Abstract
Though schizophrenia (SCZ) is classically defined based on positive symptoms and the negative symptoms of the disease prove to be debilitating for many patients, motor deficits are often present as well. A growing literature highlights the importance of motor systems and networks in the disease, and it may be the case that dysfunction in motor networks relates to the pathophysiology and etiology of SCZ. To test this and build upon recent work in SCZ and in at‐risk populations, we investigated cortical and cerebellar motor functional networks at rest in SCZ and controls using publically available data. We analyzed data from 82 patients and 88 controls. We found key group differences in resting‐state connectivity patterns that highlight dysfunction in motor circuits and also implicate the thalamus. Furthermore, we demonstrated that in SCZ, these resting‐state networks are related to both positive and negative symptom severity. Though the ventral prefrontal cortex and corticostriatal pathways more broadly have been implicated in negative symptom severity, here we extend these findings to include motor–striatal connections, as increased connectivity between the primary motor cortex and basal ganglia was associated with more severe negative symptoms. Together, these findings implicate motor networks in the symptomatology of psychosis, and we speculate that these networks may be contributing to the etiology of the disease. Overt motor deficits in SCZ may signal underlying network dysfunction that contributes to the overall disease state. Hum Brain Mapp 38:4535–4545, 2017. © 2017 Wiley Periodicals, Inc.
Keywords: resting‐state connectivity, motor, cerebellum, schizophrenia, negative symptoms
INTRODUCTION
Schizophrenia (SCZ) is characterized by debilitating symptoms that negatively impact quality of life. In addition to the more classically defined positive and negative symptom domains [Andreasen and Olsen, 1982], many patients also suffer from motor symptoms and deficits [Bernard and Mittal, 2014]. This includes but is not limited to deficits in postural control [Marvel et al., 2004], motor learning [Marvel et al., 2007], hyperkinetic movements, and force control. Together, these deficits further impact quality of life for patients with these disorders. Furthermore, it is notable that motor dysfunction is not simply a side effect of the medications used to treat the hallmark symptoms of psychosis [Walther and Strik, 2012]. When controlling for medication, patients still show deficits in motor behavior relative to controls [Walther and Strik, 2012], and these deficits are present in adolescents at ultra‐high risk (UHR) for the development of psychosis and siblings of those with SCZ, many of whom are not taking antipsychotic medications [Bernard et al., 2014; Bolbecker et al., 2013; Dean et al., 2013; Mittal et al., 2010a]. It therefore seems to be the case that motor signs and symptoms also present as a defining characteristic of SCZ. Thus, understanding motor networks and systems in individuals with SCZ is of particular interest, and may provide new insights into the networks and brain structures underlying the disease.
More broadly, the cerebello–thalamo–cortical circuit (CTCC) is of great interest in psychosis, and SCZ more specifically. From a theoretical standpoint, it has been proposed that CTCC dysfunction contributes to disorganized thought, as per the cognitive dysmetria framework [Andreasen et al., 1996, 1998]. More recently, we and others have suggested that cerebellar dysfunction more broadly results in internal model deficits that may contribute to the wide ranging signs and symptoms seen in SCZ, including in the motor domain [Bernard and Mittal, 2015; Shergill et al., 2005, 2014]. As such, in addition to investigating the primary motor cortex, inclusion of the cerebellum in our investigations of SCZ is especially important. While some subregions may relate to cognitive dysmetria, the motor areas of the cerebellum are linked with the primary motor cortex [Bernard et al., 2012; Kelly and Strick, 2003], and likely contribute to the motor deficits experienced by patients with SCZ.
SCZ has been described as a disease of dysconnectivity [Friston and Frith, 1995]. An accumulation of evidence supports this in the default mode network (DMN) in particular [Bluhm et al., 2007; Garrity et al., 2007; Öngür et al., 2010], though results are somewhat mixed [Whitfield‐Gabrieli et al., 2009]. However, other networks have been implicated as well, including those in the cerebellum [Kim et al., 2014; Liu et al., 2011; Repovs et al., 2011]. Interestingly, in recent investigations, resting‐state connectivity patterns have been used to predict conversion to psychosis in UHR populations [Anticevic et al., 2015], and positive symptom progression in UHR individuals [Bernard et al., 2017]. Notably, connectivity with motor cortical regions was associated with measures of disease progression. Anticevic et al. [2015] found this to be the case with thalamo–motor connectivity, while in our own recent work, we saw similar patterns with cerebellar–motor networks [Bernard et al., 2017]. Thus, it may be the case that connectivity with motor cortex and within the motor networks is related to disease progression and the underlying pathology resulting in the signs and symptoms experienced in SCZ. Overt motor behaviors are an additional effect of this broader circuit‐related dysfunction, which also relates to symptomatology.
To investigate the notion that motor network dysfunction is related to the disease state in SCZ, we conducted an analysis of resting‐state functional connectivity MRI (fcMRI) data in patients with SCZ, and in healthy controls (CON). These data were available through http://schizconnect.org, and resulted in a large sample of participants in both groups. We analyzed the available fcMRI data to test several hypotheses related to motor networks in patients with SCZ. First, we were interested in whether motor network dysfunction is present in SCZ, relative to CON. We tested the hypothesis that if motor networks are dysfunctional in patients with SCZ, then fcMRI will be decreased relative to CON. This is supported by research suggesting that motor connectivity is related to conversion to psychosis and positive symptom progression during the risk period [Anticevic et al., 2015; Bernard et al., 2017], the large literature showing motor deficits in these patients [Bernard and Mittal, 2014; Kent et al., 2012; Marvel et al., 2004, 2007], and fcMRI studies demonstrating decreased connectivity in SCZ [Kim et al., 2014; Liu et al., 2011; Repovs et al., 2011]. Second, the relationship between motor networks and symptom severity in SCZ is unknown. We tested the hypothesis that positive and negative symptom severity is related to connectivity in motor networks. We expected to see correlations between connectivity strength and the symptoms present in SCZ. Prior behavioral work has demonstrated links between motor behavior and symptoms in UHR individuals [Bernard et al., 2014; Dean et al., 2015; Mittal et al., 2010b], and in patients with schizophrenia [Bolbecker et al., 2009; Forsyth et al., 2012; Kent et al., 2012; Marvel et al., 2004], supporting this prediction.
