Abstract
Although emerging research suggests that pain-related anxiety may play a role in the maintenance of tobacco dependence, no previous work has examined pain-related anxiety as a predictor of smoking cessation outcomes. The goal of the current study was to test the hypothesis that pain-related anxiety would predict early lapse and relapse to cigarette smoking. These data were collected in the context of a primary study examining the role of emotional vulnerabilities in smoking cessation. The current analyses were conducted among a sample of 55 daily cigarette smokers who attempted to quit smoking without using any psychosocial or pharmacological cessation aids. Pain-related anxiety was assessed at baseline using the PASS-20. Early lapse and relapse were assessed using timeline follow-back procedures. Cox regression analyses indicated that pain-related anxiety was a significant predictor of both early smoking lapse and relapse, such that for every one-point increase on the PASS-20, the risk of early lapse increased by 3.7% and the risk of early relapse increased by 3.6%. These effects were evident above and beyond the variance accounted for by tobacco dependence, past four-week pain severity, anxiety sensitivity, and the presence of current Axis I psychopathology. Kaplan-Meier survival analyses further revealed that among early lapsers, greater pain-related anxiety predicted a more rapid trajectory to lapse. Pain-related anxiety was also shown to be a significant predictor of early lapse when the sample was limited to smokers who endorsed past four-week pain. These findings lend empirical support to the notion that pain-related anxiety may contribute to the maintenance of tobacco dependence among smokers who experience varying levels of pain intensity.
Keywords: smoking, smoking cessation, pain, pain-related anxiety
Cigarette smoking remains the leading cause of preventable death worldwide (World Health Organization, 2008). Despite known health risks and an annual economic burden in excess of $300 billion in the United States, about 42 million Americans continue to smoke tobacco cigarettes (US Department of Health & Human Services, 2014). Although about half of all smokers attempt to quit each year, nearly 70% do not utilize recommended pharmacological or behavioral cessation aids (e.g., nicotine replacement, cognitive-behavioral counseling; CDC, 2011), and the vast majority (72–85%) who engage in an unaided quit attempt relapse within the first month (Hughes, Keely, & Naud, 2004). Whereas substantial progress has been made in the identification of reliable predictors of smoking cessation (e.g., cigarette dependence, withdrawal symptoms, self-efficacy for quitting; Ditre, Zale, & Brandon, 2015), additional work is needed to identify factors that can be addressed in the context of tailored interventions.
There is increasing empirical and clinical interest in the role of pain and related factors in the maintenance of tobacco dependence (e.g., Ditre, Heckman, Butts, & Brandon, 2010; Ditre, Langdon, Kosiba, Zale, & Zvolensky, 2015). Pain has been defined as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage or defined in terms of such damage” (IASP, 1994), and pain complaints account for up to 80% of all annual U.S. physician visits (Mayo Clinic, 2001). Although about 17% of U.S. adults smoke cigarettes (Jamal et al., 2015), an estimated 30–42% of persons with pain concurrently smoke tobacco cigarettes (Zvolensky, McMillan, Gonzalez, & Asmundson, 2009), and the prevalence of smoking among clinical pain patients may be as high as 68% (Michna et al., 2004).
An evolving reciprocal model suggests that pain and smoking interact in the manner of a positive feedback loop, resulting in greater pain and the maintenance of tobacco dependence (Ditre, Brandon, Zale, & Meagher, 2011; Zale, Maisto, & Ditre, 2016). Consistent with this perspective, regular tobacco smoking has been identified as a unique risk factor in the onset and progression of several chronically painful conditions, including low back pain (OR = 1.79; Shiri, Karppinen, Leino-Arjas, Solovieva, & Viikari-Juntura, 2010) and rheumatoid arthritis (OR = 1.87 and 1.31, for men and women, respectively; Sugiyama et al., 2010), situational pain has been shown to motivate smoking urge and behavior (e.g., Ditre & Brandon, 2008), and pain patients have reliably endorsed smoking cigarettes to cope with pain (e.g., Patterson et al., 2012).
