Abstract
The vast majority of individuals initiate alcohol consumption for the first time in adolescence. Given the widespread nature of its use and evidence that adolescents may be especially vulnerable to its effects, there is concern about the long-term detrimental impact of adolescent drinking on adult functioning. While some researchers have suggested that genetic processes may confound the relationship, the mechanisms linking drinking and later adjustment remain unclear. The current study utilized a genetically-informed sample and biometric modeling to examine the nature of the familial influences on this association and identify the potential for genetic confounding. The sample was drawn from the Minnesota Twin Family Study, a longitudinal study consisting of 2,764 twins assessed in two cohorts at regular follow-ups from age 17 to age 29 (older cohort) or age 11 to age 29 (younger cohort). A broad range of adult measures was included assessing substance use, antisocial behavior, personality, socioeconomic status, and social functioning. A bivariate Cholesky decomposition was used to examine the common genetic and environmental influences on adolescent drinking and each of the measures of adult adjustment. The results revealed that genetic factors and non-shared environmental influences were generally most important in explaining the relationship between adolescent drinking and later functioning. While the presence of non-shared environmental influences on the association are not inconsistent with a causal impact of adolescent drinking, the findings suggest that many of the adjustment issues associated with adolescent alcohol consumption are best understood as genetically-influenced vulnerabilities.
Keywords: adolescence, alcohol, drinking, externalizing
Research on adolescent alcohol use has suggested that the adolescent brain may be particularly vulnerable to alcohol’s effects and that drinking during adolescence may result in altered brain development, impacting behavior and cognitive ability (Clark, Thatcher, & Tapert, 2008; Fleming, 2015; Guerri & Pascual, 2010; Jacobus & Tapert, 2013; Squeglia & Gray, 2016; Squeglia et al., 2015; Squeglia, Jacobus, & Tapert, 2014; Witt, 2010). These findings, along with a growing literature on the long-term relationship between adolescent drinking and later psychosocial functioning (see, e.g., McCambridge, McAlaney, & Rowe, 2011), have raised question about an enduring impact of adolescent drinking on adult outcomes.
Some researchers, however, have argued that current evidence of a relationship between adolescent drinking and deficits in later functioning is insufficient to argue for a causal association (see, e.g., Rossow & Kuntsche, 2013; Maimaris & McCambridge, 2013; McCambridge et al., 2011). Reliance on retrospective reporting of adolescent drinking, inconsistent statistical control for potential confounding variables, and a lack of designs that allow for more rigorous conclusions about causality have all been cited as weaknesses (e.g., Dawson, Goldstein, Chou, Ruan, & Grant, 2008; McCambridge et al., 2011).
To address these concerns, several prospective studies of the consequences of adolescent drinking have been implemented and have included covariates such as parental education, income, and other features of family environment to help alleviate concerns about environmental confounding (see, e.g., Chatterji, 2006; Kiff et al., 2012; Odgers et al., 2008; Viner & Taylor, 2007). However, research to date has not sufficiently targeted the existence of potential genetic links between adolescent drinking and later functioning (McCambridge et al., 2011). If the same genetic influences that impact drinking in adolescence also exert effects on functioning in adulthood, it is possible that the relationship is best explained by “genetic confounding” rather than a causal association. While there have been very few direct investigations into these genetic links, the extant literature does provide a basis for concern. First, heritability studies of adolescent alcohol consumption phenotypes (e.g., Fowler et al., 2007; Geels et al., 2012; Hicks, Schalet, Malone, Iacono, & McGue, 2011; Pagan et al., 2006; Rose, Dick, Viken, & Kaprio, 2001; Viken, Kaprio, Koskenvuo, & Rose, 1999) have revealed significant genetic influences on adolescent drinking. Second, studies of specific measures representing different domains of adult functioning that have been linked to adolescent drinking (adult drinking and substance use, antisocial behavior, mental health and personality, socioeconomic status, and interpersonal functioning) have also demonstrated these measures to be heritable (e.g., Althoff et al., 2012; Baker, Treloar, Reynolds, Heath, & Martin, 1996; Grant et al., 2009; Kendler et al., 2007; Kendler, Myers, Dick, & Prescott, 2010; Le, Miller, Slutske, & Martin, 2011; Levinson, 2006; Miller, Mulvey, & Martin, 1996; Miller, Mulvey, & Martin, 2001; Silventoinen, Krueger, Bouchard, Kaprio, & McGue, 2004; Tambs, Sundet, Magnus, & Berg, 1989). Third, both adolescent drinking phenotypes and (to varying degrees) the adult outcomes of interest have been shown to load on a latent, genetically-influenced externalizing dimension or have been shown to relate to specific behaviors that load on the dimension (e.g., Chassin, Pitts, & Prost, 2002; Compton, Thomas, Stinson, & Grant, 2007; Grant, 1997; Hicks et al., 2007; Hicks et al., 2011; Hills, Cox, McWilliams, & Sareen, 2005; Kendler, Gardner, & Dick, 2011; Kendler et al., 2007; Kenkel, Ribar, Cook, & Peltzman, 1994; Krueger et al., 2002; McGue & Iacono, 2005; Olino, Klein, Framer, Seeley, & Lewinsohn, 2012; Roisman, Aguilar, & Egeland, 2004; Slade, 2007; Verona, Sachs-Ericsson, & Joiner, 2004; Vrieze, Perlman, Krueger, & Iacono, 2012; Ystrom, Kendler, & Reichborn-Kjennerud, 2014). The externalizing dimension is a genetically-influenced psychiatric construct that accounts for the comorbidity between substance use issues, antisocial behavior, and disinhibited personality (Krueger et al., 2002). Taken together, these findings highlight a concern that any relationship between adolescent drinking and later functioning may comprise different manifestations of a common genetic risk rather than a causal process. Notably, the limited number of studies that directly examined the source of the covariation between alcohol consumption in adolescence and alcohol dependence in adulthood (e.g., Dick, Meyers, Rose, Kaprio, & Kendler, 2011; Grant et al., 2009; Kendler et al., 2010) identified very high genetic correlations that reflected a common genetic overlap.
Several recent studies have incorporated genetically-informed designs to more directly investigate the relationship between adolescent drinking and later functioning. Two prospective longitudinal studies have utilized the co-twin control method. Rose, Winter, Viken, and Kaprio (2014) examined the relationship between “drinking-related problems” in late-adolescence and functioning in emerging adulthood. Individuals with higher levels of drinking problems at age 18 were different from their lesser-drinking co-twins on adult substance use, social functioning, financial problems, educational status, and “life (dis)satisfaction.” Irons, Iacono, and McGue (2015) studied the effect of early initiation of drinking and early intoxication on a range of variables in emerging adulthood. These authors identified that earlier initiators of alcohol use were different from their co-twins on measures of adult substance use and stressful life events. While these investigations have added to the literature, the co-twin methodology does not specifically characterize the familial source (i.e., genetic or environmental) of the covariation or reveal the magnitude of genetic versus environmental influences; further, these investigations did not address adult functioning beyond emerging adulthood. A more recent investigation by Richmond-Rakerd et al. (2016) addressed this limitation by investigating the etiology of the covariation between early substance use initiation (including alcohol use) and adult substance use problems; however, the design was retrospective and did not investigate indicators of adult functioning aside from substance use disorders. Notably, past research has called for more prospective investigation into how adolescent drinking relates to a broader range of domains of functioning beyond adult substance use (McCambridge et al., 2011).
In summary, the research literature has not systematically addressed the extent to which genetic influences may explain the relationship between adolescent alcohol consumption and later functioning. Identifying the source of the covariation between adolescent drinking and adult adjustment outcomes illuminates the nature of this inadequately understood relationship and may also speak to the existence of a causal association, as significant genetic influences on the covariation would make it unlikely that the impact of adolescent alcohol exposure alone accounted for the relationship. Beyond elucidating the mechanisms underlying an association between adolescent drinking and later adjustment, systematic investigation of the source of the covariation might argue for the need for even more studies that incorporate genetically-informed samples (e.g., twin samples) to address genetic confounding concerns.
The first aim of this investigation was to replicate the associations between adolescent drinking and later functioning that have been documented in the literature thus far; this aim was especially important for adult outcomes outside of substance use that have received considerably less attention. The second, primary aim was to investigate the potential for genetic confounding by delineating the etiology of the covariation between adolescent drinking and adult functioning. The study was novel in extending the limited extant literature on the genetic overlap between adolescent drinking and adult alcohol dependence to a much wider range of adult outcomes. It was predicted that there would be significant shared genetic overlap between adolescent drinking and adult functioning, and genetic factors would be the most important in contributing to the phenotypic correlation.