METHODS
Participants
The final sample for analysis included a group of 82 patients with a diagnosis of schizoaffective disorder or schizophrenia (SCZ; 38.36 ± 13.78 years old, 15 female) and 88 healthy controls (CON; 38.78 ± 11.76 years old, 25 female). Patients with a diagnosis of bipolar disorder were excluded from analysis. The groups were well matched in terms of age. All participants were assessed using the Structured Clinical Interview for DSM‐IV disorders (SCID) [First et al., 1995], and the positive and negative syndrome scale (PANSS) [Kay et al., 1989; Kay and Qpjer, 1982] to determine the presence of comorbidities, and to quantify psychotic symptoms. Demographic information, symptom severity, and information regarding alcohol and marijuana usage are presented in Table 1. All SCZ participants were taking antipsychotic medications, though there was a great deal of heterogeneity in the medications used to treat these individuals. As such, we calculated chlorpromazine (CPZ) equivalents as described by Woods [2003] and Leucht et al. [2014] to use as a covariate in our analyses.
Table 1.
Participant demographics
| Patients | Controls | |
|---|---|---|
| N | 82 | 88 |
| Age | 38.36 (13.78) | 38.78 (11.76) |
| Sex | 67M, 15F | 63M, 25F |
| Participant education** | 3.83 (1.49) | 4.57 (1.28) |
| Parent education+ | 3.82 (2.15) | 4.35 (1.83) |
| Current alcohol usage* | 1.04 (0.19) | 0.98 (0.15) |
| Lifetime alcohol usage** | 1.46 (0.76) | 1.18 (0.54) |
| Current marijuana usage** | 1.02 (0.22) | 0.98 (0.15) |
| Lifetime marijuana usage | 1.46 (0.74) | 1.02 (0.30) |
| Symptoms | ||
| Positive | 15.30 (4.78, 7–29) | – |
| Negative | 15.24 (5.32, 7–29) | – |
For all variables, we provide the mean, with the standard deviation in parentheses. Positive and negative symptom severity also includes the range of scores. Education is on a scale from 1 to 8, where 1 indicates “grade 6 or less” and 8 indicates “completed graduate/professional school.” Current alcohol and marijuana usage were measured with the SCID, and scored 1, 2, or 3, where 1 was absent, 2 was abuse, and 3 was dependent. Significant group differences or trends with respect to demographic variables are indicated. +P < 0.1, *P < 0.05, **P < 0.005.
Data Acquisition
This investigation took advantage of data available as part of the SchizConnect database (http://schizconnect.org). As such, the investigators within SchizConnect provided data but did not participate in analysis or writing of this report. Within the SchizConnect database we used a search query to identify datasets that included patients with schizophrenia or psychotic disorders and healthy controls, and resting‐state fcMRI data, high‐resolution structural images, and clinical information including symptom severity. This resulted in two available datasets, one from the Functional BIRN (FBIRN) data repository, and the other was the Center of Biomedical Research Excellence (COBRE) data repository. Because the FBIRN data set was small (including only 19 scans) and the data were collected on 1.5 T and 3 T MRI machines at a different site, for analysis purposes, we only included the COBRE dataset.
The COBRE data were collected at the Mind Research Network using a Siemens 3 T MAGNETOM Tim Trio MRI scanner. Specific information regarding the data collection parameters is available in recent work published by the COBRE group [Çetin et al., 2014]. In brief, the resting‐state scan was approximately 5 min long (149 volumes, TR = 2 s), and participants were asked to keep their eyes opened and focused on a fixation cross during the scan. High‐resolution (MRPAGE) structural images were also collected.
fcMRI Analysis
All fcMRI analyses were completed using the Conn Toolbox version 16b [Whitfield‐Gabrieli and Nieto‐Castanon, 2012]. This included preprocessing procedures that were implemented in Conn using SPM. We followed a standard preprocessing pipeline which included functional realignment and unwarping, functional centering of the image to (0, 0, 0) coordinates, slice‐timing correction, structural centering to (0, 0, 0) coordinates, structural segmentation and normalization to MNI space, functional normalization to MNI space, and spatial smoothing with a smoothing kernel of 6 mm FWHM. This procedure also included processing with the Artifact Rejection Toolbox (ART). This was set using the more liberal 99th percentile settings, and allowed for the quantification or participant motion in the scanner, and the identification of outliers based on the mean signal. With these settings, the global‐signal z‐value threshold was set at 9, while the subject‐motion threshold was set at 2 mm. Motion information and framewise outliers were included as covariates in our subsequent first‐level analyses. fcMRI analysis focused on several seed regions of interest. First, given prior work demonstrating that cerebello–cortical connectivity is abnormal in UHR populations and related to symptoms and positive symptom progression [Bernard et al., 2014, 2017], and their resting connections with primary and premotor and regions [Bernard et al., 2012], we used right Lobule V and Right Crus I as seeds. These seed regions were defined based on the SUIT atlas [Diedrichsen, 2006; Diedrichsen et al., 2009]. Because primary motor connectivity (M1) is also of interest, we included a seed in M1. This seed was defined based on prior work investigating cerebello–cortical connectivity, and is where there was peak connectivity between cerebellar Lobule V and M1 [Bernard et al., 2012]. This spherical seed was centered at the coordinates −34, −18, 44 with a radius of 7 mm. We created the seed using FSL (v.5.0.7; http://fsl.fmrib.ox.ac.uk/fsl). Finally, given recent interest in the thalamus in SCZ [Anticevic et al., 2015], and findings from our own work suggesting that connectivity between the cerebellum and thalamus are related to positive symptom progression [Bernard et al., 2017], we also included a thalamic seed. The thalamic seed region was centered at −18, −18, 16, which was the peak region in the cluster showing a positive association with positive symptom progression in our recent work in UHR individuals [Bernard et al., 2017]. Importantly, this is localized to the posterior region of the thalamus. Though the resolution of the brain imaging data in our prior work was not optimized to resolve individual thalamic nuclei, this is in the approximate location of the ventral posterior nucleus. When we enter these thalamic coordinates into the Oxford probabilistic thalamic connectivity atlas [Behrens et al., 2003], the probability that this region is connected with the primary motor cortex is 0.3, and that with the somatosensory cortex is 0.29, suggesting that this is an optimal seed region given our interest in motor networks. Because the thalamus is relatively small, we used a radius of 5 mm.