To better inform the development of tailored interventions, researchers have recently turned their attention to the identification of anxiety-relevant transdiagnostic factors in the etiology, progression, and maintenance of both pain and smoking (Zale et al., 2016). Indeed, various facets of anxiety (e.g., anxiety sensitivity) have been associated with greater pain and the maintenance of tobacco dependence (e.g., McCracken & Keogh, 2009). One factor that is of increasing clinical and empirical interest is the cognitive-affective construct termed pain-related anxiety (e.g., Ditre, Langdon, et al., 2015). Pain-related anxiety reflects the tendency to respond to pain or pain-related events with anxiety or fear (McCracken, Zayfert, & Gross, 1992), and may be important to understanding the maintenance and exacerbation of tobacco dependence due to its specificity to pain-related phenomena and incorporation of pain-relevant behavioral responses (i.e., responding with escape or avoidance). Pain-related anxiety has been identified as a risk factor in the transition from acute to chronic pain (e.g., Boersma & Linton, 2006), and greater pain-related anxiety has been related to greater pain intensity, maladaptive approaches to pain coping, and increased somatic reactivity in anticipation of pain-eliciting physical activity (McCracken, Gross, Sorg, & Edmands, 1993). A growing body of evidence further suggests that pain-related anxiety can be experienced even in the absence of co-occurring clinical pain (e.g., Abrams, Carleton, & Asmundson, 2007).
Pain-related anxiety has also been implicated in the maintenance of substance use in general (e.g., Hogan, Gonzalez, Howell, Bonn-Miller, & Zvolensky, 2010), and tobacco smoking in particular (e.g., Ditre, Langdon, et al., 2015). Among individuals with chronic pain, higher levels of pain-related anxiety have been associated with current smoking (vs. non-smoking; Hooten et al., 2009; Hsu, Harden, & Houle, 2002) and the use of tobacco to cope with pain (Patterson et al., 2012). Pain-related anxiety has also been positively associated with both primary (i.e., central features of tobacco dependence, such as compulsion to smoke) and secondary (i.e., situational motivators of smoking, such as mood regulation) smoking dependence motives among smokers with chronic pain (Ditre, Zale, Kosiba, & Zvolensky, 2013), and those recruited from the local community (Ditre, Langdon, et al., 2015). Among community samples, pain-related anxiety has been positively associated with self-reported barriers to smoking cessation and expectations that smoking can alleviate negative mood (Ditre, Langdon, et al., 2015; Gonzalez, Hogan, McLeish, & Zvolensky, 2010). Collectively, these data suggest that pain-related anxiety should be considered in the maintenance of tobacco dependence among smokers with and without co-occurring pain.
The primary goal of the current study was to conduct a series of secondary analyses for the purpose of testing whether pain-related anxiety would predict early cessation outcomes among a sample of daily tobacco smokers who participated in an unaided smoking cessation attempt (i.e., they were asked to quit smoking without using any psychosocial or pharmacological cessation aids). Given evidence that pain-related anxiety may be particularly important among smokers with co-occurring pain (e.g., Ditre et al., 2013; Hooten et al., 2009), a secondary goal of this study was to examine the relationship between pain-related anxiety and cessation outcomes among a subsample of participants who endorsed past four-week pain. Specifically, we hypothesized that higher levels of pain-related anxiety would be associated with (a) an increased risk of early lapse (i.e., a lapse within 14 days post-quit) and early relapse (i.e., a relapse beginning within 28 days post-quit) to smoking, and (b) a more rapid trajectory to lapse among early-lapsers and to relapse among early-relapsers. Early abstinence outcomes were of particular interest given that participants engaged in an unaided quit attempt, and that duration of abstinence prior to the first lapse/relapse (e.g., < 1 month) is highly predictive of longer-term cessation outcomes (e.g., 6–12 months; Brown et al., 2001).
