Abstract
Ice-associated algae produce ice-binding proteins (IBPs) to prevent freezing damage. The IBPs of the three chlorophytes that have been examined so far share little similarity across species, making it likely that they were acquired by horizontal gene transfer (HGT). To clarify the importance and source of IBPs in chlorophytes, we sequenced the IBP genes of another Antarctic chlorophyte, Chlamydomonas sp. ICE-MDV (Chlamy-ICE). Genomic DNA and total RNA were sequenced and screened for known ice-associated genes. Chlamy-ICE has as many as 50 IBP isoforms, indicating that they have an important role in survival. The IBPs are of the DUF3494 type and have similar exon structures. The DUF3494 sequences are much more closely related to prokaryotic sequences than they are to sequences in other chlorophytes, and the chlorophyte IBP and ribosomal 18S phylogenies are dissimilar. The multiple IBP isoforms found in Chlamy-ICE and other algae may allow the algae to adapt to a greater variety of ice conditions than prokaryotes, which typically have a single IBP gene. The predicted structure of the DUF3494 domain has an ice-binding face with an orderly array of hydrophilic side chains. The results indicate that Chlamy-ICE acquired its IBP genes by HGT in a single event. The acquisitions of IBP genes by this and other species of Antarctic algae by HGT appear to be key evolutionary events that allowed algae to extend their ranges into polar environments.
Keywords: alga, Chlamydomonas, Antarctica, ice-binding proteins, horizontal gene transfer
Many ice-associated microorganisms secrete ice-binding proteins (IBPs) that mitigate freezing damage by modifying the external ice environment (Raymond 2014). IBPs differ from the antifreeze proteins of higher organisms in that they are present in much lower concentrations, i.e., in the μg · mL-1 range (Bayer-Giraldi et al. 2011) as opposed to the mg · mL‐1 range for fish antifreezes (Devries 1983). Their concentrations are too low to appreciably lower the freezing point and prevent freezing, but they are high enough to strongly affect the morphology and recrystallization of ice, both of which can affect the survival of microorganisms.
Among unicellular algae examined so far, two types of IBPs have been identified. Type I is the most common, being present in numerous diatoms, chlorophytes, a prymnesiophyte, and a prasinophyte (as well as in numerous bacteria and fungi; Raymond and Kim 2012). All such IBPs have at least one ∼190 a.a. domain (DUF3494) that, in organisms studied thus far, forms a triangular prism, one side of which is the ice-binding side (Kondo et al. 2012, Lee et al. 2012, Do et al. 2014). The DUF3494 domain is also present in many non-polar microbes, in which it presumably binds to other substrates. Most type I IBPs consist of just an N-terminal signal peptide followed by one or two DUF3494 domains. Several isoforms of a second type of algal IBP (type II) have been found in a chlorophyte (Raymond et al. 2009), originally identified as Chlamydomonas but possibly is a species of Chloromonas (Jung et al. 2016). The amino acid sequences of the type II isoforms have no relation to the type I sequences, although one type II IBP, like the type I IBPs, is predicted to have an ice-binding face that is rich in threonine residues (Jung et al. 2016). No other algal type II IBPs exist in any of the databases, while one homolog was found in a bacterium that experiences severe water stress, in which a water-binding protein could be an advantage (Raymond et al. 2009). Among the algae that produce type I IBPs (diatoms, a prasinophyte, a prymnesiophyte and chlorophytes), the taxonomy of the IBPs bears no relation to the taxonomy of the algae, and the IBPs are more closely related to bacterial and fungal proteins than they are to other algal IBPs (Raymond and Kim 2012). Together, these findings strongly suggest that both types of IBP genes were acquired from other microorganisms by horizontal gene transfer (HGT), allowing the algae to expand their range into polar environments. A statistical analysis of type I IBPs (Sorhannus 2011) provided further support for this idea. However, the source of the IBPs has not been identified for any of the algae. Another characteristic of IBP-producing algae, not shared with IBP-producing prokaryotes, is the presence of multiple IBP isoforms, which have several potential advantages (discussed below).
So far the IBPs of only three chlorophytes have been described, including the above-mentioned type II IBPs of a Chlamydomonas/Chloromonas species with at least four isoforms (Raymond et al. 2009), and two type I IBPs, one from a snow alga, Chloromonas brevispina, with at least 20 isoforms (Raymond 2014) and the other from an Antarctic lake alga, Chlamydomonas raudensis, recently renamed Chlamydomonas sp. UWO241 (Possmayer et al. 2016), with at least 12 isoforms (Raymond and Morgan-Kiss 2013). The IBPs of each species appear to have been acquired independently. Recently a new chlorophyte species, Chlamydomonas sp. ICE-MDV (hereafter Chlamy-ICE) was isolated from the same Antarctic lake (Lake Bonney; Li et al. 2016) and was confirmed to have ice-binding activity. With such a limited number of chlorophytes examined so far, characterizing the IBPs of Chlamy-ICE presented an opportunity to clarify the origins of algal IBPs and clarify whether multiple IBP genes is a common feature of ice-associated chlorophytes. We found that this species has as many as 50 isoforms of type I IBP genes and that the sequences more closely resemble bacterial IBP sequences than other chlorophyte IBP sequences.