We completed seed‐to‐voxel analyses using a whole‐brain approach. We investigated within‐group connectivity patterns of our seeds of interest, and we compared connectivity of the SCZ and CON groups in these regions. Because of the varying age of the participants, we controlled for age in our analyses. In addition, we included covariates related to current alcohol and marijuana use, given the impact of both these substances on cerebellar structure and function [Lopez‐Larson et al., 2012; Solowij et al., 2011; Sullivan et al., 2010a, 2010b], and CPZ equivalent dosages, given the potential impact of antipsychotic medications on brain networks. Finally, we also completed correlation analyses to investigate the relationships between resting‐state connectivity and symptom severity in the patient group alone, focusing on positive and negative symptoms. Correlation analyses were conducted directly in the Conn Toolbox using the Fisher transformed connectivity values (z scores). With recent work on the reproducibility of brain imaging results in mind [Eklund et al., 2016], we used nonparametric statistics with 5,000 permutations, implemented in the Conn Toolbox. Results were then evaluated first with a cluster‐forming threshold of P < 0.001, and then with a cluster‐level correction of P FDR < 0.05.
RESULTS
First, we compared the two groups on demographics, symptoms, and alcohol and marijuana usage. The groups were compared using t tests. Details regarding statistical significance are presented in Table 1. In brief, the two groups did not differ in age, though the SCZ group had lower education levels than the controls. Parental education was also lower in the SCZ group, but only at trend level (P = 0.08). Lifetime alcohol and marijuana usage, and current alcohol usage was higher in the SCZ group. Current marijuana usage did not differ between the two groups.
Within‐Group Connectivity Patterns
First, we investigated seed‐to‐voxel whole‐brain connectivity within the SCZ and CON groups separately. Consistent with the existing literature, in the CON group Lobule V was correlated with motor cortical regions, while Crus I was correlated with frontal and parietal cortical regions [Bernard et al., 2016, 2012; Krienen and Buckner, 2009]. Also consistent with prior work was the connectivity of the lateral M1. The network included bilateral motor, sensory, and premotor cortical regions, and the anterior cerebellum [Biswal et al., 1997; Langan et al., 2010]. Finally, the thalamus showed one large cluster of connectivity including the bilateral thalamus, caudate, putamen, and parts of the hippocampus. Strikingly, in the SCZ group, there were no areas of significant connectivity for both the thalamus and Lobule V seeds, while the lateral M1 connectivity was limited to a small cluster ipsilateral to the seed itself. Crus I was correlated with other cerebellar regions and the prefrontal cortex, though notably there were no correlations with parietal regions as has been reported in prior work on healthy controls [Bernard et al., 2016, 2012; Krienen and Buckner, 2009]. Generally, this is consistent with work showing decreased functional connectivity in SCZ [Garrity et al., 2007; Liu et al., 2011; Öngür et al., 2010]. Detailed results are presented in Supporting Information, Tables 1 and 2.
Group Differences in Cerebellar and Motor Connectivity
In our group comparisons, we looked at patterns of increased and decreased connectivity in the SCZ group relative to the CON group, when controlling for age, alcohol, marijuana, and antipsychotic medications. First, in our investigation of greater connectivity in the CON group relative to SCZ, we found this between Lobule V, the thalamus, and lateral M1. Interestingly, Lobule V was more strongly correlated with prefrontal cortical areas, as opposed to more traditional motor areas, which were expected. The thalamus seed was more strongly correlated with other regions of the thalamus, while lateral M1 showed stronger connectivity within medial motor and premotor cortical regions in the CON group. Table 2 provides the detailed coordinates and statistical information from this analysis, and the results are visualized in Figure 1.
Table 2.
Group differences in connectivity
| Seed region | Region | BA | Cluster size | MNI coordinates | T value | P (FDR) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| CON > SCZ | ||||||||
| Lobule V | Middle frontal gyrus (MFG) | 9 | 171 | −42 | 12 | 48 | 4.68 | 0.008 |
| Ventral premotor cortex | 6 | −38 | 2 | 38 | 3.51 | |||
| Anterior cingulate cortex | 32 | 428 | 10 | 50 | 22 | 4.44 | <0.001 | |
| Superior frontal gyrus (SFG) | 8 | −2 | 36 | 48 | 4.27 | |||
| 9 | −8 | 42 | 42 | 4.21 | ||||
| MFG | 9 | 189 | −22 | 46 | 32 | 4.34 | 0.007 | |
| 9 | −20 | 40 | 20 | 4.01 | ||||
| SFG | 46 | −24 | 52 | 18 | 3.65 | |||
| Thalamus | Thalamus | – | 416 | −14 | −14 | 4 | 4.35 | <0.001 |
| 14 | −12 | 12 | 4.33 | |||||
| 8 | −4 | 8 | 4.32 | |||||
| Lateral M1 | Supplementary motor area (SMA) | 6 | 595 | −6 | −14 | 58 | 4.97 | <0.001 |
| Medial motor cortex | 4 | −10 | −28 | 60 | 4.35 | |||
| −6 | −28 | 72 | 4.25 | |||||
| SCZ > CON | ||||||||
| Crus I | Parahippocampal gyrus | 27 | 328 | 16 | −30 | −4 | 4.75 | <0.001 |
| 30 | 20 | −24 | −10 | 4.02 | ||||
| Lingual gyrus | 17 | 14 | −58 | 6 | 3.99 | |||
| Thalamus | Somatosensory cortex (S1) | 3 | 854 | 50 | −16 | 30 | 5.60 | <0.001 |
| Premotor/primary motor cortex (M1) | 6 | 38 | −12 | 52 | 4.67 | |||
| 42 | −8 | 38 | 4.48 | |||||
| S1 | 3 | 981 | 24 | −40 | 54 | 5.15 | <0.001 | |
| Precuneus/S1 | 5 | −6 | −38 | 60 | 4.77 | |||
| Medial motor cortex | 4 | −8 | −26 | 58 | 4.54 | |||
| Lateral occipital cortex (LOC) | 19 | 1533 | 22 | −78 | 26 | 4.67 | <0.001 | |
| 19 | −16 | −78 | 40 | 4.61 | ||||
| Precuneus | 18 | −12 | −76 | 32 | 4.45 | |||
| Middle temporal gyrus (MTG) | 37 | 357 | −50 | −62 | 10 | 4.55 | <0.001 | |
| LOC | 37 | −40 | −64 | 8 | 4.51 | |||
| −44 | −68 | 2 | 4.36 | |||||
| S1 | 3/48 | 174 | −44 | −16 | 30 | 4.44 | 0.006 | |
| M1 | 6 | −40 | −8 | 36 | 4.15 | |||
| S1 | 3 | −34 | −18 | 34 | 3.92 | |||
| MTG | 37 | 143 | 40 | −58 | 2 | 4.27 | 0.014 | |
| 37 | 48 | −52 | 2 | 3.84 | ||||
| 48 | −66 | 4 | 3.43 | |||||
| Lateral M1 | Thalamus | – | 856 | 10 | −8 | 10 | 5.85 | <0.001 |
| – | −8 | −18 | 10 | 4.88 | ||||
| −6 | −10 | 6 | 4.55 | |||||
Results of the contrasts looking at the SCZ and CON groups relative to one another are presented above. Bolded rows indicate peak voxels within the given cluster.