Methodology
Sample
The Minnesota Twin Family Study (“MTFS”) is a prospective, longitudinal study of same-sex twin pairs born in the state of Minnesota, recruited via public birth records. Twin pairs first entered the study when they were either 11 or 17 years old and were subsequently assessed for follow-ups at approximately three year intervals. Consistent with the demographic composition of the state of Minnesota during the twins’ birth years, 98% of the sample is Caucasian. For more about the ascertainment procedure, please see Iacono, Carlson, Taylor, Elkins, and McGue (1999). Studies were conducted under protocols approved by the University of Minnesota Institutional Review Board. The sample utilized for this study combined subjects from the MTFS younger cohort, first assessed at age 11, and the older cohort, first assessed at age 17. The current study utilized data from assessments conducted when the twins were approximately 17 years old and 29 years old, which represented the intake visit and third follow-up (93.29% follow-up rate) for the older cohort and the second follow-up (87.30%) and fifth follow-up (87.57%) for the younger cohort. Notably, the study design varied from Irons et al. (2015), which also relied on the MTFS, in its inclusion of the older cohort and focus on functioning after emerging adulthood (age 29). The older cohort consisted of 1,252 individuals and the younger cohort consisted of 1,512 individuals; the combined sample totaled 2,764 individuals that comprised 486 pairs of same-sex dizygotic (DZ) twins and 896 pairs of monozygotic (MZ) twins. At each assessment, individuals presented for day-long laboratory visits or telephone interviews. Interviewers underwent extensive training and had a BA or MA in psychology. Attrition analyses revealed that level of alcohol use at the age 17 assessment was not associated with likelihood of presenting to the age 29 assessment (β = −.02, p = .77). At the age 29 assessment, approximately 56% of the sample reporting having been married. Approximately 52% of the sample had not achieved a college degree, 37% had achieved just a college degree, and 11% had achieved a master’s, professional, or doctoral degree.
Measures
In the below sections, the indicators of adolescent alcohol consumption, as well as the measures of functioning in adulthood, are delineated.
Adolescence (Age 17)
Previous studies have demonstrated that indicators of adolescent alcohol consumption, such as frequency of use and maximum number of alcohol drinks consumed in 24 hours, are highly correlated (Agrawal et al., 2009; Mason et al., 2008; Smith, McCarthy, & Goldman, 1995) and predict a range of psychosocial problems in adulthood (D’Amico, Ellickson, Collins, Martino, & Klein, 2005; Dogan, Stockdale, Widaman, & Conger, 2010; King, Meehan, Trim, & Chassin, 2006; Merline, Jager, & Schulenberg, 2008; Viner & Taylor, 2007). Given these findings, item response theory was utilized to estimate a latent dimension of alcohol consumption. It was derived by four drinking indicators assessed at the age 17 assessment: total number of lifetime intoxications (ranging from 0 to 6, with 0 = no intoxications and 6 = 50 or more), frequency of alcohol use in the previous 12 months (ranging from 0 = never to 5 = once per day), average number of drinks consumed per sitting in the previous 12 months (ranging from 0 = never to 6 = 30 or more), and maximum number of alcoholic drinks consumed in 24 hours (ranging from 0 = never to 6 = 30 or more). This formed the adolescent alcohol consumption index (“ACI”). In this analytic sample, drinking variables were assessed via the Computerized Substance Use Questionnaire (α = .95), a computerized self-report inventory that assesses use of substances, and the Substance Abuse Module of the Composite International Diagnostic Interview (“SAM”) (α = .91) (Robins, Baber, & Cottler, 1987). The correlation between scores on the CSU and the SAM was r = .88. The final index was standardized (minimum score = −1.39, maximum score = 2.11, skewness = −.22). Consistent with sex differences in adolescent drinking patterns, the mean was higher in males (.10) than in females (−.09). The correlation between the ACI and each of the CSU drinking indicators ranged from .78 to .89; correlations between the ACI and each of the SAM drinking indicators ranged from .73 to .89. The ACI has been used in previous studies examining the neurotoxic effects of alcohol use (Wilson, Malone, Thomas, & Iacono, 2015) and parent-offspring similarity in alcohol consumption (McGue, Malone, Keyes, & Iacono, 2014). For more details about the validity of this index, please see McGue et al. (2014).
Adulthood (Age 29)
The specific outcomes and scales included at age 29 were based on the previous prospective longitudinal studies of adolescent drinking which have largely examined discrete measures of adult substance use and substance use disorders (see, e.g., Kiff et al., 2012; Merline, Jager, & Schulenberg, 2008; Odgers et al., 2008; Pitkanen, Kokko, Lyyra, & Pulkkinen, 2008; Rossow & Kuntsche, 2013; Viner & Taylor, 2007), antisocial behaviors and legal problems (see, e.g., Green, Doherty, Zebrak, & Ensminger, 2011; Odgers et al. 2008; Viner & Taylor, 2007;), socioeconomic status (see, e.g., Chatterji, 2006; King, Meehan, Trim, & Chassin, 2006), mental health and personality (see, e.g., Kiff et al., 2012; Wells et al., 2004), and social functioning (see, e.g., Dogan, Stockdale, Widaman, & Conger, 2010) were included. For more detail about the comparable measures selected for this study, including discussions of their reliability and validity, please see other investigations that have used them with the current sample (Foster, Hicks, Iacono, & McGue, 2014; Hicks, Iacono, & McGue, 2010). Brief descriptions of the measures are given below.
Alcohol and drug use, as well as symptoms of substance use disorders, were assessed using the SAM. Measures of alcohol use included largest number of standard alcoholic drinks consumed in 24 hours (“Largest # Drinks Consumed”) and average number of drinks consumed per sitting (“Average # Drinks Consumed”). Measures of drug use included the number of drug classes tried (“Drug Classes Tried”) and number of marijuana uses (“Recent Marijuana Uses”) during adulthood. Substance use disorders were assessed via separate symptom counts for nicotine dependence, alcohol use disorder, and marijuana use disorder (“Nicotine Dependence SX”, “Alcohol Use Disorder SX”, “Marijuana Use Disorder SX”).2
Diagnostic kappa reliabilities for the substance use disorders were greater than .85.
Antisocial behavior was measured via DSM-IV symptoms of antisocial personality disorder (“Adult Antisocial Behavior SX”) as well as other antisocial behaviors that may not have been severe enough to be coded as a symptom. Adult antisocial behavior symptoms were identified with the Structured Clinical Interview for DSM-IV Personality Disorders (Spitzer, Williams, Gibbon, & First, 1987). Diagnostic kappa reliability was greater than .85. The other antisocial behaviors were assessed using the Life Events Interview (Billig, Hershberger, Iacono, & McGue, 1996); these included a sum of four legal problems including events like having trouble with the police for issues other than traffic violations (“Legal Problems”). The reliability of the Life Events Interview has been supported by examining twin concordance for various family events (e.g., parental divorce, family money trouble) that should be shared within members of a twin pair (Hicks, South, DiRago, Iacono, & McGue, 2009).
Socioeconomic status was represented via measures of educational stagnation, occupational stagnation, and financial problems. “Educational Stagnation” was assessed via the Social Adjustment Interview with a 12-point schedule that ranged from completion of a doctoral degree (scored 1) to failure to complete a high school degree (scored 12). Similarly, “Occupational Stagnation” was assessed with the Hollingshead system, a seven-point scale with categories ranging from “major professional” (scored 1) to “unskilled” (scored 7). Finally, the Life Events Interview was used to assess financial problems, which included a sum of five issues experienced during adulthood: losing a job, filing for bankruptcy, receiving money from the government, having financial issues, and being unemployed (“Financial Problems”).
Mental health problems were defined by symptoms of major depressive disorder, assessed via the Structured Clinical Interview for DSM-IV (Spitzer, Williams, Gibbon, & First, 1992) (“Major Depressive SX”). Diagnostic kappa reliability was greater than .85. Given recent evidence in support of dimensional models of psychopathology based on personality factors, it was also decided to include personality measures (negative emotionality and behavioral constraint) that have been shown to index risk for psychopathology (Elkins, King, McGue, & Iacono, 2006; Krueger, McGue, & Iacono, 2001), assessed using the Multidimensional Personality Questionnaire (Tellegen & Waller, 2008) (“Behavioral Disinhibition”, “Negative Emotionality”).3 For Negative Emotionality, lower order scales α ranged from .76 to .90 and 30 day test-retest reliability was .89 (Tellegen & Waller, 2008). For Behavioral Disinhibition, lower order scales α ranged from .79 to .86 and 30 day test retest reliability was .89 (Tellegen & Waller, 2008).
Measures of social functioning included a 27-item scale where individuals were asked to rate how many of their friends engaged in antisocial behaviors (“Antisocial Peers”, α = .64) and prosocial behaviors (“Prosocial Peer Disaffiliation”, α = .82).4 Number of recent sexual partners was assessed via the Life Events Interview (“Recent Sexual Partners”). Individuals who were in a romantic relationship for at least three months were asked about their partner’s use of alcohol as well as their partner’s beliefs and attitude toward substance use. From these questions, an index of partner’s drinking was computed by averaging standardized scores for average quantity consumed, frequency of drinking (10-point scale from never to 3 or more times a day), and proportion of time drunk to intoxication (5-point scale from never to every time or nearly every time) (“Partner’s Alcohol Misuse”). Beliefs about substance use were assessed via items inquiring about the degree to which the individual supported or was upset by alcohol and drug use (11-items each assessed on a four point scale), with a higher score representing more permissiveness toward substance use (“Partner’s Permissiveness Toward Substance Use”, α = .83).
Note that for the substance use measures, adult antisocial behavior symptoms, major depressive symptoms, and the measures assessed using the Life Events Interview, the ascertainment period was since the age 24 assessment (e.g., the number of drug classes tried pertained only to drug classes tried between the age 24 and age 29 assessment). The socioeconomic indicators, personality measures, assessment of peer functioning, and romantic partner’s alcohol use and permissiveness toward substance use were asked with reference to current experiences at age 29. Means and standard deviations for all of these measures prior to standardization (or log transformation, for those variables with substantial skew) are included in Table 1.
Table 1.
