Abstract
Alcohol use disorder (AUD) and major depressive disorder (MDD) are often comorbid. It is not understood how genetic risk factors for these disorders relate to each other over time and to what degree they are stable. Age-dependent characteristics of the disorders indicate that different genetic factors could be relevant at different stages of life, and MDD may become increasingly correlated with AUD over time. DSM-IV diagnoses of AUD and MDD were assessed by interviews of 2,801 young adult twins between 1999 and 2004 (T1) and 2,284 of the same twins between 2010 and 2011 (T2). Stability, change, and covariation were investigated in longitudinal biometric models. New genetic factors explained 56.4% of the genetic variance in AUD at T2. For MDD, there was full overlap between genetic influences at T1 and T2. Genetic risk factors for MDD were related to AUD, but their association with AUD did not increase over time. Thus, genetic risk factors for AUD, but not MDD, vary with age, suggesting that AUD has age-dependent heritable etiologies. Molecular genetic studies of AUD may therefore benefit from stratifying by age. The new genetic factors in AUD were not related to MDD. Environmental influences on the two disorders were correlated in middle, but not in young adulthood. The environmental components for AUD correlated over time (r=.27), but not for MDD. Environmental influences on AUD can have long-lasting effects, and the effects of preventive efforts may be enduring. Environment influences seem to be largely transient.
Keywords: Alcohol use disorder, major depressive disorder, twins, longitudinal, genetic innovation
Alcohol use disorders (AUD) and major depressive disorder (MDD) are among the most disabling mental disorders worldwide (World Health Organization, 2013), and co-occur more often than expected by chance (Hasin, Stinson, Ogburn, & Grant, 2007). AUD and MDD aggregate in families and twin studies indicate moderate heritability (Sullivan, Neale, & Kendler, 2000; Verhulst, Neale, & Kendler, 2015) that increases with age (Bergen, Gardner, & Kendler, 2007; Kendler, Schmitt, Aggen, & Prescott, 2008; van Beek et al., 2012). Despite the high comorbidity, it is not understood how genetic risk factors for AUD and MDD relate to each other over time and to what degree the etiological influences on these disorders are stable.
Candidate gene and genome-wide association studies (GWAS) have identified genetic variations that account for a small part of the phenotypic variance in AUD (Edwards et al., 2015), primarily variants related to physiological mechanisms such as alcohol metabolism (Hart & Kranzler, 2015). Similarly, molecular genetic studies have accounted for a modest proportion of the genetic risk of MDD (Geschwind & Flint, 2015; Ripke et al., 2013). This indicates that single commonly varying alleles are unlikely to have strong influences on the risk of complex disorders like AUD and MDD (Flint & Kendler, 2014). Rather, many genetic variants probably influence these disorders by exercising small effects on physiology, behavior, or exposure to different environments (Gage, Smith, Ware, Flint, & Munafò, 2016). For instance, AUD could be associated with any genetic variations associated with drinking behavior. Because there are many different reasons for drinking or for being depressed (Kendler, Gardner, & Prescott, 2011, 2002), the effect of various genetic risk factors could vary between life phases. Age-dependent genetic risks factors that emerge across the life-span are known as “genetic innovations” (e.g. Edwards, Sihvola, et al., 2011; van Beek et al., 2012). These genetic effects do not influence earlier observations of the phenotype. A common assumption in genome-wide association studies is that genetic risk factors are homogenous across all age groups (Pandey et al., 2012). If new genetic factors emerge across the lifespan, this can introduce genetic heterogeneity in age-varying GWAS samples, thereby diluting the associations between genetic variants and mental disorders.
The prevalence of AUD is highest in the early 20s (Hicks & Zucker, 2014), and previous studies have found that AUD is characterized by somewhat different traits in different phases of life (Babor, 1996). Cloninger has proposed two types of AUD (Cloninger, Sigvardsson, & Bohman, 1996), where early onset AUD (type II) is mainly related to externalizing traits and late onset AUD (type I) is more strongly related to anxiousness and harm avoidance. In line with this, internalizing disorders (anxiety and depression) increase the risk of later heavy drinking and AUD (Hicks & Zucker, 2014; Hussong, Jones, Stein, Baucom, & Boeding, 2011; Kuo, Gardner, Kendler, & Prescott, 2006). Other classifications also indicate age-dependent comorbidity profiles (Moss, Chen, & Yi, 2007). Because externalizing and internalizing traits are inherited partly independently of each other (Kendler, Aggen, et al., 2011), AUD in in young and middle adulthood could be influenced by partially different genetic factors.
Previous studies have found genetic innovation in alcohol use during adolescence (Edwards, Sihvola, et al., 2011), from adolescence to young adulthood (Edwards & Kendler, 2013), and in other externalizing phenotypes (Kendler et al., 2015). The end of young adulthood often coincides with a maturation of drinking patterns, with fewer drinks per occasion (Arria et al., 2016). It is not known whether the development in genetic effects ends with adolescence or continues in adulthood. Genetic innovation in alcohol use after the early 20s has been investigated in only one study. The results indicated stable genetic effects from age 15 to 32 on self-reported alcohol abuse (van Beek et al., 2012). We are not aware of any studies on genetic innovation in AUD after young adulthood.
MDD is less age-dependent than AUD (Ferrari et al., 2013), but the risk of MDD is still highest among young individuals (Kessler et al., 2003). As for AUD, the risk factors for depression could vary between life phases (Korten, Comijs, Lamers, & Penninx, 2012), suggesting that different genetic factors could be relevant at different stages of life. Age-dependent genetic influences on internalizing phenotypes have been reported in childhood and in adolescence (Edwards, Larsson, Lichtenstein, & Kendler, 2011; Kendler, Gardner, & Lichtenstein, 2008; Nivard et al., 2015; Waszczuk, Zavos, Gregory, & Eley, 2016). Few studies have directly investigated age-dependent genetic risks in MDD, but there are indications of small genetic innovations in internalizing disorders in adulthood, and those disorders largely share genetic risk with MDD (Cerdá, Sagdeo, Johnson, & Galea, 2010). For example, Gillespie et al. (2004) found genetic innovations among women, but not men, in mid-life and late life, although most of the relevant genetic factors were present at age 20. In line with this, age-dependent genetic influences has been found among individuals aged 75–80 (Petkus, Gatz, Reynolds, Kremen, & Wetherell, 2016). On the other hand, Nivard et al. (2015) found stable genetic influences on symptoms of anxiety and depression between the ages 18 and 63. One study found completely overlapping genetic risk factors for MDD assessed at two waves, but the follow-up time was only 1.5 years (Kendler, Neale, Kessler, Heath, & Eaves, 1993). Besides this, we are not aware of any study on genetic innovation in MDD during adulthood or genetic innovation in multiple comorbid disorders simultaneously, despite considerable evidence of overlap between genetic risk factors for AUD and MDD (Edwards et al., 2012; Kendler, Heath, Neale, Kessler, & Eaves, 1993; Tambs, Harris, & Magnus, 1997). If etiological factors for MDD become more important for AUD during adulthood, in line with an internalizing pathway to AUD, one should expect to find genetic innovation in AUD and that genetic risk of MDD in young adulthood accounts for parts of these new influences on AUD. This would have implications for both molecular genetic studies and clinical work.
In the present study, we investigate the longitudinal course of AUD and MDD in a population based twin sample with two waves of diagnostic interviews ten years apart in adulthood. Our aim was to investigate genetic and environmental sources of stability and change in AUD and MDD, including genetic innovations and longitudinal effects across disorders.