Figure 1.
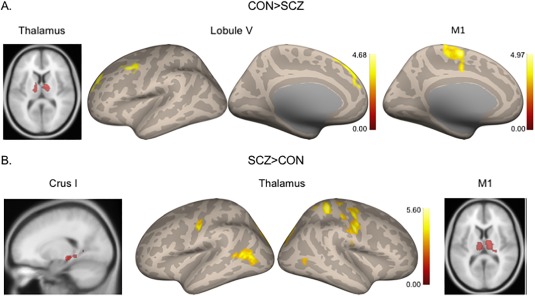
Group differences in seed‐to‐voxel connectivity. (A) Areas where connectivity is greater in the CON relative to SCZ group. (B) Areas where connectivity is greater in the SCZ relative to the CON group. [Color figure can be viewed at http://wileyonlinelibrary.com]
When investigating whether any areas show stronger connectivity in the SCZ relative to the CON group, we found several interesting patterns. First, Crus I showed increased connectivity with the parahippocampal gyrus. Interestingly, the thalamus and lateral M1 seeds showed parallel findings. Relative to the CON group, the SCZ group showed greater connectivity between the lateral M1 seed and the thalamus. Similarly, when we investigated the thalamus seed, we saw widespread areas of increased connectivity in M1 and somatosensory cortices, and in the premotor cortex, temporal, and occipital lobes. This pattern suggests hyperconnectivity of the thalamus in SCZ, particularly with motor cortical regions. These findings are visualized in Figure 1, and detailed coordinates and statistics are in Table 2.
Relationships Between Symptom Severity and Connectivity
In the SCZ group alone, we investigated correlations between connectivity strength and positive and negative symptom severity. Unlike our prior work in UHR populations[Bernard et al., 2014], we did not see any correlations between symptom severity and cerebello–cortical connectivity. However, the thalamus and lateral M1 seeds showed interesting associations with symptoms. Stronger connectivity between the thalamus and visual regions was associated with more severe positive symptoms. The lateral M1 seed was associated with negative symptoms. Stronger connectivity between M1 and regions of the putamen and thalamus was associated with more severe negative symptoms. However, stronger connectivity between M1 and other motor regions (supplementary and premotor) was associated with fewer negative symptoms (Fig. 2 and Table 3).
Figure 2.
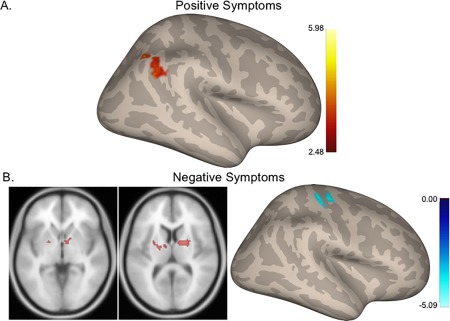
Relationships between connectivity and symptom severity in the SCZ group. (A) Higher connectivity between the thalamus and visual cortical regions is associated with more severe positive symptoms. (B) Greater connectivity between lateral M1 and basal ganglia regions is associated with more severe negative symptoms while, (C) greater connectivity within the motor cortex is associated with fewer negative symptoms. Hot colors: positive correlations. Cool colors: negative correlations. [Color figure can be viewed at http://wileyonlinelibrary.com]
Table 3.
Correlations with symptoms
| Seed region | Region | BA | Cluster size | MNI coordinates | T value | P (FDR) | ||
|---|---|---|---|---|---|---|---|---|
| X | Y | Z | ||||||
| Positive symptoms | ||||||||
| Thalamus (positive correlation) | Lateral occipital cortex | 7 | 429 | 30 | −68 | 60 | 5.98 | <0.001 |
| 7 | 38 | −66 | 52 | 4.91 | ||||
| Angular gyrus | 40 | 54 | −54 | 50 | 4.48 | |||
| Negative symptoms | ||||||||
| Lateral M1 (positive correlation) | WM/thalamus | – | 191 | 8 | 0 | 0 | 4.74 | 0.004 |
| WM/globus pallidus | – | 16 | −2 | 8 | 4.67 | |||
| Putamen | – | 133 | −20 | −8 | 10 | 4.29 | 0.012 | |
| Thalamus | – | −8 | −8 | 8 | 4.05 | |||
| Putamen | −30 | 0 | 4 | 3.76 | ||||
| Lateral M1 (negative correlation) | Medial motor cortex | 4/6 | 278 | 6 | −18 | 58 | 5.09 | <0.001 |
| Supplementary motor area | 6 | 10 | −12 | 64 | 4.70 | |||
| 6 | −12 | 72 | 4.68 | |||||
| Primary motor cortex (M1)/somatosensory cortex (S1 | 4 | 233 | 30 | −28 | 60 | 4.59 | 0.001 | |
| 4 | 38 | −24 | 60 | 4.21 | ||||
| 3 | 44 | −18 | 54 | 3.42 | ||||
Bolded rows indicate peak voxels within the given cluster.
DISCUSSION
Increasing evidence suggests that motor deficits are a core feature related to the symptoms and pathophysiology of SCZ [Walther and Strik, 2012]. However, while much support from this idea comes from the behavioral and structural brain imaging literatures, a network investigation is also of particular importance, in light of the dysconnectivity hypothesis of SCZ [Friston and Frith, 1995]. Here, we demonstrated that in SCZ, there are alterations in resting‐state motor networks. Our findings suggest an overall decrease in connectivity, though direct contrast analysis also demonstrates some notable patterns of increased connectivity relative to controls as well. Most importantly, our results demonstrate an association between motor network connectivity and negative symptom severity, further suggesting that motor systems may be at the core of the pathophysiology of SCZ. To our knowledge, this is the first investigation of patients with SCZ to directly investigate the resting‐state networks of the primary motor cortex, despite the broader implications of motor deficits in the disease.