Method
Participants
Participants were recruited from the local community at two sites (Houston, TX and Burlington, VT) to participate in a primary study of emotional vulnerabilities in smoking cessation that consisted of a 90-day unaided quit attempt (Langdon, Farris, Øverup, & Zvolensky, 2015). Participants were screened for the following inclusion criteria: between 18 and 65 years of age, smoke at least 8 cigarettes per day for at least one year (verified via expired carbon monoxide [CO] breath analysis; ≥ 8 ppm), have not decreased the number of daily cigarettes smoked by more than half in the past 6 months, and willing to engage in an unaided quit attempt. Participants were also screened for the following exclusion criteria: current use of nicotine replacement, other tobacco products, or pharmacological smoking cessation aids (e.g., varenicline), current substance dependence (excluding nicotine dependence), current or past history of psychotic spectrum symptoms or disorders, and current use of psychotropic medication.
A total of 122 participants attended a baseline session, and 83 were deemed eligible to participate in the self-guided quit attempt (those who were ineligible endorsed current use of psychotropic medications, current substance dependence, expired CO < 8ppm, and/or CPD reduction > 50% in the last 6 months; n = 39). Three participants were excluded because they did not complete the measure of pain-related anxiety. Of the remaining 80 participants, 55 (69%) attended their quit day appointment and were included in the current analyses. It is important to note that modest retention rates are expected in studies that ask daily tobacco smokers to engage in an unaided, non-incentivized quit attempt. For example, in a previous study that required an unaided quit attempt, approximately 23% of the sample withdrew prior to the quit day (Zvolensky et al., 2008). In the current sample, those who attended their quit day appointment smoked fewer cigarettes per day (M = 15.564, SD = 0.809) than those who did not attend their quit day appointment (M = 18.600, SD = 1.200), F (1, 78) = 4.399, p = .039. No differences in any other baseline sociodemographic, smoking, or pain variable were observed as a function of quit day attendance (all ps > .05).
Measures
Pain-related anxiety
Pain-related anxiety was assessed using the Pain Anxiety Symptom Scale-20 item (PASS-20; McCracken & Dhingra, 2002). The PASS-20 (range: 0–100) uses a 6-point Likert scale ranging from never (0) to always (5) to assess how often participants engage in various thoughts (e.g., “When I feel pain, I am afraid that something terrible will happen”) and behaviors (e.g., “I avoid important activities when I hurt”). The PASS-20 demonstrated excellent internal consistency in the current sample (α = .91).
Past Four-Week Pain Severity
The Short Form Health Survey-12 (SFHS; Ware, Kosinski, & Keller, 1996) is a widely used 12-item self-report measure of mental and physical health. Consistent with previous research (e.g., Ditre, Langdon, et al., 2015), a single item was used to assess the presence of past four-week bodily pain at the baseline session (i.e., “How much bodily pain have you had during the past four weeks?”; Ware et al., 1996). Response options consisted of none, very mild, mild, moderate, and severe. Given demonstrated associations between the presence of pain and numerous smoking-related factors and outcomes (e.g., Ditre et al., 2011; Zvolensky, McMillan, et al., 2009), past four-week bodily pain severity was selected as an a priori covariate for all statistical analyses.
Tobacco use and dependence
Historical and current tobacco use (e.g., number of cigarettes smoked per day) were assessed via self-report. Tobacco dependence was assessed using the Heaviness of Smoking Index (HSI; Heatherton, Kozlowski, Frecker, Rickert, & Robinson, 1989), which is comprised of two items (i.e., “How soon after you wake up do you smoke your first cigarette?” and “How many cigarettes per day do you smoke?”; Heatherton et al., 1989). HSI scores (range: 0–6) were identified as an a priori covariate, given that they have been shown to predict smoking abstinence outcomes (Courvoisier & Etter, 2010), especially during the early stages (i.e., 1 week to 1 month) of a cessation attempt (Yong et al., 2014).