Materials and Methods
Cells
Chlamydomonas sp. ICE-MDV (Chlamy-ICE) cells were collected from a depth of 15 m below the ice cover of the east lobe of Lake Bonney, Antarctica, and isolated as described previously (Li et al. 2016). To obtain a pure culture, a dilution series was used together with isolation streaking. Single colonies were then transferred to liquid cultures under constant light conditions and antibiotics were added to the growth medium to remove bacterial contamination (Streptomycin, Ampicillin, Rifampicin, Ciproflaxacin, Chloramphenicol). The purity of the strain was confirmed by fluorescence microscopy and plating on LB medium. At Miami University, the cells were grown in BBM medium supplemented with 500 mM NaCl at a temperature/light regime of 8°C/50 μmol · m-2 · s-1. Cell cultures for genomic DNA preparation were shipped to the University of Nevada Las Vegas (UNLV) and stored at 2°C with fluorescent lighting.
Sequencing and gene assembly
DNA was prepared from liquid nitrogen-ground cells with a Nucleospin kit (Clontech) using lysis buffer PL2 , and sent to the Nevada Genomics Center (NGC) at the University of Nevada Reno for sequencing. A library was prepared using the Ion Xpress Plus Fragment Library kit (Life Technologies) and sequenced on an Ion Torrent Proton platform using Hi-Q chemistry and a P1 chip (Life Technologies), yielding about 92 million reads with an average length of about 220bp. A second library was prepared from the same DNA using the Illumina TruSeq Nano DNA Library Preparation kit and sequenced on an Illumina NextSeq 500 instrument using a mid-output flow cell with paired end 2×75 sequencing, yielding 17.5 Gb of reads with Phred quality scores >30. The genome size of Chlamy-ICE is unknown. Assuming that it has a size similar to the average genome size of five other species of Chlamydomonas in GenBank (116.7 ± 20 Mb; mean ± SD), the read data indicate a coverage of about 150×. The reads were assembled into 331,087 contigs (average length 812 bp) using CLC Genomics Workbench software.
For transcriptome sequencing, cells cultured in BBM supplemented with 500 mM NaCl at 8°C were shipped overnight to UNLV at 8°C and briefly stored at the same temperature. RNA with an RIN value of about 8.0 was prepared from liquid nitrogen-ground cells with a Qiagen miniplant RNeasy kit. A library was prepared at NGC using an Illumina TruSeq Stranded mRNA Library Prep kit and sequenced on an Illumina platform as described above, yielding 54.1 Gb of sequence data. The reads were assembled into 69,392 contigs (average length 866 bp) as described above.
Databases of the raw reads and contigs were made with makeblastdb.exe (NCBI) and screened for sequences resembling representative IBPs, plant, insect and fish antifreezes and bacterial ice-nucleating genes with BLASTN and TBLASTN (NCBI). Hits were aligned with a combination of Clustal W and manual alignment in BioEdit (Hall 1999). The presence of N-terminal signal peptides was predicted with SignalP v. 3.0 (Bendtsen et al. 2004). Domains of the assembled proteins were examined with NCBI's Conserved Domains site (ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). Neighbor-joining phylogenetic trees of the protein sequences and 18S nucleic acid sequences were constructed with Mega 6 (Tamura et al. 2011).
Despite the isolation procedures described above, bacterial DNA was found in the DNA sequence data, and several of the bacterial contigs contained DUF3494-type genes. These contigs were annotated with MANATEE (University of Maryland) to identify the bacterial taxon as closely as possible.
The intron/exon structures of the IBP genes of Chlamydomonas sp. UWO241 and Chloromonas brevispina were determined for comparison with those of Chlamy-ICE. Chlamydomonas sp. UWO241 DNA, prepared for our previous cDNA study (Raymond and Morgan-Kiss 2013), was subjected to Illumina sequencing as described above. The locations of introns in the IBP genes of C. brevispina were identified from nucleotide sequences previously deposited in NCBI (accession nos. KF683599-KF683609; Raymond 2014).
The number of bacterial DUF3494 sequences in GenBank was determined with TBLASTN using a basket of 59 DUF3494 sequences from bacteria, algae and fungi as queries, yielding about 20,000 hits with E values < 1e-15. The hits were sorted by E value and duplicates with higher E values were removed with Microsoft Excel.