Methods
Sample
The sample for the current study originated from the Norwegian Institute of Public Health Twin Panel, which is thoroughly described elsewhere (Nilsen et al., 2013). Twins were identified through the national Medical Birth Registry, established January 1, 1967. Between 1999 and 2004 (T1), DSM-IV psychiatric disorders were assessed at interview in 2,801 twins born between 1967 and 1979 (44.4% response rate). Between 2010 and 2011 (T2) a second wave of interviews were conducted. Among the respondents at T1, 17 had withdrawn their consent, 14 had unknown addresses, and 12 had died. After two written reminders and a final telephone contact to non-responders, 2,284 twins (82.8% of the eligible) were interviewed at T2. The mean age was 28.1 years (SD=3.9; range 19–35) at T1, and 37.8 years (SD=3.8; range 31–44) at T2.
Zygosity was determined by a combination of questionnaire items and genotyping. The misclassification rate was estimated to be less than 1.0%, which is unlikely to bias results (Neale, 2003). At T1, there were 220 monozygotic (MZ) male pairs, 118 dizygotic (DZ) male pairs, 449 MZ female pairs, 263 DZ female pairs, 341 DZ opposite sex pairs, and 19 single twins. At T2, there were 154 MZ male pairs, 76 DZ male pairs, 358 MZ female pairs, 180 DZ female pairs, 219 DZ opposite sex pairs, and 310 single twins.
Ethics
The Regional Committees for Medical and Health Research Ethics granted ethical approval, and all participants provided written informed consent.
Measures
At T1 and T2, DSM-IV diagnoses of AUD and MDD were assessed using the computerized Composite International Diagnostic Interview (CIDI) (Wittchen & Pfister, 1997) in Norwegian translation. This is a structured diagnostic interview developed by the World Health Organization for the assessment of the DSM-IV and ICD-10 diagnoses. The interview has previously shown good test–retest and interrater reliability (Wittchen, Lachner, Wunderlich, & Pfister, 1998). The interviewers were mainly senior clinical psychology graduate students, experienced psychiatric nurses, and experienced clinical psychologists. At T1, most interviews were conducted face-to-face. For practical reasons 231 (8.3%) were done by telephone. At T2, all interviews were conducted by telephone. Different interviewers assessed each twin in a pair. The recency of the symptoms was reported. At T1 and T2, we used AUD and MDD that had occurred within the last 5 years. Preliminary analyses indicated that the results were similar if we used longer time-spans, but that statistical power was low if we used shorter time-spans. The CIDI interview assigns subthreshold diagnoses in cases where all but one of the criteria for the full disorder are met. In order to increase statistical power, subthreshold scores were included as an intermediate category.
Statistical analyses
Associations between the disorders were first estimated as polychoric correlations, crude and adjusted for sex. We then estimated a so-called cross-lagged model that was adjusted for sex and standardized. This model shows the phenotypic stability of the disorders and the cross-sectional and longitudinal associations between them.
We then conducted multivariate biometric Structural Equation Modeling (SEM) (Neale & Maes, 2004). In the classical twin model (Jinks & Fulker, 1970), differences in traits are assumed to arise from three latent sources: Additive genetic factors (A) which MZ twins share 100% and DZ twins 50%; common environmental factors (C), which contribute equally to twin similarity among MZs and DZs; and individual-specific environmental factors (E) which contribute to differences between the twins and include measurement error. We used a liability-threshold model, assuming that ordered categories, such as diagnoses, are imprecise indicators of unobserved, normally distributed liabilities and (Falconer, 1965).
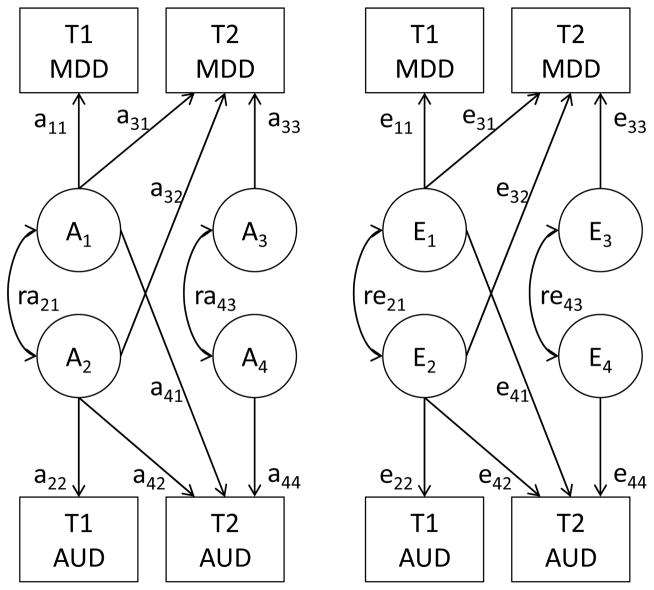
When the variables are ordered by time, the Cholesky decomposition can be interpreted as a longitudinal model (Loehlin, 1996). Each genetic and environmental component can influence observations later in time, but not earlier. Genetic innovation is in our case defined as genetic influences on T2 that were not present at T1. The Cholesky decomposition is not suited for directly interpreting results for variables that are not temporally sorted, but it can be algebraically transformed to provide correlations between the genetic and environmental components of the variables. Since cross-sectionally measured AUD and MDD do not have an a priori order, we used a combined model in which we specified correlations between variables observed simultaneously, while retaining directional paths from earlier to later observations (Figure 1). This model is algebraically equivalent to the corresponding Cholesky variant of the model.
Figure 1.
The combined longitudinal model with four variables combines two Cholesky decompositions and expresses relationships between variables measured simultaneously as correlations. C factors have the same structure and are included in the full model. The model can be extended to k variables with the number of parameters per latent source of variance being .
Initially, we tested whether different thresholds were needed for men and women. We then tested whether we could detect qualitative or quantitative sex differences in the etiology of AUD and MDD (Neale, Røysamb, & Jacobson, 2006) and the contributions of A and C variance components. In the combined model (Figure 1), both paths a41 and a44 indicate genetic influences on AUD at T2 that are not shared with AUD at T1. The former path indicates an increasing importance of genetic effects shared with MDD at T1, whereas the latter is a new T2 influence independent of both T1 AUD and T1 MDD. Paths a32 and a33 have the same function for MDD. We therefore tested the overall presence of genetic innovation at T2 in two bivariate Cholesky models, one for AUD and one for MDD at T1 and T2.
Phenotypic causality refers to direct effects between variables rather than between their biometric components. In SEM models, phenotypic causality implies associations between the outcome and all influences on the exposure. Thus, for example, if both genetic and environmental factors affect MDD at T1, and MDD at T1 causally affects AUD at T2, the finding of both genetic and environmental associations between these variables would be in line with phenotypic causality. If only a genetic association is detected, that would be inconsistent with phenotypic causality. We tested in the combined model whether genetic and environmental factors for MDD had any effect on later AUD besides the initial correlation, by dropping parameter a41, e41, and both. We then tested genetic and environmental effects from AUD to MDD by dropping parameters a32, e32, and both. Residual genetic influences in AUD at T2, that is, genetic innovations that could not be accounted for by MDD, were tested by setting a44 to zero, and similarly a33 for MDD. The stability of AUD across time was decomposed into genetic (a42) and environmental (e42) sources, and similarly for MDD (a31 and e31). The correlation between the initial factors is captured by ra21 and re21, whereas the correlation between the T2 factors is expressed by ra43 and re43. The contributions of these paths were tested.
