Abstract
Background
Despite recent insights into the pathophysiology of acute and chronic itch, chronic itch remains an often intractable condition. Among major contributors to chronic itch is dysfunction of spinal cord GABAergic inhibitory controls.
Objective
To test the hypothesis that selective GABA agonists as well as cell transplant-derived GABA are antipruritic against acute itch and in a transgenic mouse model of atopic dermatitis produced by overexpression of the TH2 cell-associated cytokine, IL-31 (IL-31Tg mice).
Methods
We injected wild-type and IL-31Tg mice with combinations of GABA-A (muscimol) or GABA-B (baclofen) receptor agonists 15–20 min prior to injection of various pruritogens (histamine, chloroquine or endothelin-1) and recorded spontaneous scratching before and after drug administration. We also tested the antipruritic properties of intraspinal transplantation of precursors of GABAergic interneurons in the IL-31Tg mice.
Results
Systemic muscimol or baclofen are antipruritic against both histamine-dependent and independent pruritogens, but the therapeutic window using either ligand alone was very small. In contrast, combined subthreshold doses of baclofen and muscimol produced a significant synergistic antipruritic effect, with no sedation. Finally, transplant-mediated long term enhancement of GABAergic signaling not only reduced spontaneous scratching in the IL-31Tg mice but also dramatically resolved the associated skin lesions.
Conclusion
Although additional research is clearly needed, existing approved GABA agonists should be considered in the management of chronic itch, notably atopic dermatitis.
Keywords: atopic dermatitis, chronic itch, baclofen, muscimol, GABA, pruritogens, GABAergic progenitor cell transplants
Introduction
Atopic dermatitis (AD), an inflammatory, relapsing chronic pruritic skin disease, is an often intractable form of chronic itch that negatively impacts the quality of life of millions of patients 1. Unfortunately, because chronic itch conditions have very different etiologies, most treatments have poor outcomes and are accompanied by unacceptable adverse side effects, notably sedation 2. Clearly, a better understanding of the pathophysiology of these chronic itch conditions is critical to designing successful therapeutic strategies.
Studies of the etiology of chronic itch 3 generally focus on changes in skin and immune dysfunction. However, there is now considerable evidence for a contribution of primary afferent pruritoceptors that transmit itch messages to spinal cord and brainstem circuits engaged by and that regulate these messages 4. Of particular interest are studies demonstrating commonalities in the mechanisms underlying nerve injury-induced neuropathic pain and itch and the possibility that comparable approaches may be appropriate for their management 5.
Although there is evidence for specificity in the transmission of itch and pain messages at the level of the primary afferent nociceptor and pruritoceptor 6, 7, both pain and itch are under spinal cord inhibitory interneuron-mediated control. For example, loss of spinal cord GABA or glycinergic function is a major contributor to the spontaneous pain and hypersensitivity that develops following nerve injury 8–10. Moreover, persistent scratching, a manifestation of chronic itch, occurs in the Bhlhb5 mutant mouse, in which there is dramatic loss of dorsal horn GABAergic inhibitory interneurons 11. Ablation of glycinergic interneurons also induces excessive scratching and pain 12. And in a model of dry skin-induced scratching in the mouse, GABA and glycine receptor antagonists can block scratching-induced inhibition of firing in superficial dorsal horn neurons 13. Finally, in patients, acute withdrawal of intrathecal baclofen, a GABA-B receptor agonist, can induce pruritus 14.
Given the evidence for a potential contribution of GABA agonists in the management of pruritus, it is surprising that there are no studies that assessed their utility, in preclinical or clinical conditions. Here, we demonstrate that both GABA-A and GABA-B agonists are not only effective in models of acute itch, but we also show that systemic administration of very low doses of these agonists has synergistic antipruritic effects in the interleukin-31 (IL-31) overexpressing transgenic mouse, a model of atopic dermatitis (AD) 15 that is refractory to anti-histamines 1, 16 and thus particularly difficult to manage. Most importantly, the antipruritic synergy could be produced without concomitant sedation. Finally, we show that sustaining high levels of GABA inhibition can be achieved using intraspinal transplantation of cortical GABAergic interneuron precursor cells. The transplants not only attenuated spontaneous scratching but also dramatically reduced skin lesions in the IL-31Tg mice.
Materials and Methods
Animals
Male C57BL6/J mice purchased from Jackson Laboratories were used for all experiments unless otherwise stated. Interleukin-31 transgenic mice were a generous gift from ZymoGenetics/Bristol-Myers Squibb. The IL-31Tg mice were generated as previously described 15. All experiments were approved by the UCSF Institutional Animal Care and Use Committee and conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals.
Pharmacology
Based on our previous studies 7 we used a minimum of 4–5 wild-type C57BL6 male mice per group in acute itch studies. For chronic itch studies, because scratching levels vary among IL-31Tg mice, to decrease variability we only used mice with skin lesions and that exhibited >100 scratching bouts (over 30 min) at the nape of the neck. Mice received intraperitoneal (i.p.) injections of baclofen (1–4.0 mg/kg in saline) or muscimol (0.3–2.0 mg/kg), and motor coordination/sedation was subsequently evaluated using the rotarod test. Only non-sedating doses were subsequently used for behavioral analyses (≤2.0mg/kg for baclofen and ≤ 1.25mg/kg for muscimol). For acute itch studies, we administered the following pruritogens subcutaneously into the nape of the neck: histamine (500 μg/100 μl), endothelin-1 (25ng/100 μl) and chloroquine (100 or 200 μg/100μl). To quantify scratching behavior, mice were habituated, individually, in plexiglass cylinders for 1h. Mice were then injected with baclofen or muscimol (i.p.) and 15 to 20 min later with the pruritogen. Behavior (scratching) was monitored by video recording over the next 30 minutes.
To assess the anti-pruritic effects of baclofen and muscimol in IL-31Tg mice, we injected these mice with baclofen (2.0 mg/kg, i.p.) or muscimol (1.25 mg/kg, i.p.) and recorded the scratching behavior up to 1h (muscimol) or 6h (baclofen). To assess the synergistic effects of the baclofen-muscimol combinations, we injected IL-31Tg mice with a subthreshold dose of baclofen (1.0 mg/kg; i.p.) 20 min prior to a subthreshold dose of muscimol (1.0 mg/kg; i.p.) and recorded scratching bouts for the next 60 min. Motor performance of the IL-31Tg mice injected with baclofen, muscimol or the combination was also evaluated with the rotarod test. In all behavioral analyses, the investigator scoring the behavior was blind to treatment and codes were only broken after all scoring was completed.
Statistical analyses
Behavioral and anatomical data are expressed as mean ± SEM, where N represents the number of mice. Raw data obtained in the course of the study were analyzed with a two-way ANOVA followed by a Bonferroni post hoc test. Asterisks (*) indicate statistically significant differences between groups, with * = p < 0.05, ** = p < 0.01, and *** = p < 0.001.
