Abstract
African American cigarette smokers have lower rates of cessation than Whites and live in communities with a higher number of tobacco advertisements. Exposure to smoking cues may promote smoking and undermine cessation. It may be possible to reduce attention to smoking cues (“attentional bias”). In this study, we investigated the effect of Attentional re-training (AR) on attentional bias and smoking in African-American smokers. Non-treatment seeking African American smokers (N = 64) were randomly assigned to an AR or Control condition. Participants were given a mobile device for 2 weeks and prompted to complete up to three AR (or control) trainings per day. Participants completed assessments of attentional bias, craving, and smoking both in the lab and in the field. Participants in the AR and Control conditions completed an average of 29.07 AR (SD = 12.48) and 30.61 control training tasks (SD = 13.07), respectively. AR reduced attentional bias assessed in the laboratory, F (1,126) = 9.20, p = .003, and field, F (1, 374) = 6.18, p = .01. This effect generalized to new stimuli, but not to new tasks. AR did not significantly reduce craving or biological measures of smoking. Smoking assessed on the mobile device declined over days in the AR group, F (1, 26) = 10.95, p = .003, but not in the Control group, F (1, 27) = 0.02, p = .89. Two weeks of AR administered on a mobile device reduced attentional bias in African American smokers and had mixed effects on smoking.
Keywords: Tobacco, Health Disparities, Cognition, Attentional Bias, African American
African American cigarette smokers have higher rates of lung cancer and lower rates of smoking cessation than White smokers (Siegel, Ward, Brawley, & Jemal, 2011; Trinidad, Pérez-Stable, White, Emery, & Messer, 2011). A recent review reported that population and community-based studies consistently indicate that African Americans are less likely to quit smoking than Whites (Kulak, Cornelius, Fong, & Giovino, 2016). It is, therefore, a priority to develop more effective interventions for this population (Piper et al., 2010).
Environmental influences are one of many factors that contribute to poor smoking cessation outcomes in African American smokers (Borrell et al., 2010; Lee, Henriksen, Rose, Moreland-Russell, & Ribisl, 2015; Manning, Catley, Harris, Mayo, & Ahluwalia, 2005). One environmental risk factor is that many African Americans live in communities with a disproportionately high density of tobacco advertisements compared to Whites (Lee et al., 2015; Widome, Brock, Noble, & Forster, 2013). Tobacco companies have a history of targeting advertisements to African American smokers (Anderson, 2011). Research indicates that point-of -sale advertising is associated with unplanned purchases of cigarettes, craving, and smoking (Clattenburg, Elf, & Apelberg, 2013; Hoek, Gifford, Pirikahu, Thomson, & Edwards, 2010; Wakefield, Germain, & Henriksen, 2008).
A related psychological factor is attentional bias to smoking cues. Attentional bias is an automatic cognitive process in which a smoker attends to a smoking-related cue without making the conscious decision to attend to the cue (Field & Cox, 2008). Attentional bias can be assessed with reaction time tasks, self-report measures, and eye movement measures (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010; Mogg, Bradley, Field, & De Houwer, 2003; Posner & Petersen, 1990). Theoretically, attentional bias is thought to capture the incentive salience of drug-related cues, which becomes sensitized in some individuals (Robinson & Berridge, 1993). Drug use is maintained by the ability of conditioned stimuli (i.e., drug cues) to trigger motivation for drugs (Robinson & Berridge, 1993). Empirically, some research indicates that attentional bias to smoking cues is associated with craving and relapse (Field, Duka, Tyler, & Schoenmakers, 2009; Janes et al., 2010; Powell, Dawkins, West, Powell, & Pickering, 2010; Waters et al., 2003), although data are inconsistent (Woud, Maas, Wiers, Becker, & Rinck, 2016).
Few studies have assessed attentional bias among predominately African American samples. However, a recent paper included two laboratory experiments that examined differences in attentional bias among African Americans and Whites during smoking and abstinent conditions. In study 1, 104 African American and 99 White non-treatment seeking smokers completed the Subjective Attentional Bias Questionnaire (SABQ; a self-report measure of attention to cues) and the Smoking Stroop task (a reaction time measure of attentional bias to smoking cues). In study 2, 74 African American and 110 White treatment-seeking smokers completed these assessments and attempted to quit. In both studies, African Americans had greater self-reported attentional bias as assessed by the SABQ but there was no between race difference by condition (i.e. abstinent vs. smoking conditions). In study 2, the between race difference in smoking cessation was partially mediated by self-reported attentional bias (Robinson et al., 2015). There is also evidence that African Americans have enhanced brain activation compared to Whites in response to smoking vs. neutral cues in the medial prefrontal cortex, and right lateral orbitofrontal cortex (Okuyemi et al., 2006).
In sum, both a cue-rich environment and attentional bias may increase the frequency at which a smoker looks at and attends to (‘exposure’to) a smoking cue thereby promoting relapse/use. African American smokers may, therefore, have greater difficulty quitting in part because: 1) some African Americans live in environments rich in tobacco cues, and/or 2) some African Americans have an attentional bias to smoking cues. While it is difficult to modify the environment, it may be possible to reduce exposure to smoking cues. Attentional Retraining (AR) refers to the use of modified cognitive tasks to change attentional bias (Hakamata et al., 2010) and is most often conducted using a modified visual probe (VP) task. Through training on a modified VP task, participants implicitly learn to attend away from motivationally salient stimuli (e.g., drug-related stimuli) and toward neutral stimuli. Researchers have theorized that through AR participants develop an implicit production rule, which specifies that if salient (e.g., smoking) and neutral stimuli are present in the environment then attention should shift to the neutral stimulus (Mathews & MacLeod, 2002). Some meta-analyses reveal that AR can reduce attentional bias and symptoms across a range of psychopathologies (Beard, Sawyer, & Hofmann, 2012; Hallion & Ruscio, 2011) while other meta-analyses indicate that AR may not consistently improve psychopathology symptoms (Cristea, Kok, & Cuijpers, 2015, 2016).
AR has also been applied to addiction (Beard et al., 2012), including nicotine addiction (Attwood, O'Sullivan, Leonards, Mackintosh, & Munafò, 2008; Field et al., 2009; Field & Eastwood, 2005; Mogoaşe, David, & Koster, 2014; Schoenmakers, Wiers, Jones, Bruce, & Jansen, 2007; Schoenmakers et al., 2010). There is currently little evidence that AR reduces smoking (e.g., (Attwood, O'Sullivan, Leonards, Mackintosh, & Munafò, 2008; Begh et al., 2015; Cristea et al., 2016; Kerst & Waters, 2014)). However, a recent study using six web-based sessions of AR indicated that AR increased abstinence in a subsample of heavy smokers (Elfeddali, de Vries, Bolman, Pronk, & Wiers, 2016). Therefore, delivering multiple sessions of AR may be a promising method for delivering AR (Elfeddali et al., 2016; Kerst & Waters, 2014; Lopes, Pires, & Bizarro, 2014).
Recently, mobile devices have been used to deliver AR in a natural setting (Enock, Hofmann, & McNally, 2014; Kerst & Waters, 2014). This approach may be beneficial because more “doses” of AR may lead to greater reductions, or more sustained reductions, in attentional bias. Kerst and Waters (2014) demonstrated that AR could be delivered for one week to smokers in the natural environment (Kerst & Waters, 2014).
Therefore, this study investigated the effect of AR delivered by a mobile device in a sample of non-treatment seeking African-American smokers. This is the first study to examine AR in African American smokers. Prior to studying the effect of AR on cessation it is important to determine if AR administered on mobile devices can reduce attentional bias in African American smokers. We proposed that AR would reduce attentional bias, and therefore exposure to smoking cues. We hypothesized that, compared to the control group, the AR group would 1) exhibit a significantly lower (more negative) attentional bias, 2) report lower craving, 3) report less smoking.
