Abstract
Identifying factors associated with the progression from recreational to pathological drinking in youth holds high clinical and theoretical importance. The present study tested cross-sectional associations of AUD severity with putative mechanisms of AUD progression among youth ages 15 to 24, namely acute subjective effects of alcohol and craving. Male (n = 44) and female (n = 42) youth completed ecological momentary assessments when not drinking, just before drinking, and while drinking, in the natural environment via handheld wireless devices. Youth were recruited from the community and were frequent and heavy drinkers, the majority (93%) with at least one AUD symptom (M = 3.4, SD = 2.4). Findings from youths’ daily lives suggested that how youth feel while they drink depends, in part, on their severity of AUD pathology. In support of hypotheses, youths with more progressed drinking pathology (i.e., those with more symptoms of AUD) reported greater reductions in craving and tension while drinking, relative to non-drinking times. In partial support of hypotheses, males with greater AUD symptomatology reported marginally attenuated increases in stimulatory states while drinking; however, contrary to hypotheses, females with greater AUD symptomatology reported enhanced increases in stimulation while drinking. This research leveraged EMA methods to study subjective responses to alcohol and craving in youths’ daily lives and to cross-sectionally test putative associations with AUD etiology. This work adds to literature supporting subjective responses to alcohol in the pathogenesis of alcoholism, specifically by highlighting their importance during the period in life when alcohol problems typically first emerge.
Keywords: subjective responses, craving, ecological momentary assessment, adolescents
Alcohol use and misuse, including the onset of alcohol use disorder (AUD), peak during adolescence and early adulthood (Johnston, O’Malley, Miech, Bachman, & Schulenberg, 2015), and nearly all contemporary perspectives conceptualize alcoholism within a developmental framework (Chassin, Sher, Hussong, & Curran, 2013). Yet, despite adolescence being a critical period for the pathogenesis of AUD, key aspects of how drinking problems advance during this developmental phase remain poorly understood. In the present study we characterized two putative mechanisms of AUD progression among adolescents and young adults, namely acute subjective effects of alcohol and craving, and we tested cross-sectional associations between these key constructs and AUD severity. Our major objective was to provide initial data on the extent to which subjective responses to alcohol and craving differ as a function of AUD severity during the period in life when alcohol problems typically first emerge.
Contemporary models of addiction etiology posit that individual differences in sensitivity to the acute subjective effects of alcohol are central to substance misuse (Schuckit et al., 2012; Volkow, Koob, & McLellan, 2016). All addictive substances, including alcohol, activate reward circuitry in the brain by causing sharp increases in the release of dopamine (Volkow et al., 2016). This potentiation in dopamine produces a host of pleasant subjective effects, such as stimulation, feelings of well-being, or euphoria, at least in the short term (de Wit & Phillips, 2012), and the abuse liability of a drug depends, in part, on its ability to alter subjective states or moods (Carter & Griffiths, 2009). On the contrary, even low doses of alcohol produce inhibitory effects on the γ-aminobutyric acid brain systems, resulting in sedating effects including drowsiness, behavioral slowing, and intoxicated feelings (Schuckit, 2009). Insensitivity to these sedative and otherwise impairing effects of alcohol as well as increased sensitivity to the stimulating and euphoric effects are both highly implicated in the development of AUD (King, Hasin, Connor, Mcnamara, & Cao, 2016; Morean & Corbin, 2010; Quinn & Fromme, 2011; Schuckit, 2014).
Human studies of adult drinkers support the role of subjective responses in AUD etiology. Retrospective accounts of early drinking experiences by adults show that a low level of response to the intoxicating effects of alcohol, especially early in life, predicts heavier drinking patterns and related problems (Schuckit, 1980, 1984, Schuckit & Smith, 1996, 2011, 2013). Human laboratory studies that involve alcohol administration with adults find support for this low level of response model (Schuckit, 2014) as well as a “differentiator” model that distinguishes between stimulatory and sedative effects at rising and falling blood alcohol levels (BAC) (Morean & Corbin, 2010; Newlin & Renton, 2010; Newlin & Thomson, 1990; Quinn & Fromme, 2011). For example, studies by King and colleagues (King, McNamara, Hasin, & Cao, 2014; King, Wit, Mcnamara, & Cao, 2011) found that heavy drinkers who reported greater stimulatory and rewarding effects (liking, wanting more) and lower sedative effects at peak BAC experienced more AUD symptoms up to 6 years later.
Although research supports the idea that particular profiles of subjective responses to alcohol reflect predispositions to alcoholism, mounting preclinical evidence suggests that associations between subjective effects of alcohol and the development of AUD are dynamic. Neurobiological adaptations associated with repeated alcohol exposure attenuate stimulatory reactions to alcohol consumption and intensify stimulatory reactions and craving to alcohol cues (Volkow et al., 2016). Consequently, AUD has been conceptualized as dynamic processes that involve progressive neurobiological dysregulation in the brain’s reward system that drives the transition from using alcohol for its pleasurable and euphoric effects (i.e., positive reinforcement) to drinking to mitigate negative affect and tension that occurs when not intoxicated (Ray, Bujarski, & Roche, 2016). Empirical evidence for this theory in humans, however, is scant. One study of intravenous alcohol administration found that alcohol dependent adults reported greater sedation, tension, and craving than non-dependent heavy drinkers at the start of the infusion procedure but had lower levels of sedation and craving over the course of alcohol administration (Bujarski and Ray, 2014). A handful of other laboratory studies directly examine subjective effects of alcohol and craving in adolescents and young adults, but do not explicitly test relations with AUD development (Behar et al., 1983; Hendershot et al., 2015; Ramirez & Miranda, 2014).
The dearth of human studies in this area underscores the need for additional research to advance our understanding of how subjective experiences of alcohol influence drinking and inform etiological models of AUD. Moreover, it is important to characterize the nature of these associations during adolescence—the key period for the transition from recreational to problem drinking. There is strong preclinical evidence that adolescents experience alcohol differently than adults in ways that may explain why youth are disproportionally affected by alcohol misuse. Adolescent rodents are less sensitive to the aversive and impairing effects of alcohol and more sensitive to the social-facilitation and rewarding effects, suggesting this developmental stage is well-suited to promote drinking (Schramm-Sapyta, Walker, Caster, Levin, & Kuhn, 2009; Spear, 2011). Adolescent animals are also less sensitive to the effects of alcohol withdrawal than adults (Crabbe, Bell, & Ehlers, 2010). Human data is scarce, however, due to ethical and legal restrictions on administering alcohol to adolescents in laboratory settings. We recently used ecological momentary assessment (EMA) methods to assess alcohol-related changes in adolescents’ subjective ratings of stimulation and sedation (Miranda et al., 2014). EMA is an in vivo approach that is well-suited to capture discrete, episodic events that are frequent and variable across situations and context, such as subjective responses while drinking (Shiffman, 2009). Our findings showed dose-dependent increases in sedation and decreases in stimulation as adolescents consumed alcohol in their usual settings. We also compared adolescent responses to a separate sample of adults who had completed a similar protocol and found adolescents reported greater stimulation at lower, but not higher, alcohol doses. It remains unknown whether youths’ subjective responses to alcohol are associated with AUD progression.
The major objective of the present study was to leverage EMA methods to characterize subjective responses to alcohol of adolescents and young adults in their daily lives and to observe putative etiological mechanisms of dependence progression. We also examined associations between craving and AUD severity. Separate from research on subjective effects of alcohol is a distinct scientific literature on the role of craving in the etiology of AUD. Craving is considered a prominent motivational determinant of drinking in contemporary theoretical models of addiction, including alcoholism (Drummond, 2001). Laboratory studies consistently show that alcohol use and alcohol cues evoke craving among adults under controlled conditions, with greater responsiveness among individuals with alcohol dependence (Carter & Tiffany, 1999; Monti et al., 1987) and heavier drinkers (Ihssen, Cox, Wiggett, Fadardi, & Linden, 2011).
