Abstract
This study investigated the serotype distribution and antimicrobial resistance of 3,820 nasopharyngeal Streptococcus pneumoniae isolates from infants and children who presented with respiratory symptoms at Seoul National University Children's Hospital from July 2010 to June 2015 after the introduction of the extended-valency pneumococcal conjugate vaccines (PCVs). Serotypes and antimicrobial susceptibility were determined using the Quellung reaction and E-test, respectively. S. pneumoniae was isolated from 397 (10.4%) specimens. The most common serotypes were 19A (14.0%), 23A (12.8%), 15B/C (10.7%), 11A (10.1%), 6C (7.8%), and 6A (6.3%) among the typeable pneumococci (n = 335). The PCV serotype proportions significantly decreased (59.1% in 2010/11 to 17.0% in 2014/15, P < 0.001), whereas the non-PCV serotype proportions significantly increased (40.9% in 2010/11 to 83.0% in 2014/15, P < 0.001). The non-susceptibility rates for penicillin (oral), penicillin (parenteral, non-meningitis), cefotaxime, and erythromycin were 97.8%, 22.8%, 27.7%, and 95.5%, respectively. The proportions of PCV serotypes responsible for non-susceptibility to penicillin (parenteral, non-meningitis) and multidrug resistance significantly decreased (80.8% to 21.1%, P < 0.001 and 64.3% to 12.3%, P < 0.001, respectively), whereas the non-PCV serotype proportions significantly increased (19.2% to 78.9%, P < 0.001 and 35.7% to 87.7%, P < 0.001, respectively). Serotypes 23A and 15B/C demonstrated significant proportional increase among the antibiotics resistant strains. Thus, the PCV serotype proportions decreased and the non-PCV serotype proportions increased among nasopharyngeal carriage pneumococci after the introduction of extended-valency PCVs in Korea. Antimicrobial non-susceptibility rates for penicillin and erythromycin remain high despite the decrease in the proportion of PCV serotypes responsible for antimicrobial resistance over time.
Keywords: Streptococcus pneumoniae, Serotype, Antimicrobial Resistance, Pneumococcal Conjugate Vaccines
Graphical Abstract
INTRODUCTION
Streptococcus pneumoniae is a leading cause of serious bacterial infections in children worldwide (1). The natural route of S. pneumoniae infection begins with colonization, which may progress to invasive disease if natural immunological barriers are compromised. Preventing nasopharyngeal (NP) colonization of S. pneumoniae may also decrease the horizontal spread of pneumococcal strains and provide herd immunity (2). The efficacy and effectiveness of the 7-valent pneumococcal conjugate vaccine (PCV7) were demonstrated in large randomized trials before licensure and during surveillance for invasive pneumococcal disease (IPD) after licensure in the United States (US) and other countries. However, an increase in IPD caused by non-vaccine serotypes has been observed (3,4). Therefore, the development and use of effective pneumococcal vaccines are required to prevent the diseases caused by these strains. Data from Active Bacterial Core surveillance in the US indicate declines in antibiotics non-susceptible IPD after the introduction of PCV7 in 2000 (5). PCV13 (Prevnar 13; Pfizer Inc., New York, NY, USA) reduced IPD across all age groups when used routinely in children and reduced colonization by 6 additional serotypes in American children (6-8).
The influence of the introduction of PCV also included the pneumococcal carriage. Most studies evaluating the effects of universal PCV7 immunization on NP carriage have shown that vaccination does not alter the overall pneumococcal carriage rate (9,10). However, the carriage of vaccine serotypes nearly disappeared, and replacement with non-vaccine serotypes was clearly observed (11,12). Similar to PCV7, PCV13 also introduced changes in NP carriage. The introduction of PCV13 for universal infant use was associated with a significant reduction in the NP carriage of the PCV13 serotypes and resistant strains (13). Universal vaccination with PCV in infants appeared to influence the antibiotic resistance of strains carried in the nasopharynx and strains causing IPD (14).
PCV7 was introduced in Korea in November 2003 as an optional vaccine, and the vaccine uptake rate increased gradually to reach 40% for the 3-primary series in 2005 and 60% in 2007 (15). PCV10 and PCV13 were introduced in July 2010 as optional vaccines. A nationwide survey of immunization in 2013 that included the PCV coverage rates of infants younger than 2 years revealed that 83.4% received 1 or more doses of PCV and 70.4% received 4 doses of PCV (16). PCV10 and PCV13 have been included in the National Immunization Program (NIP) since May 2014. Current data on the serotype distribution and antimicrobial susceptibilities of pneumococci carried in the NP from children after the introduction of PCV10 and PCV13 are limited.
This study investigated the distribution of serotypes and antimicrobial susceptibilities of pneumococcal carriage isolates from children after the introduction of extended-valency PCVs in Korea and analyzed their influence on NP carriage.
MATERIALS AND METHODS
Isolation of pneumococcal strains
NP aspirates were obtained from infants and children aged 18 years or less who presented with respiratory symptoms at the Seoul National University Children's Hospital from July 2010 to June 2015 (defined as the post-PCV10/13 period in this study). The study period was divided into 5-year groups. Each year group started in July and ended in June of the following year (e.g., 2011/12 period designates the term from July 2011 to June 2012).
NP aspirates were inoculated onto a 5% defibrinated sheep blood agar plate within 72 hours of collection and incubated overnight at 37°C in a 5% CO2 chamber. S. pneumoniae was identified based on the presence of alpha-hemolysis and inhibition by optochin. Subsequent pneumococci with the same serotype isolated from the same child were excluded from the analysis, and only the initial isolates were included in the study. Different serotypes from the same child were included in the analysis.
