Abstract
Because electronic cigarettes (“e-cigs”) containing nicotine may relieve smoking abstinence symptoms similar to NRT medication, we used within-subjects designs to test these effects with a first-generation e-cig in non-quitting and quitting smokers. In Study 1, 28 non-treatment seeking smokers abstained overnight prior to each of 3 sessions. MNWS withdrawal (and craving item) relief was assessed following 4 exposures (each 10 puffs) over 2 hrs to e-cigs that either did (36 mg/ml) or did not (i.e. placebo, 0 mg/ml) contain nicotine, or after no e-cig. Relief was greater after nicotine vs. placebo e-cig (p<.05), but not after placebo vs. no e-cig, showing relief was due to nicotine per se and not simple e-cig use behavior. Using a cross-over design in Study 2, smokers preparing to quit soon engaged in two experimental 4-day quit periods on separate weeks. In weeks 1 and 3, all received a nicotine or placebo e-cigarette on Mon to use ad lib while trying to abstain from smoking on Tues–Fri. (Week 2 involved resumption of ad lib smoking.) MNWS and QSU craving were assessed at daily visits following 24-hr abstinence. Of 17 enrolled, 12 quit for ≥ 24 hr at least once, allowing test of relief due to e-cig use on quit days. Withdrawal and craving were reduced due to nicotine vs placebo e-cig use (both p<.05). In sum, compared to placebo e-cigs, nicotine e-cigs can relieve smoking abstinence symptoms, perhaps in a manner similar to FDA-approved NRT products, although much more research with larger samples is needed.
Keywords: nicotine, e-cigarettes, tobacco abstinence, withdrawal, craving
Relevant to addressing the clinical utility of electronic cigarettes (“e-cigarettes”) in smoking cessation are their effects on abstinence symptom relief, which is an active focus of developing novel treatments for drug dependence (O’Brien, 2005). Although not approved by FDA for use to quit smoking, e-cigarettes containing nicotine may be able to relieve symptoms of tobacco abstinence in a manner similar to that of FDA-approved nicotine replacement therapy (NRT; e.g. Beard, West, Michie, & Brown, 2016; Perkins, Lerman, et al., 2008). Very few formal cessation trials with e-cigarettes have been conducted (see recent reviews by Malas et al., 2016; Hartman-Boyce et al., 2016), but some studies suggest their nicotine content may not be essential, as placebo or nicotine e-cigarette use can similarly aid reduction in tobacco cigarettes per day among those not attempting to quit (Tseng et al., 2016).
Notably, controlled studies suggest very short-term exposure to e-cigarettes may reduce withdrawal and craving after brief smoking abstinence, at least in the minutes post-use and in some smokers (e.g., Dawkins, Turner, Hasna, & Soar, 2012; Malas et al., 2016; Vansickel & Eissenberg, 2013). Yet, whether even these brief abstinence relief effects are due specifically to nicotine from the e-cigarette may not be certain (Meier et al., 2016), as nicotine delivery from early versions may have been insufficient to demonstrate symptom relief (Etter, 2015; Farsalinos et al., 2014; see also Shihadeh & Eissenberg, 2015). Moreover, while some studies showing e-cigarettes with nicotine, compared to placebo, acutely reduce craving, each was used in ad lib fashion over a brief period, allowing uncontrolled exposure (e.g. Dawkins, Turner, & Crowe, 2013). Thus, any e-cigarette use may be effective, whether or not it contains nicotine, if simple “vaping” behavior is sufficient (Meier et al., 2016). Also unclear is whether nicotine e-cigarettes can sustain relief from abstinence symptoms in smokers quit for at least 24 hrs, as in a true quit attempt, rather than during the temporary abstinence in non-quitters typical of these acute tests.
To help understand the influence of nicotine intake per se via e-cigarettes for possible efficacy in relieving withdrawal and craving during smoking abstinence, we compared changes in symptoms due to controlled use of a first-generation nicotine vs. a no-nicotine (placebo) e-cigarette (and vs. no e-cigarette use in Study 1). We used within-subjects designs to test these e-cigarettes on relief of symptoms in: 1) smokers not planning to quit permanently but were smoking abstinent overnight (Study 1), and 2) smokers already planning to quit permanently who were smoking abstinent at least 24 hours during at least one e-cigarette condition (Study 2). Both studies allowed comparison of symptom relief due to nicotine alone from the e-cigarette use (e.g. Cleophas, 1993), simulating effects when using them for temporary relief (Study 1) or for initiating an attempt to quit smoking (Study 2).