The more restricted variants of the model were tested by removing specific paths from the model by fixing their parameters to zero. The models were fitted using Full Information Maximum Likelihood (FIML) as estimation procedure to raw data in OpenMx 2.6.9 (Neale et al., 2016) within R 3.3.1. The raw data method utilizes all data, from both complete and incomplete pairs. The difference in −2 times log likelihood (Δ-2LL) is asymptotically χ2 distributed, which allows testing for significant differences in χ2 for nested submodels. In addition, we used the Akaike’s Information Criterion (AIC; Akaike, 1987) and sample size-adjusted Bayesian Information Criterion (sBIC; Sclove, 1987) as indices of parsimony. Models with low AIC and sBIC values are preferred.
Results
Descriptive results
Table 1 shows the prevalences of full and subthreshold MDD and AUD with recency the last five years among men and women at T1 and T2. More women than men satisfied criteria for MDD and subthreshold MDD at T1 and at T2. The opposite pattern was found for AUD and subthreshold AUD, which were most common among men. MDD had similar prevalence at T1 and T2, whereas AUD, and especially subthreshold AUD, were more common at T1. The average age of the most recent episode of AUD was 25.3 years at T1 and 35.1 years at T2. For MDD, the average age of the most recent episode was 25.8 years at T1 and 35.3 years at T2.
Table 1.
Prevalence of MDD and AUD among men and women, last 5 years.
| Total | Men | Women | |
|---|---|---|---|
| MDD, wave 1 | |||
| No MDD | 1937 (69.4%) | 765 (75.1%) | 1172 (66.1%) |
| Subthreshold | 543 (19.4%) | 171 (16.8%) | 372 (21.0%) |
| MDD | 312 (11.2%) | 83 (8.1%) | 229 (12.9%) |
| MDD, wave 2 | |||
| No MDD | 1684 (73.8%) | 645 (80.4%) | 1039 (70.2%) |
| Subthreshold | 310 (13.6%) | 97 (12.1%) | 213 (14.4%) |
| MDD | 288 (12.6%) | 60 (7.5%) | 228 (15.4%) |
| AUD, wave 1 | |||
| No AUD | 2143 (76.8%) | 684 (67.1%) | 1459 (82.3%) |
| Subthreshold | 456 (16.3%) | 208 (20.4%) | 248 (14.0%) |
| AUD | 193 (6.9%) | 127 (12.5%) | 66 (3.7%) |
| AUD, wave 2 | |||
| No AUD | 2018 (88.4%) | 652 (81.3%) | 1366 (92.2%) |
| Subthreshold | 156 (6.8%) | 80 (10.0%) | 76 (5.1%) |
| AUD | 109 (4.8%) | 70 (8.7%) | 39 (2.6%) |
Table 2 shows the stability of MDD and AUD and the co-occurrence of the disorders. Among individuals with MDD at T1, approximately one in three had MDD at T2, and one in two if we also consider subthreshold MDD. Most individuals with MDD at T2 did not have MDD at T1. For AUD at T1, one in four cases satisfied diagnostic criteria at T2, and one in eight had subthreshold symptoms. AUD and MDD were associated at both T1 and T2, but sex differences suppress these associations when not adjusted for.
Table 2.
Occurrence, stability, and co-occurrence of MDD and AUD, last 5 years. Percentages by row.
| Stability of MDD | |||
|---|---|---|---|
|
| |||
| T2 no MDD | T2 subth. MDD | T2 MDD | |
| T1 no MDD | 1265 (79.6%) | 178 (11.2%) | 146 (9.2%) |
| T1 subth. MDD | 285 (65.5%) | 88 (20.2%) | 62 (14.3%) |
| T1 MDD | 131 (51.6%) | 44 (17.3%) | 79 (31.1%) |
|
| |||
| Stability of AUD | |||
|
| |||
| T2 no AUD | T2 subth. AUD | T2 AUD | |
|
| |||
| T1 no AUD | 1636 (93.1%) | 83 (4.7%) | 38 (2.2%) |
| T1 subth. AUD | 284 (76.8%) | 54 (14.6%) | 32 (8.6%) |
| T1 AUD | 95 (62.5%) | 19 (12.5%) | 38 (25.0%) |
|
| |||
| Co-occurrence at T1 | |||
|
| |||
| T1 no AUD | T1 subth. AUD | T1 AUD | |
|
| |||
| T1 no MDD | 1514 (78.2%) | 321 (16.6%) | 102 (5.3%) |
| T1 subth. MDD | 410 (75.5%) | 79 (14.5%) | 54 (9.9%) |
| T1 MDD | 219 (70.2%) | 56 (17.9%) | 37 (11.9%) |
|
| |||
| Co-occurrence at T2 | |||
|
| |||
| T2 no AUD | T2 subth. AUD | T2 AUD | |
|
| |||
| T2 no MDD | 1513 (89.8%) | 105 (6.2%) | 66 (3.9%) |
| T2 subth. MDD | 264 (85.2%) | 29 (9.4%) | 17 (5.5%) |
| T2 MDD | 240 (83.3%) | 22 (7.6%) | 26 (9.0%) |
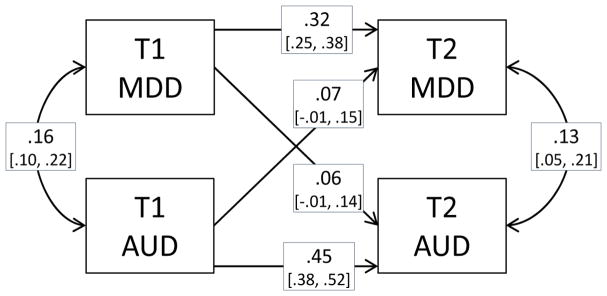
Phenotypic polychoric correlations between MDD and AUD at T1 and T2 are presented in Table 3. Adjusting for sex increased the correlation between AUD and MDD and reduced estimates of stability. AUD was more stable than MDD, and the association between the disorders seemed to be stronger at T2 than at T1. Figure 2 presents cross-lagged associations between the two disorders. The paths from one disorder at T1 to the other disorder at T2 were both positive, but not significantly different from zero.
Table 3.
Phenotypic correlations between MDD and AUD at T1 and T2 with 95% confidence intervals. Adjusted for sex below the diagonal, unadjusted above.
| T1 MDD | T1 AUD | T2 MDD | T2 AUD | |
|---|---|---|---|---|
| T1 MDD | 1 | .12 [.06, .18] | .34 [.28, .40] | .11 [.03, .20] |
| T1 AUD | .16 [.10, .22] | 1 | .07 [.00, .14] | .50 [.44, .57] |
| T2 MDD | .33 [.27, .39] | .12 [.05, .19] | 1 | .16 [.08, .24] |
| T2 AUD | .16 [.08, .24] | .46 [.39, .53] | .22 [.13, .30] | 1 |
Figure 2.
Standardized cross-lagged phenotypic associations between MDD and AUD including subthreshold disorders at T1 and T2 with 95% confidence intervals. Adjusted for sex.
Biometric models
Sex differences and variance components
Table 4 shows the cross-twin correlations for the disorders among MZs and DZs and in the five zygosity groups. The correlations were higher within MZ pairs than within DZ pairs. A breakdown into five zygosity groups yielded large confidence intervals and some inconsistent estimates, although MZ correlations tended to be stronger than DZ correlations. The biometric models were fitted to raw data that included all associations between co-twins and between traits. The mean and median cross-twin cross-trait correlations were .20 and .21 for MZs and .12 and .10 for DZ. Initial analyses indicated that the thresholds could not be set to equal for men and women (Δ7minus;2LL = 119.96; Δdf = 8; p < .001). This reflects different prevalences among men and women. The results of the biometric model fitting are shown in Table 5. We first fitted a four-variate model that included qualitative and quantitative sex differences as well as all three sources of covariance (ACE; model 1). In step 1, we tested qualitative and quantitative sex differences in etiological factors for AUD and MDD, and whether A and C factors contributed to explaining familial aggregation. Several of the models adequately represented the data without significant deterioration in −2LL. The AIC and sBIC provided positive to strong evidence favoring model 7 with no sex differences and no contributions from shared environment. Preliminary analyses with lifetime variables also favored this model. The subsequent models are therefore AE models with no sex differences other than in prevalence.