Serotype determination
The serotype was determined by the Quellung reaction using antiserum (Statens Serum Institut, Copenhagen, Denmark). All serogroup 6 strains were screened for the wciNβ and wciP6B genes using 2 simplex polymerase chain reactions (PCRs) and subsequent sequencing analyses were performed to assign serotypes 6A, 6B, 6C, and 6D, as previously described (17). Serotypes 15B and 15C were counted together because they are interconvertible, and were designated as 15B/C (18). Serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F were classified as PCV7 serotypes. Serotypes 1, 5, and 7F were classified as PCV10/13 additional serotypes. Serotypes 3, 6A, and 19A were classified as PCV13-specific serotypes. PCV serotypes were defined as any serotypes that were included in PCV13. Non-PCV serotypes included all other serotypes.
Antimicrobial susceptibility
All pneumococcal isolates obtained from children aged < 5 years with the exceptions of the non-typeable strains and 2 strains that failed to regrow were subject to tests to determine the minimal inhibitory concentrations (MICs) of 8 antimicrobials (penicillin, cefotaxime, chloramphenicol, tetracycline, clindamycin, erythromycin, trimethoprim-sulfamethoxazole, and levofloxacin) using the E-test (BioMérieux, Marcy-l'Étoile, France). The breakpoints from the Clinical and Laboratory Standards Institute 2014 Guidelines were used to assess antimicrobial susceptibility (19). Penicillin susceptibility was analyzed using 2 different breakpoints: the oral penicillin breakpoint (0.06 µg/mL) and the non-meningitis parenteral breakpoint (2.0 µg/mL). The non-meningitis parenteral breakpoint was used in this study unless otherwise specified. The non-meningitis breakpoint for cefotaxime (1.0 µg/mL) was used. Multidrug resistance (MDR) was defined as non-susceptibility to 3 or more antimicrobial drug classes using the non-meningitis parenteral breakpoint for penicillin (2.0 µg/mL).
Statistical analysis
The statistical analysis was performed using IBM SPSS Statistics for Windows, version 23.0 (IBM Corp., Armonk, NY, USA). Rates and proportions were compared using the χ2 or Fisher's exact test as appropriate. A linear-by-linear association model was used for the Pearson's χ2 test for trend analyses. A P value < 0.05 was considered significant.
Ethics statement
The Institutional Review Board of Seoul National University Hospital approved the study protocol (IRB No. H-1105-051-361). Informed consent was exempted because NP aspirates were obtained as a standard of patient care to identify the etiological agents of respiratory tract infections, including viruses.
RESULTS
Pneumococcal carriage and demographic findings
A total of 3,820 NP aspirates were collected from July 2010 through June 2015. Fifty-eight percent of the subjects (n = 2,230) were male, and the median age of the children was 2.08 years (range: 0.01–18.83 years). Three hundred and ninety-seven pneumococcal isolates were recovered, with a detection rate of 10.4%. The male-to-female ratio was 1:0.64, and the median age of the children with pneumococcal isolation was 2.00 years (range: 0.08–18.25 years). The proportions of the carriers by age group were 49.9% (< 2 years), 30.0% (2–4 years), and 20.2% (≥ 5 years). The pneumococcal detection rates of each age group were 10.7% (198/1,857) in children aged < 2 years, 13.4% (119/888) in children aged 2–4 years, and 7.4% (80/1,075) in children aged ≥ 5 years. The detection rate was significantly higher in children aged between 2 and 4 years (χ2 test, P = 0.010).
Serotype distributions in the post-PCV10/13 period
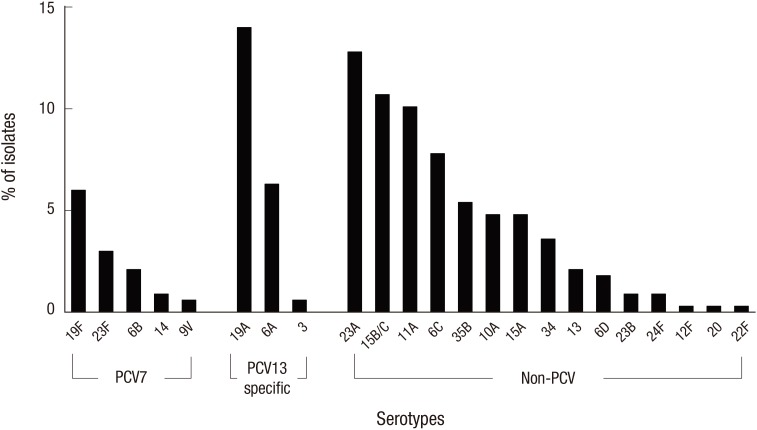
Sixty-two (15.6%) of the 397 pneumococcal isolates were non-typeable. Fig. 1 shows the serotype distribution in the post-PCV10/13 period from 2010/11 to 2014/15. The pneumococci serotype distribution revealed that the most common serotype was 19A (n = 47, 14.0%), followed by 23A (n = 43, 12.8%), 15B/C (n = 36, 10.7%), 11A (n = 34, 10.1%), 6C (n = 26, 7.8%), 6A (n = 21, 6.3%), and 19F (n = 20, 6.0%). PCV serotypes accounted for 33.4% of the strains (n = 112), and non-PCV serotypes accounted for 66.6% of the strains (n = 223). The proportion of PCV7 serotypes was 12.5% (n = 42), and PCV13-specific serotypes accounted for 20.9% of the isolates (n = 70). The most common non-PCV serotypes were 23A, 15B/C, 11A, and 6C. The proportions of PCV serotypes, non-PCV serotypes, PCV7 serotypes, and PCV13-specific serotypes did not significantly differ between the different age groups (< 2 years, 2–4 years, and ≥ 5 years) (data not shown). Examination of each specific serotype revealed that none of the individual serotypes significantly differed across the different age groups (data not shown).