IBP activity
Ice-binding activity was estimated by observing irregularities in the surface of a growing ice single crystal (a perfect crystal) submerged in cell-free culture supernatant from cells grown at approximately 2°C as described previously (Raymond and Fritsen 2001). In the case of Chlamydomonas sp. ICE-L, freeze-dried spent culture medium was kindly provided by Chenlin Liu (First Institute of Oceanography, China).
3D structure prediction
The structure of a Chlamy-ICE DUF3494 domain (the first domain in IBP-4) was predicted with Swiss Model (Arnold et al. 2006; http://swissmodel.expasy.org). The 3D structure of the DUF3494 domain of the IBP of Flavobacterium frigoris PS1 (Protein Database accession 4nu3.1.A; Do et al. 2014) was selected as the template because of its high amino acid sequence identity (33.3% identity and 50.5% similarity). The model was viewed with Jmol (http://jmol.sourceforge.net/) using a script file that colored polar side chains on the putative ice-binding side blue.
Results
The 18S ribosomal RNA gene of Chlamydomonas sp. ICE-MDV (Chlamy-ICE) was assembled from reads and deposited in GenBank (acc. no. KY314801). The sequence is 99.6% identical to that reported for Chlamydomonas sp. ICE-L (acc. no.AY31083) collected from floating ice near Prydz Bay, Antarctica (Liu and Huang 2015). Liu et al. (2016) also reported the nucleotide sequence for the helicase CiRH5 gene (acc. no. KP718765). We assembled the sequence for the same gene in our isolate and found that it was virtually identical (1730/1731 nts), so the two isolates appear to be essentially the same species.
Cell-free medium from a Chlamy-ICE culture had ice-binding activity (Fig. 1A), indicating that the cells were producing IBPs and secreting them into the medium. A sample of freeze-dried powder prepared from culture medium of the polar marine alga Chlamydomonas sp. ICE-L also had ice-binding activity (Fig. 1B). IBPs produced by bacteria in the culture (see below) may have contributed to the IBP activity, but the contribution is thought to be negligible based on the absence of visible bacteria in microscopic observations of the algal cells.
Fig. 1.
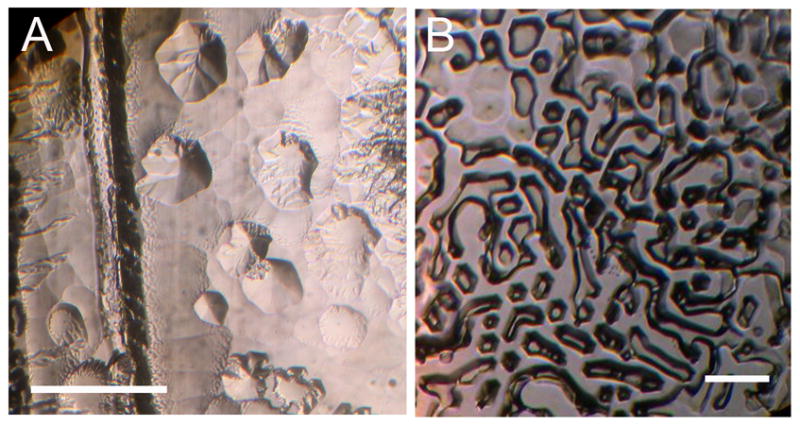
Ice-binding activity of cell-free culture medium of two Antarctic strains of Chlamydomonas sp. ICE. The photos show pitting on the basal plane of a submerged ice crystal caused by binding of ice-binding proteins. (A) Chlamydomonas sp. ICE-MDV from Lake Bonney. (B). Chlamydomonas sp. ICE from Prydz Bay, kindly provided by Chenlin Liu. The latter was freeze-dried for shipment and re-dissolved in water. Scale bars, 500 μm.
Databases made from DNA and RNA contigs were searched for homologs of known IBPs, antifreeze proteins, and ice-nucleating proteins. Many hits were obtained and all corresponded to DUF3494-type (type I) IBPs. Contigs corresponding to over 50 unique but partial IBPs (with introns) were assembled. Of the approximately 30 IBP transcripts that were assembled, none deviated from the order of exons in the corresponding genomic DNA, indicating no evidence for splice variants. Eleven complete genes, with one, two or three DUF3494 domains, were assembled and deposited in GenBank (Table 1). Three additional complete IBP genes (not shown) were considered pseudogenes because of the presence of single nucleotide deletions or an internal stop codon. Liu et al. (Liu et al. 2016) also reported finding several IBP-like transcripts in Chlamydomonas sp. ICE-L. These sequences, provided to us by C. Liu, were virtually identical to the sequences that we obtained. An example of the domain and gene structure of an IBP with two DUF3494 domains (IBP-4) is given in Figure 2. Each of the DUF3494 domains in each of the isoforms has two GT/AG-type introns at the same locations. All 11 IBPs, like all other type 1 IBPs in other microorganisms, had variations of an approximately 20-amino acid N-terminal signal peptide that is typically cleaved when the protein is secreted from the cell. In a phylogenetic tree of representative algal and bacterial DUF3494 domains that have been confirmed to have IBP activity (Fig. S1 in the Supporting Information), the eleven IBP isoforms clustered together with a bootstrap value of 97%, suggesting that they were derived from a single gene. In BLASTP searches, the closest matches to the 11 complete IBPs (with E values 9 to 57 orders of magnitude smaller than the E values of matches to other chlorophyte IBPs) were prokaryotic proteins (Table 1).
