Abstract
Iron deficiency anemia is a common clinical condition often treated with tablets containing 65 mg of elemental iron. Such doses can elicit gastrointestinal side effects lowering patient compliance. Oral iron supplements also increase hepcidin production causing decreased fractional absorption of subsequent doses. Frequent blood donors often become iron deficient. Therefore, they were enrolled in a two-year study involving continued blood donations and randomization to receive no pill, placebo, 19, or 38 mg ferrous gluconate for 60 days. Total body iron (TBI) did not change for the subset of donors in the no pill and placebo groups who completed both enrollment and final visits (p=0.21 and p=0.28, respectively). However, repeated measures regression analysis on the complete dataset estimated a significant decrease in TBI of 52 mg/year for the placebo and no pill groups (p=0.001). The effects of 19 and 38 mg iron supplementation on TBI were indistinguishable (p=0.54). TBI increased by 229 mg after the initial 60 days of iron supplementation (p<0.0001) and was maintained at this higher level with continued iron supplementation following each subsequent donation. The TBI increase was apportioned 51 mg to red cell iron (p<0.0001) and 174 mg to storage iron (p<0.0001). Changes in storage iron were negatively impacted by 57 mg due to concurrent antacid use (p=0.04). These findings in blood donors suggest that much lower doses of iron than are currently used will be effective for clinical treatment of iron deficiency anemia.
Keywords: iron deficiency, iron absorption, total body iron, ferritin, blood donor
Introduction
Iron deficiency and iron deficiency anemia are common among regular blood donors1–4 due to a loss of 200 to 250 mg of iron with each whole blood donation. The standard approach for the clinical treatment of iron deficiency is to prescribe 100 to 200 mg of elemental iron daily divided into two or three doses.5 However, many patients report gastrointestinal (GI) symptoms, such as bloating, nausea, vomiting, and constipation or diarrhea.6 Oral iron also acutely increases hepcidin synthesis, which decreases the fraction of subsequent iron doses absorbed by the GI tract.7,8 This has led to the idea of intermittent (i.e. every-other-day or weekly) dosing regimens for the treatment of iron deficiency.9,10 An additional consideration is the potential for suppression of oral iron absorption when gastric pH is altered by calcium-containing supplements 11,12 or proton pump inhibitors.13 Consequently, methods for treatment of iron deficiency may benefit from broadened consideration of how optimal iron absorption and diminished GI side effects can be harmonized. These are of increased importance to blood collecting agencies as they seek to operationalize strategies to mitigate the prevalence of iron deficiency in their donors.
The most accurate quantitative techniques for measuring iron absorption rely on careful dietary characterization, isotope labeling, and fecal analysis that are not feasible for large scale studies. Quantitative estimates of total body iron (TBI) using laboratory tests performed on peripheral blood are available for the major iron-containing compartments of the body providing a practical alternative to direct measurement. Iron present in red blood cells (RBC) represents 70–80% of TBI in iron replete individuals and is estimated using measured hemoglobin concentration and estimated total blood volume. Storage iron represents 15–20% of TBI in iron replete individuals and is estimated using soluble transferrin receptor (sTfR) and ferritin. The remaining 5–10% of iron is within myoglobin and other minor compartments that are not easily measured.
The recently completed Strategies to Reduce Iron DEficiency study (“STRIDE”, NCT02245321) was a prospective, two-year, randomized, double-blinded clinical trial investigating alternative strategies to mitigate iron deficiency in frequent blood donors.14,15 Assessment of TBI in these subjects provides a unique opportunity to compare the impact of two lower dose oral iron supplements (19 mg and 38 mg elemental iron) in a longitudinal study of an initially iron deficient population (many with ferritin <12 ng/ml) undergoing continued iron loss through repeated phlebotomy. Our initial analyses revealed that subjects receiving 19 mg of daily iron maintained essentially identical clinical measures of hemoglobin and iron over the course of the study as those receiving 38 mg.15 This finding was unexpected and indicated that much lower doses of iron than are currently used for clinical treatment may be effective for most blood donors and should be studied for patients with iron deficiency anemia. Here, quantitative estimates and longitudinal models of RBC iron, storage iron, and TBI were generated and compared to examine iron compartmentalization according to the dose received. In light of widespread availability of antacids and acid-blocking drugs, we also examined their impact on iron status.