Our knowledge of craving during adolescence is based on only a handful of studies. Initial survey data from community-based youths suggest that craving is common in this age group (Deas, Roberts, Randall, Anton, & Carolina, 2001; Deas, Roberts, & Grindlinger, 2005; Martin, Kaczynski, Maisto, Bukstein, & Moss, 1995). These early findings were supported by several laboratory studies and one EMA study of alcohol cue reactivity in adolescent drinkers, with results showing stronger effects among youths with heavier drinking histories or more alcohol-related problems (Curtin, Barnett, Colby, Rohsenow, & Monti, 2005; Ramirez & Miranda, 2014; Tapert et al., 2003; Thomas, Drobes, & Deas, 2005). The present study moves beyond cue-elicited effects by examining subjective responses to drinking and craving in the daily lives of adolescent and young adult drinkers.
Based on theroetical models of addiciton and extant findings from animal models, we hypothesized that, among youths who drink frequently, more AUD symptoms would be associated with less stimulatory and sedative effects of alcohol and greater tension reduction and relief of craving following alcohol consumption. In addition, we also examined associations between subjective reports and AUD severity during non-drinking moments to provide a more complete understanding of whether any patterns of associations observed while drinking were also present while youths were not drinking. Specifically, we examined whether AUD severity was associated with greater craving and tension and less stimulation when not drinking, as well as greater anticipatory increases in stimulation just prior to drinking.
Method
Participants and Procedure
Eligible participants were 14 to 24 years of age who reported drinking at least twice weekly in the past 30 days. The drinking criterion was intended to increase the likelihood of capturing alcohol use in the natural environment, and the age criterion capitalized on the peak developmental window for AUD. Youth were recruited from the community (e.g., recreational settings, high schools) to participate in an ongoing randomized, placebo-controlled clinical trial of whether a medication affects alcohol use. Data presented herein were collected during a baseline period occurring prior to randomization to the larger trial. Additional exclusion criteria included current Axis I psychopathology (other than alcohol, cannabis, nicotine, or disruptive behavior disorders), active suicidality or psychotic symptoms, and contraindicated medical conditions or medications. Females were ineligible if they were pregnant or nursing. Those who met provisional eligibility criteria based on a brief telephone screening completed a comprehensive, in-person interview to confirm eligibility. Written informed consent was obtained from 18- to 24-year-olds; assent was obtained from minors, and their parents provided written consent. The Brown University Institutional Review Board approved the study protocol.
Eighty-six youth (42 female, M age = 20.7 years, SD = 2.1) reported subjective responses and alcohol consumption for seven days in their natural environment. The majority identified their race as White (73.3%), Black (14.0%), Asian (4.7%) or American Indian (3.5%), and 20.9% indicated Hispanic ethnicity. Participants were frequent and heavy drinkers, with percent drinking days in the past month ranging from 10 to 97 percent (M = 37.6, SD = 20.0), and average number of drinks per drinking day in the past month ranging from 1.3 to 16.1 drinks (M = 4.9, SD = 3.1). Eighty youth (93.0%) met criteria for at least one AUD symptom (M = 3.4 symptoms, SD = 2.4; range = 0 to 9).
Participants completed assessments in their usual settings via handheld wireless devices (Omnia; Samsung Electronics, Ridgefield Park, NJ) with software developed for this study. Instructions were in simple English, and participants recorded data by tapping directly on the screen. In an initial training session, participants were oriented to the device and received age-appropriate, detailed instructions for study protocols using a graphic manual. The graphic manual depicted standard drinks by beverage type to help participants discern standard alcoholic drink volumes. During the baseline, pre-randomization EMA monitoring period, participants recorded responses at several times each day, and a combination of report types ensured adequate coverage of focal variables. Device-delivered random prompts (stratified within three-hour time blocks) and user-initiated reports prior to drinking assessed subjective states and contextual/situational factors. In order to capture instances where youth may be drinking but had not self-initiated a report, random prompts also queried whether participants were currently drinking. Device-delivered random prompts “timed out” after 2 minutes; however, participants had the option to delay the completion of random prompt assessments up to 20 minutes. Prompts that were not completed in that timeframe were marked as missed. User-initiated reports while drinking assessed subjective responses to alcohol, volume of alcohol consumed, and elapsed time since the previous report.
On average, participants completed 7 days of data collection during the baseline, pre-randomization period (SD = 2 days). Participants were compensated $5 for any interaction with the EMA device on a given study day, regardless of report type. Youth were not instructed to reduce or otherwise alter substance use patterns.
Baseline Assessments
Demographic information, including participant gender (0 = male; 1 = female), age, and racial/ethnic background, was collected at the initial training session.
Kiddie Schedule for Affective Disorders for School-Age Children (KSADS)
AUD diagnoses were determined by case consensus based on participant responses to the KSADS, a clinician-administered interview based on Diagnostic and Statistical Manual (DSM-IV-TR) criteria (Kaufman et al., 1997). Interviewers received training in diagnostic assessment and achieved high inter-rater reliability (kappa’s > 0.90).
Use of Alcohol, Nicotine, and Cannabis
A timeline follow-back interview (TLFB; Sobell & Sobell, 1992) assessed alcohol and cannabis use prior to the EMA period. Responses were used to compute percent drinking days and average number of drinks per drinking day in the past month. Participants also indicated whether or not they smoke nicotine cigarettes.
Ecological Momentary Assessment
Drink Reports
Participants were instructed to initiate a “begin-drink” report just before consuming alcohol and an “end drink” report as soon as they finished each drink. To simplify the instructional set, participants were taught to initiate begin- and end-drink reports immediately prior to and directly after each standard drink; however, assessments were delivered only for the first “begin-drink” report and first three “end-drink” reports of an episode to reduce response burden and facilitate compliance. For the first “begin-drink” report, participants rated subjective states and recorded contextual information; they also indicated whether they started drinking, and if so, how many minutes had elapsed since starting to drink their first drink. For all “end-drink” reports, participants reported how long it took them to finish their drink (elapsed minutes), selected the beverage type (e.g., beer, liquor), recorded the ounces consumed, and rated their subjective states. At begin- and end-drink reports, participants also reported concurrent smoking of nicotine cigarettes and use of marijuana.
Subjective States
A number of affective states and acute subjective responses to alcohol were assessed with sliding rulers with tick marks at discrete points from 0 to 10. Prompts stated “How [adjective] do you feel right now?” with end-point anchors “not at all” (the 0 end-point) and “extremely” (the 10 end-point). Subjective states included energized, excited, sedated, sluggish, high, tense, and stressed. Stimulation (energized, excited) and sedation (sedated, sluggish) items were based on items from the Biphasic Alcohol Effects Scale (BAES; Martin et al., 1993). All subjective states were assessed for all report types except for ‘sedated’ and ‘sluggish,’ which were assessed only for begin- and end- drink reports. As in our prior work, the average of energized and excited identified stimulatory states (α = .78), and the average of sedated and sluggish identified sedative responses (α = .72). In addition, the average of tense and stressed identified negative reinforcement from drinking (α = .83).
Craving/Urge
Prompts stated “How strong is your urge to drink alcohol right now?” Participants indicated responses on sliding rulers similar to those used for subjective states; however, end-point anchors were “no urge” (0 end-point) and “strongest ever” (10 end-point). Craving/urge was assessed for all report types.
Contextual Variables
Participants indicated their present company using multiple checkboxes (choose all that apply). Categories of friends (e.g., add examples of categories) were combined to form a “presence of peers” variable (0 = peers not present; 1 = peer present). Current location was assessed with forced choices (choose only one); see Table 2 for a list of descriptors. Time of day and weekend (0 = weekday; 1 = weekend) variables were derived from device timestamps. Contextual variables were assessed for all random prompts and upon initiation of each begin-drink report.
Table 2.