Cell transplantation
Medial ganglionic eminence (MGE) cells were dissected and transplanted into the spinal cord of IL-31 Tg mice, as previously described 17. Briefly, GFP-expressing cells from the MGE were harvested from E13.5 embryos, manually dissociated and resuspended in culture medium. One group of animals received MGE cells (MGE group) and one group received medium only (control group). After hemi-laminectomy of the C4-C8 vertebra, we transplanted cells (50,000) unilaterally over 2 segments of the cervical spinal cord. Photographs of the lesions were taken before and once a week for 4 weeks after transplantation.
Immunohistochemistry
Mice were perfused with 10 ml phosphate-buffered saline (PBS) followed by 30 ml of ice-cold 10% formalin. Spinal cord and lumbar DRGs were dissected, post-fixed 3–4 h at 4°C and cryoprotecte d overnight in phosphate-buffered (PB) 30% sucrose. Frozen cryostat sections of spinal cord and DRG were cut at 25 or 14μm, respectively. After a 1h incubation in 10% normal goat serum in PBS with 0.3% Triton (NGST), the sections were incubated overnight in primary antibody diluted in 10% NGST. The following day the sections were washed 3× with PBS, and then incubated 1h in secondary antibody (Alexa-488 or Alexa-594, diluted 1:1000 in 1% NGST). After washing 3× in PBS, sections were mounted and coverslipped with Fluoromount G. Sections were viewed with a Nikon Eclipse fluorescence microscope and images were collected with a Zeiss confocal microscope. Brightness and contrast were adjusted using Adobe Photoshop, v. 6.0 (San Jose, CA).
Antibodies
We used the following antibodies: rabbit anti-GFP (1:2000, Molecular Probe), rabbit anti-Fos (1:4000, Oncogene), chicken anti-GFP (1:2000, Abcam), rabbit anti-GABA (1:2000, Sigma), mouse anti-parvalbumin (1:2000, Sigma) and rabbit anti-Neuropeptide Y (1:2000, gift from J. Allen).
Counts of Fos+ neurons
Labeled cell bodies were counted from digitized images by an experimenter blind to treatment. The percentage of Fos+ cells was determined by counting all Fos+ cell bodies in the dorsal horn of 10 spinal cord sections. Both ipsilateral and contralateral sides were counted in 3 mice per experimental group. To calculate the percentage of Fos+ neurons we divided the number of Fos+ neurons ipsilateral to the transplant by the number of Fos+ neurons on the contralateral side and multiplied by 100. Values are presented as mean ± SEM (standard error of the mean). Statistical significance was assessed by Student’s t-test. A p-value < 0.05 was considered significant and is indicated with an asterisk (*).
Skin histology and scoring
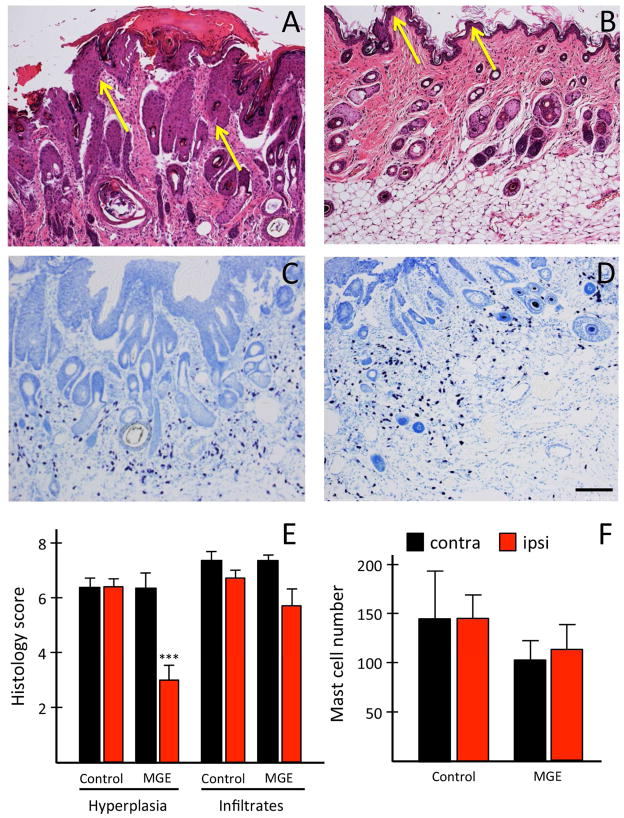
Skin biopsies were collected from transplanted and control IL-31Tg mice 4 weeks post-transplant. Skin samples were processed as previously described 18. Briefly, tissue was fixed in 4% paraformaldehyde for 1hr at room temperature and then embedded in paraffin. Six micron sections were stained with Hematoxylin-Eosin or with toluidine blue for mast cells. A dermatologist, who was blind to treatment, scored the alterations in skin structure. Epidermal thickening was scored semi-quantitatively on a 0 to 10 scale ranging from 0 (normal; orthokeratosis) to 10 (maximal pathology). A similar 0–10 point scale was used to quantify inflammatory cell infiltrate in the dermis. A score of 0 represented absence of leukocytes and a score of 10 represented a very dense leukocyte infiltration. For mast cell numbers, images of toluidine blue stained skin were taken with a Leica DM2500 microscope and counted using Image J, by an experimenter blind to treatment.