Fig. 1.
Serotype distributions of PCVs among pneumococcal carriage isolates from children in post-PCV10/13 period, 2010–2015. PCV7 serotypes (4, 6B, 9V, 14, 18C, 19F, and 23F); PCV13 specific serotypes (3, 6A, and 19A). There was no PCV10/13 additional serotype (1, 5, and 7F).
PCV = pneumococcal conjugate vaccine.
Changes in serotype distributions in the post-PCV10/13 period
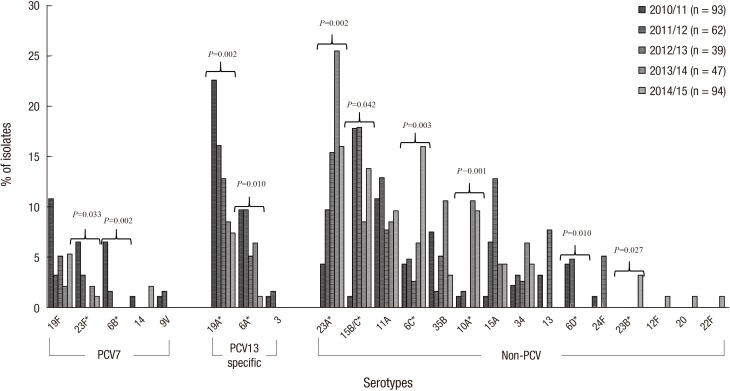
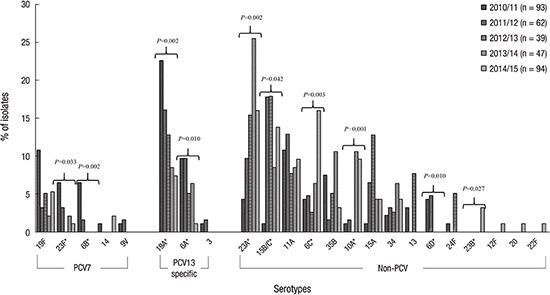
Fig. 2 shows the serotype distribution from 2010/11–2014/15 by year. The trend analysis of proportions of PCV serotypes (from 59.1% to 17.0%, P < 0.001), PCV7 serotypes (from 25.8% to 8.5%, P < 0.001), and PCV13-specific serotypes (from 33.3% to 8.5%, P < 0.001) revealed a significant decrease from 2010/11 to 2014/15. Serotypes 6B (from 6.5% to 0%, P = 0.002), 23F (from 6.5% to 1.1%, P = 0.033), 6A (from 22.6% to 1.1%, P = 0.010), and 19A (from 22.6% to 7.4%, P = 0.002) exhibited significant decreases. In contrast, the trend analysis of the proportions of non-PCV serotypes revealed a significant increase (from 40.9% to 83.0%, P < 0.001). The non-PCV serotypes 6C (from 4.3% to 16.0%, P = 0.003), 10A (from 1.1% to 9.6%, P = 0.001), 15B/C (from 1.1% to 13.8%, P = 0.042), 23A (from 4.3% to 16.0%, P = 0.002), and 23B (from 0.0% to 3.2%, P = 0.031) exhibited significant increases, whereas serotype 6D demonstrated a significant decrease (from 4.3% to 0.0%, P = 0.004).
Fig. 2.
Changes in serotype distributions of PCVs among pneumococcal carriage isolates from children in the post-PCV10/13 period from 2010–2015.
PCV = pneumococcal conjugate vaccine.
*Statistically significant (P < 0.050) using Pearson's χ2 test linear-by-linear association model.
Antimicrobial susceptibility in the post-PCV10/13 period
Table 1 shows the antimicrobial susceptibility data. A total of 267 isolates were included in the antibiotic susceptibility tests. The susceptibility rates were 2.2% for penicillin (oral), 77.2% for penicillin (parenteral, non-meningitis), 72.3% for cefotaxime, and 4.5% for erythromycin. Table 2 shows the non-susceptibility rates for penicillin (oral), penicillin (parenteral, non-meningitis), cefotaxime, and erythromycin and the MDR rates for each serotype. The most common non-susceptible serotype to the non-meningitis penicillin breakpoint was 19A (34.4%, n = 21), followed by 19F (14.8%, n = 9) and 23A (14.8%, n = 9). Serotype 19A (33.8%, n = 25) was the most common non-susceptible serotype for cefotaxime, followed by 23A (20.3%, n = 15) and 11A (16.2%, n = 12). Significant differences were observed in antibiotic non-susceptibility between the PCV and non-PCV serotypes for penicillin (non-meningitis, 41.4% vs. 13.9%, P < 0.001) and cefotaxime (40.2% vs. 21.7%, P = 0.001). The MDR rate was 91.0% and was significantly higher in the PCV serotypes than in the non-PCV serotypes (97.7% vs. 87.8%, P = 0.006).