Table 4.
Cross-twin correlations for MDD and AUD at T1 and T2 in five zygosity groups.
| T1 MDD | T1 AUD | T2 MDD | T2 AUD | |
|---|---|---|---|---|
| MZ | .30 [.18, .40] | .47 [.35, .57] | .33 [.29, .46] | .51 [.34, .66] |
| DZ | .12 [.01, .23] | .28 [.16, .40] | .09 [−.07, .25] | .35 [.14, .54] |
|
| ||||
| MZ male | .39 [.19, .59] | .48 [.32, .64] | .26 [−.03, .55] | .50 [.25, .75] |
| DZ male | .01 [−.33, .36] | .14 [−.13, .41] | −.27 [−.79, .25] | .54 [.23, .85] |
| MZ female | .27 [.14, .40] | .44 [.30, .58] | .35 [.21, .50] | .51 [.30, .72] |
| DZ female | .38 [.23, .54] | .42 [.21, .64] | .26 [.04, .48] | .46 [.04, .87] |
| DZ opposite | −.11 [−.28, .05] | .27 [.10, .44] | −.03 [−.27, .21] | .20 [−.11, .50] |
Note: MZ and DZ correlations are adjusted for different prevalences among men and women.
Table 5.
Results of biometric model fitting of AUD and MDD at T1 and T2.
| # | Model | ep | AIC | sBIC | Δ−2LL | Δdf | p |
|---|---|---|---|---|---|---|---|
| Step 1 | |||||||
| 1 | Qualitative sex differences, ACE | 86 | −7073.16 | 13455.27 | - | - | - |
| 2 | Qualitative sex differences, AE | 66 | −7084.35 | 13390.59 | 16.814 | 14 | .266 |
| 3 | Quantitative sex differences, ACE | 76 | −7092.32 | 13415.36 | 0.844 | 10 | 1.000 |
| 4 | Quantitative sex differences, AE | 56 | −7092.91 | 13361.28 | 28.25 | 24 | .250 |
| 5 | Quantitative sex differences, CE | 56 | −7083.62 | 13370.57 | 37.54 | 24 | .039 |
| 6 | No sex differences, ACE | 46 | −7080.27 | 13321.17 | 28.89 | 18 | .050 |
| 7 | No sex differences, AE | 36 | −7098.88 | 13281.81 | 30.28 | 28 | .350 |
| 8 | No sex differences, CE | 36 | −7077.79 | 13302.90 | 51.36 | 28 | .005 |
| Step 2 | |||||||
| 9 | No A from MDD to AUD (a41=0) | 35 | −7100.63 | 13277.99 | 0.26 | 1 | .611 |
| 10 | No E from MDD to AUD (e41=0) | 35 | −7100.88 | 13277.73 | 0.00 | 1 | .975 |
| 11 | No A or E from MDD to AUD | 34 | −7102.49 | 13274.05 | 0.39 | 2 | .822 |
| 12 | No A from AUD to MDD (a32=0) | 35 | −7098.13 | 13280.49 | 2.76 | 1 | .097 |
| 13 | No E from AUD to MDD (e32=0) | 35 | −7100.47 | 13278.14 | 0.41 | 1 | .521 |
| 14 | No A or E from AUD to MDD | 34 | −7100.06 | 13276.48 | 2.82 | 2 | .244 |
| 15 | No prospective AE across disorders* | 32 | −7103.26 | 13269.13 | 3.63 | 4 | .459 |
| Step 3 | |||||||
| 16 | No new A in AUD (a44=0, ra43=0) | 30 | −7096.18 | 13272.07 | 11.08 | 2 | .003 |
| 17 | No new A in MDD (a33=0, ra43=0)** | 30 | −7107.01 | 13261.23 | 0.24 | 2 | .885 |
| Step 4 | |||||||
| 18 | No genetic correlation at T1 (ra21=0) | 29 | −7084.22 | 13281.95 | 24.79 | 1 | <.001 |
| 19 | No environmental correlation at T1 (re21=0) | 29 | −7108.81 | 13257.35 | 0.20 | 1 | .656 |
| 20 | No new environmental correlation (re43=0) | 29 | −7102.46 | 13263.71 | 6.56 | 1 | .010 |
| 21 | No environmental stability in MDD (e31=0) | 29 | −7108.75 | 13257.42 | 0.26 | 1 | .608 |
| 22 | No environmental stability in AUD (e42=0) | 29 | −7100.81 | 13265.35 | 8.20 | 1 | .004 |
| 23 | Stable environmental correlation (re21=re43) | 29 | −7105.54 | 13260.63 | 3.48 | 1 | .062 |
| 24 | re21=0 and e31=0** | 28 | −7110.42 | 13253.67 | 0.59 | 2 | .744 |
Notes: ep = estimated parameters;
best fitting model in step;
overall best fitting model.
Models are compared to the best fitting model in the previous step.
Separate analyses of AUD and MDD
In the combined model, there are two possible sources of change in genetic influences on AUD at T2: Those present in MDD at T1 (a41) and those that are new at T2 (a44). For MDD, the corresponding paths are a32 and a33. Before proceeding, we therefore tested the overall degree of genetic stability in each disorder over time in separate models. In a bivariate Cholesky decomposition, AUD at T2 was influenced by genetic factors that did not influence AUD at T1 (.57, 95% CI [.39, .69]). Removing this factor led to a deterioration in model fit (Δ−2LL = 11.90; Δdf = 1; ΔAIC = 9.90; p = .001). In bivariate analyses of MDD, there were no significant genetic influences specific to T2 (Δ−2LL = 0.00; Δdf = 1; ΔAIC = 2.00; p = 1.000).
Combined analysis of alcohol and depression
Returning to the combined model, we tested in step 2 whether genetic or environmental factors for MDD at T1 had any influences on AUD at T2 beyond the initial correlation. If genetic factors for T1 MDD became increasingly relevant for AUD, path a41 would exert an influence on AUD at T2. However, dropping this parameter (model 9), estimated at −.06 in model 7, improved the model. Likewise, dropping the corresponding environmental path (e41; model 10), estimated at .00, improved the fit. Dropping both paths from T1 MDD to T2 AUD improved the fit (model 11). We also tested effects from T1 AUD to T2 MDD. Dropping a32, estimated at −.14, led to a slight increase in AIC, but to a lower sBIC and no significant deterioration in −2LL (model 12). Dropping path e32, estimated at .04, improved all fit indices (model 13), as did dropping both paths from T1 AUD to T2 MDD (model 14). The best model in this step included neither genetic nor environmental prospective paths across disorders (model 15).
In step 3 we tested whether there were new genetic effects in AUD or MDD at T2 not shared with the other disorder at T1. T2-specific genetic influences could not be removed for AUD (a44; model 16), but could be removed for MDD (a33; model 17). Thus, including genetic factors for MDD did not explain genetic innovation in AUD. In step 4, we tested whether remaining parameters could be dropped from the model. The environmental correlation between MDD and AUD at T1 was estimated at .02, and the environmental path from MDD at T1 to MDD at T2 (e31) was estimated at .02. Model fit improved when these parameters were dropped. Setting the environmental correlation to be stable led to worse fit than dropping the correlation at T1 and keeping it at T2 (model 23 vs. 19).
