Abstract
β1-Integrin is highly expressed and is a negative prognostic factor for colon and pancreatic cancer patients and the gene plays a functional role in cell migration and invasion. In this study, we demonstrate that β1-integrin expression is regulated in pancreatic and colon cancer cells by the pro-oncogenic orphan nuclear receptor 4A1 (NR4A1, Nur77, TR3) and knockdown of this receptor by RNA interference decreases β1-integrin protein and mRNA expression, α5-integrin and also expression of β1-integrin-dependent phosphorylation of FAK (pFak). Knockdown of NR4A1 also decreased migration and fibronectin-induced adhesion in pancreatic (Panc1, L3.6pL and MiaPaCa2) and colon (RKO and SW480) cancer cells. 1,1-Bis(3′-indolyl)-1-(p-substituted phenyl)methane (C-DIM) compounds containing p-hydroxy (DIM-C-pPhOH) and p-carbomethoxy (DIM-C-pPhCO2Me) groups are NR4A1 ligands that act as antagonists for this receptor. Treatment of pancreatic and colon cancer cells with DIM-C-pPhOH or DIM-C-pPhCO2Me mimics the effects of NR4A1 knockdown and decreases β1-integrin expression, β1-integrin regulated genes and responses including migration and adhesion. The results demonstrate a novel method for targeting β1-integrin in colon and pancreatic cancer cells and indicate possible clinical applications for C-DIM/NR4A1 antagonists for pancreatic and colon cancer therapy.
Keywords: β1-integrin, inhibition, NR4A1, C-DIM compounds, migration
INTRODUCTION
The nuclear receptor 4A (NR4A) subfamily of orphan receptors including NR4A1 (Nur77/TR3), NR4A2 (Nurr1) and NR4A3 (Nor1) are stress-inducible immediate early genes that play both unique and overlapping roles in metabolic, cardiovascular, neurological and immune functions, inflammation, and cancer [1,2]. Although endogenous ligands for NR4A receptors have not been identified, there is evidence that ligands for these receptors acting as agonists or antagonists are emerging as a novel class of therapeutics [2–5]. For example, Wu and coworkers have characterized cytosporone and related synthetic analogs as NR4A1 ligands [6–10] and have shown that one of their analogs inhibits endotoxin-induced sepsis in RAW 264.7 cells and in vivo [10]. The mechanism of this NR4A1-dependent antiinflammatory response is due to ligand-induced dissociation of p38-NR4A1 interactions and restoration of the p65-NR4A1 complex resulting in inhibition of NFκB signaling [10].
In double knockout animals, the loss of both NR4A1 and NR4A3 results in the rapid development of acute myeloid leukemia in mice [11] indicating a tumor suppressor-like role for these receptors. In contrast, NR4A1 is overexpressed in many solid tumors and their derived cell lines, and in breast, colon and lung tumors overexpression of NR4A1 is a negative prognostic factor [12–18]. Moreover, knockdown or overexpression of NR4A1 shows that this receptor is pro-oncogenic and regulates one or more of cell proliferation, survival and migration/invasion in lung, melanoma, lymphoma, kidney, pancreatic, colon, cervical, ovarian and gastric cancer cells [16–28]. Studies in this laboratory have identified 1,1-bis(3′-indolyl)-1-(substituted phenyl)methane (C-DIM) analogs as NR4A1 ligands that act as nuclear receptor antagonists [12,14,19,26–28]. RNAseq or array analysis have shown that treatment with specific C-DIM/NR4A1 antagonists results in both induced and repressed gene expression which contribute to the NR4A1-regulated pro-oncogenic pathways. For example, in both liver and colon cancer cells, treatment with the NR4A1 antagonist 1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl)methane (DIM-C-pPhOH) induces reactive oxygen species (ROS) through downregulation of isocitrate dehydrogenase 1 (IDH1) and thioredoxin domain containing 5 (TXNDC5) and downregulates survivin and other specificity protein (Sp)-regulated genes containing GC-rich promoters [12,14,19,26–28]. Ligand-dependent inactivation of NR4A1 activates p53 and induces ROS which induce sestrin 2 and activation of AMPKα resulting in inhibition of mTOR [14,27,28]. All of these pro-oncogenic NR4A1-regulated genes/pathways and functions are blocked by C-DIM/NR4A1 antagonists in breast, pancreatic, colon, kidney and lung cancer cell lines [12,14,19,26–28].
Recent studies have identified β;1-integrin as an NR4A1-regulated gene in breast cancer cells and the NR4A1 antagonist DIM-C-pPhOH decreases β;1-integrin expression and also inhibits β;1-integrin-dependent cell migration [29]. Since β;1-integrin is a negative prognostic factor and induces migration/invasion and metastasis in both pancreatic and colon cancer cells [30–41], this study investigated the role of NR4A1 in regulating β;1-integrin expression and subsequent inhibition of β;1-integrin-dependent migration/invasion by C-DIM/NR4A1 antagonists. The results confirm that C-DIM/NR4A1 antagonists target NR4A1 and inhibit invasion of pancreatic and colon cancer cells, demonstrating that these compounds represent a novel class of mechanism-based drugs for pancreatic and colon cancer chemotherapy.
MATERIALS AND METHODS
Cell lines and antibodies
Pancreatic cancer (Panc1 and MiaPaCa2) and colon cancer (RKO, SW480) cell lines were purchased from American Type Culture Collection (Manassas, VA) and were authenticated on April 29, 2016 by Biosynthesis (Lewisville, TX). L3.6pL cells were kindly provided by Dr. I.J. Fidler (University of Texas MD Anderson Cancer Center, Houston, TX). Cells were maintained 37°C in the presence of 5% CO2 in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium with 10% fetal bovine serum with antibiotic. NR4A1 antibody was purchased from Novus Biologicals (Littleton, CO). Sp1 antibody and glutathione (GSH) reduced free acid were purchased from Millipore (Temecula, CA); β1-integrin, p300, Sp3 and Sp4 antibodies were purchased from Santa Cruz Biotech (Santa Cruz, CA). β-Actin antibody, Dulbecco’s Modified Eagle’s Medium, and 36% formaldehyde were purchased from Sigma-Aldrich (St. Louis, MO). Hematoxylin was purchased from Vector Laboratories (Burlingame, CA). FAK, p-FAK, α5-integrin antibodies and leptomycin were purchased from Cell Signaling Technologies (Manassas, VA).
