Abstract
Background
Reduced cortical thickness is a candidate biological marker of depression, although findings are inconsistent. This could reflect analytic heterogeneity, such as use of region‐wise cortical thickness based on the Freesurfer Desikan–Killiany (DK) atlas or surface‐based morphometry (SBM). The Freesurfer Destrieux (DS) atlas (more, smaller regions) has not been utilized in depression studies. This could also reflect differential gender and age effects.
Methods
Cortical thickness was collected from 170 currently depressed adults and 52 never‐depressed adults. Visually inspected and approved Freesurfer‐generated surfaces were used to extract cortical thickness estimates according to the DK atlas (68 regions) and DS atlas (148 regions) for region‐wise analysis (216 total regions) and for SBM.
Results
Overall, except for small effects in a few regions, the two region‐wise approaches generally failed to discriminate depressed adults from nondepressed adults or current episode severity. Differential effects by age and gender were also rare and small in magnitude. Using SBM, depressed adults showed a significantly thicker cluster in the left supramarginal gyrus than nondepressed adults (P = 0.047) but there were no associations with current episode severity.
Conclusions
Three analytic approaches (i.e., DK atlas, DS atlas, and SBM) converge on the notion that cortical thickness is a relatively weak discriminator of current depression status. Differential age and gender effects do not appear to represent key moderators. Robust associations with demographic factors will likely hinder translation of cortical thickness into a clinically useful biomarker. Hum Brain Mapp, 2017. © 2017 Wiley Periodicals, Inc. Hum Brain Mapp 38:4370–4385, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: cortical thickness, MRI, depression, multisite, adults, biomarker, imaging
INTRODUCTION
Cortical thickness, a component of gray matter volume and index of cell density and health in the cerebral cortex (Rajkowska et al., 1999), has been reported to be reduced in adults with a lifetime history of major depressive disorder (MDD+) relative to adults without a lifetime history of major depressive disorder (MDD−) [Colloby et al., 2011; Jarnum et al., 2011; Koolschijn et al., 2010; Li et al., 2014; Na et al., 2016; Schmaal et al., 2016; Truong et al., 2013; Tu et al., 2012; van Eijndhoven et al., 2013; Wagner et al., 2012]. In addition to helping distinguish subjects with or without MDD for screening or diagnostic purposes, cortical thickness may also represent an etiological factor. For instance, two potent risk factors for MDD—a history of MDD in first‐degree relatives [Ozalay et al., 2016; Papmeyer et al., 2015; Peterson and Weissman, 2011] and heightened trait negative affect [Holmes et al., 2016]—have also been associated with reduced cortical thickness in certain regions. In addition, reduced cortical thickness was found to precede incidence of depression in a sample of 10–15 year olds [Foland‐Ross et al., 2015] and in a young adult sample (mean age 21 years old) [Papmeyer et al., 2015].
Altogether, cortical thickness could represent an important tool for understanding etiology of depression, inferring latent risk for depression, and guiding clinical care (e.g., risk screening, treatment planning, etc.). Results, however, have been somewhat inconsistent across studies. Depression has not been linked to reduced cortical thickness in any one particular region; rather, each report implicates one or a few frontal, temporal, parietal, and occipital areas (Table 1). Yet other studies report cortical thinning in some regions AND cortical thickening in other regions [Fallucca et al., 2011; Peterson et al., 2009; Tu et al., 2012]. For example, regions found to have cortical thinning in the largest study to date from the ENIGMA workgroup (∼1900 adult MDD subjects), such as the medial orbitofrontal cortex [Schmaal et al., 2016], have been previously reported to be thicker in MDD+ compared to MDD− in smaller studies [Qiu et al., 2014]. Furthermore, several other studies reported no difference between MDD+ and MDD− in cortical thickness in any region.
Table 1.
Selected publications investigating cortical thickness in MDD
| Author | Year | Journal | Subjects | Method | Relevant CT findings in MDDs compared to HCs |
|---|---|---|---|---|---|
| Koolschijn | 2010 | Eur Neuropsychopharmacol | 28 elderly MDDs, 38 HCs | SBM | No findings |
| Colloby | 2011 | J Affect Disord | 38 late‐life MDDs, 30 HCs | Freesurfer Region‐wise: DK | No findings |
| Han | 2014 | J Affect Disord | 20 1st ep. MDDs, 22 HCs | Freesurfer SBM | No findings |
| Lan | 2014 | Bipolar Disord | 56 MDDs, 54 HCs | Freesurfer SBM | No findings |
| Phillips | 2015 | Int J Neuropsychopharmacol | 26 treatment‐resistant MDDs, 28 HCs | Freesurfer Region‐wise: DK | No findings |
| Peterson | 2009 | Proc Natl Acad Sci USA | 66 HR children, 65 LR children | Freesurfer SBM | ↓ right lateral cerebral hemisphere in HRs vs LRs |
| Peterson & Weissman | 2011 | Annu Rev Med | 66 HR children, 65 LR children | Freesurfer SBM | ↓ cortical mantle over the lateral convexity of the right hemisphere and the medial surface of the left hemisphere in HRs vs LRs |
| Tu | 2012 | Psychiatry Res | 36 MDDs, 36 HCs | Freesurfer SBM | ↓ left anterior insula, ↓ left lOFC, par opercularis, and caudal & rostral middle frontal gyrus in MDDs with more episodes vs MDDs with less episodes |
| Wagner | 2012 | J Psychiatr Res | 15 HR for suicide MDDs, 15 not‐HR for suicide MDDs, 30 HCs | Freesurfer SBM | ↓ left dlPFC, left vlPFC, and dACC in HR MDDs vs non‐HR MDDs |
| Truong | 2013 | Psychiatry Res | 49 MDDs, 64 HCs | SBM | ↓ dlPFC, precentral and postcentral gyrus, and lingual gyrus in early onset MDDs vs HCs |
| Li | 2014 | J Affect Disord | 24 MDDs, 24 HCs | Freesurfer SBM | ↓ dACC in MDDs vs HCs. |
| Holmes | 2016 | J Neurosci | 1050 young adults without MDD | Freesurfer SBM | ↓ left sgACC and left rACC associated with heightened negative affect |
| Liu | 2015 | Depress Anxiety | 30 med‐naïve MDDs, 41 HCs | Freesurfer SBM | ↓ left lOFC in MDDs vs HCs |
| Foland‐Ross | 2015 | J Abnorm Psychol | 28 adolescent daughters of mothers with MDD (HRs), 36 adolescent LRs | Freesurfer SBM & Region‐wise: DK | ↓ right fusiform gyrus in HRs vs LRs |
| Na | 2016 | Sci Rep | 65 recurrent MDDs, 65 HCs | Freesurfer SBM | ↓ right mOFC, lingual gyrus, lateral occipital and ↓left lOFC, pars triangularis, lingual gyrus in MDDs vs HCs |
| Schmaal | 2016 | Mol Psychiatry | 1902 adult MDDs, 7658 HC; 213 adolescent MDDs, 294 HCs | Freesurfer Region‐wise: DK | ↓ mOFC, fusiform gyrus, rACC, PCC, insula, and temporal lobe in adult MDDs vs HCs. ↓ left middle temporal gyrus, right inferior temporal gyrus, and right cACC in adult MDDs vs HCs. No findings in adolescent MDDs vs adolescent HCs |
| Jaworska | 2014 | Biomed Res Int | 36 MDDs, 18 HCs | Freesurfer Region‐wise: DK | ↑ frontal pole in MDDs vs HCs |
| Qiu | 2014 | Transl Psychiatry | 46 1st ep, med‐naïve MDDs, 46 HCs | Freesurfer SBM | ↑ right mOFC, pars opercularis, rostral middle frontal gyrus, and supramarginal gyrus in MDDs vs HCs |
| Reynolds | 2014 | BMC Psychiatry | 30 adolescent MDDs, 16 adolescent HCs | Freesurfer Region‐wise: DK | ↑ right and left rostral middle frontal gyrus and left cACC in adolescent MDDs vs adolescent HCs |
| Yang | 2015 | Psychiatry Res | 27 1st‐ep MDDs, 27 HCs | Freesurfer SBM | ↑ right OFC and left inferior parietal gyrus in MDDs vs HCs |
| Szymkowicz | 2016 | Int J Geriatr Psychiatry | 43 elderly MDDs | Freesurfer SBM & Region‐wise: DK | ↑ right isthmus cingulate and left precuneus associated with depressive symptom severity |
| Fonseka | 2016 | BMC Psychiatry | 13 young adult MDDs, 14 HCs | Freesurfer Region‐wise: DK | ↑ left pars opercularis in MDDs vs HCs |
| van Eijndhoven | 2013 | Am J Psychiatry | 20 med‐naïve, 1st ep. MDDs, 31 HCs | Freesurfer SBM | ↓ left mOFC. ↑ left and right temporal pole, left cACC, and left PCC in MDDs vs HCs |
| Fallucca | 2011 | Arch Gen Psychiatry | 24 pediatric MDDs, 24 pediatric OCDs, 30 HCs | Freesurfer SBM | ↓ right pericalcarine, right postcentral, right superior parietal gyrus, and left supramarginal gyrus. ↑ left and right temporal pole in pediatric MDDs vs pediatric HCs |
| Foland‐Ross | 2015 | Int J Dev Neurosci | 33 adolescents without MDD | Freesurfer Region‐wise: DK | CT correctly predicted onset of MDD (accuracy of 70%) in adolescents. Right mOFC, right precentral, left ACC, and bilateral insula contributed most strongly |
| Papmeyer | 2015 | Biol Psychiatry | 111 HR young adults, 93 HCs, 20 HRs developed MDD | Freesurfer Region‐wise: DK | ↓ right parahippocampal and right fusiform gyrus in HRs vs HCs. ↓ parahippocampi and ↑ left inferior frontal gyrus in MDDs vs HRs who did not develop MDD. ↑ left inferior frontal and left precentral gyrus in MDDs vs HRs who did not develop MDD and vs HCs |
| Ozalay | 2016 | Psychiatry Res | 24 female MDDs & daughters (HRs), 24 HC females & daughters (LRs) | Freesurfer SBM | ↓ right supramarginal gyrus, caudal middle frontal gyrus, and lOFC in female MDDs vs female HCs. ↑left superior temporal gyrus in female MDDs vs female HCs. ↓right inferior parietal gyrus, right superior frontal gyrus, and left ventral insula, left superior posterior temporal, temporal pole, fusiform gyrus, and dACC in HRs vs LRs. ↑right precentral gyrus in HRs vs LRs. |
CT: cortical thickness; SBM: surface‐based morphometry; MDDs: persons with major depressive disorder; HCs: healthy controls; HRs: persons at high risk for MDD; LRs: persons at low risk for MDD; DK: Desikan–Killiany Atlas; DS: Destrieux atlas; lOFC, mOFC, OFC: lateral‐, medial‐, and orbitofrontal cortex; dlPFC, vlPFC: dorsolateral‐, ventrolateral‐prefrontal cortex; dACC, cACC, sgACC, rACC: dorsal‐, caudal‐, subgenual‐, rostral‐anterior cingulate cortex; PCC: posterior cingulate cortex.