Method
Participants
Sixty-four non-treatment seeking adult African American smokers were recruited from the Washington, D.C., area (see Supplementary Materials for CONSORT Flow Diagram for information about recruitment). Participants were eligible if they: were aged 18 – 65; self-identified as African American; reported smoking 5 to 10 cigarettes/day (“light” smokers), or 11+ cigarettes/day (“moderate to heavy” smokers) for the past year; had a home address and telephone number; could speak, read in English; and specified English as the first language. Exclusion criteria were: regular use of tobacco products other than cigarettes; use of bupropion, varenicline, or nicotine replacement products; currently trying to quit smoking; another household member enrolled in the study; color vision deficiency; breath carbon monoxide (CO) <8 ppm for light and <10 ppm for moderate to heavy smokers; pregnant or breastfeeding. Inclusion and exclusion criteria were assessed during the phone screening and the baseline visit. The study was approved by the Institutional Review Board of the Uniformed Services University of the Health Sciences.
Procedure
Eligible participants attended a baseline visit (Visit 1) where research staff provided a description of the study, answered questions, confirmed eligibility, and obtained written informed consent (see Supplementary Materials). Eligible participants performed cognitive assessments, completed self-report measures, provided breath and saliva samples, and received training on using a personal digital assistant (PDA). Participants were asked to record the number of cigarettes they smoked in a smoking diary. Participants were told that they could “smoke as much or as little as they liked” throughout the study.
Participants were instructed to carry a PDA with them for two weeks in the “field” (i.e., as they went about their daily life). Participants were prompted to complete four PDA field “assessments” (interactions with the PDA) per day which included the three AR tasks (AR subjects), three Control tasks (Control subjects), and one Assessment task (all subjects), which included a VP assessment (described later). Using the selected wake-up time and bed time on a given day, the program divided the day into four equal “periods”. An RA was scheduled at a random time during each period. Participants completed random assessments (RAs) and participant-initiated assessments. For RAs the participants were alerted at random times by the PDA to complete the tasks. Participants were permitted to complete a participant-initiated assessment if they missed an RA. Of the assessments completed by the AR group, 74.98% were RAs and 25.02% were participant-initiated; for Controls, 74.56 % were RAs and 25.44% were participant-initiated. The median interval between completed PDA assessments was 3.67 hours. Participants also completed an eye tracking assessment that will not be reported in this paper.
Participants attended two weekly follow-up visits in the laboratory (Visits 2 and 3). These visits occurred during the two-week period that the participants carried the PDA in the field. Cognitive, self-report, and biological assessments were re-administered at these visits (Supplementary Materials, Table S1). Participants were compensated $20 for each visit, $3 for each day in the study, and $1 for each completed RA. No compensation was provided for participant-initiated assessments.
Randomization
Participants were randomized into the AR or Control group, stratified by gender and reported level of smoking at telephone screening (light = 5–10 cigarettes per day; moderate to heavy = 11+ cigarettes per day). Participants and research assistants were blinded to group assignment; in a post-treatment questionnaire, 65.4% of AR participants and 77.3% of Control participants reported that they believed they were in the active (AR) group.
Measures
At baseline, participants completed an author constructed demographics questionnaire. The Fagerström Test for Nicotine Dependence (FTND) was used to assess nicotine dependence at the baseline session (Heatherton, Kozlowski, Frecker, & Fagerstrom, 1991). The FTND is a widely used measure in general and in studies with African American smokers (Ahluwalia, Harris, Catley, Okuyemi, & Mayo, 2002; de Meneses-Gaya, Zuardi, Loureiro, & de Souza Crippa, 2009; Okuyemi et al., 2003). In this sample, Cronbach’s alpha = .60, which is consistent with prior research (Okuyemi et al., 2007) .
Attentional Bias
Attentional bias was assessed using the standard Visual Probe (VP) task. In a VP task, a series of picture pairs (one motivationally salient and the other neutral) are presented relatively briefly (500 ms) on a computer screen, with one picture on the left and the other on the right. When the pair disappears, a probe is presented in a position formerly occupied by one of the pictures. Participants are required to indicate the location of the probe as quickly and accurately as possible. Typically, individuals are faster to respond to probes that replace motivationally salient (vs. neutral) stimuli because attention has shifted towards the salient stimuli. AR has been shown to reduce attentional bias in addiction using a stimulus duration of 500 ms (Schoenmakers et al., 2010)
Each field assessment included 80 trials; on 40 trials the probe replaced the smoking picture, and on 40 trials it replaced the neutral picture. In addition, in each laboratory assessment smoking stimuli were included on the VP task on which the participant had not received training (“new” pictures). This allowed an examination of whether the effect of AR generalizes to new (untrained) stimuli. In the field, one assessment per week involved new pictures. After the VP task, an item assessed how many times the participant was interrupted while performing the task (No times; 1 time; 2 times; 3 times; 4+ times).
To determine whether an effect of AR can be observed on a different attentional bias task, the Smoking Stroop (SS) task was used (Waters et al., 2003). In the SS task, 33 trials of smoking words and 33 trials of neutral words were administered at each laboratory visit on a PDA. Participants were told that a series of words would be presented on the screen, one after the other and that the task was to indicate the color of the word as quickly and as accurately as possible. They were told that they could ignore the meaning of the words; they were just required to respond to the colors. In the current study, the estimated internal reliability for the smoking Stroop effect calculated using split-half reliability (even vs. odd trials, using Spearman-Brown formula) was: visit 1) r = .73; visit 2) r = .54; and visit 3) r = .80 (average r = .69).
Self-reported attentional bias was assessed with the Subjective Attentional Bias Questionnaire (SABQ), an eight-item questionnaire that assesses the extent to which participants notice that their attention is captured by cigarettes and smoking cues (Leventhal et al., 2007;Robinson et al., 2015). In the current sample, Cronbach’s alpha for the SABQ was: visit 1) α = .90; visit 2) α = .88; and visit 3) α =.91.
Self-reported attentional bias was assessed on the PDA at each assessment using a single-item: “Since the last assessment, my attention has often been drawn to cigarettes” (1 = strongly disagree, 7 = strongly agree). Ratings on this item have been shown to be associated with attentional bias on the modified Stroop task (Waters et al., 2014). During each PDA field assessment, participants were asked about their exposure to tobacco advertisements since the last assessment (five response options: 1=No advertisements seen; 2=One advertisement seen; 3=Two advertisements seen; 4=Three advertisements seen; 5=Four or more advertisements seen).
Craving
The 10-item Brief Questionnaire of Smoking Urges (“QSU”) assessed craving for cigarettes in the laboratory (Cox, Tiffany, & Christen, 2001). This widely used measure is frequently used in studies with African Americans and has been validated with light African American smokers (Clausius et al., 2012). In the current sample, Cronbach’s alpha was: visit 1) α = .91; visit 2) α = .92; and visit 3) α = .94.
On the PDA, craving for cigarettes was assessed on a 7-point scale (1= no craving, 7 = extreme craving) at each PDA assessment. A second craving item was used (Kerst & Waters, 2014) to assess craving in response to smoking and neutral cues. A picture with both smoking and non-smoking content was presented for 1 second. Participants subsequently reported their craving (1–7 scale). If AR causes attention to be drawn to neutral stimuli in the picture (as predicted), then “exposure” to the smoking content should be reduced and there should be reduced craving on this item. Other research has also shown that cue-provoked craving can be assessed using photographic images on a PDA (Wray, Godleski, & Tiffany, 2011).
