Abstract
Background
The rapid clinical antidepressant effects of the glutamatergic modulator ketamine may be due to its ability to restore synaptic plasticity and related effects on sleep-wake and circadian systems. Preclinical studies indicate that ketamine alters expression of circadian clock-associated molecules, and clinical studies of ketamine on plasticity-related biomarkers further suggest an association with sleep slow waves and sleep homeostasis.
Methods
Wrist activity monitors were used to examine the pharmacologic and rapid antidepressant effects of ketamine on markers of circadian timekeeping (amplitude and timing) in mood disorders. Circadian amplitude and timing of activity at baseline, post-infusion Day1 (D1), and Day3 (D3) were measured in 51 patients with major depressive disorder (MDD) or bipolar disorder (BD).
Results
Compared with either placebo or baseline, a mood-independent decrease of the central circadian value (mesor) was present on D1 after ketamine treatment. Mood-associated circadian effects between rapid (D1) responders and non-responders were found at baseline, D1, and D3. At baseline, a phase-advanced activity pattern and lower mesor distinguished subsequent responders from non-responders. On D1, ketamine non-responders had a lower mesor and a blunted 24-hour amplitude relative to baseline. On D3, patients with a persisting clinical response exhibited a higher amplitude and mesor compared with non-responders.
Conclusions
The findings are the first to demonstrate an association between ketamine’s clinical antidepressant effects and circadian timekeeping. The results suggest that trait-like circadian activity patterns indicate rapid mood response to ketamine, and that mediators of continuing ketamine-induced mood changes include altered timing and amplitude of the circadian system.
Keywords: AMPA, neuroplasticity, slow wave sleep, clock genes, wrist activity, sleep deprivation
Introduction
Sleep deprivation (SD) and ketamine treatment both rapidly relieve symptoms in patients with major depressive disorder (MDD). The fact that both interventions affect sleep homeostasis and circadian processes suggests that the circadian and sleep-wake systems and their interactions are associated with rapid mood effects. Understanding the separate and interacting effects of these processes may provide useful clues for developing novel rapid antidepressant therapies for mood disorders.
Preclinical studies have noted that both SD and ketamine affect central circadian clock-associated molecules (1). Further, clinical ketamine studies suggest that this agent affects sleep, slow waves, and synaptic plasticity (2–4). In healthy subjects, sleep quality interacts with the circadian system to affect the temporal organization of the human transcriptome (5). Taken together, these studies suggest that interventions that restore and normalize sleep quality—such as ketamine—could correct interactions between disrupted sleep and circadian systems to enhance temporal organization of the circadian sleep wake system and ultimately improve mood and behavioral health.
Ketamine exerts its initial rapid antidepressant properties via a prolonged change in glutamatergic signaling resulting in increased synaptic strength and plasticity. Changes in glutamatergic transmission affect downstream structural changes in dendritic spines and local synaptic protein synthesis (6), including transport and release of brain-derived neurotrophic factor (BDNF) (7). BDNF secretion, activation of the tropomyosin-receptor-kinase B (TrkB) receptor, and downstream trafficking lead to further dendritic structural complexity, spine and BDNF synthesis, and synaptic plasticity (7, 8). Ultimately, changes in critical local neuronal circuits that converge via enhanced synaptic plasticity and neuronal synchronization would hypothetically produce rapid antidepressant effects, particularly in areas involved in mood and behavior (8, 9). Notably, ketamine-induced changes in BDNF levels correlate with both sleep slow waves (SWS) and mood changes, as well as with improved sleep quality in individuals with treatment-resistant depression (2, 4). Numerous interactions between sleep homeostatic and circadian systems are possible, such as ketamine’s effects on clock genes to influence circadian timing and on BDNF and SWS to affect sleep quality.
Wrist activity is a useful indirect measure of central circadian timekeeping (10), which may be an important transdiagnostic measure of circadian function. In order to advance translational research, the NIMH Research Domain Criteria (RDoC; https://www.nimh.nih.gov/research-priorities/rdoc/index.shtml) includes circadian rhythms as a core construct within the Arousal and Regulatory System domain, and actigraphy-monitored rest/activity rhythms as a paradigm to evaluate this construct. Because changes in activity levels have been linked to circadian rhythm disorders (11), seasonal affective disorder (SAD) (12), and bipolar disorder (BD) (13–15), as well as current (10, 16), sub-syndromal (17), and euthymic (18) MDD, the relationship between activity and specific diagnoses is likely complex. For example, activity patterns are phase-delayed and blunted in SAD (12) as well as blunted in both ‘at risk’ bipolar spectrum disorder (19) and in persons with elevated manic-depressive symptoms (as assessed by the Young Mania Rating Scale (YMRS)) (20). Furthermore, an evening circadian chronotype, regardless of delayed sleep phase syndrome, conveys risk for anxiety, depressive, or substance-use disorders (21).
Indeed, dysregulated circadian timekeeping measures (amplitude, phase, day versus night levels) often contribute to mood symptom severity. Correlations between clinical ratings and nighttime activity in MDD (22), as well as daytime activity in melancholic depression (23), indicate that day-night patterns of activity vary with symptom severity (24). For instance, severity of depression has been associated with amplitude and timing, particularly low amplitude and/or delayed daily peak activity (25), or circadian rhythm misalignment (26, 27).
In addition, specific markers of circadian timekeeping are often associated with effective antidepressant intervention. For example, activity patterns are phase-advanced after SD (28) and bright light (12) therapies, and altered 24-hour amplitude is often associated with increased day and decreased night activity following treatment interventions (12, 29–32). In addition, clock gene variants are often associated with diurnal preference (reviewed in (33)) and have been explored in the pathogenesis in mood disorders (34). Preclinical and clinical evidence indicates that both CLOCK (35, 36) and PER (37, 38) mutations are associated with mood disorders. The altered motor activity patterns present with these mutation (36, 39) are consistent with the possibility that mood-related temporal variations of activity are associated with genetic variants of clock-related molecules. Furthermore, disrupted patterns of activity are linked to clock-gene mutations associated with mood disorders (37, 40–42). The observations that circadian clock genes also interact to affect sleep homeostasis (43–45), and that mistimed sleep affects circadian regulation of the human transcriptome (5, 46), suggests that ketamine’s effects on sleep timing and clock gene expression might ultimately improve the underlying molecular disorganization of the circadian system.
The present study is the first to investigate the effects of a single ketamine infusion on circadian rhythm expression and clinical response in treatment-resistant mood disorders (both MDD and BD). Specifically, we analyzed the clinical evidence for ketamine’s effects on 24-hour activity patterns over the course of five days in individuals with mood disorders. The specific objectives of the study were: 1) to identify ketamine’s circadian timekeeping effects relative to placebo treatment; and 2) to assess whether ketamine’s rapid antidepressant effects (e.g., its effects on mood and relapse parameters) were associated with altered patterns of circadian timekeeping.