Quantitative real-time PCR
We used Trizol (Invitrogen, USA) to extract mRNA from cervical, thoracic and lumbar spinal cord. For transplanted animals, tissues were only collected from the cervical spinal cord (ipsilateral) 4 weeks after transplantation. We reverse-transcribed 200 ng of purified mRNA into cDNA using oligo dTs and Superscript III (Invitrogen, USA). The mRNA levels for GRP, GRPR, GAD65/67, GABA-A and GABA-B receptor subunits, PPTA and β-actin were quantified with a Realplex2 real-time PCR system (Eppendorf, Hamburg, Germany) using SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). Cycle threshold (CT) data were analyzed with a comparative CT method using β-actin as an internal standard. Ratios of gene to β-actin mRNA were compared and analyzed by Student’s t-test. The following primers were designed using NCBI Primer-Blast: (5′->3′):
GRP-F: CCGGTGTCGACAGGCGCAG
GRP-R: TCAGCCGCATACAGGGACGG
gR-F: AGTGGGGGTGTCTGTCTTCACACT
GRPR-R: TCAGGGCATGGGATGCCTGGAT
GABAARα1-F: TGGCCCACAACATGACCATGCC
GABAARα1-R: ACGGCGTGGCTCTCTGGTCC
GABAARα2-F: TGGCCCACACACATGACCATGCC
GABAARα2-R: TCGGTTCTGGCGTCGTTGCAC
GABAARβ1-F: GCCCTCAGAAAAGGGAGCGAGC
GABAARβ1-R: CTCGATGCTGGCGCTGTCGT
GABAARβ2-F: TGGCTCAAACGGTCTCGGGGT
GABAARβ2-R: ACATCAAAGGGGCAGCGGCGS
GABABR1-F: ACCCTGCCAACACCCGAAGC
GABABR1-R: CGCACTCCTGAACGGCCACC
GABABR2-F: CCGTGGGCTACACAACCGCC
GABABR2-R: TGGGTCCGGCTCCATGCTGT
GAD65-F: CAGCAGTGCCCAGGCTCATCG
GAD65-R: GGTGGTTCCAGCTGTGGCACTC
GAD67-F: CCGCCACAAACTCAGCGGCA
GAD67-R: TGGCGGCCACACTGAATCGC
Results
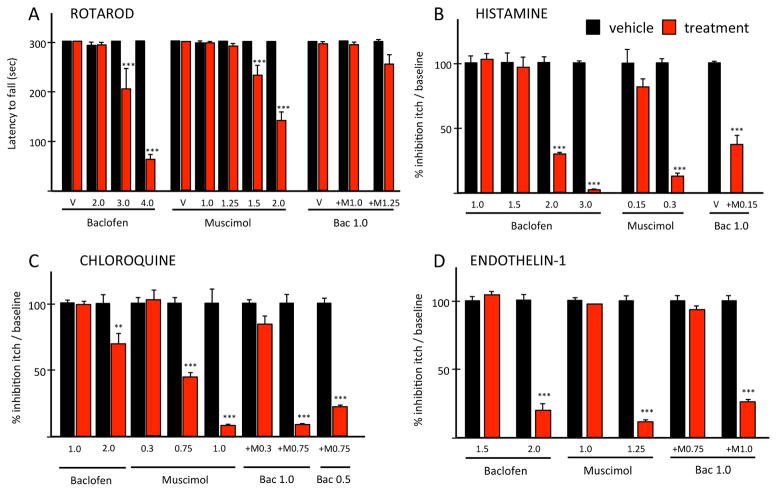
Previous studies emphasized the limitations of GABA agonists, in a variety of preclinical and clinical conditions, because of their side effects, namely sedation 19–21. For this reason, we first used the rotarod test to establish non-sedative doses of muscimol and baclofen, GABA agonists that selectively target the GABA-A and B receptors, respectively. Figure 1A illustrates that baclofen is non-sedating at doses <3.0 mg/kg and muscimol at doses <1.5 mg/kg. Next, we analyzed the anti-pruritic effect of different, non-sedating doses of baclofen or muscimol administered 15–20 min prior to a nape of the neck injection of histamine (HIS; 500 μg; Fig. 1B), or of the histamine receptor-independent pruritogens, chloroquine (CQ; 200 μg; Fig. 1C) and endothelin-1 (ET1; 25 ng; Fig. 1D). We found that the therapeutic window for the anti-pruritic effects of baclofen is small. Thus, 2.0 mg/kg baclofen significantly reduced the scratching induced by all pruritogens, but doses ≤ 1.5 mg/kg of baclofen had no antipruritic effects. The highest dose tested (3.0 mg/kg) completely blocked histamine-induced scratching. However, as this dose was sedating, a direct anti-pruritogen effect could not be concluded.
Figure 1. Synergistic anti-pruritic interaction of GABA-A and GABA-B receptor agonists.
(A) Baclofen, ≥ 3.0 mg/kg or muscimol, ≥ 1.5 mg/kg (red) are sedating. V: vehicle. Non-sedative baclofen or muscimol against histamine- (B), chloroquine- (C) and endothelin-1 (D). Co-administering non-sedative baclofen and muscimol against histamine (B) and endothelin-1 (D). Combined subthreshold baclofen and ED50 muscimol against chloroquine (C). Data: ± SEM; ** P<0.005, *** P<0.001; two-way ANOVA.
Muscimol also reduced (at 0.75 mg/kg) or largely eliminated (at 1.0 mg/kg) scratching provoked by 200 μg CQ (Fig. 1C). Even lower doses of muscimol (0.3 mg/kg) were effective against histamine-induced scratching (Fig. 1B). In contrast, only doses ≥ 1.25 mg/kg reduced endothelin-1 provoked scratching (Fig. 1D). Importantly, only doses of muscimol ≥ 1.5 mg/kg were sedating (Fig. 1A). Taken together, these results demonstrate that systemic administration of either GABA-A or GABA-B agonists has profound anti-pruritic effects against both histaminergic and non-histaminergic pruritogens, but their therapeutic window is relatively small.
Our findings indicate that muscimol and baclofen are effective at reducing acute itch that results from pruritogen-induced increased activity of primary afferent pruritoceptors. To determine whether the GABA agonists retain their therapeutic value in the setting of a chronic itch condition that is driven from the periphery, we used the IL31-overexpressor transgenic mouse (IL-31Tg mice), in which there is a persistent increase in the activity of primary afferent pruritoceptors. In the IL-31Tg mice, a lymphocyte-specific promoter drives overexpression of interleukin-31, a TH2-cell-derived cytokine that induces scratching by engaging sensory pruritoceptors that express the IL-31 receptor A 22. The chronic itch phenotype in these mice develops after 8 weeks of age and mimics atopic dermatitis, which in humans is also associated with increased atopic skin levels of IL-31 23, 24. In mice, the phenotype is manifest by significant, unremitting scratching, eventual excoriation and skin lesions.
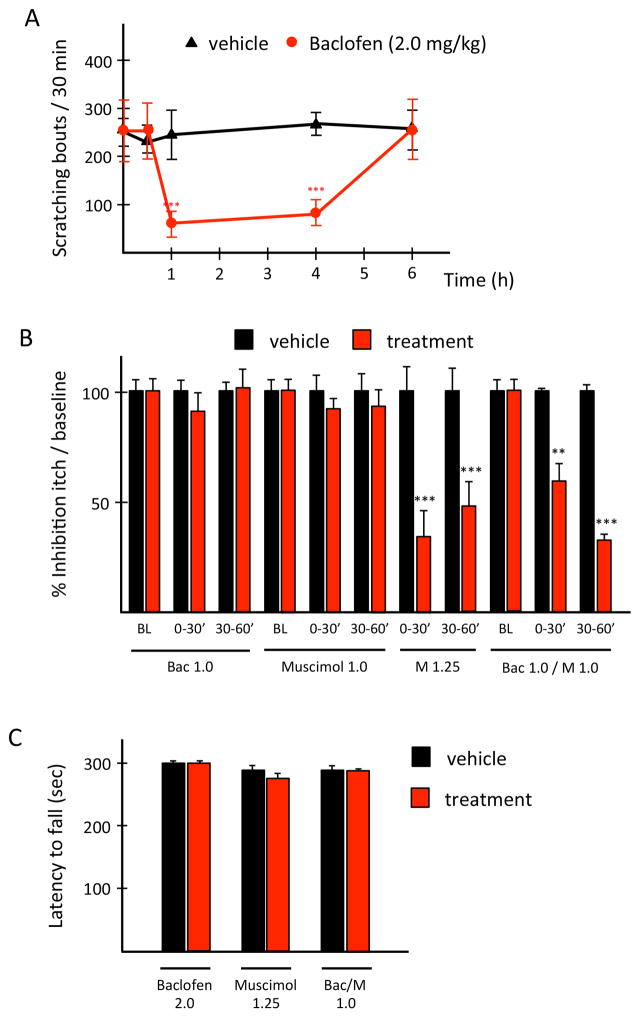
In the IL-31Tg mice, we first tested the efficacy of a single, non-sedating, systemic dose of baclofen (2.0 mg/kg) or muscimol (1.25 mg/kg) against spontaneous scratching. Compared to saline, baclofen reduced scratching after 60 min and this lasted up to 6 hours (Fig. 2A). In contrast, muscimol was anti-pruritic within 30 min of injection, but the effect lasted less than 1 hour (Fig. 2B). Importantly, in these mice, neither the muscimol nor the baclofen dose had sedating effects in the rotarod test (Fig. 2C). We conclude that pharmacological activation of either GABA-A or GABA-B receptors can significantly reduce pruritogen-evoked scratching and perhaps most importantly ameliorate spontaneous scratching in an atopic dermatitis model of chronic itch.