Cell Adhesion Assay
Panc1, L3.6pL, MiaPaCa2, RKO and SW480 cancer cells (3.0 x 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were seeded and subsequently treated with varying concentrations of DIM-C-pPhOH or DIM-C-pPhCO2Me for 24 hr or with 100 nm of siβ1-integrin or siNR4A1 for 48 hr. Cells were trypsinized, counted, then placed for 90 min on BD BioCoat Human Fibronectin Cellware 24-well plates (Bedford, MA); medium was then aspirated, wells gently washed with PBS, and stained with 0.5% Crystal Violet Stain. Cells were then counted for adhesion to fibronectin. Wells coated with BSA and poly-L-lysine were used as negative controls.
Boyden Chamber Assay
Panc1, L3.6pL, MiaPaCa2, RKO and SW480 cancer cells (3.0 x 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were seeded and subsequently treated with varying concentrations of DIM-C-pPhOH or DIM-C-pPhCO2Me for 24 hr or with 100 nm of siβ1-integrin, siNR4A1, siSp1, or sip300 for 48 hr. Cells then treated with varying concentrations of DIM-C-pPhOH or DIM-C-pPhCO2Me for 24 hr after transfection. Cells were trypsinized, counted then placed in 24-well 8.0 μm pore ThinCerts from BD Biosciences (Bedford, MA) allowed to migrate for 24 hr, fixed with formaldehyde, and then stained with hematoxylin. Cells that migrated through the pores were then counted as described [27–29].
RT PCR
RNA was isolated using Zymo Research Quick-RNA MiniPrep kit (Irvine, CA). Quantification of mRNA (β1-integrin) was performed using Bio-Rad iTaq Universal SYBER Green 1-Step Kit (Richmond, CA) using the manufacturer’s protocol with real-time PCR. TATA Binding Protein (TBP) mRNA was used as a control to determine relative mRNA expression.
Chromatin Immunoprecipitation
The chromatin immunoprecipitation (ChIP) assay was performed using the ChIP-IT Express magnetic chromatin immunoprecipitation kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. Panc1, L3.6pL, MiaPaCa2, RKO and SW480 cancer cells were treated with DMSO, DIM-C-pPhOH (15, 20 μM), or DIM-C-pPhCO2Me (15, 20 μM) for 24 hr. Cells were then fixed with 1% formaldehyde, and the cross-linking reaction was stopped by addition of 0.125 M glycine. After washing twice with phosphate-buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed, and nuclei were collected. Nuclei were then sonicated to the desired chromatin length (~200 to 1,500 bp). The sonicated chromatin was immunoprecipitated with normal IgG, p300 (Santa Cruz), Sp1 (Abcam), NR4A1 (Novus Biologicals), Sp3, Sp4 (Santa Cruz), or RNA polymerase II (pol II; Active Motif) antibodies and protein A-conjugated magnetic beads at 4°C for overnight. After the magnetic beads were extensively washed, protein-DNA cross-links were reversed and eluted. DNA was prepared by proteinase K digestion followed by PCR amplification. The primers for detection of the β1-integrin promoter region were 5′-TCACCACCCTTCGTGACAC -3′ (sense) and 5′-GAGATCCTGCATCTCGGAAG-3′ (antisense). PCR products were resolved on a 2% agarose gel in the presence of RGB-4103 GelRed Nucleic Acid Stain.
Western blot analysis
Panc1, L3.6pL, MiaPaCa2, RKO and SW480 cancer cells (3.0 x 105 per well) were seeded in Dulbecco’s modified Eagle’s medium/Ham’s F-12 medium supplemented with 2.5% charcoal-stripped fetal bovine serum and were allowed to attach for 24 hr. Cells were transfected with 100 nm of siβ1-integrin, siNR4A1, siSp1, siSp3, siSp4 (or in combination), or sip300 for 72 hr or treated with C-DIM/NR4A1 antagonists. Cells were analyzed by western blot as described previously [27–29].
Small interfering RNA interference assay
SiRNA experiments were conducted as described previously [27–29]. The siRNA complexes used in the study were as follows: siGL2-5′: CGU ACG CGG AAU ACU UCG A; siNR4A1: SASI_Hs02_00333289[1], SASI_Hs02_00333290[2]; siβ1-integrin: SASI_Hs02_00333437[1], SASI_Hs01_00159474; siSp1:SASI_Hs02_003; sip300: SASI_Hs01_00052818; siSp3, SASI_Hs01_00211941; siSp4, SASI_Hs01_00114420.
Statistical analysis
Statistical significance of differences between the treatment groups was determined as previously described [27–29].
RESULTS
1. NR4A1 regulates β1-integrin gene expression
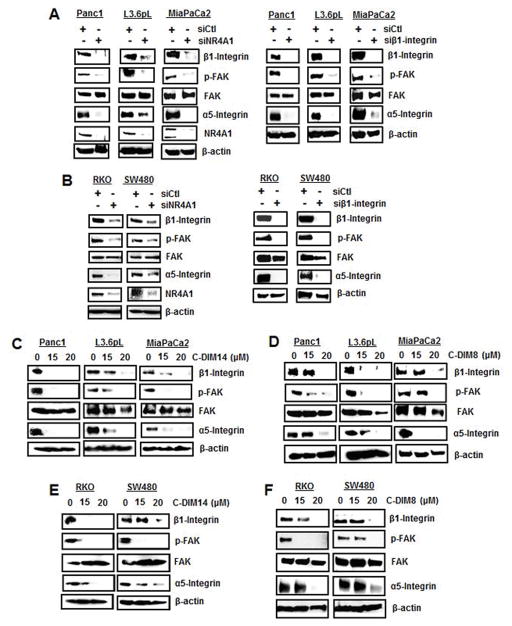
Knockdown of NR4A1 by RNAi in pancreatic (Panc1, L3.6pL and MiaPaCa2) (Fig. 1A) and colon (RKO and SW480) (Fig. 1B) cancer cells significantly decreased β1-integrin (ITGB1) mRNA levels as determined by real time PCR. Moreover, treatment of the same cell lines with 15 and 20 μM of the two C-DIM/NR4A1 antagonists DIM-C-pPhOH (C-DIM8) and the p-carboxymethyl analog (DIM-C-pPhCO2Me, C-DIM14) also decreased expression of β1-integrin mRNA levels (Figs. 1C and 1D). Western blot analysis of whole cell lysates from Panc1, L3.6pL and MiaPaCa2 cells transfected with siCtl (non-specific oligonucleotide) or siNR4A1 showed that loss of NR4A1 resulted in decreased expression of β1-integrin, phosphorylated FAK (pFAK) and 7agr;5-integrin in all three cell lines (Fig. 2A). Moreover, after knockdown of β1-integrin (siβ1-integrin), we also observed decreased expression of β1-integrin, α5-integrin and pFAK (downstream from β1-integrin). Similar results were observed in RKO and SW480 cells; knockdown of NR4A1 decreased β1-integrin, α5-integrin and pFAK, and knockdown of β1-integrin also decreased pFAK and α5-integrin (Fig. 2B). Treatment of the pancreatic cancer cell lines with 15 or 20 μM DIM-C-pPhCO2Me and DIM-C-pPhOH also decreased expression of β1-integrin, pFAK and α5-integrin (Figs. 2C and 2D) and the NR4A1 antagonists caused similar effects in RKO and SW480 cells (Figs. 2E and 2F) demonstrating that NR4A1 regulates β1-integrin in both pancreatic and colon cancer cells and that knockdown of NR4A1 (siNR4A1) or treatment with NR4A1 antagonist decreases β1-integrin expression. We also observed that DIM-C-pPhOH decreased β1-integrin and α5-integrin expression in tumor lysates from an orthotopic pancreatic cancer model using L3.6pL cells (Suppl. Fig. S1) [12].
