To the Editor
Cartilage-hair hypoplasia (CHH) is a chondrodysplasia with variable immunodeficiency. Patients’ immunologic and clinical phenotypes are highly variable and correlate weakly. Most previous studies have involved only children, and the quality of immune function in CHH adults remains largely unknown. In addition, paucity of data exists regarding T and B cell subpopulations and specific antibody responses in CHH. Here we report on the clinical and immunological phenotype in a large group of Finnish children and adults with CHH.
We invited all 110 living Finnish patients with genetically confirmed CHH. Fifty-six individuals (19 males, 37 females, median age 34, range 0.7–68 years) agreed to participate, with informed consent. The study protocol was approved by the Institutional Research Ethics Committee. Clinical data were collected, and laboratory tests performed (see Appendix E1 in the Online Repository for methods). Correlations between laboratory parameters and clinical manifestations were analyzed with the Fisher’s exact test, the Mann-Whitney or the Kruskall-Wallis tests and regression analysis, as appropriate.
All patients were homozygous (n=43) or compound heterozygous (n=13) for the RMRP g.70A>G mutation. Table I describes clinical features. We grouped patients as having: 1) no symptoms/signs of immunodeficiency (n=15, 27%), 2) features of humoral immunodeficiency only (n=26, 46%) and 3) features of combined immunodeficiency (CID, n=15, 27%) (see Table I for definitions).
Table I.
Clinical features of the 56 patients with cartilage-hair hypoplasia.
| Clinical feature | Proportion of patients | Comments |
|---|---|---|
|
| ||
| Clinical humoral immunodeficiency | 26/56 (46%) | Defined as otitis media and/or rhinosinusitis requiring surgical interventions, sepsis, pneumonia and/or bronchiectasis. |
|
| ||
| Clinical combined immunodeficiency | 15/56 (27%) | Defined as a history of severe warts, recurrent and/or severe herpes virus infections, malignancy and/or autoimmunity, with clinical signs of humoral immunodeficiency. |
|
| ||
| Pneumonia | 11/56 (20%) | |
| recurrent | 4/11 (36%) | |
| hospitalization | 5/11 (45%) | |
|
| ||
| Bronchiectasis | 10/34 (29%) | Diagnosed on lung high-resolution computed tomography. |
|
| ||
| Sinusitis | 33/56 (59%) | |
| sinus surgery | 21/33 (64%) | |
|
| ||
| Otitis media | 41/56 (73%) | |
| tympanostomy | 16/41 (39%) | |
|
| ||
| Sepsis | 3/56 (5%) | |
|
| ||
| History of warts | 19/56 (34%) | Out of two patients with severe warts, one underwent partial amputation of the toe with warts for suspected malignancy and another had protracted history of wide-spread warts. |
| warts at the time of visit | 14/19 (74%) | |
| severe warts | 2/19 (11%) | |
|
| ||
| Varicella | 42/56 (75%) | History of varicella or positive serology for varicella-zoster virus. |
| hospitalization | 5/42 (12%) | |
|
| ||
| Boils | 3/56 (5%) | |
| recurrent | 2/3 (67%) | |
|
| ||
| Mucocutaneous herpes simplex virus infection | 2/56 (4%) | One patient had required continuous acyclovir prophylaxis for 15 yrs for recurrent skin herpes simplex virus infections. |
|
| ||
| Allergy | 23/56 (41%) | History of allergic rhinoconjunctivitis. |
|
| ||
| Malignancy | 9/56 (16%) | Other malignancies included uterus carcinoma in one patient and vocal cord carcinoma in another. |
| lymphoma | 2/9 (22%) | |
| basal cell carcinoma only | 5/9 (56%) | |
| other | 2/9 (22%) | |
|
| ||
| Autoimmunity | 1/56 (2%) | Juvenile idiopathic arthritis. |
|
| ||
| Prophylactic antibiotics | 7/56 (13%) | Sulfamethoxazole/trimethoprim or azithromycin, commenced for recurrent infections or lymphopenia. |
|
| ||
| Immunoglobulin substitution | 7/56 (13%) | In four patients treatment was still ongoing at the time of the study. |
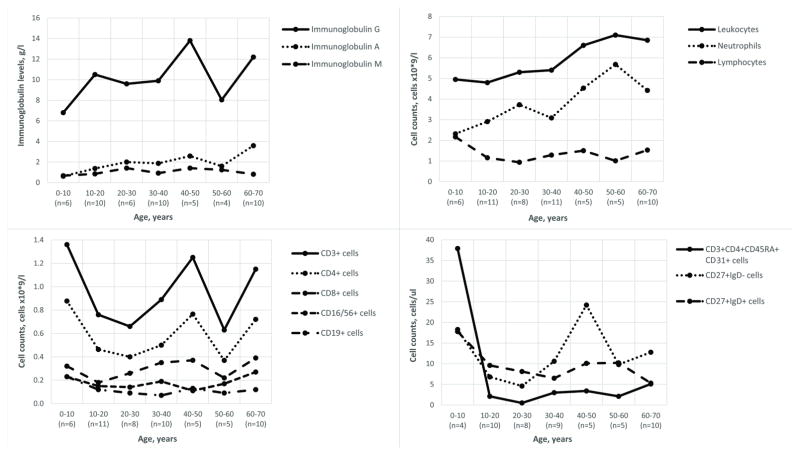
Laboratory findings are presented in Figure I and Tables E1 and E2 in the Online Repository. Common pattern of abnormalities in the B and T cell subpopulations (Table E2 in the Online Repository) included: 1) decreased CD3+CD4+CD45RA+CD31+ recent thymic emigrants (RTE), naive CD4+ and CD8+ cells; 2) increased activated CD4+, central memory CD4+ and effector memory CD8+ cells; 3) normal CD4+CD25highCD127low regulatory T cells; 4) decreased naive, transitional and CD19+CD27+ memory B cells; 5) increased activated CD21lowCD38lowCD19+ cells. The reduced counts of rapidly proliferating cells (naive T, RTE, and B) support a disturbed cell cycle as an important pathogenic mechanism1. The pattern of abnormalities in B cell subpopulations indicate B cell production and germinal center defect, while decreased CD27+ B cells suggest defective maturation or survival2.