Table 1. Antimicrobial susceptibility testing in post-PCV10/13 period*.
| Antimicrobial agent | No. (%) of isolates | ||
|---|---|---|---|
| Susceptible | Intermediate | Resistant | |
| Penicillin (oral)† | 6 (2.2) | 75 (28.1) | 186 (69.7) |
| Penicillin (non-meningitis)‡ | 206 (77.2) | 43 (16.1) | 18 (6.7) |
| Cefotaxime (non-meningitis) | 193 (72.3) | 62 (23.2) | 12 (4.5) |
| Erythromycin | 12 (4.5) | 1 (0.4) | 254 (95.1) |
| Clindamycin | 62 (23.2) | 2 (0.7) | 203 (76) |
| Tetracycline | 25 (9.4) | - | 242 (90.6) |
| Chloramphenicol | 195 (73.0) | - | 72 (27.0) |
| TMP/SMX | 92 (34.5) | 44 (16.5) | 131 (49.1) |
| Levofloxacin | 266 (99.6) | - | 1 (0.4) |
PCV = pneumococcal conjugate vaccine, TMP/SMX = trimethoprim/sulfamethoxazole, CLSI = Clinical & Laboratory Standards Institute, MIC = minimal inhibitory concentration.
*Susceptibility criteria of 2014 CLSI guideline were employed; †Breakpoints of MIC ≤ 0.06 were employed for the classification of susceptibility; ‡Breakpoints of MIC ≤ 2 were employed for the classification of susceptibility.
Table 2. Non-susceptibility rates for penicillin, cefotaxime, erythromycin, and MDR* .
| Serotype | No. (%) of non-susceptible isolates | ||||
|---|---|---|---|---|---|
| Penicillin (oral)† | Penicillin (non-meningitis)‡,∥ | Cefotaxime (non-meningitis)∥ | Erythromycin | MDR§ | |
| PCV (n = 87) | 85 (97.7) | 36 (41.4) | 35 (40.2) | 85 (97.7) | 85 (97.7) |
| PCV7 (n = 33) | 33 (100.0) | 14 (42.4) | 8 (24.2) | 32 (97.0) | 32 (97.0) |
| 6B (n = 5) | 5 (100.0) | 4 (80.0) | - | 5 (100.0) | 5 (100.0) |
| 9V (n = 2) | 2 (100.0) | - | - | 2 (100.0) | 2 (100.0) |
| 14 (n = 2) | 2 (100.0) | - | - | 1 (50.0) | 1 (50.0) |
| 19F (n = 16) | 16 (100.0) | 9 (56.3) | 7 (43.8) | 16 (100.0) | 16 (100.0) |
| 23F (n = 8) | 8 (100.0) | 1 (12.5) | 1 (12.5) | 8 (100.0) | 8 (100.0) |
| PCV13 specific (n = 54) | 52 (96.3) | 22 (40.7) | 27 (50.0) | 53 (98.1) | 53 (98.1) |
| 3 (n = 2) | - | - | - | 1 (50.0) | 1 (50.0) |
| 6A (n = 17) | 17 (100.0) | 1 (5.9) | 2 (11.8) | 17 (100.0) | 17 (100.0) |
| 19A (n = 35) | 35 (100.0) | 21 (60.0) | 25 (71.4) | 35 (100.0) | 35 (100.0) |
| Non-PCV (n = 180) | 176 (97.8) | 25 (13.9) | 39 (21.7) | 170 (94.4) | 158 (87.8) |
| 6C (n = 18) | 18 (100.0) | 1 (5.6) | 1 (5.6) | 17 (94.4) | 16 (88.9) |
| 6D (n = 3) | 3 (100.0) | 1 (33.3) | - | 3 (100.0) | 3 (100.0) |
| 10A (n = 12) | 12 (100.0) | - | 2 (16.7) | 9 (75.0) | 9 (75.0) |
| 11A (n = 31) | 30 (96.8) | 7 (22.6) | 12 (38.7) | 31 (100.0) | 30 (96.8) |
| 13 (n = 5) | 5 (100.0) | 1 (20.0) | 1 (20.0) | 5 (100.0) | 5 (100.0) |
| 15A (n = 13) | 13 (100.0) | - | 2 (15.4) | 13 (100.0) | 13 (100.0) |
| 15B/C (n = 29) | 29 (100.0) | 5 (17.2) | 6 (20.7) | 29 (100.0) | 27 (93.1) |
| 22F (n = 1) | - | - | - | 1 (100.0) | - |
| 23A (n = 36) | 36 (100.0) | 9 (25.0) | 15 (41.7) | 34 (94.4) | 32 (88.9) |
| 23B (n = 2) | - | - | - | 2 (100.0) | - |
| 24F (n = 3) | 3 (100.0) | - | - | 3 (100.0) | 3 (100.0) |
| 34 (n = 12) | 12 (100.0) | 1 (8.3) | - | 8 (66.7) | 8 (66.7) |
| 35B (n = 15) | 15 (100.0) | - | - | 15 (100.0) | 12 (80.0) |
| Total (n = 267) | 261 (97.8) | 61 (22.8) | 74 (27.7) | 255 (95.5) | 243 (91.0) |
MDR = multidrug resistance, PCV = pneumococcal conjugate vaccine, CLSI = Clinical & Laboratory Standards Institute, MIC = minimal inhibitory concentration.
*Susceptibility criteria of 2014 CLSI guideline were employed; †Breakpoints of MIC ≤ 0.06 were employed for the classification of susceptibility; ‡Breakpoints of MIC ≤ 2 were employed for the classification of susceptibility; §Multidrug resistance was defined as non-susceptibility to ≥ 3 antimicrobial drug classes; ∥Statistically significant P value < 0.050 by χ2 between PCV and non-PCV serotypes.