Current Axis I Psychopathology
All participants were administered the Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Non-Patient Edition (SCID-IV-N/P) by a trained clinician to assess whether participants met criteria for past-month Axis I psychopathology. Interviews were audio-taped, the reliability of a random selection of 10% of interviews was checked for accuracy, and there were no diagnostic disagreements between the SCID interviewer and outsider raters. Consistent with previous work, a single dichotomous variable was created to reflect the presence (1) or absence (0) of current Axis I psychopathology (e.g., Johnson, Farris, Schmidt, Smits, & Zvolensky, 2013). Given established associations between the presence of Axis I psychopathology and greater difficulty quitting (e.g., Lasser et al., 2000), this variable was identified as an a priori covariate.
Anxiety Sensitivity
The Anxiety Sensitivity Index-III is a well-established, 18-item measure of the fear that arousal-related sensations may result in adverse consequences such as death, insanity, or social rejection (ASI-3; Taylor et al., 2007). The ASI-3 (range: 0–72) uses a 5-point Likert scale ranging from very little (0) to very much (5) to assess how much participants agree with various statements (e.g., “It scares me when my heart beats rapidly”). Given that anxiety sensitivity has been positively associated with affect-regulatory smoking motives (Farris, Leventhal, Schmidt, & Zvolensky, 2015), more severe nicotine withdrawal (e.g., Johnson, Stewart, Rosenfield, Steeves, & Zvolensky, 2012), and increased risk for lapse and relapse (Assayag, Bernstein, Zvolensky, Steeves, & Stewart, 2012; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009), ASI-3 scores were selected as an a priori covariate. The ASI-3 demonstrated excellent internal consistency in the current sample (α = .93).
Time to Lapse/Relapse
Time to lapse and relapse were assessed via timeline follow-back procedures. The timeline follow-back is a widely used interview-style assessment that incorporates calendar-guided recall and anchoring of dates to significant events (Sobell & Sobell, 1992). Participants were asked to recall and record the total number of cigarettes smoked each day prior to the follow-up session. Timeline follow-back procedures for cigarette smoking have demonstrated high reliability and validity (as measured by correlations with daily monitored smoking, reports from significant others, and saliva cotinine levels; Brown et al., 1998). Consistent with previous research, a lapse (i.e., any instance of smoking after a quit attempt) during the first 14 days post-quit was classified as an ‘early lapse’ (e.g., Brown et al., 2008; Holt, Litt, & Cooney, 2012; Zvolensky, Stewart, et al., 2009), and a relapse (i.e., 7 consecutive days of smoking following a quit attempt) beginning during the first 28 days post-quit was classified as an ‘early relapse’ (e.g., al’Absi, Hatsukami, & Davis, 2005; Nakajima & al’Absi, 2011). These classifications were also employed because they tend to capture the majority of early lapses/relapses and have demonstrated utility in predicting longer-term abstinence rates (e.g., Brown et al., 2001; Brown et al., 2009).
Biochemical Verification of Smoking Status
Expired carbon monoxide (CO) was measured at all in-person visits using a Vitalograph Breathco™ CO monitor. Expired CO is measured in parts per million (ppm), and provides an indirect, non-invasive measure of blood Carboxyhemoglobin (Bittoun, 2008) that is most sensitive to recent smoking (e.g., within 24 hours; Bittoun, 2008). Recent smoking can be biochemically verified via expired CO ≥ 8 ppm (Benowitz et al., 2002).