When investigating our networks of interest in each group individually, we found connectivity patterns in the CON group that are highly consistent with the known cerebellar and motor networks in healthy adults [Bernard et al., 2016, 2012; Biswal et al., 1997, 2010; Krienen and Buckner, 2009]. Notably however, we saw very limited connectivity in the SCZ group, particularly in networks that are more strongly associated with motor functions (e.g., lateral M1, lobule V), and in Crus I connectivity [Bernard et al., 2012; Krienen and Buckner, 2009]. Together then, this provides general support for the dysconnectivity hypothesis of schizophrenia [Friston and Frith, 1995]. With that said, we may make only limited inference without the between groups comparison.
The between‐group comparisons conducted here provide much stronger support for dysconnectivity in SCZ. First, in investigating our four seeds of interest, it is striking that the most prominent motor seeds we investigated, Lobule V and lateral M1, show significantly greater connectivity with other motor areas in controls, relative to the patients. Lobule V shows stronger connectivity in an extended motor network, including premotor regions [Mayka et al., 2006], while the lateral M1 shows stronger connectivity with the supplementary motor and medial motor areas. This general decrease in cerebellar connectivity, here from Lobule V, is consistent with the limited existing literature investigating cerebellar connectivity in this patient group [Liu et al., 2011]. Together, this indicates that connectivity within these major motor networks, particularly with motor planning areas, is decreased in the SCZ group. We speculate that the decreased motor network connectivity present in SCZ may be contributing, at least in part, to the motor signs and symptoms experienced by these patients. That said, we did not have motor behavioral data from these participants. Future work is required to test this notion more directly.
Notably, there were also patterns of increased connectivity seen in the patients. Broadly speaking, this is consistent with the generally mixed resting state findings seen in patients with schizophrenia, which has primarily been documented in the default mode network [Garrity et al., 2007; Öngür et al., 2010; Whitfield‐Gabrieli et al., 2009; Whitfield‐Gabrieli and Ford, 2012]. Both increases and decreases in network connectivity relative to controls have been reported. Crus I showed greater connectivity with the medial temporal lobe, including the parahippocampal gyrus. This is particularly interesting in light of work that suggests that medial temporal lobe regions such as the hippocampus play a critical role in SCZ [Paul and Harrison, 2004]. In addition, while we demonstrated that connectivity within the broader motor networks was higher in the CON group, in the SCZ group, there was increased connectivity between the primary motor cortex and the thalamus. We also saw a reciprocal increase when looking at the lateral M1 seed. This pattern of heightened thalamo–motor connectivity at rest in the SCZ group is certainly not unprecedented [Welsh et al., 2010; Woodward et al., 2012]. However, these findings together are particularly interesting in light of recent work in patients who recently converted to psychosis [Anticevic et al., 2015]. In these individuals, thalamic hyperconnectivity with the motor cortex was associated with conversion. Additional support for the idea that hyperconnectivity in motor networks is related to disease progression comes from our investigation of positive symptom progression in UHR youth [Bernard et al., 2017]. Here, we demonstrated that hyperconnectivity between lobule V of the cerebellum and the primary motor cortex at a baseline assessment predicts more severe positive symptoms twelve months later. Together these findings suggest that hyperconnectivity with the motor cortex may be a core element to the etiology of disease in schizophrenia, and perhaps across the psychosis spectrum more broadly.
Detecting differences in the resting functional organization of the brain in SCZ provides important insight into potential contributors to the pathophysiology of the disease. However, understanding whether and to what degree these networks relate to the symptoms experienced by these patients is also of great interest and importance. Here, our results suggest that both the thalamus and motor cortex are related to the positive and negative symptoms experienced by the SCZ group. Regarding the thalamus, greater connectivity with the visual cortex was associated with more severe positive symptoms. This is perhaps not surprising given that hallucinations represent a hallmark positive symptom in SCZ. In two separate case studies of patients who experience visual hallucinations, brain activation was noted in the visual cortex [Oertel et al., 2007; Silbersweig et al., 1995]. More recently, Ford et al. [2015] found that hyperconnectivity between the visual cortex and the amygdala was associated with visual hallucinations in SCZ. The hyperconnectivity here is broadly consistent with the literature on the neural substrates of visual hallucinations, but also suggests a potential thalamic contribution.
Interestingly, we also found two diverging associations with respect to the lateral M1 seed and negative symptoms. Increased connectivity within the contralateral motor cortical regions was associated with fewer negative symptoms. Conversely, increased connectivity between lateral M1 and the putamen, globus pallidus, and thalamus was associated with more severe negative symptoms. The neural substrates of negative symptoms typically include the frontal cortex, though corticostriatal circuits have been implicated [Kring and Barch, 2014; Millan et al., 2014; Potkin et al., 2002]. Further, as noted by Walther and Strik [2012], negative symptoms are related to Parkinsonism, catatonia, and psychomotor slowing seen in SCZ. This is notable given the association between lateral M1 and the basal ganglia regions seen here. Finally, it is of note that other behavioral measures of motor behavior, including postural control and neurological soft signs in high risk populations are related to negative symptom severity [Bernard et al., 2014; Dean et al., 2015; Mittal et al., 2014]. Though not typically implicated in negative symptoms, these results do suggest that motor networks may contribute to their severity. Given the associations between broad motor behaviors and symptom severity previously reported [Walther and Strik, 2012], it is perhaps not surprising to see the associations reported here. At minimum, this finding suggests that future work looking at cortical motor and striatal motor networks with respect to negative symptom severity warrants further research. However, we speculate that these circuits may contribute at least in part to the pathophysiology and etiology of negative symptomatology in schizophrenia.
Importantly, our focus here was on cerebellar and primary motor circuits. This was motivated by our recent work suggesting that cerbello–thalamo–motor circuits may be associated with positive symptom in UHR individuals [Bernard et al., 2017], and the extensive literature implicating cerebellar circuits in SCZ more broadly [Andreasen et al., 1996; Andreasen and Pierson, 2008]. With that in mind, motor circuits certainly extend beyond the motor lobules of the cerebellum and the primary motor cortex. Indeed, there is an extensive cortical motor network including pre‐ and supplementary motor areas, and the basal ganglia also represent a crucial brain region for optimal motor function. Here, our results implicate these regions in that connectivity between M1 and putamen was associated with negative symptom severity, while greater connectivity between Lobule V and the extended motor network, including premotor cortex was seen in CON relative to SCZ individuals. However, these regions have been implicated in psychosis independently of this investigation, and outside of the context of other motor regions. In recent work, Stegmayer et al. [2014] demonstrated that supplementary motor area volume is related to aberrant motor behaviors measured with the Bern Psychiatric Scale. Furthermore, there is a long history of research implicating the basal ganglia in SCZ, including research demonstrating that volume of the basal ganglia is linked to motor symptoms in patients with SCZ [eg., Hirjak et al., 2012; for a review, see Hirjak et al., 2014], and both basal ganglia‐mediated motor symptoms are also linked to transition in UHR populations [Mittal et al., 2010b]. Our findings here further implicate these additional motor regions, but also further underscore the need for future work in patients with SCZ directly investigating the networks of these other critical motor regions. This will provide a more complete picture of motor networks in this important clinical population.