STUDY 1
Method
Participants
Minimum eligibility was age 18 or older with a smoking history of at least 10 cigarettes/day for > 1 year, presence of DSM-IV criteria for nicotine dependence (APA, 1994), and no interest in quitting in the next 6 months, but also no more than once/week e-cigarette use. The 28 participants in Study 1 (12 men, 16 women) smoked a mean (± SD) of 15.7±5.2 cigarettes/day, with 1.1±0.1 mg nicotine yield of preferred brand (n=9 for menthol, 19 for non-menthol). Age was 26.5±6.6 years old, and men and women did not differ on these characteristics. They self-identified mostly as Caucasian (75.0%), with 21.4% African American and 3.6% more than one ethnicity. None had current or past history of weekly e-cigarette use, and none had used within the prior 2 weeks of participating.
Electronic Cigarettes
These first-generation e-cigarettes were obtained from PrimeVapor LLC (Pleasant Prairie WI), labeled as containing 36 mg or 0 mg nicotine content per ml of liquid in 100% vegetable glycerin (VG), in pre-filled cartridges (each with e-liquid weights of “exactly 1 mg”; www.primevapor.com). E-cigarettes have been shown to increase plasma nicotine levels after abstinence in a manner related to these liquid concentrations of 0 and 36 mg/ml, with levels of 3.8 ng/ml (no change from abstinent baseline) versus 17.0 ng/ml, respectively, following 10 puffs on each e-cigarette by 16 naïve users (see Lopez et al., 2016). Yet, we did not obtain independent verification of nicotine delivery from ours, such as by testing the concentrations in e-liquid or in intermittent plasma samples from participants. The nicotine and placebo versions we used, “Rawhide Red (Tobacco)” for non-menthol and “Freeport (Menthol)” for menthol, were those showing nicotine’s acute reinforcement enhancing effects (Perkins, Karelitz, & Michael, 2015). We also selected them to closely match typical tobacco flavorings, given the lack of experience with e-cigarettes and unfamiliarity with other flavors in these participants. They were provided with a KR808D-1 Type automatic e-cigarette battery (650 mAh battery life, constant 3.7 V, and 2.2 Ohm resistance) from Prime Vapor.
Procedure
This research was approved by the University of Pittsburgh Institutional Review Board. Three experimental sessions, each following overnight abstinence (verified by Vitalograph BreathCO CO≤ 10 ppm; SRNT 2002), differed only in the administered e-cigarette condition: nicotine (36 mg) or placebo (0 mg) e-cigarettes (under blind conditions), or no e-cigarette. E-cigarette content was matched on menthol of the participant’s preferred tobacco brand, given the stability of menthol preference (Kasza et al. 2014) and its influence on self-report ratings of these products (e.g. Audrain-McGovern, Strasser, & Wileyto, 2016; Strasser, et al., 2013). The purpose was to compare withdrawal relief effects of nicotine vs. placebo e-cigarettes vs. no e-cigarette, each after overnight abstinence. The order of these e-cigarette conditions across sessions was counter-balanced between subjects.
Participants completed the CO check to confirm abstinence and the MNWS nicotine withdrawal measure (Hughes, 2007) upon arrival to each session. The MNWS items were each scored on 0-100 visual analog scales (VAS), anchored by “not at all” and “extremely” (as in Perkins, Lerman et al. 2008). Although QSU-brief craving (Cox, Tiffany & Christen, 2001) was not obtained in Study 1 (but see Study 2), we assessed craving via the single “Desire or Craving to Smoke” item from the MNWS (i.e. “Craving” item). All rested quietly while reading and either did, or did not (no e-cigarette session), self-administer the designated e-cigarette as described below on 4 occasions per session, 25 mins apart. The MNWS withdrawal measure was again assessed at the end of each 2-hr session to gauge differences in relief due to intermittent puffs on the nicotine and placebo e-cigarettes, compared to no e-cigarette use. Participants were compensated $20/hr for completing the entire study.