Figure 1.
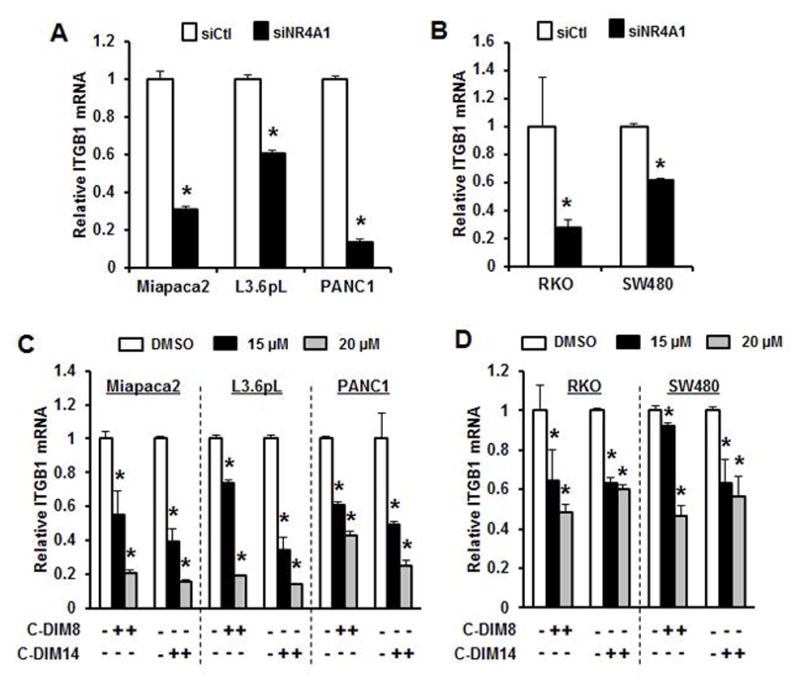
β1-Integrin is an NR4A1-regulated gene. Pancreatic (A) and colon (B) cancer cells were transfected with siNR4A1, and β1-integrin (ITGB1) mRNA levels were determined by real time PCR as outlined in the Materials and Methods. Pancreatic (C) and colon (D) cancer cells were treated with 15 and 20 μM DIM-C-pPhOH (C-DIM8) and DIM-C-pPhCO2Me (C-DIM14) for 24 hr, and mRNA levels were determined by real time PCR as outlined in the Materials and Methods. Results are expressed means ± SE for three determinations per treatment group and significantly (p<0.05) decreased expression is indicated (*).
Figure 2.
NR4A1 regulates β1-integrin and β1-integrin-regulated genes/gene activation. Pancreatic (A) and colon (B) cancer cells were transfected with siNR4A1 or siβ1-integrin, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. Pancreatic cancer cells were treated with DIM-C-pPhCO2Me (C-DIM14) (C) and DIM-C-pPhOH (C-DIM8) (D), and colon cancer cells were treated with the same C-DIMs (E and F) for 24 hr, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. Similar results were observed in more than one experiment for each panel.
2. NR4A1 antagonists inhibit cancer cell adhesion and migration
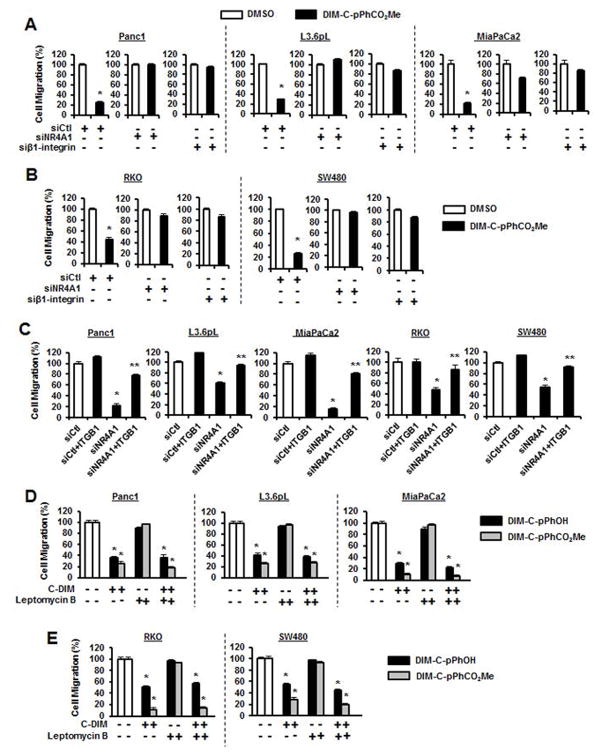
β1-Integrin regulates multiple pathways including cell migration and adhesion, and knockdown of NR4A1 by RNAi significantly decreased migration of Panc1, L3.6pL and MiaPaCa2 cells in a Boyden chamber assay (Fig. 3A) and similar results were observed in RKO and SW480 colon cancer cells transfected with siNR4A1 (Fig. 3B). Migration of Panc1, L3.6pL and MiaPaCa2 cells was also significantly decreased after treatment with the NR4A1 antagonists DIM-C-pPhOH and DIM-C-pPhCO2Me (Figs. 3C and 3D) and similar results were observed in RKO and SW480 cells treated with the same compounds (Figs. 3E and 3F).
Figure 3.