Materials and Methods
This study was conducted using data drawn from different investigations (under protocol 04-M-0222) exploring ketamine’s antidepressant mechanism of action in patients with treatment-resistant mood disorders (2, 47, 48). The studies were conducted at the National Institute of Mental Health Clinical Research Center Mood Disorders Research Unit in Bethesda, Maryland and were approved by the Combined Neuroscience Institutional Review Board of the National Institutes of Health (NIH); specific details have been previously reported (2). One study investigated the clinical effects of ketamine in MDD patients who subsequently received riluzole (another glutamatergic modulator) in an effort to extend ketamine’s antidepressant effects (47), and a second study investigated ketamine’s antidepressant effects in MDD and BD patients, some of whom received maintenance mood stabilizers. A continuing study is examining ketamine’s effects in MDD and BD in a placebo-controlled, crossover study.
Participants
Fifty-one subjects (29F, 22M), ages 20–65 years (42.6±11.8 (mean±SEM)) with confirmed clinical diagnoses of either MDD (n=30) or BD (n=21) were pooled in the analysis. All subjects had a Montgomery-Asberg Depression Rating Scale (MADRS) score of =20 and were experiencing a current major depressive episode lasting at least four weeks. In addition, all subjects had previously not responded to at least one adequate antidepressant trial (as assessed by the Antidepressant Treatment History Form, modified (49)). BD patients were required to not have responded to a prospective open trial of a mood stabilizer while at the NIMH (either lithium or valproate for at least four weeks at therapeutic levels; serum lithium, 0.6–1.2 mEq/L; or valproic acid, 50–125 μg/mL). Exclusion criteria included the presence of psychotic features, a DSM-IV diagnosis of drug or alcohol abuse or dependence in the last three months, or the presence of an unstable, serious, medical illness. Female subjects could not be pregnant or nursing. Participants were free of all psychotropic medications for two to five weeks prior to the assessment, with the exception of mood stabilizers among some BD patients (14/21 were receiving lithium, 5/21 were receiving Depakote, and 2/21 were drug-free). Cigarette use was permitted during the clinical trial, but alcohol use was not. Participants were not allowed to nap during the three days prior to and after the infusion procedure and were encouraged not to nap on all days of the protocol. All subjects provided written informed consent before entry into the study and were assigned a clinical research advocate from the NIMH Human Subjects Protection Unit to monitor the consent process and research participation throughout the study.
Experimental Design
The 51 participants wore an Actiwatch (Model AW64) for three to four days prior to, and for five days after, a scheduled ketamine or placebo infusion (ketamine n=51; placebo n=38). The watch was removed during selected procedures and bathing. A diary was used to track watch removal and replacement. Ketamine infusion was conducted as previously described (50). Briefly, at about 10:00, depressed patients received a single i.v. infusion of 0.5 mg/kg ketamine hydrochloride or placebo that lasted about 40 minutes.
Depressive symptoms were assessed via MADRS ratings conducted at baseline (60 minutes before ketamine infusion), 230 minutes post-infusion (D0), one day post-infusion (D1), and three days post-infusion (D3). At all time points, change in depressive symptoms was expressed as change in score from baseline.
D1 and D3 Ketamine Response
Patients exhibiting a 50% reduction in MADRS scores on either D0 (230 minutes post-infusion) or at 9:00 on D1 or D3 were classified as D1 (within 24 hours) or D3 (through 72 hours) ketamine responders, respectively. Patients showing a less than 50% improvement on D0, D1, or D3 were classified as D1 and D3 non-responders, respectively.
Data collection and analysis
Twenty-four hour wrist activity patterns were analyzed for differences in amplitude, phase, and mesor (the central 24-hour value of a fitted sinusoidal curve) at baseline (two days pre-infusion), D1, and D3 (where D0=infusion); ketamine- and mood-dependent effects were examined. The Supplemental Information contains a detailed description of the data collection methods used and the statistical analyses conducted.
Results
Baseline Pooled Patient Groups
Overall, the 24-hour baseline activity pattern was characterized by low nighttime values (mean <25 counts/minute) that began to rise between 05:00–06:00. Values continued to rise to peak values (200–300 counts/minute) at about 14:00, then decreased until the lowest levels were observed the next night at about 03:00 (Fig.1C; Supplemental Fig.S2, left panel). The specific parameter estimates for baseline, ketamine versus placebo, and responders versus non-responders are summarized in Tables 1–3.
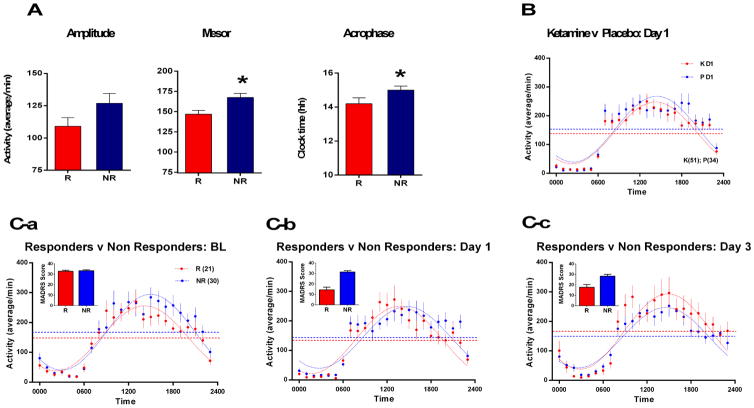
Figure 1.
A) Baseline circadian parameter estimates of amplitude, time of peak phase (acrophase), and mesor in ketamine responders and non-responders. Phase-advanced 24-hour activity and decreased central value (mesor) were associated with rapid antidepressant response to ketamine. B) Ketamine’s effects on the 24-hour pattern of wrist activity for ketamine-treated patients on Day 1 (D1) after infusion compared with placebo. Ketamine decreased the mesor without changing the amplitude or phase of activity. The mesor of the 24-hour activity pattern was lower for ketamine versus placebo treatment (p=.0317). C) Baseline (BL), D1, and D3 patterns of wrist activity for ketamine-treated patients who responded (> 50% decrease in MADRS scores) within one day of ketamine infusion compared with patterns of non-responders. Raw MADRS scores for each cohort are shown as bar chart inserts for each day.
Left panel: At baseline prior to infusion, subsequent D1 responders (Rs) are compared with D1 Non-responders (NRs). During baseline, the mesor (p=.006) and the phase (p=.019) of the baseline 24-hour activity patterns are different in D1 responders versus non-responders.
Middle Panel: Responders compared with Non-Responders on D1 after ketamine infusion. On D1, the phase of the 24-hour pattern of activity in ketamine responders differed from non-responders (p=.0038).
Right Panel: Responders who maintained the 50% decrease in MADRS score on D3 compared with patients who did not meet response criteria on D3. On D3, the mesor (p=.0202) and amplitude (p =.0488) of the 24-hour pattern significantly differed between responders and non-responders. Filled circles correspond to mean activity counts/minute in hourly bins ± SEM. The dotted sinusoidal curves correspond to the best fit line to the 24-hour data for each group. The dotted horizontal line (mesor) corresponds to the estimated 24-hour average (mesor) of the curve fits to each group. Group sizes are: baseline and D1 (responders=21, non-responders=30) and D3 (responders=13, non-responders=35).
Table 1.