Methods
Study Design
Blood donor recruitment, randomization, interventions, laboratory testing, retention, and initial findings have been previously reported.14,15 In summary, blood donors ≥18 years of age with two or more (women), or three or more (men), red cell equivalent donations in the previous year at three U.S. blood centers provided informed consent to continue making regular blood donations and were randomized to blinded doses of 0 (“placebo”), 19, or 38 mg of daily ferrous gluconate for 60 days, or, a group that received no pills. Donors already taking supplemental iron were excluded and members of an iron status information group15 were excluded from the present analysis. Each whole blood donation required fingerstick hemoglobin ≥12.5g/dL and at least 56 days elapsed time since prior donation. A venous, pre-donation blood sample was collected at each donation for measurement of complete blood count, ferritin, and sTfR. Subjects were surveyed for use of calcium-containing supplements and antacids at the beginning and end of the study. A list of antacids (such as Tagamet™, Nexium™, Prevacid™, etc.) was provided to improve recall.
Calculation of Iron Compartments
TBI was computed by adding estimates for RBC and storage iron compartments. RBC iron was calculated as the quotient of estimated blood volume16, venous hemoglobin concentration, and hemoglobin iron content17, and adjusted by 0.91 to accommodate central pooling and trapped plasma.18 Estimates for storage iron were calculated using the method by Cook, et al.19 forgoing the subsequent modification to sTfR reported by Pfeiffer20 because it was based on a relatively small study21 and the modification increased variability in our parameter estimates. Plasma ferritin was increased 5% to account for differences between plasma and serum values (Assay Manual, ADVIA Centaur, Siemens Healthcare Diagnostics, 2012). Thus,
Data Analyses
Two analytic datasets were developed including one for the 273 participants for whom venous blood samples were available at both enrollment and final visits (“paired analysis”) and another to quantify the effect of various predictors on TBI (“longitudinal analysis”, Figure 1). The longitudinal analyses included an intention-to-treat repeated measures regression model that analyzed all study visits regardless of whether or not the participant completed the study (n = 555). Repeated measures models were developed with compound symmetry covariance, as in Cable, et al.22 Covariates included race, age, gender, weight, smoking status, pregnancy history, menstrual status, blood center, number of donations in the past two years, and time since last donation, as well as an indicator variable for antacid use. Ferritin and sTfR were log base 10 transformed to better satisfy linear regression normality assumptions. All statistical analyses were performed using SAS V9.3 (SAS Institute, Inc., Cary, NC).
Figure 1.
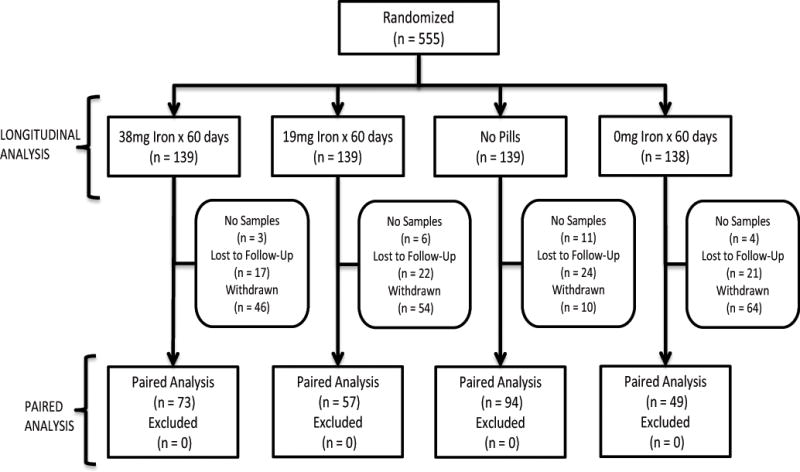
Study schematic illustrating the allocation of participants to the four study groups included in the longitudinal analysis (n = 555) and the paired analysis (n = 273). The reasons for not including participants in the paired analysis and group sizes are presented for each group.