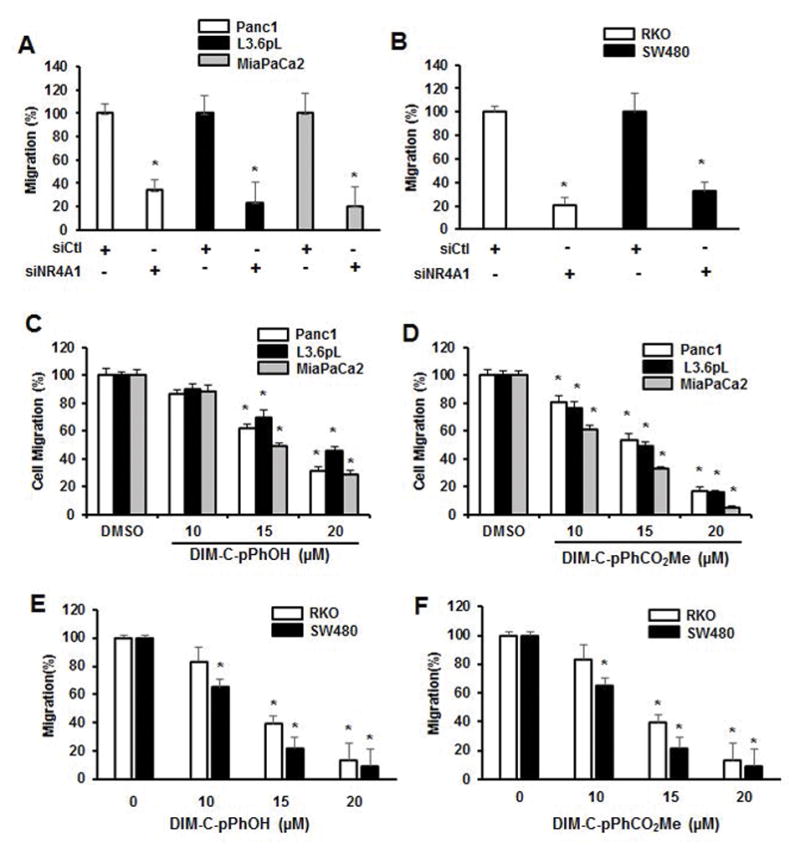
NR4A1 regulates pancreatic and colon cancer cell migration. Pancreatic (A) and colon (B) cancer cells were transfected with siCtl (control) or siNR4A1, and migration was determined in a Boyden chamber assay. Pancreatic cancer cells were treated with DIM-C-pPhOH (C) and DIM-C-pPhCO2Me (D) and colon cancer cells were treated with DIM-C-pPhOH (E) and DIM-C-pPhCO2Me (F) for 24 hr and migration was determined in a Boyden chamber assay as outlined in the Materials and Methods. Each experiment was carried out in triplicate, and results are expressed as mean ± SE and significantly (p<0.05) decreases in migration are indicated (*).
We also investigated the antimigratory effects of DIM-C-pPhCO2Me in pancreatic cancer cells after knockdown of NR4A1 or β1-integrin. DIM-C-pPhCO2Me decreased migration; however, in cells depleted of NR4A1 or β1-integrin minimum inhibition was observed in cells after treatment with DIM-C-pPhCO2Me (Fig. 4A) and similar results were observed in colon cancer cells (Fig. 4B) and also with DIM-C-pPhOH (Suppl. Fig. S2). These results not only confirm the importance of both NR4A1 and β1-integrin in cell migration but also demonstrate that in the absence of β1-integrin, DIM-C-pPhCO2Me-mediated antagonism of other NR4A1-mediated pro-oncogenic pathways has minimal effects on cell migration. Moreover, in pancreatic and colon cancer cells transfected with siNR4A1, the resulting decreased migration was rescued by β1-integrin (ITGB1) gene overexpression (Fig. 4C). Previous studies show that NR4A1-dependent anticancer activities of C-DIMs in colon and pancreatic cancer cells were due to modulation of nuclear NR4A1-mediated changes in gene expression [12,14,19,26] and this contrasted to several apoptosis-inducing agents which cause nuclear export of NR4A1 [[reviewed in 4]]. This was also confirmed in this study showing that the inhibitory effects of DIM-C-pPhCO2Me on cell migration in pancreatic (Fig. 4D) and colon (Fig. 4E) cancer cells were comparable in the absence or presence of the nuclear export inhibitor leptomycin B (LMB) demonstrating that the effects of the antagonist on NR4A1 (and β1-integrin) were nuclear effects and not associated with receptor export.
Figure 4.
β1-Integrin-dependent migration is dependent on NR4A1. Pancreatic (A) and colon (B) cancer cells were treated with DMSO or 20 μM DIM-C-pPhCO2Me alone or after transfection with siCtl (control), siNR4A1 or siβ1-integrin, and migration was determined in a Boyden chamber assay as outlined in the Materials and Methods. (C) Pancreatic and colon cancer cells were transfected with siCtl (control), siCtl+β1-integrin (ITGB1) expression plasmid, siNR4A1, and siNR4A1+β1-integrin expression plasmid, and cell migration was determined in a Boyden chamber assay. Pancreatic (D) and colon (E) cancer cells were treated with DMSO, 20 μM DIM-C-pPhOH or DIM-C-pPhCO2Me alone or in combination with leptomycin for 24 hr, and migration was determined in a Boyden chamber assay. Results are expressed as mean ± SE for three separate determinations for each treatment group and significant (p<0.05) inhibition of migration (*) and rescue by β1-integrin overexpression (**) (C) are indicated.
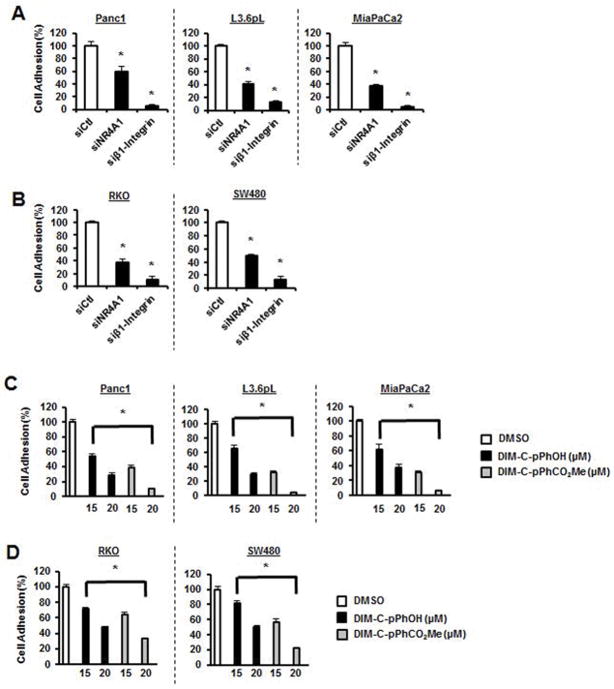
Extracellular matrix components such as fibronectin are ligands for integrins and induce α- and β-integrin heterodimer formation and activation of downstream pathways. Fibronectin-induced adhesion was investigated using BD-coated fibronectin in 24-well plates and transfection of pancreatic (Fig. 5A) and colon (Fig. 5B) cancer cells with siNR4A1 or siβ1-integrin significantly decreased adhesion demonstrating that like β1-integrin, NR4A1 plays a role in cell adhesion. This was further confirmed in pancreatic (Fig. 5C) and colon (Fig. 5D) cancer cells treated with DIM-C-pPhOH and DIM-C-pPhCO2Me which significantly inhibited fibronectin-mediated adhesion Thus, NR4A1 regulation of β1-integrin maintains adhesion in cancer cells and this can also be significantly inhibited by NR4A1 antagonists or knockdown of NR4A1.
