Abstract
With continuing disconnect between laboratory stroke treatment models and clinical stroke therapy, we propose a novel experimental model to study stroke and vessel recanalization that mirrors acute management of large vessel stroke, with concomitant directed pharmacotherapy. Using the tandem transient ipsilateral common carotid/middle cerebral artery occlusion (MCAO) model to induce stroke in mice we then added selective intra-arterial (IA) drug administration for directed pharmacotherapy. The IA model uses micro-angio tubing placed at the bifurcation of the CCA to selectively administer the drug to the internal carotid distribution. We have shown that delivery of pharmacotherapy agents selectively through an IA injection is feasible in a mouse model, which will permit studies involving pharmacotherapy, transgenic modification, and/or a combination. Our IA model has similarities to previously published models of IA injection but differs in that we do not leave an indwelling micro-port or catheter in our animals, which is not clinically relevant as it does not reflect the human condition or current clinical management. Furthermore, we optimized our model to selectively direct therapy to the ipsilateral, stroke affected hemisphere. By developing an IA drug delivery model that mirrors clinical conditions, we are bridging the gap between basic stroke research and what is standard practice in acute ischemic stroke intervention. The IA model of drug delivery can target agents directly to the site of injury while blunting systemic effects, dose penetration issues, and administration delay that have plagued the intraperitoneal and oral drug administration models.
Keywords: Stroke, Recanalization, Intra-arterial, Endovascular thrombectomy
1. Introduction
Large vessel ischemic stroke, which affects the vital arteries of the brain, is a leading cause of morbidity and mortality in the United States. At present, the only FDA-approved pharmacotherapy for thrombolysis in acute ischemic stroke is administration of tissue plasminogen activator (tPA), which acts to dissolve the clot, allowing blood flow to resume. The therapeutic window for intravenous administration of tPA is 4.5 h after onset, and when combined with additional exclusion criteria, a large number of individuals are eliminated from receiving treatment. Results from a multicenter study between 2001 and 2004 evaluating the rate of tPA administration demonstrated an increase in tPA use from 14.0% to 37.5% (Lichtman et al., 2009). While these results are positive it shows a need for alternative therapies that can be administered when an individual is not eligible for tPA. One such alternative is intra-arterial (IA) endovascular thrombectomy or mechanical removal of a clot using a retrieval device threaded through the patient’s vasculature (Investigators, 2007; Saver et al., 2012). Both forms of thrombolysis have the potential to restore blood flow to the affected area but show poor correlations with clinical outcomes (Investigators, 2007; Broderick et al., 2013; Fargen et al., 2013). Currently there is no therapy, thrombolysis included, that provides direct neuroprotective or neuroreparative effects.
The practice of endovascular thrombectomy utilized by a neurointerventionalist to remove a clot begins with the advancing of a catheter within the femoral artery in the leg. From there the catheter is threaded through the arterial vasculature of the body until it reaches the common carotid artery (CCA) which leads to the occluded middle cerebral artery (MCA) via the internal carotid artery (ICA). Navigating the catheter into the occluded internal carotid, basilar, or middle cerebral arteries provides access to not only remove the clot but to also deliver potential neuroprotective/neuroreparative compounds. Such a stroke treatment paradigm has many potential advantages over systemic routes of administration including better affected brain penetration/targeting and less systemic side-effects.
There has been a failure to bring neuroprotective agents successfully to the bedside in the treatment of acute ischemic stroke. The cause is multifactorial, but is best summarized as a failure of translation from the bench to clinic. Our approach to this topic was to retro-engineer a mouse model to mirror the clinical condition of intra-arterial (IA) thrombectomy with the opportunity for direct selective IA pharmacotherapy immediately following recanalization. In our method, a catheter is advanced through the vasculature of the mouse neck into the ICA so the potential drug delivery can be selective. Our model is different in that we initiate a stroke, recanalize and then inject IA therapeutics without leaving an indwelling microport or catheter (Chen et al., 2009; Van Winkle et al., 2013). Direct IA administration of neuroprotective agents represents a novel method of drug delivery for acute stroke. Coupled with recanalization and restoration of blood flow, this method mimics clinical practice.