Changes in penicillin non-susceptible and MDR serotypes during the post-PCV10/13 period
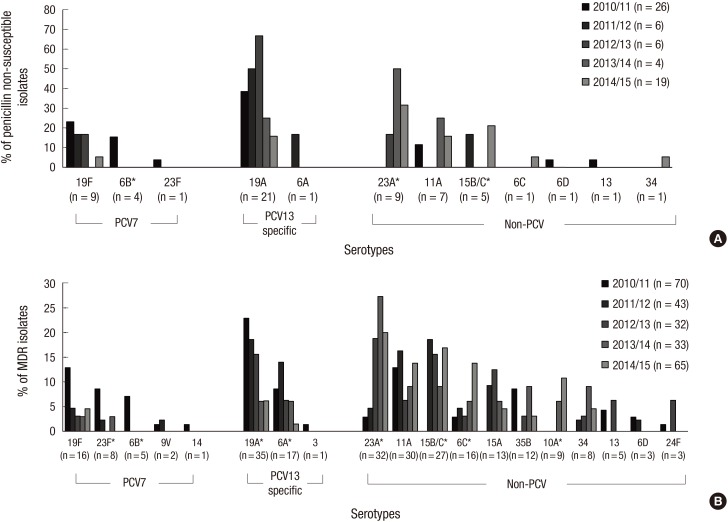
Fig. 3 shows the changes in the penicillin non-susceptible and MDR serotypes in the post-PCV10/13 period. The trend analysis of penicillin (parenteral, non-meningitis) susceptibility testing from 2010/11 to 2014/15 revealed no significant differences (non-susceptibility from 33.3% to 26.4%, P = 0.381). The proportion of PCV serotypes among isolates with non-susceptibility to penicillin decreased from 80.8% in 2010/11 to 21.1% in 2014/15 (P < 0.001), and the proportion of non-PCV serotypes increased from 19.2% in 2010/11 to 78.9% in 2014/15 (P < 0.001). The PCV7 serotypes also showed a significant decrease in the proportion of isolates with non-susceptibility (from 42.3% to 5.3%, P < 0.001). Nevertheless, the PCV13-specific serotypes did not exhibit a significant change (from 38.5% to 15.8%, P = 0.086). The PCV7 serotype 6B (from 15.4% to 0.0%, P = 0.039), exhibited significant decreases in proportion. Serotypes 23A (from 0.0% to 31.6%, P < 0.001) and 15B/C (from 0.0% to 21.1%, P = 0.026) exhibited proportional increases among the non-PCV serotypes.
Fig. 3.
Changes of penicillin non-susceptible (A) and multidrug resistant (B) serotypes in post-PCV10/13 period, 2010–2015.
*Statistically significant (P < 0.050) using Pearson's χ2 test linear-by-linear association model.
The trend analysis of MDR from 2010/11 to 2014/15 revealed no significant differences (from 89.7% to 90.3%, P = 0.998). The proportion of PCV serotypes among isolates representing MDR decreased significantly from 64.3% in 2010/11 to 12.3% in 2014/15 (P < 0.001), whereas that of the non-PCV serotypes increased from 35.7% in 2010/11 to 87.7% in 2014/15 (P < 0.001). The PCV13-specific serotypes also exhibited a significant decrease in proportion (from 32.9% to 7.7%, P < 0.001). The PCV7 serotypes 23F (from 8.6% to 0.0%, P = 0.010), 6B (from 7.1% to 0.0%, P = 0.007) and the PCV13-specific serotypes 19A (from 22.9% to 6.2%, P = 0.002), 6A (from 8.6% to 1.5%, P = 0.047) exhibited significant decreases in proportion. Serotypes 23A (from 2.9% to 20.0%, P < 0.001), 15B/C (from 0.0% to 16.9%, P = 0.014), 6C (from 2.9% to 13.8%, P = 0.013), and 10A (from 0.0% to 10.8%, P < 0.001) exhibited proportional increases among the non-PCV serotypes.
DISCUSSION
This study highlights changes in the serotype distribution of the NP carriage in children after the introduction of PCV10 and PCV13 in a country where PCVs have been in use for 12 years (since 2003). This study investigated the latter 5 years, which was the period represented by extended-valency PCVs. During the 5-year study period, the serotype distribution showed decreases in the PCV7 and PCV13-specific serotypes. Serotype 19A, which exhibited a marked increase after the introduction of PCV7 (20), was significantly decreased following the introduction of extended-valency PCVs. The decrease in serotype 6A was also significant. However, the proportion of non-PCV serotypes was significantly increased. Serotype 19F, which remained in a considerable proportion even after the introduction of PCV7, did not show a significant change.
Previous studies of the serotype distribution of the carriage pneumococcal isolates after routine PCV7 immunization reported that PCV7 reduced the carriage of PCV7 serotype pneumococci, which were replaced with non-PCV7 serotypes. Therefore, only a slight decrease or no change was observed in the overall pneumococcal carriage rate (11,21,22). This phenomenon was also observed after the introduction of PCV13 in the US (8,13). These results are consistent with the results in our study, in which the proportion of PCV10/13 serotype isolates decreased and the proportions of non-PCV10/13 serotypes increased.
The emergence of serotype 19A has been a major issue since the introduction of PCV7 in IPD and the pneumococcal carriage. One large study conducted in the US after the introduction of PCV7 in October 2000 demonstrated that IPDs caused by PCV7 serotypes declined through 2005, although the overall IPD rates leveled off beginning in 2002 primarily due to the increased incidence of IPDs caused by non-PCV7 serotype 19A (3). Regarding the pneumococcal carriage, the disappearance of vaccine serotypes occurred in young children in the US after the introduction of PCV7, with rapid replacement by penicillin non-susceptible non-vaccine serotypes, particularly 19A (11,12). The current study focused on changes in the serotype distribution after the introduction of PCV10/13 and demonstrated a decrease in serotype 19A, which was shown in many studies. We also found that the non-PCV10/13 serotypes were increased, with serotypes 23A, 15B/C, 6C, and 10A constituting a considerable proportion of the increase in Korea. One previous report 4 years after the introduction of PCV10/13 that investigated children attending daycare centers in Korea also reported that the most common serotype was 23A, which was consistent with our study (23).