Cigarette Smoking
Participants entered the number of cigarettes smoked each day on a paper and pencil smoking diary which they handed to the researcher at lab visits 2 and 3. Furthermore, smoking was assessed on the PDA using the following item: “Since the last assessment, how many cigarettes have you smoked?” (response options: 1=None; 2=one cigarette; 3=two cigarettes; 4=three cigarettes; 5=four or more cigarettes) (see Supplementary Materials, PDA Smoking item).
Smoking was also assessed with salivary cotinine (Ossip-Klein et al., 1986). Exhaled Carbon Monoxide levels were measured with a CO monitor (Bedfont Micro Smokerlyzer).
At Visit 1, if the CO monitor indicated that a participant’s expired CO level was low (<8 parts per million (ppm) for light smokers, <10 ppm for moderate to heavy smokers) (Ahluwalia et al., 2006), he or she was excluded because there is serious doubt as to whether the individual actually smokes at their stated rate (Benowitz et al., 2002).
Intervention
Participants were scheduled to complete three AR or Control tasks per day. On AR tasks, the probe always replaced the neutral picture (100% replacement). There is a perfect association between picture type and probe location. As noted above, participants also received a daily (briefer) assessment in which the probe replaced the smoking and neutral picture with equal frequency. The use of 100% replacement on AR tasks was used to minimize the impact of assessment on AR. Furthermore, this approach (100% replacement) is consistent with other AR smoking studies (e.g.,(Begh et al., 2015; Elfeddali et al., 2016; Kerst & Waters, 2014)).
On Control tasks, the probe was equally likely to replace the smoking picture and the neutral picture (50% replacement). There was no association between picture type and probe location. This control condition has been used previously (e.g. (Field et al., 2009; Kerst & Waters, 2014)). Note that the duration of AR and Control training should be similar. In addition, AR and control participants receive equal practice on the VP tasks, and they are exposed to the same pictures (smoking and neutral). Each AR and Control task had 160 trials. Stimuli for the AR and Control tasks were created by the authors and included images of exclusively African Americans.
Mobile Device Hardware and Software
The Hewlett-Packard IPAQ Personal Digital Assistant (PDA) runs on the Windows Mobile Operating system. Application programming was done in C#.NET. Participants used the touch screen to enter responses.
Stimuli
Pictures for the AR/control intervention and the VP task were selected from pictures stored on Flicker and pictures created by the author. The pictures included images of African Americans holding cigarettes, pictures of menthol cigarette packages, and brands frequently consumed by African Americans such as Newport (Anderson, 2011; Glasser et al., 2016). We also included pictures of cigarette-related stimuli including lighters and ashtrays. Permission was obtained from the photographers and a photography credits sheet was provided to participants. Pictures were assessed by two independent raters. The raters judged valence, noticeability of smoking stimulus (if present), and suitability for the current study (1–7). Based on ratings, the authors selected 160 smoking pictures and 160 neutral pictures (see Supplementary Materials, Stimuli, for more detail).
Analytic Plan
Reaction time data processing including exclusions of data are described in the Supplementary Materials. Median reaction times (on correct responses) were used to reduce the influence of outliers. Attentional bias was computed as the difference in median reaction times to respond to probes that replaced smoking and neutral pictures. A positive attentional bias score means faster responses to probes that replaced smoking pictures than probes that replaced neutral pictures, meaning that attention has shifted toward the smoking picture. A negative attentional bias score indicates faster responses to probes that replaced neutral pictures than probes that replaced smoking pictures, meaning that attention has shifted away from the smoking picture or avoidance.
Linear mixed models (LMM) (PROC MIXED in SAS) (Littell, Stroup, Milliken, Wolfinger, & Schabenberger, 2006) were used for analyses. LMMs allow for the fact that subjects differ in the number of observations available for analysis, and take into account the clustering of data by subjects. All tests were 2-tailed, and alpha was set to .05.
To analyze field data, Day in the study (a within-subject continuous variable) was entered as a continuous variable, along with Group (AR vs. control). The Group x Day interaction term was also tested. A random (subject-specific) intercept and an autoregressive model of order 1 for the residuals within subjects was used. The within-subject variable, Day, was treated as a random effect in the model if the p-value for the covariance parameter estimate (for Day) was less than 0.1 (Fitzmaurice, Laird, & Ware, 2012). Time of day (continuous variable) and Assessment Type (RAs vs. participant-initiated) were included as covariates.
To analyze laboratory data, LMMs were also used, with one between-subjects factor (Group: 2 levels) and one within-subject factor (Visit) with two levels (Visit 2 vs. Visit 3) included in all models. The Group x Visit interaction term was also tested.
For field and laboratory data, each dependent variable was tested in a separate model, and baseline (i.e., pre-intervention) measures of these variables were included as a covariate in the respective analyses. For attentional bias data, Picture Type (old vs. new) was included as an additional independent variable.
Results
AR and Control participants did not differ by age, sex, SABQ, QSU, FTND, cigarettes per day, age of smoking initiation or lifetime quit attempts (Table 1). Completion was defined as providing data at visit 3. Completers (vs. Non-completers) did not differ by age, sex, SABQ, cigarettes per day, age of smoking initiation or lifetime quit attempts (all ps > .19). Group (AR vs. Control) was not associated with Completion, χ2 (1) = 1.31, p = .25. The sample was generally of low socio-economic status. The majority of participants (50.82%) reported an income of less than $10,000 per year and that they were unemployed or unable to work (64%). Average years of education completed was 12 years (high school).
Table 1.
Participant Characteristics at Baseline
| AR (n = 31) | CON (n = 33) | t/χ2 | p | |
|---|---|---|---|---|
| Age | 43.38 (13.41) | 43.09 (12.35) | −0.09 | .92 |
| Sex (%) | 0.23 | .63 | ||
| Male | 51.61 | 48.39 | ||
| Female | 57.58 | 42.42 | ||
| FTND | 4.16 (2.35) | 4.64 (2.28) | 0.82 | .41 |
| QSU | 2.68 (1.27) | 3.18 (1.22) | 1.60 | .12 |
| SABQ | 1.33 (0.79) | 1.72 (0.93) | 1.77 | .08 |
| Cigarettes per day | 12.94 (7.27) | 13.33 (7.16) | 0.22 | .82 |
| Age when started daily smoking | 20.19 (6.40) | 20.12 (6.30) | 0.00 | .96 |
| Lifetime quit attempts (+24 hrs) | 2.94 (5.73) | 1.51 (1.97) | −1.34 | .18 |
Note. Mean (SD) for Participant Demographics. Continuous variables were examined using t-tests. Chi Square values are computed for categorical variables.
p <.05.
AR = Attention retraining, CON = Control, t = t-value, χ2 = Chi Square, FTND = Fagerström, Test for Nicotine Dependence, QSU = Questionnaire for Smoking Urges, SABQ = Subjective Attentional Bias Questionnaire.
Summary statistics for lab and field dependent variables are presented in Table 2. Participants who provided field data (n = 56) completed at least one item from 2,419 trainings/assessments, and completed (in their entirety) 2,211 (91.40%) of these trainings/assessments (AR group = 92.46%, Control group = 90.42%). Time of day of completion of trainings/assessments was similar for AR (before 12 p.m. = 28.36%; 12 p.m. to 4 p.m. = 27.53%; 4 to 8 p.m. = 26.97%; and after 8 p.m. =17.15%) and Control groups (before 12 p.m. = 22.7%; 12 p.m. to 4 p.m. = 30.65%; 4 to 8 p.m. = 30.21%; and after 8 p.m. = 16.43%).