Table 1.
Fully assembled IBP genes from Chlamy-ICE, their corresponding number of transcripts and closest matches in BLASTP searches.
| IBP no. | No. DUF3494 domains | No. exons | No. Tran-scripts | Accession No. | Closest match of DUF3494 | |
|---|---|---|---|---|---|---|
| Genus1 | E value | |||||
| 1 | 3 | 7 | 19543 | KY314784 | Methanoregula | 4.0E-73 |
| 2 | 2 | 5 | 1870 | KY314785 | Methanoregula | 4.0E-44 |
| 3 | 1 | 7 | 808 | KY314786 | Nocardioides | 2.0E-34 |
| 4 | 2 | 5 | 35424 | KY314787 | Methanoregula | 2.0E-54 |
| 5 | 3 | 7 | 4575 | KY314788 | Methanoregula | 1.0E-74 |
| 6 | 1 | 3 | 1851 | KY314789 | Peribacter | 6.0E-43 |
| 7 | 2 | 5 | 1117 | KY314790 | Methanoregula | 4.0E-43 |
| 8 | 1 | 3 | 1680 | KY314791 | Peribacter | 2.0E-45 |
| 9 | 2 | 5 | 35 | KY314792 | Methanoregula | 1.0E-45 |
| 10 | 2 | 5 | 15 | KY314793 | Methanoregula | 3.0E-44 |
| 11 | 2 | 5 | 2 | KY314794 | Peribacter | 4.0E-39 |
Methanoregula boonei (Archaea, WP_052291905); Nocardioides dokdonensis FR1436 (bacteria, ANH36626); Candidatus Peribacter riflensis (bacteria, ALM09691).
Fig 2.

Example of domain and exon structures of a Chlamy-ICE IBP (IBP-4). The gene has five exons (shown by white, gray and dark gray backgrounds) and two DUF3494 domains (underlined). Exons of the same background color are similar. Each DUF3494 domain has two exon-exon boundaries. Lower case residues indicate an N-terminal signal peptide. Other isoforms have one and three DUF3494 domains. Locations of exon-exon boundaries are the same in each DUF3494 domain. The exons were identified by comparison of the DNA and cDNA reads.
The relative expression levels of the IBPs at our culture conditions (500 mM NaCl, 8°C) could be estimated from transcript abundances. A large number (74,729) of unique ∼75-nt transcripts (representing 0.036% of the transcripts) matched the exons of the ∼50 DNA sequences to E values <1e-15. About 90% of these reads could be attributed to the above 11 sequences, although only eight of them were appreciably expressed and only two of them (IBPs 1 and 4) accounted for the bulk of the transcripts (Table 1). The three pseudogenes had hardly any transcripts. The remaining transcripts corresponded to IBP isoforms that could not be fully assembled.