Figure 2. A synergistic interaction of GABA-A and GABA-B receptors is anti-pruritic against chronic itch.
Compared to saline (black), systemic baclofen (red; A) or muscimol (B) in IL-31Tg mice is anti-pruritic. Systemic co-administration of subthreshold baclofen and muscimol synergize against spontaneous scratching and these doses are not sedating (C). Data: ± SEM; ** P<0.005, *** P<0.001; two-way ANOVA.
Synergistic interactions potentiate the anti-pruritic effects of GABA agonists
Although our results demonstrate that GABA agonists have potent antipruritic properties, their relatively small therapeutic window will likely limit their utility in patients. Indeed, clinical studies have shown that sedation is often the cause for discontinuing baclofen treatment in the management of spasticity. Therefore, with a view to overcoming this limitation, we next asked whether we could identify a non-sedating synergistic anti-pruritic interaction using a combination of subthreshold doses of baclofen and muscimol. We first examined dose combinations against various pruritogens, in wild-type mice, and found that neither 1.0 mg/kg baclofen nor 0.15 mg/kg muscimol, when administered alone, has anti-pruritic effects against any of the pruritogens (Fig. 1). However, their co-administration significantly reduced both histamine- and ET1-triggered scratching (Fig. 1B and D). Importantly, although we could not find a combination of sub-threshold dose of baclofen and muscimol that was effective against the 200 μg CQ (Fig. 1C), co-administration of a subthreshold dose of baclofen (1.0 mg/kg) with an ED50 dose of muscimol (0.75mg/kg) was anti-pruritic, and in fact, more efficacious than the same dose of muscimol administered alone. Of note, the combined dose of baclofen and muscimol was as effective as a higher dose of muscimol (1.0 mg/kg) administered alone. Interestingly, even lower doses of baclofen (0.5 mg/kg) in combination with the ED50 muscimol dose significantly retained an anti-pruritic effect against 200 μg CQ (Fig. 1C). Taken together, these results demonstrate that ineffective doses of baclofen, when combined with a subthreshold or near ED50 dose of muscimol have significant anti-pruritic effects in models of acute itch. Most importantly, these same combinations were not sedating (Fig. 1A).
Finally, we asked whether a combination of subthreshold doses of baclofen and muscimol is also effective against chronic itch. Here we co-injected baclofen (1.0 mg/kg) and muscimol (1.0 mg/kg) into the nape of the neck of the IL-31Tg mice and measured spontaneous scratching. Although these doses administered separately were completely ineffective against chronic pruritus in the IL-31Tg mice, again the combination revealed a non-sedating, synergistic interaction against spontaneous scratching (Fig. 2B–C).
Although these systemic, pharmacological approaches significantly reduced spontaneous scratching in the IL-31Tg mice, the antipruritic effects are temporary. As a result there is no resolution of the associated skin pathology characteristic of the condition. With a view to translating these pharmacological approaches to a long-term management regime, we next asked whether permanently sustaining high levels of GABA inhibition could not only reduce scratching but also ameliorate the associated skin lesions in the IL-31Tg mice. To this end, we transplanted precursors of embryonic cortical GABAergic interneurons derived from the medial ganglionic eminence (MGE) into the spinal cord of the IL-31Tg mice.
Spinal cord molecular changes in the IL-31Tg mice
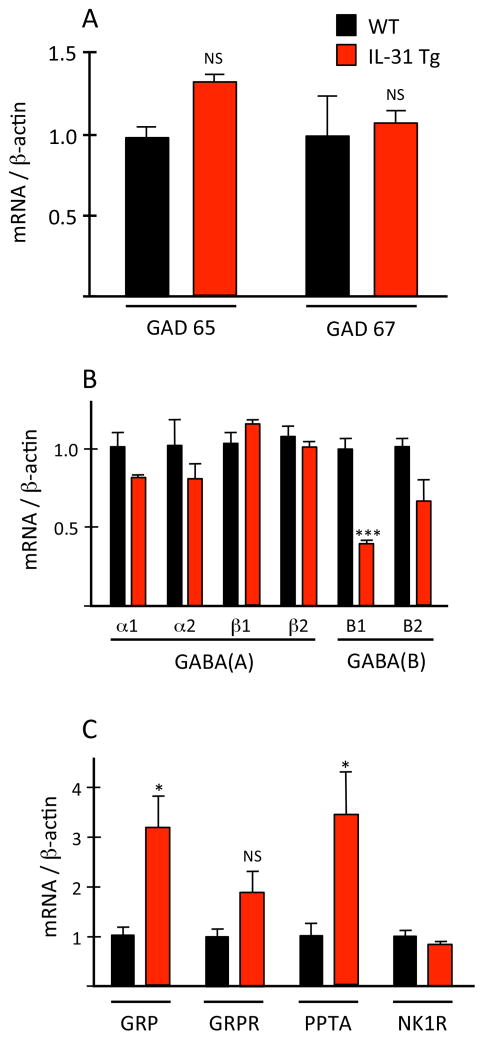
In the Bhlhb5 mutant mouse model of neuropathic itch 11, there is a profound loss of GABAergic interneurons in the dorsal horn of the spinal cord. Not surprisingly, there is a corresponding significant decrease of GAD65 and GAD67, the major biosynthetic enzymes for GABA 25. To determine whether there is any alteration in GABAergic biochemistry in the IL-31Tg mice, we used qPCR to analyze the expression levels of various receptors and enzymes involved in GABAergic signaling. In distinct contrast to the Bhlhb5 mutant mice we found no significant difference between wild type and IL-31Tg mice in spinal cord mRNA levels of GAD65 or GAD67 (Fig 3A). The major GABA-A receptor subunits (α1, α2, β1 and β2) were also unaffected (Fig 3B). On the other hand, in spinal cord segments that receive inputs from regions with skin lesions we found a significant decrease (~2 fold) in mRNA levels of the B1 subunit of the GABA-B receptor. B1 mRNA levels at other spinal cord levels did not differ compared to wild type mice. The modest decrease of the B2 subunit of the GABA-B receptor was not significant. Thus, although GABA synthesis is unchanged in the IL-31Tg mice, GABAergic signaling may, nevertheless, be reduced secondary to decreased GABA-B receptor expression.