Summarizing the cortical thickness depression literature is difficult in part because of nonuniform approaches to quantifying cortical thickness. As described in Table 1, all studies that conducted region‐wise analyses, either a priori region of interest (ROIs) or exploratory whole‐brain analyses, defined regions according to the Desikan–Killiany (DK) atlas [Desikan et al., 2006]. The DK atlas is a well‐studied atlas and is standard in FreeSurfer (http://surfer.nmr.mgh.harvard.edu/), the most utilized software package for analyzing cortical thickness data. To our knowledge, the Freesurfer‐based Destrieux (DS) atlas [Destrieux et al., 2010], which offers smaller and more regions relative to the DK atlas, has not been utilized to study depression. The reason for this absence is unclear, as psychometric properties of the DS atlas are comparable to the DK atlas [Iscan et al., 2015]. In any case, ROI analyses assume that depression will be associated with abnormalities that adhere to a priori defined regions, which may or may not correspond to underlying pathophysiology. An alternative strategy used by many studies in the literature is surface‐based morphometry (SBM), which analyzes whole‐brain cortical thickness data unconstrained by a priori structure. It is unclear if these approaches converge or diverge in understanding the link between depression and cortical thickness.
The first goal of this study is to compare 170 subjects with current major depressive disorder (MDD+) and 52 never‐depressed controls on cortical thickness as measured by these two ROI approaches (DK and DS) and by SBM. This is the first report to our knowledge to utilize these three analytic approaches, providing opportunity to describe converging vs diverging links with depression across them. A secondary goal of this study is to identify potential moderators of the link between depression and cortical thickness. Prior studies in this area utilized case–control comparisons that focused on main effects without exploration of potential moderators. In this report, we consider two demographic moderators (age and gender), which show profound links to depression in epidemiological studies of depression. We also examine a clinical feature of depression, namely current episode severity, as it may track cortical thickness.
In this study, we leveraged data collected as part of the Establishing Moderators and Biosignatures of Antidepressant Response for Clinical Care (EMBARC) project, a multisite study of depression and treatment response [Trivedi et al., 2016]. Data were available for 170 subjects with current major depressive disorder (MDD+) and 52 never‐depressed controls (MDD−) assessed at one of the four university centers in Massachusetts, Michigan, New York, and Texas. The design of EMBARC is well suited for investigating the link between cortical thickness and depression based on a relatively large sample size for a clinical imaging study, uniform multisite recording procedures, uniform data processing blind to diagnosis, and thorough clinical characterization of participants using both self‐report and diagnostic interviews. Importantly, the imaging protocol and analytic steps utilized by EMBARC, including manual inspection procedures, have been thoroughly described and investigated elsewhere [Iscan et al., 2015]. As described by Iscan et al. [2015] in a test–retest study of 40 healthy adults, cortical thickness data that is visually inspected and manually approved demonstrates higher reliability (ICC = 0.77 for DS; ICC = 0.81 for DK) compared to when this is skipped (ICC = 0.59 for DS; ICC = 0.62 for DK). Because visual inspection is burdensome and time consuming, many studies are unable to implement this processing step. To our knowledge, this is the largest study to date that uses identical processing conditions and manual inspection procedures to compare Freesurfer‐derived cortical thickness measurements between depressed and nondepressed cohorts.
METHODS AND MATERIALS
Details about the EMBARC study, including ascertainment and randomization, have been reported elsewhere [Trivedi et al., 2016; Webb et al., 2016; Delaparte et al., 2017; Olvet et al., 2016]. All participants were of the age between 18 and 65 years and provided signed consent. Inclusion criteria for MDD+ subjects were to be in a current depressive episode, verified by a semi‐structured clinical interview conducted by a trained interviewer, and to have a clinically significant score of at least 14 on the Quick Inventory of Depressive Symptoms (QIDS‐SR; [Rush et al., 2003]). MDD+ and MDD− subjects were excluded for current pregnancy, lifetime history of psychosis or bipolar disorder, substance dependence within the previous 6 months, substance abuse within the past 2 months, or any factor that would obscure treatment response in the randomization trial (recent treatment for depression involving other medications, somatic treatments, or psychotherapy) or contraindicate use of study medication (i.e., risk of interaction with ongoing medication, clinically significant laboratory results, etc.).
Data Acquisition
Specific details about scanning, processing, and sequence parameters have been published elsewhere [Iscan et al., 2015]. To summarize, T1w images were acquired with 3T MRI scanners at each site. An MPRAGE sequence was used at the University of Texas Southwestern Medical Center (TX: Philips Achieva, 8‐channel (ch) head coil), University of Michigan (UM: Philips Ingenia, 15‐ch), and Massachusetts General Hospital and Stony Brook University (MGH & SBU: Siemens TrioTim, 12‐ch), while an IR‐FSPGR sequence was used at Columbia University Medical Center (CU: GE Signa HDx, 8‐ch). The following MR parameters were consistent across sites: TR (repetition time): 5.9–8.2 ms, TE (echo time): 2.4–4.6 ms, flip angle: 8–12°, slice thickness: 1 mm, field of view: 256 × 256 mm, voxel dimensions: 1 mm isotropic, acquisition matrix: 256 × 256 or 256 × 243, acceleration factor: 2, and 174–178 sagittal slices. Acquisition times ranged from 4.4 to 5.5 min.
Processing
Cortical thickness was computed for 34 bilateral Desikan‐Killiany (DK) atlas regions [Desikan et al., 2006] and 74 bilateral Destrieux (DS) atlas regions [Destrieux et al., 2010] using FreeSurfer 5.3.0's standard, automated cortical reconstruction pipeline (http://surfer.nmr.mgh.harvard.edu/) on a Linux‐based computing cluster. The pipeline's subroutines have been described in previous publications [Iscan et al., 2015]; the processing steps include skull‐stripping [Segonne et al., 2004], Talairach transformation, subcortical grey/white matter segmentation [Fischl et al., 2002], intensity normalization [Sled et al., 1998], grey/white matter tessellation, topology correction [Fischl et al., 2001; Segonne et al., 2007], and intensity gradient‐based surface deformation to generate grey/white and grey/cerebrospinal fluid surface models [Dale et al., 1999; Fischl et al., 2001; Segonne et al., 2007]. The resulting surface models were then inflated and registered to a spherical surface atlas, allowing parcellation of cortical regions of interest [Fischl et al., 1999a, 1999b, 2004]. Finally, regional cortical thicknesses were computed by taking the mean of the white‐pial distance at all vertices within each parcellated region [Fischl and Dale, 2000]. The surface models (used to calculate cortical thickness) then underwent an empirical, systematic inspection process [Iscan et al., 2015] in which a trained technician carefully inspected 2D sections of the pial and white surface models overlaid on the T1w image for fidelity to visible tissue class boundaries. Cases where inaccurate tissue delineation persisted for ≥6 consecutive coronal and axial slices were deemed inaccurate and thus disqualified from further analyses. Of 293 eligible cases inspected, 222 (76%) passed inspection. All technicians were blinded to subject diagnoses.
Statistical Analysis
Region‐wise analysis: The region‐wise cortical thickness estimates from the DS and DK atlases were analyzed in R (R Development Core Team, Vienna, Austria. http://www.R-project.org). Bivariate associations were described using Pearson correlations. Group differences (i.e., MDD+ vs MDD−) were tested using hierarchical linear models. First, extraneous factors were modeled as covariates in Step 1: age and age2 [Salat et al., 2004; Sowell et al., 2007], gender [Luders et al., 2006; Savic and Arver, 2014], education [Kim et al., 2015], and recording site [Iscan et al., 2015]. Then, group status (MDD+ vs MDD−) was added in Step 2 to determine the amount of variance associated with diagnosis over and above Step 1. Next, the interactions for diagnosis by age (Step 3a) and diagnosis by gender (Step 3b) were added to determine the amount of variance accounted for over and above Step 2. Analysis of current depression severity (QIDS score) excluded controls, and one depressed case for which QIDS score was not available (n = 169).
In all region‐wise analyses, the magnitude of effect (i.e., change in r 2 from Step 1 to Step 2, change in r 2 from Step 2 to Step 3a/3b) and P value level were reported. Pearson's r were reported for the association between depression severity and cortical thickness to convey the direction of the association. When interpreting the results, we also considered the number of significant effects relative to number of tests. We note that r 2 is easily converted to other effect size metrics for comparison with other studies or use in meta‐analysis (Rosenthal, 1994).
SBM analysis: All SBM analyses were performed in Freesurfer 5.3.0. The approved cortical thickness maps for each subject were first registered to a common spherical atlas [Fischl et al., 1999b] and smoothed with a 10 mm Gaussian kernel. A general linear model (GLM) was used to examine the vertex‐wise differences in cortical thickness: (1) between the MDD+ and MDD− groups and (2) associated with QIDS score, controlling for age, age2, gender, education, and site. Right and left hemispheres were examined separately. A Monte‐Carlo Null‐Z simulation cluster analysis with 10,000 iterations and cluster‐forming threshold of P < 0.001 was used to correct for multiple comparisons and is explained in detail elsewhere [Wagner et al., 2012]. In short, the family‐wise error significance threshold was set at P < 0.05 and through a combination of probability and cluster‐size thresholding, cluster‐wise probability (CWP) P values are obtained for resulting clusters. This CWP result represents the overall alpha significance level for the cluster. The Monte‐Carlo simulation and clustering is based on the AlphaSim algorithm [Ward, 2000].
RESULTS
Demographics: Table 2 presents the sample characteristics by imaging site. The sites were similar in proportion of MDD+ vs MDD−, course of depression, gender, age, education, QIDS score, and total brain volume. There was a significant site effect for number of discreet episodes of depression reported by MDD+ subjects (log10 transformed due to skew; winsorized to 20 as maximum due to skew). Post hoc comparisons revealed that MDD+ subjects at Michigan reported more discrete episodes of depression than MDD+ subjects at Columbia (P = 0.006) and Harvard (P = 0.04). The MDD+ subjects did not differ by site in lifetime rates of Panic Disorder, Obsessive Compulsive Disorder, Specific Phobia, Social Phobia, Anorexia Nervosa, Bulimia Nervosa, or Any Illicit Substance Use Disorder (a composite category that excluded nicotine and alcohol). MDD+ subjects at Michigan were more likely to meet criteria for Post‐traumatic Stress Disorder and Alcohol Use Disorder than MDD+ subjects at other sites.
Table 2.
