition and Conditioned Attention Theory. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 54.Lubow RE, Gewirtz JC. Latent inhibition in humans: data, theory, and implications for schizophrenia. Psychological bulletin. 1995;117:87–103. doi: 10.1037/0033-2909.117.1.87. [DOI] [PubMed] [Google Scholar]
- 55.Hall G, Channell S. A comparison of intradimensional and extradimensional shift learning in pigeons. Behav Processes. 1985;10:285–95. doi: 10.1016/0376-6357(85)90075-0. [DOI] [PubMed] [Google Scholar]
- 56.Reiss S, Wagner AR. CS habituation produces a “latent inhibition effect” but no active “conditioned inhibition”. Learning and Motivation. 1972;3:237–45. [Google Scholar]
- 57.Pearce JM, Hall G. A model for Pavlovian learning: variations in the effectiveness of conditioned but not of unconditioned stimuli. Psychol Rev. 1980;87:532–52. [PubMed] [Google Scholar]
- 58.Weiner I, Feldon J. The switching model of latent inhibition: an update of neural substrates. Behav Brain Res. 1997;88:11–25. doi: 10.1016/s0166-4328(97)02314-0. [DOI] [PubMed] [Google Scholar]
- 59.Gray NS, Snowden RJ. The relevance of irrelevance to schizophrenia. Neuroscience and biobehavioral reviews. 2005;29:989–99. doi: 10.1016/j.neubiorev.2005.01.006. [DOI] [PubMed] [Google Scholar]
- 60.Lubow RE, Rifkin B, Alek M. The context effect: The relatioship between stimulus preexposure and environmental preexposure determines subsequent learning. J Exp Psychol Anim Behav Process. 1976;2:38–47. [Google Scholar]
- 61.Good M, Honey RC. Conditioning and contextual retrieval in hippocampal rats. Behav Neurosci. 1991;105:499–509. doi: 10.1037//0735-7044.105.4.499. [DOI] [PubMed] [Google Scholar]
- 62.Honey RC, Good M. Selective hippocampal lesions abolish the contextual specificity of latent inhibition and conditioning. Behav Neurosci. 1993;107:23–33. doi: 10.1037//0735-7044.107.1.23. [DOI] [PubMed] [Google Scholar]
- 63.Coutureau E, Galani R, Gosselin O, Majchrzak M, Di Scala G. Entorhinal but not hippocampal or subicular lesions disrupt latent inhibition in rats. Neurobiology of learning and memory. 1999;72:143–57. doi: 10.1006/nlme.1998.3895. [DOI] [PubMed] [Google Scholar]
- 64.Lodge DJ, Grace AA. Hippocampal dysfunction and disruption of dopamine system regulation in an animal model of schizophrenia. Neurotoxicity research. 2008;14:97–104. doi: 10.1007/BF03033801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schiller D, Weiner I. Basolateral amygdala lesions in the rat produce an abnormally persistent latent inhibition with weak preexposure but not with context shift. Behav Brain Res. 2005;163:115–21. doi: 10.1016/j.bbr.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 66.Weiner I, Gal G, Feldon J. Disrupted and undisruptable latent inhibition following shell and core lesions. Ann N Y Acad Sci. 1999;877:723–7. doi: 10.1111/j.1749-6632.1999.tb09310.x. [DOI] [PubMed] [Google Scholar]
- 67.Johnstone EC, Owens DG, Crow TJ, Frith CD, Alexandropolis K, Bydder G, et al. Temporal lobe structure as determined by nuclear magnetic resonance in schizophrenia and bipolar affective disorder. J Neurol Neurosurg Psychiatry. 1989;52:736–41. doi: 10.1136/jnnp.52.6.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Suddath RL, Christison GW, Torrey EF, Casanova MF, Weinberger DR. Anatomical abnormalities in the brains of monozygotic twins discordant for schizophrenia. N Engl J Med. 1990;322:789–94. doi: 10.1056/NEJM199003223221201. [DOI] [PubMed] [Google Scholar]
- 69.Andreasen NC, Ehrhardt JC, Swayze VW, 2nd, Alliger RJ, Yuh WT, Cohen G, et al. Magnetic resonance imaging of the brain in schizophrenia. The pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry. 1990;47:35–44. doi: 10.1001/archpsyc.1990.01810130037006. [DOI] [PubMed] [Google Scholar]
- 70.Mion CC, Andreasen NC, Arndt S, Swayze VW, 2nd, Cohen GA. MRI abnormalities in tardive dyskinesia. Psychiatry Res. 1991;40:157–66. doi: 10.1016/0925-4927(91)90007-d. [DOI] [PubMed] [Google Scholar]
- 71.McCarley RW, Wible CG, Frumin M, Hirayasu Y, Levitt JJ, Fischer IA, et al. MRI anatomy of schizophrenia. Biol Psychiatry. 1999;45:1099–119. doi: 10.1016/s0006-3223(99)00018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Baiano M, Perlini C, Rambaldelli G, Cerini R, Dusi N, Bellani M, et al. Decreased entorhinal cortex volumes in schizophrenia. Schizophr Res. 2008;102:171–80. doi: 10.1016/j.schres.2007.11.035. [DOI] [PubMed] [Google Scholar]