Figure 3. Upregulation of pro-pruritic and downregulation of anti-pruritic genes in the spinal cord of IL-31Tg mice.
(A–C) In IL-31Tg mice (red), spinal cord mRNA levels of the GABA-B1 receptor (B) decreased; GRP and PPTA mRNA (C) increased. Expression of GAD 65/67 (A), GABA-A receptor subunits (B) or GRP- and NK1-receptors (C) did not change. Data: ± SEM; * P<0.05; ** P<0.005; NS = not significant; Student’s t-test.
We also analyzed spinal cord mRNA levels of gastrin-releasing peptide (GRP) and its receptor (GRPR), both of which have been implicated in the transmission of “itch” signals carried by sensory pruritoceptors 26–30. Compared to wild type mice, in the IL-31Tg mice, GRP mRNA levels are significantly increased (~4 fold, p=0.047) in segments of cervical spinal cord corresponding to dermatomes with lesions (Fig 3C). GRPR levels did not change. We also observed a significant increase in the spinal cord expression levels of preprotachykinin-A (PPTA) mRNA, the precursor of substance P (SP), a peptide that provokes significant scratching after intrathecal administration [31]. Levels of the SP receptor, NK1R, did not change. Taken together, our analysis indicates that peripheral overexpression of IL-31 results in spinal cord upregulation of the pro-pruritoceptive peptides, GRP and SP, and concurrent downregulation of a putative anti-pruritoceptive receptor, the B1 subunit of the GABA-B receptor. We hypothesize that these biochemical changes together contribute to chronic itch in the IL-31Tg mice.
Long term GABAergic inhibition ameliorates skin lesions in IL-31Tg mice
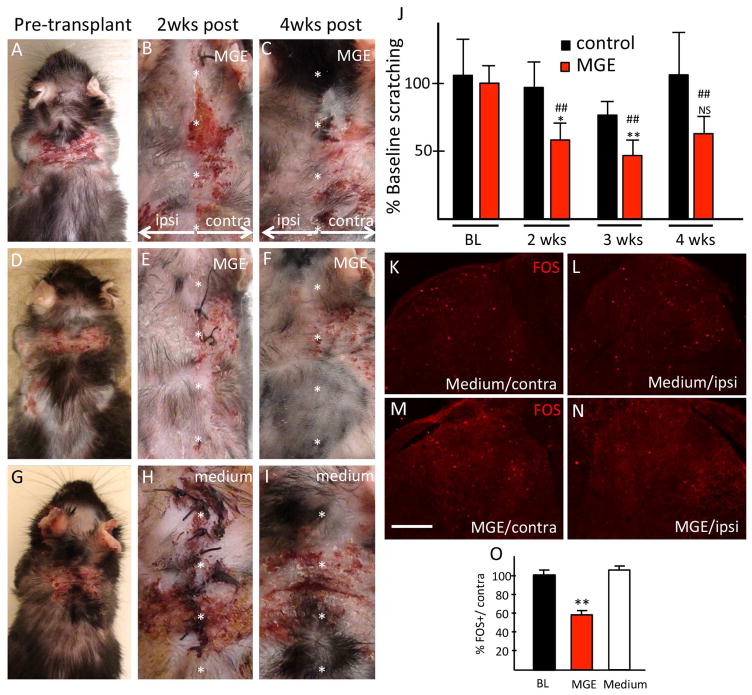
Given the association between chronic itch and decreased GABA-B receptor expression in the IL-31Tg mice, we next tested whether the IL-31Tg mice respond to MGE cell transplantation. Here, we studied IL-31Tg mice with bilateral lesions in the nape of the neck. Importantly, lesions never resolve spontaneously. We transplanted MGE cells unilaterally into the cervical spinal cord (C4-C8), so that scratching of the skin ipsilateral and contralateral to the transplants could be compared. A control group received transplant medium only. We monitored spontaneous scratching and severity of the skin lesions for 4 weeks post-transplantation.
Within two weeks of MGE transplantation, we recorded substantially reduced skin lesions ipsilateral to the transplant (Fig 4A–F). In several IL-31Tg mice there was complete resolution of the skin lesions and regrowth of hair, of note only ipsilateral to the transplant. Neither the severity of lesions contralateral to the transplant, nor lesions in control animals ever decreased (Fig 4G–I). Interestingly, skin lesions often persisted in dermatomes immediately adjacent to recovered areas, demonstrating that the MGE transplants exert a topographic, rather than systemic effect. The improvement of the skin ipsilateral to the transplant was associated with a significant (~50%) reduction of scratching in the MGE-transplanted mice (Fig 4J), which began 2 weeks post-transplant and remained low for the following 2 weeks. In contrast, spontaneous scratching did not change in control mice. We conclude that intraspinal MGE transplantation in IL-31Tg mice markedly reduces spontaneous scratching, a likely consequence of reduced itch, which in turn leads to gradual resolution of the skin lesions.
Figure 4. Transplant-mediated reduction of scratching, skin lesions and spinal cord activity in IL-31Tg mice.
Skin lesions (A–I) and scratching (J; N=10 vs 9 controls) reduced only after MGE-transplant. Data: ± SEM; * relative to baseline; # relative to control; * P<0.05; ** P<0.005; ## P<0.05; NS = not significant; two-way ANOVA and Bonferroni post-hoc. (K–O) MGE transplants reduced scratching-induced dorsal horn Fos+ neurons (N; red bar in O). BL= baseline. Data: ± SEM; * P<0.05; Student’s t-test. Scale bar: 100 μm.
Skin histology
Compared to the contralateral side (arrows in Fig 5A), skin from the transplanted side had a thinner stratum corneum, with reduced epidermal thickening (arrows in Fig 5B), which was confirmed histologically (scores: contralateral: 6 ± 0.56 vs ipsilateral: 3 ± 0.58; Fig 5E; left). Scratching-induced wounds were also reduced, as was erythrocyte extravasation. However, regardless of treatment, mast cell number did not change (Fig 5C, D, F) and there was no difference in inflammatory cell infiltrate (Fig 5E, right). Importantly, in medium-injected, control mice, we found no difference in the histology between ipsilateral and contralateral skin (data not shown). We conclude that intraspinal transplant of MGE cells results in an overall improvement, but not complete normalization of previously lesional skin structure, a likely consequence of MGE-induced reduction of scratching.