Descriptive statistics by imaging site
| Columbia | Harvard | Michigan | UTSW | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MDD+ | MDD− | MDD+ | MDD− | MDD+ | MDD− | MDD+ | MDD− | ||
| n | n | n | n | n | n | N | n | Statistic | |
| Group | 44 | 12 | 34 | 17 | 33 | 9 | 59 | 14 | χ site (3) = 3.74, P = 0.29 |
| Course | χ site (3) = 6.18, P = 0.10 | ||||||||
| Recurrent | 36 | – | 26 | – | 32 | – | 51 | – | |
| Single episode | 8 | – | 8 | – | 1 | – | 8 | – | |
| Gender | χ site (3) = 2.59, P = 0.47 | ||||||||
| M | 21 | 4 | 16 | 8 | 11 | 3 | 20 | 8 | |
| F | 23 | 8 | 18 | 9 | 22 | 6 | 39 | 6 | |
| Education level | χ site (3) = 5.78, P = 0.12 | ||||||||
| ≥16 years | 26 | 7 | 15 | 9 | 14 | 5 | 21 | 8 | |
| < 16 years | 16 | 5 | 19 | 8 | 19 | 4 | 38 | 6 | |
| Lifetime psychopathology | |||||||||
| Panic disorder | 9 | 0 | 6 | 0 | 5 | 0 | 7 | 0 | χ site (3) = 1.40, P = 0.71 |
| OCD | 0 | 0 | 2 | 0 | 0 | 0 | 4 | 0 | χ site (3) = 5.20, P = 0.16 |
| Specific phobia | 8 | 0 | 6 | 0 | 11 | 0 | 7 | 0 | χ site (3) = 6.46, P = 0.09 |
| Social phobia | 2 | 0 | 0 | 0 | 4 | 0 | 9 | 0 | χ site (3) = 4.99, P = 0.17 |
| PTSD | 6 | 0 | 1 | 0 | 11 | 0 | 9 | 0 | χ site (3) = 11.97, P = 0.01 |
| Anorexia | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | χ site (3) = 1.39, P = 0.71 |
| Bulimia | 0 | 1 | 3 | 0 | 0 | 0 | 1 | 0 | χ site (3) = 5.51, P = 0.14 |
| Alcohol use disorder | 6 | 0 | 2 | 1 | 10 | 0 | 15 | 0 | χ site (3) = 8.78, P = 0.03 |
| Illicit use disorder | 5 | 1 | 3 | 0 | 6 | 0 | 12 | 0 | χ site (3) = 3.00, P = 0.39 |
|
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
Mean (SD) Range |
||
| Number of episodes^& |
5.32 (6.23) 1–20 |
– |
6.18 (6.31) 1–20 |
– |
9.36 (6.74) 1–20 |
– |
9.14 (7.60) 1–20 |
– |
F site (3,166) = 2.85, P = 0.04, Post Hoc: M > C, M > H |
| Age |
34.45 (10.52) 18–61 |
36.92 (13.23) 18–64 |
35.44 (14.69) 18–60 |
39.24 (18.46) 18–65 |
34.36 (13.11) 18–58 |
43.78 (15.19) 23–62 |
41.37 (11.40) 19–63 |
32.71 (11.75) 20–57 |
F site (3,214) = 0.37, P = 0.77, F DX (1,214) = 0.69, P = 0.41, F int (3,214) = 3.30, P = 0.02 |
| Total brain volume* |
1.15 (0.14) 0.85–1.54 |
1.14 (0.13) 0.99–1.44) |
1.12 (0.10) 0.91–1.41 |
1.12 (0.11) 0.87–1.29 |
1.12 (0.12) 0.92–1.43 |
1.09 (0.15) 0.90–1.28 |
1.11 (0.11) 0.85–1.39 |
1.19 (0.16) 0.97–1.54 |
F site (3,214) = 1.23, P = 0.30, F DX (1,214) = 0.24, P = 0.62, F int (3,214) = 1.82, P = 0.14 |
| Quick inventory of depression symptoms |
19.00 (2.98) 14–24 |
1.50 (1.13) 0–4 |
17.65 (2.89) 14–27 |
1.12 (1.05) 0–3 |
18.67 (3.27) 14–27 |
2.22 (1.99) 0–6 |
17.28 (2.43) 14–24 |
1.14 (1.29) 0–4 |
F site (3, 213) = 2.11, P = 0.10, F DX (1,213) = 1,561, P < 0.01, F int (3,213) = 0.51, P = 0.68 |
Note: ^ = for ANOVA, data was log10 +1 transformed due to positive skew; & = data were windsored to 20 for reporting descriptive statistics. * = 105 and millimeters3 2 participants at Columbia declined to report education.
Freesurfer Desikan–Killiany Atlas: Table 3 presents the proportion of variance in cortical thickness accounted for by age, age2, gender, education, and site. On average, these factors accounted for approximately 23% of individual differences in left hemisphere and right hemisphere cortical thickness. The incremental main effect of depression (Model 2) was weakly related to cortical thickness (all P values > 0.05). The interaction between depression and age (Model 3a; while controlling for main effects) was weakly related to cortical thickness (all P values > 0.05). The interaction between depression and gender (Model 3b; while controlling for main effects) identified 2 significant effects at P ≤ 0.01 (2.94% of 68 interactions; see Supporting Information, Figs. 1 and 2). The largest effect was the left cuneus, which accounted for 2.8% of variance in that region over and above covariates and main effect of depression. In both cases, greater cortical thickness was observed in depressed males compared to depressed females and less cortical thickness was observed in nondepressed males compared to nondepressed females.
Table 3.
Effect of depression on cortical thickness derived from DK atlas
| MDD+ | MDD− | Model 1: Covariates only | Model 2: Covariates + MDD | Model 3a: Model 2 + MDD × Age | Model 3b: Model 2 + MDD × Gender | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | |||||
| Region adapted from atlas | Mean | SD | Mean | SD | Mean | SD | Mean | SD | r 2% | Δr 2% | Δr 2% | Δr 2% | ||||
| Bankssts | 2.51 | 0.17 | 2.60 | 0.18 | 2.52 | 0.18 | 2.59 | 0.18 | 21.06 | 21.47 | 0.04 | 0.02 | 0.02 | 0.00 | 0.27 | 0.25 |
| Caudal anterior cingulate | 2.58 | 0.26 | 2.54 | 0.21 | 2.64 | 0.25 | 2.57 | 0.29 | 9.56 | 10.26 | 0.29 | 0.22 | 0.52 | 0.07 | 0.04 | 1.06 |
| Caudal middle frontal | 2.54 | 0.17 | 2.54 | 0.16 | 2.55 | 0.15 | 2.52 | 0.16 | 25.46 | 24.2 | 0.02 | 0.35 | 0.00 | 0.06 | 0.03 | 0.80 |
| Cuneus | 1.75 | 0.15 | 1.78 | 0.15 | 1.77 | 0.12 | 1.82 | 0.14 | 21.43 | 30.36 | 0.04 | 0.09 | 0.20 | 0.00 | 2.83** | 1.15 |
| Entorhinal | 3.37 | 0.35 | 3.47 | 0.40 | 3.33 | 0.30 | 3.40 | 0.37 | 8.36 | 19.59 | 0.63 | 0.75 | 0.35 | 0.05 | 0.86 | 0.11 |
| Frontal pole | 2.82 | 0.30 | 2.78 | 0.30 | 2.83 | 0.33 | 2.78 | 0.28 | 22.25 | 12.27 | 0.15 | 0.02 | 0.54 | 0.14 | 1.53* | 0.74 |
| Fusiform | 2.72 | 0.16 | 2.70 | 0.18 | 2.72 | 0.19 | 2.72 | 0.16 | 28.2 | 26.23 | 0.03 | 0.03 | 0.35 | 0.29 | 0.04 | 0.04 |
| Inferior parietal | 2.45 | 0.14 | 2.47 | 0.13 | 2.45 | 0.15 | 2.47 | 0.14 | 17.67 | 24.25 | 0.03 | 0.03 | 0.22 | 0.08 | 0.44 | 1.18 |
| Inferior temporal | 2.81 | 0.17 | 2.81 | 0.18 | 2.82 | 0.16 | 2.86 | 0.16 | 17.46 | 16.87 | 0.01 | 0.77 | 0.24 | 0.01 | 0.78 | 0.12 |
| Insula | 3.03 | 0.18 | 2.99 | 0.19 | 3.02 | 0.17 | 3.04 | 0.16 | 22.51 | 27.01 | 0.22 | 0.51 | 0.28 | 0.14 | 0.53 | 0.27 |
| Isthmus cingulate | 2.54 | 0.24 | 2.47 | 0.22 | 2.51 | 0.21 | 2.43 | 0.23 | 17.86 | 15.71 | 0.33 | 0.23 | 0.01 | 0.03 | 0.15 | 0.41 |
| Lateral occipital | 2.14 | 0.14 | 2.20 | 0.15 | 2.13 | 0.13 | 2.20 | 0.15 | 11.48 | 8.38 | 0.24 | 0.07 | 0.20 | 0.03 | 0.40 | 0.47 |
| Lateral orbitofrontal | 2.68 | 0.18 | 2.65 | 0.19 | 2.68 | 0.18 | 2.65 | 0.22 | 25.91 | 25.97 | 0.02 | 0.01 | 0.05 | 0.63 | 0.20 | 0.35 |
| Lingual | 1.96 | 0.16 | 2.01 | 0.16 | 1.98 | 0.15 | 2.03 | 0.12 | 37.13 | 34.01 | 0.01 | 0.00 | 0.00 | 0.07 | 1.41* | 2.43** |
| Medial orbitofrontal | 2.43 | 0.19 | 2.42 | 0.21 | 2.38 | 0.18 | 2.45 | 0.24 | 26.44 | 30.89 | 0.92 | 0.44 | 0.52 | 0.07 | 1.27 | 1.75* |
| Middle temporal | 2.88 | 0.16 | 2.91 | 0.17 | 2.89 | 0.16 | 2.93 | 0.17 | 28.67 | 24.97 | 0.10 | 0.49 | 0.01 | 0.32 | 1.17 | 1.31 |
| Paracentral | 2.30 | 0.17 | 2.32 | 0.19 | 2.34 | 0.14 | 2.32 | 0.17 | 26.02 | 33.72 | 0.22 | 0.29 | 0.07 | 0.32 | 0.12 | 0.16 |
| Parahippocampal | 2.77 | 0.36 | 2.70 | 0.30 | 2.75 | 0.34 | 2.70 | 0.25 | 10.79 | 17.52 | 0.22 | 0.28 | 0.05 | 0.07 | 1.17 | 0.62 |
| Pars opercularis | 2.58 | 0.16 | 2.59 | 0.18 | 2.59 | 0.17 | 2.61 | 0.19 | 31.82 | 25.11 | 0.15 | 0.21 | 0.16 | 0.33 | 0.32 | 0.52 |
| Pars orbitalis | 2.77 | 0.25 | 2.74 | 0.24 | 2.72 | 0.26 | 2.75 | 0.26 | 30.97 | 25.4 | 0.29 | 0.21 | 0.26 | 1.10 | 0.44 | 0.04 |
| Pars triangularis | 2.48 | 0.18 | 2.50 | 0.17 | 2.47 | 0.20 | 2.52 | 0.20 | 36.51 | 30.99 | 0.00 | 0.81 | 0.15 | 0.10 | 0.03 | 0.36 |
| Pericalcarine | 1.50 | 0.16 | 1.52 | 0.16 | 1.51 | 0.16 | 1.54 | 0.14 | 36.04 | 34.35 | 0.33 | 0.09 | 0.00 | 0.00 | 1.38* | 0.63 |
| Postcentral | 2.03 | 0.12 | 2.02 | 0.13 | 2.04 | 0.13 | 2.00 | 0.12 | 18.53 | 13.86 | 0.07 | 0.94 | 0.14 | 0.01 | 0.38 | 0.73 |
| Posterior cingulate | 2.52 | 0.18 | 2.49 | 0.17 | 2.52 | 0.19 | 2.49 | 0.16 | 27.30 | 23.20 | 0.01 | 0.03 | 0.17 | 0.12 | 0.38 | 0.43 |
| Precentral | 2.51 | 0.15 | 2.48 | 0.14 | 2.50 | 0.15 | 2.49 | 0.14 | 32.00 | 24.94 | 0.38 | 0.02 | 0.65 | 0.03 | 0.15 | 1.01 |
| Precuneus | 2.34 | 0.15 | 2.36 | 0.15 | 2.34 | 0.14 | 2.36 | 0.14 | 23.26 | 23.44 | 0.00 | 0.08 | 0.15 | 0.00 | 0.37 | 0.84 |
| Rostral anterior cingulate | 2.83 | 0.22 | 2.81 | 0.22 | 2.89 | 0.25 | 2.85 | 0.28 | 13.69 | 17.31 | 1.02 | 0.45 | 0.16 | 1.47 | 2.14* | 0.45 |
