Abstract
The overall premise of this review is that cerebrospinal fluid (CSF) is transported within a dedicated peri-vascular network facilitating metabolic waste clearance from the central nervous system while we sleep. The anatomical profile of the network is complex and has been defined as a peri-arterial CSF influx pathway and peri-venous clearance routes, which are functionally coupled by interstitial bulk flow supported by astrocytic aquaporin 4 water channels. The role of the newly discovered system in the brain is equivalent to the lymphatic system present in other body organs and has been termed the “glymphatic pathway” or “(g)lymphatics” because of its dependence on glial cells. We will discuss and review the general anatomy and physiology of CSF from the perspective of the glymphatic pathway, a discovery which has greatly improved our understanding of key factors that control removal of metabolic waste products from the central nervous system in health and disease and identifies an additional purpose for sleep. A brief historical and factual description of CSF production and transport will precede the ensuing discussion of the glymphatic system along with a discussion of its clinical implications.
Keywords: glymphatic pathway, cerebrospinal fluid, brain, microcirculation, transport
Introduction
The lymphatic system is present throughout most of the body and is designed to transport lymph and remove metabolic waste. It is also important for tissue fluid homeostasis, the immune system and for facilitating absorption of fatty substances from the digestive system (Ikomi and others 2012). Anatomically, the lymphatic system includes lymphatic capillaries, larger transporting lymph vessels, and lymph nodes. Lymph is formed by the microcirculation (capillaries and post-capillary venules) because of hydrostatic and oncotic pressure differences between blood plasma and the abluminal side of glycocalyx residing on endothelial cells driving plasma with solutes through transendothelial junctions and pores (Levick and Michel 2010; Weinbaum and others 2007). The classical Starling principle assumes complete symmetry between ultrafiltration in the arterial part and reabsorption in the venous part of the microcirculation; however, there is growing consensus that fluid homeostasis in most tissues relies critically on lymphatic function and not on venous reabsorption (Levick and Michel 2010). Instead, the ultrafiltrate generated from the microcirculation is propelled into the tissue—a process facilitating removal of cellular waste products—and carried by lymphatic vessels to lymph nodes; from where it flows into larger efferent lymph vessels that ultimately drain into the circulation via the subclavian veins. The brain parenchyma is exceptional to this principle because it is devoid of lymph vessels; though a recent study identified authentic lymphatic vessels residing in the meninges that drain into cervical lymph nodes and are involved in the drainage of solutes from the brain (Louveau and others 2015).
The brain is protected from the systemic circulation by the blood-brain barrier (BBB), which is formed by capillary endothelial cells characterized by tight junctions and almost no pores making it impermeable even to small ions. One could make the argument that because the BBB prohibits generation of an ultrafiltrate there is no need for a lymphatic system to remove excess fluids from the brain. However, the brain still has a need for removal of toxic substances and macromolecules. Decades of research and important new developments in the field have revealed another process by which cerebrospinal fluid (CSF) facilitates removal of metabolic waste in the brain (Cserr 1974; Ghersi-Egea and others 1996; Kida and Weller 1993; Rennels and others 1985). This review is a summary of the past and current information that led to the conceptualization of the glymphatic pathway, including the physiological and physical factors that govern its functional state.
Sources of CSF
The major part of CSF is produced by the choroid plexuses located in the lateral, third and fourth ventricles (Box 1). The process of choroidal CSF formation is complex (Damkier and others 2013; Johanson and others 2011). Briefly ventricular CSF production is a two-step process: a pressure-driven passive filtration of plasma across the choroidal capillaries into the choroidal interstitial space and an active secretion component across the choroidal epithelium into the ventricles controlled by carbonic anhydrase and membrane ion carrier proteins (Damkier and others 2010). Although still debated and incompletely understood, other sources contribute to CSF production including the cerebral microcirculation (Bulat and Klarica 2011; Crone 1986; Cserr 1974; Kimelberg 2004; Milhorat and others 1971) and metabolic water production (Rapoport 1978). Thirty years ago, Cserr and others introduced the concept that interstitial fluid (ISF) surrounding all brain cells was secreted by endothelial cells of the BBB (Fig. 1) and communicated with CSF via bulk flow (Cserr 1988; Cserr and others 1977; Cserr and others 1981).
Box 1. Sources of Cerebrospinal Fluid.
Choroid plexus: 70% to 80%
Endothelial cells: 10%
Metabolic water: 10%
Figure 1.
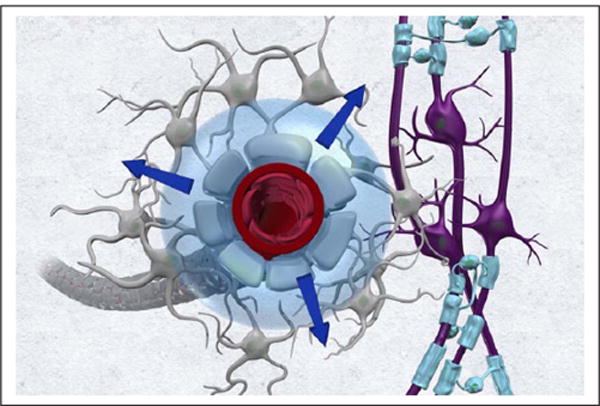
The brain microcirculation contributes to cerebrospinal fluid (CSF) production. It has been proposed that the capillary unit secretes fluid which enters the interstitial space and communicates with ventricular and subarachnoid CSF via bulk flow. The figure illustrates this concept from the perspective of the capillary and astrocytic end-foot.
She further suggested that the rate of endothelial fluid secretion into ISF was in steady state with its drainage through “a series of patent extracellular channels,” which included peri-vascular, peri-ventricular, and white matter locations (Cserr and others 1977); and the clearance of solutes from the ISF was reflective of the rate of endothelial cell fluid secretion (Cserr 1988). This concept was supported by Crone who argued that the BBB could be thought of as an “epithelial membrane” with fluid secretion (Crone 1986). Putting data from these studies into a quantitative perspective the various production sources of CSF can be estimated. In 3-month-old rats, the CSF formation rate has been measured and calculated (using dye-dilution and ventriculo-cisternal perfusion) to be ~2 μL/min (Chiu and others 2012). Further, the solute drainage rate from the rat ISF of the caudate nucleus was estimated to be 0.2 μL/min (for a 1.8 g rat brain (Cserr 1988)), which signifies that the microcirculation contributes ~10% to overall CSF production. In this context, it is noteworthy that invertebrates such as the cuttlefish Sepia has no choroid plexus, yet is capable of draining solutes from the brain interstitial space at a rate of 0.1 μL/min/g brain (Abbott and others 1985) indicating that in some species there is considerable CSF derived from the cerebral microvasculature. In human brain, however, it is still unclear how much the microcirculation quantitatively contributes to CSF production. It is stated that 70% to 80% of CSF is directly secreted by the choroid plexus, 12% from metabolism (Rapoport 1978) and the remaining stems from the microcirculation (reviewed by Brinker and others 2014; Sakka and others 2011). The contribution of the brain microcirculation to CSF is likely to involve the aquaporin-4 (AQP4) water channels; the location of which, is restricted to glia and ependymocytes in the brain (reviewed by Nagelhus and Ottersen 2013).