Furthermore, because our mouse model is based on the clinical treatment of stroke, it was also essential to verify that our model could be effectively utilized to deliver compounds directly and specifically to the site of ischemia. The efficacy of selective delivery of potential pharmacotherapies is dependent upon optimized flow rate and injection volume. The study of flow rate and injection volume through carbon black injection allowed us to demonstrate that a compound administered in such a fashion could reach the affected area and its injection rate and volume could be optimized for our model. Through this retro-engineered model, flow rate and injection volume study we hope to overcome many of the hurdles that have long plagued potential pharmacologic agents in acute ischemic stroke.
2. Materials and methods
2.1. Nylon suture, metal wire, micro-angio tubing and syringe preparation
Two different sized sutures were used: one 2-0 nylon monofilament suture cut 2 cm in length for occlusion of the CCA and three 6-0 nylon braided silk sutures cut 1 cm in length for permanent occlusion of the external carotid artery (ECA) and securing of the micro-angio tubing. Metal wire (Small Parts, Logansport, IN) 0.0127 cm in diameter were cut to a length of 0.1–0.15 cm. Using micro forceps under a dissecting microscope, a 34 gauge needle (Hamilton Syringe Co., Reno, NV) was fitted with a 10 cm length of micro-angio tubing (MRE 010-Braintree Scientific, Braintree, MA), the combined needle and tubing were then attached to a 100 μl Hamilton Gas Tight syringe (Fig. 1C).
Fig. 1.
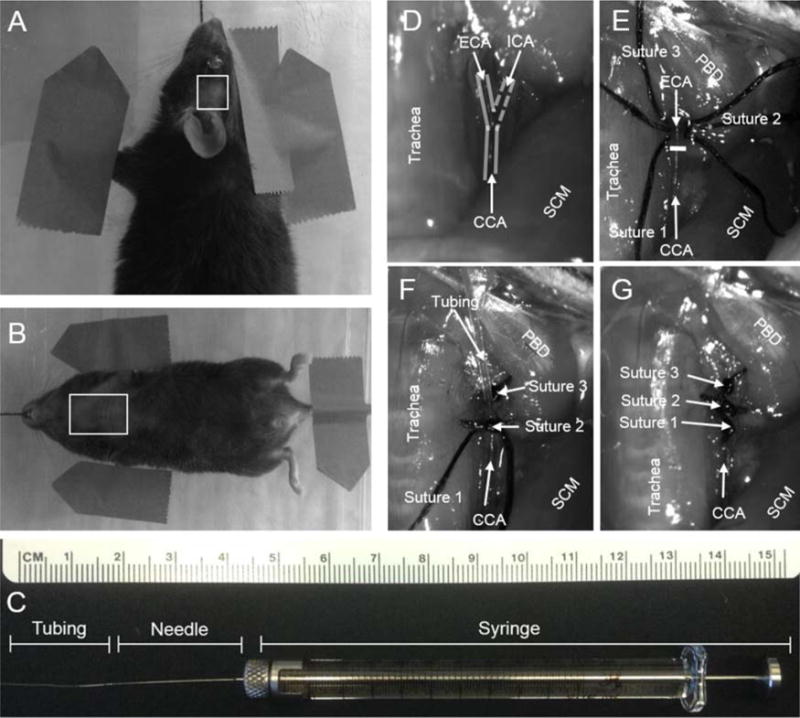
(A) Mouse in pronated position with box outlining shaved temporal region, temporal incision from lateral corner of left eye to medial region of left ear. (B) Mouse in supine position with box outlining shaved thoracic region. A midline incision is made from apex of ribcage to angle of mandible. (C) 100 μl syringe with 34 gauge and micro-angio tubing attached. (D) Outline of CCA/ECA/ICA with trachea and sternocleidomastoid (SCM) as landmarks. (E) Sutures 1–3 placement and white line marking CCA bifurcation, region framed by trachea, SCM and posterior belly of the digastric (PBD). (F) Micro-angio tubing inserted into ECA toward the CCA bifurcation and secured with suture 2. (G) ECA suture ligated.