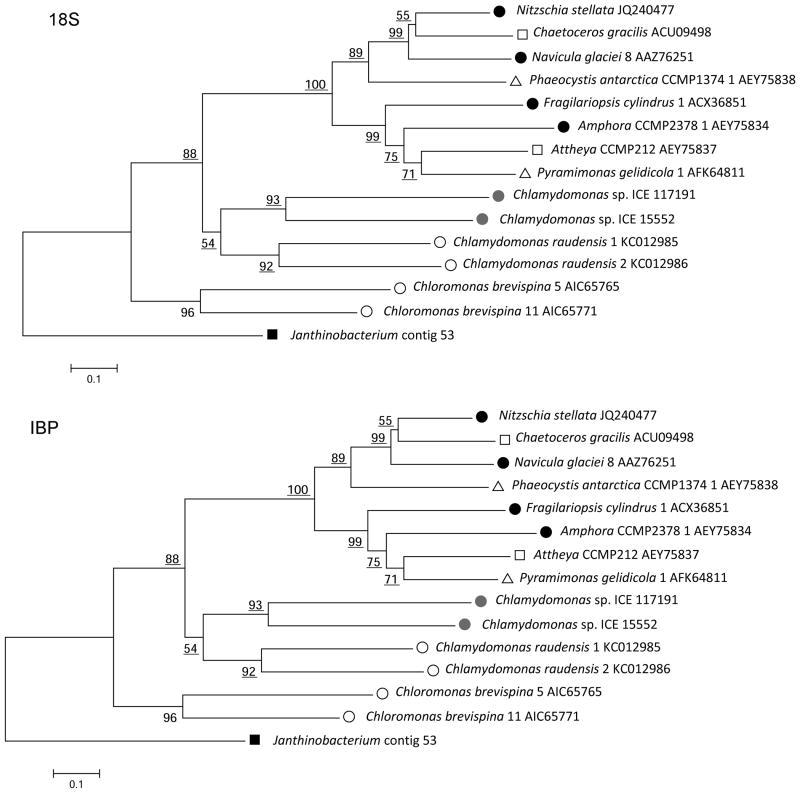
In a comparison of the IBP and 18S ribosomal RNA trees of IBP-producing algae (Fig. 3), the portions of the trees for non-chlorophytes are highly incongruent, indicating that the IBP genes for these algae were probably acquired by HGT, as previously reported (Raymond and Kim 2012). The chlorophytes clustered in the 18S tree as expected, but were split into two divergent groups in the IBP tree. Chlamydomonas sp. CCMP681, shown in the 18S tree, provides further evidence of a mismatch between the trees as it has IBPs that have no similarity to DUF3494-type IBPs (Raymond et al. 2009).
Fig. 3.
Neighbor-Joining phylogenetic trees of IBP-producing algae. (Top) 18S ribosomal RNA nucleotide sequences. (Bottom) Amino acid sequences of DUF3494 domains of the IBPs. Symbols: closed circles, pennate diatoms; open squares, centric diatoms; gray circles, Chlamy-ICE; open circles, other chlorophytes; open triangles, other algae. The trees were rooted on a red alga (top) and a bacterium (bottom) as outgroups. The chlorophytes are closely related in the 18S tree but distantly related in the IBP tree. Similar incongruities are present in the diatoms and other algae. Bootstrap values less than 50 are not shown.
Although the algal cells were originally thought to be axenic (see Methods), many of the longest contigs (some over 200 kb) assembled from the DNA sequence data were clearly of bacterial origin as shown by annotation by MANATEE. Several of the bacterial contigs encoded complete genes with full DUF3494 domains (Table S1 in the Supporting Information), none of which showed strong similarity to the Chlamy-ICE IBPs.
Discussion
Of 16 chlorophyte genomes that have been sequenced and deposited in public databases, genes for known IBPs have been found only in polar and ice-associated species. In fact, IBP genes have been found in all ice-associated algae examined so far. The present finding of as many as 50 IBP isoforms in Chlamy-ICE extend these observations and provides further evidence that IBPs are essential for survival of algae living in icy environments. Similarly, the snow alga Chloromonas brevispina has genes for over 20 IBP isoforms (Raymond 2014), the sea ice diatom Fragilariopsis cylindris has genes for 19 IBP and IBP-like isoforms (Mock et al. 2017) and the marine alga Phaeocystis antarctica appears to have genes for dozens of IBP-like proteins based on a BLASTN search of transcript data in NCBI's Trace Archives (http://www.ncbi.nlm.nih.gov/Traces/home/index.cgi). In contrast to the algae, ice-associated prokaryotes typically have a single IBP gene. There are several potential advantages for the presence of multiple IBP isoforms in the algae. They may provide higher concentrations of secreted IBPs. Because not all the IBPs have secretion signals, they may also have other functions such as anchoring the cell membrane to an ice substrate. This has recently been shown to occur with a non-DUF3494-type IBP (Bar Dolev et al. 2016). Their expressions may also be induced by different ice conditions, such as in lake ice, sub-zero brine pockets or newly forming ice crystals in the water column, providing the algae with a greater phenotypic plasticity than is found in ice-associated prokaryotes. High frequencies of IBP genes were also found relative to some photosynthesis genes in the metatranscriptome of Antarctic sea ice (Uhlig et al. 2015) and relative to a housekeeping gene (GAPDH) in a metagenome of epiphytic bacteria on an Antarctic moss (Raymond 2016).
The Chlamy-ICE IBPs all have DUF3494 domains, which is the type (type I) that has been found most widely so far in microorganisms as well as in sea ice (Uhlig et al. 2015). IBPs with two DUF3494 domains have previously been reported in bacteria (Raymond et al. 2008) and algae (Raymond and Morgan-Kiss 2013), but this is the first report of IBPs (IBPs 1 and 5) with three DUF3494 domains. All the fully assembled IBP genes encode N-terminal signal peptides, suggesting that their activity is on extracellular ice.