Descriptive Statistics of Adult Outcome Measures Used and Regression of Age 17 Adolescent Alcohol Consumption Index (“ACI”) on Age 29 Adult Outcomes
| Outcome Variable | Descriptive Statistics | Regression of Adult Outcome on Adolescent Alcohol Consumption Index | |||
|---|---|---|---|---|---|
|
| |||||
| Mean | Standard Dev. | Min | Max | B | |
| Substance Use and Substance Use Disorders | |||||
| Largest # Drinks Consumed | 9.26 | 7.29 | 0 | 72 | .36(.02)*** |
| Avg # Drinks Consumed | 3.16 | 2.36 | 0 | 24 | .37(.02)*** |
| Drug Classes Tried | .53 | 1.01 | 0 | 5+ | .33(.02)*** |
| Recent Marijuana Uses | 17.47 | 63.99 | 0 | 400 | .29(.02)*** |
| Nicotine Dependence SX | .82 | 1.39 | 0 | 6 | .30(.02)*** |
| Alcohol Use Disorder SX | .57 | 1.34 | 0 | 11 | .25(.02)*** |
| Marijuana Use Disorder SX | .24 | .99 | 0 | 10 | .21(.02)*** |
| Antisocial Behavior and Legal Problems | |||||
| Adult Antisocial Behavior SX | .73 | .87 | 0 | 6 | .28(.02)*** |
| Legal Problems | .28 | .77 | 0 | 4 | .16(.02)*** |
| Socioeconomic Status | |||||
| Educational Stagnation | 5.32 | 2.69 | 0 | 12 | .18(.02)*** |
| Occupational Stagnation | 3.47 | 1.58 | 0 | 7 | .17(.02)*** |
| Financial Problems | .63 | .98 | 0 | 5 | .09(.02)*** |
| Mental Health and Personality | |||||
| Major Depressive SX | 1.14 | 2.41 | 0 | 9 | .05(.02)* |
| Negative Emotionality | 79.41 | 13.57 | 40.90 | 131.80 | .09(.02)*** |
| Behavioral Disinhibition | 125.91 | 15.63 | 74.60 | 195.00 | .17(.02)*** |
| Social Functioning | |||||
| Antisocial Peers | 33.79 | 4.79 | 23 | 60 | .32(.02)*** |
| Prosocial Peer Disaffiliation | 20.86 | 2.77 | 11 | 36 | .14(.02)*** |
| Recent Sexual Partners | 1.93 | 2.21 | 0 | 20 | .21(.02)*** |
| Partner’s Alcohol Misuse | .00 | .82 | −1.56 | 3.25 | .22(.02)*** |
| Partner’s Permissiveness | 26.87 | 6.31 | 11 | 44 | .33(.03)*** |
| Toward Substance Use | |||||
Note. Means, standard deviation, and range of raw scores on each variable (prior to standardization or log transformation, for those variables with substantial skew) are shown above. In the right-most column, measures of age 29 functioning were separately regressed on the age 17 ACI via linear mixed models. All variables were z-scored. All analyses adjusted for gender, cohort, zygosity, and ages at the age 17 and age 29 assessments. Models included random intercepts to account for the correlated nature of twin observations. Ns for these analyses range from 1762 (for age 29 outcomes relating to participant’s romantic partner) to 2332.
p < .05,
p < .01,
p < .001
Data Analyses
Each of the adult outcomes was standardized and separately regressed on the ACI via a linear mixed model to determine if there was an association between the ACI and the adult variable. These models were fit utilizing PROC MIXED in SAS. Family group was included as a random effect to account for the fact that observations within twin pairs are not independent. Covariates included cohort membership, zygosity, gender, and specific ages at the age 17 adolescent follow-up (average age at assessment was 17.83 with a standard deviation of .69) and the adult age 29 follow-up (average age at assessment was 29.43 with a standard deviation of .67).
For the biometric analyses, twin models were fit utilizing the structural equation model-fitting statistical package OpenMx in R (Boker et al., 2011, Boker et al., 2012). Missing data was addressed using full information maximum-likelihood estimation. First, standard maximum likelihood-based biometric models were implemented to examine the univariate influences on the variance of each of the adult variables and the ACI (Neale & Cardon, 1992). It was assumed that the variance in the phenotypes could be partitioned into additive genetic (A), shared environmental (C), and non-shared environmental components (E). Shared environment reflects environmental influences that make members of a twin pair more similar to each other, while non-shared environment reflects influences that are specific to the individual as well as measurement error. Theory predicts that the MZ covariance is equivalent to A + C and the DZ covariance is equivalent to (1/2)A + C. The fit of univariate AE, CE, and E submodels was assessed based on a Chi squared goodness-of-fit test as well as model parsimony. The Akaike Information Criterion (AIC) provided an index of the optimal balance of goodness-of-fit and parsimony (Akaike, 1987).
If there was a statistically significant relationship between the ACI and the adult measure, a bivariate Cholesky decomposition model was utilized to determine the strength of the genetic, shared environmental, or non-shared environmental covariation between adolescent drinking and each of the adult outcomes (Neale & Cardon, 1992). The Cholesky decomposition is a saturated model that allows one to identify the levels on which adolescent drinking and adult functioning covary (see Figure 1 for a depiction of the model). It estimates six latent factors (A1, C1, E1, A2, C2, and E2). The model specifies that all of the variance in adolescent drinking, as well as the covariation between adolescent drinking and adult functioning, load on the first factors (A1, C1, E1). The residual variance in the adult outcome variable load on the second factors (A2, C2, E2). In this instance, the first variable entered in the model was adolescent drinking, and the second variable was the specific indicator of adult functioning being examined. If the genetic or shared environmental influences on adolescent drinking accounted for substantial variance in the adult measure, there would be greater concern about the potential for familial confounding. In contrast, non-shared environmental overlap would indicate that within sibling pairs, the twin who drank more alcohol tended to have more problems in adult adjustment than their co-twin, despite the pair’s environmental similarities and shared genes. Results of the Cholesky decomposition were then transformed to yield a mathematically equivalent standardized solution (correlated factors model) (Loehlin, 1996), which provides correlations reflecting the extent to which the genetic, shared environmental, or non-shared environmental influences on adolescent drinking and adult functioning overlap with each other (Posthuma, 2009). The amount of the phenotypic correlation attributable to each of these sources is a function of these correlations as well as the univariate heritability of each variable.
Figure 1. Bivariate Cholesky Model.
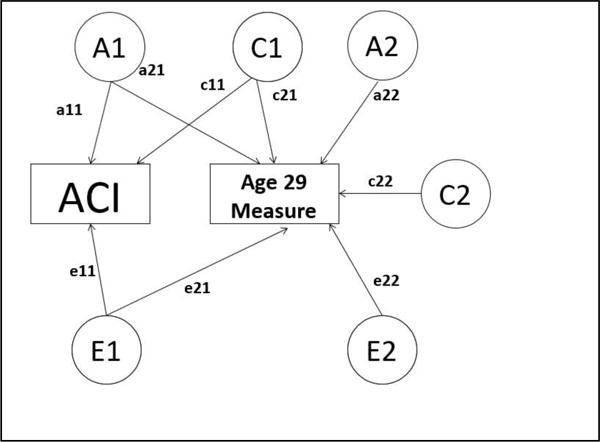
Note. In the bivariate Cholesky model, genetic and environmental contributions to the age 17 adolescent alcohol consumption index (“ACI”) can also contribute to the measure of age 29 functioning. This figure is a path diagram for the bivariate Cholesky decomposition, where observed phenotypes are shown in squares and latent factors (A, C, and E) are shown in circles. A = additive genetic influences; C = shared environmental influences; E = non-shared environmental influences; a11 = genetic path for the ACI; c11 = shared environmental path for the ACI; e11 = non-shared environmental path for the ACI; a12 = genetic covariance between the ACI and the age 29 outcome; c12 = shared environmental covariance between the ACI and the age 29 outcome; e12 = non-shared environmental covariance between the ACI and the age 29 outcome; a22 = genetic path unique to the age 29 outcome; c22 = shared environmental path unique to the age 29 outcome; e22 = non-shared environmental path unique to the age 29 outcome.
Subsequently, three additional submodels were completed that dropped shared environmental influences common to both the ACI and the adult outcome (AE model), dropped genetic influences common to both the ACI and the adult outcome (CE model), or dropped both genetic and shared environmental influences. As in the univariate case, the viability of these submodels was based on the AIC.
Results
For the regressions in the first stage of analyses (see Table 1), adolescent drinking was significantly associated with each of the adult outcome measures. Consistently, the largest effect sizes were present for the substance use and social functioning measures.
Univariate Analyses
Table 2 reports the twin correlations for the ACI and all of the adult outcomes. Correlations for all of the variables were statistically significant within MZ twins, ranging from .23 to .69. With the exception of legal problems, correlations were also significant within DZ twins, ranging from .03 to .56. With one exception (financial problems), the MZ correlation was higher than the DZ correlation, indicating genetic influences.
Table 2.