Circadian Rhythm of CSF Production Rate
CSF production varies with circadian rhythm and in humans appears to peak during sleep after midnight as documented by magnetic resonance imaging (MRI) techniques (Nilsson and others 1992). Circadian variability in CSF production rate might be attributed to autonomic innervation of the choroid plexus (the vascular bed as well as the secretory epithelium) (Lindvall and Owman 1981). Physiological studies documented that increased sympathetic tone exerts an inhibitory action on carbonic anhydrase associated with the choroidal epithelium and likely therefore also reduces CSF production (Damkier and others 2013). During non–rapid eye movement sleep sympathetic tone is low and parasympathetic tone is high (Bonnet and Arand 1997) which may explain peak production from the choroid plexus during sleep. Alternatively, CSF production may increase during sleep because of changes pertaining to CSF volume shifts, which will be discussed in the context of the glymphatic pathway.
CSF Transport in the Central Nervous System
The CSF transport process involves several anatomical compartments including the ventricles, the subarachnoid space, brain, and spine parenchyma; and the drainage sites. In reviewing preclinical and clinical CSF transport studies it is important to consider that results are sensitive to pressure leaks, body position, tracer injection technique and site of tracer administration (Klarica and others 2006; Klarica and others 2014). In particular, the site of injection has been emphasized by kinetic modeling studies of CSF drug transport (Shafer and Shafer 1999); since CSF is not as well-stirred as blood in the systemic circulation. Rodents, for example, are characterized by partial obliteration of the subarachnoid CSF space above the hemispheric convexities which impacts mixing with CSF from the other compartments (Cserr and others 1981; Morse and Low 1972).
The amount of CSF produced daily in humans is 400 to 500 mL (Cutler and others 1968; Rubin and others 1966), but at any given time only 150 mL of CSF fills the subarachnoid space of the cranium and spine, including the ventricles (Schroth and Klose 1992a). CSF is reabsorbed at dedicated anatomical drainage sites; and is replaced several times daily, 4 to 5 times in humans and 11 times in the rat brain (Johanson and others 2008). Heinrich Quincke was among the first to gain direct access to CSF and to study transport of solutes (Bechter and Benveniste 2015; Benveniste and others 2015). He administered an emulsion of cinnabar granules (mercury(II) sulfide, HgS; granule size ~1 μm) into CSF via the lateral ventricles, the subarachnoid space (basal cisterns) and the lumbar intrathecal space of live animals (dog, cats, and rabbits) (Bechter and others 2015). When injecting the cinnabar dye into the lateral ventricles he observed (post mortem) that it had travelled through the central canal of the spine all the way down to the lumbar region (Bechter and others 2015). Since then numerous investigators have repeated this experiment using state-of-the-art technologies and smaller molecular weight contrast dyes, and we now have a good understanding of CSF circulation in brain and spine. Ventricular and subarachnoid CSF communicates; and there is free flow between the subarachnoid space of the brain and spinal cord. There is also consensus that choroidal CSF moves from the sites of production through the interventricular foramina to the third and the fourth ventricles via the aqueduct and into the subarachnoid space of the basal cisterns (Fig. 2).
Figure 2.
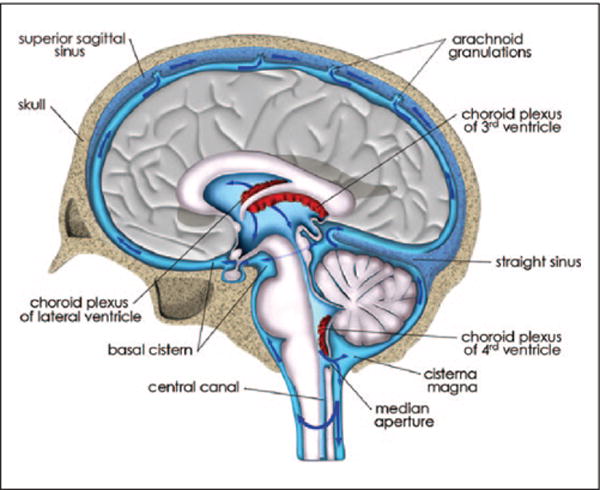
Cerebrospinal fluid (CSF) transport and reabsorption from ventricles and subarachnoid space: CSF transport from the site of formation in the choroid plexuses in the lateral, 3rd and 4th ventricle and out into the subarachnoid space via the median aperture is illustrated in an anatomical drawing of the human brain at the level of the superior sagittal sinus. The blue arrows in the subarachnoid space indicate CSF transport above the hemispheric convexities toward the arachnoid granulations; from where CSF exits into the dural sinuses. There is also CSF transport from the subarachnoid space and down toward the spine.
Transfer of CSF out of the ventricles is governed by pressure-driven gradients created by cardiac and respiratory movements and likely also by local currents generated by beating cilia on ependymal cells lining the ventricles (Box 2). Non-invasive MRI in combination with specialized pulse-sequences has been used in humans and laboratory animals to interpret how cardiac pulsation and respiratory effort influence ventricular CSF transport (Friese and others 2004; Schroth and Klose 1992a, 1992b; Wagshul and others 2011). For example, in the aqueduct, CSF movement was observed to be oscillatory and in sync with cardiac pulsation (Schroth and Klose 1992a). In particular, ventricular CSF was documented to flow from the third to fourth ventricle shortly after the R-wave of the electrocardiogram and immediately thereafter in the opposite direction (Schroth and Klose 1992a). The influence of respiration on CSF transport was recently investigated in normal human subjects from the lateral ventricles all the way down to the cervical subarachnoid space using ultra-fast MRI imaging (Dreha-Kulaczewski and others 2015). This study employed very fast acquisitions (20 frames/s or 1000 images over 50 seconds), which enabled differentiation of cardiac and respiratory modulation of CSF flow (Dreha-Kulaczewski and others 2015) demonstrating that forced inspiration was a main driver of CSF flow in the ventricles (Dreha-Kulaczewski and others 2015). The actual direction of CSF movement in the ventricular spaces during forced inspiration is currently unknown but likely creates large currents in the ventricular CSF compartment contributing to outward transport.
Box 2. Drivers of Cerebrospinal Fluid Transport.
Intracerebral pressure differentials
Arterial pressure and pulsatility
Respiratory forces (intrathoracic pressure changes)
Interstitial fluid volume
The dependence of CSF transport on intact intracranial hydrostatic pressure is the foundation for clinical diagnosis of CSF leakage using radionuclide cisternography, or contrast enhanced MRI. Thus, in patients with dural tears CSF transport is significantly reduced not only because of the CSF leakage itself but also because of hydrostatic pressure changes. Radionuclide cisternography uses metabolically inert tracers like 99mTc- or 111In-labeled diethylenetriamine pentaacetic acid (DTPA) delivered into the lumbar intrathecal space in combination with dynamic SPECT (single-photon emission computed tomography) or SPECT/CT imaging. From such studies, it has been documented that lumbar CSF reaches the foramen magnum in ~1 hour and the cranial subarachnoid space in a few hours (2–4 hours.) (Edsbagge and others 2004). Furthermore, physical activity accelerates CSF transport dynamics in humans (Edsbagge and others 2004), which may be secondary to greater respiratory effort (Dreha-Kulaczewski and others 2015). Figure 3 shows a SPECT/CT scan from a 63-year-old woman 24 hours after lumbar intrathecal delivery of 111In DTPA; and demonstrates the distribution pattern of the inert tracer in the brain and cervical spine. The 111In-DTPA uptake is high in the cervical spine, frontoorbital cortex and cerebellum; and above the cerebral hemispheres in agreement with the expected normal distribution and consistent with no CSF leakage (Fig. 3). Other imaging techniques and tracers (e.g., paramagnetic contrast; Algin and Turkbey 2013) or water-soluble iodinated contrast (Sage 1983) reveal the same CSF transport pattern under normal conditions.