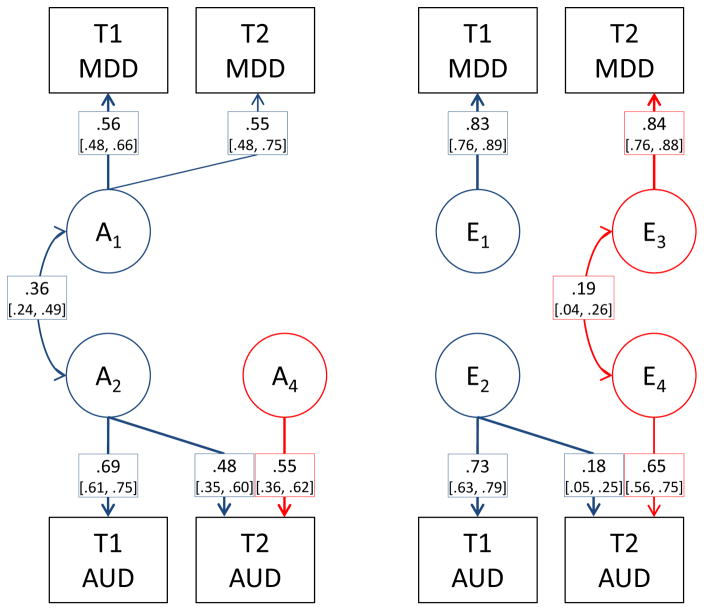
The best fitting model is shown in Figure 3 with path estimates and confidence intervals. Genetic innovations (A4) explained 56.4% of the genetic variance in AUD at T2. These numbers correspond to a correlation of .66 (95% CI [.56, .85]) between the genetic influences on AUD at T1 and T2. The estimated heritability of AUD was .47 (95% CI [.37, .57]) at T1 and .53 (95% CI [.38, .69]) at T2. For MDD, there was full overlap between genetic influences at T1 and T2. The heritability of MDD was .31 (95% CI [.26, .37]) at T1 and .30 (95% CI [.20, .41]) at T2. The environmental influences on AUD correlated .27 (95% CI [.08, .42]) across time. The environmental factors for MDD were uncorrelated, indicating that all the environmental variance in MDD was new at T2. The initial correlation between genetic risk factors for AUD and MDD was r = .36 (95% CI [.24, .49]), with no initial correlation in E-factors. At T2 the correlation in E-factors for AUD and MDD was r = .18 (95% CI [.05, .31]).
Figure 3.
Best fitting model with genetic (left) and individual-specific environmental (right) influences on AUD and MDD.
Discussion
The most important findings in this population-based longitudinal twin study are the evidence of the genetic innovation in AUD, but not in MDD. Genetic risk factors for MDD were related to AUD, but the genetic association did not increase over time. The environmental influences were more stable for AUD than for MDD, and the environmental associations between the disorders increased over time.
We have demonstrated genetic innovation in AUD after young adulthood. The finding of different genetic risk factors for AUD at different phases of life is in line with theoretical expectations of two distinct, partially heritable age-dependent etiologies (Cloninger et al., 1996). In line with previous studies (Hicks & Zucker, 2014), we found that the prevalence of AUD and subthreshold AUD dropped between young and middle adulthood. This indicates that changes in drinking patterns took place. The end of young adulthood is characterized by transitions in several life domains, including employment and family life. At baseline, the average age of the most recent AUD episode was 25 years, whereas it was 35 years at follow-up. Several important changes in life take place between these ages. The average person becomes a parent and gets married (Statistics Norway, 2015, 2016a), and there are changes in level of employment (Statistics Norway, 2016b). This shift can lead to a different set of social and psychological risk factors becoming relevant for AUD. If genetic factors influence some of these life-events and risk factors, that will be reflected by new genetic influences on AUD. Results from previous studies on alcohol use in adolescence and young adulthood are conflicting with regard to presence (Edwards & Kendler, 2013; Edwards, Sihvola, et al., 2011) or absence (Rose, Dick, Viken, & Kaprio, 2001; van Beek et al., 2012) of genetic innovation. If the genetic innovation in AUD is related to maturation between young and middle adulthood, the older age of the participants may explain why the genetic innovation was more prominent in the present study. In addition, the aforementioned studies used short self-reported measures of symptoms or consumption, whereas we used diagnostic interviews.
The changing genetic influences on AUD detected in the current study indicate that a single set of genetic factors relevant at all ages cannot be found. This can have implications for molecular genetic studies. GWAS are based on the assumption that genetic variations have the same additive effects in all age groups. Violation of this assumption will dilute associations between specific loci and AUD in age-heterogeneous samples and may be a partial explanation of the “missing heritability”. Samples therefore need to be age stratified, or interactions with age explicitly studied. The heritability of AUD was estimated slightly higher at T2 (53%) than at T1 (47%). Increasing heritability of externalizing behaviors are in line with previous studies (Bergen et al., 2007; Kendler, Schmitt, et al., 2008).
We did not find genetic innovation in MDD. Although no previous studies have investigated genetic innovation in diagnostically assessed MDD over similar periods of time, the findings are primarily in line with previous studies of depressive symptoms. Nivard et al. (2015) found genetic innovations on symptoms of anxiety and depression in childhood and adolescence, but not between age 18 and 63. Gillespie et al. (2004) found that genetic influences on depression in the early 20s accounted for most of the risk throughout life, although there were small genetic innovations among women. We cannot exclude the possibility that such effects were not detected in the present study due to lower statistical power. In any case, both the present and previous studies indicate that the genetic influences on MDD are largely stable after adolescence.
Genetic risk factors for AUD were shared with MDD, which is in line with previous twin studies (Kendler, Heath, et al., 1993; Tambs et al., 1997) and molecular genetic studies (Edwards et al., 2012). We found no indications that the genetic risk factors for MDD in young adulthood indicated an increased risk for AUD in middle adulthood, beyond the initial genetic association between the two disorders. Nor did we find new genetic factors that influenced both disorders. Thus, the genetic innovations in AUD were not related to MDD. Our results fit with the hypothesized existence of several classes of alcohol abusers, but are only partially in line with Cloninger’s classification, in which AUD at higher ages should be more strongly characterized by anxious and avoidant traits and less heritable (Cloninger et al., 1996). Previous studies have described internalizing pathways to AUD (Hussong et al., 2011; Kuo et al., 2006), and our results indicate that those are driven by an increasing environmental correlation. Although genetic factors for MDD did not explain the genetic innovations in AUD, the genetic factors associated with AUD in middle adulthood may have manifested as other measurable phenotypes earlier in life. We have described a method to identify components of genetic innovations by applying data on other phenotypes. Identifying these phenotypes is important in order to understand how AUD develops, and thus making it possible to design rational interventions. Possible candidates to investigate in future research include personality, psychopathology, education, and measures of social success.
Genetic and environment factors contributed approximately equally to the stability of AUD from T1 to T2. Thus, etiological factors for T1 AUD were still relevant at T2, regardless of whether they were genetic or environmental. This is in line with a causal interpretation where AUD in young adulthood leads to AUD in middle adulthood. Thus, young adulthood could be a period where enduring alcohol use patterns are shaped. Preventing AUD in this life-phase can be particularly important as it could have long-lasting effects. One of the most common finding in behavioral genetic studies is that age-to-age stability is primarily due to genetic factors (Plomin, DeFries, Knopik, & Neiderhiser, 2016). The present study suggests that AUD is an exception to that rule, possibly reflecting the “vicious cycle” of addiction. Given that measurement error is included in the E component in twin studies, the true environmental correlations may be even larger. For MDD, the stability was attributable to genetic factors. Despite a genetic risk persistent across life-phases, the environmental triggers of MDD were largely transient and not of importance ten years later. The lack of environmental stability indicates that psychological strains, negative life-events, and other risk factors for depression that occur in young adulthood or earlier do not have a significant lasting impact. Thus, work to prevent MDD may need to be more continuous and ongoing. This may be different in other age groups, as a previous twin study indicates that the stability of environmental factors for depression increases with age (Kendler, Eaves, et al., 2011).