Alcohol Use Disorder (AUD) Severity Effects on Craving, Stimulation, and Tension just before drinking (Begin-drink Reports) and while drinking (End-drink reports), relative to Non-drinking times
| Parameters | Craving
|
Stimulation
|
Tension
|
||||
|---|---|---|---|---|---|---|---|
| Fixed Effect Estimates
| |||||||
| b | 95% CI | b | 95% CI | b | 95% CI | ||
| Intercept (Non-drinking)a | 2.51 *** | (1.93, 3.08) | 4.27 *** | (3.86, 4.69) | 3.01 *** | (2.37, 3.65) | |
| Begin-drink | 3.25 *** | (2.79, 3.71) | 0.84 *** | (0.43, 1.25) | 0.09 | (− 0.31, 0.49) | |
| End-drink/s | 1.70 *** | (1.36, 2.04) | 0.69 *** | (0.39, 0.99) | − 0.71 *** | (− 1.01, − 0.41) | |
| AUD severity effects | |||||||
| AUDb | 0.14 | (− 0.05, 0.33) | 0.02 | (− 0.11, 0.16) | 0.08 | (− 0.14, 0.30) | |
| AUD × Begin-drink | − 0.04 | (− 0.24, 0.17) | 0.14 | (− 0.05, 0.32) | − 0.10 | (− 0.28, 0.08) | |
| AUD × End-drink/s | − 0.19 ** | (− 0.31, −0.07) | 0.17 ** | (0.06, 0.28) | − 0.11 † | (− 0.22, 0.0008) | |
| Estimated BAC | − 0.14 | (− 3.68, 3.40) | 0.98 | (− 2.17, 4.14) | 2.87 † | (− 0.23, 5.97) | |
| Time of day | |||||||
| Midnight to 6am | − 0.45 * | (− 0.86, −0.03) | − 0.93 *** | (− 1.30, −0.56) | 0.00 | (− 0.36, 0.36) | |
| 6am to noon | − 1.41 *** | (− 1.67, −1.15) | − 0.17 | (− 0.40, 0.06) | − 0.27 * | (− 0.49, −0.04) | |
| Noon to 6pm | − 0.84 *** | (− 1.06, −0.62) | 0.27 ** | (0.07, 0.47) | − 0.14 | (− 0.34, 0.05) | |
| 6pm to midnight (reference) | |||||||
| Location | |||||||
| Public place | 0.41 * | (0.07, 0.74) | 0.72 *** | (0.42, 1.01) | − 0.01 | (− 0.30, 0.29) | |
| Friend’s home | 0.19 | (− 0.24, 0.62) | 0.67 *** | (0.29, 1.04) | − 0.37 † | (− 0.75, 0.00) | |
| Elsewhere | 0.12 | (− 0.48, 0.71) | 0.28 | (− 0.25, 0.81) | − 0.35 | (− 0.87, 0.17) | |
| Party/Club | 1.05 * | (0.22, 1.88) | 1.92 *** | (1.19, 2.65) | − 0.54 | (− 1.27, 0.19) | |
| School | 0.09 | (− 0.47, 0.64) | 0.60 * | (0.11, 1.09) | 0.00 | (− 0.48, 0.49) | |
| Car | 0.19 | (− 0.25, 0.62) | 0.70 *** | 0.32, 1.09 | 0.17 | (− 0.20, 0.56) | |
| Dorm | 0.22 | (− 0.32, 0.76) | 0.17 | (− 0.29, 0.64) | 0.15 | (− 0.33, 0.63) | |
| Work | 0.24 | (− 0.21, 0.68) | − 0.03 | (− 0.43, 0.36) | 0.98 *** | (0.59, 1.37) | |
| Home (reference) | |||||||
| Weekend | 0.54 *** | (0.31, 0.77) | 0.09 | (− 0.11, 0.29) | − 0.03 | (− 0.23, 0.18) | |
| Peers present | 0.42 *** | (0.18, 0.66) | 0.47 *** | (0.26, 0.68) | − 0.18 | (− 0.39, 0.03) | |
| Age | − 0.07 | (− 0.27, 0.12) | 0.07 | (− 0.07, 0.20) | 0.12 | (− 0.11, 0.34) | |
| Gender | − 0.57 | (− 1.36, 0.22) | − 0.30 | (− 0.85, 0.24) | 0.41 | (− 0.49, 1.31) | |
| Percent Drinking Days | 0.02 | (− 0.005, 0.04) | 0.002 | (− 0.01, 0.02) | 0.003 | (− 0.02, 0.03) | |
| Drinks per Drinking Day | − 0.004 | (− 0.14, 0.13) | − 0.08 | (− 0.17, 0.02) | 0.09 | (− 0.07, 0.25) | |
| Random Effect Estimates
|
|||||||
| σ2 | 95% CI | σ2 | 95% CI | σ2 | 95% CI | ||
| Person Intercept Variance | 2.30 *** | (1.47, 3.13) | 1.02 *** | (0.62, 1.43) | 3.20 *** | (2.10, 4.30) | |
| Day Intercept Variance | 1.05 *** | (0.77, 1.33) | 0.66 *** | (0.45, 0.87) | 0.87 *** | (0.66, 1.09) | |
| Moment Residual Variance | 3.46 *** | (3.02, 3.71) | 2.82 *** | (2.61, 3.03) | 2.61 *** | (2.42, 2.81) | |
Note. b = unstandardized parameter estimate; CI = confidence interval; σ2 = random variance estimate; BAC = blood alcohol content; AUD = Alcohol Use Disorder. Begin-drink reports occurring after initiation of drinking were excluded, and including these reports did not alter the pattern of results. Person and contextual covariates are included to account for variability in subjective states attributable to sources other than AUD severity. Inclusion of interactive effects with report type did not alter the pattern of significant focal results for AUD symptoms.
Due to the coding scheme for the categorical report-type variable, the intercept (when all predictors in the model = 0) is the level of subjective states at non-drinking random prompts for Craving, Stimulation, and Tension outcomes. Thus, begin-drink and end-drink effects are relative to non-drinking random prompts.
This is the effect of AUD symptoms on the intercept, and thus the effect of AUD symptoms on subjective states at non-drinking random prompts for Craving, Stimulation, and Tension outcomes.
Time of day is a categorical variable with four categories and Location is a categorical variable with nine categories. To conserve space, type III tests of fixed effects are shown for these two variables, and individual effects of each category are not tabled.
p < .10,
p < .05;
p < .01;
p < .001
Estimated Blood Alcohol Content (eBAC)
Weight and gender, as well as timestamps and participant-reported standard drinks from end-drink reports were used to calculate eBAC. Consistent with prior EMA research (Miranda et al., 2014; H. Treloar, Piasecki, McCarthy, Sher, & Heath, 2015), we used the Matthews & Miller formula (Matthews & Miller, 1979). This eBAC calculation produces estimates that correlate strongly with actual BACs (Hustad & Carey, 2005).
Analytic Approach
Three-level, mixed models with momentary reports (level 1) nested within study days (level 2) nested within participants (level 3) were estimated in SAS 9.3 PROC MIXED (SAS Institute Inc. 2012). This analytic approach is robust to unique timing of reports and variable numbers of reports for each youth in the study (Gibbons, Hedeker, & DuToit, 2010; Raudenbush & Bryk, 2002; Singer & Willett, 2003). Including a level for study day allowed for examination of change in subjective responses within study days. Random intercepts were included to allow levels of subjective states and craving to vary across participants and study days. Random slope terms were excluded due to lack of significant improvement in model fit and increased model complexity. For all analyses, random-prompt and begin-drink assessments recorded after alcohol use on a given day were excluded to avoid confounding non- and post-drinking subjective states1.
Initial unconditional means models (without any predictors) identified the relative variability accounted for by participant characteristics (level 3), day-to-day fluctuations (level 2), and momentary contextual influences (level 1). For each outcome, fit statistics (−LL, AIC, BIC) for two- and three-level models were compared and significance of the random intercept term for the day level was verified to support use of the three-level model. Next, report type was added as a categorical predictor to characterize changes in subjective states and craving/urge, within participants and study days, in the natural environment2. For stimulation, tension, and craving/urge, the report type predictor examined the change in subjective responses at begin-drink reports and end-drink reports, relative to non-drinking reports. Since sedative and “high” responses were conceptualized as alcohol effects, and thus not measured at non-drinking random prompt reports, the report-type predictor in models predicting these alcohol responses examined the change in subjective responses at end-drink reports, relative to begin-drink reports.