Model Specification
STRIDE was designed to detect long term (i.e. two-year) effects of group assignment on TBI; not to evaluate a specific temporal effect of group assignment on TBI. A repeated measures ANOVA model with constant (intercept) and slope (temporal) effects for each group was deemed unduly complex. First, non-supplemented participants could be expected to have no immediate change from baseline (i.e. no constant effect) since they essentially continued their pre-STRIDE enrollment donation behavior. Second, iron supplemented participants could be expected to have no decline over time (i.e. no slope effect), since they received additional iron supplementation after each subsequent donation. Thus, rather than a comparison of groups by the pair of constant and slope effects, the comparison of groups might be achieved by the two slope effects of the non-supplemented groups and the two constant effects of the iron supplemented groups. Therefore, iron status in the non-supplemented groups was modeled as a continual, gradual rate of decline parameterized as a rate of change per year. In contrast, iron status in the two iron supplemented groups was modeled as a change in iron stores immediately after enrollment parameterized as change in mg from baseline with persistence through the two-year study. While other models were entertained, since STRIDE was not designed to differentiate among nuances of any temporal effect, other models were statistically indistinguishable from the model presented.
Results
Paired Analyses of Enrollment and Final Visits Reveals Equal Effects of 19 and 38 mg Iron
Mean enrollment TBI was approximately 2,500 mg with no difference across groups (Table I). Approximately 85% of iron was in the red cell compartment and 15% in the storage compartment. TBI decreased approximately 55 mg in the No Pills and Placebo groups; consisting of decreases of 16 mg for RBC iron and 39 mg for storage iron (Table II). However, these were not statistically different from enrollment. The proportion of iron in the RBC and storage iron compartments did not change, nor did measures of RBC iron (p=0.99), storage iron (p=0.96), or TBI (p=0.97). Ferritin and hemoglobin remained essentially constant despite continued blood donation. Decreased TBI was driven by the previously reported15 significant increase in sTfR. Equal increases were observed for the 19 mg and 38 mg iron groups including 49 mg for RBC iron (p=0.003), 191 mg for storage iron (p<0.0001), and 240 mg for TBI (p<0.0001) with an increase in the relative proportion of storage iron from approximately 15% to 20%. Increases between the 19 and 38 mg iron groups were indistinguishable for RBC iron (p=0.54), storage iron (p=0.61), and TBI (p=0.54).
Table I.
RBC iron, storage iron, and TBI at enrollment and final visits by group for the 273 subjects included in the paired analysis.
| RBC Iron | Storage Iron | Total Body Iron | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||
| Group (n) | Enrollment, mg (%) | Final, mg (%) | ∆, mg (95%C.I.) | p | Enrollment, mg (%) | Final, mg (%) | ∆, mg (95%C.I.) | p | Enrollment, mg | Final, mg | ∆, mg (95%C.I.) | p |
| No Pills (94) | 2172 (84%) | 2156 (85%) | −16 (−47, 17) | 0.34 | 418 (16%) | 379 (15%) | −38 (−100, 24) | 0.22 | 2589 | 2535 | −54 (−140, 31) | 0.21 |
| Placebo (49) | 2064 (85%) | 2048 (86%) | −16 (−54, 22) | 0.41 | 366 (15%) | 325 (14%) | −41 (−122, 41) | 0.33 | 2429 | 2373 | −56 (−160, 47) | 0.28 |
| 19mg (57) | 2176 (87%) | 2236 (80%) | 60 (11, 109) | 0.02 | 335 (13%) | 541 (20%) | 206 (136, 275) | <0.0001 | 2511 | 2778 | 266 (116, 324) | <0.0001 |
| 38mg (73) | 2147 (85%) | 2187 (80%) | 40 (−1, 82) | 0.06 | 375 (15%) | 555 (20%) | 180 (107, 253) | <0.0001 | 2523 | 2743 | 220 (159, 373) | <0.0001 |
n = 273 is a subset of the larger cohort (n=555) and is comprised of donors with baseline and final visit venous samples available for testing
Table II.