2.2. Animal preparation for surgery
In accordance with University of Kentucky guidelines, 3 month old C57/Bl6 (Jackson Laboratory) mice were anesthetized via intraperitoneal injection with a combination Ketamine/Xylazine mixture (1:1.33) in a saline solution using a weight based dosing scale. The mice were shaved on the left temporal region of the head from the lateral corner of the left eye to the medial region of the left ear with the resultant shaved region being 0.75 cm by 0.75 cm (Fig. 1A). The shaved area of the cervical and thoracic region started at the angle of the mandible and extended to the apex of the ribcage with a width spanning from forelimb axillary region to the opposite forelimb axillary region (Fig. 1B). The shaved areas were cleaned 3 times with alcohol prep pads followed by a cotton tipped applicator moistened with betadine.
2.3. Surgical preparation
Once the mouse was fully anesthetized it was placed on a heated (to control body temperature) elevated platform. With the mouse in the supine position, the head was secured using teeth restraints and the forelimbs and tail were secured to the platform using surgical tape (Fig. 1A and B).
2.4. MCAO stroke surgery
To induce focal cerebral ischemia, we used the previously described transient tandem ipsilateral CCA/MCA occlusion stroke model (MCAO) (Aronowski et al., 1999; Lee et al., 2011). With the mouse in the supine position a midline incision was made allowing us to isolate and elevate the CCA to place a 2-0 suture underneath. It is important to note that the vagus nerve runs parallel to the CCA and should be separated so that it is not clamped with the CCA. We then removed the surgical tape and rotated the mouse so that it was in a pronated position and re-secured. Another incision was made in the left temporal region for the lateral corner of the left eye to the medial region of the left ear exposing the temporal region. The temporalis muscle was reflected, the MCA verified and a small burr hole drilled to allow the metal wire to be placed under the MCA. The mouse was repositioned to the supine position for temporary clamping of the CCA, (the occlusion time of the CCA/MCA can be varied; our lab occludes for 60 min). Before the head was sutured, blood flow measurements using the Laser Doppler and Speckle were taken using the process detailed in the following section. All surgical sites were temporarily sutured with 4-0 pre-needled nylon suture.
2.5. Laser Doppler and Laser Speckle for blood flow measurement
To measure blood flow in the ipsilateral MCA, a Perimed Laser Doppler flow meter (Periflux System 5000, Perimed) probe was placed directly over the MCA touching the skull, a reading was taken pre-occlusion and the probe was placed in the same spot to take the post-occlusion measurements after insertion of the metal wire under the MCA to confirm a decrease in blood flow. The ipsilateral hemisphere total blood flow was measured using a Laser Speckle (Pericam PSI HR, Perimed) which was positioned 15 cm above the exposed skull of the mouse. The laser sighting system was employed to ensure consistent measurements, a permanent marker was used to make a landmark dot along the sagittal suture to ensure centering of the sighting system to confirm pre and post occlusion measurements were consistent. Post-occlusion measurements were taken at 5, 10 and 15 min post occlusion.