The IBP gene sequences of Chlamy-ICE are far more closely related to prokaryotic genes than they are to other algal genes. Furthermore, the position of the Chlamy-ICE IBPs in the IBP tree relative to other chlorophyte IBPs does not match the position of Chlamy-ICE in the 18S tree relative to other chlorophytes (Fig. 3). Together, these observations strongly suggest that Chlamy-ICE acquired the IBPs by horizontal gene transfer, probably from a prokaryote. All of the Chlamy-ICE IBP isoforms had two introns in the same locations in each DUF3494 domain, suggesting that the different isoforms were created by gene duplication events after the creation of these introns in the originally acquired gene. The conservation of exon-intron junctions, together with the similarity of the different Chlamy-ICE isoforms (Fig. S1) suggest that the HGT occurred in a single event.
Similar HGT events are thought to be responsible for the acquisitions of IBP genes in other algae (Raymond and Kim 2012, Raymond and Morgan-Kiss 2013, Raymond 2014). The unrelatedness of the IBPs in different algal species, strongly suggest that the HGT events occurred independently in each species. Consistent with this idea is the finding that the intron locations in the DUF3494 domains of the IBPs of C. brevispina and Chlamydomonas sp. UWO241 (J. Raymond unpublished data; see methods) were unrelated to those in Chlamy-ICE.
Potential donors of IBP genes are legion: GenBank alone (representing a tiny fraction of all bacteria) has over 900 unique bacterial proteins that match existing DUF3494 sequences to E values <1e-15. In addition to the IBPs, there is evidence of horizontal transfer of other genes in the closely related Chlamydomonas sp. ICE-L, including genes involved in PUFA synthesis, molecular chaperone proteins and cell membrane transport proteins, all of which have high similarity to genes in Antarctic bacteria (Liu et al. 2016).
The RNA-seq data indicate that just a few of the dozens of the Chlamy-ICE IBP genes were responsible for most of the IBP transcripts under our culture conditions (motile cells, absence of ice, 8°C), conditions which presented no danger of freezing damage. This suggests that the alga constitutively produces a low level of IBPs even in the absence of freezing temperatures. The IBP activity of the culture medium (Fig. 1A) took longer to develop than it did with other chlorophytes (J. Raymond unpublished data). In a more stressful environment, such as in porous lake ice or sub-zero brine pockets, the cells might express additional IBP isoforms and express them more strongly.
The structure of the first DUF3494 domain of IBP-4 was predicted from the known structure of the IBP from the sea ice bacterium Flavobacterium frigoris (Fig. S2, A and B in the Supporting Information). In the model, all of the hydrophilic side chains on the presumed ice-binding side of the molecule (shown in blue) form three ordered rows, as was found to be the case in other IBPs whose structures were determined by X-ray diffraction (see fig. 4 of Raymond 2014). This similarity and orderliness of the arrays suggests that the model in Figure S2A is largely correct.
Organisms that produce DUF3494 proteins have been found in virtually all environments, including snow, tropical beaches, hot springs, and acid mine drainage. The predicted structures of DUF3494 proteins from different environments as well as a DUF3494 consensus sequence all had similar arrays of hydrophilic residues, suggesting that this arrangement is a characteristic of all DUF3494 proteins (Raymond 2014). The ordered array of hydrophilic residues suggests that these proteins serve to bind to an ordered hydrophilic substrate. It seems likely that this structure first developed in mesophilic microorganisms and was later modified to be specific for ice (Raymond 2014). This would have provided a survival advantage to microorganisms living in icy habitats, and as a result of horizontal gene transfer, allowed many other microorganisms to expand their ranges into these habitats.
Supplementary Material
Fig. S1. Neighbor-Joining phylogenetic tree of amino acid sequences of DUF3494 domains of IBPs of Chlamy-ICE and other representative microorganisms. Symbols: light green, Chlamy-ICE; dark green, other chlorophytes; light blue, pennate diatoms; dark blue, centric diatoms; olive, other algae; black, fungus; red; bacteria; gray, archaeon. The tree is rooted on a bacterium as the outgroup. Bootstrap values less than 50 are not shown.
Fig. S2. Predicted structure of first DUF3494 domain of Chlamy-ICE IBP-4. The structure is based on the structure of the IBP of Flavobacterium frigoris (Do et al. 2014). The domain has the shape of a triangular prism in which the ice-binding face forms one of the three sides. (A) 3D Jmol views of the ice-binding face (left) and along the axis of the prism (right). Ordered rows of hydrogen-bonding side chains on the ice-binding face are shown in dark blue. Color codes: red, α-helices; purple, 310-helices; yellow, β-sheets; light blue, β-turns. (B) Alignment of ice-binding side chains (blue residues) and β-sheets (yellow background) on the ice-binding face in (A). In the predicted structure, the N-terminus (denoted by n-) wraps around to form the bottom row. Red residues indicate the alpha helix.