| Rostral middle frontal | 2.38 | 0.17 | 2.37 | 0.16 | 2.38 | 0.16 | 2.36 | 0.17 | 34.76 | 35.68 | 0.06 | 0.07 | 0.40 | 0.24 | 0.20 | 0.27 |
| Superior frontal | 2.73 | 0.16 | 2.72 | 0.16 | 2.71 | 0.16 | 2.71 | 0.18 | 36.92 | 37.9 | 0.21 | 0.00 | 0.01 | 0.46 | 0.31 | 0.20 |
| Superior parietal | 2.14 | 0.13 | 2.14 | 0.14 | 2.14 | 0.12 | 2.15 | 0.12 | 16.1 | 12.91 | 0.06 | 0.01 | 0.20 | 0.16 | 0.53 | 0.08 |
| Superior temporal | 2.79 | 0.16 | 2.82 | 0.18 | 2.79 | 0.18 | 2.83 | 0.16 | 26.9 | 36.09 | 0.00 | 0.02 | 0.89 | 0.01 | 1.46* | 0.21 |
| Supramarginal | 2.55 | 0.14 | 2.56 | 0.15 | 2.57 | 0.15 | 2.56 | 0.14 | 24.61 | 19.55 | 0.29 | 0.00 | 0.09 | 0.01 | 0.77 | 0.11 |
| Temporal pole | 3.68 | 0.32 | 3.78 | 0.33 | 3.64 | 0.31 | 3.73 | 0.31 | 4.92 | 2.60 | 0.34 | 0.46 | 0.01 | 0.01 | 1.29 | 0.39 |
| Transverse temporal | 2.31 | 0.23 | 2.35 | 0.24 | 2.28 | 0.21 | 2.33 | 0.25 | 15.46 | 14.92 | 0.87 | 0.33 | 0.17 | 0.35 | 2.07* | 1.74* |
| Mean | 2.54 | 0.19 | 2.55 | 0.19 | 2.54 | 0.19 | 2.55 | 0.19 | 22.95 | 22.70 | 0.23 | 0.24 | 0.21 | 0.20 | 0.75 | 0.62 |
Model 1 presents total r squared for a model including age, age2 gender, education, and recording site. Model 2 presents the delta r‐squared when adding a group term (MDD+ vs MDD−). Model 3a and 3b present the increment in r 2 from the interaction term. *P < 0.05, **P < 0.01. Cortical thickness is expressed in millimeters.
Among depressed cases only, covariates accounted for approximately 22% of individual differences in left hemisphere and right hemisphere cortical thickness on average (Supporting Information, Table I). Current depression severity (Model 2) was negatively associated with cortical thickness in the right middle temporal gyrus at P < 0.01 (1.47% of 68 tests). This effect accounted for 4.28% of variance in that region over and above covariates. The interaction between depression severity and age (Model 3a) was significant in the left bankssts at P < 0.01 (1.47% of 68 interactions), which accounted for 3.53% of variance in that region over covariates and main effect of depression severity (Supporting Information, Fig. 3). As shown in Supporting Information, Figure 3, higher QIDS scores were associated with less cortical thickness in this region at older ages. The interaction between depression severity and gender (Model 3b) was weakly related to cortical thickness (all P values > 0.01).
Freesurfer Destrieux Atlas: Table 4 presents the proportion of variance in cortical thickness accounted for by age, age2, gender, and site. On average, these factors accounted for approximately 19% (left hemisphere) and 20% (right hemisphere) of individual differences in cortical thickness. The incremental main effect of depression (Model 2) was weakly related to cortical thickness (all P values > 0.01). One interaction between depression and age (Model 3a) identified the left middle occipital gyrus as significant at P < 0.01 (0.06% of 148 interactions; Supporting Information, Fig. 4). In this region, cortical thinning with age appears attenuated in currently depressed compared to never depressed adults. The interaction between depression and gender (Model 3b) identified 4 significant effects at P < 0.01 (2.7% of 148 interactions; Supporting Information, Figs. 5–8). The largest magnitude of effect was in the right temporal transverse sulcus, which accounted for 3.7% of variance in cortical thickness in that region. As shown in Supporting Information, Figure 8, never‐depressed males exhibit less cortical thickness in this region than never‐depressed females, whereas similar cortical thickness was observed between currently depressed males and females.
Table 4.
Effect of depression on cortical thickness derived from DS Atlas
| MDD+ | MDD− |
Model 1: Covariates only |
Model 2 Adds MDD |
Model 3a Adds Age × MDD |
Model 3b Adds Gender × MDD |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | R | L | R | L | R | L | R | L | R | L | R | |||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | r 2% | Δr 2% | Δr 2% | Δr 2% | |||||
| Ins lg S cent ins | 3.20 | 0.25 | 3.36 | 0.29 | 3.22 | 0.29 | 3.31 | 0.34 | 11.16 | 17.70 | 0.28 | 0.19 | 0.03 | 0.61 | 0.53 | 0.19 |
| S Cingul Ant | 2.64 | 0.18 | 2.61 | 0.24 | 2.63 | 0.20 | 2.61 | 0.21 | 19.34 | 31.51 | 0.10 | 0.14 | 0.02 | 0.47 | 0.44 | 1.22 |
| S Cingul MidAnt | 2.65 | 0.20 | 2.70 | 0.20 | 2.62 | 0.21 | 2.68 | 0.20 | 26.58 | 30.11 | 0.13 | 0.15 | 0.05 | 0.12 | 0.24 | 0.37 |
| S Cingul MidPost | 2.53 | 0.18 | 2.54 | 0.17 | 2.51 | 0.17 | 2.55 | 0.17 | 34.71 | 36.12 | 0.01 | 0.36 | 0.21 | 0.07 | 0.03 | 0.34 |
| S frontomargin | 2.35 | 0.21 | 2.38 | 0.23 | 2.36 | 0.24 | 2.40 | 0.24 | 21.53 | 22.15 | 0.00 | 0.01 | 0.18 | 0.17 | 0.07 | 0.58 |
| S Occipital Inf | 2.41 | 0.23 | 2.56 | 0.19 | 2.40 | 0.20 | 2.55 | 0.24 | 6.41 | 6.88 | 0.02 | 0.00 | 0.02 | 0.65 | 0.66 | 0.14 |
| S Paracentral | 2.26 | 0.18 | 2.20 | 0.19 | 2.22 | 0.18 | 2.19 | 0.19 | 20.02 | 25.52 | 0.05 | 0.22 | 0.12 | 0.40 | 0.01 | 0.01 |
| S Subcentral | 2.68 | 0.19 | 2.72 | 0.19 | 2.70 | 0.18 | 2.68 | 0.18 | 25.40 | 19.37 | 0.19 | 0.71 | 0.25 | 0.05 | 0.19 | 0.06 |
| S Transv Fronto pol | 2.59 | 0.31 | 2.55 | 0.24 | 2.60 | 0.22 | 2.56 | 0.23 | 17.60 | 21.73 | 0.01 | 0.00 | 0.03 | 0.14 | 1.09 | 0.00 |
| Cingul Postdorsal | 2.94 | 0.22 | 2.89 | 0.18 | 2.90 | 0.21 | 2.91 | 0.19 | 25.07 | 19.43 | 0.16 | 0.51 | 0.80 | 0.31 | 1.88* | 1.79* |
| Cingul Postventral | 2.38 | 0.30 | 2.71 | 0.31 | 2.44 | 0.31 | 2.70 | 0.30 | 8.69 | 15.85 | 0.82 | 0.01 | 0.02 | 0.07 | 0.01 | 2.19* |
| Cuneus | 1.71 | 0.14 | 1.74 | 0.14 | 1.69 | 0.14 | 1.71 | 0.15 | 24.11 | 32.74 | 0.02 | 0.01 | 0.37 | 0.03 | 1.68* | 1.82* |
| Front Inf Opercular | 2.77 | 0.18 | 2.79 | 0.21 | 2.76 | 0.18 | 2.77 | 0.19 | 31.47 | 26.59 | 0.05 | 0.13 | 0.32 | 0.38 | 0.24 | 1.24 |
| Front Inf Orbital | 2.76 | 0.24 | 2.83 | 0.24 | 2.79 | 0.26 | 2.77 | 0.25 | 11.88 | 18.56 | 0.38 | 0.90 | 0.04 | 0.82 | 0.09 | 0.10 |
| Front Inf Triangul | 2.64 | 0.22 | 2.71 | 0.22 | 2.67 | 0.20 | 2.68 | 0.20 | 32.99 | 22.28 | 0.08 | 1.21 | 0.16 | 0.10 | 0.00 | 0.23 |
| Front Middle | 2.63 | 0.18 | 2.62 | 0.19 | 2.63 | 0.18 | 2.64 | 0.17 | 34.63 | 33.64 | 0.06 | 0.02 | 0.01 | 0.34 | 0.35 | 1.06 |
| Front Sup | 2.84 | 0.17 | 2.83 | 0.18 | 2.85 | 0.18 | 2.84 | 0.17 | 36.76 | 35.43 | 0.13 | 0.05 | 0.01 | 0.30 | 0.64 | 0.21 |
| Insular Short | 3.58 | 0.22 | 3.58 | 0.25 | 3.60 | 0.23 | 3.52 | 0.22 | 9.41 | 14.85 | 0.52 | 0.22 | 0.03 | 0.01 | 1.17 | 1.26 |
| Octemp Lat Fusifor | 2.82 | 0.22 | 2.83 | 0.21 | 2.80 | 0.20 | 2.78 | 0.23 | 23.99 | 24.15 | 0.04 | 0.24 | 0.40 | 0.63 | 0.01 | 0.08 |
| Octemp Med Lingual | 1.93 | 0.16 | 2.04 | 0.13 | 1.91 | 0.17 | 2.00 | 0.18 | 30.73 | 32.05 | 0.00 | 0.21 | 0.08 | 0.08 | 0.95 | 2.39** |
| Octemp Med Parahip | 3.06 | 0.27 | 3.08 | 0.27 | 3.10 | 0.29 | 3.09 | 0.30 | 15.00 | 16.84 | 0.84 | 0.26 | 0.11 | 0.15 | 0.89 | 0.00 |
| Occipital Middle | 2.52 | 0.18 | 2.57 | 0.16 | 2.52 | 0.17 | 2.58 | 0.17 | 6.20 | 7.77 | 0.03 | 0.07 | 3.24** | 0.00 | 0.00 | 1.38 |
| Occipital Sup | 2.06 | 0.18 | 2.14 | 0.17 | 2.05 | 0.20 | 2.14 | 0.20 | 8.68 | 6.66 | 0.01 | 0.01 | 0.00 | 0.02 | 1.43 | 0.06 |
| Orbital | 2.80 | 0.20 | 2.82 | 0.24 | 2.80 | 0.20 | 2.80 | 0.20 | 35.28 | 30.70 | 0.02 | 0.12 | 0.01 | 0.93 | 0.28 | 0.08 |
| Pariet Inf Angular | 2.62 | 0.19 | 2.67 | 0.18 | 2.64 | 0.19 | 2.69 | 0.17 | 14.81 | 25.74 | 0.09 | 0.04 | 0.88 | 0.00 | 0.03 | 0.37 |
| Pariet Inf Supramar | 2.72 | 0.17 | 2.72 | 0.17 | 2.72 | 0.16 | 2.73 | 0.18 | 20.57 | 22.15 | 0.04 | 0.01 | 0.29 | 0.59 | 0.54 | 0.00 |
| Parietal Sup | 2.31 | 0.13 | 2.32 | 0.15 | 2.31 | 0.14 | 2.31 | 0.15 | 15.67 | 11.92 | 0.14 | 0.01 | 0.99 | 0.59 | 0.16 | 0.00 |
| Postcentral | 2.13 | 0.17 | 2.06 | 0.17 | 2.11 | 0.16 | 2.11 | 0.17 | 13.44 | 10.11 | 0.01 | 2.03* | 0.06 | 0.05 | 0.35 | 1.03 |
| Precentral | 2.74 | 0.19 | 2.72 | 0.21 | 2.75 | 0.18 | 2.74 | 0.18 | 22.01 | 17.22 | 0.30 | 0.65 | 1.29 | 0.26 | 0.23 | 1.94* |
| Precuneus | 2.47 | 0.16 | 2.46 | 0.17 | 2.47 | 0.17 | 2.45 | 0.17 | 17.73 | 15.55 | 0.00 | 0.02 | 0.07 | 0.19 | 0.60 | 0.31 |