2.6. Placement of micro-angio tubing into the ECA
2.6.1. ECA suture placement
With the anesthetized mouse in the supine position the temporary sutures were removed and the previously isolated CCA was exposed, moving rostrally along the CCA (lateral to the trachea) to the bifurcation of the ECA and ICA. With the bifurcation located, it is next necessary to further expose the ECA and ICA by 0.50–0.75 cm using angled or straight fine tipped micro forceps for suture placement and clamp insertion (Fig. 1D). Using a pair of fine tipped curved forceps, the ECA was gently lifted allowing three 1.0 cm lengths of 6-0 suture to be threaded underneath, the sutures were transferred to the fine tipped curved forceps from a pair of fine straight tipped forceps. The sutures were then separated so that the first suture was at the bifurcation of the CCA, the second suture was placed midway between the bifurcation and the superior most exposed portion of the ECA and the third suture was placed at the superior most exposed area of the ECA near the muscle (Posterior Belly of the Digastric-PBD) (Fig. 1E). Once all the sutures were placed, the superior most suture was tied to permanently occlude the ECA. The superior thyroid artery should be identified, if it arises proximal to the distal permanent ECA suture (Fig. 1F), then a fourth suture should be threaded underneath to achieve permanent occlusion.
2.6.2. Nicking the ECA and micro-angio tubing placement
With the ECA (and superior thyroid) artery permanently occluded, a curved removable clamp (Micro Serrefines-Fine Science, Foster City, CA) was attached to the ICA forming a closed system between the clamped CCA, the suture ligated ECA and the clamped ICA. The ECA was then nicked just above the second suture with a pair of micro-scissors (Vannas Spring Scissors-Fine Science, Foster City, CA) to allow for the micro-angio tubing (MRE010 Braintree, Braintree, MA) attached to the Hamilton Syringe to be inserted using a pair of angled forceps (for easier tubing insertion it is recommended to cut the tip of the inserted tubing at a 45° angle) (Fig. 1F). A pair of angled micro forceps were used to grasp the tubing 0.5 cm from the tip and a pair of curved forceps to grasp the tied suture of the ECA, which helped prevent the ECA from rolling and gave better stability for tubing insertion. Once the tubing was inserted and the tip placed at the bifurcation of the CCA, the second suture was tied securing the tubing and forming a tight seal between the tubing and ECA vessel wall (Fig. 1I and G). Removal of the clamped ICA occurred after ensuring a tight seal around the suture tubing. Given the small blood volume of a mouse, care must be taken to minimize blood loss during these steps, as a significant blood loss could significantly reduce cerebral perfusion and confound our results of evaluating infarct volume of ischemic stroke in the brain and study outcomes.
2.6.3. CCA clamp and MCA metal wire removal
At the end of the predetermined occlusion time, the CCA clamp and the metal wire were removed with the mouse in the supine position to restore blood flow to the previously occluded area. To better visualize and remove the temporary sutures and MCA metal wire, the tape securing the left forelimb was removed and the body angled to the contralateral side.
2.6.4. Carbon black ink flow rate and injection volume
After a 5 min waiting period for reperfusion, the 100 μl Hamilton syringe with a 34 gauge needle and micro-angio tubing was loaded with a mixture of fountain inks (Pelikan-Fount India & Higgins Fountain Pen India) in a 1:9 ratio (Hasan et al., 2012). The syringe was attached to a syringe pump (BS-8000, Braintree Scientific, Braintree, MA) with flow rate settings of 1 μl, 2.5 μl, 5 μl and 10 μl per minute at volumes of 10 μl, 25 μl, 50 μl and 100 μl for each flow rate.
2.6.5. Removal of micro-angio tubing and permanent ECA occlusion
To remove the micro-angio tubing, the suture on the proximal ECA was first secured at the bifurcation of the CCA ensuring that the ECA was permanently occluded for the remainder of the experiment. With the first suture tied, the tubing was gently removed from the vessel with a pair of fine tipped forceps holding the second suture acting as an anchor to better assist in tubing removal. When the tubing was fully removed from the ECA, the suture was rechecked to ensure no blood leakage and the incision in the neck and head were permanently closed using pre-needled 4-0 nylon monofilament suture (Fig. 1G).
2.7. Reperfusion
Reperfusion of the MCA was confirmed using the Laser Doppler flow meter at 15 min post occlusion and Laser Speckle imaging at 5 min intervals starting at reperfusion to 15 min post occlusion.