Comparison of baseline wrist activity levels on Day 1 (D1) following ketamine or placebo
| CONTRAST | Individual Parameters | Combined | ||||
|---|---|---|---|---|---|---|
| Mesor (counts) | Amplitude (counts) | Acrophase (hh:mm) | P | F | ||
| Ketamine: Baseline vs D1 (n = 51) | Baseline | 158.9 ± 3.7 (a) | 118.8 ± 5.24 | 14:40 ± 0:09 | 0.0005*** | 5.940 (3,2404) |
| D1 | 140.4 ± 3.69 ††† | 107.5 ± 5.23 | 14:14 ± 0:11 | |||
| Placebo: Baseline vs D1 | Baseline (n = 38) | 159.8 ± 3.95 | 117.6 ± 5.59 | 14:50 ± 0:11 | 0.3917 | 1.000 (3,1690) |
| D1 (n = 34) | 153.4 ± 4.84 | 114.4 ± 6.82 | 14:26 ± 0:14 | |||
| Ketamine vs Placebo: D1 | Ketamine Infusion (n =51) | 140.4 ± 3.69 | 107.5 ± 5.23 | 14:14 ± 0:11 | 0.123 | 1.928 (3,1974) |
| Placebo Infusion (n= 34) | 153.4 ± 4.84 | 114.4 ± 6.82 | 14:26 ± 0:14 | |||
Mean Estimate ± SEM
For overall curve fit difference:
p < .05
p < .01
p < .001
For independent parameter tests:
p < .05
p < .01
p < .001
Table 3.
Baseline 24-hour patterns of wrist activity in subsequent Day 1 (D1) ketamine responders versus non-responders
| CONTRAST | Individual Parameters | Combined | ||||
|---|---|---|---|---|---|---|
| Mesor (counts) | Amplitude (counts) | Acrophase (hh:mm) | P | F | ||
| Baseline Activity: Responders vs Non- Responders | Responders (n = 21) | 146.9 ± 4.77(a) | 109.1 ± 6.74 | 14:09 ± 0:14 | 0.0011* | 5.373 (3,1210) |
| Non- Responders (n = 30) | 167.4 ± 5.30 †† | 127 ± 7.51 | 14:59 ± 0:14 † | |||
Mean Estimate ± SEM
For overall curve fit difference:
p < .05
p < .01
p < .001
For independent parameter tests:
p < .05
p < .01
p < .001
Ketamine versus Placebo
Ketamine infusion decreased hourly activity levels and significantly decreased the estimated mesor relative to baseline and to placebo on D1 (Fig.1B; Supplemental Fig.S2), with no differences on baseline days (Supplemental Fig.S1, left panel). The overall fit of the D1 24-hour activity pattern showed significant overall effects for ketamine (p=.0005, Table1). Relative to baseline, ketamine decreased the mesor of the circadian pattern (p<.001; F=12.41; df=1,240; Table1) with a trend (p=.0823; F=3.02; df=1,2404) to phase-advance the activity pattern on D1. Compared to D1 placebo, D1 ketamine produced a trend to decrease the mesor (p=.0317; F=4.62; df=1,1974). Placebo infusion had no significant D1 effects on the circadian pattern relative to baseline days.
Ketamine D1: Responders versus Non-Responders
The overall 24-hour pattern of D1 activity in the ketamine responders group differed significantly (p<.0144; F=3.53; df=3,1188) relative to non-responders on D1, with activity higher in the early part of the day (00:00–06:00) and lower in the afternoon (12:00–18:00). Specifically, the timing of activity was phase-advanced in responders versus non-responders (p<.0038; F=8.42; df=1,1188; Fig.1C, center panel; Table2), although the timing did not differ from baseline. Relative to baseline, D1 non-responders, but not responders, showed a decrease in the mesor (p=.0017; F=9.843; df=1,1188), and a trend toward decrease in the amplitude (p=.0328; F=4.57; df=1,1399; Table2) of the 24-hour activity pattern. Similar results were obtained when excluding ketamine open-label subjects (n=12), and including MDD-only subjects (for further analyses, see Supplemental Information).
Table 2.
Day 1 (D1) patterns of wrist activity in D1 ketamine responders and non-responders
| CONTRAST | Individual Parameters | Combined | ||||
|---|---|---|---|---|---|---|
| Mesor (counts) | Amplitude (counts) | Acrophase (hh:mm) | P | F | ||
| D1: Responders vs Non- Responders | Responders (n = 21) | 135.2 ± 5.06(a) | 113.9 ± 7.16 | 13:38 ± 0:14 | 0.0144 * | 3.533 (3,1188) |
| Non- Responders (n = 30) | 144.2 ± 5.18 | 104.6 ± 7.33 | 14:43 ± 0:16 †† | |||
| Responders: Baseline vs D1 (n=21) | Baseline | 146.9 ± 4.77 | 109.1 ± 6.74 | 14:09 ± 0:14 | 0.139 | 1.835 (3,999) |
| D1 | 135.2 ± 5.06 | 113.9 ± 7.16 | 13:38 ± 0:14 | |||
| Non-Responders: Baseline vs D1 (n = 30) | Baseline | 167.4 ± 5.30 | 127 ± 7.51 | 14:59 ± 0:14 | 0.0019** | 5.004 (3,1399) |
| D1 | 144.2 ± 5.18 †† | 104.6 ± 7.33 † | 14:09 ± 0:14 | |||
Mean Estimate ± SEM
For overall curve fit difference:
p < .05
p < .01
p < .001
For independent parameter tests:
p < .05
p < .02
p < .001
Ketamine D1: Responders versus Placebo
Activity timing was advanced (p<.0208; F=5.357; df=1,1281), and the 24-hour mesor was lower (p=.012; F=6.238; df=1,1281), in D1 ketamine responders versus D1 for those who received placebo (for further analyses, see Supplemental Information).
Ketamine D3: Responders versus Non-Responders
Morning and afternoon activity levels were higher on D3 in ketamine responders (patients with a 50% reduction in MADRS scores on D3 compared with D3 non-responders). The overall 24-hour activity pattern of D3 responders differed significantly from non-responders (p=.0218; F=3.230; df=3,111), with the mesor (p=.0202; F=5.411; df=1,1111) and amplitude (p=.0488; F=3.890; df=1,1111) each contributing to the D3 group difference in activity patterns (Fig.1C, right panel). Increased mesor and amplitude parameters distinguished ketamine D3 responders from the placebo-treated group on D3 (for further analyses, see Supplemental Information).
Baseline Activity Indicators of Rapid D1 Clinical Response
Baseline 24-hour patterns of activity indicated the subsequent D1 clinical response of responders and non-responders to ketamine infusion (Table3; p=.0011; F=5.373; df=3,1210). Specifically, at baseline (i.e., before ketamine infusion), next-day responders to ketamine had less activity relative to non-responders between 12:00–23:00 (Fig.1C, left panel), an effect consistent with a lower mesor (p=.006; F=7.575; df=1,1210) of the fitted curve. In addition, the baseline timing (acrophase) of the 24-hour pattern was significantly earlier in ketamine responders than non-responders (p=.019; F=5.516; df=1,1210; Fig.1A and 1C, left panel).