| Rectus | 2.55 | 0.23 | 2.54 | 0.31 | 2.59 | 0.26 | 2.50 | 0.27 | 40.31 | 45.00 | 0.67 | 0.42 | 0.22 | 0.14 | 0.18 | 1.44* |
| Subcallosal | 2.68 | 0.48 | 2.55 | 0.42 | 2.61 | 0.39 | 2.49 | 0.41 | 8.87 | 11.05 | 0.38 | 0.15 | 0.37 | 0.00 | 0.01 | 0.00 |
| Temp sup G T Transv | 2.36 | 0.20 | 2.41 | 0.27 | 2.37 | 0.23 | 2.41 | 0.26 | 11.71 | 13.88 | 0.16 | 0.05 | 0.09 | 0.29 | 1.79* | 1.70* |
| Temp Sup Lateral | 3.07 | 0.19 | 3.10 | 0.21 | 3.08 | 0.20 | 3.09 | 0.20 | 17.13 | 28.51 | 0.11 | 0.00 | 0.16 | 0.19 | 1.03 | 0.34 |
| Temp Sup Plan polar | 3.46 | 0.32 | 3.28 | 0.23 | 3.39 | 0.28 | 3.26 | 0.29 | 10.86 | 13.26 | 0.43 | 0.00 | 0.05 | 0.10 | 2.42* | 0.03 |
| Temp Sup Plan tempo | 2.56 | 0.21 | 2.57 | 0.19 | 2.57 | 0.19 | 2.58 | 0.20 | 10.03 | 16.55 | 0.04 | 0.04 | 0.65 | 0.03 | 3.15** | 0.40 |
| Temporal Inf | 2.96 | 0.20 | 3.00 | 0.20 | 2.94 | 0.21 | 2.94 | 0.22 | 21.09 | 21.02 | 0.00 | 0.79 | 0.32 | 0.02 | 0.25 | 0.08 |
| Temporal Middle | 3.06 | 0.19 | 3.10 | 0.19 | 3.04 | 0.18 | 3.09 | 0.19 | 27.58 | 26.79 | 0.08 | 0.22 | 0.14 | 0.04 | 0.57 | 2.13* |
| Lat Fis Ant Horizont | 2.25 | 0.32 | 2.26 | 0.24 | 2.24 | 0.28 | 2.26 | 0.23 | 16.09 | 16.09 | 0.27 | 0.00 | 0.31 | 0.14 | 0.05 | 0.13 |
| Lat Fis Ant Vertical | 2.42 | 0.28 | 2.48 | 0.34 | 2.37 | 0.28 | 2.45 | 0.31 | 20.99 | 17.02 | 0.49 | 0.16 | 0.06 | 0.00 | 0.11 | 0.22 |
| Lat Fispost | 2.38 | 0.18 | 2.46 | 0.17 | 2.37 | 0.18 | 2.44 | 0.18 | 20.76 | 24.93 | 0.05 | 0.49 | 0.04 | 0.42 | 2.02* | 0.17 |
| Pole Occipital | 1.89 | 0.14 | 1.92 | 0.17 | 1.91 | 0.17 | 1.93 | 0.15 | 14.69 | 18.35 | 0.63 | 0.70 | 0.00 | 0.03 | 0.48 | 0.52 |
| Pole Temporal | 3.31 | 0.21 | 3.35 | 0.23 | 3.38 | 0.25 | 3.39 | 0.25 | 8.75 | 7.35 | 1.99* | 0.94 | 1.39 | 0.01 | 1.65* | 0.12 |
| S calcarine | 1.78 | 0.17 | 1.82 | 0.14 | 1.79 | 0.17 | 1.82 | 0.16 | 40.30 | 36.53 | 0.59 | 0.16 | 0.01 | 0.02 | 1.98** | 1.09 |
| S central | 1.80 | 0.17 | 1.78 | 0.15 | 1.81 | 0.15 | 1.77 | 0.16 | 31.20 | 27.13 | 0.74 | 0.10 | 1.54* | 0.00 | 0.01 | 0.22 |
| S Cingul Marginalis | 2.19 | 0.15 | 2.20 | 0.17 | 2.19 | 0.17 | 2.20 | 0.18 | 19.82 | 23.37 | 0.08 | 0.09 | 0.07 | 0.00 | 0.41 | 0.09 |
| S Circular Insula Ant | 2.80 | 0.30 | 2.85 | 0.31 | 2.78 | 0.26 | 2.84 | 0.28 | 14.71 | 18.54 | 0.03 | 0.00 | 0.01 | 0.09 | 0.02 | 0.50 |
| S Circular Insula Inf | 2.76 | 0.22 | 2.67 | 0.18 | 2.75 | 0.20 | 2.70 | 0.23 | 22.15 | 16.98 | 0.06 | 0.26 | 0.68 | 0.10 | 0.44 | 0.62 |
| S Circular Insula Sup | 2.56 | 0.16 | 2.62 | 0.21 | 2.56 | 0.17 | 2.60 | 0.19 | 33.46 | 29.08 | 0.02 | 0.47 | 0.00 | 0.05 | 0.00 | 0.16 |
| S Collat Transv Ant | 2.72 | 0.35 | 2.68 | 0.27 | 2.74 | 0.32 | 2.67 | 0.31 | 9.97 | 15.47 | 0.00 | 0.03 | 2.73* | 0.37 | 0.82 | 0.05 |
| S Collat Transv Post | 2.06 | 0.24 | 2.10 | 0.27 | 2.02 | 0.25 | 2.08 | 0.25 | 13.10 | 11.28 | 0.09 | 0.16 | 0.67 | 0.03 | 0.55 | 0.03 |
| S Front Inf | 2.24 | 0.17 | 2.22 | 0.17 | 2.23 | 0.16 | 2.21 | 0.16 | 22.93 | 22.98 | 0.54 | 0.27 | 0.44 | 0.24 | 0.06 | 0.14 |
| S Front Middle | 2.15 | 0.18 | 2.16 | 0.18 | 2.17 | 0.20 | 2.16 | 0.18 | 20.44 | 26.18 | 0.03 | 0.31 | 0.21 | 0.01 | 0.06 | 0.26 |
| S Front Sup | 2.38 | 0.15 | 2.38 | 0.15 | 2.40 | 0.16 | 2.38 | 0.16 | 20.45 | 16.33 | 0.67 | 0.01 | 0.00 | 0.15 | 0.48 | 0.07 |
| S Interm Prim Jensen | 2.52 | 0.43 | 2.24 | 0.18 | 2.38 | 0.35 | 2.28 | 0.20 | 5.48 | 8.95 | 2.62* | 0.59 | 1.34 | 0.96 | 0.04 | 0.00 |
| S Intrapariet P Trans | 2.09 | 0.15 | 2.10 | 0.13 | 2.09 | 0.14 | 2.08 | 0.14 | 9.05 | 7.23 | 0.14 | 0.57 | 0.06 | 0.02 | 0.59 | 0.07 |
| S Octemp Lat | 2.55 | 0.22 | 2.56 | 0.23 | 2.51 | 0.20 | 2.50 | 0.20 | 6.31 | 12.11 | 0.76 | 1.05 | 0.00 | 1.03 | 0.14 | 0.01 |
| S Octemp Med Lingual | 2.40 | 0.20 | 2.37 | 0.18 | 2.39 | 0.20 | 2.35 | 0.22 | 31.71 | 26.58 | 0.00 | 0.01 | 0.00 | 0.02 | 0.03 | 0.33 |
| S OC Middle Lunatus | 1.96 | 0.21 | 2.04 | 0.25 | 1.99 | 0.19 | 2.05 | 0.20 | 9.69 | 1.14 | 0.48 | 0.04 | 0.01 | 0.29 | 0.03 | 1.55 |
| S OC Sup Transversal | 2.03 | 0.19 | 2.05 | 0.16 | 2.01 | 0.16 | 2.04 | 0.17 | 11.01 | 10.42 | 0.11 | 0.00 | 0.06 | 0.06 | 0.40 | 0.33 |
| S Occipital Ant | 2.26 | 0.16 | 2.31 | 0.18 | 2.27 | 0.17 | 2.32 | 0.19 | 7.23 | 10.55 | 0.05 | 0.00 | 0.01 | 0.05 | 0.13 | 0.46 |
| S Orbital H Shaped | 2.56 | 0.27 | 2.57 | 0.25 | 2.61 | 0.26 | 2.62 | 0.26 | 14.86 | 28.13 | 0.32 | 0.20 | 0.65 | 1.01 | 0.09 | 0.29 |
| S Orbital Lateral | 2.17 | 0.36 | 2.16 | 0.32 | 2.19 | 0.32 | 2.21 | 0.30 | 19.62 | 23.83 | 0.17 | 0.03 | 0.03 | 0.02 | 0.23 | 0.80 |
| S Orbital Med olfact | 2.37 | 0.29 | 2.23 | 0.32 | 2.40 | 0.33 | 2.20 | 0.29 | 16.18 | 19.84 | 0.09 | 0.57 | 0.26 | 0.03 | 0.00 | 0.00 |
| S Parieto Occipital | 2.13 | 0.15 | 2.18 | 0.18 | 2.10 | 0.19 | 2.15 | 0.19 | 15.16 | 17.47 | 0.09 | 0.26 | 0.10 | 0.00 | 1.09 | 1.34 |
| S Pericallosal | 2.31 | 0.33 | 2.19 | 0.28 | 2.30 | 0.34 | 2.18 | 0.26 | 11.27 | 15.24 | 0.02 | 0.16 | 0.55 | 0.00 | 0.07 | 0.13 |
| S Postcentral | 2.08 | 0.13 | 2.01 | 0.14 | 2.06 | 0.14 | 2.02 | 0.15 | 18.41 | 11.13 | 0.28 | 0.22 | 0.07 | 0.00 | 0.86 | 0.75 |
| S Precentral Inf Part | 2.41 | 0.16 | 2.44 | 0.15 | 2.43 | 0.18 | 2.42 | 0.17 | 31.73 | 21.71 | 0.14 | 0.38 | 0.51 | 0.96 | 0.64 | 0.04 |
| S Precentral Sup Part | 2.32 | 0.15 | 2.34 | 0.19 | 2.34 | 0.19 | 2.33 | 0.21 | 21.86 | 28.85 | 0.26 | 0.01 | 0.00 | 0.17 | 0.02 | 0.28 |
| S Suborbital | 2.30 | 0.28 | 2.40 | 0.43 | 2.40 | 0.31 | 2.41 | 0.43 | 12.55 | 7.37 | 1.78* | 0.03 | 0.02 | 0.04 | 2.54* | 0.26 |
| S Subparietal | 2.30 | 0.20 | 2.35 | 0.21 | 2.34 | 0.19 | 2.40 | 0.19 | 16.72 | 22.21 | 1.06 | 1.78* | 0.24 | 0.00 | 0.06 | 0.34 |
| S Temporal Inf | 2.48 | 0.19 | 2.55 | 0.17 | 2.50 | 0.18 | 2.48 | 0.22 | 15.06 | 6.84 | 0.03 | 1.83* | 0.17 | 0.49 | 0.24 | 0.25 |
| S Temporal Sup | 2.45 | 0.15 | 2.50 | 0.15 | 2.44 | 0.14 | 2.48 | 0.14 | 29.28 | 30.38 | 0.27 | 0.26 | 0.00 | 0.24 | 0.82 | 0.19 |
| S Temporal Transverse | 2.37 | 0.36 | 2.55 | 0.28 | 2.43 | 0.32 | 2.52 | 0.32 | 15.90 | 12.20 | 0.70 | 0.11 | 0.11 | 0.22 | 0.64 | 3.66** |
| Mean | 2.49 | 0.21 | 2.50 | 0.22 | 2.49 | 0.22 | 2.51 | 0.21 | 19.17 | 19.96 | 0.30 | 0.30 | 0.33 | 0.22 | 0.57 | 0.57 |
Model 1 presents total r squared for a model including age, age2 gender, education, and recording site. Model 2 presents the delta r‐squared when adding a group term (MDD+ vs MDD−). Model 3a and 3b present the increment in r 2 from the interaction term. *P < 0.05, **P < 0.01. Cortical thickness is expressed in millimeters.
Among depressed cases only, covariates accounted for approximately 18% (left hemisphere) and 19% (right hemisphere) of individual differences in cortical thickness. Current depression severity (Model 2) was negatively associated with cortical thickness in 4 regions at P < 0.01, the strongest of which was in the right occipital anterior sulcus (5.29% of variance; 2.7% of 148 interactions). Depression severity interacted with age (Model 3a) in one region at P < 0.01, the left anterior occipital sulcus and accounted for 4.41% of variance in that region (Supporting Information, Fig. 9). As shown, higher QIDS score was associated with less cortical thickness in this region at older ages. The interaction between depression severity and gender (Model 3b) was weakly related to cortical thickness (all P values > 0.01).
Surface‐based morphometry: After multiple comparisons correction, vertex‐wise whole‐brain comparisons of MDD+ to MDD− revealed that the MDD+ group had thicker left supramarginal gyri than the MDD− group, with a cluster size of 177.86 mm2 and a cluster‐wise, corrected P value of 0.047 (Figure 1). This region is denoted as supramarginal in Freesurfer, although the cluster extends into the inferior parietal region. The analysis of current depression severity (QIDS score) in the MDD+ group did not identify any clusters after multiple comparisons correction.
Figure 1.
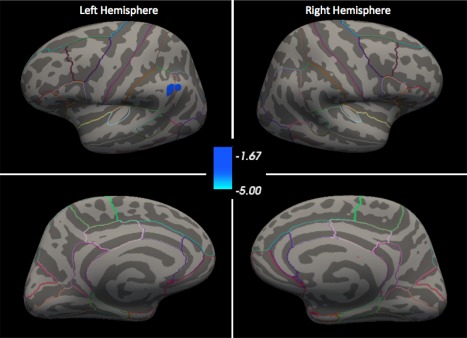