During the two e-cigarette sessions, subjects self-administered 10 puffs over 5 mins from the designated e-cigarette, one puff every 30 sec, as in other studies of acute e-cigarette effects (e.g. Dawkins & Corcoran 2014; Lopez et al. 2016; Spindle et al. 2015; Vansickel & Eissenberg 2013). Similar to prior research standardizing tobacco cigarette topography (Perkins, Karelitz, Giedgowd, & Conklin, 2012), the specific timing and duration of each puff inhalation by participants were guided by computer-displayed instructions, with a 4-sec puff hold duration. To assess topography and confirm equal exposure between the e-cigarettes, all puffs were via portable Clinical Research Support System (“CReSS Pocket”), with an adapter for use with e-cigarettes (“E-Cig Adaptor 9.00 mm”) obtained from Borgwaldt KC, Inc. (Richmond VA).
Data Analyses
A one-way repeated-measures analysis of variance (ANOVA), conducted using IBM SPSS 24.0, tested the change in MNWS withdrawal score between time points (i.e., arrival baseline and end of session) as a function of the within-subjects independent variable e-cigarette condition (nicotine, placebo, no e-cigarette). Follow-up a priori pairwise comparisons compared the change from baseline between the nicotine vs. placebo e-cigarette conditions (isolating acute nicotine effects alone), the placebo e-cigarette vs no e-cigarette conditions (isolating effects of e-cigarette use behavior alone), and the nicotine e-cigarette vs no e-cigarette conditions (testing combined effects of acute nicotine and e-cigarette use behavior). We also conducted the same analyses just on responses to the single “Craving” item from the MNWS (Hughes, 2007), to assess effects on craving (again, since we did not obtain the QSU-brief in Study 1). A paired t-test was conducted to verify no differences in puff topography responses to each e-cigarette condition (nicotine vs. placebo). No differences were significant due to order of the e-cigarette conditions across sessions, and so responses were collapsed across order. Cohen’s d and partial eta-squared values (ŋp2), were computed as measures of effect size for analyses of abstinence symptom relief (Cohen 1988).
Results
In preliminary comparisons, mean (±SEM) puff volume was not different between the nicotine vs. placebo e-cigarette, 102.5 (±7.6) vs 110.8 (±6.6) ml/puff, respectively, t(27) = 1.13, p>.25, d=0.21. When examining withdrawal change scores, primary analyses found a significant main effect of e-cigarette condition, F(2, 54) = 6.92, p<.005, η2p= .20. As shown in Figure 1, follow-ups indicated the decline in withdrawal was significantly greater during the nicotine e-cigarette condition, when compared to no e-cigarette, t(27)=3.57, p<.005, d=0.68, and to the placebo e-cigarette condition, t(27)=2.17, p<.05, d=0.41. There was no significant difference in withdrawal relief between the placebo and no e-cigarette conditions, t(27)=1.50, p=.14, d=0.28. Perhaps not surprisingly, virtually identical results were seen for the change from baseline on the single MNWS “craving” item, with a significant main effect of e-cigarette condition, F(2,54)=4.38, p<.05, η2p=.14. A significantly greater reduction in craving was found for the nicotine e-cigarette condition vs. no e-cigarette, t(27)=3.08, p=.005, d=0.58, and vs. placebo, t(27)=2.17, p<.05, d=0.41, with no difference between the placebo and no e-cigarette conditions, t(27)=0.65, p>.50, d=0.12.
Figure 1.