In young adulthood, the overlap between AUD and MDD was entirely due to shared genetic risk factors. The environmental association increased over time and was significant in middle adulthood. The environmental association could indicate that some environmental risk factors could lead to both AUD and MDD, but mainly at higher ages. Examples could be divorce, unemployment (Pirkola et al., 2005), or other stressful life-events that may result in gradual changes towards depression-prone personality (Rosenström et al., 2015) and self-medication with alcohol. Alternatively, the environmental correlation could be due to causal effects between AUD and MDD in either direction. Such possible causal effects must be of relatively short duration; otherwise we would have detected effects between the disorders across waves of measurement. A longitudinal study found that the association was best explained by a causal model in which AUD led to MDD, but not vice versa (Fergusson, Boden, & Horwood, 2009). However, their model relied on the assumption that the comorbidity between AUD and MDD was caused by a stable process. We have shown that the relationship is not stable. A possible, although speculative explanation for the lack of environmental association in young adulthood is that drinking is normative in environments typically encountered in young adulthood, and therefore less reflective of a tendency to psychopathology. Future studies could benefit from studying causal mechanisms between AUD and MDD in longitudinal biometric models with shorter follow-up time and while accounting for measurement error.
Limitations
Despite strengths such as repeated diagnostic interviews in a population-based genetically informative sample, the following limitations must be considered: First, we could not distinguish between alcohol abuse and alcohol dependence due to relatively low prevalence. However, the criteria for these disorders form a continuum (Saha, Chou, & Grant, 2006), and the merging of the two diagnoses as AUD is in line with the DSM-5 (American Psychiatric Association, 2013). Second, due to the sample size, we had low power to detect sex differences and influences from shared environment, which have been observed in previous studies (Prescott, Aggen, & Kendler, 2000; Verhulst et al., 2015). A power analysis suggest that a sample of our size is too low to detect moderate sex differences in continuous data (Verhulst, 2017). The cross-twin correlations were in line with multiple solutions for sex differences, and the model fitting favored the more parsimonious model. However, we cannot conclude on the presence or absence of sex differences. Third, there was high attrition from the Medical Birth Registry until T2. Women were more likely to participate than men were. Despite relatively equal birth rates, the net sample included almost twice as many women as men. The overrepresentation of women can have biased the results. If there are sex differences in etiology, the results are more representative for women than for men. Monozygotic twins were more likely to participate than dizygotic twins were. This can lead to lower statistical power. Attrition of 17% of the sample from T1 to T2 also leads to lower power, but is unlikely to influence parameter estimates (Reichborn-Kjennerud et al., 2015). The use of FIML ensures that all available data are being utilized, and can sometimes correct for bias even when data are not missing completely at random (Enders & Bandalos, 2001). Fourth, we studied relatively young Norwegian adult twins. Whereas twins are largely representative for singletons with regard to personality and other psychological traits (Johnson, Krueger, Bouchard, & McGue, 2002), the generalization may be limited to individuals of similar age and ethnic background as the participants.
Conclusion
Genetic risk factors for AUD, but not MDD, depend on age, as new genetic risk factors for AUD emerge in middle adulthood. If replicated, these findings could have consequences for molecular genetic studies. There was initially not a correlation between environmental factors leading to AUD and MDD, but a correlation became apparent with higher age, suggesting either shared environmental triggers or causal effects between the disorders. The genetic innovation in AUD was not related to genetic factors influencing MDD. Environmental triggers of AUD seem to have long-lasting effects whereas MDD is primarily stable due to the constant genetic risk factors. Thus, efforts to prevent AUD in young adulthood may have long-lasting effects.
General scientific summary.
Alcohol use disorder (AUD) and major depressive disorder (MDD) are influenced by genetic risk factors, and these may vary between life phases. This study suggests that the genetic risk factors for MDD are stable from young to middle adulthood, but vary across time for AUD. Environmental triggers of MDD were transient, but environmental triggers of AUD had lasting effects, suggesting that preventing AUD in young adulthood may be particularly important. The environmental aspects of AUD and MDD became more strongly associated with higher age, suggesting that some environments can lead to both disorders or that the disorders develop together.
Acknowledgments
This project was supported by Research Council of Norway (RCN) (grant 240061). Previous collections and analyses of twin data from this project were in part supported by the National Institutes of Health (grant MH-068643) and grants from the RCN, the Norwegian Foundation for Health and Rehabilitation, and the Norwegian Council for Mental Health. The results and ideas in this manuscript have not previously been disseminated at conferences, websites, or elsewhere.
References
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–332. http://dx.doi.org/10.1007/BF02294359. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5. Washington, DC: American Psychiatric Publishing; 2013. http://dx.doi.org/10.1176/appi.books.9780890425596.744053. [Google Scholar]
- Arria AM, Caldeira KM, Allen HK, Vincent KB, Bugbee BA, O’Grady KE. Drinking like an adult? Trajectories of alcohol use patterns before and after college graduation. Alcoholism: Clinical and Experimental Research. 2016;40:583–590. doi: 10.1111/acer.12973. http://dx.doi.org/10.1111/acer.12973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babor TF. Classification of alcoholics: Typology theories from the 19th century to the present. Alcohol Health & Research World. 1996;20:6–14. [PMC free article] [PubMed] [Google Scholar]
- Bergen SE, Gardner CO, Kendler KS. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: a meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. http://dx.doi.org/10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Cerdá M, Sagdeo A, Johnson J, Galea S. Genetic and environmental influences on psychiatric comorbidity: A systematic review. Journal of Affective Disorders. 2010;126:14–38. doi: 10.1016/j.jad.2009.11.006. http://dx.doi.org/10.1016/j.jad.2009.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cloninger CR, Sigvardsson S, Bohman M. Type I and type II alcoholism: An Update. Alcohol Health & Research World. 1996;20:18–23. [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Aliev F, Bierut LJ, Bucholz KK, Edenberg H, Hesselbrock V, … Dick DM. Genome-wide association study of comorbid depressive syndrome and alcohol dependence. Psychiatric Genetics. 2012;22:31–41. doi: 10.1097/YPG.0b013e32834acd07. http://dx.doi.org/10.1097/YPG.0b013e32834acd07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Aliev F, Wolen AR, Salvatore JE, Gardner CO, McMahon G, … Kendler KS. Genomic influences on alcohol problems in a population-based sample of young adults. Addiction. 2015;110:461–470. doi: 10.1111/add.12822. http://dx.doi.org/10.1111/add.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Kendler KS. Alcohol consumption in men is influenced by qualitatively different genetic factors in adolescence and adulthood. Psychological Medicine. 2013;43:1857–1868. doi: 10.1017/S0033291712002917. http://dx.doi.org/10.1017/S0033291712002917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Larsson H, Lichtenstein P, Kendler KS. Early environmental influences contribute to covariation between internalizing symptoms and alcohol intoxication frequency across adolescence. Addictive Behaviors. 2011;36:175–182. doi: 10.1016/j.addbeh.2010.10.001. http://dx.doi.org/10.1016/j.addbeh.2010.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Sihvola E, Korhonen T, Pulkkinen L, Moilanen I, Kaprio J, … Dick DM. Depressive symptoms and alcohol use are genetically and environmentally correlated across adolescence. Behavior Genetics. 2011;41:476–487. doi: 10.1007/s10519-010-9400-y. http://dx.doi.org/10.1007/s10519-010-9400-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling. 2001;8:430–457. http://dx.doi.org/10.1207/S15328007SEM0803_5. [PubMed] [Google Scholar]