In order to test whether change in subjective responses varied as a function of AUD severity, main and interactive effects of AUD symptoms were added. Main effects (when all other predictors in the model = 0) represented the effect of AUD severity on subjective states when not drinking (i.e., with either non-drinking reports or begin-drink reports as the reference, depending on the assessment frame of the outcome). Interactive effects evaluated the influence of AUD severity on change in subjective states just before drinking and subjective responses while drinking (relative to non-drinking reports for stimulation, tension, and craving, and relative to begin-drink reports for sedation and high). Interactive effects were probed by calculating the model-based (empirical Bayes) estimates for the conditional regression of subjective responses on AUD symptoms. To test whether AUD effects on subjective states were better accounted for by contextual effects (i.e., time of day, weekend, presence of peers, location) and person-level individual differences (i.e., age, gender, baseline percent drinking days, baseline drinks per drinking day), these variables were included.3
Results
Ecological Momentary Assessment Reports and Compliance
Youth completed a total of 1721 non-drinking random-prompt reports, 218 begin-drink reports, and 333 end-drink reports over the course of the 1-week EMA monitoring period. Random prompt reports made after a drink report on a given day were flagged by the device and excluded from the analysis (n = 76; 4.4%). Drink reports for multiple drinking episodes within a given day were also excluded (n = 16 begin-drink reports and 26 end-drink reports). A number of begin-drink reports were logged after drinking had already been initiated (n = 104; 47.7%); the majority of these were within 10 minutes of initiating drinking (n = 77; 74.0%). To preserve interpretation of findings for begin-drink reports as subjective states prior to consumption of any alcohol on a given day, begin-drink reports occurring after initiation of drinking were excluded; however, the pattern of results was consistent when all begin-drink reports were retained. Overall random prompt compliance was defined by total random prompts completed by all participants divided by total random prompts completed plus missed random prompts. There were 1721 completed random prompts and 2330 combined completed + missed random prompts; thus, overall random prompt compliance was 73.9%.
Baseline Demographics
Descriptive information for baseline participant characteristics by AUD symptoms are shown in Table 1. Although AUD symptoms were normally distributed and treated as a continuous variable in analyses, participant information organized by common AUD categories is provided for descriptive purposes. The only participant-level characteristic that significantly differed across these categories was baseline grams of cannabis per use day, χ2 = 3.40, p = .024, with significant post-hoc contrasts only between the Mild and Moderate AUD categories. The bivariate Pearson correlation between the AUD symptom count variable used in the focal analyses and baseline grams per use day was not significant, r = − 0.04, p = .773, however. Marginally significant differences were shown for baseline average drinks per drinking day, and the bivariate Pearson correlation with AUD count was significant, r = .25, p = .021. Gender differences across AUD categories were also marginally significant; however, the bivariate association between the AUD symptom count variable used in our analyses and gender, as assessed with an independent samples t-test, was not significant. Specifically, there was not a significant gender difference in symptom count for males (M = 3.73, SD = 1.87) and females (M = 3.14, SD = 2.27), t(84) = 1.31, p = .125. The number of youth indicating smoking (nicotine) or use of cannabis did not differ across AUD categories.
Table 1.
Summary of Baseline Characteristics by Alcohol Use Disorder Severity
| Variable | Alcohol Use Disorder (AUD) Symptoms
|
Overall (N = 86) |
F or χ2 | p | |||
|---|---|---|---|---|---|---|---|
| None (0 to 1 symptom) (n = 17) |
Mild (2 to 3 symptoms) (n = 26) |
Moderate (4 to 5 symptoms) (n = 28) |
Severe (6+ symptoms) (n = 15) |
||||
| Age | |||||||
| Observed Range | 18 − 23 | 17 − 24 | 14 − 24 | 18 − 24 | 14 − 24 | ||
| M (SD) | 21.3 (1.8) | 20.9 (1.8) | 20.4 (2.5) | 20.4 (2.1) | 20.7 (2.1) | 0.85 | .472 |
| Gender, # (%) female | 12 (70.6) | 13 (50.0) | 9 (32.1) | 8 (53.3) | 42 (48.8) | 6.48 | .091 |
| Race, # (%) | |||||||
| White or Caucasian | 10 (62.5) | 23 (88.5) | 20 (71.4) | 10 (71.) | 63 (75.0) | 11.18 | .513 |
| Black or African-American | 3 (18.8) | 1 (3.8) | 5 (17.9) | 3 (21.4) | 12 (14.3) | ||
| Asian | 2 (12.) | 0 | 2 (7.1) | 0 | 4 (4.8) | ||
| American Indian or Native Alaskan | 1 (6.3) | 1 (3.8) | 0 | 1 (7.1) | 3 (3.6) | ||
| Other | 0 | 1 (3.8) | 1 (3.6) | 0 | 2 (2.4) | ||
| Hispanic, # (%) | 4 (23.5) | 2 (7.7) | 8 (28.6) | 4 (26.7) | 18 (20.9) | 4.11 | .250 |
| Baseline drinking levels | |||||||
| % drinking days, M (SD) | 32.9 (20.2) | 37.2 (18.4) | 40.6 (24.0) | 37.8 (13.7) | 37.6 (20.0) | 0.52 | .673 |
| Drinks per drinking day, M (SD) | 3.3 (1.8) | 4.6 (3.1) | 5.6 (3.1) | 5.8 (3.5) | 4.9 (3.1) | 2.51 | .065 |
| Baseline cannabis use levels | |||||||
| % use days, M (SD) | 22.7 (37.9) | 20.1 (28.1) | 41.8 (43.1) | 16.4 (25.8) | 27.0 (36.2) | 2.50 | .065 |
| Grams per use day, M (SD) | 0.67 (0.77) | 0.29 (0.20)a | 0.81 (0.78)a | 0.34 (0.24) | 0.55 (0.61) | 3.40 | .024 |
| Baseline Smoking Status, # (%) smokers | 3 (17.6) | 5 (19.2) | 12 (42.9) | 4 (26.7) | 24 (27.9) | 4.98 | .173 |
| Baseline Cannabis Status, # (%) users | 8 (47.1) | 18 (69.2) | 23 (82.1) | 11 (73.3) | 60 (69.8) | 6.28 | .099 |
Note. Baseline drinking levels derived from the Timeline Follow-Back interview administered at baseline.
Significantly different Bonferroni contrasts, p < .05.
Mixed Modeling
Unconditional means models including random intercept terms without any additional predictors were used to estimate the amount of variability in subjective states accounted for by each level of the model. Significant random effects for each level supported the three-level model including random intercepts for participants (level 3) and days (level 2), as well as momentary residuals (level 1). Intraclass correlation coefficients (ICCs) were calculated from the variability at each level, divided by the total variability, and were converted to percentages to further describe the relative variability accounted for by participant, day, and momentary influences. ICCs for participant, day, and moment levels were as follows: craving = .29, .19, .51; stimulation = .21, .18, .61; tension = .46, .14, .39; sedation = .47 .13 .40; high = .40, .21, .39. Overall, the majority of variability in stimulation and craving was reflected in the momentary effects, accounting for 61% and 51% of the variance in stimulation and craving subjective states, respectively. In contrast, more of the variability in tension, sedation, and high was attributable to participant-level characteristics, accounting for 46%, 47%, and 40% of the variance, respectively.
Models including only the effect of report type were important to show general trends in subjective states and craving. Overall, craving increased significantly just before drinking, b = 4.05, p < .001, and while drinking, b = 2.62, p < .001, relative to non-drinking times. Similarly, stimulation increased significantly before drinking, b = 1.10, p < .001, and while drinking, b = 1.03, p < .001, relative to non-drinking times. In contrast, tension did not significantly change just before drinking, relative to non-drinking times, p = .925, but did significantly decrease while drinking, b = − 0.68, p < .001. Since sedation and high were not assessed at non-drinking times, end-drink reports could only be compared to begin-drink reports. Relative to just before drinking, sedation significantly increased, b = 0.50, p = .011; however, subjective ratings of ‘high’ did not significantly change while drinking, p = .187.