Mean values at enrollment for variables used in the computation of RBC iron, storage iron, and TBI for 555 subjects included in longitudinal regression analysis.
| No Pills | Placebo | 19mg | 38mg | Total | |
|---|---|---|---|---|---|
|
| |||||
| N (% female) | 139 (49.6%) | 138 (49.3%) | 139 (48.9%) | 139 (49.6%) | 555 (49.4%) |
| Body Size, mean (SD) | |||||
| Height, inches | 67.6 (3.9) | 67.2 (3.4) | 67.9 (3.6) | 67.3 (3.7) | |
| Weight, pounds | 182.1 (44.6) | 179.1 (47.1) | 184.4 (46.1) | 182.4 (49.1) | |
| Estimated Blood Volume, L1 | 4.96 (0.93) | 4.91 (0.89) | 5.05 (0.85) | 4.98 (0.96) | |
| Venous Hemoglobin, g/dL | 13.95 (1.33) | 14.10 (1.36) | 13.89 (1.24) | 14.00 (1.39) | |
| RBC Iron, mg | 2148 (533) | 2155 (528) | 2178 (467) | 2168 (540) | |
|
| |||||
| Ferritin, ng/mL | 29.4 (28.2) | 25.9 (20.6) | 23.6 (25.9) | 27.0 (29.2) | |
| sTfR, mg/L | 3.57 (1.34) | 3.53 (1.31) | 3.60 (1.19) | 4.11 (5.34) | |
| log (sTfR/ferritin) | 2.21 (0.47) | 2.22 (0.41) | 2.30 (0.41) | 2.27 (0.44) | |
| Storage Iron, mg | 426 (382) | 422 (319) | 361 (309) | 394 (353) | |
|
| |||||
| Total Body Iron, mg | 2572 (808) | 2575 (754) | 2547 (678) | 2570 (795) | |
|
| |||||
| RBC Iron/TBI | 85.7% (11.2) | 85.3% (9.7) | 87.2% (9.8) | 86.4% (10.4) | |
| Storage Iron/TBI | 14.3% (11.2) | 14.7% (9.7) | 12.8% (9.8) | 13.6% (10.4) | |
estimated blood volume computed using formula from Nadler, et al. 1962.
Longitudinal Analyses of Iron Status at All Visits Reveals Equal Effects of 19 and 38 mg Iron
Longitudinal multivariable regression analysis of 555 enrolled subjects across 3,168 follow-up visits over two years was performed to examine changes in iron balance between groups in more detail than the paired analysis allowed. Biometric indicators were equivalent at enrollment across randomization groups (Table II). Declines in TBI were observed in the No Pills (43 mg/year, p = 0.03) and Placebo groups (68 mg/year, p=0.004) (Table III), and, were indistinguishable between the two groups for RBC iron (16 mg/year, p=0.78), storage iron (39 mg/year, p=0.38), and TBI (52 mg/year, p=0.42). Increases in TBI of 216 and 240 mg occurred in the 19 and 38 mg iron groups, respectively (p<0.0001 for both). RBC iron (p = 0.95), storage iron (p = 0.36), and TBI (p = 0.54) were indistinguishable in the 19 and 38 mg iron groups and demonstrated immediate increases from enrollment of 51 mg for RBC iron (p<0.0001), 174 mg for storage iron (p<0.0001), and 229 mg for TBI (p<0.0001).
Table III.
Multivariable longitudinal regression results based on 3,168 observed study visits from 555 donors and adjusting for race, age, gender, weight, smoking status, pregnancy history, menstrual status, and blood center.