Changes in serotype distribution may differ by region, time, the PCV being implemented, and immunization policies. One study performed in Atlanta, Georgia, in the US demonstrated that serotype 35B was the most increased non-vaccine serotype in the pneumococcal carriage after the introduction of PCV13 (13). The study also demonstrated a steeper decrease (from 25.8% to 3%, P < 0.001) in the PCV13 serotypes and an increase (from 68.4% to 97%, P < 0.001) in the non-PCV13 serotypes compared to our observations. Another study from the US demonstrated the emergence of serotypes 35B, 23B, 21, and 15A/B/C (24). A French study collected NP carriage isolates from young children with acute otitis media after the introduction of PCV13 and detected serotypes 15A and 11A in 5% of the NP carriages, but an increase in the non-vaccine serotypes was not clearly observed (25). A study in the United Kingdom also demonstrated a continued reduction in the carriage of the PCV7 serotypes and additional protection against the carriage of PCV13-specific serotypes across all age groups within 2 years of PCV13 replacing PCV7; additionally, serotypes 11A, 23B, 24F, and 35F were the most frequent in 2012/2013 (26). An Italian study assessed NP carriages 6 and 12 months after PCV13 vaccination in healthy Italian children aged 3–59 months and observed a decrease in NP colonization caused by the PCV13 serotypes and a substantial increase in 15A (27).
A previous study from the same institute investigated the NP carriage after the introduction of PCV7 (20). Serotype 19F, which was the most common PCV7 serotype in this study, exhibited a modest reduction during the study period. The explanation for this result was that serotype 19F demonstrated the lowest geometric mean titer (GMT) in the opsonophagocytic assay (OPA) among the PCV7 serotypes, which might indicate weaker vaccine-induced mucosal immunity (28). A recent study demonstrated the decreased acquisition of 19F and significantly higher immunoglobulin G (IgG) responses elicited by PCV13 for this specific serotype (29). PCV13 may have enhanced activity against serotype 19F due to cross-reactive antibodies induced by 19A (30). Although a trend of a decrease in serotype 19F was observed in this study, our findings did not demonstrate a statistically significant decrease in serotype 19F. Instead, our study demonstrated a marked reduction in serotype 6A. A study conducted in Massachusetts, USA, demonstrated a reduction in serotype 6A in the pneumococcal carriage after the introduction of PCV7, which was not observed in a previous study conducted in Korea (20,31). Because the OPA GMT of serotype 6A reached levels similar to serotype 19F, there might be a delayed reduction resulting where PCV7 coverage rate is modest. Therefore, the effect of the delayed reduction by PCV7 may have added to the direct effect of PCV13.
Non-susceptibility to penicillin remains steady in the post-PCV7 and pre-PCV10/13 era despite the decrease in penicillin non-susceptible PCV7 serotype pneumococci in many previous studies. The most likely explanation is an increased proportion of penicillin non-susceptible non-PCV7 serotype pneumococci, particularly serotype 19A (11,12,21). Several studies demonstrated a decrease in non-susceptibility to penicillin or MDR in the post-PCV10/13 era. A recent study from 42 medical centers in the US investigated antimicrobial susceptibilities for pneumococci from invasive and non-invasive diseases among all age groups from 2008/09 to 2012/13. The study revealed a significant decrease in the overall non-susceptibility to penicillin and MDR in pneumococcal isolates after the introduction of PCV13 (14). This study also demonstrated that the increasing proportion of non-PCV13 serotypes exhibited non-susceptibility to penicillin or MDR, with the penicillin non-susceptible or MDR serotypes 35B, 23A, 23B, and 15B replacing the decreasing serotype 19A.
The current study revealed unique changes in the serotype distribution of penicillin non-susceptible and MDR isolates. The major serotypes of isolates non-susceptible to penicillin were the PCV serotypes during the 2010/11 period and the non-PCV serotypes in 2014/15. This study demonstrated a significant decrease in the proportions of serotypes 6B, 6A, 19A, and 23F, which exhibited high levels of antibiotic resistance. However, serotypes 23A and 15B/C accounted for increasing proportions of penicillin non-susceptible pneumococci, and serotypes 23A, 15B/C, 6C, and 10A accounted for increasing proportions of pneumococci exhibiting MDR. This result was partially consistent with a previous study in Korea that demonstrated that 23A, 15B, and 15C were antibiotic non-susceptible serotypes with high rates of carriage following the introduction of extended-valency PCVs in Korea (23). A study conducted in the US assessed the potential additional impact of PCV13 over PCV7 and revealed that the highest proportions of non-susceptible isolates of the most commonly isolated non-vaccine serotypes were represented by 10B, 11A, 15A, 15B/C, 16F, 17F, 21, 23B, 34, 35B, and 38 (32). We did not identify one dominant non-PCV10/13 serotype that was highly associated with antibiotic non-susceptibility among the NP carriage isolates (e.g., serotype 19A in the post-PCV7 and pre-PCV10/13 period). Continued surveillance is needed to monitor trends in non-vaccine serotypes that may emerge as highly associated with antibiotic non-susceptibility.
This study has several limitations. First, pneumococcal carriage isolates were collected at a single center in a large city, and thus this sample may not represent the national data. Multicenter-based data collection is needed to investigate changes in the pneumococcal carriage and to evaluate the impact of newly introduced PCVs on the serotype distribution. Second, this study did not include an individual's vaccination status or previous history of antibiotic use.