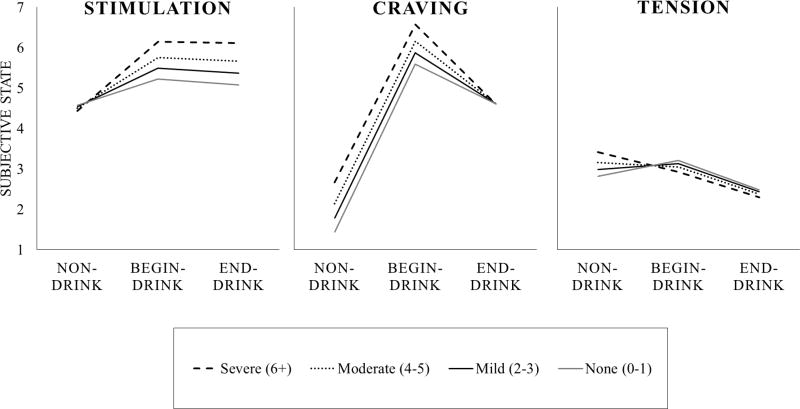
Models including the interactive effect of AUD symptom count with report type tested whether the aforementioned general trends were altered based on youths’ level of AUD severity. Results from models predicting craving, stimulation, and tension are shown in Table 2. Age, gender, percent drinking days, and drinks per drinking day were included to test whether other person-level variables accounted for model results. Non-significant main effects of AUD symptom count suggested that AUD severity was not associated with craving, stimulation, or tension at non-drinking times. Craving. The significant negative interactive effect for AUD × End-drink/s showed that increases in craving while drinking were attenuated for youth with more symptoms of AUD. Specifically, each additional symptom of AUD was associated with a one fifth of a point attenuation in craving while drinking, relative to non-drinking times, b = − 0.19, p = .003, even after accounting for average drinking levels, age, gender, presence of peers, location, time of day, and eBAC. This finding supported our hypothesis that youth with more AUD symptoms would experience greater relief of craving following alcohol consumption. The non-significant interactive effect for AUD × Begin-drink showed that increases in craving just before drinking were not related to AUD severity, p = .717. Stimulation. The significant positive interactive effect for AUD × End-drink/s showed that increases in stimulation while drinking were enhanced for youth with more symptoms of AUD. Specifically, each additional symptom of AUD was associated with nearly one fifth of a point increase in stimulation while drinking, relative to non-drinking times, b = 0.17, p = .003, even after accounting for average drinking levels, age, gender, presence of peers, location, time of day, and eBAC. This finding opposed our hypothesis that youth with more AUD symptoms would experience reduced stimulation from alcohol consumption, suggesting instead that AUD severity is associated with greater stimulation while drinking. The non-significant interactive effect for AUD × Begin-drink suggested that increases in stimulation just before drinking were not related to AUD severity, p = .146. Tension. The marginally significant negative interactive effect for AUD × End-drink/s showed that decreases in tension while drinking trended toward being enhanced for youth with more symptoms of AUD. Specifically, each additional symptom of AUD was associated with an additional one tenth of a point decrease in tension while drinking, relative to non-drinking times, b = − 0.11, p = .052, even after accounting for average drinking levels, age, gender, presence of peers, location, time of day, and eBAC. This finding partially supported our hypothesis that youth with more AUD symptoms would experience greater relief of tension from alcohol consumption; however, the substantive meaning of this effect was perhaps too small to be of clinical significance. The non-significant interactive effect for AUD × Begin-drink showed that the lack of change in tension just before drinking, relative to non-drinking times, was not altered by AUD severity, p = .286. Sedation and High. Findings for sedation and high were not significant and are not tabled. Thus, our hypothesis that youth with more AUD symptoms would report less sedative effects of alcohol was not supported.
Exploratory Analyses
Participant-level interactive effects of baseline drinking levels and gender
Although baseline drinks per drinking day and gender covariates did not account for these results, potential associations of these variables with AUD severity supported additional analyses testing whether the interactive effect of AUD symptom count with report type varied by gender or baseline drinking levels. Two significant three-way interactive effects were found. For the stimulation outcome, there was a significant gender × AUD count × report type interaction suggesting gender differences in the relation of AUD severity and stimulation for end-drink reports, b = 0.49, 95%CI (0.25, 0.73), p < .001. Follow-up mixed models run separately for females and males suggested that AUD-related enhancements in stimulation for end-drink reports were only found in female participants, b = 0.32, 95%CI 9 0.19, 0.45), p < .001. In contrast, male youth with more AUD symptoms reported marginally decreased stimulatory responses at end-drink reports, b = − 0.08, 95%CI (− 0.38, 0.03), p = .088, consistent with our original hypotheses. For the craving outcome, there was a significant baseline drinks per drinking day × AUD count × report type interaction suggesting that youth who drink more heavily and with more AUD symptoms reported the greatest attenuation of craving while drinking, b = − 0.07, 95%CI (− 0.1 − 0.02), p = .009.
Momentary interactive effects of concurrent smoking (nicotine) and cannabis use
There were 13 begin-drink reports (13.3%) and 42 end-drink reports (13.7%) where concurrent smoking (nicotine) was indicated. There were 3 begin-drink reports (3.1%) and 27 end-drink reports (8.8%) where concurrent cannabis use was indicated. Of these, 2 begin-drink reports an 8 end-drink reports indicated combined use of both nicotine and cannabis. To test whether concurrent use of nicotine or cannabis influenced subjective response findings, we identified days as smoking = 1 or non-smoking = 1 and cannabis use = 1 or non-cannabis use = 0. These binary variables were included as covariates and evaluated as interactive effects with report type and AUD symptom count. For the craving outcome, a three-way interactive effect of cigarette (nicotine) use × AUD count × report type was significant, p = .010. This was probed by testing models separately for smoking and non-smoking days. On non-smoking days, youth with greater AUD symptoms reported lower levels of craving at end-drink reports relative to non-drinking reports, b = − 0.13, SE = 0.06, p = .038, similar to our original findings. On smoking days, this effect was more pronounced, b = − 2.33, SE = 0.26, p < .001, and craving was also reduced prior to drinking at begin-drink reports relative to non-drinking reports for youth with more greater AUD symptoms, b = − 1.93, SE = 0.54, p < .001. Interactive effects of cannabis use for the craving outcome were not significant, and no other interactive effects were significant for stimulation, tension, high, or sedation for either smoking or cannabis use.
Discussion
This cross-sectional study indicated that how youth feel while they drink in their natural environment depends on the severity of their AUD pathology. Our findings add to the body of literature supporting subjective responses to alcohol in the pathogenesis of alcoholism, specifically by highlighting their importance during adolescence, the peak period for AUD development. In support of hypotheses, youths with more progressed drinking pathology (i.e., those with more symptoms of AUD) reported greater reductions in craving from drinking, relative to non-drinking times, with effects more pronounced for the heaviest drinking youth. In partial support of hypotheses, male youth with more AUD symptoms reported marginally attenuated increases in stimulatory states while drinking, relative to youth with less symptomatology; however, contrary to hypotheses, female youth with more AUD symptoms reported enhanced increases in stimulation while drinking, relative to youth with less symptomatology. Results also partially supported the hypothesis that drinking results in greater tension reduction for those with more advanced AUD pathology, although the effect was quite small.
Researchers have posited myriad possible explanations for the heightened vulnerability for alcohol misuse among youth, with emphasis on the considerable biological, cognitive, and psychosocial changes that mark this developmental period. On the whole, there is ample evidence that liability factors for AUD are age specific and that the cascading processes associated with alcohol misuse are multifaceted and complex. This study advances the scientific literature in several important ways. First, although most individuals with AUD transition to pathological drinking in their late teens and early twenties, core concepts of AUD development, namely craving and subjective affective responses to alcohol, are rarely directly studied in humans during this period. Few studies to date have examined subjective states and craving as they relate to drinking among human adolescents (Behar et al., 1983; Hendershot et al., 2015), and only one prior study from our group examined these in youths’ daily lives (Miranda Jr. et al., 2014). Further, despite the theoretical importance, associations of AUD development with subjective responses and craving among youth had not previously been explicitly tested. The present ecological research builds on prior work by leveraging youths’ drinking behavior in the natural environment to examine subjective responses to alcohol in the daily lives of youth. Our work provides the first direct human evidence, albeit cross-sectional, to support a key tenet of contemporary etiological models of addiction, i.e., acute effects of alcohol are related to addiction progression.