MADRS ratings for Baseline, D1, and D3 In Ketamine Responders versus Non-Responders
No difference was noted between baseline MADRS ratings of prospective ketamine responders and non-responders (32.8±0.98 versus 33.4±0.98 (mean±sem), respectively). In contrast, D1 responders had significantly lower MADRS ratings than non-responders (11.9±1.99 versus 30.0±1.51; df=49, t=7.3, p<.0001), and D3 responders had lower MADRS ratings than non-responders (15.8±2.42 versus 27.5±1.72, t=4.05, p<.0001, respectively) (Fig.1C, bar-graph inserts). AM activity (midnight-06:00) and MADRS scores were positively correlated on D1 (p<.05, Pearson correlation), but not on D3. PM activity (12:00–18:00) and MADRS scores were not correlated on D1, but showed significantly negative correlations on D3 (p<.001). Correlations between change in MADRS score (baseline minus D1 and D3) and corresponding amplitude, mesor, and acrophase (D1 and D3 minus baseline) change scores were significant on D3 for amplitude (p=.016). Trends towards significance were also seen for D1 amplitude (p=0.32) and D3 mesor (p=.018) (see Table4 and Supplemental Materials).
Table 4.
Correlations between Treatment Response and Individual Circadian Parameter Estimates
| Circadian Parameter 3 | |||
|---|---|---|---|
| Contrast | Amplitude | Mesor | Acrophase |
| MADRS BL minus Day 1 | .3012*† | .1855 | .1364 |
| MADRS BL minus Day 3 | .3483††† | .3430†† | −.0027 |
Pearson correlation coefficient; 3 Day 1 and Day 3 minus baseline
p<0.1;
p<0.06;
p<0.05
MADRS: Montgomery-Asberg Depression Rating Scale; BL: baseline.
Discussion
The present study found that ketamine had distinct mood-dependent effects on wrist-activity markers of circadian timekeeping. In those who responded to ketamine, treatment was associated with advanced timing on both baseline and D1 as well as increased amplitude on D3. In non-responders, ketamine treatment was associated with decreased amplitude on D1 and D3 (Table2, Fig.1C). Independent of mood, ketamine had only small effects on circadian timekeeping parameters on D1: a trend to phase advance timing, but no effects on amplitude. The current findings, which are specific to individuals with treatment-resistant depression, represent the first clinical evidence linking the circadian system to ketamine’s rapid antidepressant effects. The fact that: 1) clinical response was related to baseline circadian differences, and 2) altered circadian timekeeping on D1 and D3 was mood-dependent, suggests that underlying circadian rhythm-related mechanisms predict and contribute to ketamine-mediated mood effects.
Taken together, the evidence suggests that activity markers of circadian timekeeping may be useful for identifying both the underlying mechanisms of ketamine’s rapid antidepressant effects as well as target populations of patients likely to benefit from this agent. Further, because ketamine’s rapid effects on mood are linked to markers of circadian timekeeping (i.e., phase and amplitude), the fact that these markers have previously been shown to be altered by circadian clock gene-related variants suggests a possible connection to clock gene machinery (33, 36). Alternately, the measured effects on these markers might be influenced by external factors, such as light or behavior.
Baseline Differences Between Responders and Non-Responders
In this study, baseline differences in the estimated circadian parameter values distinguished ketamine responders from non-responders. Relative to non-responders, responders showed phase-advanced circadian timing (~50 minutes), a lower mesor (~88%), and decreased amplitude (~86%) at baseline (Table 3). Baseline differences in the sleep-wake patterns of ketamine responders versus non-responders have been described (2, 3), but the possible contribution of circadian-related circuits to clinical response has not.
The baseline phase differences between ketamine responders and non-responders suggest a functional difference between circadian circuits controlling the timing of activity rhythms that subsequently influence mood response to ketamine. Thus, the phase-delayed and elevated activity pattern seen during pre-treatment in non-responders may provide important clues to the mechanisms underlying ketamine’s rapid antidepressant effects (see below). CLOCK gene variants are associated with increased motor activity (33, 36), analogous to baseline increased amplitude in non-responders. The fact that increased circadian clock amplitude is associated with greater redundancy of clock gene variants and greater resistance to phase change after a change of external lighting cues (51) may be relevant to the treatment-resistant status of the ketamine non-responders in this study. Further research with a larger sample is needed to understand the relationship between circadian biomarkers, clock gene variants, and ketamine’s rapid antidepressant effects.
Rapid Antidepressant Effects in Responders and Non-Responders
The relationship between ketamine’s effects on activity levels and mood is complex, involving both central circadian clock-controlled (52, 53), and treatment-associated (24) mood effects on motor activity. In this study, D1 and D3 activity patterns appear to have been driven by ketamine’s effects on underlying circadian mechanisms rather than by links between mood and activity level. For example, on D1, the responder and non-responder groups did not differ in activity levels or amplitude, and no correlation between mood change and PM activity level was observed despite significant differences in mood (MADRS scores declined by >50% in responders; Fig.1C, center panel and bar-graph inset). The fact that responders had an earlier acrophase of 24-hour activity at baseline and D1 (Table3) suggests that the phase-advanced activity-rest pattern observed here was associated with potential rapid response, similar to SD and light therapies (28).
Responder and non-responder groups had different timing and amplitude parameters (Fig.1A and 1C) at baseline, D1, and D3, further suggesting biological differences in the organization of their circadian system, consistent with different sleep-wake effects for these groups with regard to ketamine’s effects on slow wave sleep and BDNF (2, 3). Delayed circadian phase, which was present in the non-responders, was previously linked to elevated glutamatergic levels in emerging depression (54), suggesting that these levels may persist in ketamine non-responders.
CLOCK gene variants alter circadian amplitude and phase (55, 56), biomarkers also associated with morning-evening variation in chronotypes (57), suggesting that such gene mutations might mediate rapid antidepressant response to ketamine (Table 3), and that their markers (amplitude and phase) may also be useful for predicting ketamine response per se.
Interacting Sleep and Circadian Processes Affect Mood Change
The observation that circadian amplitude progressively increased from D1 to D3 in responders indicates a strengthening of the circadian system consistent with progressively greater interaction between sleep homeostatic and circadian processes between D0, D1, and D3. This interaction may involve a cascade of events initiated by plasticity-associated molecules (e.g., BDNF (2, 4, 58)) as well as improved sleep quality. Accordingly, D1 MADRS scores correlated with decreased activity counts and increased SWS the first night post-infusion. In contrast, markers of circadian timekeeping (amplitude and acrophase) were not affected on D1.
Clinical ketamine studies show that this agent affects sleep, SWS, and synaptic plasticity (2–4), while preclinical SD studies and ketamine interventions have identified their effects on central circadian clock-associated molecules (1, 59). It has been proposed that circadian and sleep-related processes interact to mediate the rapid and continued features of mood response through a progressive temporal re-organization of the human transcriptome (5) that involves properly synchronizing sleep with phase of the circadian clock to positively affect the human transcriptome (5, 46). Circadian clock genes affect sleep homeostasis (43–45), suggesting that ketamine’s effects on sleep timing and/or clock gene expression may improve the underlying molecular organization of the circadian system. Both ketamine and SD rapidly (within 24 hours) improve mood, alter cellular/molecular events associated with plasticity and sleep homeostasis, and alter clock gene-associated molecules. In contrast, while SSRIs and light therapy rapidly alter clock gene-associated molecules, the beneficial mood effects are more delayed (days to weeks). This suggests that ketamine’s plasticity and sleep-associated effects are more important than clock-gene effects as initiating factors for rapid mood benefits, and that the later interaction of these early plasticity changes with circadian clock-associated effects could be linked to the extended mood benefits (Fig.2).