Correlations within MZ and DZ Twin Pairs and Univariate Heritability of Age 17 Adolescent Alcohol Consumption Index and Age 29 Adult Outcomes
| Adolescent Measures | MZ | DZ | A | C | E |
|---|---|---|---|---|---|
| Adolescent Alcohol Consumption Index (“ACI”) | .72(.69, .75) | .55(.48, .60) | .34(.22, .48) | .38(.24, .49) | .28(.25, .31) |
| Age 29 Measures | |||||
|
| |||||
| Substance Use and Substance Use | |||||
| Largest # Drinks Consumed | .51(.45, .56) | .29(.21, .37) | .43(.24, .56) | .08(.00, .24) | .49(.44, .55) |
| Avg # Drinks Consumed | .50(.45, .55) | .29(.21, .37) | .42(.23, .55) | .08(.00, .25) | .50(.45, .55) |
| Drug Classes Tried | .53(.48, .58) | .24(.15, .33) | .53(.42, .58) | .00(.00, .13) | .47(.42, .52) |
| Recent Marijuana Uses | .50(.45, .55) | .28(.20, .36) | .44(.25, .55) | .06(.00, .22) | .50(.45, .55) |
| Nicotine Dependence SX | .53(.47, .57) | .32(.24, .40) | .40(.23, .57) | .12(.00, .28) | .48(.43, .53) |
| Alcohol Use Disorder SX | .24(.17, .30) | .17(.08, .26) | .13(.00, .30) | .11(.00, .26) | .76(.70, .83) |
| Marijuana Use Disorder SX | .48(.42, .53) | .24(.16, .31) | .48(.29, .53) | .00(.00, .15) | .52 (.47, .58) |
| Antisocial Behavior and Legal Problems | |||||
| Adult Antisocial Behavior SX | .37(.31, .43) | .19(.10, .28) | .36(.15, .43) | .01(.00, .19) | .63(.57, .69) |
| Legal Problems | .23(.16, .29) | .03(.00, .13) | .21(.10, .27) | .00(.00, .09) | .79(.73, .85) |
| Socioeconomic Status | |||||
| Educational Stagnation | .69(.66, .73) | .56(.50, .62) | .26(.14, .40) | .43(.30, .54) | .31(.27, .34) |
| Occupational Stagnation | .49(.43, .55) | .15(.05, .24) | .48(.42, .53) | .00(.00, .08) | .52(.47, .58) |
| Financial Problems | .23(.16, .30) | .29(.20, .37) | .00(.00, .13) | .25(.20, .31) | .75(.69, .80) |
| Mental Health and Personality | |||||
| Major Depressive SX | .23(.16, .30) | .11(.03, .20) | .23(.11, .30) | .00(.00, .18) | .77(.70, .82) |
| Negative Emotionality | .44(.38, .50) | .19(.10, .28) | .44(.28, .49) | .00(.00, .14) | .56(.51, .62) |
| Behavioral Disinhibition | .50(.45, .55) | .15(.06, .24) | .49(.43, .54) | .00(.00, .07) | .51(.46, .57) |
| Social Functioning | |||||
| Antisocial Peers | .50(.45, .55) | .27(.18, .35) | .47(.28, .55) | .03(.00, .20) | .50(.45, .55) |
| Prosocial Peer Disaffiliation | .39(.33, .45) | .24(.14, .33) | .32(.10, .45) | .08(.00, .26) | .60(.55, .67) |
| Recent Sexual Partners | .39(.32, .45) | .25(.16, .33) | .29(.08, .45) | .10(.00, .28) | .61(.55, .68) |
| Partner’s Alcohol Misuse | .30(.21, .37) | .17(.04, .29) | .25(.00, .37) | .05(.00, .29) | .70(.63, .79) |
| Partner’s Permissiveness Toward Substance Use | .41(.33, .48) | .25(.13, .36) | .32(.08, .48) | .09(.00, .32) | .59(.52, .67) |
Note. The 95% confidence intervals of the correlations and variance component estimates are shown in parentheses. A = additive genetic influences; C = shared environmental influences; E = non-shared environmental influences.
The results of the univariate biometric models are presented in Table 2. There were significant genetic, shared environmental, and non-shared environmental influences on adolescent drinking; estimates were comparable to other investigations of familial influences on alcohol consumption that have been completed with the MTFS (see, e.g., Hicks et al., 2011). Adult measures varied on the contributions of genetic and shared environmental influences. For the majority of the variables, including most of the substance use variables, genetic influences were significant while shared environmental contributions were not. Shared environmental contributions were significant for financial problems and educational stagnation. For each measure, the CE, AE, and E submodels were compared with the ACE model. Fit indices for the submodels are available as supplementary materials (Supplementary Table 1). For most of the measures, constraining the shared environmental influence to be zero did not result in a significant loss of model fit, and the AE model was the best model for the majority of the measures. In summary, the univariate analyses suggested that genetic influences account for a significant portion of the variability in most of the adult adjustment measures, which provides a foundation for the hypothesis that shared genetic influences could explain the association between adolescent drinking and later adjustment.
Bivariate Analyses
Cross-twin cross-trait correlations between the ACI and each of the adult variables are reported in Table 3. MZ cross-twin cross-trait correlations were generally larger than the DZ correlations, suggesting genetic influences on the covariation between adolescent drinking and adult functioning, although the confidence intervals for MZ and DZ twin pairs were overlapping for almost all of the measures, except for recent marijuana uses, drug classes tried, and adult antisocial behavior symptoms. Results for the bivariate analyses are discussed below by domain of functioning. Standardized path coefficients for each model are presented in the supplementary materials (Supplementary Figure 1), while the genetic, shared environmental, and non-shared environmental correlations from the correlated factors solution are reported in Table 4. Fit indices of submodels appear in supplementary materials (Supplementary Table 2).
Table 3.
Cross-Twin Cross-Trait Correlations between Age 17 Adolescent Alcohol Consumption Index and Age 29 Adult Outcomes
| MZ | DZ | |
|---|---|---|
| Substance Use and Substance Use Disorders | ||
| Largest # Drinks Consumed | .35(.30, .39) | .24(.18, .30) |
| Avg # Drinks Consumed | .34(.29, .38) | .26(.20, .31) |
| Drug Classes Tried | .29(.25, .34) | .17(.11, .23) |
| Recent Marijuana Uses | .26(.21, .30) | .14(.08, .20) |
| Nicotine Dependence SX | .28(.23, .32) | .19(.13, .25) |
| Alcohol Use Disorder SX | .21(.16, .26) | .14(.08, .20) |
| Marijuana Use Disorder SX | .19(.15, .24) | .12(.07, .18) |
| Antisocial Behavior and Legal Problems | ||
| Adult Antisocial Behavior SX | .26(.22, .31) | .15(.08, .21) |
| Legal Problems | .13(.08, .17) | .10(.03, .16) |
| Socioeconomic Status | ||
| Educational Stagnation | .19(.14, .23) | .16(.10, .22) |
| Occupational Stagnation | .14(.09, .19) | .14(.07, .20) |
| Financial Problems | .06(.01, .11) | .08(.02, .14) |
| Mental Health and Personality | ||
| Major Depressive SX | .01(−.04, .06) | .04(−.02, .10) |
| Negative Emotionality | .06(.01, .11) | .08(.02, .14) |
| Behavioral Disinhibition | .15(.10, .20) | .09(.03, .15) |
| Social Functioning | ||
| Antisocial Peers | .31(.26, .35) | .20(.14, .26) |
| Prosocial Peer Disaffiliation | .16(.11, .20) | .07(.01, .13) |
| Recent Sexual Partners | .19(.14, .23) | .12(.06, .18) |
| Partner’s Alcohol Misuse | .20(.15, .25) | .15(.08, .22) |
| Partner’s Permissiveness Toward Substance Use | .28(.23, .33) | .20(.13, .27) |
Note. The 95% confidence intervals of the correlations are shown in parentheses.
Table 4.