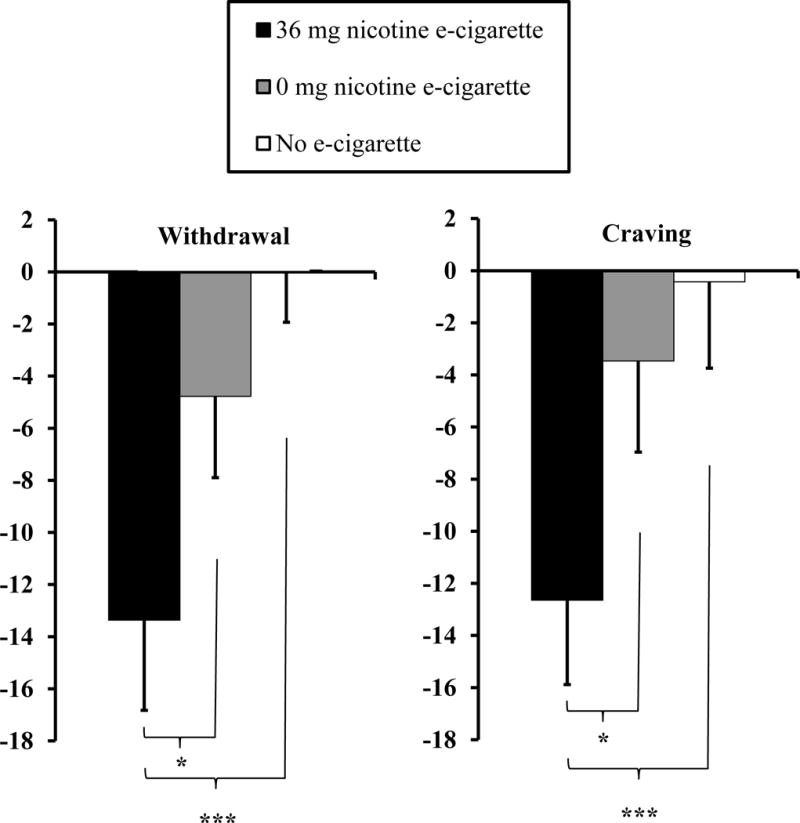
Mean±SEM change in MNWS withdrawal (and in the single “craving” item) from overnight abstinent baseline (CO≤10 ppm) to the end of each 2-hr session, following controlled use of a nicotine or placebo e-cigarette, or no e-cigarette, in a within-subjects design (N=28). * p<.05, *** p<.005
In sum, e-cigarettes with nicotine, but not simple use of one without nicotine, relieved withdrawal (including the MNWS item on craving) during exposures after overnight abstinence in smokers not preparing to quit. Study 2 aimed to explore these effects during separate 4-day periods of ad lib nicotine vs. placebo e-cigarette exposure after ≥24 hr abstinence in those who were preparing to quit soon. (A third period involving quit days with no e-cigarette use, as in Study 1, was not feasible.)
STUDY 2
Method
Participants
Eligible for Study 2 were adult dependent smokers who did intend to quit tobacco permanently within the next 2 months (in contrast to those in Study 1). Those currently taking medications to treat serious psychological problems (e.g. psychosis, major depression) were excluded. Recruitment procedures were similar to those described in our earlier comparable cross-over study on abstinence relief effects of week-long use of active NRT vs. placebo patch (Perkins et al. 2008). Of the 17 who enrolled, 12 (7 M, 5 F) were able to quit for at least 24 hr during one of the testing weeks, so that symptom relief on quit days due to the e-cigarette’s nicotine content could be compared. Mean (SD) characteristics of these 12 participants were 29.4 (11.3) years of age, self-report of smoking an average of 11.8 (2.3) cigs/day, 1.0±0.2 mg nicotine yield of preferred brand (n=8 for menthol, n=4 for non-menthol), with 7 identifying as Caucasian, 4 as African-American, and 1 as Asian. As in Study 1, none had current or past history of regular e-cigarette use (mean±SD of 7±9 months since last use in the 6 who reported any prior exposure). Participants were compensated by $15/visit over 10 visits (see Procedures), plus the offer of 8 weeks of NRT patch treatment and brief cessation counseling to make a permanent quit attempt after the end of this study, all at no cost. (An offer of post-study cessation treatment was a component of compensation specifically intended to attract those smokers already planning to quit soon.)
Procedures
This study was described to participants as “not a treatment study” but as a test of the effects of ad lib use of two different e-cigarettes “that may or may not contain nicotine” while trying to quit tobacco use during week-long periods. Exactly the same nicotine vs. placebo e-cigarettes described in Study 1 were used in Study 2, but use was ad lib over days in Study 2 (rather than under tightly controlled conditions over 2-hr sessions in Study 1). To maximize statistical power (Cleophas, 1993), we used a cross-over design similar to our prior within-subject comparison of symptom relief during cessation due to nicotine vs. placebo patch (Perkins et al., 2008). Thus, participants engaged in two separate 4-day quit attempt periods while using e-cigarettes that either did (36 mg/ml) or did not (i.e. placebo, 0 mg/ml) contain nicotine. During their 3 weeks of study participation, all were given their designated nicotine or placebo e-cigarette on Mon of weeks 1 and 3 and instructed to try and quit tobacco on Tues–Fri. Order of the e-cigarette conditions between weeks 1 and 3 was counter-balanced, and each was provided without identifying labels (i.e. blind). Week 2 involved resumption of ad lib tobacco smoking (i.e. “washout”) and no study visits, to equate prior smoking intake before both periods of using an e-cigarette while attempting to quit tobacco.