Table 2.
Summary Statistics on Lab and Field Dependent Variables by Training Group and Time
| Group ↓ |
Lab Visit → Day → |
Visit 1 | Visit 2 | Visit 3 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14+ | |||||
| AR | VP Bias (ms) | −6.35 (34.76) | −10.50 (38.43) | 9.32 (31.78) | 4.77 (52.65) | −16.05 (69.14) | −9.43 (62.16) | −29.13 (121.63) | −9.63 (63.26) | 0.09 (26.27) | −22.66 (40.86) | −53.35 (215.22) | −15.44 (81.30) | −15.82 (46.14) | −50.23 (131.53) | 31.92 (85.05) | 20.70 (85.45) | −15.13 (32.06) |
| Reported Attention (1–7) | 3.58 (1.95) | 3.16 (1.68) | 2.73 (1.86) | 3.18 (1.92) | 3.71 (2.01) | 3.12 (2.07) | 3.05 (2.03) | 3.18 (1.93) | 3.05 (2.02) | 3.06 (2.17) | 2.97 (2.16) | 2.75 (1.91) | 2.79 (1.92) | 3.25 (2.20) | ||||
| Stroop (ms) | 33.56 (116.92) | 34.83 (92.04) | 38.40 (187.19) | |||||||||||||||
| SABQ (0–4) | 1.33 (0.79) | 1.19 (0.74) | 0.94 (0.76) | |||||||||||||||
| Advertisements (1–5) | 2.30 (1.36) | 1.91 (1.33) | 1.65 (1.19) | 1.73 (1.22) | 1.76 (1.29) | 1.91 (1.37) | 1.86 (1.40) | 1.88 (1.39) | 1.86 (1.52) | 1.98 (1.42) | 1.50 (1.40) | 1.48 (1.08) | 1.82 (1.45) | 1.89 (1.47) | ||||
| Cued Craving (1–7) | 3.60 (1.96) | 3.54 (1.94) | 3.01 (2.05) | 3.10 (2.04) | 3.55 (2.01) | 3.00 (2.12) | 3.24 (2.05) | 3.25 (1.95) | 3.16 (2.25) | 3.08 (2.23) | 2.95 (2.21) | 2.65 (1.89) | 2.45 (1.75) | 2.88 (2.18) | ||||
| Craving (1–7) | 3.69 (1.90) | 3.31 (1.75) | 2.84 (1.84) | 3.18 (1.93) | 3.58 (1.95) | 2.94 (3.05) | 3.20 (2.00 | 3.21 (2.10) | 3.21 (2.10) | 3.04 (2.24) | 3.13 (2.23) | 2.55 (1.87) | 2.82 (1.95) | 3.32 (2.15) | ||||
| QSU (0–5) | 2.69 (1.27) | 2.23 (1.25) | 2.08 (1.45) | |||||||||||||||
| Smoking (1–5) | 3.42 (1.44) | 3.80 (1.44) | 3.46 (1.47) | 3.25 (1.48) | 3.32 (1.53) | 3.53 (1.52) | 3.38 (1.54) | 3.46 (1.54) | 3.38 (1.53) | 3.17 (1.64) | 3.25 (1.53) | 3.33 (1.50) | 3.20 (1.61) | 3.38 (1.39) | 3.28 (1.37) | |||
| No. Cigs (Diary) | 10.85 (6.77) | 11.30 (7.06) | 11.56 (7.64) | 12.48 (8.45) | 12.11 (8.59) | 11.67 (8.33) | 10.19 (8.89) | 10.54 (7.28) | 10.13 (7.38) | 9.96 (7.74) | 10.79 (9.15) | 10.82 (9.29) | 10.22 (7.80) | 12.44 (10.79) | ||||
| Cotinine (ng/mL) | 366.06 (39.36) | 415.83 (261.50) | 377.03 (259.35) | |||||||||||||||
| CO (ppm) | 12.16 (5.21) | 9.21 (5.98) | 9.15 (5.29) | |||||||||||||||
| CON | VP Bias (ms) | −1.66 (44.87) | −15.19 (45.75) | 17.29 (50.60) | 35.77 (101.32) | 18.93 (39.93) | −1.13 (106.93) | 10.10 (82.20) | 4.03 (77.72) | 15.82 (35.64) | 40.32 (118.51) | 4.14 (27.15) | 2.29 (55.99) | −16.61 (58.42) | 51.78 (200.65) | 15.75 (43.67) | 21.44 (208.19) | 10.33 (29.76) |
| Reported Attention (1–7) | 3.72 (2.00) | 3.53 (1.82) | 3.75 (1.83) | 3.28 (1.80) | 3.40 (1.82) | 3.36 (1.92) | 3.24 (1.943) | 3.45 (2.07) | 3.48 (2.01) | 2.77 (1.71) | 2.97 (1.71) | 2.97 (1.85) | 3.13 (2.06) | 2.99 (1.73) | ||||
| Advertisements (1–5) | 1.91 (1.13) | 1.88 (1.21) | 1.65 (1.11) | 1.76 (1.32) | 1.62 (1.14) | 1.59 (1.02) | 1.56 (.94) | 1.70 (1.07) | 1.66 (.99) | 1.58 (1.06) | 1.70 (1.27) | 1.63 (1.09) | 1.65 (1.13) | 1.74 (1.27) | ||||
| Stroop (ms) | 20.86 (120.40) | −5.38 (135.66) | −21.08 (129.62) | |||||||||||||||
| SABQ (0–4) | 1.75 (0.92) | 1.51 (0.81) | 1.29 (0.82) | |||||||||||||||
| Cued Craving (1–7) | 3.83 (2.05) | 4.22 (2.09) | 3.94 (1.86) | 3.43 (1.88) | 3.34 (1.97) | 3.37 (1.99) | 3.45 (2.21) | 3.49 (2.02) | 3.70 (2.17) | 2.94 (1.94) | 3.26 (2.00) | 3.11 (2.10) | 3.24 (2.15) | 3.13 (1.98) | ||||
| Craving (1–7) | 3.93 (2.11) | 3.95 (2.06) | 3.84 (1.86) | 3.42 (1.83) | 3.44 (1.96) | 3.48 (1.97) | 3.38 (2.10) | 3.76 (2.12) | 3.71 (2.16) | 2.98 (1.88) | 3.06 (1.98) | 3.22 (2.04) | 3.16 (2.11) | 3.14 (1.82) | ||||
| QSU (0–5) | 3.23 (1.27) | 3.93 (2.03) | 2.54 (1.21) | |||||||||||||||
| Smoking (1–5) | 3.28 (1.50) | 3.31 (1.51) | 3.31 (1.49) | 3.33 (1.51) | 3.17 (1.44) | 3.58 (1.48) | 3.29 (1.59) | 3.42 (1.34) | 3.34 (1.43) | 3.48 (1.47) | 3.59 (1.49) | 3.5 (1.34) | 3.53 (1.42) | 3.38 (1.44) | ||||
| No. Cigs (Diary) | 10.74 (5.73) | 12.07 (5.90) | 10.63 (6.52) | 10.00 (6.66) | 10.11 (6.20) | 10.77 (7.20) | 10.07 (6.91) | 9.88 (5.78) | 11.00 (6.54) | 10.58 (6.07) | 10.83 (6.88) | 10.56 (6.81) | 10.27 (6.95) | 10.53 (7.17) | ||||
| Cotinine (ng/mL) | 388.89 (67.94) | 419.1 (237.90) | 451.06 (294.60) | |||||||||||||||
| CO (ppm) | 12.19 (4.33) | 10.62 (5.52) | 10.30 (7.34) |
Note. Mean (SD) for study measures. Data derive from PDA assessments Days 1 – 14 or smoking diaries and Lab visits 1–3. AR= Attention Retraining, CON = control training, No. Cigs (Diary) = Number of cigarettes reported in smoking diary, per day, VP Bias = Visual Probe attentional bias score, CO = expired carbon monoxide, CON = control training, Questionnaire, QSU = Questionnaire for Smoking Urges, SABQ = Subjective Attentional Bias, Stroop = Smoking Stroop Task.