Genetic and Environmental Influences on the Relationship between Age 17 Adolescent Alcohol Consumption Index and Age 29 Adult Functioning
| Adult Outcome | ra | rc | re | Phenotypic Correlation | Contribution of Familial & Non-shared Environmental Influences to Phenotypic Correlation | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| A | C | E | |||||
| Substance Use and Substance Use Disorders | |||||||
| Largest # Drinks Consumed | .55(.28, .84) | .79(.19, 1.00) | .12(.05, .20) | .39(.35, .43) | .21 | .13 | .05 |
| Avg # Drinks Consumed | .43(.16, .72) | .92(.39, 1.00) | .14(.06, .21) | .39(.35, .43) | .16 | .18 | .05 |
| Drug Classes Tried | .56(.32, .80) | 1.00(−1.00, 1.00) | .15(.08, .23) | .35(.31, .39) | .23 | .06 | .06 |
| Recent Marijuana Uses | .61(.33, .91) | .16(−1.00, 1.00) | .12(.04, .19) | .30(.26, .35) | .23 | .03 | .04 |
| Nicotine Dependence SX | .45(.16, .74) | .52(−.04, 1.00) | .12(.05, .19) | .32(.28, .36) | .17 | .11 | .04 |
| Alcohol Use Disorder SX | .61(.06, 1.00) | .38(−1.00, 1.00) | .11(.03, .18) | .26(.22, .30) | .14 | .07 | .05 |
| Marijuana Use Disorder SX | .35(.07, .64) | 1.00(−1.00, 1.00) | .07(−.01, .14) | .22(.18, .26) | .14 | .05 | .03 |
| Antisocial Behavior and Legal Problems | |||||||
| Adult Antisocial Behavior SX | .67(.34, 1.00) | .34 (−1.00, 1.00) | .09(.02, .16) | .30(.26, .34) | .23 | .03 | .04 |
| Legal Problems | .24(−.25, .67) | 1.00(−1.00, 1.00) | .10(.02, .17) | .17(.13, .21) | .06 | .06 | .05 |
| Socioeconomic Status | |||||||
| Educational Stagnation | .17(−.15, .48) | .33(.10, .57) | .09(.02, .16) | .21(.17, .26) | .05 | .13 | .03 |
| Occupational Stagnation | .07(−.22, .33) | 1.00(.28, 1.00) | .08(.004, .16) | .17(.12, .22) | .03 | .11 | .03 |
| Financial Problems | −1.00(−1.00, 1.00) | .30(−.04, .71) | .06(−.01, .13) | .09(.05, .14) | −.03 | .09 | .03 |
| Mental Health and Personality | |||||||
| Major Depressive SX | −.22(−1.00, .23) | 1.00(−1.00, 1.00) | .07(−.001, .14) | .04(.001, .09) | −.06 | .07 | .03 |
| Negative Emotionality | −.07(−.42, .23) | 1.00(−1.00, 1.00) | .06(−.02, .13) | .09(.04, .13) | −.02 | .09 | .02 |
| Behavioral Disinhibition | .29(.04, .54) | 1.00(−1.00, 1.00) | .09(.01, .16) | .18(.14, .23) | .12 | .03 | .03 |
| Social Functioning | |||||||
| Antisocial Peers | .55(.29, .82) | 1.00(−1.00, 1.00) | .10(.03, .18) | .35(.31, .39) | .22 | .09 | .04 |
| Prosocial Peer | .52(.15, 1.00) | −.11(−1.00, 1.00) | −.01(−.08, .06) | .15(.11, .20) | .17 | −.02 | −.003 |
| DReiscaefnfitl Si aetxiouna l Partners | .41(.02, 87) | .29(−1.00, 1.00) | .08(.01, .16) | .22(.18, .26) | .13 | .06 | .03 |
| Partner’s Alcohol | .35(−.16, 1.00) | 1.00(−1.00, 1.00) | .05(−.03, .14) | .23(.18, .27) | .11 | .10 | .02 |
| MPairstunseer’ s Permissiveness | .46(.07, .96) | .76(−1.00, 1.00) | .11(.03, .19) | .33(.28, .37) | .16 | .12 | .05 |
| Toward Substance Use | |||||||
Note. rg = genetic correlation; rc = shared environmental correlation; re = nonshared environmental correlation. The 95% confidence intervals of the correlations are shown in parentheses. A = contribution of additive genetic influences to phenotypic correlation; C = contribution of shared environmental influences to phenotypic correlation; E = contribution of non-shared environmental influences to phenotypic correlation.
Substance use variables
For all of the substance use measures, except average drinks consumed, genes accounted for most of the relationship with adolescent drinking — the percentage of the phenotypic correlation accounted for by genes ranged from 42 to 77%. The variables where genes contributed the most to the overlap with adolescent drinking were all indicators of illicit substance use: recent marijuana uses, drug classes tried, and marijuana use disorder symptoms (the percentage of the covariation account for by genes ranged from 64 to 77% for these variables). Genetic correlations were statistically significant for all of the substance use measures and were the largest for alcohol use disorder symptoms and recent marijuana uses (rg = .61). Further, the shared environmental correlation was significant for largest drinks consumed and average number of drinks consumed. There were small but significant non-shared environmental correlations for all of the substance use measures except for marijuana use disorder symptoms.
Antisocial behavior and legal problems
Symptoms of adult antisocial behavior had significant genetic overlap with adolescent drinking (rg = .67), with genes contributing to the majority of the covariation (approximately 77%). In contrast, genes, shared, and non-shared environment contributed roughly equally to the covariation with legal problems. Genetic and shared environmental correlations for legal problems were each nonsignificant, although dropping both the a21 and c21 paths resulted in a marked loss of fit. Small but significant non-shared environmental correlations were present for both legal problems and adult antisocial behavior symptoms.
Socioeconomic status
For occupational stagnation, educational stagnation, and financial problems, shared environmental influences were most important in explaining the relationship with adolescent drinking, accounting for over half of the covariation. The shared environmental correlation reached unity for occupational stagnation and was also significant for educational stagnation (rc = .33). Neither the genetic nor the shared environmental correlation reached significance for financial problems, but setting both the c21 and a21 paths to zero resulted in a significant loss of model fit. Significant non-shared environmental correlations were present for both educational and occupational stagnation.
Mental health and personality
There was a significant genetic correlation between adolescent drinking and behavioral disinhibition (rg = .29), and genetic factors accounted for most of the overlap between the ACI and this measure (approximately 64%). The non-shared environmental correlation was also statistically significant. In contrast, shared environment accounted for most of the relationship with both major depressive symptoms and negative emotionality; however, for depression, the a21 and c21 pathways could be dropped without a significant loss of model fit.
Social functioning
Genetic influences contributed the most variance to the phenotypic overlap with each of the indicators of social functioning, contributing the most to the phenotypic correlation with prosocial and antisocial peers. With the exception of partner’s alcohol misuse, genetic correlations were significant for each of these measures, ranging from rg = .41 to .55. Notably, there were significant non-shared environmental correlations for partner’s permissiveness toward substance use, recent sexual partners, and antisocial peers.
Unique variance in adult functioning
As evident in the model results in Supplementary Figure 1, almost without exception, the most variance in each of the adult measures was explained by non-shared environmental influences that were not shared with adolescent drinking, ranging from 46% (drug classes tried) to 79% (legal problems). The sole exception was educational attainment, where the most variance in the measure was explained by shared environmental influences that were not common with adolescent drinking (38%). By comparison, across all of the measures, genetic effects shared with adolescent drinking contributed at most 16% of the total variance in the adult measures (for drug classes tried, recent marijuana uses, and adult antisocial behavior symptoms), and shared environmental effects overlapping with adolescent drinking contributed at most 8% of the total variance (for average drinks consumed).
Results Summary
In summary, consistent with the hypothesis, there was significant genetic overlap between adolescent drinking and multiple measures of adult functioning. Out of the 20 variables examined, there were significant genetic correlations with the ACI for 13 measures that spanned each of the areas of adult functioning, with the exception of adult socioeconomic status. For fourteen measures from each of the areas of adult functioning assessed, there were significant non-shared environmental correlations with adolescent drinking. Finally, for four measures spanning adult substance use and socioeconomic status, significant shared environmental correlations were present.
Similarly, as hypothesized, for most of the adult measures (13 out of 20), genes accounted for the most of the phenotypic correlation with adolescent drinking, while shared environment was the most important for the remaining measures (note that for legal problems, genes and shared environment were equal contributors). There was a fairly consistent pattern where genetic influences accounted for the majority of the covariance with the adult substance use measures (present for six of the seven adult measures) and for adult social functioning (present for all five measures), while shared environmental influences accounted for the majority of the covariance with the adult socioeconomic measures.
Discussion
The purpose of this study was to delineate the extent of the genetic overlap between adolescent drinking and a range of domains of adult functioning. Identification of the mechanisms linking adolescent drinking to later functioning can help elucidate the nature of this inadequately understood relationship. The analyses revealed several key findings that warrant further discussion. First, adolescent alcohol consumption was associated with a wide array of adult adjustment variables across each of the domains assessed. Most prospective studies have focused on adult substance use variables with less emphasis on other domains (see, e.g., Marshall, 2014; McCambridge et al., 2011). The current findings provide additional support for the previously limited literature demonstrating a relationship between adolescent drinking and non-substance use adult outcomes. Identifying associations between adolescent drinking and multiple domains of adult functioning provides a rationale for increased focus on the etiology and impact of adolescent drinking.
Second, consistent with the hypothesis, the genetic influences exercising effects on adolescent drinking behavior at age 17 were also exercising effects 12 years later on a number of adult adjustment variables. As such, since the phenotypic correlation between adolescent drinking and adult functioning largely reflects shared genetic influences, it is unlikely that the relationship can be attributed solely to the consequences of exposure to alcohol during adolescence, a finding that has important public health implications for understanding the impact that interventions designed to reduce adolescent drinking will have on functional outcomes.
Notably, the pattern of shared genetic influences on adolescent drinking and adult functioning was present for a number of measures that have been demonstrated to load on the genetically-influenced externalizing dimension, including adult substance use (Hicks et al., 2011), adult antisocial behavior (Krueger et al., 2002; Vrieze et al., 2012), and behavioral disinhibition (Hicks et al., 2011). Given the stability of the externalizing dimension across the lifespan (Vrieze et al., 2012), it is not surprising that the variables loading the most highly on this construct at age 29 would be linked to adolescent drinking through genetic mechanisms.
Further, the presence of common genetic influences on the covariation between adolescent drinking and adult adjustment is consistent with the concern that genetic risk could confound the relationship (see, e.g., Bornovalova, Huibregtse, Hicks, Keyes, McGue, & Iacono, 2013; Goodnight et al., 2016, for additional discussions of this interpretation). These findings underscore that researchers interested in the causal impact of alcohol consumption in adolescence should utilize methodology (e.g., propensity score-matching, co-twin control design) to address possible confounding.