Figure 3.
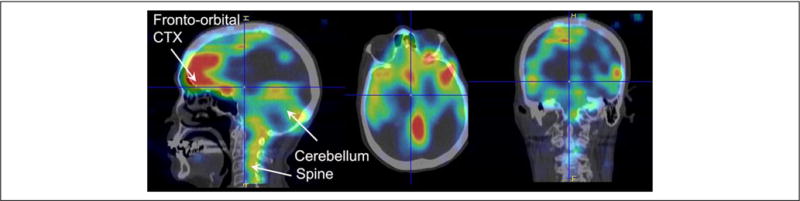
Cerebrospinal fluid (CSF) transport of metabolically inert tracer in human brain The distribution pattern of 111In-labeled diethylenetriamine pentaacetic acid (111In-DTPA) in brain and cervical spine in three orthogonal planes from a 63-year-old female, 24 hrs. after lumbar intrathecal injection of the radiotracer. The images were acquired using combined SPECT/CT. The radiotracer in CSF was transported from the lumbar intrathecal space to the cranial subarachnoid space and into brain parenchyma over 24 hrs. There is evidence of the radiotracer over the hemispheric convexities as well as in parenchyma. The uptake in the brain in inhomogeneous with excessive uptake in the cerebellum, frontoorbital cortex (CTX) and pons. There was no evidence of dural leak in this patient and the brain 111In-DTPA uptake pattern was read as normal. Data courtesy: Professor Dinko Franceschi, MD, Department of Nuclear Medicine, Radiology Stony Brook University.
Conceptualization of the Glymphatic Pathway
Early studies that quantified the ISF volume fraction around brain cells were in part obtained from measures of solute transport in brain tissue (reviewed in Chodobski and others 2015). In the 1970s, Cserr and coworkers administered extracellular solutes into rat brain parenchyma and showed that they were transported faster than what could be explained by their respective diffusion coefficients and their drainage pathways, including peri-vascular spaces (Cserr 1974; Cserr and others 1977). The focus on peri-vascular channels was later extensively investigated in studies that injected horseradish peroxidase (HRP) into the subarachnoid space of cats (Rennels and others 1985). They documented rapid (4–10 min) peri-arterial influx of HRP brain-wide and also highlighted arterial pulsations as a convective driver of HRP transport in CSF (Rennels and others 1985). Recently, a team of investigators headed by Iliff and Nedergaard (Iliff and others 2012) repeated these experiments (Rennels and others 1985). Instead of HRP, they administered fluorescently tagged tracers of different molecular weight (MW) into the CSF of mice either via the lateral ventricles or the cisterna magna (CM); and tracked movement of the dyes into the cortex using optical imaging techniques. In agreement with Rennels and others, they observed slow absorption of the dyes across the ventricle lining into brain parenchyma and very rapid influx into the peri-arterial spaces when the dyes were delivered into the cisterna magna (Iliff and others 2012). They distinguished arteries and arterioles from veins and venules using a transgenic double reporter mouse, Tie2-GFP:NG2-DsRed mice, in which arteries and arterioles can be readily identified due to a dense labeling of DsRed. Therefore, it was determined that dyes were transported rapidly into the brain from the subarachnoid space along the peri-arterial but not peri-venous spaces (Iliff and others 2012). In addition, in vivo two-photon optical imaging allowed direct visualization of the rapid peri-arterial solute influx into the cortex and demonstrated that larger MW dyes were trapped because the gap between astrocytic end-feet constituted a physical barrier (gap width ~20–30 nm) to larger molecules whereas small MW dyes escaped from the peri-arterial space. Other experiments in which solutes, including Aβ1–40 (amyloid-β) were injected directly into brain parenchyma showed that clearance from the interstitial space occurred at least partly along large central veins (Iliff and others 2012). The peri-venous Aβ1–40 clearance routes from the brain observed by Iliff and colleagues (Iliff and others 2012) is in disagreement with previous studies (Carare and others 2008) and will be discussed later. However, one of the truly novel discoveries was the demonstration of the importance of AQP4 water channels polarized on glial end-feet, for parenchymal CSF transport and drainage of solutes including Aβ (Iliff and others 2012). For this purpose, they used AQP4−/− mice, which are global APQ4 gene knockouts signifying that AQP4 channels are absent everywhere; and showed that glymphatic clearance was significantly reduced compared with normal littermates. The importance of AQP4−/− channels in facilitating efficient glymphatic transport and clearance was recently supported by evidence of parenchymal retention of adeno-associated viruses in aging mice which coincided with loss of AQP4 channel polarization on glial cell end-feet (Murlidharan and others 2016). Collectively these experiments led to the conceptualization of the “glymphatic” pathway as a macroscopic system for waste clearance comprising a network of peri-arterial and peri-venous conduits coupled by AQP4 water channels on glial cells’ end-feet (Fig. 4).
Figure 4.
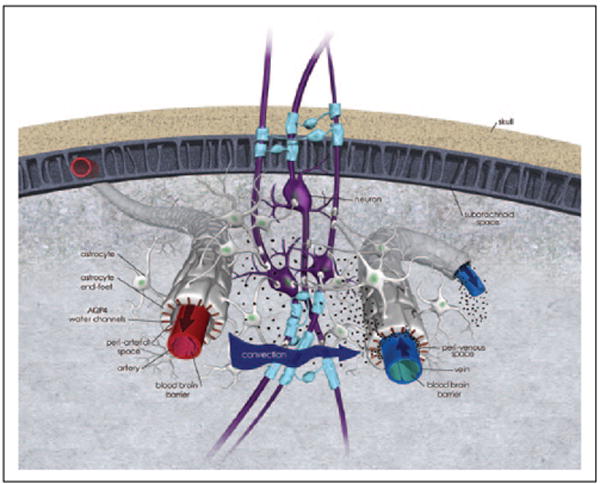
Glymphatic pathway function conceptualized. The principle of cerebrospinal fluid (CSF) transport and waste solute removal via the glymphatic pathway is illustrated on this 3-dimensional anatomical drawing. First, CSF enters along the peri-arterial space; the outer perimeter of the peri-arterial space is made up by astrocytic end-feet on which AQP4 water channels are strategically positioned to facilitate rapid water exchange across the capillary-endfeet complex. Second, CSF is transported from the peri-arterial space into brain parenchyma; a process driven by diffusion, convection (arrow indicate convective force) and local mixing whereby CSF exchanges with interstitial fluid (ISF). The CSF-ISF mixing facilitates waste removal. The CSF-ISF and waste leaves the brain parenchyma along peri-venous channels (large central veins). The CSF and solute transport system illustrated has been designed “glymphatic” pathway or (g)lymphatics due to its dependence on glial cells.
The transport of CSF and solutes through this system was also determined to be “bulk-flow” driven because it was dependent on arterial pulsations (Iliff and others 2013) and solute transport in the peri-vascular space was independent of MW. Several theoretical modeling papers are debating the forces that drive CSF transport through this system, and recently it was suggested that diffusion and local mixing is dominant driver ISF solute transport (Asgari and others 2016).