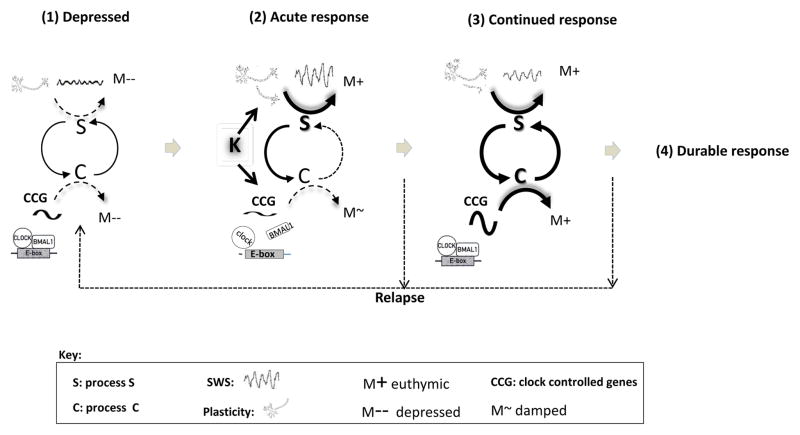
Figure 2.
A model of rapid mood response to ketamine is shown that incorporates the temporal interactions of sleep, plasticity, and clock-associated genes. In this schematic, ketamine’s rapid antidepressant effects and individuals’ subsequent relapse are related to the interaction of sleep homeostasis (process S) and circadian (process C) processes. While depressed (1), sleep loss, stress, environmental, and genetic factors are associated with diminished sleep homeostatic (S) and circadian (C) mechanisms, weakening S-C interactions, and promoting depressed mood (M-). (2) Acutely (four to 24 hours) after ketamine infusion, ketamine rapidly increases plasticity and improves mood (M+), as well as increases sleep slow waves (SWS) and sleep quality. Simultaneously, ketamine acts on clock controlled gene (CCG) associated molecules to alter timing and diminish circadian output, thus weakening the S-C interaction and lessening the circadian mood component. (3) During a continued antidepressant response, the interaction between S and C is strengthened as increased S acts on C, thus facilitating and re-establishing a more functional S-C interaction, greater temporal organization of the transcriptome, continued mood improvement (M+), and potentially contributing to a durable response (4). Alternatively a weakened S-C interaction is associated with relapse.
Conceptual Organization and Localization of Ketamine’s Circadian Timekeeping Effects
Several conceptual levels may be considered in discussing post-infusion reorganization of circadian timekeeping. First, ketamine may alter timekeeping of the central clock itself by acting on clock gene-related molecules within the central clock, on transmitters, or on cellular coupling within the central clock. The fact that ketamine alters ectopically expressed CLOCK:BMAL1-mediated expression as well as transcription of BMAL1, PER2, and Cry1 (1)—which are also present within the suprachiasmatic nucleus (SCN)—is consistent with central clock effects. If central clock genes were altered by ketamine, markers and output rhythms generated by the central clock (activity, 24-hour body temperature, melatonin, cortisol, and gene expression) might be similarly affected. This is therefore a testable hypothesis in future experiments.
However, when its effects were examined independent of mood in the current analysis, ketamine did not alter amplitude or phase on D1. This suggests that ketamine, when administered at about 10:00, did not alter central clock function when effects were examined independent of mood change.
A second possibility is that ketamine might affect entrainment circuits that synchronize the central clock to external lighting cycles. Interestingly, N-methyl-D-aspartate (NMDA) antagonism alters glutamate-mediated light input to the central clock as well as PER expression (60, 61), which would be consistent with ketamine-altered light input to the central clock. However, the fact that ketamine has a half-life of 2.5 to three hours, and was infused mid-morning when the phase-shifting effects of light are negligible, argues against a direct effect of ketamine on light input per se. While the presence of psychoactive ketamine metabolites with more extended presence (62, 63) might implicate these metabolites in light-input pathways, to our knowledge, their circadian system properties have not been evaluated.
A third possibility is that ketamine affects mood-related circuitry in a way critical to both its rapid and/or enduring mood effects. Ketamine, acting outside of the central clock, might thus affect non-central clock gene expression (such as PER2 and BMAL1) within reward circuits of the ventral tegmental area and ventral striatum (37, 38). This interpretation would require ketamine to act on abnormal clock gene patterns within regions and circuits implicated in both mood and motor activity (64), thus linking ketamine’s effects to the timing and amplitude of activity. It has been suggested that CLOCK (65) and PER2 (37, 38) may affect monoamine oxidase-a (MAO-A) transcription, dopamine levels (37, 38), and mood (39). Ketamine is known to enhance dopamine turnover (66), which is closely linked to known antidepressant mechanisms of action. Thus, while the current results do not allow a distinction between ketamine acting at the central clock versus a non-central site, the results support the possibility that it could act on clock genes within circuits also implicated in mood and activity.
Study Limitations and Strengths
This study is associated with several limitations. First, due to clinical considerations, only one measure of circadian clock output was used; future studies would benefit by selecting more specific markers of the central clock, such as 24-hour melatonin, cortisol, and core body temperature, as well as measurement of light exposure, which is critical to external synchronization. In addition, the convenience marker used here, wrist activity, does not necessarily mark the expression of the underlying clock, although it is optimized for studying sleep and circadian rhythms (11) by using multiple days (67, 68). The current study required us to compare single days to examine rapid mood changes. Second, while the sample size of the pooled subject groups was large for a study of this kind, if increased, it could enable researchers to distinguish between MDD and BD cohorts and/or the effects of mood stabilizers. As discussed earlier, the cohort sizes are small, and cohort results should thus be regarded as preliminary. Third, the study lacked a healthy control group needed for comparing pharmacological, phase, and amplitude markers of circadian timekeeping with the patient cohorts.
This study is currently in progress. The study is also associated with several strengths. First, the overall results are based on populations in which significant clinical depression is a core symptom, which strengthens the finding as it relates to treatment-resistant depression and significant clinical depression per se. This strength informs a broader interpretation of the biological underpinnings of ketamine response. Second, the study had several controls, including a baseline, placebo arm, and responder versus non-responder contrasts for ketamine infusion.
Taken together, the results indicate that wrist activity markers are linked to drug and mood-dependent effects of ketamine. As initiatives such as RDOC purport to understand the neural basis of human functioning, rapid-acting treatments such as ketamine can provide key insights into the relationship between circadian rhythms, activity, and mood response.