| RBC Iron, mg | Storage Iron, mg | Total Body Iron, mg | ||||
|---|---|---|---|---|---|---|
|
|
||||||
| Δ (95% C.I.) | p-value | Δ (95% C.I.) | p-value | Δ (95% C.I.) | p-value | |
| Group1 | <0.0001 | <0.0001 | <0.0001 | |||
| No Pills | −15 (−31, 3) | −31 (−56, −5) | −43 (−84, −3) | |||
| Placebo | −18 (−35, 0) | −48 (−80, −17) | −68 (−114, −21) | |||
| 19mg | 50 (25, 75) | 162 (127, 198) | 216 (162, 270) | |||
| 38mg | 51 (27, 75) | 186 (146, 226) | 240 (180, 300) | |||
|
| ||||||
| time since last donation | <0.0001 | <0.0001 | <0.0001 | |||
| 8–13 weeks | −50 (−71, −30) | −186 (−214, −158) | −236 (−280, −193) | |||
| 14–19 weeks | −23 (−43, −2) | −117 (146, −88) | −138 (−181, −94) | |||
| 20–25 weeks | −18 (−42, 6) | −61(−94, −28) | −76 (−125, −28) | |||
| 26+ weeks | REF | REF | REF | |||
|
| ||||||
| # of blood donations in previous 2 years | 0.008 | 0.002 | 0.002 | |||
| 0 | REF | REF | REF | |||
| 1–3 | −8 (−235, 219) | −9 (−298, 280) | −20 (−538, 497) | |||
| 4–9 | −39 (−266, 187) | −88 (−375, 199) | −130 (−694, 385) | |||
| 10+ | −63 (−290, 164) | −111 (−400, 178) | −173 (−689, 344) | |||
|
| ||||||
| antacids2 | −30 (−85, 25) | 0.27 | −57 (−114, −1) | 0.04 | −88 (−186, 10) | 0.07 |
group effect modeled as rate of change per year for No Pills and Placebo groups, and as change in first study inter-donation interval in 19 mg and 38 mg iron pill groups
either or both baseline and final questionnaire reported use of antacids
Regression Quantifies Effects of Iron Supplementation Relative to Iron Loss and Antacid Use
Continued blood donation over the two-year study period maintained a state of iron deficiency among most subjects in the No Pills and Placebo groups. The longitudinal model included known covariates of iron status in blood donors including the time since last donation and the number of donations made in the two years prior to the current donation.22 The time since last donation parameter was a significant contributor to iron status (p<0.0001). It was inversely proportional to the magnitude of TBI deficit and its effect on iron status could be quantified (Table III). For example, delaying the time since last donation from 20–25 weeks to over 26 weeks corresponded to a 76 mg increase in TBI; while delaying it from 8–13 weeks to over 26 weeks increased TBI by 236 mg. The number of donations in the previous two years was also a significant contributor to iron status (p=0.002). Subjects with 10 or more donations in the previous two years had a TBI deficit of 153 mg compared to subjects donating one to three times. Finally, the prevalence of antacid use was approximately 20% corresponding to a 30 mg deficit in RBC iron, a 57 mg deficit in storage iron, and an 88 mg deficit in TBI (Table III). This effect was significant only for storage iron (p=0.04).
Discussion
Iron balance was assessed in 555 iron deficient subjects over 3,168 encounters as they underwent continued iron loss during two years of frequent blood donation. In both paired and repeated measures analyses, subjects randomized to receive 19 or 38 mg of elemental iron had statistically equivalent increases in RBC iron, storage iron, and TBI. These findings are similar to those of Rimon and colleagues,24 who observed equivalent increases in hemoglobin and ferritin in a prospective, randomized study of elderly hospitalized patients with iron deficiency anemia receiving 15, 50, or 150 mg of daily elemental iron over a 60 day interval. Therefore, it appears that iron deficiency among frequent blood donors can be mitigated by use of just 19 mg elemental iron daily for 60 days following each donation, especially because pill compliance was equivalent across groups.14,15 More broadly, these data suggest that much lower doses of iron than are currently used will effectively treat clinical iron deficiency and iron deficiency anemia.
Current voluntary whole blood donation standards in the U.S. require a minimum of eight weeks between donations. Results from the present analysis indicate that donation every eight weeks produces an average decrement of 236 mg in TBI when compared to donation at >26 weeks. This finding is consistent with several recent studies indicating that recovery of iron stores following a single whole blood donation requires more than 26 weeks in many donors.23,25,26 Oral iron supplementation of frequent blood donors produced a large increase in storage iron associated with a more modest relative increase in RBC iron in the time between blood donations. The differential increase within the two iron compartments resulted in a shift in the proportion of TBI allocated to storage iron from 15% to 20%. This is likely a result of the hemoglobin screening during qualification for blood donation, which prevents donation by those with severe anemia, but not by those with severely depleted iron stores.