Completion of Trainings
Participants were scheduled to complete 42 trainings and 14 assessments. AR and Control participants (n = 28, respectively) completed a mean of 29.07 (SD = 12.48) and 30.61 training tasks (SD = 13.07) respectively, F (1, 54) = 0.20, p = .65, and a mean of 9.46 (SD = 4.53) and 9.82 assessment tasks (SD = 4.57) respectively, F (1, 54) = 0.09, p = .77. Mean duration of completed trainings was 10.58 minutes (SD = 0.90) and 6.25 minutes (SD = 0.68) for completed assessments (AR group), and 10.68 minutes (SD = 0.82) and 7.44 minutes (SD = 1.98) for completed assessments (Control group).
Effect of AR on Attentional Bias
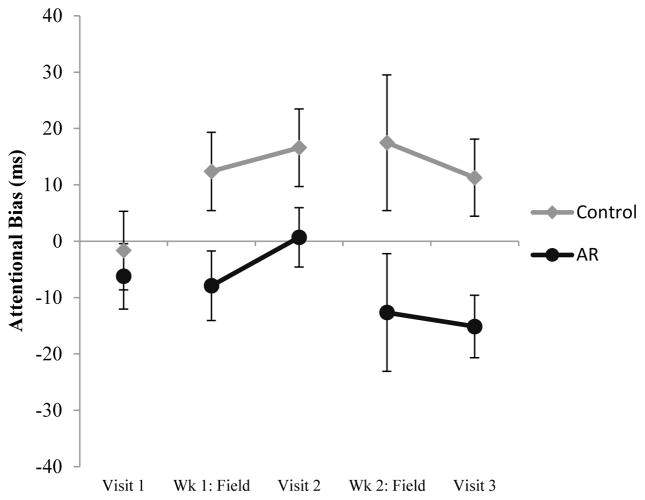
LMMs on laboratory and field data revealed a significant main effect of Group (Table 3, Figure 1). Attentional bias was 19.24 ms lower in the AR group (vs. Control) in the laboratory, and 24.06 ms lower in the field. There was no evidence that the effect of Group was greater in old (vs. new) pictures (no Group x Picture Type interaction) either in the laboratory, F (1, 125) = 1.46, p = .23, or in the field, F (1, 372) = 0.22, p = .64.
Table 3.
Results of Linear Mixed Models for Laboratory and Field data
| n1 | n2 | Group | Group x Time | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| df | PE | SE | F | p | df | PE | SE | F | P | |||
| Lab Data | ||||||||||||
| VP Bias (ms) | 183 | 55 | 1,126 | −19.24 | 6.34 | 9.20 | .003 | 1,125 | 10.02 | 12.14 | 0.68 | .41 |
| Stroop (ms) | 93 | 55 | 1,37 | 51.39 | 32.94 | 2.43 | .13 | 1,36 | −28.73 | 49.35 | 0.34 | .56 |
| SABQ (0–4) | 101 | 56 | 1,44 | −0.07 | 0.15 | 0.25 | .62 | 1,43 | −0.02 | 0.21 | 0.01 | .93 |
| QSU (0–5) | 101 | 56 | 1,44 | 0.13 | 0.24 | 0.28 | .60 | 1,43 | 0.04 | 0.25 | 0.03 | .86 |
| CO (ppm) | 105 | 57 | 1,47 | −0.54 | 1.25 | 0.19 | .67 | 1,46 | 0.02 | 1.50 | 0.00 | .99 |
| Cotinine (ng/mL) | 101 | 57 | 1,43 | −11.39 | 46.87 | 0.06 | .81 | 1,42 | 51.25 | 50.58 | 0.32 | .57 |
| Field Data | ||||||||||||
| VP Bias (ms) | 481 | 53 | 1, 374 | −24.06 | 9.68 | 6.18 | .01 | 1, 374 | 0.34 | 2.63 | 0.02 | .90 |
| Self-Reported Attention (1–7) | 2249 | 56 | 1, 2081 | −0.23 | 0.23 | 0.95 | .33 | 1, 2081 | 0.02 | 0.02 | 1.12 | .29 |
| Advertisements (1–5) | 2241 | 56 | 1, 2075 | 0.05 | 0.12 | 0.20 | .65 | 1, 2075 | 0.00 | 0.12 | 0.00 | .96 |
| Cued Craving (1–7) | 2276 | 56 | 1, 2108 | −0.45 | 0.28 | 2.50 | .11 | 1, 2108 | 0.02 | 0.03 | 0.44 | .51 |
| Craving (1–7) | 2276 | 56 | 1, 2108 | −0.39 | 0.28 | 2.00 | .16 | 1, 2108 | 0.03 | 0.03 | 1.20 | .27 |
| Smoking (PDA) (1–5) | 2225 | 56 | 1, 2057 | 0.02 | 0.24 | 0.01 | .93 | 1, 2057 | −0.04 | 0.02 | 3.91 | .04 |
| No. Cigs (Diary) | 738 | 54 | 1, 683 | 1.25 | 1.07 | 1.38 | .24 | 1, 682 | −0.01 | 0.05 | 0.05 | .82 |
Note. n1 = number of level 1 units, i.e., assessments, visits, or days; n2 = number of subjects (level two units) who completed at least one assessment at visit 2 or visit 3 (lab data) and at least one PDA assessment (field data). Columns labeled Group show the results for the main effect of Group. The comparison category is CON (Negative parameter estimates indicate lower values for AR vs. CON). Columns labeled Group x Time shows the results for the Group x Visit (lab data) or Group x Day (field data) interaction term. Visit is a categorical variable with two levels (visit 2 vs. visit 3). All models include main effects for Group and Visit. The LMM for VP Bias also includes Picture Type (old vs. new) as an additional within-subject factor (lab data). In addition, the baseline (pre-intervention) measure for each dependent variable was included as a covariate. Data for analysis of VP Bias use all assessments with no more than one reported interruption (see text). Key: CO = expired carbon monoxide, QSU = Brief Questionnaire for Smoking Urges, SABQ = Subjective Attentional Bias Questionnaire, VP Bias = Visual Probe attentional bias score, PE = (unstandardized) parameter estimate; SE = standard error; F = F value from mixed model
Figure 1.
Attentional bias assessed during PDA field assessments and at laboratory visits. Note: Attentional Bias = Visual Probe attentional bias score, Wk = Week. Data for PDA field assessments are aggregated over all assessments during Wk 1 or Wk 2. Data are also aggregated over old and new pictures (both field assessments and laboratory assessments). Error bars are ±1 SE.
An attentional bias score can also be computed on Control trainings, because on control trainings the probe is equally likely to replace smoking and neutral pictures. (Attentional bias scores cannot be computed on AR trainings because the probe never replaces smoking pictures). Control participants exhibited a significant attentional bias (attentional bias significantly different from 0) on Assessments, PE = 14.88, SE = 6.85, p = .039, which was not significantly different (p = .18) from attentional bias assessed at Control trainings, PE = 7.31, SE = 3.34, p = .037. In sum, in the field Control participants exhibited a significant (positive) attentional bias at both Assessments and Trainings. In contrast, at Assessments AR participants exhibited a (non-significant) negative attentional bias, PE = −8.80, SE = 7.10, p = .22. At laboratory visit 3 only, AR participants did exhibit significant avoidance (p = .03).