Supplementary Material
Supplemental Figure S1: Ketamine’s (K) effects on the 24-hour pattern of wrist activity are shown for ketamine treatment at baseline (BL; one day before ketamine treatment; left panel) versus placebo (P) treatment on the day of ketamine treatment (right panel). At baseline there was no difference between the 24-hour patterns of placebo and ketamine activity (left panel). Both placebo and ketamine infusions (indicated by arrow at 10:00 on D0) were associated with an eight-hour infusion- and recovery-related decrease in the level of activity (right panel; ketamine and placebo are shown relative to the pre-ketamine baseline mean ± SEM (shaded area)). On D0 there was no difference between ketamine and placebo treatments from 12:00–00:00; (F1,94 = 0.79, p=.37). Filled circles correspond to mean activity counts/minute in hourly bins ± SEM. The dotted sinusoidal curve corresponds to the best fit line to the 24-hour data for both placebo and ketamine baseline days.
Supplemental Figure S2: Ketamine’s effects on the 24-hour pattern of wrist activity are shown for ketamine-treated patients one day after infusion (D1) versus their prior baseline (BL) (left panel), and compared with D1 placebo treatment (right). On D1, the central value (mesor) of the 24-hour activity pattern was lower for ketamine versus baseline (p=.0004, left panel), and there was a trend for ketamine to be lower than placebo treatment (p=.0317, right panel). There was also a trend (p=.0823) for ketamine to advance the phase of wrist activity relative to prior baseline days (left panel). Filled circles correspond to mean activity counts/minute in hourly bins ± SEM. The dotted sinusoidal curves correspond to the best fit line to the 24-hour data for each group. The dotted horizontal line (mesor) corresponds to the estimated 24-hour average (mesor) of the curve fits to each group.
Supplemental Table S1: Comparisons of 24-hour patterns of wrist activity in patients with mood disorders who did and did not respond to ketamine infusion on Day 1 (D1).
Acknowledgments
The authors thank the 7SE research unit and staff for their support, and the patients for their invaluable contributions. Ioline Henter (NIMH) provided excellent editorial assistance.
Footnotes
Clinical Trials Registration Information: www.clinicaltrials.gov, NCT00088699, “Investigation of the Rapid (Next Day) Antidepressant Effects of an NMDA Antagonist”
Financial Disclosures Funding for this work was supported by the Intramural Research Program at the National Institute of Mental Health, National Institutes of Health (IRP-NIMH-NIH; ZIA MH002927; NCT00088699), by a NARSAD Independent Investigator Award to Dr. Zarate, and by a Brain and Behavior Mood Disorders Research Award to Dr. Zarate. Dr. Zarate is listed as a co-inventor on a patent for the use of (2R,6R)-hydroxynorketamine, (S)-dehydronorketamine, and other stereoisomeric dehydro and hydroxylated metabolites of (R,S)-ketamine metabolites in the treatment of depression and neuropathic pain. Dr. Zarate is listed as co-inventor on a patent application for the use of (2R,6R)-hydroxynorketamine and (2S,6S)-hydroxynorketamine in the treatment of depression, anxiety, anhedonia, suicidal ideation, and post-traumatic stress disorders; he has assigned his patent rights to the U.S. government but will share a percentage of any royalties that may be received by the government. All other authors report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bellet MM, Vawter MP, Bunney BG, Bunney WE, Sassone-Corsi P. Ketamine influences CLOCK:BMAL1 function leading to altered circadian gene expression. PLoS One. 2011;6:e23982. doi: 10.1371/journal.pone.0023982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Duncan WC, Sarasso S, Ferrarelli F, Selter J, Riedner BA, Hejazi NS, et al. Concomitant BDNF and sleep slow wave changes indicate ketamine-induced plasticity in major depressive disorder. Int J Neuropsychopharmacol. 2013;16:301–311. doi: 10.1017/S1461145712000545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Duncan WC, Jr, Selter J, Brutsche N, Sarasso S, Zarate CA., Jr Baseline delta sleep ratio predicts acute ketamine mood response in major depressive disorder. J Affect Disord. 2013;145:115–119. doi: 10.1016/j.jad.2012.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duncan WC, Jr, Zarate CA., Jr Ketamine, sleep, and depression: current status and new questions. Curr Psychiatry Rep. 2013;15:394. doi: 10.1007/s11920-013-0394-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Archer SN, Laing EE, Moller-Levet CS, van der Veen DR, Bucca G, Lazar AS, et al. Mistimed sleep disrupts circadian regulation of the human transcriptome. Proc Natl Acad Sci U S A. 2014;111:E682–691. doi: 10.1073/pnas.1316335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329:959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338:68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maeng S, Zarate CA., Jr The role of glutamate in mood disorders: results from the ketamine in major depression study and the presumed cellular mechanism underlying its antidepressant effects. Curr Psychiatry Rep. 2007;9:467–474. doi: 10.1007/s11920-007-0063-1. [DOI] [PubMed] [Google Scholar]
- 9.Machado-Vieira R, Ibrahim L, Henter ID, Zarate CA., Jr Novel glutamatergic agents for major depressive disorder and bipolar disorder. Pharmacol Biochem Behav. 2012;100:678–687. doi: 10.1016/j.pbb.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teicher MH. Actigraphy and motion analysis: new tools for psychiatry. Harv Rev Psychiatry. 1995;3:18–35. doi: 10.3109/10673229509017161. [DOI] [PubMed] [Google Scholar]
- 11.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–392. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 12.Winkler D, Pjrek E, Praschak-Rieder N, Willeit M, Pezawas L, Konstantinidis A, et al. Actigraphy in patients with seasonal affective disorder and healthy control subjects treated with light therapy. Biol Psychiatry. 2005;58:331–336. doi: 10.1016/j.biopsych.2005.01.031. [DOI] [PubMed] [Google Scholar]
- 13.Kupfer DJ, Weiss BL, Foster G, Detre TP, Delgado J, McPartland R. Psychomotor activity in affective states. Arch Gen Psychiatry. 1974;30:765–768. doi: 10.1001/archpsyc.1974.01760120029005. [DOI] [PubMed] [Google Scholar]
- 14.Wehr TA, Muscettola G, Goodwin FK. Urinary 3-methoxy-4-hydroxyphenylglycol circadian rhythm. Early timing (phase-advance) in manic-depressives compared with normal subjects. Arch Gen Psychiatry. 1980;37:257–263. doi: 10.1001/archpsyc.1980.01780160027002. [DOI] [PubMed] [Google Scholar]
- 15.Wolff Ed, Putnam FW, Post RM. Motor activity and affective illness. The relationship of amplitude and temporal distribution to changes in affective state. Arch Gen Psychiatry. 1985;42:288–294. doi: 10.1001/archpsyc.1985.01790260086010. [DOI] [PubMed] [Google Scholar]
- 16.Volkers AC, Tulen JH, van den Broek WW, Bruijn JA, Passchier J, Pepplinkhuizen L. Motor activity and autonomic cardiac functioning in major depressive disorder. J Affect Disord. 2003;76:23–30. doi: 10.1016/s0165-0327(02)00066-6. [DOI] [PubMed] [Google Scholar]