While this investigation provides important new insights regarding resting state motor networks in SCZ, there are several limitations that must be considered. First, while we controlled for antipsychotic medication as well as current alcohol and marijuana usage, it is not possible to completely eliminate their potential impact. Further, this does not account for effects of long‐term use or abuse. Second, the patients included in this analysis were chronic, and we did not include disease duration or duration of untreated illness. Both these factors could certainly impact the results presented here, and warrant further consideration in future work. That said, we have a relatively large sample, and though there is heterogeneity with respect to these factors, this allows for broader generalizability across populations of patients with SCZ, and perhaps psychosis more broadly. Relatedly, the averages of total positive and negative symptoms are somewhat low, though scores in both domains ranged from 7 to 29. This is likely due, at least in part, to the chronic nature of this sample, and the fact that all patients are taking antipsychotic medications. Though there is a relatively large range in symptom severity in the sample, this may have impacted our correlation analyses investigating brain networks and symptom severity. Finally, with respect to our thalamic connectivity analysis, it is important to note that the thalamus is a very small structure. Here, we used a 5 mm radius to account for this and minimize the inclusion of signal from surrounding nuclei, and aimed to optimize our localization as much as possible with respect to thalamic regions that project to motor and somatosensory cortex. However, we cannot completely eliminate the possibility that we are also including signal from adjacent nuclei, with connections to other cortical regions.
Using publically available data, we have demonstrated that in patients with schizophrenia, there are alterations in motor networks, both those of the primary motor cortex, and also the cerebellum. Notably, the thalamus seems to be an important node, particularly with respect to the primary motor cortex. Furthermore, we demonstrated that connectivity within motor regions was related to negative symptom severity, suggesting that motor systems may play a role in the etiology and symptomatology of the disease. This work builds off of prior resting state findings in psychosis risk populations [Anticevic et al., 2015], and investigations of motor behaviors in both at‐risk and schizophrenia populations [Bernard et al., 2014; Dean et al., 2013; Kent et al., 2012; Marvel et al., 2007; Mittal et al., 2010b]. As our understanding and appreciation for the motor signs and symptoms seen in SCZ increases, so does our need for better understanding of the underlying brain networks. The presence of motor network deficits, when controlling for medication, and their implications in symptom severity further suggest that motor systems may represent an important area of study, and target of intervention in schizophrenia, and psychosis more broadly.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
J.A.B. was supported in part by a Brain and Behavior Research Foundation NARSAD Young Investigator Award as the Donald and Janet Boardman Family Investigator. Data used in preparation of this article were obtained from the SchizConnect database. Data collection and sharing for this project was funded by NIMH cooperative agreement 1U01MH097435. COBRE data were downloaded from the Collaborative Informatics and Neuroimaging Suite Data Exchange tool (COINS; http://coins.mrn.org.dx) and data collection was performed at the Mind Research Network, and funded by a Centre for Biomedical Research Excellence Grant 5P20RR021938/P20GM103472 from the NIH to Dr Vince Calhoun. We thank Dr Joseph Orr for critical comments and feedback on this manuscript.
REFERENCES
- Andreasen NC, O'Leary DS, Cizadlo T, Arndt S, Rezai K, Ponto LL, Watkins GL, Hichwa RD (1996): Schizophrenia and cognitive dysmetria: A positron‐emission tomography study of dysfunctional prefrontal‐thalamic‐cerebellar circuitry. Proc Natl Acad Sci USA 93:9985–9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen NC, Paradiso S, O'Leary DS (1998): “Cognitive dysmetria” as an integrative theory of schizophrenia: A dysfunction in cortical‐subcortical‐cerebellar circuitry?. Schizophr Bull 24:203–218. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Olsen S (1982): Negative v positive schizophrenia. Arch Gen Psychiatry 39:789–794. [DOI] [PubMed] [Google Scholar]
- Andreasen NC, Pierson R (2008): The role of the cerebellum in schizophrenia. Biol Psychiatry 64:81–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anticevic A, Haut K, Murray JD, Repovs G, Yang GJ, Diehl C, McEwen SC, Bearden CE, Addington J, Goodyear B, Cadenhead KS, Mirzakhanian H, Cornblatt B. a, Olvet D, Mathalon DH, McGlashan TH, Perkins DO, Belger A, Seidman LJ, Tsuang MT, van Erp TGM, Walker EF, Hamann S, Woods SW, Qiu M, Cannon TD (2015): Association of thalamic dysconnectivity and conversion to psychosis in youth and young adults at elevated clinical risk. JAMA Psychiatry 6519:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Johansen‐Berg H, Woolrich MW, Smith SM, Wheeler‐Kingshott C. a M, Boulby P. a, Barker GJ, Sillery EL, Sheehan K, Ciccarelli O, Thompson AJ, Brady JM, Matthews PM (2003): Non‐invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Dean DJ, Kent JS, Orr JM, Pelletier‐Baldelli A, Lunsford‐Avery J, Gupta T, Mittal VA (2014): Cerebellar networks in individuals at ultra high‐risk of psychosis: Impact on postural sway and symptom severity. Hum Brain Mapp 35:4064–4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Mittal VA (2015): Dysfunctional activation of the cerebellum in schizophrenia: A functional neuroimaging meta‐analysis. Clin Psychol Sci 3:545–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA (2017): Cerebello‐thalamo‐cortical networks predict positive symptom progression in individuals at ultra‐high risk for psychosis. NeuroImage Clin 14:622–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Mittal VA (2014): Cerebellar‐motor dysfunction in schizophrenia and psychosis‐risk: The importance of regional cerebellar analysis approaches. Front Psychiatry Schizophr 5:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Orr JM, Mittal VA (2016): Differential motor and prefrontal cerebello‐cortical network development: Evidence from multimodal neuroimaging. Neuroimage 124:291–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, Hassevoort KM, Benson BL, Welsh RC, Wiggins JL, Jaeggi SM, Buschkuehl M, Monk CS, Jonides J, Peltier SJ (2012): Resting state cortico‐cerebellar functional connectivity networks: A comparison of anatomical and self‐organizing map approaches. Front Neuroanat 6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS (1997): Simultaneous assessment of flow and BOLD signals in resting‐state functional connectivity maps. NMR Biomed 10:165–170. [DOI] [PubMed] [Google Scholar]