Figure 5.
NR4A1 inactivation decreases cancer cell adhesion. Pancreatic (A) and colon (B) cancer cells were transfected with siCtl (control), siNR4A1 and siβ1-integrin, and cell adhesion was determined as outlined in the Materials and Methods. Pancreatic (C) and colon (D) cancer cells were treated with DIM-C-pPhOH or DIM-C-pPhCO2Me for 24 hr, and cell adhesion was determined as outlined in the Materials and Methods. Results are expressed as means ± SE for three replicate determinations for each treatment group and significant (p<0.05) inhibition of adhesion is indicated (*).
3. Mechanism of NR4A1 regulation of β1-integrin
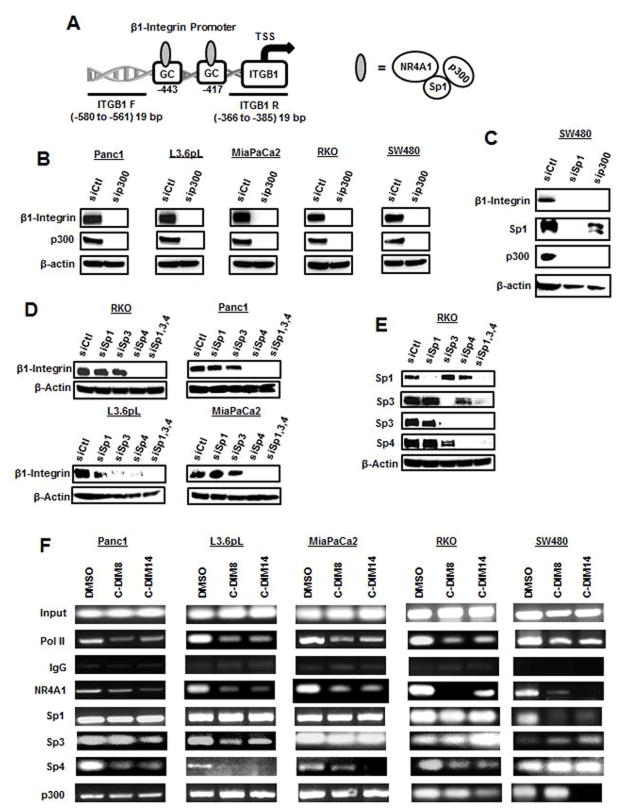
Previous studies in breast cancer cells showed that β1-integrin is regulated by a NR4A1/p300/Sp1 complex bound to a proximal GC-rich region of the β1-integrin promoter (Fig. 6A) [29] and this same complex also regulates survivin expression in pancreatic and colon cancer cells [12,26]. The functional role of these complexes was investigated by RNAi, and knockdown of p300 decreased β1-integrin protein expression (Fig. 6B) in the five pancreatic and colon cancer cell lines and this paralleled the effects observed after knockdown of NR4A1 or treatment with NR4A1 antagonists (Fig. 2). In a separate experiment, cells were transfected with siSp1, and decreased expression of β1-integrin was observed only in SW480 cells (Fig. 6C) and this corresponded with the role of Sp1 in regulating β1-integrin in breast cancer cells [29]. Interestingly, knockdown of Sp1 also decreased expression of p300 in SW480 cells and this may also contribute to the decreased expression of β1-integrin. However, since Sp1 knockdown did not affect β1-integrin expression in Panc1, L3.6pL, MiaPaCa2 and RKO cells (data not shown), we further investigated the role of Sp3 and Sp4 in regulation of β1-integrin using lysates from a previous study on suppression of Sp proteins [42]. Figure 6D shows that knockdown of Sp4 and all three Sp proteins combined (siSp1/3/4) decreased β1-integrin expression in all four cell lines and in L3.6pL cells, knockdown of Sp3 also decreased β1-integrin expression. Thus, the role of Sp1, Sp3 and Sp4 in NR4A1-dependent regulation of β1-integrin is cell context dependent. The effects of Sp1, Sp3 and Sp4 (singly and combined) knockdown in RKO cells were not previously determined and it was evident that Sp3 and Sp4 but not Sp1 coregulated each other (Fig. 6E) from their respective GC-rich promoters [43,44]. Figure 6F summarizes results of ChIP assays on interactions with the GC-rich sequence of the β1-integrin promoter in all five pancreatic and colon cancer cell lines. In untreated cells, pol II, NR4A1, Sp1, Sp3, Sp4 and p300 were bound to the GC-rich region with one exception; Sp3 binding was barely detectable in SW480 cells. Treatment with the two NR4A1 antagonists (C-DIM8 and C-DIM14) induced similar effects on promoter interactions but differed in the magnitude of the response for some factors. Treatment with NR4A1 antagonists decreased pol II and NR4A1 interaction to the β1-integrin promoter in all five cell lines and Sp1 was decreased only in SW480 cells. Sp3 interactions were variable (increased, decreased and unchanged) and Sp4 was decreased in all cells with the exception of SW480 cells. The NR4A1 antagonists decreased p300 interactions with the β1-integrin promoter in SW480 and RKO colon cancer cells but not in the pancreatic cancer cell lines. These results demonstrate that the effects of NR4A1 antagonists on the NR4A1/p300/Sp interactions with the β1-integrin promoter are complex and cell context dependent with DNA-bound Sp4 being a major factor based on the knockdown and ChIP assays (Fig. 6D). This is the first example of an important role for NR4A1/Sp4-mediated transactivation, and examples of other genes regulated by this complex are currently being investigated.