Surface‐based morphometry comparison of MDD+ to MDD− after multiple comparisons correction. Colorbar represents log(p), multiple comparisons corrected, where blue represents cortical thickening in MDD+ compared to MDD−. All maps thresholded at P < 0.05. Outlines of all DK regions are mapped onto the common atlas. [Color figure can be viewed at http://wileyonlinelibrary.com]
DISCUSSION
In this report, we examined the association between cortical thickness and major depressive disorder using data from 170 currently depressed adults and 52 never‐depressed adults collected as part of a multisite study. To our knowledge, this is the first depression study to examine three strategies for assessing cortical thickness and is the largest to employ a previously validated, slice‐wise visual‐inspection method on every participant's cortical thickness data [Iscan et al., 2015]. This essential step is often impractical due to the burden of staff time required, but improves data quality (i.e., test–retest reliability) relative to fully automated processing steps without manual approval [Iscan et al., 2015]. Moreover, this is also the first study to our knowledge to compare depressed and nondepressed adults using regions defined by the DS atlas. Compared to the DK atlas, the DS atlas offers more, smaller targets, and has also demonstrated strong test–retest reliability [Iscan et al., 2015]. Finally, this is the first cortical thickness depression study to explore differential age and gender effects.
Essentially, our main result is that currently depressed adults and never‐depressed adults exhibited comparable levels of cortical thickness. The DK atlas—which is notable for fewer, larger regions and use in all prior a priori, region‐based studies of depression—did not discriminate subjects with depression from never‐depressed adults in any region. A few effects were observed with the smaller, more regions defined by the DS atlas; however, effect sizes were small and consistent with the false positive rate. The third analytic method, surface‐based morphometry, identified a small area of cortical thickening in the left supramarginal gyrus that survived correction for multiple comparisons. Thus, if present in depressed adults, reduced cortical thickness likely reflects a small effect size in affected regions. A similar conclusion was reached in prior reports that utilized the DK atlas to measure cortical thickness [Phillips et al., 2015; Schmaal et al., 2016]. This study extends this conclusion to cortical thickness analyzed using the SBM approach and the DS atlas.
That we identified the left supramarginal gyrus as thicker in depression is intriguing because it replicates two previous SBM studies of depression [Qiu et al., 2014; Yang et al., 2015] and contradicts a third [Ozalay et al., 2016]. It is worth noting that the DS and DK atlases each cover this area. Unfortunately, we can only speculate as to why SBM but not region‐wise analysis identify this region as tracking increased cortical thickness in depression. The inconsistency of results across methods may reflect that atlases define regions according to structural boundaries that do not necessarily correspond to indicators of cortical pathophysiology in depression. That is, SBM may be better for detecting cortical thickness effects that do not fit neatly into predefined regions. Of note, previous ROI studies using the DK atlas have not always reported results for regions corresponding to the supramarginal gyrus. This “missing data” in the literature significantly hinders efforts to integrate findings in either a qualitative review of the literature or a meta‐analysis. Thus, focused hypothesis‐testing designs keep the false positive rate low, but also hinder efforts to integrate findings across studies.
Depression is a heterogeneous phenotype subject to wide intra‐ and intervariability in course, symptoms, response to treatment, and etiology. This heterogeneity may obscure the link between depression and cortical thickness, at least to the extent that only specific features of depression track cortical thickness. We pursued this question via exploratory age and gender interactions with depression. Overall, these effects provided no more than a few small magnitude effects, and it remains unclear which features of current depression best track cortical thickness. It is important to emphasize that our study examined a cohort that was relatively homogenous for chronic depression, which includes many who reported a single‐episode of depression that continued unremitted or with only partial remission for many years, and many who report recurrent depression characterized by full interepisode recovery. Therefore, we cannot rule out the possibility that cortical thickness better tracks depression in other kinds of depressed cohorts. In addition, more powerful within‐subject designs may yet reveal that depression is associated with differential rate of change in cortical thickness across adulthood.
That cortical thickness appears no more than weakly related to current depression should inform the design and implementation of future studies. Strategies to combine datasets or results (e.g., meta‐analysis), such as that employed by ENIGMA [Schmaal et al., 2016], may be more effective than single studies in confirming the presence of subtle thinning in specific regions. Second, cortical thickness appears highly sensitive to several extraneous sources of variance. In this study, the combined effects of age, age2, sex, education, and site differences accounted for nearly a fifth of the total individual differences in cortical thickness. This is much larger than estimates of the effect of depression on cortical thickness. Thus, the strategies by which studies adjust for these factors may have a significant impact on the results. Future research may benefit from establishing cortical thickness normative data to enhance compatibility between recording sites and studies and optimally control for these factors.
There are a number of limitations to this study. First, although we examined a large sample of depressed subjects relative to many other studies, larger samples may be needed to detect subtle associations and identify moderators. In particular, inclusion of fewer nondepressed adults than depressed adults necessarily lowered statistical power to detect group differences. However, larger samples would not be expected to yield larger magnitude associations [Phillips et al., 2015; Schmaal et al., 2016]. Second, site differences may have masked an association between cortical thickness and depression. However, considerable effort was made to create and apply uniform procedures for sample ascertainment and recording; sites were matched on proportion of cases and controls, gender, mean age, and current depressive severity; and site differences were statistically controlled for in our analyses to minimize impact on results. Third, we were unable to conduct exploratory analyses of all candidate moderators of the association between MDD and cortical thickness. This is because the sample was relatively homogenous for long‐duration, chronic depression. Future studies of moderators may benefit from examining a more heterogeneous depressed cohort, including remitted cases and recent first‐onset cases. The list of potential moderators of the link between cortical thickness and MDD is lengthy, as it includes any trait or disease previously correlated with cortical thickness. This list includes health‐related phenotypes, such as diabetes [Ajilore et al., 2010; Franc et al., 2011] and obesity [Kim et al., 2015]; and individual difference traits, such as cognitive ability [Burzynska et al., 2012; Klein et al., 2014], religiosity [Miller et al., 2014], meditation experience [Lazar et al., 2005], and negative affect [Holmes et al., 2016]. Along these lines, diagnostic comorbidity is a potential moderator of the link between cortical thickness and depression. While comorbidity is common in clinical samples of depressed adults, this study was not designed to parse unique effects of comorbidity patterns on cortical thickness.