- Falconer DS. The inheritance of liability to certain diseases, estimated from the incidence among relatives. Annals of Human Genetics. 1965;29:51–76. http://dx.doi.org/10.1111/j.1469-1809.1965.tb00500.x. [Google Scholar]
- Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Archives of General Psychiatry. 2009;66:260–266. doi: 10.1001/archgenpsychiatry.2008.543. http://dx.doi.org/10.1001/archgenpsychiatry.2008.543. [DOI] [PubMed] [Google Scholar]
- Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJL, … Whiteford HA. Burden of depressive disorders by country, sex, age, and year: Findings from the Global Burden of Disease Study 2010. PLoS Medicine. 2013;10:e1001547. doi: 10.1371/journal.pmed.1001547. http://dx.doi.org/10.1371/journal.pmed.1001547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flint J, Kendler KS. The genetics of major depression. Neuron. 2014;81:484–503. doi: 10.1016/j.neuron.2014.01.027. http://dx.doi.org/10.1016/j.neuron.2014.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage SH, Davey Smith G, Ware JJ, Flint J, Munafò MR. G = E: What GWAS can tell us about the environment. PLOS Genetics. 2016;12:e1005765. doi: 10.1371/journal.pgen.1005765. http://dx.doi.org/10.1371/journal.pgen.1005765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Flint J. Genetics and genomics of psychiatric disease. Science. 2015;349:1489–1494. doi: 10.1126/science.aaa8954. http://dx.doi.org/10.1126/science.aaa8954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Kirk KM, Evans DM, Heath AC, Hickie IB, Martin NNG. Do the genetic or environmental determinants of anxiety and depression change with age? A longitudinal study of australian twins. Twin Research and Human Genetics. 2004;7:39–53. doi: 10.1375/13690520460741435. http://dx.doi.org/10.1375/twin.7.1.39. [DOI] [PubMed] [Google Scholar]
- Hart AB, Kranzler HR. Alcohol dependence genetics: lessons learned from genome-wide association studies (GWAS) and post-GWAS analyses. Alcoholism: Clinical and Experimental Research. 2015;39:1312–1327. doi: 10.1111/acer.12792. http://dx.doi.org/10.1111/acer.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–42. doi: 10.1001/archpsyc.64.7.830. http://dx.doi.org/10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Zucker RA. Alcoholism: A life span and course. In: Lewis M, Rudolph KD, editors. Handbook of Developmental Psychopathology, third edition. New York, NY: Springer; 2014. pp. 583–599. http://dx.doi.org/10.1007/978-1-4614-9608-3. [Google Scholar]
- Hussong AM, Jones DJ, Stein GL, Baucom DH, Boeding S. An internalizing pathway to alcohol use and disorder. Psychology of Addictive Behaviors. 2011;25:390–404. doi: 10.1037/a0024519. http://dx.doi.org/10.1037/a0024519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jinks JL, Fulker DW. Comparison of the biometrical genetical, MAVA, and classical approaches to the analysis of the human behavior. Psychological Bulletin. 1970;73:311–349. doi: 10.1037/h0029135. http://dx.doi.org/10.1037/h0029135. [DOI] [PubMed] [Google Scholar]
- Johnson W, Krueger RF, Bouchard TJ, McGue M. The personalities of twins: just ordinary folks. Twin Research. 2002;5:125–31. doi: 10.1375/1369052022992. http://dx.doi.org/10.1375/1369052022992. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Aggen SH, Knudsen GP, Røysamb E, Neale MC, Reichborn-Kjennerud T. The structure of genetic and environmental risk factors for syndromal and subsyndromal common DSM-IV axis I and all axis II disorders. The American Journal of Psychiatry. 2011;168:29–39. doi: 10.1176/appi.ajp.2010.10030340. http://dx.doi.org/10.1176/appi.ajp.2010.10030340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ, Loken EK, Pedersen NL, Middeldorp CM, Reynolds C, … Gardner CO. The impact of environmental experiences on symptoms of anxiety and depression across the life span. Psychological Science. 2011;22:1343–52. doi: 10.1177/0956797611417255. http://dx.doi.org/10.1177/0956797611417255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Lichtenstein P. A developmental twin study of symptoms of anxiety and depression: Evidence for genetic innovation and attenuation. Psychological Medicine. 2008;38:1567. doi: 10.1017/S003329170800384X. http://dx.doi.org/10.1017/S003329170800384X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for major depression in women. The American Journal of Psychiatry. 2002;159:1133–1145. doi: 10.1176/appi.ajp.159.7.1133. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Prescott CA. Toward a comprehensive developmental model for alcohol use disorders in men. Twin Research and Human Genetics. 2011;14:1–15. doi: 10.1375/twin.14.1.1. http://dx.doi.org/10.1375/twin.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Heath AC, Neale MC, Kessler RC, Eaves LJ. Alcoholism and major depression in women. Archives of General Psychiatry. 1993;50:690. doi: 10.1001/archpsyc.1993.01820210024003. http://dx.doi.org/10.1001/archpsyc.1993.01820210024003. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Lönn SL, Maes HH, Morris NA, Lichtenstein P, Sundquist J, Sundquist K. A national Swedish longitudinal twin-sibling study of criminal convictions from adolescence through early adulthood. Twin Research and Human Genetics. 2015;18:227–233. doi: 10.1017/thg.2015.25. http://dx.doi.org/10.1017/thg.2015.25. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Kessler RC, Heath AC, Eaves LJ. A longitudinal twin study of 1-year prevalence of major depression in women. Archives of General Psychiatry. 1993;50:843–852. doi: 10.1001/archpsyc.1993.01820230009001. http://dx.doi.org/10.1001/archpsyc.1993.01820230009001. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–82. doi: 10.1001/archpsyc.65.6.674. http://dx.doi.org/10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, … Wang PS. The epidemiology of major depressive disorder. JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. http://dx.doi.org/10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- Korten NCM, Comijs HC, Lamers F, Penninx BWJH. Early and late onset depression in young and middle aged adults: Differential symptomatology, characteristics and risk factors? Journal of Affective Disorders. 2012;138:259–267. doi: 10.1016/j.jad.2012.01.042. http://dx.doi.org/10.1016/j.jad.2012.01.042. [DOI] [PubMed] [Google Scholar]
- Kuo PH, Gardner CO, Kendler KS, Prescott CA. The temporal relationship of the onsets of alcohol dependence and major depression: Using a genetically informative study design. Psychological Medicine. 2006;36:1153–1162. doi: 10.1017/S0033291706007860. http://dx.doi.org/10.1017/S0033291706007860. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26:65–69. [Google Scholar]