Third, while non-shared environmental factors specific to each of the adult measures were the greatest source of variance for most of the measures, non-shared environmental factors were generally only minor contributors to the covariation with adolescent drinking. It appears that while there are significant factors that contribute to alcohol use in adolescence that are not shared within twin pairs (i.e., significant non-shared environmental influences), they have limited overlap with the non-shared environmental factors that contribute to adult outcomes. Nonetheless, the analyses still detected small significant non-shared environmental correlations between adolescent drinking and most of the adult measures. (In other words, within sibling pairs, the heavier drinking twin tended to demonstrate more adjustment issues than their lesser drinking co-twins). This pattern was evident for multiple substance use indicators, antisocial behavior symptoms, legal problems, behavioral disinhibition, socioeconomic status, partner’s permissiveness toward substance use, recent sexual partners, and antisocial peer engagement. As discussed in Bornovalova et al. (2013), small non-shared environmental overlap would be consistent with (although not direct evidence for) a subtle causal impact of adolescent drinking. In short, while the common genetic influences on the relationship between adolescent drinking and most of the adult adjustment indicators would suggest that the phenotypic correlation likely cannot be accounted for exclusively by the impact of adolescent alcohol exposure, the current findings do not rule out the presence of a causal process.
Fourth, shared environmental factors were also important in explaining the relationship between adolescent drinking and multiple indicators of adult functioning. While genetic risk associated with adolescent drinking has been discussed due to its relationship with the externalizing dimension, this result provides a reminder that researchers must also consider shared environmental influences as a potential explanatory mechanism linking adolescent alcohol exposure and adult adjustment, and in studies of the causal impact of adolescent drinking, shared environmental factors may represent a confounding influence that must be addressed methodologically through designs like the co-twin control analysis that can account for these family influences.
In sum, this study suggests that the relationship between adolescent drinking and adult functioning is best understood by focusing on genetic influences. The pattern of results would be consistent with a scenario where common genetic factors contribute to both adolescent drinking and adult functioning, and it emphasizes the need for research designs that address confounding familial influences when investigating the causal consequences of adolescent drinking on later functioning.
This study is not without its limitations. As discussed, the Cholesky decomposition does not provide direct evidence of specific confounding or causal processes. The sample utilized was largely Caucasian, which limits generalizability. Further, for some of the bivariate models, submodels that dropped common genetic influences were not significantly different from submodels that dropped shared environmental influences, making it difficult to firmly disentangle the contributions of genes and environment to the relationship between adolescent drinking and later functioning. Finally, the scope of this study and power considerations limited analyses to one indicator of adolescent alcohol consumption; it is also possible that studying other indicates of use, including problematic drinking, would be informative.
Several avenues of future research would be useful in further assessing the mechanisms linking adolescent drinking and adult functioning. First, researchers might focus on specific developmental precursors to adolescent drinking and test the degree to which they account for genetic or environmental overlap between adolescent drinking and adult functioning. Once these factors are established, it would be possible to investigate how moderating influences in the environment affect the familial contributions to the relationship. For example, an initial step might be investigating whether the genetic risk for childhood or adolescent externalizing behavior accounts for the genetic risk that underlies the association between adolescent drinking and adult functioning. Given recent research suggesting the existence of a general factor of psychopathology “p” (Caspi et al., 2014), it would also be important to distinguish whether the relationship is best accounted for by the externalizing dimension or by the p factor, which is characterized by poor self-control and executive functioning deficits in childhood that could plausibly link impulsive behavior in adolescence (like substance use) with later problems.
Second, studies with greater power to more clearly disentangle the roles of genetic versus shared environmental factors and examine a wider range of adolescent drinking measures would be important to expand upon this investigation. Third, while this investigation was not aimed at delineating concrete evidence of a causal process between adolescent drinking and adult functioning, understanding the presence of such a causal link would have very significant public health implications. This study’s findings regarding common familial influences on adolescent drinking and adult adjustment raises question about methodology used in past studies that were aimed at causally linking adolescent drinking and adult functioning. The findings speak to the need for future research studies investigating causality using methodological approaches that systematically account for the role of familial confounding influences, such as the co-twin control design. Relatedly, future research should attempt to identify whether the genetic overlap between adolescent drinking and adult outcomes is truly explained by confounding influences (consistent with biological pleiotropy, where the same genetic variants influence different phenotypes) or mediated pleiotropy (when one phenotype causes another phenotype, and a genetic variant is associated directly with the first phenotype) (Solovieff, Cotsapas, Lee, Purcell, & Smoller, 2013).
The current study utilized a helpful statistical method to examine the relationship between adolescent drinking and adult functioning. Results suggest the importance of genetic factors in explaining the relationship; they further suggest that many other factors beyond adolescent drinking are important in understanding contributors to adult functioning. The findings speak to the importance of utilizing research designs that systematically address the role of unmeasured familial confounding influences that may be pertinent in understanding the relationship between adolescent drinking and later consequences.
Supplementary Material
Acknowledgments
This research was supported by National Institute on Drug Abuse Awards R37 DA005147 and National Institute on Alcohol Abuse and Alcoholism Award R01AA 009367.
Footnotes
Some of the data appearing in this submission previously appeared in a poster presentation at the 17th Annual Meeting of the Society for Personality and Social Psychology (January 2017). Specifically, the contents of Table 3 and Table 4 appeared on the poster. No other results were included.
Symptoms of alcohol abuse and alcohol dependence were summed, as were symptoms of marijuana abuse and marijuana dependence. The diagnostic conceptualization system at the time of the assessment was DSM-IV. These variables were labeled as “use disorder” symptoms consistent with the recent revision in DSM-V that eliminated the distinction between dependence and abuse.
These variables were coded such that a higher score on the behavioral constraint measure represented reduced levels of behavioral constraint, (or, as referred to henceforth, higher levels of behavioral disinhibition).
These variables were coded such that a higher score on the antisocial peer index represented increased affiliation with antisocial peers, while a higher score on the prosocial index represented less affiliation with prosocial peers.
Contributor Information
Jordan Sparks Waldron, School of Psychological Sciences, University of Indianapolis.
Stephen M. Malone, Department of Psychology, University of Minnesota
Matt McGue, Department of Psychology, University of Minnesota.
William G. Iacono, Department of Psychology, University of Minnesota
References
- Agrawal A, Grant JD, Littlefield A, Waldron M, Pergadia ML, Lynskey MT, Heath AC. Developing a quantitative measure of alcohol consumption for genomic studies on prospective cohorts. Journal of Studies on Alcohol and Drugs. 2009;70(2):157–168. doi: 10.15288/jsad.2009.70.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52(3):317–332. doi: 10.1007/BF02294359. [DOI] [Google Scholar]
- Althoff RR, Hudziak JJ, Willemsen G, Hudziak V, Bartels M, Boomsma DI. Genetic and environmental contributions to self-reported thoughts of self- harm and suicide. American Journal of Medical Genetics. 2012;159B(1):120–127. doi: 10.1002/ajmg.b.32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker LA, Treloar SA, Reynolds CA, Heath AC, Martin NG. Genetics of educational attainment in Australian twins: Sex differences and secular changes. Behavior Genetics. 1996;26(2):89–102. doi: 10.1007/BF02359887. [DOI] [PubMed] [Google Scholar]
- Billig JP, Hershberger SL, Iacono WG, McGue M. Life events and personality in late adolescence: genetic and environmental relations. Behavior Genetics. 1996;26(6):543–554. doi: 10.1007/BF02361227. [DOI] [PubMed] [Google Scholar]
- Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Brandmaier A. OpenMx 1.2 user guide 2012 [Google Scholar]