Assessed at daily visits in weeks 1 and 3 were expired-air CO and measures of withdrawal (MNWS) and craving (QSU-brief; Cox et al. 2001), both using items scored on 0-100 VAS, following 24-hr tobacco smoking abstinence (verified by Vitalograph BreathCO CO<5 ppm; Perkins, Karelitz, & Jao, 2013). Abstinence ≥24 hr was required here so that symptom relief responses to ad lib e-cigarette use when trying to quit tobacco would not be confounded by any recent smoking exposure. Amount of use was also assessed daily by subtracting the cartridge weight from that on the prior day. Participants received two new cartridges on the first day of each period. Empty cartridges were exchanged for new cartridges at each daily visit, as needed. To aid smoking cessation initiation during each brief quit period, all were given written suggestions on avoiding tobacco products, how to cope with urges, etc. (adapted from Perkins, Conklin, & Levine, 2008). They were also offered additional payment of $15 per quit day as motivation to abstain for >24 hrs, beyond the daily compensation for study participation (noted above). This study was approved by the University of Pittsburgh Institutional Review Board.
Data Analyses
Multilevel modeling was performed using the MIXED command in SPSS 24.0 (Raudenbush & Bryk, 2001; Singer & Willett, 2003). For Study 2, lower-level within-condition days (Level-1) were nested within higher-level nicotine conditions (Level-2). Following best-practice recommendations (Aguinis, Gottfredson, & Culpepper, 2013), two models were estimated per dependent variable: 1) a null model estimated without predictors from either level, and 2) a random intercept and fixed slope model. An intraclass correlation coefficient (ICC) was computed using parameters estimated in Model 1 to quantify the proportion of total variance in each dependent variable due to nicotine condition. Model 2 assessed differences between the nicotine vs. placebo e-cigarette conditions on responding to each symptom measure, controlling for effects of Level-1 day. Means for each dependent variable by nicotine condition were estimated controlling for the effect of quit week day. Symptom data were only included for days in which 24-hr abstinence from tobacco smoking was verified (CO<5 ppm). No differences were found for the order of e-cigarette conditions between quit weeks 1 vs. 3 in preliminary analyses.
Results
For the 12 participants able to quit ≥ 24 hrs at least once during e-cigarette conditions, the number of days quit (mean±SEM), controlling for effect of day, did not differ due to nicotine versus placebo (2.9±0.3 vs. 2.3±0.3, respectively), t(82)=1.6, ns. Similarly, in preliminary comparisons, daily amount of ad lib use of each e-cigarette cartridge was not different for nicotine versus placebo, 0.25±0.05 vs. 0.30±0.05 mg, t (81.1)=1.1, ns (or about 25–30% each day of the 1 mg e-liquid per cartridge; www.primevapor.com). Therefore, the amount of exposure to each e-cigarette across quit days was similar, allowing controlled comparison of ad lib nicotine versus placebo e-cigarette use on ability to attenuate abstinence symptoms.
Shown in Figure 2 are MNWS withdrawal and QSU craving by the nicotine and placebo e-cigarette conditions, controlling for the effect of day within condition. The ICC’s (calculated using parameters estimated in each symptom’s respective Model 1) indicated 37% of variation in MNWS withdrawal and 62% of variation in QSU craving were each due to nicotine condition. Mean MNWS withdrawal, controlling for the effect of day, was lower in the nicotine vs. placebo e-cigarette condition (23.0 vs 31.3; t (53.1) = 2.2, p<.05; difference of −8.3±3.8), as was QSU craving (28.4 vs 35.6; t (51.0) = 2.2, p<.05; difference of −7.1±3.3). In follow-ups of the QSU factor scores controlling for the effect of day, QSU factor 1 (reflecting positive smoking reward anticipation) was significantly decreased by nicotine vs placebo e-cigarette (34.2 vs 43.3; t (50.9) = 2.4, p<.05; difference of −9.1±3.8), but QSU factor 2 (anticipation of negative affect relief) was not significantly decreased (21.7 vs 26.7; t (51.4) = 1.6, p>.10; difference of −5.0±3.2).
Figure 2.