Procedure
All procedures for this study, titled “Anxiety Vulnerability and Smoking Cessation”, were approved by the University of Vermont Institutional Review Board (IRB # CHRBS B09-058) and the University of Houston Institutional Review Board (IRB # 12389-02-[7513]). Participants were recruited to take part in a study examining barriers to successful smoking cessation (Langdon et al., 2015). Upon arrival to the baseline session, smoking status was biochemically verified by expired CO, the SCID-IV/NP was administered, and self-report questionnaires were completed. Participants were compensated $20 for completion of the baseline session. Eligible respondents were then invited to participate in an unaided smoking cessation attempt. Each participant selected his/her own quit date, which typically occurred within two weeks of the baseline assessment (M = 12.8 days, SD = 5.7). Participants were instructed to quit smoking on their own, without any assistance (i.e., pharmacolgical or psychosocial treatment; Langdon et al., 2015). In-person follow-up appointments were scheduled for the quit day, and days 3, 7, 14, 28, and 90 post-quit. Each follow-up visit consisted of self-report assessments, timeline follow-back procedures, and biochemical verification of smoking status via expired CO. Participants were compensated $10 for completing each follow-up assessment, and could earn an additional $20 for completing all of them. Participants were not incentivized to remain abstinent.
Data Analytic Plan
All analyses were conducted using SPSS Statistics 21 (IBM Corp, 2012). First, we ran a series of bivariate correlations to test zero-order associations between PASS-20 total scores (pain-related anxiety), number of days to lapse and relapse, HSI scores (tobacco dependence), severity of past four-week bodily pain, presence of current Axis I psychopathology, ASI-3 scores (anxiety sensitivity), and sociodemographic factors.
Next, we used the Cox proportional hazards model to estimate the risk of both early lapse and early relapse as a function of pain-related anxiety. The Cox model is a well-established statistical procedure that has frequently been used to examine predictors of lapse/relapse to cigarette smoking (e.g., al’Absi, Nakajima, Allen, Lemieux, & Hatsukami, 2015; Zvolensky et al., 2008). This semiparametric model estimates hazard ratios by examining the pattern of covariation of predictor variables with the event of interest (Cox & Oakes, 1984). Consistent with previous research, individuals who maintained abstinence during the given time period (i.e., did not report an early lapse or early relapse) or withdrew from the study before having lapsed/relapsed were censored (e.g., al’Absi et al., 2015). Established procedures for the Cox proportional hazards model indicate that a minimum of 5 events should be included per predictor variable to increase confidence interval coverage, and decrease relative bias and type I error (Vittinghoff & McCulloch, 2007). After ensuring that our models were consistent with this recommendation (our models included 5 predictor variables, and we observed 44 events for early lapse and 34 events for early relapse), covariates (i.e., ASI-3 total scores, past four-week pain severity, presence of current Axis I psychopathology, and HSI scores) were entered into the first step of each model, and continuous PASS-20 scores were entered at the second step.
We used Kaplan Meier survival curves to compare the trajectories to early lapse and relapse between participants with high vs. low levels of pain-related anxiety. Consistent with previous research, PASS-20 scores were dichotomized via median split (e.g., Evans, Seidman, Lung, Zeltzer, & Tsao, 2013). The Kaplan Meier survival curve represents the probability of maintaining smoking abstinence for a given length of time while considering time in many small intervals (Kaplan & Meier, 1958). Two survival curves can be compared statistically using a log-rank test to challenge the null hypothesis that the survival curves do not differ by group (Goel, Khanna, & Kishore, 2010). If a significant log-rank result is observed (p < .05), it can be concluded that the trajectory to lapse/relapse differs based on group status. These procedures are well-established and have been used in numerous studies examining differences in time to lapse/relapse to cigarette smoking (e.g., Wong, Chan, & Lam, 2016).
Results
Participant Characteristics
Participants included 55 current daily tobacco smokers (66% male; Mage = 34.8, SD = 14.6), who reported smoking approximately 16 cigarettes per day (SD = 5.5) for an average of 16 years (SD = 14.0). Mean expired CO at baseline was 18.93 ppm (SD = 9.30), and the mean HSI score was 2.6 (SD = 1.4), indicating a moderate level of tobacco dependence (e.g., Chaiton, Cohen, McDonald, & Bondy, 2007). The sample was predominantly white (87%) and fairly well-educated (27% completed four years of college). Structured clinical interviews (SCID-IV-N/P) revealed that 38% of the sample met criteria for current (past-month) Axis I psychopathology. PASS-20 total scores ranged from 0 to 68 (M = 28.4, SD = 16.5).