Figure 5. MGE transplants ameliorate skin pathology.
A–F: Scratching associated hyperparakeratosis, epidermal thickening (hyperplasia) and looser upper dermis, (A: yellow arrows; E; N=3) are reduced after MGE-transplantation (B). Neither inflammatory infiltrate (A, B, E; N=3), nor mast cell number differed in control (C; N=3) and transplanted mice (D, F; N=4). Data: ± SEM; *** P<0.001; Student’s t-test. Scale bar: 145 μm.
MGE cells differentiate into GABAergic interneurons
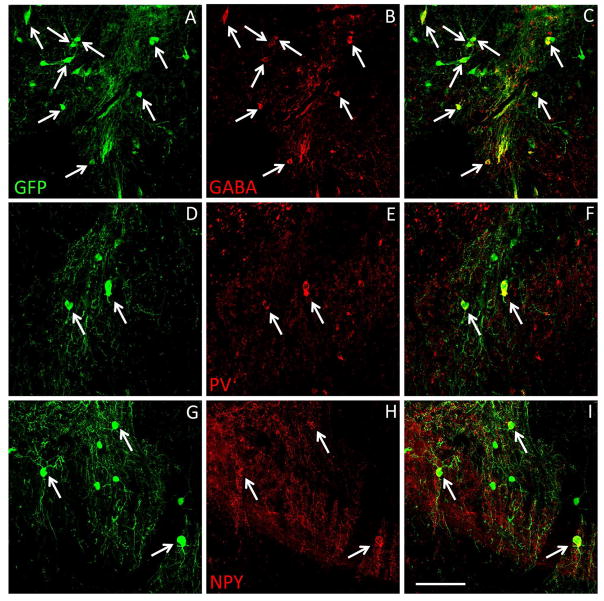
In mice that received an MGE transplant in the cervical spinal cord, we detected a large number of GFP+ MGE cells throughout the cervical enlargement (segments C4-C8; Fig 6), as well as GFP+ processes that extended rostrally and caudally, well beyond the transplant site (see Figure S1 in the Online Repository). Many MGE cells also expressed GABA (Fig 6A–C) and markers of subpopulations of GABAergic interneurons, namely parvalbumin (PV; Fig 6D–F) and neuropeptide Y (NPY; Fig 6G–I). Together, our results indicate that MGE transplants survived, differentiated into GABAergic interneurons and integrated in the spinal cord of the IL-31Tg mice.
Figure 6. Spinal cord transplanted MGE cells differentiate into GABAergic interneurons.
(A–I) GFP+ (green) MGE cells survived in the spinal cord of IL-31Tg mice (A, D, G) and expressed markers of subpopulations of GABAergic interneurons, including GABA (B), parvalbumin (E) and neuropeptide Y (H). Merged imaged are shown in C, F and I. Scale bar equals 100 μm.
GABA signaling and neuronal activity in the IL-31Tg mice
To better understand the mechanisms that underlie the MGE-mediated antipruritic effects, we assessed the effects of the MGE cell transplants in the spinal cord biochemistry of the IL-31Tg mice. We hypothesize that scratching-induced activation of MGE cells in the IL-31Tg mice results in spinal cord release of the MGE-derived GABA, which in turn should reduce the levels of neuronal activity in the spinal cord. To test this hypothesis, we immunostained spinal cord for the immediate early gene Fos, a marker of activated neurons, in both transplanted and control IL-31Tg mice. Figures 4K–N illustrate that scratching indeed dramatically induced Fos in the dorsal horn of the IL-31Tg mice, particularly in cord segments that receive inputs from skin with lesions. MGE cell transplants significantly reduced Fos expression (by ~50 %, p< 0.003; Fig 4N, O) ipsilateral to the transplant, indicating reduced neuronal activity and thus less drive in itch circuitry. In contrast, there was no reduction in the number of Fos+ neurons in the control IL-31Tg mice (Fig 4L,O).
Next, we measured dorsal horn GAD65 and 67 mRNA before and after MGE transplantation and found comparable levels in transplanted and non-transplanted mice, indicating that the MGE transplants do not increase GAD mRNA levels above normal (see Figure S2 in the Online Repository). Thus, although release of GABA from MGE cells is critical to the presumptive GABA-B receptor signaling deficit in the IL-31Tg mice, our results suggest that the transplants do not act as therapeutic pumps that continuously release GABA, which could be concluded if GAD levels were increased. Rather we suggest that it is the integration of the cells into the spinal cord circuits that underlies their inhibitory effects.
Discussion
As there is considerable preclinical evidence that GABAergic circuits regulate the transmission of pruritoceptive messages at the level of the spinal cord and that dysfunction of GABA signaling contributes to chronic itch 4, 11, 32, it is surprising, to our knowledge, that neither preclinical nor clinical studies examined the therapeutic effects of GABA agonists against either acute or chronic itch. Baclofen, a prototypical GABA-B agonist, has proven effective in several preclinical pain studies 33, 34 and by the intrathecal route is routinely used in patients with multiple sclerosis, for its anti-spasticity properties [35]. Baclofen is also occasionally prescribed, in France 36 to treat addictive disorders. Muscimol, on the other hand, although not FDA-approved, has been evaluated in a recent clinical trial to treat epilepsy (NCT00005925). Our present preclinical findings are, therefore, the first to show that baclofen and muscimol, in fact, have profound antipruritic properties in models of both acute and chronic itch and that their anti-pruritic effects can be produced without concomitant sedation. We also show that sustaining high levels of GABA-mediated inhibition by MGE cell transplantation has significant utility in the management of a chronic inflammatory itch condition (namely atopic dermatitis) as the transplants not only reduced spontaneous scratching, but also dramatically reduced the incidence and severity of the associated skin lesions.
Although baclofen and muscimol have different onset latency, duration of action and potency, we found that both agonists effectively reduce the scratching evoked by pruritogens that engage both histamine-dependent and -independent pathways. It appears, therefore, that GABA receptor-mediated inhibitory controls can regulate the transmission of “itch” messages generated by most subset of afferents, a conclusion consistent with the fact that loss of dorsal horn GABAergic interneurons increases the scratching evoked by a wide variety of pruritogens 11. Given that baclofen and muscimol were administered systemically, it remains to be determined whether their site of action is spinal and/or supraspinal. Interestingly, a recent study reported that direct microinjection of muscimol into the central nucleus of the amygdala has anti-pruritic effects against both acute (serotonin) and chronic (dry skin model) itch 37. As supraspinal antinociceptive actions of GABA receptor agonists have been reported 38, it is likely that systemic administration of GABA agonists has concurrent spinal and supraspinal actions. Somewhat disappointingly, but consistent with studies reporting baclofen-related side effects, we found a very small therapeutic window using systemic GABA agonists, due to sedation. Importantly, however, we showed that this limitation was mitigated with synergistic combinations of low doses of baclofen and muscimol. Indeed, doses near the ED50 or even subthreshold doses of muscimol potentiated the anti-pruritic effects of non-sedating, subthreshold doses of baclofen in acute pruritus.