Figure 6.
Mechanism of NR4A1 regulation of β1-integrin gene expression. (A) β1-Integrin promoter and proximal GC-rich sequence. (B) Pancreatic and colon cancer cells were transfected with siCtl (control) and sip300, and whole cell lysates were analyzed by western blots as outlined in the Materials and Methods. (C) SW480 cells were transfected with siCtl (control), siSp1 and sip300, and whole cell lysates were analyzed by western blots. (D) RKO, Panc1, L3.6pL and MiaPaCa2 lysates from cells transfected with siCtl (control), siSp1, siSp3, siSp4 or siSp1,3,4 were obtained from a previous study (42) and analyzed by western blots. (E) Using a similar protocol, Sp proteins were also knockdowned in RKO cells and analyzed for different Sp proteins by western blots. [Note: RKO cells were not included in a previous study on the effects of Sp knockdown in multiple cancer cell lines (42).] (F) Pancreatic and colon cancer cells were analyzed in ChIP assays to determine interactions with the GC-rich region of the β1-integrin promoter as outlined in the Materials and Methods. C-DIM8 = DIM-C-pPhOH; C-DIM14 = DIM-C-pPhCO2Me.
DISCUSSION
Integrins are heterodimeric cell surface receptors that consist of an α- and β-subunit and these receptors play critical roles in maintaining cellular homeostasis and also in several human pathologies. There are 18 different α and 8 different β integrin subunits and among the 24 α/β-integrin heterodimers, β1-integrin forms the largest subgroup of 12 receptor complexes [45–49]. β1-Integrin heterodimers interact with other signaling pathways and activate multiple genes and kinases such as focal adhesion kinase resulting in the induction of cell adhesion, migration and invasion. The role of integrins in multiple diseases has spurred studies on development of drugs that target integrins and these include antibodies, peptides that inhibit heterodimer formation, and other small molecules [47–49]. However, the success of these agents in cancer chemotherapy has been limited and integrin inhibitors have not been approved for clinical applications in cancer chemotherapy.
C-DIMs have been identified as a novel class of NR4A1 ligands that modulate nuclear NR4A1-mediated gene expression and associated pathways [12,14,15,26–29]. DIM-C-pPhOH and DIM-C-pPhCO2Me inhibit mTOR, decrease cell proliferation and survival, and induce ROS in multiple cancer cell lines where these compounds act as NR4A1 antagonists [14,19]. Our recent study showed that these NR4A1 ligands also inhibit breast cancer cell migration through inhibition of β1-integrin expression which is an NR4A1-regulated gene in breast cancer cells [29]. There is also evidence that β1-integrin is a negative prognostic factor for colon and pancreatic patients and plays an important role in cancer cell migration/invasion and metastasis [30–41]. It was evident from the results of RNAi studies that decreased expression of NR4A1 resulted in decreased expression of β1-integrin RNA and protein, α5-integrin and a β1-integrin-activated FAK gene phosphorylation (Figs. 1A, 1B, 2A and 2E). Moreover, siNR4A1 also decreased β1-integrin-regulated migration and adhesion (Figs. 3 and 5) and the effects of NR4A1 or β1-integrin knockdown were comparable indicating that NR4A1 regulates β1-integrin in pancreatic and colon cancer cells as previously observed for breast cancer cell lines [29]. We also observed that knockdown of NR4A1 by RNAi or treatment with DIM-C-pPhOH and DIM-C-pPhCO2Me gave comparable results demonstrating that these C-DIMs act as NR4A1 antagonists to decrease β1-integrin expression and thereby represent a novel class of agents targeting β1-integrin in pancreatic and colon cancer.
Although NR4A1 regulates expression of genes through binding as a monomer or dimer to cognate response elements in breast cancer cells, NR4A1 regulates expression of β1-integrin by cooperative interactions of NR4A1 and p300 with promoter DNA (GC-rich)-bound Sp1 [29]. NR4A1/p300 also induced survivin expression through interactions with Sp1 bound to the GC-rich survivin promoter [12]. Results of RNAi and ChIP assays showed that in SW480 colon cancer cells, β1-integrin was regulated by an NR4A1/p300/Sp1 complex bound to the proximal GC-rich site of the β1-integrin promoter (Fig. 6A). ChIP assays showed that Sp1, Sp3 and Sp4 bound the proximal GC-rich site in the β1-integrin promoter; however, it is clear from Sp knockdown studies that the functional Sp protein was cell context dependent (Fig. 6C and 6D). Thus, Sp4 and to a lesser extent Sp3 in combination with NR4A1 and p300 also regulate β1-integrin expression is some colon and pancreatic cancer cell lines. The reason for the cell selective and functional role of Sp1, Sp3 and Sp4 in regulating β1-integrin expression via an NR4A1/p300/Sp complex is unclear and is currently being investigated.
In summary, results of this study demonstrate the role of NR4A1 in regulation of pancreatic and colon cancer cell migration through transcriptional activation of β1-integrin which is an NR4A1/p300/Sp-regulated gene. This observation is consistent with previous studies demonstrating that NR4A1 regulates multiple pro-oncogenic genes/pathways in solid tumors. We also show that C-DIM/NR4A1 antagonists represent a novel class of drugs that target β1-integrin in pancreatic and colon cancer, and current studies are focused on developing these compounds for future clinical applications.
Supplementary Material
Acknowledgments
Funding: The financial assistance of the National Institutes of Health (P30-ES023512, S. Safe), Texas AgriLife Research and Sid Kyle endowment, is gratefully acknowledged.
Abbreviations
- C-DIM
1,1-bis(3′-indolyl)-1-(p-substituted phenyl)methane
- DIM-C-pPhCO2Me
1,1-bis(3′-indolyl)-1-(p-carboxymethylphenyl)methane
- DIM-C-pPhOH
1,1-bis(3′-indolyl)-1-(p-hydroxyphenyl)methane
- IDH1
isocitrate dehydrogenase 1
- NR4A1
nuclear receptor 4A1
- TXNDC5
thioredoxin domain containing 5
Footnotes
Conflict of Interest: There are no conflicts of interest to declare.