- Boker SM, Neale MC, Maes HH, Wilde MJ, Spiegel M, Brick TR, Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76(2):306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornovalova MA, Huibregtse BM, Hicks BM, Keyes M, McGue M, Iacono W. Tests of a direct effect of childhood abuse on adult borderline personality disorder traits: A longitudinal discordant twin design. Journal of Abnormal Psychology. 2013;122(1):180–194. doi: 10.1037/a0028328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Moffitt TE. The p factor: one general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science. 2014;2(2):119–137. doi: 10.1177/2167702613497473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chassin L, Pitts SC, Prost J. Binge drinking trajectories from adolescence to emerging adulthood in a high-risk sample: Predictors and substance abuse outcomes. Journal of Consulting and Clinical Psychology. 2002;70(1):67. doi: 10.1037/0022-006X.70.1.67. [DOI] [PubMed] [Google Scholar]
- Chatterji P. Does alcohol use during high school affect educational attainment?: Evidence from the National Education Longitudinal Study. Economics of Education Review. 2006;25(5):482–497. doi: 10.1016/j.econedurev.2005.05.005. [DOI] [Google Scholar]
- Clark DB, Thatcher DL, Tapert SF. Alcohol, psychological dysregulation, and adolescent brain development. Alcoholism: Clinical and Experimental Research. 2008;32(3):375–385. doi: 10.1111/j.1530-0277.2007.00601.x. [DOI] [PubMed] [Google Scholar]
- Compton WM, Thomas YF, Stinson FS, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV drug abuse and dependence in the United States: Results from the national epidemiologic survey on alcohol and related conditions. Archives of General Psychiatry. 2007;64(5):566. doi: 10.1001/archpsyc.64.5.566. [DOI] [PubMed] [Google Scholar]
- D’Amico EJ, Ellickson PL, Collins RL, Martino S, Klein DJ. Processes linking adolescent problems to substance-use problems in late young adulthood. Journal of Studies on Alcohol and Drugs. 2005;66(6):766. doi: 10.15288/jsa.2005.66.766. [DOI] [PubMed] [Google Scholar]
- Dawson D, Goldstein R, Chou SP, Ruan WJ, Grant B. Age at first drink and the first incidence of adult-onset DSM-IV alcohol use disorders. Alcoholism: Clinical and Experimental Research. 2008;32(12):2149–2160. doi: 10.1111/j.1530-0277.2008.00806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick DM, Meyers JL, Rose RJ, Kaprio J, Kendler KS. Measures of current alcohol consumption and problems: Two independent twin studies suggest a complex genetic architecture. Alcoholism: Clinical and Experimental Research. 2011;35(12):2152–2161. doi: 10.1111/j.1530-0277.2011.01564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan SJ, Stockdale GD, Widaman KF, Conger RD. Developmental relations and patterns of change between alcohol use and number of sexual partners from adolescence through adulthood. Developmental Psychology. 2010;46(6):1747–1759. doi: 10.1037/a0019655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins IJ, King SM, McGue M, Iacono WG. Personality traits and the development of nicotine, alcohol, and illicit drug disorders: prospective links from adolescence to young adulthood. Journal of Abnormal Psychology. 2006;115(1):26. doi: 10.1037/0021-843X.115.1.26. [DOI] [PubMed] [Google Scholar]
- Englund MM, Egeland B, Oliva EM, Collins WA. Childhood and adolescent predictors of heavy drinking and alcohol use disorders in early adulthood: a longitudinal developmental analysis. Addiction. 2008;103(s1):23–35. doi: 10.1111/j.1360-0443.2008.02174.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming R. Does alcohol damage the adolescent brain? Neuroanatomical and neuropsychological consequences of adolescent drinking. Neuroscience and Neuroeconomics. 2015;1:51–60. [Google Scholar]
- Foster KT, Hicks BM, Iacono WG, McGue M. Alcohol use disorder in women: Risks and consequences of an adolescent onset and persistent course. Psychology of Addictive Behaviors. 2014;28(2):322. doi: 10.1037/a0035488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, van den Bree MB. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102(3):413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geels LM, Bartels M, van Beijsterveldt TC, Willemsen G, van der Aa N, Boomsma DI, Vink JM. Trends in adolescent alcohol use: Effects of age, sex and cohort on prevalence and heritability. Addiction. 2012;107(3):518–527. doi: 10.1111/j.1360-0443.2011.03612.x. [DOI] [PubMed] [Google Scholar]
- Goodnight JA, Donahue KL, Waldman ID, Van Hulle CA, Rathouz PJ, Lahey BB, D’Onofrio BM. Genetic and environmental contributions to associations between infant fussy temperament and antisocial behavior in childhood and adolescence. Behavior Genetics. 2016;46(5):680–692. doi: 10.1007/s10519-016-9794-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF. Prevalence and correlates of alcohol use and DSM-IV alcohol dependence in the United States: Results of the national longitudinal alcohol epidemiologic survey. Journal of Studies on Alcohol and Drugs. 1997;58(5):464. doi: 10.15288/jsa.1997.58.464. [DOI] [PubMed] [Google Scholar]
- Grant JD, Agrawal A, Bucholz KK, Madden PA, Pergadia ML, Nelson EC, Heath AC. Alcohol consumption indices of genetic risk for alcohol dependence. Biological Psychiatry. 2009;66(8):795–800. doi: 10.1016/j.biopsych.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green KM, Doherty EE, Zebrak KA, Ensminger ME. Association between adolescent drinking and adult violence: Evidence from a longitudinal study of urban African Americans. Journal of Studies on Alcohol and Drugs. 2011;72(5):701–710. doi: 10.15288/jsad.2011.72.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerri C, Pascual M. Mechanisms involved in the neurotoxic, cognitive, and neurobehavioral effects of alcohol consumption during adolescence. Alcohol. 2010;44(1):15–26. doi: 10.1016/j.alcohol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Hicks BM, Blonigen DM, Kramer MD, Krueger RF, Patrick CJ, Iacono WG, McGue M. Gender differences and developmental change in externalizing disorders from late adolescence to early adulthood: A longitudinal twin study. Journal of Abnormal Psychology. 2007;116(3):433–447. doi: 10.1037/0021-843X.116.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Iacono WG, McGue M. Consequences of an adolescent onset and persistent course of alcohol dependence in men: Adolescent risk factors and adult outcomes. Alcoholism: Clinical and Experimental Research. 2010;34(5):819–833. doi: 10.1111/j.1530-0277.2010.01154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, Schalet BD, Malone SM, Iacono WG, McGue M. Psychometric and genetic architecture of substance use disorder and behavioral disinhibition measures for gene association studies. Behavior Genetics. 2011;41(4):459–475. doi: 10.1007/s10519-010-9417-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks BM, South SC, DiRago AC, Iacono WG, McGue M. Environmental adversity and increasing genetic risk for externalizing disorders. Archives of General Psychiatry. 2009;66(6):640–648. doi: 10.1001/archgenpsychiatry.2008.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hills AL, Cox BJ, McWilliams LA, Sareen J. Suicide attempts and externalizing psychopathology in a nationally representative sample. Comprehensive Psychiatry. 2005;46(5):334–339. doi: 10.1016/j.comppsych.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Iacono WG, Carlson SR, Taylor J, Elkins IJ, McGue M. Behavioral disinhibition and the development of substance-use disorders: Findings from the Minnesota Twin Family Study. Development and Psychopathology. 1999;11(04):869–900. doi: 10.1017/S0954579499002369. [DOI] [PubMed] [Google Scholar]
- Irons DE, Iacono WG, McGue M. Tests of the effects of adolescent early alcohol exposures on adult outcomes. Addiction. 2015;110(2):269–278. doi: 10.1111/add.12747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobus J, Tapert SF. Neurotoxic effects of alcohol in adolescence. Annual Review of Clinical Psychology. 2013;9:703–721. doi: 10.1146/annurev-clinpsy-050212-185610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Gardner C, Dick DM. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011;41(7):1507–1516. doi: 10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Jacobson KC, Gardner CO, Gillespie N, Aggen SA, Prescott CA. Creating a social world: A developmental twin study of peer-group deviance. Archives of General Psychiatry. 2007;64(8):958. doi: 10.1001/archpsyc.64.8.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler KS, Myers J, Dick D, Prescott CA. The relationship between genetic influences on alcohol dependence and on patterns of alcohol consumption. Alcoholism: Clinical and Experimental Research. 2010;34(6):1058–1065. doi: 10.1111/j.1530-0277.2010.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenkel DS, Ribar DC, Cook PJ, Peltzman S. Alcohol consumption and young adults’ socioeconomic status. Brookings Papers on Economic Activity Microeconomics. 1994:119–175. [Google Scholar]
- Kiff CJ, Cortes RC, Lengua LJ, Kosterman R, Hawkins JD, Mason WA. Effects of timing of adversity on adolescent and young adult adjustment. Journal of Research on Adolescence. 2012;22(2):284–300. doi: 10.1111/j.1532-7795.2012.00781.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KM, Meehan BT, Trim RS, Chassin L. Marker or mediator? The effects of adolescent substance use on young adult educational attainment. Addiction. 2006;101(12):1730–1740. doi: 10.1111/j.1360-0443.2006.01507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, Patrick CJ, Carlson SR, Iacono WG, McGue M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. Journal of Abnormal Psychology. 2002;111(3):411–424. doi: 10.1037/11855-003. [DOI] [PubMed] [Google Scholar]
- Krueger RF, McGue M, Iacono WG. The higher-order structure of common DSM mental disorders: Internalization, externalization, and their connections to personality. Personality and Individual Differences. 2001;30(7):1245–1259. doi: 10.1016/S0191-8869(00)00106-9. [DOI] [Google Scholar]
- Le AT, Miller PW, Slutske WS, Martin NG. Opportunity and educational outcomes in Australia. Economic Record. 2011;87(s1):125–135. doi: 10.1111/j.1475-4932.2011.00749.x. [DOI] [Google Scholar]
- Levinson DF. The genetics of depression: A review. Biological Psychiatry. 2006;60(2):84–92. doi: 10.1016/j.biopsych.2005.08.024. [DOI] [PubMed] [Google Scholar]
- Loehlin JC. The Cholesky approach: A cautionary note. Behavior Genetics. 1996;26(1):65–69. doi: 10.1007/BF02361160. [DOI] [Google Scholar]