- 17.Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7:176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- 18.Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162:50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- 19.Ankers D, Jones SH. Objective assessment of circadian activity and sleep patterns in individuals at behavioural risk of hypomania. J Clin Psychol. 2009;65:1071–1086. doi: 10.1002/jclp.20608. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez R, Tamminga CA, Tohen M, Suppes T. The relationship between affective state and the rhythmicity of activity in bipolar disorder. J Clin Psychiatry. 2014;75:e317–322. doi: 10.4088/JCP.13m08506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reid KJ, Jaksa AA, Eisengart JB, Baron KG, Lu B, Kane P, et al. Systematic evaluation of Axis-I DSM diagnoses in delayed sleep phase disorder and evening-type circadian preference. Sleep Med. 2012;13:1171–1177. doi: 10.1016/j.sleep.2012.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemke MR, Puhl P, Broderick A. Motor activity and perception of sleep in depressed patients. J Psychiatr Res. 1999;33:215–224. doi: 10.1016/s0022-3956(98)00067-3. [DOI] [PubMed] [Google Scholar]
- 23.Lemke MR, Broderick A, Zeitelberger M, Hartmann W. Motor activity and daily variation of symptom intensity in depressed patients. Neuropsychobiology. 1997;36:57–61. doi: 10.1159/000119362. [DOI] [PubMed] [Google Scholar]
- 24.Burton C, McKinstry B, Szentagotai Tatar A, Serrano-Blanco A, Pagliari C, Wolters M. Activity monitoring in patients with depression: A systematic review. J Affect Disord. 2013;145:21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 25.Robillard R, Naismith SL, Smith KL, Rogers NL, White D, Terpening Z, et al. Sleep-wake cycle in young and older persons with a lifetime history of mood disorders. PLoS One. 2014;9:e87763. doi: 10.1371/journal.pone.0087763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasler BP, Buysse DJ, Kupfer DJ, Germain A. Phase relationships between core body temperature, melatonin, and sleep are associated with depression severity: further evidence for circadian misalignment in non-seasonal depression. Psychiatry Res. 2010;178:205–207. doi: 10.1016/j.psychres.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Emens J, Lewy A, Kinzie JM, Arntz D, Rough J. Circadian misalignment in major depressive disorder. Psychiatry Res. 2009;168:259–261. doi: 10.1016/j.psychres.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Benedetti F, Dallaspezia S, Fulgosi MC, Barbini B, Colombo C, Smeraldi E. Phase advance is an actimetric correlate of antidepressant response to sleep deprivation and light therapy in bipolar depression. Chronobiol Int. 2007;24:921–937. doi: 10.1080/07420520701649455. [DOI] [PubMed] [Google Scholar]
- 29.Kasper S, Hajak G, Wulff K, Hoogendijk WJ, Montejo AL, Smeraldi E, et al. Efficacy of the novel antidepressant agomelatine on the circadian rest-activity cycle and depressive and anxiety symptoms in patients with major depressive disorder: a randomized, double-blind comparison with sertraline. J Clin Psychiatry. 2010;71:109–120. doi: 10.4088/JCP.09m05347blu. [DOI] [PubMed] [Google Scholar]
- 30.Todder D, Caliskan S, Baune BT. Longitudinal changes of day-time and nighttime gross motor activity in clinical responders and non-responders of major depression. World J Biol Psychiatry. 2009;10:276–284. doi: 10.3109/15622970701403081. [DOI] [PubMed] [Google Scholar]
- 31.Todder D, Caliskan S, Baune BT. Night locomotor activity and quality of sleep in quetiapine-treated patients with depression. J Clin Psychopharmacol. 2006;26:638–642. doi: 10.1097/01.jcp.0000239798.59943.77. [DOI] [PubMed] [Google Scholar]
- 32.Raoux N, Benoit O, Dantchev N, Denise P, Franc B, Allilaire J, et al. Circadian pattern of motor activity in major depressed patients undergoing antidepressant therapy: relationship between actigraphic measures and clinical course. Psychiatry Res. 1994;52:85–98. doi: 10.1016/0165-1781(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 33.McClung CA. Circadian genes, rhythms and the biology of mood disorders. Pharmacol Ther. 2007;114:222–232. doi: 10.1016/j.pharmthera.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Partonen T. Clock gene variants in mood and anxiety disorders. J Neural Transm. 2012;119:1133–1145. doi: 10.1007/s00702-012-0810-2. [DOI] [PubMed] [Google Scholar]
- 35.Roybal K, Theobold D, Graham A, DiNieri J, Russo SJ, Krishnan V, et al. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci USA. 2007;104:6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McClung CA. Circadian rhythms and mood regulation: insights from pre-clinical models. Eur Neuropsychopharmacol. 2011;21(Suppl 4):S683–693. doi: 10.1016/j.euroneuro.2011.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampp G, Albrecht U. The circadian clock and mood-related behavior. Commun Integr Biol. 2008;1:1–3. doi: 10.4161/cib.1.1.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hampp G, Ripperger JA, Houben T, Schmutz I, Blex C, Perreau-Lenz S, et al. Regulation of monoamine oxidase A by circadian-clock components implies clock influence on mood. Curr Biol. 2008;18:678–683. doi: 10.1016/j.cub.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Schnell A, Albrecht U, Sandrelli F. Rhythm and mood: relationships between the circadian clock and mood-related behavior. Behav Neurosci. 2014;128:326–343. doi: 10.1037/a0035883. [DOI] [PubMed] [Google Scholar]
- 40.Milhiet V, Etain B, Boudebesse C, Bellivier F. Circadian biomarkers, circadian genes and bipolar disorders. J Physiol Paris. 2011;105:183–189. doi: 10.1016/j.jphysparis.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 41.Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165:820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- 42.Benedetti F, Dallaspezia S, Fulgosi MC, Lorenzi C, Serretti A, Barbini B, et al. Actimetric evidence that CLOCK 3111 T/C SNP influences sleep and activity patterns in patients affected by bipolar depression. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:631–635. doi: 10.1002/ajmg.b.30475. [DOI] [PubMed] [Google Scholar]
- 43.Franken P, Dijk DJ. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29:1820–1829. doi: 10.1111/j.1460-9568.2009.06723.x. [DOI] [PubMed] [Google Scholar]
- 44.Franken P. A role for clock genes in sleep homeostasis. Curr Opin Neurobiol. 2013;23:864–872. doi: 10.1016/j.conb.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Franken P, Thomason R, Heller HC, O'Hara BF. A non-circadian role for clock-genes in sleep homeostasis: a strain comparison. BMC Neurosci. 2007;8:87. doi: 10.1186/1471-2202-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moller-Levet CS, Archer SN, Bucca G, Laing EE, Slak A, Kabiljo R, et al. Effects of insufficient sleep on circadian rhythmicity and expression amplitude of the human blood transcriptome. Proc Natl Acad Sci U S A. 2013;110:E1132–1141. doi: 10.1073/pnas.1217154110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ibrahim L, Diazgranados N, Franco-Chaves J, Brutsche N, Henter ID, Kronstein P, et al. Course of improvement in depressive symptoms to a single intravenous infusion of ketamine vs add-on riluzole: results from a 4-week, double-blind, placebo-controlled study. Neuropsychopharmacology. 2012;37:1526–1533. doi: 10.1038/npp.2011.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ionescu DF, Luckenbaugh DA, Niciu MJ, Richards EM, Zarate CAJ. A single infusion of ketamine improves depression scores in patients with anxious bipolar depression. Bipolar Disord. 2015;17:438–443. doi: 10.1111/bdi.12277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sackeim HA. The definition and meaning of treatment-resistant depression. J Clin Psychiatry. 2001;62(Suppl 16):10–17. [PubMed] [Google Scholar]