In summary, to our knowledge, this was the second largest study of cortical thickness in depression, the first to examine three separate analytic methods and differential age and gender effects, and the first large study to employ strict quality control procedures during data processing. Although previous reports describe robust associations between major depressive disorder and reduced cortical thickness, this study finds such an association to be relatively weak at best. Larger sample sizes and more comprehensive searches for moderators may yield more robust effects.
FINANCIAL DISCLOSURES
Dr Perlman reported no biomedical financial interests or potential conflicts of interest.
Elizabeth Bartlett reports no biomedical financial interests or potential conflicts of interest.
Dr DeLorenzo reports no biomedical financial interests or potential conflicts of interest.
Dr Weissman receives royalties from the Oxford University Press, Perseus Press, the American Psychiatric Association Press, and MultiHealth Systems.
Dr McGrath has received research support from National Institute of Mental Health, New York State Department of Mental Hygiene, Research Foundation for Mental Hygiene (New York State), Forest Research Laboratories, Sunovion Pharmaceuticals, Naurex Pharmaceuticals (now Allergan).
Tony Jin reported no biomedical financial interests or potential conflicts of interest.
Dr Adams reported no biomedical financial interests or potential conflicts of interest.
Dr Trivedi is or has been an advisor/consultant and received fee from Alkermes, AstraZeneca, Cerecor, Eli Lilly & Company, Lundbeck, Naurex, Neuronetics, Otsuka Pharmaceuticals, Pamlab, Pfizer Inc., SHIRE Development and Takeda.
Dr Kurian has received grant support from Targacept, Inc.; Pfizer, Inc.; Johnson & Johnson; Evotec; Rexahn; Naurex; and Forest Pharmaceuticals.
Dr Fava has received research support from Abbot Laboratories; Alkermes, Inc.; American Cyanamid;Aspect Medical Systems; AstraZeneca; Avanir Pharmaceuticals; BioResearch; BrainCells Inc.; Bristol‐Myers Squibb; CeNeRx BioPharma; Cephalon; Cerecor; Clintara, LLC; Covance; Covidien; Eli Lilly and Company;EnVivo Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Forest Pharmaceuticals, Inc.; Ganeden Biotech, Inc.; GlaxoSmithKline; Harvard Clinical Research Institute; Hoffman‐LaRoche; Icon Clinical Research; i3 Innovus/Ingenix; Janssen R&D, LLC; Jed Foundation; Johnson & Johnson Pharmaceutical Research & Development; Lichtwer Pharma GmbH; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante; Methylation Sciences Inc; National Alliance for Research on Schizophrenia & Depression (NARSAD); National Center for Complementary and Alternative Medicine (NCCAM); National Institute of Drug Abuse (NIDA); National Institute of Mental Health (NIMH); Neuralstem, Inc.; Novartis AG; Organon Pharmaceuticals; PamLab, LLC.; Pfizer Inc.; Pharmacia‐Upjohn; Pharmaceutical Research Associates., Inc.; Pharmavite® LLC;PharmoRx Therapeutics; Photothera; Reckitt Benckiser; Roche Pharmaceuticals; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Sanofi‐Aventis US LLC; Shire; Solvay Pharmaceuticals, Inc.; Stanley Medical Research Institute (SMRI); Synthelabo; Tal Medical; Wyeth‐Ayerst Laboratories; has served on an advisory board or consulted for Abbott Laboratories; Acadia; Affectis Pharmaceuticals AG; Alkermes, Inc.; Amarin Pharma Inc.; Aspect Medical Systems; AstraZeneca; Auspex Pharmaceuticals; Avanir Pharmaceuticals; AXSOME Therapeutics; Bayer AG; Best Practice Project Management, Inc.; Biogen; BioMarin Pharmaceuticals, Inc.; Biovail Corporation; BrainCells Inc; Bristol‐Myers Squibb; CeNeRx BioPharma; Cephalon, Inc.; Cerecor; CNS Response, Inc.; Compellis Pharmaceuticals; Cypress Pharmaceutical, Inc.; DiagnoSearch Life Sciences (P) Ltd.; Dinippon Sumitomo Pharma Co. Inc.; Dov Pharmaceuticals, Inc.; Edgemont Pharmaceuticals, Inc.; Eisai Inc.; Eli Lilly and Company; EnVivo Pharmaceuticals, Inc.; ePharmaSolutions; EPIX Pharmaceuticals, Inc.; Euthymics Bioscience, Inc.; Fabre‐Kramer Pharmaceuticals, Inc.; Forest Pharmaceuticals, Inc.; Forum Pharmaceuticals; GenOmind, LLC; GlaxoSmithKline; Grunenthal GmbH; i3 Innovus/Ingenis; Intracellular; Janssen Pharmaceutica; Jazz Pharmaceuticals, Inc.; Johnson & Johnson Pharmaceutical Research & Development, LLC; Knoll Pharmaceuticals Corp.; Labopharm Inc.; Lorex Pharmaceuticals; Lundbeck Inc.; MedAvante, Inc.; Merck & Co., Inc.; MSI Methylation Sciences, Inc.; Naurex, Inc.; Nestle Health Sciences; Neuralstem, Inc.; Neuronetics, Inc.; NextWave Pharmaceuticals; Novartis AG;Nutrition 21; Orexigen Therapeutics, Inc.; Organon Pharmaceuticals; Osmotica; Otsuka Pharmaceuticals; Pamlab, LLC.; Pfizer Inc.; PharmaStar; Pharmavite® LLC.; PharmoRx Therapeutics; Precision Human Biolaboratory; Prexa Pharmaceuticals, Inc.; PPD; Puretech Ventures; PsychoGenics; Psylin Neurosciences, Inc.; RCT Logic, LLC (formerly Clinical Trials Solutions, LLC); Rexahn Pharmaceuticals, Inc.; Ridge Diagnostics, Inc.; Roche; Sanofi‐Aventis US LLC.; Sepracor Inc.; Servier Laboratories; Schering‐Plough Corporation; Solvay Pharmaceuticals, Inc.; Somaxon Pharmaceuticals, Inc.; Somerset Pharmaceuticals, Inc.; Sunovion Pharmaceuticals; Supernus Pharmaceuticals, Inc.; Synthelabo; Taisho Pharmaceutical; Takeda Pharmaceutical Company Limited; Tal Medical, Inc.; Tetragenex Pharmaceuticals, Inc.; TransForm Pharmaceuticals, Inc.; Transcept Pharmaceuticals, Inc.; Vanda Pharmaceuticals, Inc.; VistaGen; speaking/publishing from Adamed, Co; Advanced Meeting Partners; American Psychiatric Association; American Society of Clinical Psychopharmacology; AstraZeneca; Belvoir Media Group; Boehringer Ingelheim GmbH; Bristol‐Myers Squibb; Cephalon, Inc.; CME Institute/Physicians Postgraduate Press, Inc.; Eli Lilly and Company; Forest Pharmaceuticals, Inc.; GlaxoSmithKline; Imedex, LLC; MGH Psychiatry Academy/Primedia; MGH Psychiatry Academy/Reed Elsevier; Novartis AG; Organon Pharmaceuticals; Pfizer Inc.; PharmaStar; United BioSource,Corp.; Wyeth‐Ayerst Laboratories; has equity holdings from: Compellis; PsyBrain, Inc.; holds patents for Sequential Parallel Comparison Design (SPCD), licensed by MGH to Pharmaceutical Product Development, LLC (PPD); and patent application for a combination of Ketamine plus Scopolamine in Major Depressive Disorder (MDD), licensed by MGH to Biohaven; .
Dr Ogden reported no biomedical financial interests or potential conflicts of interest.
Dr Cooper reported no biomedical financial interests or potential conflicts of interest.
Dr Malchow reported no biomedical financial interests or potential conflicts of interest.
Dr Weyandt reported no biomedical financial interests or potential conflicts of interest.
Dr Oquendo owns stock in Bristol Myers Squibb.
Dr McInnis has consulted with Janssen pharmaceuticals in the past 3 years.
Dr Parsey reports no biomedical financial interests or potential conflicts of interest.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Valeant Pharmaceuticals donated the Wellbutrin XL used in the study. This work was supported by the EMBARC National Coordinating Center at UT Southwestern Medical Center, Madhukar H. Trivedi, MD, Coordinating PI, and the Data Center at Columbia and Stony Brook Universities. We thank Randy Buckner for providing comments on earlier draft of the manuscript.