There were no significant effects involving Group for Stroop (laboratory), SABQ (laboratory), self-reported attentional bias (field) and self-reported exposure to advertisements (field) (Table 3).
Effect of AR on Craving
There were no significant effects involving Group for QSU (laboratory), craving (field), or cued craving (field) (Table 3).
Effect of AR on Smoking
There were no significant effects involving Group for CO and cotinine levels (laboratory) or reported cigarettes smoked in the diary (Table 3).
LMM analyses on cigarettes smoked revealed a significant Group by Day interaction (Table 3) (see Supplementary Materials for additional analyses). Smoking rate declined over days in the AR group, PE = −0.04, SE = 0.01, F (1, 26) = 10.95, p = .003, but not the Control group, PE = 0.00, SE = 0.02, F (1, 27) = 0.02, p = .89.
Discussion
The main findings were as follows. First, AR (vs. Control) significantly reduced attentional bias to smoking cues on the VP task in the laboratory and field. The effect of AR generalized to new pictures on the VP task, but not to attentional bias assessed on the smoking Stroop task, or self-report measures of attentional bias. Second, AR (vs. Control) did not reduce craving. Third, AR, but not Control training, significantly reduced the number of cigarettes smoked over time when assessed on the PDA. There was no effect of AR on self-reported cigarettes smoked in the smoking diary or on biological measures of smoking.
As expected, there was consistent evidence that Control, but not AR, participants exhibited attentional bias (attention toward smoking cues) in the field. In contrast, AR participants tended to exhibit a negative attentional bias (attention away from smoking cues, or “avoidance”) although this bias was not significantly different from zero in the field data. In contrast to a previous study (Kerst & Waters, 2014), a Group x Day interaction was not observed, indicating that the effect of AR did not get larger over time.
Another complication is that, for reasons that are not clear, neither group exhibited significant attentional bias at baseline. Speculatively, attentional bias may be less robust in the current sample because it included a mix of light and moderate to heavy smokers. A prior study indicated significant pre-treatment bias in heavy smokers only and an effect of AR on smoking in the heavy smokers only (Elfeddali et al., 2016). The absence of attentional bias at baseline is also inconsistent with some previous AR studies that have reported significant attentional bias at baseline (e.g.,(Attwood et al., 2008; Field et al., 2009; Kerst & Waters, 2014; Lopes et al., 2014)). On the other hand, two AR studies reported no significant attentional bias at baseline (Begh et al., 2015; McHugh, Murray, Hearon, Calkins, & Otto, 2010), and no effect of AR on attentional bias (Begh et al., 2015; McHugh et al., 2010). However, as noted by Begh and colleagues, AR may still be effective for smokers without a baseline attentional bias because AR can train smokers to focus their attention away from smoking cues (Begh et al., 2015). This hypothesis is supported by studies in the anxiety literature which show an effect of AR in individuals without pre-treatment bias (Amir, Beard, Burns, & Bomyea, 2009; Amir, Weber, Beard, Bomyea, & Taylor, 2008).
Moreover, significant avoidance, which may be a desired outcome (Peuker & Bizarro, 2014), was observed at the final laboratory visit, consistent with the results of a multi-session laboratory AR protocol (Lopes et al., 2014). Not all laboratory AR studies have demonstrated avoidance in the AR group (Attwood et al., 2008; McHugh et al., 2010). Notably, these studies involved a single session of AR, and multiple sessions may be necessary to produce avoidance. Overall, there is modest evidence that AR can produce avoidance in smokers.
The effect of AR generalized to new pictures, consistent with some (Lopes et al., 2014) but not all previous research (Field et al., 2009). Field et al. (2009) used a single training session. It is possible that multiple training sessions are necessary for generalization to new pictures.
There was no evidence that the effect of AR generalized to other measures of attentional bias. These results are consistent with previous studies that have found no effect of AR on the modified Stroop task (Field et al., 2007; Field et al., 2009). Although the VP and modified Stroop are both reaction time measures, the modified Stroop involves different cognitive processes (Mogg & Bradley, 2002), and another type of AR intervention may be necessary to target the attentional bias assessed by this task (Fadardi & Cox, 2009). The effect of AR may not generalize to self-reported exposure to tobacco advertisements because these stimuli were not included in the training. Obtaining pictures of the tobacco point-of-sale environment representative of participants’ neighborhoods is a challenge but may be necessary to obtain an effect. Additional training may also be required to observe transfer effects.
AR did not reduce craving. For cued craving, this finding was counter to hypothesis and different from previous findings (Kerst & Waters, 2014). In addition, there is currently little evidence that AR reduces non-cued craving in the laboratory with several studies reporting null findings (Field et al., 2009; Kerst & Waters, 2014; Lopes et al., 2014; Schoenmakers et al., 2010).
AR had mixed effects on smoking. The AR, but not Control group, reported smoking significantly fewer cigarettes over time on the PDA item. However, there was no effect of AR on biological measures of smoking or reported cigarettes smoked on the smoking diary. Furthermore, previous research has not demonstrated an effect of AR on non-treatment seeking smokers (Kerst & Waters, 2014; Lopes et al., 2014) suggesting that any effect of AR on smoking in non-treatment seeking smokers is likely to be modest.
These points notwithstanding, the effect of AR may be more apparent on the PDA smoking item (vs. smoking diary) because it assesses smoking more frequently and with less opportunity for recall bias. Reported smoking in diaries is subject to biases and participant non-compliance (Stone, Shiffman, Schwartz, Broderick, & Hufford, 2002). In addition, biological measures assess smoking primarily over the past 24 hours (carbon monoxide) or 80–100 hours (cotinine) (Benowitz et al., 2002), indicating that the PDA assessments in this study may have provided a more comprehensive measure of smoking during the study. Notably, the current study included more training sessions than previous studies, and more AR may be necessary to reduce smoking.
The limitations of the study were as follows. First, participant attrition could lead to subtle differences in the characteristics of the individuals in the two groups, potentially undermining the internal validity of the study. Second, the study sample was non-treatment seeking and therefore could not examine the effect of AR on smoking cessation in African American smokers seeking to quit smoking. Third, study measures did not provide data on participants’ attention to smoking cues in their daily lives. Wearable technologies such as Google Glass can capture high-resolution videos, images and head movements which may allow for the collection a participant’s actual exposure to cues (Paxton, Rodriguez, & Dale, 2015). Last, there were multiple dependent variables and no correction for multiple tests. Replication is required to bolster confidence in these findings.
In summary, this was the first study to develop and administer an AR intervention to a group of ethnic minority smokers. The study comprehensively assessed attentional bias by including cognitive measures, self-reported attentional bias, and self-reported exposure to tobacco advertisements both in the lab and field. This study suggests that multiple sessions of AR can be implemented using mobile technology to African American smokers. The clinical utility of AR requires further investigation in the form of randomized controlled trials (RCTs) with African American smokers wishing to quit.
Supplementary Material
Acknowledgments
This study was funded by National Cancer Institute F31CA180625-01A1 to Cendrine Robinson. The authors thank Daniel Melvin and Faiza Coleman-Salako for assistance with data collection.
Footnotes
The authors have no conflicts of interest to declare.
Preliminary data and ideas from this manuscript were presented previously. Data were presented as a poster at the Society for Research on Nicotine and Tobacco 2015 Annual meeting in Philadelphia, PA.