This study may have several implications for future pneumococcal immunization programs. The replacement phenomenon that was demonstrated in the era of PCV10/13 is challenging for the current vaccine strategy in Korea and suggests that polysaccharide-based PCV may not be the fundamental solution to combat IPD and carriage.
Footnotes
Funding: This study was partly supported by Seoul National University Hospital grants (06-2011-2180 and 06-2014-1010), which were underwritten by Pfizer Pharmaceuticals Korea Ltd.
CONFLICT OF INTEREST: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Lee JK, Yun KW. Investigation: Lee JK, Yun KW, Choi EH, Kim SJ, Lee SY. Supervision: Choi EH, Lee HJ.
References
- 1.Nuorti JP, Whitney CG, Centers for Disease Control and Prevention (CDC) Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine - recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 2.Bogaert D, De Groot R, Hermans PW. Streptococcus pneumoniae colonisation: the key to pneumococcal disease. Lancet Infect Dis. 2004;4:144–154. doi: 10.1016/S1473-3099(04)00938-7. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC) Invasive pneumococcal disease in children 5 years after conjugate vaccine introduction--eight states, 1998–2005. MMWR Morb Mortal Wkly Rep. 2008;57:144–148. [PubMed] [Google Scholar]
- 4.Miller E, Andrews NJ, Waight PA, Slack MP, George RC. Herd immunity and serotype replacement 4 years after seven-valent pneumococcal conjugate vaccination in England and Wales: an observational cohort study. Lancet Infect Dis. 2011;11:760–768. doi: 10.1016/S1473-3099(11)70090-1. [DOI] [PubMed] [Google Scholar]
- 5.Hampton LM, Farley MM, Schaffner W, Thomas A, Reingold A, Harrison LH, Lynfield R, Bennett NM, Petit S, Gershman K, et al. Prevention of antibiotic-nonsusceptible Streptococcus pneumoniae with conjugate vaccines. J Infect Dis. 2012;205:401–411. doi: 10.1093/infdis/jir755. [DOI] [PubMed] [Google Scholar]
- 6.Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, et al. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis. 2015;15:301–309. doi: 10.1016/S1473-3099(14)71081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gounder PP, Bruce MG, Bruden DJ, Singleton RJ, Rudolph K, Hurlburt DA, Hennessy TW, Wenger J. Effect of the 13-valent pneumococcal conjugate vaccine on nasopharyngeal colonization by Streptococcus pneumoniae--Alaska, 2008–2012. J Infect Dis. 2014;209:1251–1258. doi: 10.1093/infdis/jit642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee GM, Kleinman K, Pelton SI, Hanage W, Huang SS, Lakoma M, Dutta-Linn M, Croucher NJ, Stevenson A, Finkelstein JA. Impact of 13-valent pneumococcal conjugate vaccination on Streptococcus pneumoniae carriage in young children in Massachusetts. J Pediatric Infect Dis Soc. 2014;3:23–32. doi: 10.1093/jpids/pit057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Huang SS, Platt R, Rifas-Shiman SL, Pelton SI, Goldmann D, Finkelstein JA. Post-PCV7 changes in colonizing pneumococcal serotypes in 16 Massachusetts communities, 2001 and 2004. Pediatrics. 2005;116:e408–e413. doi: 10.1542/peds.2004-2338. [DOI] [PubMed] [Google Scholar]
- 10.Millar EV, O'Brien KL, Watt JP, Bronsdon MA, Dallas J, Whitney CG, Reid R, Santosham M. Effect of community-wide conjugate pneumococcal vaccine use in infancy on nasopharyngeal carriage through 3 years of age: a cross-sectional study in a high-risk population. Clin Infect Dis. 2006;43:8–15. doi: 10.1086/504802. [DOI] [PubMed] [Google Scholar]
- 11.Park SY, Moore MR, Bruden DL, Hyde TB, Reasonover AL, Harker-Jones M, Rudolph KM, Hurlburt DA, Parks DJ, Parkinson AJ, et al. Impact of conjugate vaccine on transmission of antimicrobial-resistant Streptococcus pneumoniae among Alaskan children. Pediatr Infect Dis J. 2008;27:335–340. doi: 10.1097/INF.0b013e318161434d. [DOI] [PubMed] [Google Scholar]
- 12.Huang SS, Hinrichsen VL, Stevenson AE, Rifas-Shiman SL, Kleinman K, Pelton SI, Lipsitch M, Hanage WP, Lee GM, Finkelstein JA. Continued impact of pneumococcal conjugate vaccine on carriage in young children. Pediatrics. 2009;124:e1–e11. doi: 10.1542/peds.2008-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola SW, et al. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J. 2015;34:1168–1174. doi: 10.1097/INF.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 14.Richter SS, Diekema DJ, Heilmann KP, Dohrn CL, Riahi F, Doern GV. Changes in pneumococcal serotypes and antimicrobial resistance after introduction of the 13-valent conjugate vaccine in the United States. Antimicrob Agents Chemother. 2014;58:6484–6489. doi: 10.1128/AAC.03344-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013;28:1283–1288. doi: 10.3346/jkms.2013.28.9.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang HI, Park EY, Kim MY. National immunization survey in South Korea, 2013. Public Health Wkly Rep. 2013;7:449–454. [Google Scholar]