REFERENCES
- Ajilore O, Narr K, Rosenthal J, Pham D, Hamilton L, Watari K, Elderkin‐Thompson V, Darwin C, Toga A, Kumar A (2010): Regional cortical gray matter thickness differences associated with type 2 diabetes and major depression. Psychiatry Res 184:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Nagel IE, Preuschhof C, Gluth S, Backman L, Li SC, Lindenberger U, Heekeren HR (2012): Cortical thickness is linked to executive functioning in adulthood and aging. Hum Brain Mapp 33:1607–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloby SJ, Firbank MJ, Vasudev A, Parry SW, Thomas AJ, O'Brien JT (2011): Cortical thickness and VBM‐DARTEL in late‐life depression. J Affect Disord 133:158–164. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI (1999): Cortical surface‐based analysis. I. Segmentation and surface reconstruction. NeuroImage 9:179–194. [DOI] [PubMed] [Google Scholar]
- Delaparte L, Yeh FC, Adams P, Malchow A, Trivedi MH, Oquendo MA, … Cooper C (2017): A comparison of structural connectivity in anxious depression versus non‐anxious depression. J Psychiatric Res 89:38–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ (2006): An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 31:968–980. [DOI] [PubMed] [Google Scholar]
- Destrieux C, Fischl B, Dale A, Halgren E (2010): Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. NeuroImage 53:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fallucca E, MacMaster FP, Haddad J, Easter P, Dick R, May G, Stanley JA, Rix C, Rosenberg DR (2011): Distinguishing between major depressive disorder and obsessive‐compulsive disorder in children by measuring regional cortical thickness. Arch Gen Psychiatry 68:527–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM (2000): Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci USA 97:11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM (2001): Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imag 20:70–80. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S, Montillo A, Makris N, Rosen B, Dale AM (2002): Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron 33:341–355. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM (1999a): Cortical surface‐based analysis. II: Inflation, flattening, and a surface‐based coordinate system. NeuroImage 9:195–207. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM (1999b): High‐resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp 8:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, Caviness V, Makris N, Rosen B, Dale AM (2004): Automatically parcellating the human cerebral cortex. Cereb Cortex 14:11–22. [DOI] [PubMed] [Google Scholar]
- Foland‐Ross LC, Sacchet MD, Prasad G, Gilbert B, Thompson PM, Gotlib IH (2015): Cortical thickness predicts the first onset of major depression in adolescence. Int J Develop Neurosci 46:125–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franc DT, Kodl CT, Mueller BA, Muetzel RL, Lim KO, Seaquist ER (2011): High connectivity between reduced cortical thickness and disrupted white matter tracts in long‐standing type 1 diabetes. Diabetes 60:315–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes AJ, Hollinshead MO, Roffman JL, Smoller JW, Buckner RL (2016): Individual differences in cognitive control circuit anatomy link sensation seeking, impulsivity, and substance use. J Neurosci 36:4038–4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iscan Z, Jin TB, Kendrick A, Szeglin B, Lu H, Trivedi M, Fava M, McGrath PJ, Weissman M, Kurian BT, Adams P, Weyandt S, Toups M, Carmody T, McInnis M, Cusin C, Cooper C, Oquendo MA, Parsey RV, DeLorenzo C (2015): Test‐retest reliability of freesurfer measurements within and between sites: Effects of visual approval process. Hum Brain Mapp 36:3472–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarnum H, Eskildsen SF, Steffensen EG, Lundbye‐Christensen S, Simonsen CW, Thomsen IS, Frund ET, Theberge J, Larsson EM (2011): Longitudinal MRI study of cortical thickness, perfusion, and metabolite levels in major depressive disorder. Acta Psychiatr Scand 124:435–446. [DOI] [PubMed] [Google Scholar]
- Kim H, Kim C, Seo SW, Na DL, Kim HJ, Kang M, Shin HY, Cho SK, Park SE, Lee J, Hwang JW, Jeon S, Lee JM, Kim GH, Cho H, Ye BS, Noh Y, Yoon CW, Guallar E (2015): Association between body mass index and cortical thickness: Among elderly cognitively normal men and women. Int Psychogeriatrics 27:121–130. [DOI] [PubMed] [Google Scholar]
- Klein D, Mok K, Chen JK, Watkins KE (2014): Age of language learning shapes brain structure: A cortical thickness study of bilingual and monolingual individuals. Brain Lang 131:20–24. [DOI] [PubMed] [Google Scholar]
- Koolschijn PC, van Haren NE, Schnack HG, Janssen J, Hulshoff Pol HE, Kahn RS (2010): Cortical thickness and voxel‐based morphometry in depressed elderly. Eur Neuropsychopharmacol 20:398–404. [DOI] [PubMed] [Google Scholar]
- Lazar SW, Kerr CE, Wasserman RH, Gray JR, Greve DN, Treadway MT, McGarvey M, Quinn BT, Dusek JA, Benson H, Rauch SL, Moore CI, Fischl B (2005): Meditation experience is associated with increased cortical thickness. Neuroreport 16:1893–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Metzger CD, Li W, Safron A, van Tol MJ, Lord A, Krause AL, Borchardt V, Dou W, Genz A, Heinze HJ, He H, Walter M (2014): Dissociation of glutamate and cortical thickness is restricted to regions subserving trait but not state markers in major depressive disorder. J Affect Disord 169:91–100. [DOI] [PubMed] [Google Scholar]
- Luders E, Narr KL, Thompson PM, Rex DE, Woods RP, Deluca H, Jancke L, Toga AW (2006): Gender effects on cortical thickness and the influence of scaling. Hum Brain Mapp 27:314–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller L, Bansal R, Wickramaratne P, Hao X, Tenke CE, Weissman MM, Peterson BS (2014): Neuroanatomical correlates of religiosity and spirituality: A study in adults at high and low familial risk for depression. JAMA Psychiatry 71:128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Na KS, Won E, Kang J, Chang HS, Yoon HK, Tae WS, Kim YK, Lee MS, Joe SH, Kim H, Ham BJ (2016): Brain‐derived neurotrophic factor promoter methylation and cortical thickness in recurrent major depressive disorder. Sci Rep 6:21089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olvet DM, Delaparte L, Yeh FC, DeLorenzo C, McGrath PJ, Weissman MM, … Carmody TJ (2016): A comprehensive examination of white matter tracts and connectometry in major depressive disorder. Depression Anxiety 33:56–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozalay O, Aksoy B, Tunay S, Simsek F, Chandhoki S, Kitis O, Eker C, Gonul AS (2016): Cortical thickness and VBM in young women at risk for familial depression and their depressed mothers with positive family history. Psychiatry Res 252:1–9. [DOI] [PubMed] [Google Scholar]
- Papmeyer M, Giles S, Sussmann JE, Kielty S, Stewart T, Lawrie SM, Whalley HC, McIntosh AM (2015): Cortical thickness in individuals at high familial risk of mood disorders as they develop major depressive disorder. Biol Psychiatry 78:58–66. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Warner V, Bansal R, Zhu H, Hao X, Liu J, Durkin K, Adams PB, Wickramaratne P, Weissman MM (2009): Cortical thinning in persons at increased familial risk for major depression. Proc Natl Acad Sci USA 106:6273–6278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Weissman MM (2011): A brain‐based endophenotype for major depressive disorder. Annu Rev Med 62:461–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips JL, Batten LA, Tremblay P, Aldosary F, Blier P (2015): A prospective, longitudinal study of the effect of remission on cortical thickness and hippocampal volume in patients with treatment‐resistant depression. Int J Neuropsychopharmacol 18: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu L, Lui S, Kuang W, Huang X, Li J, Li J, Zhang J, Chen H, Sweeney JA, Gong Q (2014): Regional increases of cortical thickness in untreated, first‐episode major depressive disorder. Transl Psychiatry 4:e378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel‐Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Stockmeier CA (1999): Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry 45:1085–1098. [DOI] [PubMed] [Google Scholar]
- Rosenthal R (1994): Parametric measures of effect size. The handbook of research synthesis 231–244. [Google Scholar]
- Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, Markowitz JC, Ninan PT, Kornstein S, Manber R, Thase ME, Kocsis JH, Keller MB (2003): The 16‐item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS‐C), and self‐report (QIDS‐SR): A psychometric evaluation in patients with chronic major depression. Biol Psychiatry 54:573–583. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B (2004): Thinning of the cerebral cortex in aging. Cereb Cortex 14:721–730. [DOI] [PubMed] [Google Scholar]
- Savic I, Arver S (2014): Sex differences in cortical thickness and their possible genetic and sex hormonal underpinnings. Cereb Cortex 24:3246–3257. [DOI] [PubMed] [Google Scholar]
- Schmaal L, Hibar DP, Samann PG, Hall GB, Baune BT, Jahanshad N, Cheung JW, van Erp TG, Bos D, Ikram MA, Vernooij MW, Niessen WJ, Tiemeier H, Hofman A, Wittfeld K, Grabe HJ, Janowitz D, Bulow R, Selonke M, Volzke H, Grotegerd D, Dannlowski U, Arolt V, Opel N, Heindel W, Kugel H, Hoehn D, Czisch M, Couvy‐Duchesne B, Renteria ME, Strike LT, Wright MJ, Mills NT, de Zubicaray GI, McMahon KL, Medland SE, Martin NG, Gillespie NA, Goya‐Maldonado R, Gruber O, Kramer B, Hatton SN, Lagopoulos J, Hickie IB, Frodl T, Carballedo A, Frey EM, van Velzen LS, Penninx BW, van Tol MJ, van der Wee NJ, Davey CG, Harrison BJ, Mwangi B, Cao B, Soares JC, Veer IM, Walter H, Schoepf D, Zurowski B, Konrad C, Schramm E, Normann C, Schnell K, Sacchet MD, Gotlib IH, MacQueen GM, Godlewska BR, Nickson T, McIntosh AM, Papmeyer M, Whalley HC, Hall J, Sussmann JE, Li M, Walter M, Aftanas L, Brack I, Bokhan NA, Thompson PM, Veltman DJ (2016): Cortical abnormalities in adults and adolescents with major depression based on brain scans from 20 cohorts worldwide in the ENIGMA Major Depressive Disorder Working Group. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B (2004): A hybrid approach to the skull stripping problem in MRI. NeuroImage 22:1060–1075. [DOI] [PubMed] [Google Scholar]
- Segonne F, Pacheco J, Fischl B (2007): Geometrically accurate topology‐correction of cortical surfaces using nonseparating loops. IEEE Trans Med Imag 26:518–529. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC (1998): A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Trans Med Imag 17:87–97. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Peterson BS, Kan E, Woods RP, Yoshii J, Bansal R, Xu D, Zhu H, Thompson PM, Toga AW (2007): Sex differences in cortical thickness mapped in 176 healthy individuals between 7 and 87 years of age. Cereb Cortex 17:1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi MH, McGrath PJ, Fava M, Parsey RV, Kurian BT, Phillips ML, Oquendo MA, Bruder G, Pizzagalli D, Toups M, Cooper C, Adams P, Weyandt S, Morris DW, Grannemann BD, Ogden RT, Buckner R, McInnis M, Kraemer HC, Petkova E, Carmody TJ, Weissman MM (2016): Establishing moderators and biosignatures of antidepressant response in clinical care (EMBARC): Rationale and design. J Psychiatric Res 78:11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Truong W, Minuzzi L, Soares CN, Frey BN, Evans AC, MacQueen GM, Hall GB (2013): Changes in cortical thickness across the lifespan in major depressive disorder. Psychiatry Res 214:204–211. [DOI] [PubMed] [Google Scholar]
- Tu PC, Chen LF, Hsieh JC, Bai YM, Li CT, Su TP (2012): Regional cortical thinning in patients with major depressive disorder: A surface‐based morphometry study. Psychiatry Res 202:206–213. [DOI] [PubMed] [Google Scholar]
- van Eijndhoven P, van Wingen G, Katzenbauer M, Groen W, Tepest R, Fernandez G, Buitelaar J, Tendolkar I (2013): Paralimbic cortical thickness in first‐episode depression: Evidence for trait‐related differences in mood regulation. Am J Psychiatry 170:1477–1486. [DOI] [PubMed] [Google Scholar]
- Wagner G, Schultz CC, Koch K, Schachtzabel C, Sauer H, Schlosser RG (2012): Prefrontal cortical thickness in depressed patients with high‐risk for suicidal behavior. J Psychiatric Res 46:1449–1455. [DOI] [PubMed] [Google Scholar]
- Ward BD (2000): AlphaSim: Simultaneous Inference for fMRI Data. Wisconsin: Biophysics Research Institute, Medical College of Wisconsin. [Google Scholar]
- Webb CA, Dillon DG, Pechtel P, Goer FK, Murray L, Huys QJ, … Kurian BT (2016): Neural correlates of three promising endophenotypes of depression: Evidence from the EMBARC study. Neuropsychopharmacology 41:454–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang XH, Wang Y, Huang J, Zhu CY, Liu XQ, Cheung EF, Xie GR, Chan RC (2015): Increased prefrontal and parietal cortical thickness does not correlate with anhedonia in patients with untreated first‐episode major depressive disorders. Psychiatry Res. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