The majority of participants (n = 44; 80%) endorsed an early lapse to smoking, and about 62% of the sample (n = 34) endorsed an early relapse to smoking. Participants who reported continued abstinence provided a lower CO reading at both the day 14 follow-up appointment (M = 2.78ppm, SD = 3.96) and the day 28 follow-up appointment (M = 2.43, SD = 3.05), compared to participants who reported having smoked (M = 8.00ppm, SD = 7.35 and M = 11.66, SD = 7.94 respectively; ps < .05). Although expired CO could not be used to verify prolonged abstinence (expired CO is most sensitive to recent smoking; Bittoun, 2008), readings at the in-person follow-up appointments were consistent with self-reported smoking status. We observed a moderate correlation (r = .543) between early lapse and early relapse, which indicates that although there was some overlap between early lapsers and early relapsers, these outcomes were not redundant (e.g., 25% of participants who endorsed an early lapse to smoking did not subsequently endorse an early relapse).
Approximately 73% (n = 40) of the sample endorsed at least very mild past four-week pain, and smokers who endorsed past four-week pain reported higher levels of pain-related anxiety (M = 31.68, SD = 16.06) than those who did not endorse past four-week pain (M = 19.60, SD = 14.68; F [1, 53] = 6.449, p = .014). Among smokers who reported past four-week pain, 82.5% (n = 33) endorsed an early lapse to smoking, and 65% (n = 26) endorsed an early relapse. Sociodemographic and clinical data are presented in Table 1.
Table 1.
Sociodemographic, smoking, and pain characteristics
| Pain-Related Anxiety | Past Four-Week Pain Status | Total Sample |
|||
|---|---|---|---|---|---|
|
|
|||||
| Low | High | No Pain | Pain | ||
|
| |||||
| n (%) | n (%) | n (%) | n (%) | n (%) | |
| Gender | |||||
| Male | 22 (78.6%) | 14 (51.9%) | 9 (60.0%) | 27 (67.5%) | 36 (65.5%) |
| Race | |||||
| White | 25 (89.3%) | 23 (85.2%) | 14 (93.3%) | 34 (85.0%) | 48 (87.3%) |
| Black or African American | 0 (0.0%) | 3 (11.1%) | 0 (0.0%) | 3 (7.5%) | 3 (5.5%) |
| Other | 3 (10.7%) | 1 (3.7%) | 1 (6.7%) | 3 (7.5%) | 4 (7.3%) |
| Marital Status | |||||
| Single | 13 (46.4%) | 17 (63.0%) | 9 (60.0%) | 21 (52.5%) | 30 (54.5%) |
| Married/Living with Someone | 10 (35.7%) | 5 (18.5%) | 3 (20.0%) | 12 (30.0%) | 15 (27.3%) |
| Separated/Divorced/Annulled | 5 (17.9%) | 5 (18.5%) | 3 (20.0%) | 7 (17.5%) | 10 (18.2%) |
| Education | |||||
| 12 Years | 2 (7.1%) | 4 (14.8%) | 2 (13.3%) | 4 (10.0%) | 6 (10.9%) |
| 12–15 Years | 20 (71.4%) | 14 (51.8%) | 9 (60.0%) | 25 (62.5%) | 33 (61.9%) |
| ≥ 16 Years | 6 (21.4%) | 9 (33.3%) | 4 (26.7%) | 11 (27.5%) | 15 (27.3%) |
| Current Axis I Psychopathologya | 8 (28.6%) | 13 (48.1%) | 5 (33.3%) | 16 (40.0%) | 21 (38.2%) |
| Past Four-Week Pain Severityb | |||||
| None | 11 (39.3%) | 4 (14.8%) | 15 (100%) | 0 (0.0%) | 15 (27.3%) |