Table S1. DUF3494 genes in bacteria associated with Chlamy-ICE.
Acknowledgments
Collection, isolation and culture of the algae were supported by National Science Foundation grant PLR-1056396 to RM-K. We thank Amber Teufel for assistance in the isolation of Chlamydomonas sp. ICE-MDV. We thank the School of Life Sciences, University of Nevada, Las Vegas for providing laboratory facilities for conducting this work, Kristina Kruse and Craig Osborn (University of Nevada Reno) for sequencing analyses and Suvarna Nadendla (University of Maryland) for annotation analysis. Sequencing and protein analyses at the University of Nevada were supported by grant #P20GM103440 from the National Institute of General Medical Sciences. Additional funding for this work was provided by JR.
List of abbreviations
- IBP
ice-binding protein
- Chlamy-ICE
Chlamydomonas-ICE-MDV
- HGT
horizontal gene transfer
- DUF
domain of unknown function
- UNLV
University of Nevada, Las Vegas
- GADPH
glyceraldehyde 3-phosphate dehydrogenase
- MDV
McMurdo Dry Valleys
Footnotes
The authors have no conflict of interest to declare
Contributor Information
James A. Raymond, School of Life Sciences, University of Nevada, Las Vegas, Las Vegas, Nevada 89154 USA.
Rachael Morgan-Kiss, Department of Microbiology, Miami University, Oxford, Ohio 45056 USA.
References
- Arnold K, Bordoli L, Kopp J, Schwede T. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201. doi: 10.1093/bioinformatics/bti770. [DOI] [PubMed] [Google Scholar]
- Bar Dolev M, Bernheim R, Guo SQ, Davies PL, Braslavsky I. Putting life on ice: bacteria that bind to frozen water. J R Soc Interface. 2016;13 doi: 10.1098/rsif.2016.0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer-Giraldi M, Weikusat I, Besir H, Dieckmann G. Characterization of an antifreeze protein from the polar diatom Fragilariopsis cylindrus and its relevance in sea ice. Cryobiology. 2011;63:210–19. doi: 10.1016/j.cryobiol.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Bendtsen JD, Nielsen H, von Heijne G, Brunak S. Improved prediction of signal peptides: SignalP 3.0. J Molec Biol. 2004;340:783–95. doi: 10.1016/j.jmb.2004.05.028. [DOI] [PubMed] [Google Scholar]
- Devries AL. Antifreeze peptides and glycopeptides in cold-water fishes. Ann Rev Physiol. 1983;45:245–60. doi: 10.1146/annurev.ph.45.030183.001333. [DOI] [PubMed] [Google Scholar]
- Do H, Kim SJ, Kim HJ, Lee JH. Structure-based characterization and antifreeze properties of a hyperactive ice-binding protein from the Antarctic bacterium Flavobacterium frigoris PS1. Acta Cryst D. 2014;70:1061–73. doi: 10.1107/S1399004714000996. [DOI] [PubMed] [Google Scholar]
- Hall TA. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp Ser. 1999;41:95–98. [Google Scholar]
- Jung W, Campbell RL, Gwak Y, Kim JI, Davies PL, Jin E. New cysteine-rich ice-binding protein secreted from Antarctic microalga, Chloromonas sp. PLoS One. 2016;11:e0154056. doi: 10.1371/journal.pone.0154056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo H, Hanada Y, Sugimoto H, Hoshino T, Garnham CP, Davies PL, Tsuda S. Ice-binding site of snow mold fungus antifreeze protein deviates from structural regularity and high conservation. Proc Natl Acad Sci USA. 2012;109:9360–65. doi: 10.1073/pnas.1121607109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Park AK, Do H, Park KS, Moh SH, Chi YM, Kim HJ. Structural basis for antifreeze activity of ice-binding protein from arctic yeast. J Biol Chem. 2012;287:11460–68. doi: 10.1074/jbc.M111.331835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Podar M, Morgan-Kiss RM. Ultrastructural and single-cell-level characterization reveals metabolic versatility in a microbial eukaryote community from an ice-covered Antarctic lake. Appl Environ Microbiol. 2016;82:3659–70. doi: 10.1128/AEM.00478-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CL, Huang XH. Transcriptome-wide analysis of DEAD-box RNA helicase gene family in an Antarctic psychrophilic alga Chlamydomonas sp ICE-L. Extremophiles. 2015;19:921–31. doi: 10.1007/s00792-015-0768-8. [DOI] [PubMed] [Google Scholar]
- Liu CL, Wang XL, Wang XN, Sun CJ. Acclimation of Antarctic Chlamydomonas to the sea-ice environment: a transcriptomic analysis. Extremophiles. 2016;20:437–50. doi: 10.1007/s00792-016-0834-x. [DOI] [PubMed] [Google Scholar]