- Moss HB, Chen CM, Yi H. Subtypes of alcohol dependence in a nationally representative sample. Drug and Alcohol Dependence. 2007;91:149–158. doi: 10.1016/j.drugalcdep.2007.05.016. http://dx.doi.org/10.1016/j.drugalcdep.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC. A finite mixture distribution model for data collected from twins. Twin Research. 2003;6:235–239. doi: 10.1375/136905203765693898. http://dx.doi.org/10.1375/136905203765693898. [DOI] [PubMed] [Google Scholar]
- Neale MC, Hunter MD, Pritikin JN, Zahery M, Brick TR, Kirkpatrick RM, … Boker SM. OpenMx 2.0: Extended structural equation and statistical modeling. Psychometrika. 2016;81:535–549. doi: 10.1007/s11336-014-9435-8. http://dx.doi.org/10.1007/s11336-014-9435-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neale MC, Maes HHM. Methodology for Genetic Studies of Twins and Families. Dordrecht, The Netherlands: Kluwer; 2004. [Google Scholar]
- Neale MC, Røysamb E, Jacobson K. Multivariate genetic analysis of sex limitation and G × E interaction. Twin Research and Human Genetics. 2006;9:481–489. doi: 10.1375/183242706778024937. http://dx.doi.org/10.1375/183242706778024937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsen TS, Knudsen GP, Gervin K, Brandt I, Røysamb E, Tambs K, … Harris JR. The Norwegian twin registry from a public health perspective: A research update. Twin Research and Human Genetics. 2013;16:285–295. doi: 10.1017/thg.2012.117. http://dx.doi.org/10.1017/thg.2012.117. [DOI] [PubMed] [Google Scholar]
- Nivard MG, Dolan CV, Kendler KS, Kan KJ, Willemsen G, van Beijsterveldt CEM, … Boomsma DI. Stability in symptoms of anxiety and depression as a function of genotype and environment: a longitudinal twin study from ages 3 to 63 years. Psychological Medicine. 2015;45:1039–49. doi: 10.1017/S003329171400213X. http://dx.doi.org/10.1017/S003329171400213X. [DOI] [PubMed] [Google Scholar]
- Pandey A, Davis NA, White BC, Pajewski NM, Savitz J, Drevets WC, McKinney BA. Epistasis network centrality analysis yields pathway replication across two GWAS cohorts for bipolar disorder. Translational Psychiatry. 2012;2:e154. doi: 10.1038/tp.2012.80. http://dx.doi.org/10.1038/tp.2012.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Gatz M, Reynolds CA, Kremen WS, Wetherell JL. Stability of genetic and environmental contributions to anxiety symptoms in older adulthood. Behavior Genetics. 2016;46:492–505. doi: 10.1007/s10519-015-9772-0. http://dx.doi.org/10.1007/s10519-015-9772-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkola SP, Isometsä E, Suvisaari J, Aro H, Joukamaa M, Poikolainen K, … Lönnqvist JK. DSM-IV mood-, anxiety- and alcohol use disorders and their comorbidity in the Finnish general population--results from the Health 2000 Study. Social Psychiatry and Psychiatric Epidemiology. 2005;40:1–10. doi: 10.1007/s00127-005-0848-7. http://dx.doi.org/10.1007/s00127-005-0848-7. [DOI] [PubMed] [Google Scholar]
- Plomin R, DeFries JC, Knopik VS, Neiderhiser JM. Top 10 Replicated Findings From Behavioral Genetics. Perspectives on Psychological Science. 2016;11:3–23. doi: 10.1177/1745691615617439. http://dx.doi.org/10.1177/1745691615617439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott CA, Aggen SH, Kendler KS. Sex-specific genetic influences on the comorbidity of alcoholism and major depression in a population-based sample of US twins. Archives of General Psychiatry. 2000;57:803–811. doi: 10.1001/archpsyc.57.8.803. http://dx.doi.org/10.1001/archpsyc.57.8.803. [DOI] [PubMed] [Google Scholar]
- Reichborn-Kjennerud T, Czajkowski N, Ystrøm E, Ørstavik R, Aggen SH, Tambs K, … Kendler KS. A longitudinal twin study of borderline and antisocial personality disorder traits in early to middle adulthood. Psychological Medicine. 2015;45:3121–3131. doi: 10.1017/S0033291715001117. http://dx.doi.org/10.1017/S0033291715001117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ripke S, Wray NR, Lewis CM, Hamilton SP, Weissman MM, Breen G … Major Depressive Disorder Working Group of the Psychiatric GWAS Consortium. A mega-analysis of genome-wide association studies for major depressive disorder. Molecular Psychiatry. 2013;18:497–511. doi: 10.1038/mp.2012.21. http://dx.doi.org/10.1038/mp.2012.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: regional residency moderates longitudinal influences on alcohol use. Alcoholism, Clinical and Experimental Research. 2001;25:637–643. http://dx.doi.org/10.1111/j.1530-0277.2001.tb02261.x. [PubMed] [Google Scholar]
- Rosenström T, Jylhä P, Pulkki-Råback L, Holma M, Raitakari OT, Isometsä E, Keltikangas-Järvinen L. Long-term personality changes and predictive adaptive responses after depressive episodes. Evolution and Human Behavior. 2015;36:337–344. http://dx.doi.org/10.1016/j.evolhumbehav.2015.01.005. [Google Scholar]
- Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychological Medicine. 2006;36:931–941. doi: 10.1017/S003329170600746X. http://dx.doi.org/10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- Sclove SL. Application of model-selection criteria to some problems in multivariate analysis. Psychometrika. 1987;52:333–343. http://dx.doi.org/10.1007/BF02294360. [Google Scholar]
- Statistics Norway. Marriage and divorce, 2014. Ekteskap og skilsmisser, 2014. 2015 Retrieved January 10, 2017, from https://www.ssb.no/befolkning/statistikker/ekteskap/aar-detaljerte-tal/2015-08-20.
- Statistics Norway. Births, 2015. Fødte, 2015. 2016a Retrieved January 10, 2017, from https://www.ssb.no/befolkning/statistikker/fodte.
- Statistics Norway. Workforce survey. Arbeidskraftundersøkelsen, 3. kvartal 2016. 2016b Retrieved January 10, 2017, from https://www.ssb.no/arbeid-og-lonn/statistikker/aku.
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: Review and meta-analysis. American Journal of Psychiatry. 2000;157:1552–1562. doi: 10.1176/appi.ajp.157.10.1552. http://dx.doi.org/10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Tambs K, Harris JR, Magnus P. Genetic and environmental contributions to the correlation between alcohol consumption and symptoms of anxiety and depression. Results from a bivariate analysis of Norwegian twin data. Behavior Genetics. 1997;27:241–250. doi: 10.1023/a:1025662114352. [DOI] [PubMed] [Google Scholar]
- van Beek JHDA, Kendler KS, de Moor MHM, Geels LM, Bartels M, Vink JM, … Boomsma DI. Stable genetic effects on symptoms of alcohol abuse and dependence from adolescence into early adulthood. Behavior Genetics. 2012;42:40–56. doi: 10.1007/s10519-011-9488-8. http://dx.doi.org/10.1007/s10519-011-9488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B. A Power Calculator for the Classical Twin Design. Behavior Genetics. 2017;47:255. doi: 10.1007/s10519-016-9828-9. http://dx.doi.org/10.1007/s10519-016-9828-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhulst B, Neale MC, Kendler KS. The heritability of alcohol use disorders: a meta-analysis of twin and adoption studies. Psychological Medicine. 2015;45:1061–1072. doi: 10.1017/S0033291714002165. http://dx.doi.org/10.1017/S0033291714002165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waszczuk MA, Zavos HMS, Gregory AM, Eley TC. The stability and change of etiological influences on depression, anxiety symptoms and their co-occurrence across adolescence and young adulthood. Psychological Medicine. 2016;46:161–75. doi: 10.1017/S0033291715001634. http://dx.doi.org/10.1017/S0033291715001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen HU, Lachner G, Wunderlich U, Pfister H. Test-retest reliability of the computerized DSM-IV version of the Munich-Composite International Diagnostic Interview (M-CIDI) Social Psychiatry and Psychiatric Epidemiology. 1998;33:568–578. doi: 10.1007/s001270050095. [DOI] [PubMed] [Google Scholar]
- Wittchen H-U, Pfister H. DIA-X Interviews (M-CIDI) Frankfurt, Germany: Swets & Zeitlinger; 1997. [Google Scholar]
- World Health Organization. WHO methods and data sources for global burden of disease estimates 2000–2011. Geneva: World Health Organization; 2013. Retrieved from www.who.int/healthinfo/global_burden_disease/estimates/en/index2.html. [Google Scholar]