2.8. Animal recovery
The mouse was allowed to recover in a heated recovery cage to regulate body temperature until it met or exceeded the guidelines set forth in our university approved IUCAC protocol. Once the mouse was fully recovered it was returned to its home cage with its littermates in standard laboratory housing at the University of Kentucky.
2.9. Stroke volume assessment
2.9.1. Euthanasia
On post stroke day 3 the mice were euthanized via cervical dislocation and decapitated for removal of brain (Lee et al., 2011).
2.9.2. Removal of brain
A midline incision was made using a pair of blunted scissors on the scalp of the decapitated head exposing the calvarium. Next a pair of fine tipped scissors were inserted at the base of the skull and used to cut the calvarium and thereby expose the brain. The brain was lifted from the skull base using a pair of blunted curved forceps and placed in a 1X PBS solution for a quick 10–15 s wash.
2.9.3. Brain slicing and staining
The removed brain was transferred to a brain mold and sliced into 2 mm sections with a surgical blade. The brain sections were then transferred to a petri dish and stained using a 1% solution of 2,3,5-triphenlytetrazolium chloride (TTC) for 10 min to visualize ischemic infarct (Lee et al., 2011).
2.9.4. Infarct measurement
TTC stained brain sections were placed horizontally from frontal to occipital in a 3 cm wide petri dish and were scanned using a HP Scanjet G4050. The scanned images were input into NIH Image J for infarct analysis (Lee et al., 2011).
3. Results
3.1. Surgical outcome
The animals tolerated the procedure well with surgical effects (lethargy, decreased grooming and eating) subsiding within 24 h and resumption of normal behavior and eating habits. There was a decrease in body weight over 2 days averaging 2–3 g (if stoke is carried out to post stroke day 7, weight is regained). The MCAO and IA injection model has a low mortality rate of less than 5% with death being attributed to ruptured MCA or CCA. Exclusions from the IA study occurred when the clamp perforated the CCA upon clamp removal or when the metal wire pierced the MCA as it was being inserted or removed from underneath the MCA. These animals were excluded based on failure to guarantee recanalization and targeted drug delivery.
3.2. Laser Speckle and Laser Doppler
Laser Speckle imaging (n = 7) showed a significant (P = 0.017) 25.26% decrease in hemisphere blood flow from baseline to occlusion and an increase of 5.23% from occlusion to 15 min reperfusion (Fig. 2A). Laser Doppler (n = 22) showed a significant (P < 0.001) 83.81% decrease in MCA flow from baseline to occlusion and an increase of 59.15% from occlusion to 15 min reperfusion when compared to baseline (Fig. 2B).
Fig. 2.
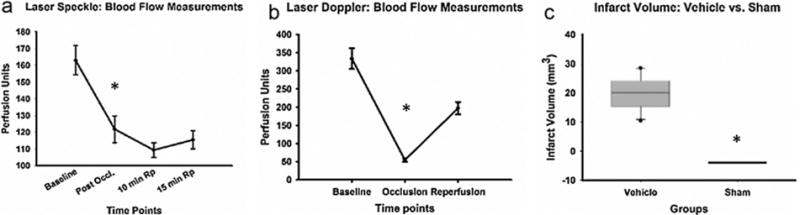
(A) Laser Speckle (n = 7): blood flow measurements of the ipsilateral hemisphere at time points baseline (before occlusion), post occlusion (immediately after occlusion), 10 min reperfusion (Rp) and 15 min Rp. * indicates P = 0.017. (B) Laser Doppler (n = 22) MCA blood flow measurement at time points baseline (before occlusion), occlusion (immediately after occlusion) and reperfusion (5 min reperfusion). * indicates P = <0.001. (C) Infarct volume measurements vehicle (n = 10) versus sham (n = 8) after MCAO surgery PSD 3. * indicates P = <0.001.