Figure I.
Selected immunologic features in 56 patients with cartilage-hair hypoplasia. All laboratory values are shown as medians. Patients are grouped by age and the number of subjects is shown in each age group.
Pre-existing tetanus toxoid antibody levels were below 0.1 IU/ml in only 3/43 patients (Table E1 in the Online Repository). Seven out of eight (88%) patients who agreed to receive Pneumovax® vaccine demonstrated specific antibody deficiency (SAD) (see Table E3 in the Online Repository). Clinical features of the seven subjects with SAD included rhinosinusitis (n=6), OM (n=5), warts (n=3), malignancies (n=3; lymphoma, basal cell carcinoma, uterus carcinoma), severe varicella requiring hospitalization (n=2) and recurrent herpes simplex virus infection (n=1). Patients with SAD showed clinical signs of humoral (n=2) and combined (n=4) immunodeficiency. SAD may therefore be a marker of more severe disease and vaccine responses should be routinely studied after infancy.
Patients with no clinical signs of immunodeficiency showed no specific pattern of laboratory abnormalities. No correlation was observed between history of pneumonia or rhinosinusitis and immunologic defects. Genotype did not correlate with any clinical or laboratory parameter.
Patients with clinically suggested CID showed decreased median CD3+, CD8+ and RTE counts (see Table E4 in the Online Repository). Of these parameters, RTE counts demonstrated the strongest trend towards significance in multiple regression analysis (p=0.094, B coefficient −15.628). Lymphocyte proliferative responses were not performed, since previous studies have demonstrated that they are abnormal in the majority of patients and show no correlation with clinical manifestations in CHH1.
Lower median IgG2 concentrations (1.08 vs 1.96 g/l, p=0.016) were observed in patients who required hospitalization for VZV infection. Four out of five hospitalized patients (80%) were IgG2 deficient (IgG2 range 0.55–1.47 g/l) compared with 9 out of 37 (24%) non-hospitalized patients (p=0.011). In multiple regression analysis, only low IgG2 showed a trend towards significance (p=0.055, B coefficient −1.734). The association between IgG2 deficiency and severe varicella has been described previously3, suggesting that CHH patients with low IgG2 should be regarded at risk for severe VZV infection. However, these findings require validation in further studies, as low IgG2 may only represent a marker of a more profound immunodeficiency.
We then analyzed laboratory results in various age groups: children <18 years (n=15), young adults 18–44 years (n=23) and adults >45 years (n=18) (see Table E4 in the Online Repository). Patients aged >45 years demonstrated higher neutrophil and CD4+ cell counts, as well as higher median IgA, IgG and IgG2 levels than younger patients. In addition, children showed higher CD19+ cell counts and lower median concentrations of antibodies to tetanus toxoid than adult patients. While lower immunoglobulin and vaccine antibody levels in pediatric patients may reflect slow maturation of immune system, lower CD19+ and higher CD4+ cell numbers in CHH adults deserve further studies. As mortality due to infections and malignancies is increased in CHH4, patients surviving into adulthood represent individuals with milder disease. Furthermore, while some RMRP gene mutations induce a more severe immunodeficiency5, the predominance of g.70A>G mutation in Finnish patients can result in milder presentations.