| Very Mild | 10 (35.7%) | 12 (44.4%) | 0 (0.0%) | 22 (55.0%) | 22 (40.0%) |
| Mild | 4 (14.3%) | 5 (18.5%) | 0 (0.0%) | 9 (22.5%) | 9 (16.4%) |
| Moderate | 1 (3.6%) | 5 (18.5%) | 0 (0.0%) | 6 (15.0%) | 6 (10.9%) |
| Severe | 2 (7.1%) | 1 (3.7%) | 0 (0.0%) | 3 (7.5%) | 3 (5.5%) |
|
| |||||
| M (SD) | M (SD) | M (SD) | M (SD) | M (SD) | |
|
| |||||
| Age | 34.46 (15.58) | 35.11 (13.68) | 29.87 (12.64) | 36.63 (14.93) | 34.78 (14.55) |
| Cigarettes per Day | 16.32 (6.40) | 14.78 (4.45) | 16.27 (6.87) | 15.30 (5.01) | 15.56 (5.53) |
| Years of Smoking | 15.75 (14.72) | 16.78 (13.44) | 12.80 (13.68) | 17.55 (14.04) | 16.25 (13.98) |
| HSI Tobacco Dependencec | 2.75 (1.48) | 2.37 (1.31) | 2.73 (1.44) | 2.50 (1.40) | 2.56 (1.40) |
| Anxiety Sensitivityd1 | 8.61 (6.15) | 21.26 (14.05) | 11.47 (8.53) | 16.08 (13.50) | 14.82 (12.44) |
| Pain-Related Anxietye12 | 15.71 (8.16) | 41.52 (11.95) | 19.60 (14.68) | 31.68 (16.06) | 28.38 (16.48) |
| Days to First Lapse | 18.48 (31.86) | 6.92 (18.42) | 17.14 (29.04) | 11.37 (25.98) | 12.92 (26.67) |
| Days to First Relapse | 28.18 (35.91) | 15.66 (25.69) | 27.55 (33.49) | 19.80 (30.84) | 21.65 (31.29) |
Note.
Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-IV-N/P);
assessed using a single item from the Short Form Health Survey – 12;
Heaviness of Smoking Index;
Anxiety Sensitivity Index – III;
Pain Anxiety Symptoms Scale – 20 item.
Significant (p < .05) difference as a function of pain-related anxiety;
Significant (p < .05) difference as a function of past four-week pain status.
Bivariate Correlations
PASS-20 total scores were positively correlated with past four-week pain severity (r = .37, p < .01), ASI-3 total scores (r = .70, p < .01), and the presence of current Axis I psychopathology (r = .30, p < .05). The presence of current Axis I psychopathology was negatively associated with number of days to first smoking lapse (r = −.29; p < .05). No additional covariates were identified via bivariate analyses.
Pain-Related Anxiety and Early Lapse and Early Relapse to Smoking
As hypothesized, Cox regression analysis revealed that pain-related anxiety was a significant predictor of early smoking lapse (HR = 1.037, p = .013; see Table 2). Examination of the hazard ratio revealed that for every one-point increase on the PASS-20, the risk of early lapse increased by 3.7%. These effects were evident above and beyond the variance accounted for by tobacco dependence scores, past four-week pain severity, anxiety sensitivity, and the presence of current Axis I psychopathology. Also as hypothesized, Cox regression analysis revealed that pain-related anxiety was a significant predictor of early smoking relapse after accounting for the same covariates, such that for every one-point increase on the PASS-20, the risk of early relapse increased by 3.6% (HR = 1.036, p = .027; see Table 2).
Table 2.