Sleep and Glymphatic Transport
Another novel finding was the demonstration that glymphatic transport and clearance of soluble Aβ was enhanced during slow wave sleep or general anesthesia due to a 60% increase of the cortical ISF space (Xie and others 2013). The volume changes in ISF space from wakefulness to sleep were documented by the “point” source paradigm developed by Nicholson and others, whereby tetramethylammonium (TMA+) is released iontophoretically into the brain and the concentration changes are measured dynamically with an ion-selective microelectrode positioned ~100 to 150 μm away (reviewed in Chodobski and others 2015). From the TMA+ diffusion curves the volume fraction α and the tortuosity factor, λ, of the local ISF can be calculated. In awake mice, the average cortical α was ~14% and during sleep it increased by 60% to ~23%; while the tortuosity remained unchanged (Xie and others 2013). The same enlargement of ISF space occurred during general anesthesia with ketamine/xylazine (Xie and others 2013). The enlargement of α during sleep or anesthesia increases solute transport (influx as well as clearance) via the glymphatic pathway (Xie and others 2013). Sleep position also influence glymphatic transport efficiency as demonstrated in a recent study using rodents anesthetized with ketamine/xylazine (Lee and others 2015). It was shown that the lateral recumbent position was the most efficient body posture for glymphatic transport including clearance of Aβ1–40 when compared with prone and supine positions (Lee and others 2015).
Because sleep and general anesthesia are characterized by changes in central arousal in part controlled via the locus coeruleus and noradrenergic (NA) tone (Aston-Jones and Bloom 1981) it was hypothesized that NA blockade during wakefulness would produce the same changes in ISF volume fraction α as normal deep wave sleep. Indeed, this was confirmed using a cocktail of NA antagonists applied directly onto cortex; which also evoked a sleep-like EEG (Xie and others 2013). A recent follow-up study by the same investigators demonstrated that the sleep-wake cycle was also accompanied by significant changes in extracellular K+ (~3.6 mM during sleep or isoflurane anesthesia, that rises to 4.1 mM during wakefulness) (Ding and others 2016).
Drainage Sites for Brain Waste
A major role of CSF is to facilitate removal of waste solutes in order to prevent accumulation of toxic levels of soluble Aβ (Carare and others 2008; Iliff and others 2012), tau (Iliff and others 2014), and lactate (Lundgaard and others 2016) from the brain. The exact drainage routes are still debated (Bakker and others 2016), emanating in part due to use of different experimental techniques. There is, however, general agreement, that a prerequisite for the brain waste removal process is that CSF must exchange with parenchymal ISF and subsequently exit to the systemic circulation. Anatomically, CSF drainage sites include the arachnoid villi (Pollay 2010), conduits along cranial and peripheral nerves (Fig. 5), peri-vascular routes (Carare and others 2008; Cserr 1988; Iliff and others 2012) and meningeal lymphatic vessels (Louveau and others 2015).
Figure 5.
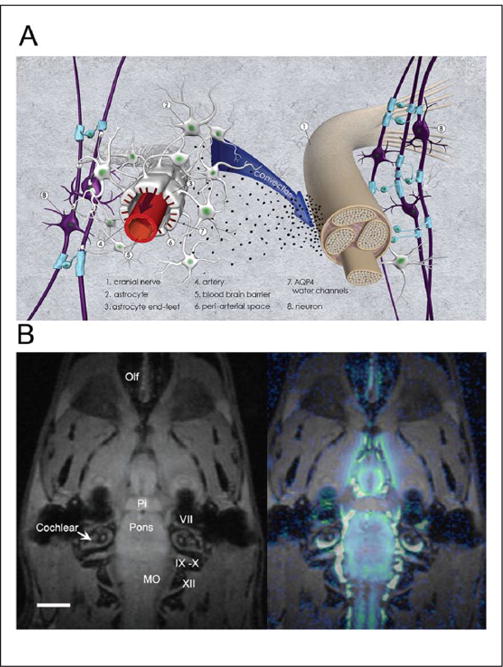
Cerebrospinal fluid (CSF) and waste drainage along cranial and peripheral nerves A: Anatomical drawing (3-dimensional perspective) of CSF and waste drainage along cranial or peripheral nerves. Contrast agents administered into CSF can exit along cranial and peripheral nerves. However, it is currently unknown how CSF and waste solutes exit from the subarachnoid space around the nerve roots. Studies from the olfactory nerves show that CSF and large contrast molecules are transported along the nerve (i.e., the space defined by the epineurium and perineurium) and then drain into veins and/or lymph vessels outside of the nerve. There is also some evidence in the literature (optic nerve studies) indicating that CSF and waste solutes can access the nerve itself. B: In vivo MRI study of rat after injection of paramagnetic contrast (Gd-DTPA) into the CSF via the cisterna magna demonstrating drainage along the cranial nerve. The MRI on the left shows the anatomical template from the rat at the level of the pituitary gland and cranial nerves exiting from pons and medulla oblongata (MO). Pi = pituitary gland; MO = medulla oblongata; Olf = olfactory nerves. Scale bar = 3 mm. The MRI on the right shows a color-coded map overlaid on the anatomical template, which represents distribution of Gd-DTPA 1 hour after administration into the cisterna magna. It is evident that CSF tagged with contrast has exited along the cranial nerves. The spatial resolution of the MRI (0.0013 mm3) is insufficient to document contrast within the nerve sheaths.
In humans, arachnoid villi are present along the cerebral venous sinuses and intercavernous sinuses (Pollay 2010) and can also be observed in the spine region along the spinal nerve roots. The arachnoid villi are constructed as one-way valves permitting CSF and solutes (particles up to 7.5 μm in size) to cross into the dural sinuses (Pollay 2010). In rats and rabbit, the arachnoid villi are absent at the superior sagittal sinus but present at the skull base (Pollay 2010). Importantly, the arachnoid villi occlude in senescence, which might affect CSF transport dynamics. It is general assumed that tracers administered into the subarachnoid space are cleared in part via reabsorption across the arachnoid villi although this cannot be directly visualized in real time due to insufficient spatial and temporal resolution.
Drainage of CSF and waste solutes along peri-vascular conduits have been documented by multiple investigators and a major argument has arisen in the literature regarding the anatomical substrate of the peri-vascular drainage pathways. In the original work, CSF solute transport via the glymphatic pathway was described as peri-arterial influx and peri-venous efflux; and clearance of Aβ1–40 administered into the parenchyma was observed to clear peri-venously (Iliff and others 2012). In contrast, work by Carare and coworkers showed that small MW solutes administered into striatum of mice cleared rapidly along the basement membrane of capillaries and arteries (Bakker and others 2016; Carare and others 2008) inferring that drainage can occur in reverse direction of the arterial pulse. These data are important for understanding pathological Aβ deposition as observed in cerebral amyloid angiopathy.
The spine can be a significant site of CSF tracer reabsorption as shown in Figure 6. A static whole-body positron emission tomography (PET) scan acquired from an anesthetized female baboon 4.5 hours after administration of 18F (4.5 mCi) into the lumbar intrathecal space is shown (Benveniste, Fowler, and Volkow, 2013, unpublished). The 18F radiotracer uptake is high in the entire spine while uptake in the brain appears minimal (Fig. 6). The 18F tracer is also evident in bone, including skull, jaw, and bladder indicating that slow transfer from CSF to blood and bone has occurred.
Figure 6.