Since no differences in iron, hemoglobin status, or compliance between the 19 and 38 mg iron groups were identified, the combined amount of dietary and supplemental iron absorbed between blood donations was equivalent. Equal absorption in the two groups may have occurred because fractional iron absorption of the 19 mg dose is increased, producing the same net iron absorption as the 38 mg dose. In a previous study where donors received 38 mg daily iron for six months following a single whole blood donation, approximately 75% of TBI recovered in the first four weeks after blood donation with an additional 9–15% recovery between 4 and 8 weeks after donation.23 Therefore, an alternative possibility is that iron absorption was initially greater among individuals taking 38 mg iron, but total iron absorbed over the entire interval between donations was equivalent between 19 and 38 mg doses. STRIDE was not designed or powered to detect differences in iron absorption kinetics between donors taking 19 and 38 mg iron to differentiate these two possibilities.
The prevalence of antacid use in the prior 30 days in the United States is nearly 8%.27 One in five participants reported antacid use at some time during STRIDE. When antacid use was included as a binary term in the repeated measures model, there was a non-significant (p=0.07) decline of 88 mg in TBI, comprised of a non-significant (p=0.27) decline of 30 mg in RBC iron and a significant (p=0.04) decline of 57 mg in storage iron. The observation that antacid use was associated with a decrease in storage iron suggests that absorbed iron is preferentially utilized for erythropoiesis. This finding is consistent with other studies11–13 suggesting that use of antacids or proton pump inhibitors modestly interferes with iron absorption. However, due to the low number of donors (n=90) reporting antacid use, the statistical power to detect an antacid effect was low. It is possible that users of antacids were less likely to comply with taking study pills; however, subjects randomized to iron were no more likely to experience gastrointestinal discomfort than subjects randomized to placebo14 thereby diminishing the potential for confounding.
The randomization groups had indistinguishable iron status at enrollment and repeated measures regression analysis of all 3,168 encounters during the study period decreases the potential for selection bias associated with the previously reported differential losses of follow-up among randomization group.14,15 Nevertheless, there are limitations to this study. Calculation of TBI using estimates of RBC iron and storage iron suffers from the underlying assumption that there is neutral iron balance in minor iron compartments, or that these minor compartments do not meaningfully alter temporal changes in TBI estimates. This methodology also assumes that the daily loss of iron can be classified as an ordinal variable, and though commonly applied in the clinical setting28 this approach does not allow for potential individual-level variation. These limitations are evidenced in the broad confidence intervals associated with reported parameter estimates. However, the direction and magnitude of change, as well as the variability around the point estimates for 19 and 38 mg iron-supplemented groups, are essentially identical. Also, only frequent blood donors were enrolled. Blood donors are screened for hemoglobin, but not ferritin. Therefore, non-anemic iron deficiency was common at enrollment with 76% of females and 52% of males having plasma ferritin <26 ng/mL.14 Since anemia was infrequent, the results from this analysis cannot be directly applied to patients with iron deficiency anemia. Although the two iron doses were equivalent in efficacy, the proportion of individuals with ferritin <26 ng/mL ranged between 30–40% at the end of the study. This persistent iron deficiency among some subjects may reflect non-compliance with pills or an inadequate inter-donation interval to correct iron deficiency in some frequent donors.
Oral iron induces synthesis of the central iron regulatory hormone, hepcidin, which then blocks subsequent dietary iron absorption.7,8,29 The relationship between iron dose and hepcidin production is direct and dose-dependent 9 providing a potential physiological basis for repeated higher doses of iron to be absorbed less readily than lower doses. It has also been shown that consecutive iron doses given on the same day are not absorbed as efficiently.9 It has therefore been proposed that every-other-day9,10, or even weekly dosing30, could be a superior treatment regimen for iron deficiency. Another alternative supported by the findings presented here, as well as those of Rimon and colleagues,24 is daily supplementation with low (19 mg) iron. This is the amount of iron present in many over-the-counter multiple vitamins with iron and is not associated with more adverse events than placebo.14,31–33 Together, these findings indicate that low dose iron should harmonize the need for maximal benefit on TBI while mitigating side effects in the treatment of iron deficiency in blood donors and perhaps iron deficiency anemia in patients.