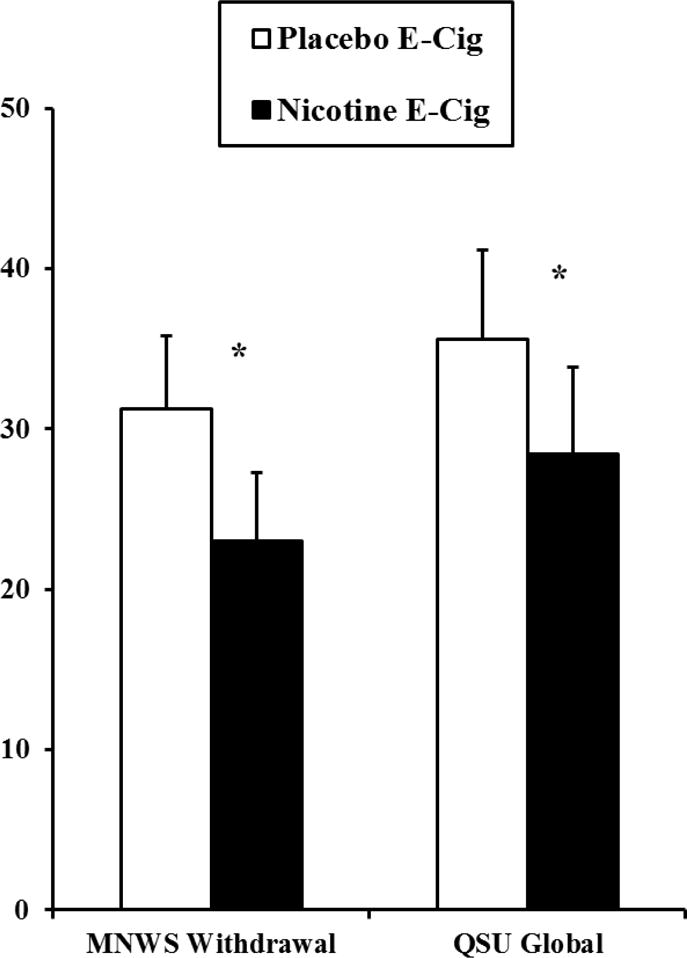
Mean±SEM MNWS withdrawal and QSU craving, controlling for effect of day, due to ad lib use of nicotine versus placebo e-cigarette following >24 hr smoking abstinence (CO< 5 ppm; N=12). * p<.05 for relief due to the nicotine vs. placebo e-cigarette.
Discussion
These results indicate that, compared to use of placebo e-cigarettes, use of nicotine-containing e-cigarettes may be more effective in reducing some tobacco abstinence symptoms, under controlled short-term conditions when not trying to quit smoking (Study 1) or under ad lib use after ≥24 hr quit (Study 2). Specific comparisons in Study 1 showed relief was due to nicotine per se, as use of placebo e-cigarettes vs. the no use condition was ineffective in relieving MNWS withdrawal (including the “craving” item), suggesting no influence of simple e-cigarette use behavior. Very similarly, in Study 2 MNWS withdrawal and QSU craving, especially factor 1, were relieved by the nicotine vs. placebo e-cigarette. Far more study is needed on e-cigarette efficacy in relieving craving and withdrawal during periods of >24 hr abstinence, but our findings suggest the potential for nicotine e-cigarettes to alleviate abstinence symptoms in a manner similar to that due to FDA-approved nicotine replacement treatments (e.g. Jorenby, Keehn, & Fiore, 1995).
Study 1 strengths include carefully controlled exposure to nicotine vs. placebo e-cigarettes, and the novel comparison of placebo versus a no e-cigarette condition to test simple behavioral effects of e-cigarette use. No effect of e-cigarette use without acute nicotine intake in Study 1 perhaps helps to address some of the uncertainty about the importance of nicotine intake from e-cigarettes in prior studies (cited in the Introduction). We also documented comparable use of both e-cigarettes in Study 2 via daily change in cartridge weight, ruling out greater use of the nicotine vs. placebo e-cigarette as a contribution to the difference in craving response. Yet, lack of verification of the nicotine delivery here is a limitation in both studies, as is uncertainty about other potential constituents in the e-liquid. Despite the further limitation of a small abstinent sample in Study 2, that study used a stringent criterion of CO<5 ppm to biochemically verify 24-hr smoking abstinence every day during each period, allowing for careful comparison of symptom relief from nicotine vs. placebo e-cigarette use during complete smoking abstinence. Finally, use of a within-subject design in both studies clearly isolated the influence of nicotine intake alone from e-cigarette use while maximizing the statistical power provided by small samples (Cleophas 1993).