- Maimaris W, McCambridge J. Age of first drinking and adult alcohol problems: systematic review of prospective cohort studies. Journal of Epidemiology and Community Health. 2013 doi: 10.1136/jech-2013-203402. jech-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall EJ. Adolescent alcohol use: risks and consequences. Alcohol and Alcoholism. 2014;49(2):160–164. doi: 10.1093/alcalc/agt180. [DOI] [PubMed] [Google Scholar]
- Mason WA, Kosterman R, Haggerty KP, Hawkins JD, Redmond C, Spoth RL, Shin C. Dimensions of adolescent alcohol involvement as predictors of young-adult major depression. Journal of Studies on Alcohol and Drugs. 2008;69(2):275–285. doi: 10.15288/jsad.2008.69.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCambridge J, McAlaney J, Rowe R. Adult consequences of late adolescent alcohol consumption: A systematic review of cohort studies. PLoS Medicine. 2011;8(2):e1000413. doi: 10.1371/journal.pmed.1000413.t001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG. The association of early adolescent problem behavior with adult psychopathology. The American Journal of Psychiatry. 2005;162(6):1118–1124. doi: 10.1007/s10519-006-9061-z. [DOI] [PubMed] [Google Scholar]
- McGue M, Malone S, Keyes M, Iacono WG. Parent—Offspring Similarity for Drinking: A Longitudinal Adoption Study. Behavior Genetics. 2014;44(6):620–628. doi: 10.1007/s10519-014-9672-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merline A, Jager J, Schulenberg JE. Adolescent risk factors for adult alcohol use and abuse: Stability and change of predictive value across early and middle adulthood. Addiction. 2008;103(s1):84–99. doi: 10.1111/j.1360-0443.2008.02178.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller P, Mulvey C, Martin N. Earnings and schooling: An overview of economic research based on the Australian twin register. Acta Geneticae Medicae Et Gemellologiae. 1996;45(4):417–429. doi: 10.1017/s0001566000000817. [DOI] [PubMed] [Google Scholar]
- Miller P, Mulvey C, Martin N. Genetic and environmental contributions to educational attainment in Australia. Economics of Education Review. 2001;20(3):211–224. doi: 10.1016/S0272-7757(00)00018-2. [DOI] [Google Scholar]
- Neale M, Cardon L, editors. Methodology for genetic studies of twins and families. Springer Science & Business Media; 1992. (No. 67). [Google Scholar]
- Odgers CL, Caspi A, Nagin DS, Piquero AR, Slutske WS, Milne BJ, Moffitt TE. Is it important to prevent early exposure to drugs and alcohol among adolescents? Psychological Science. 2008;19(10):1037–1044. doi: 10.1111/j.1467-9280.2008.02196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olino TM, Klein DN, Farmer RF, Seeley JR, Lewinsohn PM. Examination of the structure of psychopathology using latent class analysis. Comprehensive Psychiatry. 2012;53(4):323–332. doi: 10.1016/j.comppsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagan JL, Rose RJ, Viken RJ, Pulkkinen L, Kaprio J, Dick DM. Genetic and environmental influences on stages of alcohol use across adolescence and into young adulthood. Behavior Genetics. 2006;36(4):483–497. doi: 10.1007/s10519-006-9062-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck SC, Vida M, Eccles JS. Adolescent pathways to adulthood drinking: Sport activity involvement is not necessarily risky or protective. Addiction. 2008;103(s1):69–83. doi: 10.1111/j.1360-0443.2008.02177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitkanen T, Kokko K, Lyyra AL, Pulkkinen L. A developmental approach to alcohol drinking behaviour in adulthood: a follow-up study from age 8 to age 42. Addiction. 2008;103(s1):48–68. doi: 10.1111/j.1360-0443.2008.02176.x. [DOI] [PubMed] [Google Scholar]
- Posthuma D. Handbook of Behavior Genetics. New York, NY: Springe; 2009. Multivariate genetic analysis; pp. 47–59. [Google Scholar]
- Richmond-Rakerd LS, Slutske WS, Lynskey MT, Agrawal A, Madden PA, Bucholz KK, Martin NG. Age at first use and later substance use disorder: Shared genetic and environmental pathways for nicotine, alcohol, and cannabis. Journal of Abnormal Psychology. 2016;125(7):946. doi: 10.1037/abn0000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Babor TF, Cottler LB. Composite International Diagnostic Interview: Expanded Substance Abuse Module. St. Louis: Authors; 1987. [Google Scholar]
- Roisman GI, Aguilar B, Egeland B. Antisocial behavior in the transition to adulthood: The independent and interactive roles of developmental history and emerging developmental tasks. Development and Psychopathology. 2004;16(4):857–871. doi: 10.1017/S0954579404040040. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Dick DM, Viken RJ, Kaprio J. Gene-environment interaction in patterns of adolescent drinking: Regional residency moderates longitudinal influences on alcohol use. Alcoholism: Clinical and Experimental Research. 2001;25(5):637–643. doi: 10.1111/j.1530-0277.2001.tb02261.x. [DOI] [PubMed] [Google Scholar]
- Rose RJ, Winter T, Viken RJ, Kaprio J. Adolescent alcohol abuse and adverse adult outcomes: Evaluating confounds with drinking-discordant twins. Alcoholism: Clinical and Experimental Research. 2014;38(8):2314–2321. doi: 10.1111/j.1530-0277.2001.tb02261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossow I, Kuntsche E. Early Onset of Drinking and Risk of Heavy Drinking in Young Adulthood—A 13-Year Prospective Study. Alcoholism: Clinical and Experimental Research. 2013;37(s1):E297–E304. doi: 10.1111/j.1530-0277.2012.01924.x. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Krueger RF, Bouchard TJ, Kaprio J, McGue M. Heritability of body height and educational attainment in an international context: Comparison of adult twins in Minnesota and Finland. American Journal of Human Biology. 2004;16(5):544–555. doi: 10.1002/ajhb.20060. [DOI] [PubMed] [Google Scholar]
- Slade T. The descriptive epidemiology of internalizing and externalizing psychiatric dimensions. Social Psychiatry and Psychiatric Epidemiology. 2007;42(7):554–560. doi: 10.1007/s00127-007-0200-5. [DOI] [PubMed] [Google Scholar]
- Smith GT, McCarthy DM, Goldman MS. Self-reported drinking and alcohol-related problems among early adolescents: Dimensionality and validity over 24 months. Journal of Studies on Alcohol. 1995;56(4):383–394. doi: 10.15288/jsa.1995.56.383. [DOI] [PubMed] [Google Scholar]
- Solovieff N, Cotsapas C, Lee PH, Purcell SM, Smoller JW. Pleiotropy in complex traits: challenges and strategies. Nature Reviews Genetics. 2013;14(7):483–495. doi: 10.1038/nrg3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R (SCID) New York: New York State Psychiatric Institute; 1987. (Biometrics Research, 11). [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID): I: history, rationale, and description. Archives of General Psychiatry. 1992;49(8):624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Squeglia LM, Gray KM. Alcohol and drug use and the developing brain. Current Psychiatry Reports. 2016;18(5):1–10. doi: 10.1007/s11920-016-0689-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Jacobus J, Tapert SF. The effect of alcohol use on human adolescent brain structures and systems. Handbook of Clinical Neurology. 2014;125:501–510. doi: 10.1016/B978-0-444-62619-6.00028-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Tapert SF, Sullivan EV, Jacobus J, Meloy MJ, Rohlfing T, Pfefferbaum A. Brain development in heavy-drinking adolescents. American Journal of Psychiatry. 2015;172(6):531–542. doi: 10.1176/appi.ajp.2015.14101249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambs K, Sundet JM, Magnus P, Berg K. Genetic and environmental contributions to the covariance between occupational status, educational attainment, and IQ: A study of twins. Behavior Genetics. 1989;19(2):209–222. doi: 10.1007/BF01065905. [DOI] [PubMed] [Google Scholar]
- Tellegen A, Waller NG. Exploring personality through test construction: Development of the Multidimensional Personality Questionnaire. The SAGE Handbook of Personality Theory and Assessment. 2008;2:261–292. doi: 10.4135/9781849200479.n13. [DOI] [Google Scholar]
- Verona E, Sachs-Ericsson N, Joiner TE. Suicide attempts associated with externalizing psychopathology in an epidemiological sample. American Journal of Psychiatry. 2004;161(3):444–451. doi: 10.1176/appi.ajp.161.3.444. [DOI] [PubMed] [Google Scholar]
- Viken RJ, Kaprio J, Koskenvuo M, Rose RJ. Longitudinal analyses of the determinants of drinking and of drinking to intoxication in adolescent twins. Behavior Genetics. 1999;29(6):455–461. doi: 10.1023/A:1021631122461. [DOI] [PubMed] [Google Scholar]
- Viner R, Taylor B. Adult outcomes of binge drinking in adolescence: Findings from a UK national birth cohort. Journal of Epidemiology and Community Health. 2007;61(10):902–907. doi: 10.1136/jech.2005.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrieze SI, Perlman G, Krueger RF, Iacono WG. Is the continuity of externalizing psychopathology the same in adolescents and middle-aged adults? A test of the externalizing spectrum’s developmental coherence. Journal of Abnormal Child Psychology. 2012;40(3):459–470. doi: 10.1007/s10802-011-9571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells JE, Horwood LJ, Fergusson DM. Drinking patterns in mid-adolescence and psychosocial outcomes in late adolescence and early adulthood. Addiction. 2004;99(12):1529–1541. doi: 10.1111/j.1360-0443.2004.00918.x. [DOI] [PubMed] [Google Scholar]
- Wilson S, Malone SM, Thomas KM, Iacono WG. Adolescent drinking and brain morphometry: A co-twin control analysis. Developmental Cognitive Neuroscience. 2015;16:130–138. doi: 10.1016/j.dcn.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ED. Research on alcohol and adolescent brain development: opportunities and future directions. Alcohol. 2010;44(1):119–124. doi: 10.1016/j.alcohol.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Ystrom E, Kendler KS, Reichborn-Kjennerud T. Early age of alcohol initiation is not the cause of alcohol use disorders in adulthood, but is a major indicator of genetic risk. A population-based twin study. Addiction. 2014;109(11):1824–1832. doi: 10.1111/add.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakrajsek JS, Shope JT. Longitudinal examination of underage drinking and subsequent drinking and risky driving. Journal of Safety Research. 2006;37(5):443–451. doi: 10.1016/j.jsr.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