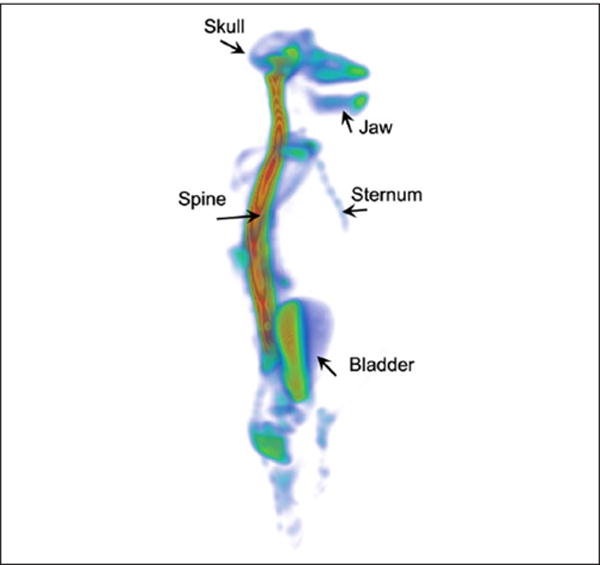
Cerebrospinal fluid (CSF) transport of 18F in nonhuman primate visualized by positron emission tomography (PET). Static PET whole body image of anesthetized female non-human primate (baboon/Papio) 4.5 hours after intrathecal administration of 18F via the L3/L4 lumbar level. The baboon was intubated but allowed to breathe spontaneously during the study. Normal physiological parameters where maintained throughout the study. The 18F distribution pattern is shown in the whole baboon body, and visualized as a volume rendered, color-coded map where red and blue colors represent high and low tracer uptake, respectively. The area associated with the spine has the highest uptake of 18F. The 18F tracer has reached the cranium but penetration into the brain parenchyma is not apparent. There is also 18F uptake in bony structures, including jaw, skull, and vertebrae. Color scale: Arbitrary units. Data courtesy: This study was performed by Helene Benveniste, Joanna Fowler, and Nora Volkow on May 3, 2013 at Brookhaven National Laboratory, Upton, New York; after approval by the Institutional Animal Use and Care Committee (unpublished data). After the conclusion of the study anesthesia was discontinued and the baboon recovered without problems and was returned to the non-human primate housing colony.
Clinical Relevance of the Glymphatic System
There are several clinical conditions that are likely to disrupt glymphatic brain function, including disease states that disturb intracranial pressure, conditions that damage the vascular system and/or disorders that interfere with sleep mechanisms. There are also disorders that are likely to arise because of glymphatic system dysfunction (i.e., neurodegenerative diseases and autoimmune diseases). Here we highlight some of these disorders.
Disorders of CSF Pressure
Increases as well as decreases in CSF pressure are likely to jeopardize normal CSF transport and therefore the function of the glymphatic system. Increases in intracranial CSF pressure can result from brain or spine occupying lesions (tumor, hemorrhage, infection). Decreased CSF pressure occurs with leaks in the dura secondary to trauma (surgical, head trauma, lumbar puncture). Increases in CSF pressure can also be sporadic in which case it is referred to as “idiopathic” intracranial hypertension (IIH) and/or spontaneous intracranial hypotension (SIH) (Hoffmann and Goadsby 2013) and commonly present as headaches and visual disturbances. A recent study examined the association with IIH and the AQP4 gene given its implication in brain water homeostasis; however, the 24 AQP4 gene SNPs revealed no relationship to IIH (Kerty and others 2013).
Hydrocephalus
Hydrocephalus are a group of conditions that result in increased dilation of the cerebral ventricles (i.e., ventriculomegaly) often linked with an increase in intracranial pressure that if untreated can result in brain herniation and death (Kahle and others 2016). Although the majority of hydrocephalus cases have been ascribed to obstruction of CSF flow, recent evidence now highlights the role of disrupted intracranial pulsations (Wagshul and others 2011) and/or increased CSF production (Karimy and others 2016) in the etiology of hydrocephalus. In adults, the most common form is idiopathic normal pressure hydrocephalus (iNPH); it occurs with higher frequency in the elderly (>70 years) and presents as impaired gait, cognition, and urinary urgency and incontinence (Williams and Malm 2016). Though there are currently no available techniques to assess glymphatic system function in the human brain such techniques will in the future allow for exploration of how hydrocephalus interferes with glymphatic transport and help determine, for example, if glymphatic transport dysfunction might be one mechanism underlying the high comorbidity reported between iNPH and Alzheimer’s disease (Williams and Malm 2016).
Traumatic Brain Injury
Traumatic brain injury (TBI) accounts for more than 30% of all injury related deaths mostly from mechanical impact or blast waves and results in disruption of the structure and function of the brain, including cerebral blood vessels (Jullienne and others 2016). Disruption of blood vessels by TBI, which can present as intracranial hemorrhages, cerebral blood flow deficits, BBB disruption and/or edema could interfere with glymphatic function. Indeed, preclinical studies showed that global APQ4−/− knockout mice, had accentuated neurodegeneration following head trauma (Iliff and others 2014); thus suggesting that disruption of glymphatic CSF transport with TBI might be a contributory mechanism to the increased risk of neurodegenerative conditions associated with TBI including Alzheimer’s disease (Gupta and Sen 2016).
Cerebrovascular Diseases
Hypertension and atherosclerosis are among the most frequent risk factors associated with AD (de la Torre 2012). Though the consequences of hypertension and cerebral atherosclerosis on glymphatic function still have to be evaluated there is evidence from preclinical studies that hypertension whether spontaneous (Schreiber and others 2014) or induced (Carnevale and Lembo 2011) is associated with a higher accumulation of amyloid in brain. Preclinical studies recently showed that middle-aged rats with type 2 diabetes exhibit a sharp decrease in glymphatic clearance, and that the reduction in glymphatic transport is negatively correlated with cognitive and behavioral performance (Jiang and others 2016). Furthermore, induction of type-2 diabetes in a senescence-accelerated mice triggers AD-like pathology and memory deficits (Mehla and others 2014).
Sleep Disruption
Chronic sleep impairments in healthy subjects have been associated with a higher risk for subsequent development of Alzheimer’s disease (Musiek and Holtzman 2016). Moreover, the relevance of sleep in brain Aβ accumulation has been documented in mouse models of Aβ deposition in whom sleep deprivation significantly accelerates brain amyloid accumulation whereas orexin antagonist drugs, which promote sleep (Kang and others 2009) or genetic deletion of orexin that increases sleep time prevent brain amyloid accumulation (Roh and others 2014). Since sleep serves multiple physiological functions various factors probably contribute to the increased risk for neurodegenerative conditions associated with sleep deprivation but disruption of glymphatic function is likely to be one of them.
Alzheimer’s Disease
Alzheimer’s disease (AD) is the most prevalent dementia and is associated with extracellular accumulation of Aβ and intracellular accumulation of hyperphosphorylated tau protein in the brain. Most AD cases are sporadic and the etiology for the cause of amyloid and tau accumulation is not understood. This is in contrast to familial AD for which mutations in the amyloid precursor protein or presenilin 1 and 2 underlie amyloid accumulation (Louveau and others 2016). Age, which is the main risk factor for sporadic AD, is associated with a reduction in CSF production that is likely to interfere with glymphatic clearance in brain. Aging is also associated with a loss of perivascular expression of AQP4, which was recently shown to correlate with the severity of Aβ accumulation in post-mortem brains of healthy controls and AD patients (Zeppenfeld and others 2017). Similarly, the genetic variants that increase the risk for sporadic AD such as apoliproprotein E4, Apo-J, or phosphatidylinositol-binding clathrin assembly protein (PICALM) have been implicated in mechanisms involved in the removal of Aβ from brain (Louveau and others 2016). Moreover, findings of impaired Aβ clearance in patients with sporadic AD further highlights the relevance of brain clearance mechanisms (Louveau and others 2016).