LITERATURE CITED
- 1.Pearen MA, Muscat GE. Minireview: Nuclear hormone receptor 4A signaling: implications for metabolic disease. Molecular Endocrinology. 2010;24(10):1891–1903. doi: 10.1210/me.2010-0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Safe S, Jin UH, Morpurgo B, Abudayyeh A, Singh M, Tjalkens RB. Nuclear receptor 4A (NR4A) family - orphans no more. Journal of Steroid Biochemistry and Molecular Biology. 2015 doi: 10.1016/j.jsbmb.2015.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurakula K, Koenis DS, van Tiel CM, de Vries CJ. NR4A nuclear receptors are orphans but not lonesome. Biochimica et Biophysica Acta. 2014;1843(11):2543–2555. doi: 10.1016/j.bbamcr.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 4.Zhang XK. Targeting Nur77 translocation. Expert Opinion on Therapeutic Targets. 2007;11(1):69–79. doi: 10.1517/14728222.11.1.69. [DOI] [PubMed] [Google Scholar]
- 5.Safe S, Jin UH, Hedrick E, Reeder A, Lee SO. Minireview: role of orphan nuclear receptors in cancer and potential as drug targets. Molecular Endocrinology. 2014;28(2):157–172. doi: 10.1210/me.2013-1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhan Y, Du X, Chen H, et al. Cytosporone B is an agonist for nuclear orphan receptor Nur77. Nature Chemical Biology. 2008;4(9):548–556. doi: 10.1038/nchembio.106. [DOI] [PubMed] [Google Scholar]
- 7.Wang WJ, Wang Y, Chen HZ, et al. Orphan nuclear receptor TR3 acts in autophagic cell death via mitochondrial signaling pathway. Nature Chemical Biology. 2014;10(2):133–140. doi: 10.1038/nchembio.1406. [DOI] [PubMed] [Google Scholar]
- 8.Liu JJ, Zeng HN, Zhang LR, et al. A unique pharmacophore for activation of the nuclear orphan receptor Nur77 in vivo and in vitro. Cancer Research. 2010;70(9):3628–3637. doi: 10.1158/0008-5472.CAN-09-3160. [DOI] [PubMed] [Google Scholar]
- 9.Zhan YY, Chen Y, Zhang Q, et al. The orphan nuclear receptor Nur77 regulates LKB1 localization and activates AMPK. Nature Chemical Biology. 2012;8(11):897–904. doi: 10.1038/nchembio.1069. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Liu Y, Chen HZ, et al. Impeding the interaction between Nur77 and p38 reduces LPS-induced inflammation. Nature Chemical Biology. 2015;11(5):339–346. doi: 10.1038/nchembio.1788. [DOI] [PubMed] [Google Scholar]
- 11.Mullican SE, Zhang S, Konopleva M, et al. Abrogation of nuclear receptors Nr4a3 and Nr4a1 leads to development of acute myeloid leukemia. Nature Medicine. 2007;13(6):730–735. doi: 10.1038/nm1579. [DOI] [PubMed] [Google Scholar]
- 12.Lee SO, Abdelrahim M, Yoon K, et al. Inactivation of the orphan nuclear receptor TR3/Nur77 inhibits pancreatic cancer cell and tumor growth. Cancer Research. 2010;70(17):6824–6836. doi: 10.1158/0008-5472.CAN-10-1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Muscat GE, Eriksson NA, Byth K, et al. Research resource: nuclear receptors as transcriptome: discriminant and prognostic value in breast cancer. Molecular Endocrinology. 2013;27(2):350–365. doi: 10.1210/me.2012-1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee SO, Andey T, Jin UH, Kim K, Singh M, Safe S. The nuclear receptor TR3 regulates mTORC1 signaling in lung cancer cells expressing wild-type p53. Oncogene. 2012;31(27):3265–3276. doi: 10.1038/onc.2011.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho SD, Yoon K, Chintharlapalli S, et al. Nur77 agonists induce proapoptotic genes and responses in colon cancer cells through nuclear receptor-dependent and nuclear receptor-independent pathways. Cancer Research. 2007;67(2):674–683. doi: 10.1158/0008-5472.CAN-06-2907. [DOI] [PubMed] [Google Scholar]
- 16.Wang JR, Gan WJ, Li XM, et al. Orphan nuclear receptor Nur77 promotes colorectal cancer invasion and metastasis by regulating MMP-9 and E-cadherin. Carcinogenesis. 2014;35(11):2474–2484. doi: 10.1093/carcin/bgu157. [DOI] [PubMed] [Google Scholar]
- 17.Zhou F, Drabsch Y, Dekker TJ, et al. Nuclear receptor NR4A1 promotes breast cancer invasion and metastasis by activating TGF-beta signalling. Nat Commun. 2014;5:3388. doi: 10.1038/ncomms4388. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Lin Y, Li W, et al. Regulation of Nur77 expression by beta-catenin and its mitogenic effect in colon cancer cells. FASEB Journal. 2011;25(1):192–205. doi: 10.1096/fj.10-166462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SO, Jin UH, Kang JH, et al. The orphan nuclear receptor NR4A1 (Nur77) regulates oxidative and endoplasmic reticulum stress in pancreatic cancer cells. Molecular Cancer Research. 2014;12(4):527–538. doi: 10.1158/1541-7786.MCR-13-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith AG, Lim W, Pearen M, Muscat GE, Sturm RA. Regulation of NR4A nuclear receptor expression by oncogenic BRAF in melanoma cells. Pigment Cell Melanoma Res. 2011;24(3):551–563. doi: 10.1111/j.1755-148X.2011.00843.x. [DOI] [PubMed] [Google Scholar]
- 21.Zhan YY, He JP, Chen HZ, Wang WJ, Cai JC. Orphan receptor TR3 is essential for the maintenance of stem-like properties in gastric cancer cells. Cancer Letters. 2013;329(1):37–44. doi: 10.1016/j.canlet.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Bras A, Albar JP, Leonardo E, de Buitrago GG, Martinez AC. Ceramide-induced cell death is independent of the Fas/Fas ligand pathway and is prevented by Nur77 overexpression in A20 B cells. Cell Death and Differentiation. 2000;7(3):262–271. doi: 10.1038/sj.cdd.4400653. [DOI] [PubMed] [Google Scholar]
- 23.Li QX, Ke N, Sundaram R, Wong-Staal F. NR4A1, 2, 3--an orphan nuclear hormone receptor family involved in cell apoptosis and carcinogenesis. Histology and Histopathology. 2006;21(5):533–540. doi: 10.14670/HH-21.533. [DOI] [PubMed] [Google Scholar]
- 24.Kolluri SK, Bruey-Sedano N, Cao X, et al. Mitogenic effect of orphan receptor TR3 and its regulation by MEKK1 in lung cancer cells. Molecular and Cellular Biology. 2003;23(23):8651–8667. doi: 10.1128/MCB.23.23.8651-8667.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ke N, Claassen G, Yu DH, et al. Nuclear hormone receptor NR4A2 is involved in cell transformation and apoptosis. Cancer Research. 2004;64(22):8208–8212. doi: 10.1158/0008-5472.CAN-04-2134. [DOI] [PubMed] [Google Scholar]