3.3. Infarct volume
Using NIH Image J software, the sectioned and stained brains had an average infarct measurement of 19.89 mm3, which was significantly (P = <0.001) different from the sham measurements (n = 10 and 8, respectively, Fig. 2C).
3.4. Flow rate and injection volume
Flow rate and injection volume studies (n = 3) were performed to optimize selectivity of infusion to the ipsilateral hemispheric cerebral circulation (Table 1). Injection volumes were 100 μl, 50 μl, 25 μl and 10 μl with flow rates of 1.0 μl, 2.5 μl, 5.0 μl, 7.5 μl and 10 μl/min. The 100 μl volume showed contralateral staining at all flow rates except the 1 μl/min flow rate which did not provide enough force to overcome the opposing blood pressure of the CCA to begin ink injection immediately. A time lapse of around 1 min was observed resulting in its exclusion from further testing. The 50 μl volume showed contralateral staining for the 5.0, 7.5 and 10 μl/min infusion rates but little contralateral staining at 2.5 μl/min. The 25 μl volume showed contralateral staining for the 5.0, 7.5 and 10 μl/min flow rates but little to no contralateral staining at 2.5 μl/min. The 10 μl volume at flow rate 2.5 μl/min showed no contralateral staining, leading us to determine the optimal flow rate as 2.5 μl/min at a volume between 10 and 25 μl. Cross sectional liver images of flow rate 2.5 μl/min at volumes listed above show carbon black in the 25 and 50 μl but not in the 10 μl (Fig. 3A–H). It is of note that with a different gauge needle and micro-angio tubing the opposing blood pressure of the CCA could be overcome by varying the above measurements (Lees et al., 2006; Savitz, 2007).
Table 1.
Injection rates at 10, 7.5, 5.0, 2.5 and 1.0 μl/min at Injection Volumes 100, 50, 25 and 10 μl. Ipsilateral and Contralateral staining of the Middle Cerebral Artery (MCA) at specific injection rates and volumes, no staining at the 1.0 μl/min injection rate(*) due to opposing pressure from the CCA. No further testing of injection rate 1.0 μl/min due to inability to overcome opposing pressure. Little to no contralateral staining was seen at the injection rate 2.5 μl/min at volumes 10 and 25 μl. Slight staining (**) was seen in the frontal region of both hemispheres due to mice having an azygous Anterior Cerebral Artery. Carbon black ink was found throughout the liver and vasculature at injection rates 10, 7.5 and 5.0 μl/min and injection volumes 100 and 50 μl with slight staining at 25 μl and no staining at 10 μl.
| Injection rate (μl/min) | Injection volume (μl) | MCA staining
|
Hemispheric staining
|
Systemic staining | ||
|---|---|---|---|---|---|---|
| Ipsilateral | Contralateral | Ipsilateral | Contralateral | Liver | ||
| 10.0 | 100 | Yes | Yes | Yes | Yes | Yes |
| 7.5 | 100 | Yes | Yes | Yes | Yes | Yes |
| 5.0 | 100 | Yes | Yes | Yes | Yes | Yes |
| 2.5 | 100 | Yes | Yes | Yes | Yes | Yes |
| 1.0* | 100 | N/A | N/A | N/A | N/A | N/A |
| 10.0 | 50 | Yes | Yes | Yes | Yes | Yes |
| 7.5 | 50 | Yes | Yes | Yes | Yes | Yes |
| 5.0 | 50 | Yes | Yes | Yes | Yes | Yes |
| 2.5 | 50 | Yes | Yes | Yes | Yes | Yes |
| 2.5 | 25 | Yes | Slight | Yes | Slight | Slight |
| 2.5 | 10 | Yes | No | Yes | Slight** | No |
Fig. 3.