The phenotypic expression of diminished sedative responses to alcohol associated with AUD is well documented (Schuckit & Smith, 2011), but whether these differences typify youth at risk of AUD or vary across stages of AUD development remains unknown. Contrary our hypothesis, we did not find support for an association of AUD symptom development and acute sedative effects of alcohol or subjective ‘high’. Thus, although the use of EMA offered the advantage of testing a component of low level of response directly during adolescence, when it matters most, without relying on retrospective reports from older drinkers or inferring human adolescents’ subjective states from preclinical analogues, findings were inconclusive. One key limitation is that these states were considered acute alcohol effects, and as such, these were only measured at begin- and end-drink reports and not at other non-drinking times. It is possible that a comparison of sedative effects and subjective high at end-drink reports, relative to other times rather than relative to just before drinking, would have yielded different results.
Although alcohol’s stimulant effects reflect a well-studied counterpart to its sedative effects, the nature and role of stimulatory responses in AUD development is poorly understood. From a purely behavioral learning perspective, greater euphoric or stimulating effects of alcohol should promote continued use; however, a progressive model of addiction neurobiology posits that stimulatory responses to alcohol are heightened in the early stages of addiction and decrease with repeated alcohol exposure, thus reflecting the advancing stages of brain reorganization in response to repeated drinking (Volkow et al., 2016). Our hypotheses were made in line with the latter, but gender differences in findings added further complexity and supported the former for female youth. Female youth with more symptoms of AUD reported greater stimulatory effects of drinking. Thus, results for females reflected alcohol sensitivity indicative of early stages of addiction, akin to results of King and colleagues’ (2011, 2014, 2016) laboratory studies in adult men and women who drink heavily (gender differences in stimulatory responses were not examined these prior studies). These findings lend support to a relatively straightforward theory for how acute effects of alcohol relate to drinking—youth who experience more pleasant, stimulatory effects are more likely to drink repeatedly and at levels that make them susceptible to developing problems (Ray et al., 2016). In contrast, findings for male youth reflected sensitivity indicative of the latter stages of addiction. Male youth with more symptoms of AUD reported marginally decreased stimulatory effects of drinking. Gender differences highlight the need for studies explicitly designed to test potential disparities in hypothesized effects and the importance of testing gender differences in post-hoc analyses in studies with the sample size to support such tests.
In addition to adding to research on subjective responses, this study also advances our understanding of craving among youth and its association with AUD severity. On the whole, our findings showed that youths reported higher levels of craving just prior to and immediately following alcohol use, as compared to non-drinking moments. Heightened craving levels while drinking were moderated, however, by level of AUD pathology, such that youth with more AUD symptoms reported greater attenuation of craving while drinking. Put in the vernacular, these results may show that drinking “scratched the itch” more for youth with more progressed AUD pathology, and particularly those youth who drink the most heavily. Existing research is generally consistent with this result, but the lack of association of AUD severity and craving levels in non-drinking moments contrasts with related studies in this area. A recent study examined whether alcohol cues evoke craving among adolescent drinkers in the laboratory and in the natural environment, and tested the clinical relevance of craving during this developmental period by examining the prospective association between craving and alcohol use (Ramirez & Miranda, 2014). Laboratory results replicated previous findings that adolescent drinkers experience increases in craving when exposed to alcohol cues (Curtin et al., 2005; Thomas et al., 2005), with a large magnitude effect size (d = 0.86). A central finding of this study was that severity of alcohol-related problems moderated the effects of alcohol cues on craving in the natural environment. Specifically, alcohol cues were robust predictors of craving among adolescents with more severe pathological drinking habits but had negligible effects on cue-elicited craving among youths with few alcohol problems. Our findings extend prior work by characterizing craving among youth during drinking as well as non-drinking moments.
Our results must be considered in the context of important limitations of this work. First and foremost, the cross-sectional nature of this study precludes definitive inferences regarding directionality or causality of these findings. Only a longitudinal study following youths over time can truly test the causal pathways posited to underlie the developmental unfolding of alcohol addiction. Short of that, our work provides a cross-sectional evaluation of differences in subjective alcohol responses and craving posited to mark the progression of AUD. Although the present research takes us an important step closer to understanding the period of peak risk for the still-developing brain in humans—adolescence and young adulthood—conclusions remain limited to cross-sectional associations, and causal processes should not be inferred. Our cross-sectional findings raise the question of whether AUD-related differences in subjective states and craving while drinking are a function of the pathogenesis of addiction—where addicted youth become hypersensitive to reinforcement with repeated drinking—or whether these differences pre-existed and possibly conferred liability for AUD. These and other alternative explanations for the current findings should be addressed in future prospective longitudinal research.
In addition, our EMA protocol characterized male and female youths’ subjective responses to alcohol at the low range of eBAC levels. It is possible that associations between AUD symptom severity and subjective response profiles are different at higher levels of intoxication. Additionally, our EMA approach focused exclusively on the ascending limb of the blood alcohol curve. As such, we were unable to examine associations between AUD severity and the full spectrum of biphasic alcohol effects. Other considerations include the fact that participants were heavy drinkers recruited for a larger clinical trial testing the combined effects of pharmacotherapy and a behavioral intervention on alcohol use. In addition, the majority of our sample met diagnostic criteria for AUD and all participants reported some clinically significant alcohol-related problems. Consequently, our findings may not generalize to lighter drinkers or individuals not looking to reduce their alcohol use. Likewise, we studied adolescents and young adults. Whether these results generalize to older drinkers is unknown and warrants future research.
A final consideration is the limited ability of an EMA protocol to definitively control external factors in the natural environment, such as differential exposure to alcohol cues and other contextual factors that may be associated with alcohol craving. Whereas controlled laboratory research can systematically account for potential confounding influences, this in vivo study in participants’ daily lives is subject to a host of external influences that are important areas of research in and of themselves and may or may not be accounted for (e.g., type of bar setting, number and gender composition of present company). Although we included covariates to explain variance in subjective states due to location, time of day, weekend, and presence of peers, other possible influences were not assessed. For example, other than distinguishing between locations such as “bar” and “home,” our assessment of location did not provide sufficient information to ascertain the degree to which extraneous alcohol cues were present while youths were drinking. Consequently, we are unable to rule out the possibility that specific alcohol cues altered subjective responses, and the inclusion of such variables in future research would help bridge findings from the human laboratory to participants’ daily lives in their natural environments. In addition, it is possible that participants' expectations about alcohol effects may also have influenced their subjective states and craving, especially while drinking. Research shows that alcohol outcome expectancies may be a mechanism by which other more distal factors (e.g., family history, AUD) influence immediate experiences (Treloar, Pedersen, & McCarthy, 2016). While the present study hypothesized cross-sectional associations between AUD severity and subjective responses to alcohol and craving, consideration of expectancies and other social-cognitive variables in future research would provide a more complete and comprehensive understanding of the factors that interact with craving and acute alcohol effects.
In conclusion, this work further highlights the utility of EMA methods for testing alcohol effects in underage drinkers and for testing alcohol use in real time, in real-world settings. Indeed, researchers have applied EMA to study subjective responses to alcohol, nicotine, and caffeine among adults (Buckner, Zvolensky, & Ecker, 2013; Piasecki et al., 2011; Piasecki, McCarthy, Fiore, & Baker, 2008; Serre et al., 2012; H. R. Treloar, Piasecki, McCarthy, & Baker, 2014), and to a lesser extent among adolescents (Gwaltney, Bartolomei, Colby, & Kahler, 2008; Miranda et al., 2014). Identifying factors associated with the progression from recreational to pathological drinking in youth holds high clinical and theoretical importance. This study leveraged EMA methods with adolescents and young adults to study subjective responses to alcohol in youths’ daily lives and to observe putative etiological mechanisms of AUD progression.
Figure 1.
Model-based (empirical Bayes) estimates representing the conditional change in subjective states across report types as a function of Alcohol Use Disorder (AUD) symptom count are shown. Response ranges for stimulation, craving, and tension were 1 to 10. Solid gray lines reflect no AUD diagnosis (0 to 1 symptoms), solid black lines indicate mild AUD (2 to 3 symptoms), dotted lines reflect moderate AUD (4 to 5 symptoms), and dashed lines reflect severe AUD (6+ symptoms). AUD symptom counts ranged from 0 to 9 in this sample (M = 3.4; SD = 2.2).