Perhaps more importantly, we showed that the combination of low doses of GABA-A and GABA-B agonists is also significantly anti-pruritic in a model of chronic inflammatory itch. Chronic itch in the IL-31Tg mice differs considerably from the neuropathic itch that develops in Bhlhb5 mutant mice, in which there is a selective loss of spinal cord GABAergic inhibitory interneurons. In contrast, the AD-like condition produced in the IL-31Tg mice involves both peripheral and central mechanisms. First, there is enhanced release of IL-31 in the skin 15, which we previously reported exerts its effect by activating a small subset of TRPV1-positive primary afferent pruritoceptors that express the IL-31 receptor (RA). As capsaicin-mediated deletion of these pruritoceptors significantly reduced scratching provoked by injection of IL-31 22, we conclude that a peripheral mechanism contributes to the IL-31-mediated itch phenotype. Here, we also uncovered significant changes in spinal cord circuits in the IL-31Tg mice. Not only is there a significant increase in message levels of GRP, a neuropeptide expressed by excitatory interneurons that engage pro-pruritic dorsal horn circuitry 27, but we also recorded a significant decrease of postsynaptic GABA-B1 receptor subunit message. We suggest that these pathophysiological changes in the IL-31Tg mice result in an enhanced excitatory and decreased inhibitory “drive” that underlies a central hyperexcitability state that is critical to the chronic itch phenotype, including that observed in patients with atopic dermatitis 39.
Consistent with this observation, spinal cord Fos expression in the absence of an applied stimulus, was significantly increased in segments that receive inputs from skin with lesions. As the MGE cell transplants significantly reduced the Fos-expression as well as the excessive scratching, we suggest that the inhibitory control exerted by the transplanted cells reduced the activity of hyperactive spinal cord circuits. Interestingly, despite the reduced scratching in transplanted animals, neither inflammatory infiltrate nor mast cell numbers changed. We conclude, therefore, that the MGE transplants can overcome both the increased peripheral primary afferent and central excitatory drive that occurs in this model of chronic itch. This approach differs considerably from the more commonly prescribed treatments for AD, namely anti-inflammatory medications that have a predominant peripheral site of action. Taken together with our previous studies, the present findings suggest that MGE transplantation is a disease-modifying approach that can repair a GABAergic neuronal dysfunction. Our study also demonstrates that sustaining high levels of spinal cord GABAergic inhibition, whether pharmacologically or using cell-based therapies, can be very effective against chronic itch conditions with a variety of etiologies. Importantly, as there are reports that long-term use of baclofen can increase sedation liability, future preclinical studies should determine whether repeated administration of GABA agonists, either alone or in combination, can mimic the prolonged effects of the transplants without concomitant increase in sedation. Of course, although baclofen is approved for clinical use, the choice of GABA-A agonist will require additional study to determine which is most appropriate for a clinical trial. Of interest are possible combinations with benzodiazepines, which bind to and regulate GABA-A receptors.
Conclusion
Although there are reports of synergistic actions of GABA agonists and morphine in rodents 38, 40 and humans 41, this is the first demonstration that GABA agonists acting at different GABA receptors potentiate and the first report of their preclinical use in the treatment of acute and chronic itch. We believe that a comparable pharmacotherapeutic approach should be considered in the clinical management of acute and chronic itch. Most importantly, as sedation is the major cause for discontinuing baclofen treatment in patients 42, it is particularly significant that a synergistic combination of very low doses of baclofen and subthreshold doses of other GABA agonists retain profound antipruritic effects, without concomitant sedation.
Supplementary Material
Clinical implications.
Preclinical data encourage the design of human studies to explore the use of GABA-agonists, such as FDA-approved baclofen in the management of acute and chronic itch.
Acknowledgments
Source of funding: This work was supported by grants from the National Institutes of Health AR059402 (MS), NS78326 (JMB) NS14627 (AIB), a grant from the UK Wellcome Trust (AIB) and the Science foundation Ireland (IvP) (MS).
FC, JMB and XW performed pharmacological and behavioral analyses. JMB performed and analyzed transplant integration. FC, JMB and CS performed qPCR. MS and TB performed histological scoring. FC, JMB, MS and AIB designed experiments and wrote the manuscript. AIB supervised all studies. The authors have no conflict of interests.
List of Abbreviations
- AD
atopic dermatitis
- CQ
chloroquine
- ET1
endothelin 1
- GABA
gamma aminobutyric acid
- GAD
glutamic acid decarboxylase
- GFP
green fluorescent protein
- GRP
gastrin releasing peptide
- GRPR
gastrin releasing peptide receptor
- HIS
histamine
- IL-31
interleukin 31
- IL-31Tg
IL-31 overexpressing transgenic mouse
- MGE
medial ganglionic eminence
- NPY
neuropeptide Y
- PV
parvalbumin
- SP
substance P
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hong J, Buddenkotte J, Berger TG, Steinhoff M. Management of itch in atopic dermatitis. Semin Cutan Med Surg. 2011;30:71–86. doi: 10.1016/j.sder.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhoff M, Cevikbas F, Ikoma A, Berger TG. Pruritus: management algorithms and experimental therapies. Semin Cutan Med Surg. 2011;30:127–137. doi: 10.1016/j.sder.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leslie TA, Greaves MW, Yosipovitch G. Current topical and systemic therapies for itch. Handb Exp Pharmacol. 2015;226:337–356. doi: 10.1007/978-3-662-44605-8_18. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Carstens E. Neural processing of itch. Neuroscience. 2013;250:697–714. doi: 10.1016/j.neuroscience.2013.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Basbaum AI, Braz JM. Cell transplants to treat the “disease” of neuropathic pain and itch. Pain. 2016;157(Suppl 1):S42–47. doi: 10.1097/j.pain.0000000000000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han L, Ma C, Liu Q, et al. A subpopulation of nociceptors specifically linked to itch. Nat Neurosci. 2013;16:174–182. doi: 10.1038/nn.3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Imamachi N, Park GH, Lee H, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibuki T, Hama AT, Wang XT, et al. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- 9.Lever I, Cunningham J, Grist J, et al. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- 10.Petitjean H, Pawlowski SA, Fraine SL, et al. Dorsal Horn Parvalbumin Neurons Are Gate-Keepers of Touch-Evoked Pain after Nerve Injury. Cell Rep. 2015;13:1246–1257. doi: 10.1016/j.celrep.2015.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ross SE, Mardinly AR, McCord AE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foster E, Wildner H, Tudeau L, et al. Targeted ablation, silencing, and activation establish glycinergic dorsal horn neurons as key components of a spinal gate for pain and itch. Neuron. 2015;85:1289–1304. doi: 10.1016/j.neuron.2015.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akiyama T, Iodi Carstens M, Carstens E. Transmitters and pathways mediating inhibition of spinal itch-signaling neurons by scratching and other counterstimuli. PLoS One. 2011;6:e22665. doi: 10.1371/journal.pone.0022665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ben Smail D, Hugeron C, Denys P, Bussel B. Pruritus after intrathecal baclofen withdrawal: A retrospective study. Arch Phys Med Rehabil. 2005;86:494–497. doi: 10.1016/j.apmr.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Dillon SR, Sprecher C, Hammond A, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. 2004;5:752–760. doi: 10.1038/ni1084. [DOI] [PubMed] [Google Scholar]
- 16.Klein PA, Clark RA. An evidence-based review of the efficacy of antihistamines in relieving pruritus in atopic dermatitis. Arch Dermatol. 1999;135:1522–1525. doi: 10.1001/archderm.135.12.1522. [DOI] [PubMed] [Google Scholar]
- 17.Braz JM, Sharif-Naeini R, Vogt D, et al. Forebrain GABAergic neuron precursors integrate into adult spinal cord and reduce injury-induced neuropathic pain. Neuron. 2012;74:663–675. doi: 10.1016/j.neuron.2012.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seeliger S, Derian CK, Vergnolle N, et al. Proinflammatory role of proteinase-activated receptor-2 in humans and mice during cutaneous inflammation in vivo. FASEB J. 2003;17:1871–1885. doi: 10.1096/fj.02-1112com. [DOI] [PubMed] [Google Scholar]
- 19.Zeilhofer HU, Benke D, Yevenes GE. Chronic pain states: pharmacological strategies to restore diminished inhibitory spinal pain control. Annu Rev Pharmacol Toxicol. 2012;52:111–133. doi: 10.1146/annurev-pharmtox-010611-134636. [DOI] [PubMed] [Google Scholar]
- 20.Alabed S, Latifeh Y, Mohammad HA, Rifai A. Gamma-aminobutyric acid agonists for neuroleptic-induced tardive dyskinesia. Cochrane Database Syst Rev. 2011:CD000203. doi: 10.1002/14651858.CD000203.pub3. [DOI] [PubMed] [Google Scholar]
- 21.Walker P, Watanabe S, Bruera E. Baclofen, a treatment for chronic hiccup. J Pain Symp Man. 1998;16:125–132. doi: 10.1016/s0885-3924(98)00039-6. [DOI] [PubMed] [Google Scholar]
- 22.Cevikbas F, Wang X, Akiyama T, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. 2014;133:448–460. doi: 10.1016/j.jaci.2013.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boguniewicz M, Leung DY. Atopic dermatitis: a disease of altered skin barrier and immune dysregulation. Immunol Rev. 2011;242:233–246. doi: 10.1111/j.1600-065X.2011.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer EM, Shin DB, Nattkemper LA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. 2013;133:2783–2785. doi: 10.1038/jid.2013.227. [DOI] [PubMed] [Google Scholar]
- 25.Braz JM, Juarez-Salinas D, Ross SE, Basbaum AI. Transplant restoration of spinal cord inhibitory controls ameliorates neuropathic itch. J Clin Invest. 2014;124:3612–3616. doi: 10.1172/JCI75214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. 2013;340:968–971. doi: 10.1126/science.1233765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Solorzano C, Villafuerte D, Meda K, et al. Primary afferent and spinal cord expression of gastrin-releasing peptide: message, protein, and antibody concerns. J Neurosci. 2015;35:648–657. doi: 10.1523/JNEUROSCI.2955-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 29.Sun YG, Zhao ZQ, Meng XL, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhang J, Eberhart D, et al. Excitatory superficial dorsal horn interneurons are functionally heterogeneous and required for the full behavioral expression of pain and itch. Neuron. 2013;78:312–324. doi: 10.1016/j.neuron.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wilcox GL. Pharmacological studies of grooming and scratching behavior elicited by spinal substance P and excitatory amino acids. Ann NY Acad Sci. 1988;525:228–236. doi: 10.1111/j.1749-6632.1988.tb38608.x. [DOI] [PubMed] [Google Scholar]
- 32.Kardon AP, Polgar E, Hachisuka J, et al. Dynorphin acts as a neuromodulator to inhibit itch in the dorsal horn of the spinal cord. Neuron. 2014;82:573–586. doi: 10.1016/j.neuron.2014.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levy RA, Proudfit HK. The analgesic action of baclofen [beta-(4-chlorophenyl)-gamma-aminobutyric acid] J Pharmacol Exp Ther. 1977;202:437–445. [PubMed] [Google Scholar]
- 34.Wilson PR, Yaksh TL. Baclofen is antinociceptive in the spinal intrathecal space of animals. Eur J Pharmacol. 1978;51:323–330. doi: 10.1016/0014-2999(78)90423-5. [DOI] [PubMed] [Google Scholar]
- 35.Natale M, D’Oria S, Nero VV, et al. Long-term effects of intrathecal baclofen in multiple sclerosis. Clin Neurol Neurosurg. 2016;143:121–125. doi: 10.1016/j.clineuro.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 36.Rolland B, Paille F, Fleury B, et al. Off-label baclofen prescribing practices among French alcohol specialists: results of a national online survey. PLoS One. 2014;9:e98062. doi: 10.1371/journal.pone.0098062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen L, Wang W, Tan T, et al. GABA(A) Receptors in the central nucleus of the amygdala are involved in pain- and itch-related responses. J Pain. 2016;17:181–189. doi: 10.1016/j.jpain.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 38.Levy RA, Proudfit HK. Analgesia produced by microinjection of baclofen and morphine at brain stem sites. Eur J Pharmacol. 1979;57:43–55. doi: 10.1016/0014-2999(79)90102-x. [DOI] [PubMed] [Google Scholar]
- 39.Steinhoff M, Cevikbas F, Yeh I, et al. Evaluation and management of a patient with chronic pruritus. J Allergy Clin Immunol. 2012;130:1015–1016. e1017. doi: 10.1016/j.jaci.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Hara K, Saito Y, Kirihara Y, et al. The interaction of antinociceptive effects of morphine and GABA receptor agonists within the rat spinal cord. Anesth Analg. 1999;89:422–427. doi: 10.1097/00000539-199908000-00032. [DOI] [PubMed] [Google Scholar]
- 41.Singh PN, Sharma P, Gupta PK, Pandey K. Clinical evaluation of diazepam for relief of postoperative pain. Br J Anaesth. 1981;53:831–836. doi: 10.1093/bja/53.8.831. [DOI] [PubMed] [Google Scholar]
- 42.Gracies JM, Nance P, Elovic E, et al. Traditional pharmacological treatments for spasticity. Part II: General and regional treatments. Muscle Nerve Suppl. 1997;6:S92–120. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.