- Biswal BB, Mennes M, Zuo X‐N, Gohel S, Kelly C, Smith SM, Beckmann CF, Adelstein JS, Buckner RL, Colcombe S, Dogonowski A‐M, Ernst M, Fair D, Hampson M, Hoptman MJ, Hyde JS, Kiviniemi VJ, Kötter R, Li S‐J, Lin C‐P, Lowe MJ, Mackay C, Madden DJ, Madsen KH, Margulies DS, Mayberg HS, McMahon K, Monk CS, Mostofsky SH, Nagel BJ, Pekar JJ, Peltier SJ, Petersen SE, Riedl V, Rombouts S. a RB, Rypma B, Schlaggar BL, Schmidt S, Seidler RD, Siegle GJ, Sorg C, Teng G‐J, Veijola J, Villringer A, Walter M, Wang L, Weng X‐C, Whitfield‐Gabrieli S, Williamson P, Windischberger C, Zang Y‐F, Zhang H‐Y, Castellanos FX, Milham MP (2010): Toward discovery science of human brain function. Proc Natl Acad Sci USA 107:4734–4739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bluhm RL, Miller J, Lanius RA, Osuch EA, Boksman K, Neufeld RWJ, Théberge J, Schaefer B, Williamson P (2007): Spontaneous low‐frequency fluctuations in the BOLD signal in schizophrenic patients: Anomalies in the default network. Schizophr Bull 33:1004–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Kent JS, Petersen IT, Klaunig MJ, Forsyth JK, Howell JM, Westfall DR, O'Donnell BF, Hetrick WP (2013): Impaired cerebellar‐dependent eyeblink conditioning in first‐degree relatives of individuals with schizophrenia. Schizophr Bull 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolbecker AR, Mehta CS, Edwards CR, Steinmetz JE, O'Donnell BF, Hetrick WP (2009): Eye‐blink conditioning deficits indicate temporal processing abnormalities in schizophrenia. Schizophr Res 111:182–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çetin MS, Christensen F, Abbott CC, Stephen JM, Mayer AR, Cañive JM, Bustillo JR, Pearlson GD, Calhoun VD (2014): Thalamus an posterior temporal lobe show greater inter‐network connectivity at rest and across sensory paradigms in schizophrenia. Neuroimage 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Bernard JA, Orr JM, Pelletier‐Baldelli a, Gupta T, Carol EE, Mittal V. a (2013): Cerebellar morphology and procedural learning impairment in neuroleptic‐naive youth at ultrahigh risk of psychosis. Clin Psychol Sci 2:152–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean DJ, Kent JS, Bernard JA, Orr JM, Gupta T, Pelletier‐Baldelli A, Carol EE, Mittal V. a (2015): Increased postural sway predicts negative symptom progression in youth at ultrahigh risk for psychosis. Schizophr Res 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J (2006): A spatially unbiased atlas template of the human cerebellum. Neuroimage 33:127–138. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N (2009): A probabilistic MR atlas of the human cerebellum. Neuroimage 46:39–46. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, Knutsson H (2016): Cluster failure: Why fMRI inferences for spatial extent have inflated false‐positive rates. Proc Natl Acad Sci 113:201602413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J (1995): Structured Clinical Interview for the DSM‐IV Axis I Disorders (SCID‐I), Patient Edition. Washington DC: American Psychiatric Press. [Google Scholar]
- Ford JM, Palzes VA, Roach BJ, Potkin SG, Van Erp TGM, Turner JA, Mueller BA, Calhoun VD, Voyvodic J, Belger A, Bustillo J, Vaidya JG, Preda A, McEwen SC, Mathalon DH (2015): Visual hallucinations are associated with hyperconnectivity between the amygdala and visual cortex in people with a diagnosis of schizophrenia. Schizophr Bull 41:223–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth JK, Bolbecker AR, Mehta CS, Klaunig MJ, Steinmetz JE, O'Donnell BF, Hetrick WP (2012): Cerebellar‐dependent eyeblink conditioning deficits in schizophrenia spectrum disorders. Schizophr Bull 38:751–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD (1995): Schizophrenia: A disconnection syndrome? Clin Neurosci. [PubMed] [Google Scholar]
- Garrity AG, Pearlson GD, Mckiernan K, Ph D, Lloyd D, Ph D, Kiehl KA, Ph D, Calhoun VD Ph D (2007): Aberrant “default mode” functional connectivity in schizophrenia. Am J Psychiatry 450–457. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Wolf RC, Stieltjes B, Seidl U, Schröder J, Thomann P. a (2012): Neurological soft signs and subcortical brain morphology in recent onset schizophrenia. J Psychiatr Res 46:533–539. [DOI] [PubMed] [Google Scholar]
- Hirjak D, Wolf RC, Wilder‐Smith EP, Kubera KM, Thomann P. a (2014): Motor abnormalities and basal ganglia in schizophrenia: Evidence from structural magnetic resonance imaging. Brain Topogr 42–43. [DOI] [PubMed] [Google Scholar]
- Kay SR, Opler LA, Lindenmayer J‐P (1989): The Positive and Negative Syndrome Scale (PANSS): Rationale and standarisation. Br J Psychiatry 115. [PubMed] [Google Scholar]
- Kay SR, Qpjer LA (1982): The Positive and Negative Syndrome Scale (PANSS) for schizophrenia. Schizophr Bull 13:261–276. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL (2003): Cerebellar loops with motor cortex and prefrontal cortex of a nonhuman primate. J Neurosci 23:8432–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JS, Hong SL, Bolbecker AR, Klaunig MJ, Forsyth JK, O'Donnell BF, Hetrick WP (2012): Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One 7:e41808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D‐J, Kent JS, Bolbecker AR, Sporns O, Cheng H, Newman SD, Puce A, O'Donnell BF, Hetrick WP (2014): Disrupted modular architecture of cerebellum in schizophrenia: A graph theoretic analysis. Schizophr Bull 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krienen FM, Buckner RL (2009): Segregated fronto‐cerebellar circuits revealed by intrinsic functional connectivity. Cereb Cortex 19:2485–2497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM, Barch DM (2014): The motivation and pleasure dimension of negative symptoms: Neural substrates and behavioral outputs. Eur Neuropsychopharmacol 24:725–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langan J, Peltier SJ, Bo J, Fling BW, Welsh RC, Seidler RD (2010): Functional implications of age differences in motor system connectivity. Front Syst Neurosci 4:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Samara M, Heres S, Patel MX, Woods SW, Davis JM (2014): Dose equivalents for second‐generation antipsychotics: The minimum effective dose method. Schizophr Bull 40:314–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fan G, Xu K, Wang F (2011): Changes in cerebellar functional connectivity and anatomical connectivity in schizophrenia: A combined resting‐state functional MRI and diffusion tensor imaging study. J Magn Reson Imaging 34:1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez‐Larson MP, Rogowska J, Bogorodzki P, Bueler CE, McGlade EC, Yurgelun‐Todd D. a (2012): Cortico‐cerebellar abnormalities in adolescents with heavy marijuana use. Psychiatry Res 202:224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvel CL, Schwartz BL, Rosse RB (2004): A quantitative measure of postural sway deficits in schizophrenia. Schizophr Res 68:363–372. [DOI] [PubMed] [Google Scholar]