- 26.Lee SO, Li X, Hedrick E, et al. Diindolylmethane analogs bind NR4A1 and are NR4A1 antagonists in colon cancer cells. Molecular Endocrinology. 2014;28(10):1729–1739. doi: 10.1210/me.2014-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hedrick E, Lee SO, Kim G, et al. Nuclear receptor 4A1 (NR4A1) as a drug target for renal cell adenocarcinoma. PloS One. 2015;10(6):e0128308. doi: 10.1371/journal.pone.0128308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. Nuclear receptor 4A1 as a drug target for breast cancer chemotherapy. Endocrine-Related Cancer. 2015;22(5):831–840. doi: 10.1530/ERC-15-0063. [DOI] [PubMed] [Google Scholar]
- 29.Hedrick E, Lee SO, Doddapaneni R, Singh M, Safe S. NR4A1 antagonists inhibit β1-integrin-dependent breast cancer cell migration. Molecular and Cellular Biology. 2016;36(9):1383–1394. doi: 10.1128/MCB.00912-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grzesiak JJ, Tran Cao HS, Burton DW, et al. Knockdown of the β (1) integrin subunit reduces primary tumor growth and inhibits pancreatic cancer metastasis. International Journal of Cancer. 2011;129(12):2905–2915. doi: 10.1002/ijc.25942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu F, Li H, Bu X, Zhang Y. Effects and mechanism of integrin-β1 gene expression inhibited by shRNA in invasion of pancreatic carcinoma PANC-1 cells. Hepato-Gastroenterology. 2012;59(114):561–564. doi: 10.5754/hge10760. [DOI] [PubMed] [Google Scholar]
- 32.Vogelmann R, Kreuser ED, Adler G, Lutz MP. Integrin α6β1 role in metastatic behavior of human pancreatic carcinoma cells. International Journal of Cancer. 1999;80(5):791–795. doi: 10.1002/(sici)1097-0215(19990301)80:5<791::aid-ijc25>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 33.Grzesiak JJ, Bouvet M. The α2β1 integrin mediates the malignant phenotype on type I collagen in pancreatic cancer cell lines. British Journal of Cancer. 2006;94(9):1311–1319. doi: 10.1038/sj.bjc.6603088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou G, Chiu D, Qin D, et al. Detection and clinical significance of CD44v6 and integrin-β1 in pancreatic cancer patients using a triplex real-time RT-PCR assay. Applied Biochemistry and Biotechnology. 2012;167(8):2257–2268. doi: 10.1007/s12010-012-9752-2. [DOI] [PubMed] [Google Scholar]
- 35.Bottger TC, Maschek H, Lobo M, Gottwohl RG, Brenner W, Junginger T. Prognostic value of immunohistochemical expression of β-1 integrin in pancreatic carcinoma. Oncology. 1999;56(4):308–313. doi: 10.1159/000011984. [DOI] [PubMed] [Google Scholar]
- 36.Sun C, Zargham R, Shao Q, et al. Association of CD98, integrin β1, integrin β3 and Fak with the progression and liver metastases of colorectal cancer. Pathology, Research and Practice. 2014;210(10):668–674. doi: 10.1016/j.prp.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 37.Su JM, Gui L, Zhou YP, Zha XL. Expression of focal adhesion kinase and α5 and β1 integrins in carcinomas and its clinical significance. World Journal of Gastroenterology. 2002;8(4):613–618. doi: 10.3748/wjg.v8.i4.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Peng L, Xing X, Li W, et al. PRL-3 promotes the motility, invasion, and metastasis of LoVo colon cancer cells through PRL-3-integrin β1-ERK1/2 and-MMP2 signaling. Molecular Cancer. 2009;8:110. doi: 10.1186/1476-4598-8-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kryczka J, Stasiak M, Dziki L, Mik M, Dziki A, Cierniewski C. Matrix metalloproteinase-2 cleavage of the β1 integrin ectodomain facilitates colon cancer cell motility. Journal of Biological Chemistry. 2012;287(43):36556–36566. doi: 10.1074/jbc.M112.384909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wiktorska M, Sacewicz-Hofman I, Stasikowska-Kanicka O, Danilewicz M, Niewiarowska J. Distinct inhibitory efficiency of siRNAs and DNAzymes to β1 integrin subunit in blocking tumor growth. Acta Biochimica Polonica. 2013;60(1):77–82. [PubMed] [Google Scholar]
- 41.Bartolome RA, Barderas R, Torres S, et al. Cadherin-17 interacts with α2β1 integrin to regulate cell proliferation and adhesion in colorectal cancer cells causing liver metastasis. Oncogene. 2014;33(13):1658–1669. doi: 10.1038/onc.2013.117. [DOI] [PubMed] [Google Scholar]
- 42.Hedrick E, Cheng Y, Jin UH, Kim K, Safe S. Specificity protein (Sp) transcription factors Sp1, Sp3 and Sp4 are non-oncogene addiction genes in cancer cells. Oncotarget. 2016;7(16):22245–22256. doi: 10.18632/oncotarget.7925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song J, Mangold M, Suske G, et al. Characterization and promoter analysis of the mouse gene for transcription factor Sp4. Gene. 2001;264(1):19–27. doi: 10.1016/s0378-1119(01)00328-6. [DOI] [PubMed] [Google Scholar]
- 44.Tapias A, Monasterio P, Ciudad CJ, Noe V. Characterization of the 5′-flanking region of the human transcription factor Sp3 gene. Biochimica et Biophysica Acta. 2005;1730(2):126–136. doi: 10.1016/j.bbaexp.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 45.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110(6):673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 46.Brakebusch C, Fassler R. β1-Integrin function in vivo: adhesion, migration and more. Cancer and Metastasis Reviews. 2005;24(3):403–411. doi: 10.1007/s10555-005-5132-5. [DOI] [PubMed] [Google Scholar]
- 47.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nature Reviews: Cancer. 2010;10(1):9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goodman SL, Picard M. Integrins as therapeutic targets. Trends in Pharmacological Sciences. 2012;33(7):405–412. doi: 10.1016/j.tips.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 49.Barkan D, Chambers AF. β1-integrin: a potential therapeutic target in the battle against cancer recurrence. Clinical Cancer Research. 2011;17(23):7219–7223. doi: 10.1158/1078-0432.CCR-11-0642. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.