- Mock T, Otillar RP, Strauss J, McMullan M, Paajanen P, Schmutz J, Salamov A, et al. Extensive genetic diversity and differential bi-allelic expression in a Southern Ocean diatom. Nature. 2017;541:536–40. [Google Scholar]
- Possmayer M, Gupta RK, Szyszka-Mroz B, Maxwell DP, Lachance MA, Huner NPA, Smith DR. Resolving the phylogenetic relationship between Chlamydomonas sp UWO 241 and Chlamydomonas raudensis SAG 49.72 (Chlorophyceae) with nuclear and plastid DNA sequences. J Phycol. 2016;52:305–10. doi: 10.1111/jpy.12383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JA. The ice-binding proteins of a snow alga, Chloromonas brevispina: probable acquisition by horizontal gene transfer. Extremophiles. 2014;18:987–94. doi: 10.1007/s00792-014-0668-3. [DOI] [PubMed] [Google Scholar]
- Raymond JA. Dependence on epiphytic bacteria for freezing protection in an Antarctic moss, Bryum argenteum. Environ Microbiol Rep. 2016;8:14–19. doi: 10.1111/1758-2229.12337. [DOI] [PubMed] [Google Scholar]
- Raymond JA, Christner BC, Schuster SC. A bacterial ice-binding protein from the Vostok ice core. Extremophiles. 2008;12:713–17. doi: 10.1007/s00792-008-0178-2. [DOI] [PubMed] [Google Scholar]
- Raymond JA, Fritsen CH. Semipurification and ice recrystallization inhibition activity of ice-active substances associated with Antarctic photosynthetic organisms. Cryobiology. 2001;43:63–70. doi: 10.1006/cryo.2001.2341. [DOI] [PubMed] [Google Scholar]
- Raymond JA, Janech MG, Fritsen CH. Novel ice-binding proteins from a psychrophilic Antarctic alga (Chlamydomonadaceae, Chlorophyceae) J Phycol. 2009;45:130–36. doi: 10.1111/j.1529-8817.2008.00623.x. [DOI] [PubMed] [Google Scholar]
- Raymond JA, Kim HJ. Possible role of horizontal gene transfer in the colonization of sea ice by algae. PLoS One. 2012;7:e35968. doi: 10.1371/journal.pone.0035968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond JA, Morgan-Kiss R. Separate origins of ice-binding proteins in antarctic Chlamydomonas species. PLoS One. 2013;8:e59186. doi: 10.1371/journal.pone.0059186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorhannus U. Evolution of antifreeze protein genes in the diatom genus Fragilariopsis: evidence for horizontal gene transfer, gene duplication and episodic diversifying selection. Evol Bioinform. 2011;7:279–89. doi: 10.4137/EBO.S8321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular Evolutionary Genetics Analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molec Biol Evol. 2011;28:2731–39. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig C, Kilpert F, Frickenhaus S, Kegel JU, Krell A, Mock T, Valentin K, Beszteri B. In situ expression of eukaryotic ice-binding proteins in microbial communities of Arctic and Antarctic sea ice. ISME J. 2015;9:2537–40. doi: 10.1038/ismej.2015.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Neighbor-Joining phylogenetic tree of amino acid sequences of DUF3494 domains of IBPs of Chlamy-ICE and other representative microorganisms. Symbols: light green, Chlamy-ICE; dark green, other chlorophytes; light blue, pennate diatoms; dark blue, centric diatoms; olive, other algae; black, fungus; red; bacteria; gray, archaeon. The tree is rooted on a bacterium as the outgroup. Bootstrap values less than 50 are not shown.
Fig. S2. Predicted structure of first DUF3494 domain of Chlamy-ICE IBP-4. The structure is based on the structure of the IBP of Flavobacterium frigoris (Do et al. 2014). The domain has the shape of a triangular prism in which the ice-binding face forms one of the three sides. (A) 3D Jmol views of the ice-binding face (left) and along the axis of the prism (right). Ordered rows of hydrogen-bonding side chains on the ice-binding face are shown in dark blue. Color codes: red, α-helices; purple, 310-helices; yellow, β-sheets; light blue, β-turns. (B) Alignment of ice-binding side chains (blue residues) and β-sheets (yellow background) on the ice-binding face in (A). In the predicted structure, the N-terminus (denoted by n-) wraps around to form the bottom row. Red residues indicate the alpha helix.
Table S1. DUF3494 genes in bacteria associated with Chlamy-ICE.