Public Significance Statement.
This study suggests that how youth feel when they drink in their usual environments relates to their severity of Alcohol Use Disorder. This initial finding points to the need for studies that track changes in youths’ experiences while drinking over time as alcohol problems develop.
Acknowledgments
The National Institute on Alcohol Abuse and Alcoholism at the National Institutes of Health supported this work (AA07850, AA07459, K23AA024808).
The authors wish to thank Alexander Blanchard for his significant contribution to the data collection and database management supporting this work.
Footnotes
The same pattern of results is observed when all begin-drink reports were retained, regardless of whether drinking had already been initiated.
Specification of a categorical “report type” variable (indicating subjective states at non-drinking random prompts, begin-drink reports, and end-drink reports) in the Class statement of SAS PROC MIXED creates a series of dummy-coded variables and avoids imposing a linear structure for change in subjective response states.
Although the relative size of effects changed with the addition or removal of covariates, the same pattern of significant results remained when all covariates were excluded. The same pattern of significant results also remained when interactive effects of time-varying covariates with report type were included.
Disclosures
Both authors contributed significantly to the manuscript and have read and approved the manuscript in its final form.
Neither author has a conflict of interest to report.
Findings reported herein were previously presented at the 39th Annual Scientific Meeting of the Research Society on Alcoholism, June, 2016, in New Orleans, LA.
References
- Behar D, Berg C, L RJ, Nelson W, Linnoila M, Cohen M, Marshall T. Behavioral and Physiological Effects of Ethanol in High-Risk and Control Children: A Pilot Study. Alcoholism: Clinical & Experimental Research. 1983;7(4):404–410. doi: 10.1111/j.1530-0277.1983.tb05495.x. https://doi.org/10.1111/j.1530-0277.1983.tb05495.x. [DOI] [PubMed] [Google Scholar]
- Buckner JD, Zvolensky MJ, Ecker AH. Cannabis use during a voluntary quit attempt: an analysis from ecological momentary assessment. Drug and Alcohol Dependence. 2013;132(3):610–6. doi: 10.1016/j.drugalcdep.2013.04.013. https://doi.org/10.1016/j.drugalcdep.2013.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter B, Tiffany S. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94(3):327–340. [PubMed] [Google Scholar]
- Carter LP, Griffiths RR. Principles of laboratory assessment of drug abuse implications for clinical development. Drug Alcohol Dependence. 2009;105(Suppl):S14–S25. doi: 10.1016/j.drugalcdep.2009.04.003. https://doi.org/10.1016/j.drugalcdep.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Sher KJ, Hussong A, Curran P. The developmental psychopathology of alcohol use and alcohol disorders: research achievements and future directions. Development and Psychopathology. 2013;25(4 Pt 2):1567–84. doi: 10.1017/S0954579413000771. https://doi.org/10.1017/S0954579413000771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addiction Biology. 2010;15(2):125–44. doi: 10.1111/j.1369-1600.2009.00191.x. https://doi.org/10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtin JJ, Barnett NP, Colby SM, Rohsenow DJ, Monti PM. Cue Reactivity in Adolescents: Measurement of Separate Approach and Avoidance Reactions*. Journal of Studies on Alcohol and Drugs. 2005;66:332–343. doi: 10.15288/jsa.2005.66.332. [DOI] [PubMed] [Google Scholar]
- Deas D, Roberts J, Randall C, Anton R, Carolina S. Adolescent Obsessive Compulsive Drinking Scale: An Assessment Tool for Problem Drinking. Jounal of the National Medical Association. 2001;93:92–103. [PMC free article] [PubMed] [Google Scholar]
- Deas D, Roberts JS, Grindlinger D. The utility of DSM-IV criteria in diagnosing substance abuse/dependence in adolescents. Journal of Substance Use. 2005;10(1):10–21. https://doi.org/10.1080/1465989042000271200. [Google Scholar]
- de Wit H, Phillips TJ. Do initial responses to drugs predict future use or abuse? Neuroscience and Biobehavioral Reviews. 2012;36(6):1565–76. doi: 10.1016/j.neubiorev.2012.04.005. https://doi.org/10.1016/j.neubiorev.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond DC. Theories of drug craving , ancient and modern. Addiction. 2001;96:33–46. doi: 10.1046/j.1360-0443.2001.961333.x. https://doi.org/10.1080/09652140020016941. [DOI] [PubMed] [Google Scholar]
- Gibbons RD, Hedeker D, DuToit S. Advances in analysis of longitudinal data. Annual Review of Clinical Psychology. 2010;6:79–107. doi: 10.1146/annurev.clinpsy.032408.153550. https://doi.org/10.1146/annurev.clinpsy.032408.153550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwaltney CJ, Bartolomei R, Colby SM, Kahler CW. Ecological momentary assessment of adolescent smoking cessation: a feasibility study. Nicotine & Tobacco Research. 2008;10(7):1185–90. doi: 10.1080/14622200802163118. https://doi.org/10.1080/14622200802163118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Wardell JD, Strang NM, Markovich MSD, Claus ED, Ramchandani VA. Application of an Alcohol Clamp Paradigm to Examine Inhibitory Control, Subjective Responses and Acute Tolerance in Late Adolescence. Experimental and Clinical Psychopharmacology. 2015;23(3):147–158. doi: 10.1037/pha0000017. https://doi.org/10.1037/pha0000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hustad JTP, Carey KB. Using calculations to estimate blood alcohol concentrations for naturally occurring drinking episodes: a validity study. Journal of Studies on Alcohol. 2005;66(1):130–8. doi: 10.15288/jsa.2005.66.130. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15830913. [DOI] [PubMed] [Google Scholar]
- Ihssen N, Cox WM, Wiggett A, Fadardi JS, Linden DEJ. Differentiating Heavy from Light Drinkers by Neural Responses to Visual Alcohol Cues and Other Motivational Stimuli. Cerebral Cortex. 2011;21:1408–1415. doi: 10.1093/cercor/bhq220. https://doi.org/10.1093/cercor/bhq220. [DOI] [PubMed] [Google Scholar]
- Johnston LD, O’Malley PM, Miech RA, Bachman JG, Schulenberg JE. Monitoring the Future results on drug use: 1975’2014: Overview, key findings on adolescent drug use 2015 [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Ryan N. Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36(7):980–8. doi: 10.1097/00004583-199707000-00021. https://doi.org/10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- King AC, Hasin D, Connor SJO, Mcnamara PJ, Cao D. Archival Report A Prospective 5-Year Re-examination of Alcohol Response in Heavy Drinkers Progressing in Alcohol Use Disorder. Biological Psychiatry. 2016;79(6):489–498. doi: 10.1016/j.biopsych.2015.05.007. https://doi.org/10.1016/j.biopsych.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, McNamara PJ, Hasin DS, Cao D. Alcohol challenge responses predict future alcohol use disorder symptoms: a 6-year prospective study. Biological Psychiatry. 2014;75(10):798–806. doi: 10.1016/j.biopsych.2013.08.001. https://doi.org/10.1016/j.biopsych.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AC, Wit HDe, Mcnamara PJ, Cao D. Rewarding, Stimulant, and Sedative Alcohol Responses and Relationship to Future Binge Drinking. Archives of General Psychiatry. 2011;68(4):389–399. doi: 10.1001/archgenpsychiatry.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CS, Kaczynski NA, Maisto SA, Bukstein OM, Moss HB. Patterns of DSM-IV Alcohol Abuse and Dependence Symptoms in Adolescent Drinkers. Journal of Studies on Alcohol & Drugs. 1995;56:672–680. doi: 10.15288/jsa.1995.56.672. [DOI] [PubMed] [Google Scholar]
- Matthews DB, Miller WR. Estimating Blood Alcohol Concentration: Two Computer Programs and Their Applications. Addictive Behaviors. 1979;4:55–60. doi: 10.1016/0306-4603(79)90021-2. [DOI] [PubMed] [Google Scholar]