- 17.Choi EH, Lee HJ, Cho EY, Oh CE, Eun BW, Lee J, Kim MJ. Prevalence and genetic structures of Streptococcus pneumoniae serotype 6D, South Korea. Emerg Infect Dis. 2010;16:1751–1753. doi: 10.3201/eid1611.100941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meats E, Brueggemann AB, Enright MC, Sleeman K, Griffiths DT, Crook DW, Spratt BG. Stability of serotypes during nasopharyngeal carriage of Streptococcus pneumoniae . J Clin Microbiol. 2003;41:386–392. doi: 10.1128/JCM.41.1.386-392.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clinical and Laboratory Standards Institute (US) Performance Standards for Antimicrobial Susceptibility testing: Twenty-Fourth Informational Supplement (CLSI Document M100-S24) Wayne, PA: National Committee for Clinical and Laboratory Standards; 2014. [Google Scholar]
- 20.Cho EY, Kang HM, Lee J, Kang JH, Choi EH, Lee HJ. Changes in serotype distribution and antibiotic resistance of nasopharyngeal isolates of Streptococcus pneumoniae from children in Korea, after optional use of the 7-valent conjugate vaccine. J Korean Med Sci. 2012;27:716–722. doi: 10.3346/jkms.2012.27.7.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore MR, Hyde TB, Hennessy TW, Parks DJ, Reasonover AL, Harker-Jones M, Gove J, Bruden DL, Rudolph K, Parkinson A, et al. Impact of a conjugate vaccine on community-wide carriage of nonsusceptible Streptococcus pneumoniae in Alaska. J Infect Dis. 2004;190:2031–2038. doi: 10.1086/425422. [DOI] [PubMed] [Google Scholar]
- 22.Dunais B, Bruno-Bazureault P, Carsenti-Dellamonica H, Touboul P, Pradier C. A decade-long surveillance of nasopharyngeal colonisation with Streptococcus pneumoniae among children attending day-care centres in south-eastern France: 1999–2008. Eur J Clin Microbiol Infect Dis. 2011;30:837–843. doi: 10.1007/s10096-011-1154-9. [DOI] [PubMed] [Google Scholar]
- 23.Choe YJ, Lee HJ, Lee H, Oh CE, Cho EY, Choi JH, Kang HM, Yoon IA, Jung HJ, Choi EH. Emergence of antibiotic-resistant non-vaccine serotype pneumococci in nasopharyngeal carriage in children after the use of extended-valency pneumococcal conjugate vaccines in Korea. Vaccine. 2016;34:4771–4776. doi: 10.1016/j.vaccine.2016.08.030. [DOI] [PubMed] [Google Scholar]
- 24.Kaur R, Casey JR, Pichichero ME. Emerging Streptococcus pneumoniae strains colonizing the nasopharynx in children after 13-valent pneumococcal conjugate vaccination in comparison to the 7-valent era, 2006–2015. Pediatr Infect Dis J. 2016;35:901–906. doi: 10.1097/INF.0000000000001206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cohen R, Levy C, Bingen E, Koskas M, Nave I, Varon E. Impact of 13-valent pneumococcal conjugate vaccine on pneumococcal nasopharyngeal carriage in children with acute otitis media. Pediatr Infect Dis J. 2012;31:297–301. doi: 10.1097/INF.0b013e318247ef84. [DOI] [PubMed] [Google Scholar]
- 26.van Hoek AJ, Sheppard CL, Andrews NJ, Waight PA, Slack MP, Harrison TG, Ladhani SN, Miller E. Pneumococcal carriage in children and adults two years after introduction of the thirteen valent pneumococcal conjugate vaccine in England. Vaccine. 2014;32(9):4349–4355. doi: 10.1016/j.vaccine.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 27.Mameli C, Fabiano V, Daprai L, Bedogni G, Faccini M, Garlaschi ML, Penagini F, Dilillo D, Torresani E, Gramegna M, et al. A longitudinal study of Streptococcus pneumoniae carriage in healthy children in the 13-valent pneumococcal conjugate vaccine era. Hum Vaccin Immunother. 2015;11:811–817. doi: 10.1080/21645515.2015.1010945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bryant KA, Block SL, Baker SA, Gruber WC, Scott DA, PCV13 Infant Study Group Safety and immunogenicity of a 13-valent pneumococcal conjugate vaccine. Pediatrics. 2010;125:866–875. doi: 10.1542/peds.2009-1405. [DOI] [PubMed] [Google Scholar]
- 29.Dagan R, Patterson S, Juergens C, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Comparative immunogenicity and efficacy of 13-valent and 7-valent pneumococcal conjugate vaccines in reducing nasopharyngeal colonization: a randomized double-blind trial. Clin Infect Dis. 2013;57:952–962. doi: 10.1093/cid/cit428. [DOI] [PubMed] [Google Scholar]
- 30.Grant LR, O’Brien SE, Burbidge P, Haston M, Zancolli M, Cowell L, Johnson M, Weatherholtz RC, Reid R, Santosham M, et al. Comparative immunogenicity of 7 and 13-valent pneumococcal conjugate vaccines and the development of functional antibodies to cross-reactive serotypes. PLoS One. 2013;8:e74906. doi: 10.1371/journal.pone.0074906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nahm MH, Lin J, Finkelstein JA, Pelton SI. Increase in the prevalence of the newly discovered pneumococcal serotype 6C in the nasopharynx after introduction of pneumococcal conjugate vaccine. J Infect Dis. 2009;199:320–325. doi: 10.1086/596064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dagan R, Juergens C, Trammel J, Patterson S, Greenberg D, Givon-Lavi N, Porat N, Gurtman A, Gruber WC, Scott DA. Efficacy of 13-valent pneumococcal conjugate vaccine (PCV13) versus that of 7-valent PCV (PCV7) against nasopharyngeal colonization of antibiotic-nonsusceptible Streptococcus pneumoniae . J Infect Dis. 2015;211:1144–1153. doi: 10.1093/infdis/jiu576. [DOI] [PubMed] [Google Scholar]