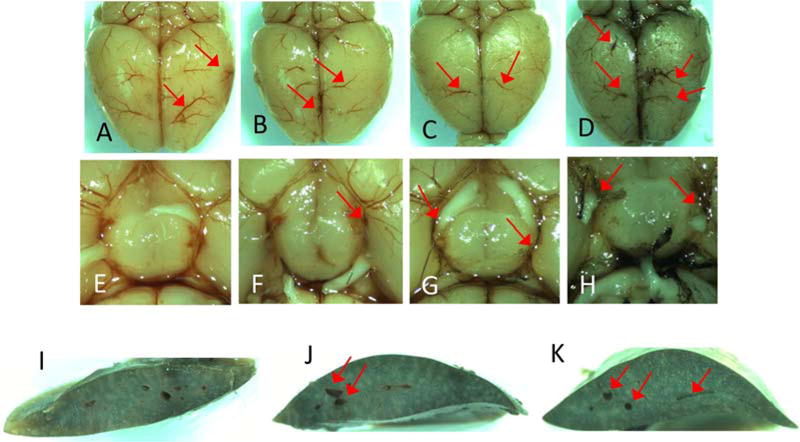
Red arrows signify carbon black ink staining in cerebral and liver vasculature. (A)–(D) Dorsal view of brain at injection rate 2.5 μL/min at volumes 10 μL, 25 μL, 50 μL and 100 μL. (E)–(H) Circle of Willis at injection rate 2.5 μL/min at volumes 10 μL, 25 μL, 50 μL and 100 μL. (I)–(K) Cross section of liver at injection rate 2.5 μL/min at volumes 10 μL, 25 μL and 50 μL. (For interpretation of the references to color in this text, the reader is referred to the web version of the article.)
4. Discussion
The advancement of neuroprotective pharmacotherapy for ischemic stroke has been complicated by failures in translation from the laboratory to the clinic. Many promising neuroprotective agents have failed in both basic research and clinical trials due to multiple factors, including failure to combine pharmacotherapy with vessel reperfusion, and failure to direct therapy to the affected tissue in a timely fashion. With our modified version of the MCAO model and potential for selective delivery of agents that could be neuroprotective, neuroreparative, barrier-protective, or vasculo-protective, each of these issues are addressed.
Imitating a thrombus in a large vessel occlusion by kinking the MCA and clamping the CCA mimics the large vessel occlusion that is observed clinically. Removing the MCA metal wire and CCA clamp restores blood flow to the occluded vessel as would be seen clinically with the mechanical retrieval of the thrombus. Rapid recanalization of an occluded vessel in acute ischemic stroke is paramount. Indeed, speed of recanalization is required by Comprehensive Stroke Centers to be reported as a ‘core measure’ to the Joint Commission (JCAHO 07-22-2014). Retro-engineering our mouse model from the clinical condition while incorporating flow rate and injection volume optimization allows us to mimic current neurosurgical practices. By bringing our mouse model one step closer to what is seen clinically through the addition of intra-arterial drug delivery, we are demonstrating current practices for stroke treatment. The flow rate and injection volume studies further mimic what is seen clinically with superselective drug delivery but through optimization of flow rate and injection volume we also demonstrated that potential therapeutic compounds could selectively reach the site of ischemia for maximal effect and potentially mitigate systemic side effects.
In this study, we employed an easily visible carbon black ink mixture to optimize volume and flow rates to maximally target ipsilateral stroke brain tissue. One potential limitation of this study is that this ink is more dense than typical pharmacologic agents in solution. While this may confound the applicability of rate/volume studies, lower density solutions would be expected to travel farther with a wider potential distribution (contralateral/systemic); thus, the validated 2.5 μl/min flow rate and 10–25 μl volume would serve as an absolute maximum using this technique. However, additional localization experiments for each target drug should be conducted.
5. Conclusion
By modifying an already well-established mouse stroke model and adapting it to mimic current clinical stroke treatment, we have developed a novel method of intra-arterial drug delivery for potential therapeutic compounds that may limit systemic side effects, enable early pharmacotherapy, and provide methodology for testing the role of acutely administered compounds for neuroprotection and neuro-repair in acute ischemic stroke.
highlights.