Autoimmune Diseases
The immune privilege in the brain was believed to reflect the presence of the BBB barrier, which restricts entry of immune cells into the brain and the absence of a lymphatics system in the brain parenchyma. However, the recent documentation of lymphatic vessels in the meninges (Louveau and others 2015) that allow trafficking of immune cells from the brain has raised the question of its potential role in autoimmune diseases such as multiple sclerosis (Louveau and others 2015).
Clinical Evidence of a Glymphatic Pathway
Currently, only a few clinical research studies support the existence of a sleep-dependent CSF-ISF metabolic waste removal system in the human brain, including (1) evidence of circadian variation in the CSF concentration of Aβ and tau (Roh and others 2012); (2) evidence that chronic sleep deprivation increases CSF Aβ (Ooms and others 2014); (3) evidence that among older adults, shorter sleep duration and poorer sleep quality are associated with greater Aβ burden (Spira and others 2013); and (4) neuroimaging studies find that the human brain is slightly larger in the morning compared with the evening hours (Nakamura and others 2015). A major step toward understanding the implication of AQP4 for AD pathology was recently shown in post-mortem human brain where significant loss of peri-vascular expression of AQP4 was demonstrated in AD human brain tissue (Fig. 7) in comparison with normal aging brain (Zeppenfeld and others 2017).
Figure 7.
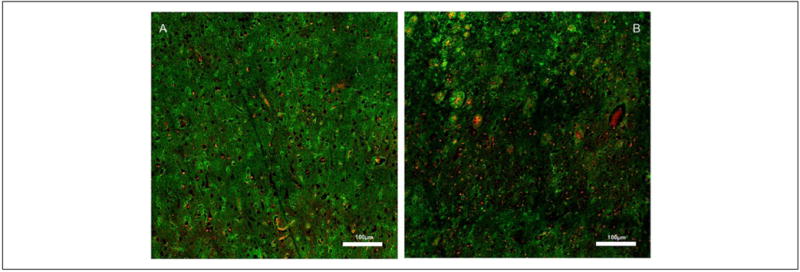
Wide field confocal images showing AQP4 immunoreactivity from postmortem cortical tissue of a young, cognitively intact subject (A) and of a subject with Alzheimer’s disease (B). In the young subject, there is uniform AQP4 expression (green) throughout; whereas in the Alzheimer’s disease (AD) subject the distribution is inhomogeneous within the cortex. The inhomogeneous AQP4 expression pattern in the AD post-mortem tissue is associated with amyloid beta plaques (red) and reactive astrocytes. Data courtesy: Dr. Jeffrey Iliff, PhD, Oregon Health & Science University. For more details, see (Zeppenfeld and others 2017).
As of now there are no diagnostic tests to measure the function of the glymphatic system in the human brain.
Though there are radiological scintigraphy procedures that can measure the transport of radiotracers in the CSF of humans these are used to diagnose CSF leaks, normal pressure hydrocephalus, or intraventricular shunt malfunction. Analyses of CSF through lumbar punctures is being proposed as a biomarker for monitoring reduced clearance of beta amyloid in patients with early AD that is interpreted to reflect reduce clearance rates (Zhou and others 2015).
Conclusions
The concept of the glymphatic pathway as a waste removal system in the central nervous system has been strengthened considerably by recent preclinical research. Nevertheless, considerable gaps remain in our knowledge with regard to its existence in the human brain. The key to demonstrate the system’s existence and functionality in the live human brain will be to confirm that a similar peri-vascular CSF transport and CSF-ISF exchange system dependent on peri-vascular AQP4 channels removes waste solutes; and further that transport efficiency is dependent on sleep-wake states.
Acknowledgments
We are grateful to Professor Anker Jon Hansen, MD, PhD, at the Center for Basic and Translational Neuroscience, University of Copenhagen, Denmark, for essential comments and valuable insights. We also acknowledge 3D animation artist Elena Nikanorova for the medical illustrations of the glymphatic pathway and other medical figures in this review.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The research was generously supported by Grants: NIA 5R01AG048769 and NIA 1RF1AG053991-01.
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbott NJ, Bundgaard M, Cserr HF. Tightness of the blood-brain barrier and evidence for brain interstitial fluid flow in the cuttlefish, Sepia officinalis. J Physiol. 1985;368:213–26. doi: 10.1113/jphysiol.1985.sp015854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Algin O, Turkbey B. Intrathecal gadolinium-enhanced MR cisternography: a comprehensive review. AJNR Am J Neuroradiol. 2013;34:14–22. doi: 10.3174/ajnr.A2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asgari M, de Zelicourt D, Kurtcuoglu V. Glymphatic solute transport does not require bulk flow. Sci Rep. 2016;6:38635. doi: 10.1038/srep38635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci. 1981;1:876–86. doi: 10.1523/JNEUROSCI.01-08-00876.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker EN, Bacskai BJ, Arbel-Ornath M, Aldea R, Bedussi B, Morris AW, et al. Lymphatic clearance of the brain: perivascular, paravascular and significance for neurodegenerative diseases. Cell Mol Neurobiol. 2016;36:181–94. doi: 10.1007/s10571-015-0273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K, Benveniste H. Quinckes’ pioneering 19th centuries CSF studies may inform 21th centuries research. Neurol Psychiatry Brain Res. 2015;21:79–81. doi: 10.1016/j.npbr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechter K, Hof PR, Benveniste H. On the flow dynamics of cerebrospinal fluid. Neurol Psychiatry Brain Res 2015. 2015;21:96–103. doi: 10.1016/j.npbr.2015.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benveniste H, Hof PR, Nedergaard M, Bechter K. Modern cerebrospinal fluid flow research and Heinrich Quincke’s seminal 1872 article on the distribution of cinnabar in freely moving animals. J Comp Neurol. 2015;523:2017–8. doi: 10.1002/cne.23850. [DOI] [PubMed] [Google Scholar]
- Bonnet MH, Arand DL. Heart rate variability: sleep stage, time of night, and arousal influences. Electroencephalogr Clin Neurophysiol. 1997;102:390–6. doi: 10.1016/s0921-884x(96)96070-1. [DOI] [PubMed] [Google Scholar]
- Brinker T, Stopa E, Morrison J, Klinge P. A new look at cerebrospinal fluid circulation. Fluids Barriers CNS. 2014;11:10. doi: 10.1186/2045-8118-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulat M, Klarica M. Recent insights into a new hydrodynamics of the cerebrospinal fluid. Brain Res Rev. 2011;65:99–112. doi: 10.1016/j.brainresrev.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Carare RO, Bernardes-Silva M, Newman TA, Page AM, Nicoll JA, Perry VH, et al. Solutes, but not cells, drain from the brain parenchyma along basement membranes of capillaries and arteries: significance for cerebral amyloid angiopathy and neuroimmunology. Neuropathol Appl Neurobiol. 2008;34:131–44. doi: 10.1111/j.1365-2990.2007.00926.x. [DOI] [PubMed] [Google Scholar]
- Carnevale D, Lembo G. ‘Alzheimer-like’ pathology in a murine model of arterial hypertension. Biochem Soc Trans. 2011;39:939–44. doi: 10.1042/BST0390939. [DOI] [PubMed] [Google Scholar]