- 73.Joyal CC, Laakso MP, Tiihonen J, Syvalahti E, Vilkman H, Laakso A, et al. A volumetric MRI study of the entorhinal cortex in first episode neuroleptic-naive schizophrenia. Biol Psychiatry. 2002;51:1005–7. doi: 10.1016/s0006-3223(01)01368-3. [DOI] [PubMed] [Google Scholar]
- 74.Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, et al. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58:173–83. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- 75.Velakoulis D, Wood SJ, Wong MT, McGorry PD, Yung A, Phillips L, et al. Hippocampal and amygdala volumes according to psychosis stage and diagnosis: a magnetic resonance imaging study of chronic schizophrenia, first-episode psychosis, and ultra-high-risk individuals. Arch Gen Psychiatry. 2006;63:139–49. doi: 10.1001/archpsyc.63.2.139. [DOI] [PubMed] [Google Scholar]
- 76.Bois C, Levita L, Ripp I, Owens DC, Johnstone EC, Whalley HC, et al. Hippocampal, amygdala and nucleus accumbens volume in first-episode schizophrenia patients and individuals at high familial risk: A cross-sectional comparison. Schizophr Res. 2015;165:45–51. doi: 10.1016/j.schres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 77.Schmidt MJ, Mirnics K. Neurodevelopment, GABA system dysfunction, and schizophrenia. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2015;40:190–206. doi: 10.1038/npp.2014.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gur RE, Maany V, Mozley PD, Swanson C, Bilker W, Gur RC. Subcortical MRI volumes in neuroleptic-naive and treated patients with schizophrenia. Am J Psychiatry. 1998;155:1711–7. doi: 10.1176/ajp.155.12.1711. [DOI] [PubMed] [Google Scholar]
- 79.Lauer M, Senitz D, Beckmann H. Increased volume of the nucleus accumbens in schizophrenia. J Neural Transm. 2001;108:645–60. doi: 10.1007/s007020170042. [DOI] [PubMed] [Google Scholar]
- 80.Rascle C, Mazas O, Vaiva G, Tournant M, Raybois O, Goudemand M, et al. Clinical features of latent inhibition in schizophrenia. Schizophr Res. 2001;51:149–61. doi: 10.1016/s0920-9964(00)00162-6. [DOI] [PubMed] [Google Scholar]
- 81.Hemsley DR. A simple (or simplistic?) cognitive model for schizophrenia. Behaviour research and therapy. 1993;31:633–45. doi: 10.1016/0005-7967(93)90116-c. [DOI] [PubMed] [Google Scholar]
- 82.Pratte M, Jamon M. Impairment of novelty detection in mice targeted for the Chl1 gene. Physiology & behavior. 2009;97:394–400. doi: 10.1016/j.physbeh.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 83.Liu Q, Dwyer ND, O’Leary DD. Differential expression of COUP-TFI, CHL1, and two novel genes in developing neocortex identified by differential display PCR. J Neurosci. 2000;20:7682–90. doi: 10.1523/JNEUROSCI.20-20-07682.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Buhusi M, Midkiff BR, Gates AM, Richter M, Schachner M, Maness PF. Close homolog of L1 is an enhancer of integrin-mediated cell migration. The Journal of biological chemistry. 2003;278:25024–31. doi: 10.1074/jbc.M303084200. [DOI] [PubMed] [Google Scholar]
- 85.Katic J, Loers G, Kleene R, Karl N, Schmidt C, Buck F, et al. Interaction of the cell adhesion molecule CHL1 with vitronectin, integrins, and the plasminogen activator inhibitor-2 promotes CHL1-induced neurite outgrowth and neuronal migration. J Neurosci. 2014;34:14606–23. doi: 10.1523/JNEUROSCI.3280-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wright AG, Demyanenko GP, Powell A, Schachner M, Enriquez-Barreto L, Tran TS, et al. Close homolog of L1 and neuropilin 1 mediate guidance of thalamocortical axons at the ventral telencephalon. J Neurosci. 2007;27:13667–79. doi: 10.1523/JNEUROSCI.2888-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Demyanenko GP, Siesser PF, Wright AG, Brennaman LH, Bartsch U, Schachner M, et al. L1 and CHL1 Cooperate in Thalamocortical Axon Targeting. Cereb Cortex. 2011;21:401–12. doi: 10.1093/cercor/bhq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schlatter MC, Buhusi M, Wright AG, Maness PF. CHL1 promotes Sema3A-induced growth cone collapse and neurite elaboration through a motif required for recruitment of ERM proteins to the plasma membrane. Journal of neurochemistry. 2008;104:731–44. doi: 10.1111/j.1471-4159.2007.05013.x. [DOI] [PubMed] [Google Scholar]
- 89.Ren J, Zhao T, Xu Y, Ye H. Interaction between DISC1 and CHL1 in regulation of neurite outgrowth. Brain Res. 2016;1648:290–7. doi: 10.1016/j.brainres.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 90.Demyanenko GP, Schachner M, Anton E, Schmid R, Feng G, Sanes J, et al. Close homolog of L1 modulates area-specific neuronal positioning and dendrite orientation in the cerebral cortex. Neuron. 2004;44:423–37. doi: 10.1016/j.neuron.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 91.Nikonenko AG, Sun M, Lepsveridze E, Apostolova I, Petrova I, Irintchev A, et al. Enhanced perisomatic inhibition and impaired long-term potentiation in the CA1 region of juvenile CHL1-deficient mice. The European journal of neuroscience. 2006;23:1839–52. doi: 10.1111/j.1460-9568.2006.04710.x. [DOI] [PubMed] [Google Scholar]