Contributor Information
Cendrine D. Robinson, Uniformed Services University of the Health Sciences
Christine Muench, National Institute on Alcohol Abuse and Alcoholism.
Emily Brede, Uniformed Services University of the Health Sciences.
Romano Endrighi, Boston University.
Edwin H. Szeto, Uniformed Services University of the Health Sciences
Joanna R. Sells, Uniformed Services University of the Health Sciences
John P. Lammers, Uniformed Services University of the Health Sciences
Kolawole S. Okuyemi, University of Minnesota
Andrew J. Waters, Uniformed Services University of the Health Sciences
References
- Ahluwalia JS, Harris KJ, Catley D, Okuyemi KS, Mayo MS. Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA. 2002;288(4):468–474. doi: 10.1001/jama.288.4.468. [DOI] [PubMed] [Google Scholar]
- Ahluwalia JS, Okuyemi K, Nollen N, Choi WS, Kaur H, Pulvers K, Mayo MS. The effects of nicotine gum and counseling among African American light smokers: a 2× 2 factorial design. Addiction. 2006;101(6):883–891. doi: 10.1111/j.1360-0443.2006.01461.x. [DOI] [PubMed] [Google Scholar]
- Amir N, Beard C, Burns M, Bomyea J. Attention modification program in individuals with generalized anxiety disorder. Journal of Abnormal Psychology. 2009;118(1):28. doi: 10.1037/a0012589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir N, Weber G, Beard C, Bomyea J, Taylor CT. The Effect of a Single-Session Attention Modification Program on Response to a Public-Speaking Challenge in Socially Anxious Individuals. Journal of Abnormal Psychology. 2008;117(4):860–868. doi: 10.1037/a0013445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson SJ. Marketing of menthol cigarettes and consumer perceptions: a review of tobacco industry documents. Tobacco Control. 2011;20(Suppl 2):ii20–ii28. doi: 10.1136/tc.2010.041939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attwood AS, O'Sullivan H, Leonards U, Mackintosh B, Munafò MR. Attentional bias training and cue reactivity in cigarette smokers. Addiction. 2008;103(11):1875–1882. doi: 10.1111/j.1360-0443.2008.02335.x. [DOI] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG. Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behavior Therapy. 2012;43(4):724–740. doi: 10.1016/j.beth.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begh R, Munafò MR, Shiffman S, Ferguson SG, Nichols L, Mohammed MA, … Aveyard P. Lack of attentional retraining effects in cigarette smokers attempting cessation: A proof of concept double-blind randomised controlled trial. Drug and Alcohol Dependence. 2015;149:158–165. doi: 10.1016/j.drugalcdep.2015.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, Hall S, Tsoh J, Ahijevych K, Jarvis M, … Henningfield J. Biochemical verification of tobacco use and cessation. Nicotine and Tobacco Research. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Borrell LN, Roux AVD, Jacobs DR, Shea S, Jackson SA, Shrager S, Blumenthal RS. Perceived racial/ethnic discrimination, smoking and alcohol consumption in the Multi-Ethnic Study of Atherosclerosis (MESA) Preventive Medicine. 2010;51(3):307–312. doi: 10.1016/j.ypmed.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clattenburg EJ, Elf JL, Apelberg BJ. Unplanned cigarette purchases and tobacco point of sale advertising: a potential barrier to smoking cessation. Tobacco Control. 2013;22(6):376–381. doi: 10.1136/tobaccocontrol-2012-050427. [DOI] [PubMed] [Google Scholar]
- Clausius RL, Krebill R, Mayo MS, Bronars C, Martin L, Ahluwalia JS, Cox LS. Evaluation of the brief questionnaire of smoking urges in Black light smokers. Nicotine & Tobacco Research. 2012;14(9):1110–1114. doi: 10.1093/ntr/ntr267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief questionnaire of smoking urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3(1):7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P. Efficacy of cognitive bias modification interventions in anxiety and depression: meta-analysis. The British Journal of Psychiatry. 2015;206(1):7–16. doi: 10.1192/bjp.bp.114.146761. [DOI] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P. The Effectiveness of Cognitive Bias Modification Interventions for Substance Addictions: A Meta-Analysis. PloS One. 2016;11(9):e0162226. doi: 10.1371/journal.pone.0162226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meneses-Gaya IC, Zuardi AW, Loureiro SR, de Souza Crippa JA. Systematic Review. Brazilian Journal of Pulmonology. 2009;35(1):73–82. [Google Scholar]
- Elfeddali I, de Vries H, Bolman C, Pronk T, Wiers RW. A randomized controlled trial of Web-based Attentional Bias Modification to help smokers quit. Health Psychology. 2016;35(8):870. doi: 10.1037/hea0000346. [DOI] [PubMed] [Google Scholar]
- Enock PM, Hofmann SG, McNally RJ. Attention bias modification training via smartphone to reduce social anxiety: a randomized, controlled multi-session experiment. Cognitive Therapy and Research. 2014;38(2):200–216. [Google Scholar]
- Fadardi JS, Cox WM. Reversing the sequence: reducing alcohol consumption by overcoming alcohol attentional bias. Drug and Alcohol Dependence. 2009;101(3):137–145. doi: 10.1016/j.drugalcdep.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and Alcohol Dependence. 2008;97(1):1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Eastwood B, Child R, Santarcangelo M, Gayton M. Experimental manipulation of attentional biases in heavy drinkers: do the effects generalise? Psychopharmacology. 2007;192(4):593–608. doi: 10.1007/s00213-007-0760-9. [DOI] [PubMed] [Google Scholar]
- Field M, Duka T, Tyler E, Schoenmakers T. Attentional bias modification in tobacco smokers. Nicotine & Tobacco Research. 2009;11(7):812–822. doi: 10.1093/ntr/ntp067. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Vol. 998. John Wiley & Sons; 2012. [Google Scholar]
- Glasser AM, Johnson AL, Rath JM, Williams VF, Vallone DM, Villanti AC. Tobacco Product Brand Preference among US Young Adults, 2011–2014. Tobacco Regulatory Science. 2016;2(1):44–55. [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, … Pine DS. Attention bias modification treatment: a meta-analysis toward the establishment of novel treatment for anxiety. Biological Psychiatry. 2010;68(11):982–990. doi: 10.1016/j.biopsych.2010.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM. A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychological Bulletin. 2011;137(6):940. doi: 10.1037/a0024355. [DOI] [PubMed] [Google Scholar]
- Heatherton T, Kozlowski L, Frecker R, Fagerstrom K. Fagerstrom Test for Nicotine Dependence. Journal of Addictions. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hoek J, Gifford H, Pirikahu G, Thomson G, Edwards R. How do tobacco retail displays affect cessation attempts? Findings from a qualitative study. Tobacco Control. 2010;19(4):334–337. doi: 10.1136/tc.2009.031203. [DOI] [PubMed] [Google Scholar]
- Janes AC, Pizzagalli DA, Richardt S, Chuzi S, Pachas G, Culhane MA, … Kaufman MJ. Brain reactivity to smoking cues prior to smoking cessation predicts ability to maintain tobacco abstinence. Biological Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerst WF, Waters AJ. Attentional retraining administered in the field reduces smokers’ attentional bias and craving. Health Psychology. 2014;33(10):1232. doi: 10.1037/a0035708. [DOI] [PubMed] [Google Scholar]