Consistent with previous reports6, measured laboratory values correlated poorly with any assessed symptoms and despite clearly abnormal laboratory indices, few patients developed serious complications. Worldwide, of the 30 subjects with CHH and hematopoietic stem cell transplantation (HSCT) whose genotype has been reported, only six patients were homozygous for g.70A>G mutation6,7,8,9. Only one Finnish child with CHH, homozygote for g.70A>G mutation, has required HSCT for severe hypoplastic anemia - not for immunodeficiency. Thus selection of patients who would benefit from HSCT based on routinely available laboratory parameters is highly cumbersome in a cohort carrying predominantly RMRP g.70A>G mutations.
The major limitation of this study is the retrospective nature of clinical data. Inclusion of only living patients left out those with CHH who had died of cancer or severe infections. Another limitation is the use of a single laboratory measurement per patient. Results of immunologic tests fluctuate over time and predicting any clinical course based on cross-sectional laboratory values remains challenging.
The observed high number of asymptomatic patients and individuals with clinical signs of humoral immunodeficiency only should be interpreted with caution, as clinical features may occur and cumulate with time. Follow-up studies should assess the applicability of clinical and immunological phenotype correlations, including the potential to predict a more severe course (CID) in patients with lower CD3+, CD8+ and RTE counts.
In summary, we demonstrated that approximately one fourth of the surviving Finnish patients with CHH included in this study manifested clinical signs of CID, while another fourth showed no signs of immunodeficiency despite laboratory immunologic abnormalities. This is the first report describing high prevalence of SAD and a specific pattern of abnormalities in B and T cell compartments in patients with CHH.
Supplementary Material
Acknowledgments
Funding
The study was funded by the Sigrid Jusélius Foundation (OM), the Academy of Finland (OM), the Folkhälsan Research Foundation (OM), the Helsinki University Hospital Research Funds (OM, MT), the Swedish Childhood Cancer Foundation (OM), the Foundation for Pediatric Research (OM, MT), the Finnish Medical Foundation (SK) and the Doctoral School in Health Sciences at the University of Helsinki (SK). The research was supported in part by the Intramural Research program of the NIH, NIAID.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.de la Fuente MA, Recher M, Rider NL, Strauss KA, Morton DH, Adair M, et al. Reduced thymic output, cell cycle abnormalities, and increased apoptosis of T lymphocytes in patients with cartilage-hair hypoplasia. J Allergy Clin Immunol. 2011;128:139–46. doi: 10.1016/j.jaci.2011.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Driessen GJ, van Zelm MC, van Hagen PM, Hartwig NG, Trip M, Warris A, et al. B-cell replication history and somatic hypermutation status identify distinct pathophysiologic backgrounds in common variable immunodeficiency. Blood. 2011;118:6814–23. doi: 10.1182/blood-2011-06-361881. [DOI] [PubMed] [Google Scholar]
- 3.Sugita K, Owada Y, Ozawa T, Sakakibara H, Eguchi M, Furukawa T, et al. An infant with both autoimmune neutropenia and idiopathic thrombocytopenia with IgG2/IgA deficiency. Int J Hematol. 1993;57:27–30. [PubMed] [Google Scholar]
- 4.Mäkitie O, Pukkala E, Kaitila I. Increased mortality in cartilage–hair hypoplasia. Arch Dis Child. 2001;84:65–7. doi: 10.1136/adc.84.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thiel CT, Mortier G, Kaitila I, Reis A, Rauch A. Type and level of RMRP functional impairment predicts phenotype in the cartilage hair hypoplasia-anauxetic dysplasia spectrum. Am J Hum Genet. 2007;81:519–29. doi: 10.1086/521034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rider NL, Morton DH, Puffenberger E, Hendrickson CL, Robinson DL, Strauss KA. Immunologic and clinical features of 25 Amish patients with RMRP 70 A-->G cartilage hair hypoplasia. Clin Immunol. 2009;131:119–28. doi: 10.1016/j.clim.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Guggenheim R, Somech R, Grunebaum E, Atkinson A, Roifman CM. Bone marrow transplantation for cartilage-hair-hypoplasia. Bone Marrow Transplant. 2006;38:751–6. doi: 10.1038/sj.bmt.1705520. [DOI] [PubMed] [Google Scholar]
- 8.Bordon V, Gennery AR, Slatter MA, Vandecruys E, Laureys G, Veys P, et al. Inborn Error Working Party of the European Bone Marrow Transplantation (EBMT) group. Clinical and immunologic outcome of patients with cartilage hair hypoplasia after hematopoietic stem cell transplantation. Blood. 2010;116:27–35. doi: 10.1182/blood-2010-01-259168. [DOI] [PubMed] [Google Scholar]
- 9.Ip W, Gaspar HB, Kleta R, Chanudet E, Bacchelli C, Pitts A, et al. Variable Phenotype of Severe Immunodeficiencies Associated with RMRP Gene Mutations. J Clin Immunol. 2015;35:147–57. doi: 10.1007/s10875-015-0135-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.