- 92.Maras PM, Baram TZ. Sculpting the hippocampus from within: stress, spines, and CRH. Trends in neurosciences. 2012;35:315–24. doi: 10.1016/j.tins.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Leuner B, Shors TJ. Stress, anxiety, and dendritic spines: what are the connections? Neuroscience. 2013;251:108–19. doi: 10.1016/j.neuroscience.2012.04.021. [DOI] [PubMed] [Google Scholar]
- 94.McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron. 2013;79:16–29. doi: 10.1016/j.neuron.2013.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chang CH, Grace AA. Amygdala-ventral pallidum pathway decreases dopamine activity after chronic mild stress in rats. Biological psychiatry. 2014;76:223–30. doi: 10.1016/j.biopsych.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hu W, Zhang M, Czeh B, Flugge G, Zhang W. Stress impairs GABAergic network function in the hippocampus by activating nongenomic glucocorticoid receptors and affecting the integrity of the parvalbumin-expressing neuronal network. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2010;35:1693–707. doi: 10.1038/npp.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Scheggi S, Leggio B, Masi F, Grappi S, Gambarana C, Nanni G, et al. Selective modifications in the nucleus accumbens of dopamine synaptic transmission in rats exposed to chronic stress. Journal of neurochemistry. 2002;83:895–903. doi: 10.1046/j.1471-4159.2002.01193.x. [DOI] [PubMed] [Google Scholar]
- 98.Pacchioni AM, Cador M, Bregonzio C, Cancela LM. A glutamate-dopamine interaction in the persistent enhanced response to amphetamine in nucleus accumbens core but not shell following a single restraint stress. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2007;32:682–92. doi: 10.1038/sj.npp.1301080. [DOI] [PubMed] [Google Scholar]
- 99.Young AM, Moran PM, Joseph MH. The role of dopamine in conditioning and latent inhibition: what, when, where and how? Neuroscience and biobehavioral reviews. 2005;29:963–76. doi: 10.1016/j.neubiorev.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 100.Braunstein-Bercovitz H, Dimentman-Ashkenazi I, Lubow RE. Stress affects the selection of relevant from irrelevant stimuli. Emotion. 2001;1:182–92. doi: 10.1037/1528-3542.1.2.182. [DOI] [PubMed] [Google Scholar]
- 101.Hellman PA, Crider A, Solomon PR. Interaction of tail-pressure stress and d-amphetamine in disruption of the rat’s ability to ignore an irrevelant stimulus. Behav Neurosci. 1983;97:1017–21. doi: 10.1037//0735-7044.97.6.1017. [DOI] [PubMed] [Google Scholar]
- 102.Melo LL, Ferrari EA, Teixeira NA, Sandner G. Enhancement of latent inhibition by chronic mild stress in rats submitted to emotional response conditioning. Neural Plast. 2003;10:327–33. doi: 10.1155/NP.2003.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Knapman A, Heinzmann JM, Holsboer F, Landgraf R, Touma C. Modeling psychotic and cognitive symptoms of affective disorders: Disrupted latent inhibition and reversal learning deficits in highly stress reactive mice. Neurobiology of learning and memory. 2010;94:145–52. doi: 10.1016/j.nlm.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 104.Bisaz R, Conboy L, Sandi C. Learning under stress: a role for the neural cell adhesion molecule NCAM. Neurobiology of learning and memory. 2009;91:333–42. doi: 10.1016/j.nlm.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 105.Sandi C. Stress, cognitive impairment and cell adhesion molecules. Nat Rev Neurosci. 2004;5:917–30. doi: 10.1038/nrn1555. [DOI] [PubMed] [Google Scholar]
- 106.Schmalbach B, Lepsveridze E, Djogo N, Papashvili G, Kuang F, Leshchyns’ka I, et al. Age-dependent loss of parvalbumin-expressing hippocampal interneurons in mice deficient in CHL1, a mental retardation and schizophrenia susceptibility gene. Journal of neurochemistry. 2015;135:830–44. doi: 10.1111/jnc.13284. [DOI] [PubMed] [Google Scholar]
- 107.Kleene R, Chaudhary H, Karl N, Katic J, Kotarska A, Guitart K, et al. Interaction between CHL1 and serotonin receptor 2c regulates signal transduction and behavior in mice. Journal of cell science. 2015;128:4642–52. doi: 10.1242/jcs.176941. [DOI] [PubMed] [Google Scholar]