- Marvel CL, Turner BM, O'Leary DS, Johnson HJ, Pierson RK, Boles Ponto LL, Andreasen NC (2007): The neural correlates of implicit sequence learning in schizophrenia. Neuropsychology 21:761–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE (2006): Three‐dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: A meta‐analysis. Neuroimage 31:1453–1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millan MJ, Fone K, Steckler T, Horan WP (2014): Negative symptoms of schizophrenia: Clinical characteristics, pathophysiological substrates, experimental models and prospects for improved treatment. Eur Neuropsychopharmacol 24:645–692. [DOI] [PubMed] [Google Scholar]
- Mittal VA, Daley M, Shiode MF, Bearden CE, O'Neill J, Cannon TD (2010a): Striatal volumes and dyskinetic movements in youth at high‐risk for psychosis. Schizophr Res 123:68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Dean DJ, Bernard JA, Orr JM, Pelletier‐Baldelli A, Carol EE, Gupta T, Turner J, Leopold DR, Robustelli BL, Millman ZB (2014): Neurological soft signs predict abnormal cerebellar‐thalamic tract development and negative symptoms in adolescents at high risk for psychosis: A longitudinal perspective. Schizophr Bull 50:1204–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal VA, Walker EF, Bearden CE, Walder D, Trottman H, Daley M, Simone A, Cannon TD (2010b): Markers of basal ganglia dysfunction and conversion to psychosis: Neurocognitive deficits and dyskinesias in the prodromal period. Biol Psychiatry 68:93–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oertel V, Rotarska‐Jagiela A, van de Ven VG, Haenschel C, Maurer K, Linden DEJ (2007): Visual hallucinations in schizophrenia investigated with functional magnetic resonance imaging. Psychiatry Res Neuroimag 156:269–273. [DOI] [PubMed] [Google Scholar]
- Öngür D, Lundy M, Greenhouse I, Shinn AK, Menon V, Cohen BM, Renshaw PF (2010): Default mode network abnormalities in bipolar disorder and schizophrenia. Psychiatry Res Neuroimag 183:59–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul EW, Harrison J (2004): The hippocampus in schizophrenia: A review of the neuropathological evidence and its pathophysiological implications. Psychopharmacology (Berl) 174:151–162. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Alva G, Fleming K, Anand R, Keator D, Carreon D, Doo M, Jin Y, Wu JC, Fallon JH (2002): A PET study of the pathophysiology of negative symptoms in schizophrenia. Am J Psychiatry 159:227–237. [DOI] [PubMed] [Google Scholar]
- Repovs G, Csernansky JG, Barch DM (2011): Brain network connectivity in individuals with schizophrenia and their siblings. Biol Psychiatry 69:967–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM (2005): Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry 162:2384–2386. [DOI] [PubMed] [Google Scholar]
- Shergill SS, White TP, Joyce DW, Bays PM, Wolpert DM, Frith CD (2014): Functional magnetic resonance imaging of impaired sensory prediction in schizophrenia. JAMA Psychiatry 71:28–35. [DOI] [PubMed] [Google Scholar]
- Silbersweig DA, Stern E, Frith C, Cahill C, Holmes A, Grootoonk S, Seaward J, McKenna P, Chua SE, Schnorr L, Jones T, Frackowiak RSJ (1995): A functional neuroanatomy of hallucinations in schizophrenia. Nat Lond 378:176–179. [DOI] [PubMed] [Google Scholar]
- Solowij N, Yücel M, Respondek C, Whittle S, Lindsay E, Pantelis C, Lubman DI (2011): Cerebellar white‐matter changes in cannabis users with and without schizophrenia. Psychol Med 41:2349–2359. [DOI] [PubMed] [Google Scholar]
- Stegmayer K, Horn H, Federspiel A, Razavi N, Bracht T, Laimböck K, Strik W, Dierks T, Wiest R, Müller TJ, Walther S (2014): Supplementary motor area (SMA) volume is associated with psychotic aberrant motor behaviour of patients with schizophrenia. Psychiatry Res Neuroimag 223:49–51. [DOI] [PubMed] [Google Scholar]
- Sullivan EV, Rohlfing T, Pfefferbaum A (2010a): Pontocerebellar volume deficits and ataxia in alcoholic men and women: No evidence for “telescoping”. Psychopharmacology (Berl) 208:279–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Rose J, Pfefferbaum A (2010b): Physiological and focal cerebellar substrates of abnormal postural sway and tremor in alcoholic women. Biol Psychiatry 67:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther S, Strik W (2012): Motor symptoms and schizophrenia. Neuropsychobiology 66:77–92. [DOI] [PubMed] [Google Scholar]
- Welsh RC, Chen AC, Taylor SF (2010): Low‐frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36:713–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Ford JM (2012): Default mode network activity and connectivity in psychopathology. Annu Rev Clin Psychol 8:49–76. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Nieto‐Castanon A (2012): Conn: A functional connectivity toolbox for correlated and anticorrelated brain networks. Brain Connect 2:125–141. [DOI] [PubMed] [Google Scholar]
- Whitfield‐Gabrieli S, Thermenos HW, Milanovic S, Tsuang MT, Stephen V, Mccarley RW, Shenton ME, Alan I, Nieto‐castanon A, Laviolette P, Gabrieli JDE, Seidman LJ (2009): Hyperactivity and hyperconnectivity of the default network in schizophrenia and in first‐degree relatives of persons with schizophrenia. Proc Natl Acad Sci USA 106:1279–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SW (2003): Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 64:663–667. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Karbasforoushan H, Heckers S (2012): Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169:1092–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