In sum, our studies show relief due to nicotine in e-cigarette use, both during temporary abstinence in non-quitting smokers (Study 1) as well as during > 24 hr abstinence in smokers planning to quit permanently (Study 2). Nevertheless, substantially more controlled research is needed on the effects of nicotine from e-cigarette use in relieving abstinence symptoms, with larger and diverse study samples assessed over longer durations of tobacco cessation. Because abstinence symptom relief only partly aids ability to quit tobacco (e.g., Kenford et al. 1994), long-term testing of nicotine e-cigarettes on success of continuous abstinence from smoking is needed to more fully determine their potential clinical efficacy (Malas et al. 2016).
PUBLIC HEALTH SIGNIFICANCE.
E-cigarette relief of withdrawal and craving during smoking abstinence depends on its nicotine content, both from acute use in non-quitting smokers and over days of use in smokers planning a permanent quit attempt. Yet, because symptom relief does not fully predict smoking quit success, long-term testing of nicotine e-cigarettes on continuous smoking abstinence is needed to adequately gauge their clinical efficacy.
Acknowledgments
This research was supported by NIH Grants DA035774 (KAP) and T32 HL7560 (JLK). Preliminary findings from this research were presented at the annual meeting of the Society for Research on Nicotine and Tobacco (SRNT) in Florence, Italy, March 2017. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
All authors played a significant role in the conduct of this research and in the statistical analyses and preparation of the manuscript. The authors thank an anonymous journal reviewer for helpful comments on analyses.
Footnotes
DISCLOSURES
No author has any potential conflicts of interest to report.
References
- Aguinis H, Gottfredson RK, Culpepper SA. Best-practice recommendations for estimating cross-level interaction effects using multilevel modeling. Journal of Management. 2013;39:1490–1528. doi: 10.1177/0149206313478188. [DOI] [Google Scholar]
- American Psychiatric Association (APA) Diagnostic and statistical manual of mental disorders. 4th. Arlington, VA: American Psychiatric Publishing; 1994. [Google Scholar]
- Audrain-McGovern J, Strasser AA, Wileyto EP. The impact of flavoring on the rewarding and reinforcing value of e-cigarettes with nicotine among young adult smokers. Drug & Alcohol Dependence. 2016;166:263–267. doi: 10.1016/j.drugalcdep.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard E, West R, Michie S, Brown J. Association between electronic cigarette use and changes in quit attempts, success of quit attempts, use of smoking cessation pharmacotherapy, and use of stop smoking services in England: time series analysis of population trends. British Medical Journal. 2016;354:i4645. doi: 10.1136/bmj.i4645. [DOI] [PubMed] [Google Scholar]
- Cleophas TJM. Cross-over studies: a modified analysis with more power. Clinical Pharmacology & Therapeutics. 1993;53:515–520. doi: 10.1038/clpt.1993.64. [DOI] [PubMed] [Google Scholar]
- Cohen J. Statistical power analysis for the behavioral sciences. 2nd. Hillsdale, NJ: Lawrence Earlbaum Associates; 1988. [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology. 2014;231:401–407. doi: 10.1007/s00213-013-3249-8. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Hasna S, Soar K. The electronic cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors. 2012;37:970–973. doi: 10.1016/j.addbeh.2012.03.004. [DOI] [PubMed] [Google Scholar]
- Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology. 2013;227:377–384. doi: 10.1007/s00213-013-2983-2. [DOI] [PubMed] [Google Scholar]
- Etter J-F. Explaining the effects of electronic cigarettes on craving for tobacco in recent quitters. Drug & Alcohol Dependence. 2015;148:102–108. doi: 10.1016/j.drugalcdep.2014.12.030. [DOI] [PubMed] [Google Scholar]
- Farsalinos KE, Spyrou A, Tsimopoulou K, Stefapoulos C, Romagna G, Voudris V. Nicotine absorption from electronic cigarette use: comparison between first and new-generation devices. Scientific Reports. 2014;4:4133. doi: 10.1038/srep04133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database Syst Rev. 2016;(9):CD010216. doi: 10.1002/14651858.CD010216.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes JR. Effects of abstinence from tobacco: valid symptoms and time course. Nicotine & Tobacco Research. 2007;9:315–327. doi: 10.1080/14622200701188919. [DOI] [PubMed] [Google Scholar]