- Miranda R, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. Journal of Abnormal Psychology. 2014;123(1):117–29. doi: 10.1037/a0035328. https://doi.org/10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda R, Jr, Monti PM, Ray L, Treloar HR, Reynolds EK, Ramirez J, Magill M. Characterizing subjective responses to alcohol among adolescent problem drinkers. Journal of Abnormal Psychology. 2014;123(1):117–129. doi: 10.1037/a0035328. https://doi.org/10.1037/a0035328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti PM, Binkoff Ja, Abrams DB, Zwick WR, Nirenberg TD, Liepman MR. Reactivity of alcoholics and nonalcoholics to drinking cues. Journal of Abnormal Psychology. 1987;96(2):122–126. doi: 10.1037//0021-843x.96.2.122. https://doi.org/10.1037/0021-843X.96.2.122. [DOI] [PubMed] [Google Scholar]
- Morean ME, Corbin WR. Subjective response to alcohol: A critical review of the literature. Alcoholism: Clinical and Experimental Research. 2010;34:385–395. doi: 10.1111/j.1530-0277.2009.01103.x. https://doi.org/10.1111/j.1530-0277.2009.01103.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Renton RM. High risk groups often have higher levels of alcohol response than low risk: the other side of the coin. Alcoholism, Clinical and Experimental Research. 2010;34(2) doi: 10.1111/j.1530-0277.2009.01081.x. 199-202-5. https://doi.org/10.1111/j.1530-0277.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: A critical review and analysis. Psychological Bulletin. 1990;108(3):383–402. doi: 10.1037/0033-2909.108.3.383. https://doi.org/10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- Piasecki TM, Jahng S, Wood PK, Robertson BM, Epler AJ, Cronk NJ, Sher KJ. The subjective effects of alcohol-tobacco co-use: an ecological momentary assessment investigation. Journal of Abnormal Psychology. 2011;120(3):557–71. doi: 10.1037/a0023033. https://doi.org/10.1037/a0023033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB. Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: an ecological study. Psychology of Addictive Behaviors: Journal of the Society of Psychologists in Addictive Behaviors. 2008;22(2):230–9. doi: 10.1037/0893-164X.22.2.230. https://doi.org/10.1037/0893-164X.22.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn PD, Fromme K. Subjective response to alcohol challenge: A quantitative review. Alcoholism: Clinical and Experimental Research. 2011;35:1759–1770. doi: 10.1111/j.1530-0277.2011.01521.x. https://doi.org/10.1111/j.1530-0277.2011.01521.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez J, Miranda R. Alcohol craving in adolescents: Bridging the laboratory and natural environment. Psychopharmacology. 2014;231:1841–1851. doi: 10.1007/s00213-013-3372-6. https://doi.org/10.1007/s00213-013-3372-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raudenbush SW, Bryk AS. Hierarchical Linear Models: Applications and Data Analysis Methods. 2. Thousand Oaks, CA: Sage Publications, Inc; 2002. [Google Scholar]
- Ray LA, Bujarski S, Roche DJO. Subjective Response to Alcohol as a Research Domain Criterion. Alcoholism: Clinical & Experimental Research. 2016;40(1):6–17. doi: 10.1111/acer.12927. https://doi.org/10.1111/acer.12927. [DOI] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Walker QD, Caster JM, Levin ED, Kuhn CM. Are adolescents more vulnerable to drug addiction than adults? Evidence from animal models. Psychopharmacology. 2009;206(1):1–21. doi: 10.1007/s00213-009-1585-5. https://doi.org/10.1007/s00213-009-1585-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA. Self-rating of alcohol intoxication by young men with and without family histories of alcoholism. Journal of Studies on Alcohol. 1980;41(3):242–249. doi: 10.15288/jsa.1980.41.242. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Subjective Responses to Alcohol in Sons of Alcoholics and Control Subjects. Archives of General Psychiatry. 1984;41(9):879–884. doi: 10.1001/archpsyc.1984.01790200061008. https://doi.org/10.1001/archpsyc.1984.01790200061008. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. Alcohol-use disorders. Lancet. 2009;373(9662):492–501. doi: 10.1016/S0140-6736(09)60009-X. https://doi.org/10.1016/S0140-6736(09)60009-X. [DOI] [PubMed] [Google Scholar]
- Schuckit MA. The Answer You Get Depends on the Question You Ask. Biological Psychiatry. 2014;75:754–755. doi: 10.1016/j.biopsych.2014.03.018. https://doi.org/10.1016/j.biopsych.2014.03.018. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Archives of General Psychiatry. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Onset and course of alcoholism over 25 years in middle class men. Drug and Alcohol Dependence. 2011;113:21–28. doi: 10.1016/j.drugalcdep.2010.06.017. https://doi.org/10.1016/j.drugalcdep.2010.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL. Stability of scores and correlations with drinking behaviors over 15 years for the Self-Report of the Effects of Alcohol Questionnaire. Drug and Alcohol Dependence. 2013;128(3):194–9. doi: 10.1016/j.drugalcdep.2012.08.022. https://doi.org/10.1016/j.drugalcdep.2012.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J, Trim RS, Cesario E, Saunders G, Campbell N. Comparison Across Two Generations of Prospective Models of How the Low Level of Response to Alcohol. Journal of Studies on Alcohol and Drugs. 2012;73:195–204. doi: 10.15288/jsad.2012.73.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serre F, Fatseas M, Debrabant R, Alexandre J-M, Auriacombe M, Swendsen J. Ecological momentary assessment in alcohol, tobacco, cannabis and opiate dependence: a comparison of feasibility and validity. Drug and Alcohol Dependence. 2012;126(1’2):118–23. doi: 10.1016/j.drugalcdep.2012.04.025. https://doi.org/10.1016/j.drugalcdep.2012.04.025. [DOI] [PubMed] [Google Scholar]
- Shiffman S. Ecological momentary assessment (EMA) in studies of substance use. Psychological Assessment. 2009;21(4):486–97. doi: 10.1037/a0017074. https://doi.org/10.1037/a0017074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied Longitudinal Data Analysis. New York, NY: Oxford University Press, Inc; 2003. [Google Scholar]
- Sobell LC, Sobell MB. In: Measuring Alcohol Consumption: Psychosocial and biochemical methods. Litten RZ, Allen JP, editors. Totowa, NJ: Humana Press; 1992. https://doi.org/10.1007/978-1-4612-0357-5. [Google Scholar]
- Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Developmental Cognitive Neuroscience. 2011;1(4):390–403. doi: 10.1016/j.dcn.2011.08.001. https://doi.org/10.1016/j.dcn.2011.08.001.Rewards. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Cheung EH, Brown GG, Frank LR, Paulus MP, Schweinsburg AD, Brown SA. Neural Response to Alcohol Stimuli in Adolescents With Alcohol Use Disorder. Archives of General Psychiatry. 2003;60:727–735. doi: 10.1001/archpsyc.60.7.727. [DOI] [PubMed] [Google Scholar]
- Thomas SE, Drobes DJ, Deas D. Alcohol cue reactivity in alcohol-dependent adolescents. Journal of Studies on Alcohol. 2005;66:354–360. doi: 10.15288/jsa.2005.66.354. [DOI] [PubMed] [Google Scholar]
- Treloar H, Pedersen SL, McCarthy DM. The Role of Expectancy in Substance-use Progression. In: Kopetz CE, Lejuez CW, editors. Addictions: A Social Psycholoigcal Perspective. New York, NY: Routledge; 2016. pp. 120–147. [Google Scholar]
- Treloar H, Piasecki TM, McCarthy DM, Sher KJ, Heath AC. Ecological Evidence that Affect and Perceptions of Drink Effects Depend on Alcohol Expectancies. Addiction. 2015 doi: 10.1111/add.12982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treloar HR, Piasecki TM, McCarthy DE, Baker TB. Relations Among Caffeine Consumption, Smoking, Smoking Urge, and Subjective Smoking Reinforcement in Daily Life. Journal of Caffeine Research. 2014;4(3):93–99. doi: 10.1089/jcr.2014.0007. https://doi.org/10.1089/jcr.2014.0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, McLellan AT. Neurobiologic Advances from the Brain Disease Model of Addiction. New England Journal of Medicine. 2016;374(4):363–371. doi: 10.1056/NEJMra1511480. https://doi.org/10.1056/NEJMra1511480. [DOI] [PMC free article] [PubMed] [Google Scholar]