- Chiu C, Miller MC, Caralopoulos IN, Worden MS, Brinker T, Gordon ZN, et al. Temporal course of cerebrospinal fluid dynamics and amyloid accumulation in the aging rat brain from three to thirty months. Fluids Barriers CNS. 2012;9:3. doi: 10.1186/2045-8118-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chodobski A, Ghersi-Egea JF, Nicholson C, Nagaraja TN, Szmydynger-Chodobska J. The quest for a better insight into physiology of fluids and barriers of the brain: the exemplary career of Joseph D. Fenstermacher. Fluids Barriers CNS. 2015;12:1. doi: 10.1186/2045-8118-12-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone C. The blood-brain barrier as a tight epithelium: where is information lacking? Ann N Y Acad Sci. 1986;481:174–85. doi: 10.1111/j.1749-6632.1986.tb27149.x. [DOI] [PubMed] [Google Scholar]
- Cserr HF. Relationship between cerebrospinal fluid and interstitial fluid of brain. Fed Proc. 1974;33:2075–8. [PubMed] [Google Scholar]
- Cserr HF. Role of secretion and bulk flow of brain interstitial fluid in brain volume regulation. Ann N Y Acad Sci. 1988;529:9–20. doi: 10.1111/j.1749-6632.1988.tb51415.x. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, Milhorat TH. Flow of cerebral interstitial fluid as indicated by the removal of extracellular markers from rat caudate nucleus. Exp Eye Res. 1977;25(Suppl):461–73. doi: 10.1016/s0014-4835(77)80041-9. [DOI] [PubMed] [Google Scholar]
- Cserr HF, Cooper DN, Suri PK, Patlak CS. Efflux of radiolabeled polyethylene glycols and albumin from rat brain. Am J Physiol. 1981;240:F319–28. doi: 10.1152/ajprenal.1981.240.4.F319. [DOI] [PubMed] [Google Scholar]
- Cutler RW, Page L, Galicich J, Watters GV. Formation and absorption of cerebrospinal fluid in man. 1968;91:707–20. doi: 10.1093/brain/91.4.707. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Brown PD, Praetorius J. Epithelial pathways in choroid plexus electrolyte transport. Physiology (Bethesda) 2010;25:239–49. doi: 10.1152/physiol.00011.2010. [DOI] [PubMed] [Google Scholar]
- Damkier HH, Brown PD, Praetorius J. Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev. 2013;93:1847–92. doi: 10.1152/physrev.00004.2013. [DOI] [PubMed] [Google Scholar]
- de la Torre JC. Cerebral hemodynamics and vascular risk factors: setting the stage for Alzheimer’s disease. J Alzheimers Dis. 2012;32:553–67. doi: 10.3233/JAD-2012-120793. [DOI] [PubMed] [Google Scholar]
- Ding F, O’Donnell J, Xu Q, Kang N, Goldman N, Nedergaard M. Changes in the composition of brain interstitial ions control the sleep-wake cycle. Science. 2016;352:550–5. doi: 10.1126/science.aad4821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreha-Kulaczewski S, Joseph AA, Merboldt KD, Ludwig HC, Gartner J, Frahm J. Inspiration is the major regulator of human CSF flow. J Neurosci. 2015;35:2485–91. doi: 10.1523/JNEUROSCI.3246-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edsbagge M, Tisell M, Jacobsson L, Wikkelso C. Spinal CSF absorption in healthy individuals. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1450–5. doi: 10.1152/ajpregu.00215.2004. [DOI] [PubMed] [Google Scholar]
- Friese S, Hamhaber U, Erb M, Kueker W, Klose U. The influence of pulse and respiration on spinal cerebrospinal fluid pulsation. Invest Radiol. 2004;39:120–30. doi: 10.1097/01.rli.0000112089.66448.bd. [DOI] [PubMed] [Google Scholar]
- Ghersi-Egea JF, Gorevic PD, Ghiso J, Frangione B, Patlak CS, Fenstermacher JD. Fate of cerebrospinal fluid-borne amyloid beta-peptide: rapid clearance into blood and appreciable accumulation by cerebral arteries. J Neurochem. 1996;67:880–3. doi: 10.1046/j.1471-4159.1996.67020880.x. [DOI] [PubMed] [Google Scholar]
- Gupta R, Sen N. Traumatic brain injury: a risk factor for neurodegenerative diseases. Rev Neurosci. 2016;27:93–100. doi: 10.1515/revneuro-2015-0017. [DOI] [PubMed] [Google Scholar]
- Hoffmann J, Goadsby PJ. Update on intracranial hypertension and hypotension. Curr Opin Neurol. 2013;26:240–7. doi: 10.1097/WCO.0b013e328360eccc. [DOI] [PubMed] [Google Scholar]
- Ikomi F, Kawai Y, Ohhashi T. Recent advance in lymph dynamic analysis in lymphatics and lymph nodes. Ann Vasc Dis. 2012;5:258–68. doi: 10.3400/avd.ra.12.00046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Chen MJ, Plog BA, Zeppenfeld DM, Soltero M, Yang L, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Liao Y, Plogg BA, Peng W, Gundersen GA, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, Wang M, Zeppenfeld DM, Venkataraman A, Plog BA, Liao Y, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190–9. doi: 10.1523/JNEUROSCI.1592-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Q, Zhang L, Ding G, Davoodi-Bojd E, Li Q, Li L, et al. Impairment of the glymphatic system after diabetes. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16654702. Epub June 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson C, Stopa E, McMillan P, Roth D, Funk J, Krinke G. The distributional nexus of choroid plexus to cerebrospinal fluid, ependyma and brain: toxicologic/pathologic phenomena, periventricular destabilization, and lesion spread. Toxicol Pathol. 2011;39:186–212. doi: 10.1177/0192623310394214. [DOI] [PubMed] [Google Scholar]
- Johanson CE, Duncan JA, 3rd, Klinge PM, Brinker T, Stopa EG, Silverberg GD. Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jullienne A, Obenaus A, Ichkova A, Savona-Baron C, Pearce WJ, Badaut J. Chronic cerebrovascular dysfunction after traumatic brain injury. J Neurosci Res. 2016;94:609–22. doi: 10.1002/jnr.23732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahle KT, Kulkarni AV, Limbrick DD, Jr, Warf BC. Hydrocephalus in children. Lancet. 2016;387:788–99. doi: 10.1016/S0140-6736(15)60694-8. [DOI] [PubMed] [Google Scholar]
- Kang JE, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-beta dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326:1005–7. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimy JK, Duran D, Hu JK, Gavankar C, Gaillard JR, Bayri Y, et al. Cerebrospinal fluid hypersecretion in pediatric hydrocephalus. Neurosurg Focus. 2016;41:E10. doi: 10.3171/2016.8.FOCUS16278. [DOI] [PubMed] [Google Scholar]
- Kerty E, Heuser K, Indahl UG, Berg PR, Nakken S, Lien S, et al. Is the brain water channel aquaporin-4 a pathogenetic factor in idiopathic intracranial hypertension? Results from a combined clinical and genetic study in a Norwegian cohort. Acta Ophthalmol. 2013;91:88–91. doi: 10.1111/j.1755-3768.2011.02231.x. [DOI] [PubMed] [Google Scholar]
- Kida S, Weller RO. Morphological basis for fluid transport through and around ependymal, arachnoidal and glial cells. In: Raimondi A, editor. Principles of pediatric neuroscurgery, IV: intracranial cyste lesions. Berlin, Germany: Springer-Verlag; 1993. pp. 37–52. [Google Scholar]
- Kimelberg HK. Water homeostasis in the brain: basic concepts. Neuroscience 2004. 2004;129:851–60. doi: 10.1016/j.neuroscience.2004.07.033. [DOI] [PubMed] [Google Scholar]
- Klarica M, Rados M, Draganic P, Erceg G, Oreskovic D, Marakovic J, et al. Effect of head position on cerebrospinal fluid pressure in cats: comparison with artificial model. Croat Med J 2006. 2006;47:233–8. [PMC free article] [PubMed] [Google Scholar]