Demonstrate reliable and reproducible mouse stroke model similar to human condition.
Retro-engineered intra-arterial drug delivery model for mice from human condition.
Optimized drug injection volume and flow rate for intra-arterial delivery model.
Acknowledgments
We would like to thank Jill Roberts PhD for her guidance on the MCAO procedure, Leon de Hoog for his technical assistance with the Flow Rate and Injection Volume studies and Adam Bachstetter PhD for his insight. Funding was provided through the Department of Neurosurgery at the University of Kentucky Medical Center.
References
- Aronowski J, Cho KH, Strong R, Grotta JC. Neurofilament proteolysis after focal ischemia; when do cells die after experimental stroke? J Cereb Blood Flow Metab: Off J Int Soc Cereb Blood Flow Metab. 1999;19:652–60. doi: 10.1097/00004647-199906000-00008. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Palesch YY, Demchuk AM, Yeatts SD, Khatri P, Hill MD, et al. Interventional management of stroke IIII (2013) endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med. 2013;368:893–903. doi: 10.1056/NEJMoa1214300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Swartz KR, Toborek M. Vessel microport technique for applications in cerebrovascular research. J Neurosci Res. 2009;87:1718–27. doi: 10.1002/jnr.21973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fargen KM, Meyers PM, Khatri P, Mocco J. Improvements in recanalization with modern stroke therapy: a review of prospective ischemic stroke trials during the last two decades. J Neurointerv Surg. 2013;5:506–11. doi: 10.1136/neurintsurg-2012-010541. [DOI] [PubMed] [Google Scholar]
- Hasan MR, Herz J, Hermann DM, Doeppner TR. Visualization of macroscopic cerebral vessel anatomy – a new and reliable technique in mice. J Neurosci Methods. 2012;204:249–53. doi: 10.1016/j.jneumeth.2011.11.024. [DOI] [PubMed] [Google Scholar]
- Investigators IIT. The interventional management of stroke (IMS) II study. Stroke: J Cereb Circ. 2007;38:2127–35. doi: 10.1161/STROKEAHA.107.483131. [DOI] [PubMed] [Google Scholar]
- Lee B, Clarke D, Al Ahmad A, Kahle M, Parham C, Auckland L, et al. Perlecan domain V is neuroprotective and proangiogenic following ischemic stroke in rodents. J Clin Invest. 2011;121:3005–23. doi: 10.1172/JCI46358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lees KR, Zivin JA, Ashwood T, Davalos A, Davis SM, Diener HC, et al. Stroke-acute ischemic NXYTTI (2006) NXY-059 for acute ischemic stroke. N Engl J Med. 2006;354:588–600. doi: 10.1056/NEJMoa052980. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Watanabe E, Allen NB, Jones SB, Dostal J, Goldstein LB. Hospital arrival time and intravenous t-PA use in US Academic Medical Centers, 2001–2004. Stroke: J Cereb Circ. 2009;40:3845–50. doi: 10.1161/STROKEAHA.109.562660. [DOI] [PubMed] [Google Scholar]
- Saver JL, Jahan R, Levy EI, Jovin TG, Baxter B, Nogueira RG, et al. Solitaire flow restoration device versus the Merci Retriever in patients with acute ischaemic stroke (SWIFT): a randomised, parallel-group, non-inferiority trial. Lancet. 2012;380:1241–9. doi: 10.1016/S0140-6736(12)61384-1. [DOI] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–5. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Van Winkle JA, Chen B, Lei IF, Pereira B, Rajput PS, Lyden PD. Concurrent middle cerebral artery occlusion and intra-arterial drug infusion via ipsilateral common carotid artery catheter in the rat. J Neurosci Methods. 2013;213:63–9. doi: 10.1016/j.jneumeth.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