- Kulak JA, Cornelius ME, Fong GT, Giovino GA. Differences in Quit Attempts and Cigarette Smoking Abstinence Between Whites and African Americans in the United States: Literature Review and Results From the International Tobacco Control US Survey. Nicotine & Tobacco Research. 2016;18(suppl 1):S79–S87. doi: 10.1093/ntr/ntv228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JG, Henriksen L, Rose SW, Moreland-Russell S, Ribisl KM. A systematic review of neighborhood disparities in point-of-sale tobacco marketing. American Journal of Public Health. 2015;105(9):e8–e18. doi: 10.2105/AJPH.2015.302777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15(1):21. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors. 2010;35(12):1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littell RC, Stroup WW, Milliken GA, Wolfinger RD, Schabenberger O. SAS for mixed models. SAS institute; 2006. [Google Scholar]
- Lopes FM, Pires AV, Bizarro L. Attentional bias modification in smokers trying to quit: a longitudinal study about the effects of number of sessions. Journal of Substance Abuse Treatment. 2014;47(1):50–57. doi: 10.1016/j.jsat.2014.03.002. [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L. Selective attention and emotional vulnerability: assessing the causal basis of their association through the experimental manipulation of attentional bias. Journal of Abnormal Psychology. 2002;111(1):107. [PubMed] [Google Scholar]
- Manning BK, Catley D, Harris KJ, Mayo MS, Ahluwalia JS. Stress and quitting among African American smokers. Journal of Behavioral Medicine. 2005;28(4):325–333. doi: 10.1007/s10865-005-9004-9. [DOI] [PubMed] [Google Scholar]
- McHugh RK, Murray HW, Hearon BA, Calkins AW, Otto MW. Attentional bias and craving in smokers: the impact of a single attentional training session. Nicotine & Tobacco Research. 2010;12(12):1261–1264. doi: 10.1093/ntr/ntq171. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP. Selective processing of smoking-related cues in smokers: manipulation of deprivation level and comparison of three measures of processing bias. Journal of Psychopharmacology. 2002;16(4):385–392. doi: 10.1177/026988110201600416. [DOI] [PubMed] [Google Scholar]
- Mogg K, Bradley BP, Field M, De Houwer J. Eye movements to smoking-related pictures in smokers: relationship between attentional biases and implicit and explicit measures of stimulus valence. Addiction. 2003;98(6):825–836. doi: 10.1046/j.1360-0443.2003.00392.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Ahluwalia JS, Ebersole-Robinson M, Catley D, Mayo MS, Resnicow K. Does menthol attenuate the effect of bupropion among African American smokers? Addiction. 2003;98(10):1387–1393. doi: 10.1046/j.1360-0443.2003.00443.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Powell JN, Savage CR, Hall SB, Nollen N, Holsen LM, … Ahluwalia JS. Clinical and Imaging Study: Enhanced cue-elicited brain activation in African American compared with Caucasian smokers: an fMRI study. Addiction biology. 2006;11(1):97–106. doi: 10.1111/j.1369-1600.2006.00007.x. [DOI] [PubMed] [Google Scholar]
- Okuyemi KS, Pulvers KM, Cox LS, Thomas JL, Kaur H, Mayo MS, … Ahluwalia JS. Nicotine dependence among African American light smokers: a comparison of three scales. Addictive Behaviors. 2007;32(10):1989–2002. doi: 10.1016/j.addbeh.2007.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ossip-Klein DJ, Bigelow G, Parker SR, Curry S, Hall S, Kirkland S. Task Force 1: Classification and assessment of smoking behavior. Health Psychology. 1986 [PubMed] [Google Scholar]
- Paxton A, Rodriguez K, Dale R. PsyGlass: Capitalizing on Google Glass for naturalistic data collection. Behavior Research Methods. 2015;47(3):608–619. doi: 10.3758/s13428-015-0586-z. [DOI] [PubMed] [Google Scholar]
- Peuker AC, Bizarro L. Attentional avoidance of smoking cues in former smokers. Journal of Substance Abuse Treatment. 2014;46(2):183–188. doi: 10.1016/j.jsat.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Piper ME, Cook JW, Schlam TR, Jorenby DE, Smith SS, Bolt DM, Loh WY. Gender, race, and education differences in abstinence rates among participants in two randomized smoking cessation trials. Nicotine & Tobacco Research. 2010;12(6):647–657. doi: 10.1093/ntr/ntq067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner ML, Petersen SE. The Attention System of the Human Brain. Annu Rev Neurosci. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Powell J, Dawkins L, West R, Powell J, Pickering A. Relapse to smoking during unaided cessation: clinical, cognitive and motivational predictors. Psychopharmacology. 2010;212(4):537–549. doi: 10.1007/s00213-010-1975-8. [DOI] [PubMed] [Google Scholar]
- Robinson CD, Pickworth WB, Heishman SJ, Wetter DW, Cinciripini PM, Li Y, … Waters AJ. Black Cigarette Smokers Report More Attention to Smoking Cues Than White Smokers: Implications for Smoking Cessation. Nicotine & Tobacco Research. 2015;17(8):1022–1028. doi: 10.1093/ntr/ntu263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson T, Berridge K. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Research Reviews. 1993;18(3):247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Schoenmakers TM, de Bruin M, Lux IF, Goertz AG, Van Kerkhof DH, Wiers RW. Clinical effectiveness of attentional bias modification training in abstinent alcoholic patients. Drug and Alcohol Dependence. 2010;109(1):30–36. doi: 10.1016/j.drugalcdep.2009.11.022. [DOI] [PubMed] [Google Scholar]
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011. CA: A Cancer Journal for Clinicians. 2011;61(4):212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- Stone AA, Shiffman S, Schwartz JE, Broderick JE, Hufford MR. Patient non-compliance with paper diaries. BMJ. 2002;324(7347):1193–1194. doi: 10.1136/bmj.324.7347.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinidad DR, Pérez-Stable EJ, White MM, Emery SL, Messer K. A nationwide analysis of US racial/ethnic disparities in smoking behaviors, smoking cessation, and cessation-related factors. American Journal of Public Health. 2011;101(4):699–706. doi: 10.2105/AJPH.2010.191668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield M, Germain D, Henriksen L. The effect of retail cigarette pack displays on impulse purchase. Addiction. 2008;103(2):322–328. doi: 10.1111/j.1360-0443.2007.02062.x. [DOI] [PubMed] [Google Scholar]
- Waters AJ, Shiffman S, Sayette MA, Paty JA, Gwaltney CJ, Balabanis MH. Attentional bias predicts outcome in smoking cessation. Health Psychology. 2003;22(4):378. doi: 10.1037/0278-6133.22.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AJ, Szeto EH, Wetter DW, Cinciripini PM, Robinson JD, Li Y. Cognition and craving during smoking cessation: an ecological momentary assessment study. Nicotine & Tobacco Research. 2014;16(Suppl 2):S111–S118. doi: 10.1093/ntr/ntt108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widome R, Brock B, Noble P, Forster JL. The relationship of neighborhood demographic characteristics to point-of-sale tobacco advertising and marketing. Ethnicity and Health. 2013;18(2):136–151. doi: 10.1080/13557858.2012.701273. [DOI] [PubMed] [Google Scholar]
- Woud ML, Maas J, Wiers R, Becker ES, Rinck M. Assessment of tobacco-related approach and attentional biases in smokers, cravers, ex-smokers, and non-smokers. Frontiers in Psychology. 2016;7(172) doi: 10.3389/fpsyg.2016.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray JM, Godleski SA, Tiffany ST. Cue-reactivity in the natural environment of cigarette smokers: the impact of photographic and in vivo smoking stimuli. Psychology of Addictive Behaviors. 2011;25(4):733. doi: 10.1037/a0023687. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.