- Jorenby DE, Keehn DS, Fiore MC. Comparative efficacy and tolerability of nicotine replacement therapies. CNS Drugs. 1995;3:227–236. doi: 10.2165/00023210-199503030-00007. [DOI] [Google Scholar]
- Kasza KA, Hyland AJ, Bansal-Travers M, Vogl LM, Chen J, et al. Switching between menthol and nonmenthol cigarettes: findings from the U.S. cohort of the interational Tobacco Control Four Country survey. Nicotine & Tobacco Research. 2014;16:1255–1265. doi: 10.1093/ntr/ntu098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenford SL, Fiore MC, Jorenby DE, Smith SS, Wetter D, Baker TB. Predicting smoking cessation: who will quit with and without the nicotine patch. JAMA. 1994;271:589–594. doi: 10.1001/jama.1994.03510320029025. [DOI] [PubMed] [Google Scholar]
- Lopez AA, Hiler MM, Soule EK, Ramoa CP, Karaoghlanian NV, Lipato T, et al. Effects of electronic cigarette liquid nicotine concentration on plasma nicotine and puff topography in tobacco cigarette smokers: a preliminary report. Nicotine & Tobacco Research. 2016;18:720–723. doi: 10.1093/ntr/ntv182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malas M, van der Tempel J, Schwartz R, Minichiello A, Lightfoot C, Noormohamed A, et al. Electronic cigarettes for smoking cessation: a systematic review. Nicotine & Tobacco Research. 2016;18:1926–1936. doi: 10.1093/ntr/ntw119. [DOI] [PubMed] [Google Scholar]
- Meier E, Wahlquist AE, Heckman BW, Cummings KM, Froeliger B, Carpenter MJ. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine & Tobacco Research. 2017;19:176–182. doi: 10.1093/ntr/ntw157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien CP. Anticraving medications for relapse prevention: a possible new class of psychoactive medications. American Journal of Psychiatry. 2005;162:1423–1431. doi: 10.1176/appi.ajp.162.8.1423. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Conklin CA, Levine MD. Cognitive-behavioral therapy for smoking cessation. New York: Routledge; 2008. [Google Scholar]
- Perkins KA, Karelitz JL, Giedgowd GE, Conklin CA. The reliability of puff topography and subjective responses during ad lib smoking of a single cigarette. Nicotine & Tobacco Research. 2012;14:490–494. doi: 10.1093/ntr/ntr150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Jao NC. Optimal carbon monoxide criteria to confirm 24-hr smoking abstinence. Nicotine & Tobacco Research. 2013;15:578–582. doi: 10.1093/ntr/nts205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Michael VC. Reinforcement enhancing effects of acute nicotine via electronic cigarettes. Drug & Alcohol Dependence. 2015;153:104–108. doi: 10.1016/j.drugalcdep.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkins KA, Lerman C, Stitzer ML, Fonte CA, Briski JL, Scott JA, Chengappa KNR. Development of procedures for early human screening of smoking cessation medications. Clinical Pharmacology & Therapeutics. 2008;84:216–221. doi: 10.1038/clpt.2008.30. [DOI] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: SAGE Pub; 2001. [Google Scholar]
- Shihadeh A, Eissenberg T. Electronic cigarette effectiveness and abuse liability: predicting and regulating nicotine flux. Nicotine & Tobacco Research. 2015;15:158–162. doi: 10.1093/ntr/ntu175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. New York: Oxford Univ. Press; 2003. [Google Scholar]
- Spindle TR, Breland AB, Karaoghlanian NV, Eng M, Shihadeh AL, Eissenberg T. Preliminary results of an examination of electronic cigarette user puff topography: the effect of a mouthpiece-based topography measurement device on plasma nicotine and subjective effects. Nicotine & Tobacco Research. 2015;17:142–149. doi: 10.1093/ntr/ntu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SRNT. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Strasser AA, Ashare RL, Kaufman M, Tang KZ, Mesaros AC, Blair IA. The effect of menthol on cigarette smoking behaviors, biomarkers and subjective responses. Cancer Epidemiology, Biomarkers & Prevention. 2013;22:382–389. doi: 10.1158/1055-9965.EPI-12-1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rostrosen J, Shelley D. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine & Tobacco Research. 2016;18:1937–1943. doi: 10.1093/ntr/ntw017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Eissenberg TE. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine & Tobacco Research. 2013;15:267–270. doi: 10.1093/ntr/ntr316. [DOI] [PMC free article] [PubMed] [Google Scholar]