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute (NHLBI) grant 1R01HL105809.
Footnotes
Conflict of Interest Disclosure: AEM has received honoraria from Siemens and receives research grant funding from Novo Nordisk. The other authors have no competing financial interests to report.
References
- 1.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: analysis of enrollment data from the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51(3):511–522. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salvin HE, Pasricha SR, Marks DC, Speedy J. Iron deficiency in blood donors: a national cross-sectional study. Transfusion. 2014;54(10):2434–2444. doi: 10.1111/trf.12647. [DOI] [PubMed] [Google Scholar]
- 3.Baart AM, van Noord PA, Vergouwe Y, et al. High prevalence of subclinical iron deficiency in whole blood donors not deferred for low hemoglobin. Transfusion. 2013;53(8):1670–1677. doi: 10.1111/j.1537-2995.2012.03956.x. [DOI] [PubMed] [Google Scholar]
- 4.Lee CK, Wong HK, Hong J, Leung JN, Tsoi WC, Lin CK. A study of the predonation hemoglobin and iron status among Hong Kong Chinese blood donors. Transfusion. 2013;53(2):322–327. doi: 10.1111/j.1537-2995.2012.03788.x. [DOI] [PubMed] [Google Scholar]
- 5.Camaschella C. Iron-Deficiency Anemia. N Engl J Med. 2015;373(5):485–486. doi: 10.1056/NEJMc1507104. [DOI] [PubMed] [Google Scholar]
- 6.Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS One. 2015;10(2):e0117383. doi: 10.1371/journal.pone.0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Piperno A, Girelli D, Nemeth E, et al. Blunted hepcidin response to oral iron challenge in HFE-related hemochromatosis. Blood. 2007;110(12):4096–4100. doi: 10.1182/blood-2007-06-096503. [DOI] [PubMed] [Google Scholar]
- 8.Lin L, Valore EV, Nemeth E, Goodnough JB, Gabayan V, Ganz T. Iron transferrin regulates hepcidin synthesis in primary hepatocyte culture through hemojuvelin and BMP2/4. Blood. 2007;110(6):2182–2189. doi: 10.1182/blood-2007-04-087593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moretti D, Goede JS, Zeder C, et al. Oral iron supplements increase hepcidin and decrease iron absorption from daily or twice-daily doses in iron-depleted young women. Blood. 2015;126(17):1981–1989. doi: 10.1182/blood-2015-05-642223. [DOI] [PubMed] [Google Scholar]
- 10.Schrier SL. So you know how to treat iron deficiency anemia. Blood. 2015;126(17):1971. doi: 10.1182/blood-2015-09-666511. [DOI] [PubMed] [Google Scholar]
- 11.Minihane AM, Fairweather-Tait SJ. Effect of calcium supplementation on daily nonheme-iron absorption and long-term iron status. Am J Clin Nutr. 1998;68(1):96–102. doi: 10.1093/ajcn/68.1.96. [DOI] [PubMed] [Google Scholar]
- 12.Benkhedda K, L’Abbe MR, Cockell KA. Effect of calcium on iron absorption in women with marginal iron status. Br J Nutr. 2010;103(5):742–748. doi: 10.1017/S0007114509992418. [DOI] [PubMed] [Google Scholar]
- 13.Hutchinson C, Geissler CA, Powell JJ, Bomford A. Proton pump inhibitors suppress absorption of dietary non-haem iron in hereditary haemochromatosis. Gut. 2007;56(9):1291–1295. doi: 10.1136/gut.2006.108613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bialkowski W, Bryant BJ, Schlumpf KS, et al. The strategies to reduce iron deficiency in blood donors randomized trial: design, enrolment and early retention. Vox Sang. 2014 doi: 10.1111/vox.12210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mast AE, Bialkowski W, Bryant BJ, et al. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion. 2016 doi: 10.1111/trf.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nadler SB, Hidalgo JH, Bloch T. Prediction of blood volume in normal human adults. Surgery. 1962;51(2):224–232. [PubMed] [Google Scholar]