- 50.Zarate CAJ, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, et al. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry. 2006;63:856–864. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 51.Erzberger A, Hampp G, Granada AE, Albrecht U, Herzel H. Genetic redundancy strengthens the circadian clock leading to a narrow entrainment range. J R Soc Interface. 2013;10:20130221. doi: 10.1098/rsif.2013.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Boivin DB, Czeisler CA, Dijk DJ, Duffy JF, Folkard S, Minors DS, et al. Complex interaction of the sleep-wake cycle and circadian phase modulates mood in healthy subjects. Arch Gen Psychiatry. 1997;54:145–152. doi: 10.1001/archpsyc.1997.01830140055010. [DOI] [PubMed] [Google Scholar]
- 53.Wirz-Justice A. Diurnal variation of depressive symptoms. Dialogues Clin Neurosci. 2008;10:337–343. doi: 10.31887/DCNS.2008.10.3/awjustice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Naismith SL, Lagopoulos J, Hermens DF, White D, Duffy SL, Robillard R, et al. Delayed circadian phase is linked to glutamatergic functions in young people with affective disorders: A proton magnetic resonance spectroscopy study. BMC Psychiatry. 2014;14:345. doi: 10.1186/s12888-014-0345-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Allebrandt KV, Teder-Laving M, Akyol M, Pichler I, Muller-Myhsok B, Pramstaller P, et al. CLOCK gene variants associate with sleep duration in two independent populations. Biol Psychiatry. 2010;67:1040–1047. doi: 10.1016/j.biopsych.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 56.Novakova M, Sladek M, Sumova A. Human chronotype is determined in bodily cells under real-life conditions. Chronobiol Int. 2013;30:607–617. doi: 10.3109/07420528.2012.754455. [DOI] [PubMed] [Google Scholar]
- 57.Baehr EK, Revelle W, Eastman CI. Individual differences in the phase and amplitude of the human circadian temperature rhythm: with an emphasis on morningness-eveningness. J Sleep Res. 2000;9:117–127. doi: 10.1046/j.1365-2869.2000.00196.x. [DOI] [PubMed] [Google Scholar]
- 58.Haile CN, Murrough JW, Iosifescu DV, Chang LC, Al Jurdi RK, Foulkes A, et al. Plasma brain derived neurotrophic factor (BDNF) and response to ketamine in treatment-resistant depression. Int J Neuropsychopharmacol. 2014;17:331–336. doi: 10.1017/S1461145713001119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bunney BG, Li JZ, Walsh DM, Stein R, Vawter MP, Cartagena P, et al. Circadian dysregulation of clock genes: clues to rapid treatments in major depressive disorder. Mol Psychiatry. 2015;20:48–55. doi: 10.1038/mp.2014.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cao R, Lee B, Cho HY, Saklayen S, Obrietan K. Photic regulation of the mTOR signaling pathway in the suprachiasmatic circadian clock. Mol Cell Neurosci. 2008;38:312–324. doi: 10.1016/j.mcn.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao R, Li A, Cho HY, Lee B, Obrietan K. Mammalian target of rapamycin signaling modulates photic entrainment of the suprachiasmatic circadian clock. J Neurosci. 2010;30:6302–6314. doi: 10.1523/JNEUROSCI.5482-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zarate CA, Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, et al. Relationship of ketamine's plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry. 2012;72:331–338. doi: 10.1016/j.biopsych.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, et al. NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 2016;533:481–486. doi: 10.1038/nature17998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li JZ, Bunney BG, Meng F, Hagenauer MH, Walsh DM, Vawter MP, et al. Circadian patterns of gene expression in the human brain and disruption in major depressive disorder. Proc Natl Acad Sci U S A. 2013;110:9950–9955. doi: 10.1073/pnas.1305814110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mukherjee S, Coque L, Cao JL, Kumar J, Chakravarty S, Asaithamby A, et al. Knockdown of Clock in the ventral tegmental area through RNA interference results in a mixed state of mania and depression-like behavior. Biol Psychiatry. 2010;68:503–511. doi: 10.1016/j.biopsych.2010.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tan S, Lam WP, Wai MS, Yu WH, Yew DT. Chronic ketamine administration modulates midbrain dopamine system in mice. PLoS One. 2012;7:e43947. doi: 10.1371/journal.pone.0043947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Someren EJ, Swaab DF, Colenda CC, Cohen W, McCall WV, Rosenquist PB. Bright light therapy: improved sensitivity to its effects on rest-activity rhythms in Alzheimer patients by application of nonparametric methods. Chroniobiol Int. 1999;16:505–518. doi: 10.3109/07420529908998724. [DOI] [PubMed] [Google Scholar]
- 68.Wirz-Justice A. How to measure circadian rhythms in humans. Medicographia. 2007;29:84–90. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1: Ketamine’s (K) effects on the 24-hour pattern of wrist activity are shown for ketamine treatment at baseline (BL; one day before ketamine treatment; left panel) versus placebo (P) treatment on the day of ketamine treatment (right panel). At baseline there was no difference between the 24-hour patterns of placebo and ketamine activity (left panel). Both placebo and ketamine infusions (indicated by arrow at 10:00 on D0) were associated with an eight-hour infusion- and recovery-related decrease in the level of activity (right panel; ketamine and placebo are shown relative to the pre-ketamine baseline mean ± SEM (shaded area)). On D0 there was no difference between ketamine and placebo treatments from 12:00–00:00; (F1,94 = 0.79, p=.37). Filled circles correspond to mean activity counts/minute in hourly bins ± SEM. The dotted sinusoidal curve corresponds to the best fit line to the 24-hour data for both placebo and ketamine baseline days.
Supplemental Figure S2: Ketamine’s effects on the 24-hour pattern of wrist activity are shown for ketamine-treated patients one day after infusion (D1) versus their prior baseline (BL) (left panel), and compared with D1 placebo treatment (right). On D1, the central value (mesor) of the 24-hour activity pattern was lower for ketamine versus baseline (p=.0004, left panel), and there was a trend for ketamine to be lower than placebo treatment (p=.0317, right panel). There was also a trend (p=.0823) for ketamine to advance the phase of wrist activity relative to prior baseline days (left panel). Filled circles correspond to mean activity counts/minute in hourly bins ± SEM. The dotted sinusoidal curves correspond to the best fit line to the 24-hour data for each group. The dotted horizontal line (mesor) corresponds to the estimated 24-hour average (mesor) of the curve fits to each group.
Supplemental Table S1: Comparisons of 24-hour patterns of wrist activity in patients with mood disorders who did and did not respond to ketamine infusion on Day 1 (D1).