- Klarica M, Rados M, Erceg G, Petosic A, Jurjevic I, Oreskovic D. The influence of body position on cerebrospinal fluid pressure gradient and movement in cats with normal and impaired craniospinal communication. PLoS One. 2014;9:e95229. doi: 10.1371/journal.pone.0095229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Xie L, Yu M, Kang H, Feng T, Deane R, et al. The effect of body posture on brain glymphatic transport. J Neurosci. 2015;35:11034–44. doi: 10.1523/JNEUROSCI.1625-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levick JR, Michel CC. Microvascular fluid exchange and the revised Starling principle. Cardiovasc Res. 2010;87:198–210. doi: 10.1093/cvr/cvq062. [DOI] [PubMed] [Google Scholar]
- Lindvall M, Owman C. Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J Cereb Blood Flow Metab. 1981;1:245–66. doi: 10.1038/jcbfm.1981.30. [DOI] [PubMed] [Google Scholar]
- Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neurolympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron. 2016;91:957–73. doi: 10.1016/j.neuron.2016.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louveau A, Smirnov I, Keyes TJ, Eccles JD, Rouhani SJ, Peske JD, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337–41. doi: 10.1038/nature14432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgaard I, Lu ML, Yang E, Peng W, Mestre H, Hitomi E, et al. Glymphatic clearance controls state-dependent changes in brain lactate concentration. J Cereb Blood Flow Metab. 2016 doi: 10.1177/0271678X16661202. Epub Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehla J, Chauhan BC, Chauhan NB. Experimental induction of type 2 diabetes in aging-accelerated mice triggered Alzheimer-like pathology and memory deficits. J Alzheimers Dis. 2014;39:145–62. doi: 10.3233/JAD-131238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milhorat TH, Hammock MK, Fenstermacher JD, Levin VA. Cerebrospinal fluid production by the choroid plexus and brain. Science. 1971;173:330–2. doi: 10.1126/science.173.3994.330. [DOI] [PubMed] [Google Scholar]
- Morse DE, Low FN. The fine structure of the pia mater of the rat. Am J Anat. 1972;133:349–67. doi: 10.1002/aja.1001330309. [DOI] [PubMed] [Google Scholar]
- Murlidharan G, Crowther A, Reardon RA, Song J, Asokan A. Glymphatic fluid transport controls paravascular clearance of AAV vectors from the brain. JCI Insight. 2016;1:e88034. doi: 10.1172/jci.insight.88034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–8. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagelhus EA, Ottersen OP. Physiological roles of aquaporin-4 in brain. Physiol Rev. 2013;93:1543–62. doi: 10.1152/physrev.00011.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Brown RA, Narayanan S, Collins DL, Arnold DL. Alzheimer’s disease neuroimaging I. Diurnal fluctuations in brain volume: statistical analyses of MRI from large populations. Neuroimage. 2015;118:126–32. doi: 10.1016/j.neuroimage.2015.05.077. [DOI] [PubMed] [Google Scholar]
- Nilsson C, Stahlberg F, Thomsen C, Henriksen O, Herning M, Owman C. Circadian variation in human cerebrospinal fluid production measured by magnetic resonance imaging. Am J Physiol. 1992;262(1 Pt 2):R20–4. doi: 10.1152/ajpregu.1992.262.1.R20. [DOI] [PubMed] [Google Scholar]
- Ooms S, Overeem S, Besse K, Rikkert MO, Verbeek M, Claassen JA. Effect of 1 night of total sleep deprivation on cerebrospinal fluid beta-amyloid 42 in healthy middle-aged men: a randomized clinical trial. JAMA Neurol. 2014;71:971–7. doi: 10.1001/jamaneurol.2014.1173. [DOI] [PubMed] [Google Scholar]
- Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoport SI. A mathematical model for vasogenic brain edema. J Theor Biol. 1978;74:439–67. doi: 10.1016/0022-5193(78)90224-2. [DOI] [PubMed] [Google Scholar]
- Rennels ML, Gregory TF, Blaumanis OR, Fujimoto K, Grady PA. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4 doi: 10.1126/scitranslmed.3004291. 150ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh JH, Jiang H, Finn MB, Stewart FR, Mahan TE, Cirrito JR, et al. Potential role of orexin and sleep modulation in the pathogenesis of Alzheimer’s disease. J Exp Med. 2014;211:2487–96. doi: 10.1084/jem.20141788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin RC, Henderson ES, Ommaya AK, Walker MD, Rall DP. The production of cerebrospinal fluid in man and its modification by acetazolamide. J Neurosurg. 1966;25:430–6. doi: 10.3171/jns.1966.25.4.0430. [DOI] [PubMed] [Google Scholar]
- Sage MR. Kinetics of water-soluble contrast media in the central nervous system. AJR Am J Roentgenol. 1983;141:815–24. doi: 10.2214/ajr.141.4.815. [DOI] [PubMed] [Google Scholar]
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011;128:309–16. doi: 10.1016/j.anorl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Schreiber S, Drukarch B, Garz C, Niklass S, Stanaszek L, Kropf S, et al. Interplay between age, cerebral small vessel disease, parenchymal amyloid-beta, and tau pathology: longitudinal studies in hypertensive stroke-prone rats. J Alzheimers Dis. 2014;42(Suppl 3):S205–15. doi: 10.3233/JAD-132618. [DOI] [PubMed] [Google Scholar]
- Schroth G, Klose U. Cerebrospinal fluid flow. I. Physiology of cardiac-related pulsation. Neuroradiology. 1992a;35:1–9. doi: 10.1007/BF00588270. [DOI] [PubMed] [Google Scholar]
- Schroth G, Klose U. Cerebrospinal fluid flow. II. Physiology of respiration-related pulsations. Neuroradiology. 1992b;35:10–5. doi: 10.1007/BF00588271. [DOI] [PubMed] [Google Scholar]
- Shafer SL, Shafer A. Spinal pharmacokinetics. In: Yaksh TL, editor. Spinal drug delivery. New York: Elsevier; 1999. pp. 271–95. [Google Scholar]
- Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurol. 2013;70:1537–43. doi: 10.1001/jamaneurol.2013.4258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagshul ME, Eide PK, Madsen JR. The pulsating brain: a review of experimental and clinical studies of intracranial pulsatility. Fluids Barriers CNS. 2011;8:5. doi: 10.1186/2045-8118-8-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu Rev Biomed Eng. 2007;9:121–67. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Williams MA, Malm J. Diagnosis and treatment of idiopathic normal pressure hydrocephalus. Continuum (Minneap Minn) 2016;22(2):579–99. doi: 10.1212/CON.0000000000000305. Dementia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeppenfeld DM, Simon M, Haswell JD, D’Abreo D, Murchison C, Quinn JF, et al. Association of perivascular localization of aquaporin-4 with cognition and Alzheimer disease in aging brains. JAMA Neurol. 2017;74:91–9. doi: 10.1001/jamaneurol.2016.4370. [DOI] [PubMed] [Google Scholar]
- Zhou B, Wen M, Yu WF, Zhang CL, Jiao L. The diagnostic and differential diagnosis utility of cerebrospinal fluid alpha-synuclein levels in Parkinson’s disease: a meta-analysis. Parkinsons Dis. 2015;2015:567386. doi: 10.1155/2015/567386. [DOI] [PMC free article] [PubMed] [Google Scholar]