- 17.Bernhart FWaS, Leonard The Iron Content of Crystalline Human Hemoglobin. J Biol Chem. 1943;147:19–22. [Google Scholar]
- 18.Chaplin H, Jr, Mollison PL, Vetter H. The body/venous hematocrit ratio: its constancy over a wide hematocrit range. J Clin Invest. 1953;32(12):1309–1316. doi: 10.1172/JCI102859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cook JD, Flowers CH, Skikne BS. The quantitative assessment of body iron. Blood. 2003;101(9):3359–3364. doi: 10.1182/blood-2002-10-3071. [DOI] [PubMed] [Google Scholar]
- 20.Pfeiffer CM, Cook JD, Mei Z, Cogswell ME, Looker AC, Lacher DA. Evaluation of an automated soluble transferrin receptor (sTfR) assay on the Roche Hitachi analyzer and its comparison to two ELISA assays. Clin Chim Acta. 2007;382(1–2):112–116. doi: 10.1016/j.cca.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Skikne BS, Flowers CH, Cook JD. Serum transferrin receptor: a quantitative measure of tissue iron deficiency. Blood. 1990;75(9):1870–1876. [PubMed] [Google Scholar]
- 22.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52(4):702–711. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kiss JE, Brambilla D, Glynn SA, et al. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;313(6):575–583. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rimon E, Kagansky N, Kagansky M, et al. Are we giving too much iron? Low-dose iron therapy is effective in octogenarians. Am J Med. 2005;118(10):1142–1147. doi: 10.1016/j.amjmed.2005.01.065. [DOI] [PubMed] [Google Scholar]
- 25.Schotten N, Pasker-de Jong PC, Moretti D, et al. The donation interval of 56 days requires extension to 180 days for whole blood donors to recover from changes in iron metabolism. Blood. 2016 doi: 10.1182/blood-2016-04-709451. [DOI] [PubMed] [Google Scholar]
- 26.Mast AE, Lee TH, Schlumpf KS, et al. The impact of HFE mutations on haemoglobin and iron status in individuals experiencing repeated iron loss through blood donation. Br J Haematol. 2012;156(3):388–401. doi: 10.1111/j.1365-2141.2011.08952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. Trends in Prescription Drug Use Among Adults in the United States From 1999–2012. JAMA. 2015;314(17):1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brittenham G. Disorders of Iron Homeostasis. In: Hoffman R, Benz EJ Jr, Silberstein LE, Heslop H, Weitz J, Anastasi J, editors. Hematology: Basic Principles and Practice. 6. Elsevier; 2013. pp. 437–449. [Google Scholar]
- 29.Girelli D, Trombini P, Busti F, et al. A time course of hepcidin response to iron challenge in patients with HFE and TFR2 hemochromatosis. Haematologica. 2011;96(4):500–506. doi: 10.3324/haematol.2010.033449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cook JD, Reddy MB. Efficacy of weekly compared with daily iron supplementation. Am J Clin Nutr. 1995;62(1):117–120. doi: 10.1093/ajcn/62.1.117. [DOI] [PubMed] [Google Scholar]
- 31.Bryant BJ, Yau YY, Arceo SM, Daniel-Johnson J, Hopkins JA, Leitman SF. Iron replacement therapy in the routine management of blood donors. Transfusion. 2012;52(7):1566–1575. doi: 10.1111/j.1537-2995.2011.03488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Radtke H, Tegtmeier J, Rocker L, Salama A, Kiesewetter H. Daily doses of 20 mg of elemental iron compensate for iron loss in regular blood donors: a randomized, double-blind, placebo-controlled study. Transfusion. 2004;44(10):1427–1432. doi: 10.1111/j.1537-2995.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 33.Makrides M, Crowther CA, Gibson RA, Gibson RS, Skeaff CM. Efficacy and tolerability of low-dose iron supplements during pregnancy: a randomized controlled trial. Am J Clin Nutr. 2003;78(1):145–153. doi: 10.1093/ajcn/78.1.145. [DOI] [PubMed] [Google Scholar]