Cox Proportional Hazards Regressions
| Unadjusted Hazard Ratio 95% Confidence Interval |
p | Adjusted Hazard Ratio 95% Confidence Interval |
p | |
|---|---|---|---|---|
| Early Lapse among Entire Samplea | ||||
| Past Four-Week Pain Severityc | 1.160 (.915–1.469) | .219 | 1.172 (.901–1.525) | .238 |
| Anxiety Sensitivityd | .999 (.976–1.022) | .938 | .940 (.899–.982) | .005** |
| Current Axis I Psychopathologye | 2.232 (1.183–4.210) | .013* | 3.567 (1.641–7.755) | .001** |
| Tobacco Dependencef | .988 (.815–1.197) | .900 | 1.129 (.897–1.421) | .302 |
| Pain-Related Anxietyg | 1.011 (.995–1.028) | .174 | 1.037 (1.008–1.068) | .013* |
|
| ||||
| Early Relapse among Entire Sampleb | ||||
| Past Four-Week Pain Severity | 1.066 (.804–1.412) | .658 | 1.041 (.748–1.449) | .812 |
| Anxiety Sensitivity | 1.007 (.981–1.033) | .597 | .958 (.913–1.004) | .071 |
| Current Axis I Psychopathology | 1.895 (.947–3.789) | .071 | 3.033 (1.276–7.207) | .012* |
| Tobacco Dependence | 1.175 (.926–1.492) | .185 | 1.379 (1.032–1.842) | .030* |
| Pain-Related Anxiety | 1.014 (.995–1.033) | .154 | 1.036 (1.004–1.069) | .027* |
|
| ||||
| Early Lapse among Participants with Pain | ||||
| Past Four-Week Pain Severity | 1.043 (.748–1.453) | .804 | .993 (.687–1.434) | .968 |
| Anxiety Sensitivity | 1.007 (.986–1.029) | .517 | .944 (.898–.992) | .023* |
| Current Axis I Psychopathology | 1.863 (.896–3.876) | .096 | 2.767 (.977–7.833) | .055 |
| Tobacco Dependence | .965 (.772–1.205) | .752 | 1.055 (.820–1.357) | .677 |
| Pain-Related Anxiety | 1.019 (1.000–1.039) | .046* | 1.050 (1.012–1.089) | .009** |
|
| ||||
| Early Relapse among Participants with Pain | ||||
| Past Four-Week Pain Severityb | .884 (.583–1.340) | .562 | .862 (.549–1.352) | .517 |
| Anxiety Sensitivityc | 1.010 (.985–1.036) | .443 | .966 (.914–1.021) | .216 |
| Current Axis I Psychopathologyd | 1.816 (.825–3.995) | .138 | 2.443 (.818–7.292) | .109 |
| Tobacco Dependencee | 1.141 (.866–1.503) | .350 | 1.234 (.910–1.673) | .176 |
| Pain-Related Anxietyf | 1.017 (.995–1.040) | .122 | 1.036 (.996–1.078) | .075 |
Note.
First lapse within 14 days;
relapse within 28 days;
assessed using a single item from the Short Form Health Survey – 12;
Anxiety Sensitivity Index – III;
Structured Clinical Interview for DSM-IV Non-Patient Edition (SCID-IV-N/P);
Heaviness of Smoking Index;
Pain Anxiety Symptoms Scale – 20;
p < .05;
p < .01.
Pain-Related Anxiety and Trajectories to Early Lapse and Early Relapse to Smoking
Kaplan Meier survival analysis further revealed that, among early-lapsers, greater pain-related anxiety predicted a more rapid trajectory to lapse (p = .037; Figure 1). All participants (100%) high in pain-related anxiety lapsed by day 7, compared to just 77.3% of those low in pain-related anxiety. Among early-relapsers, 84.2% of participants who reported high pain-related anxiety relapsed by day 7, compared to only 66.7% of those low in pain-related anxiety. However, no significant differences in relapse trajectories were observed as a function of pain-related anxiety (p = .322; Figure 1).
Figure 1.
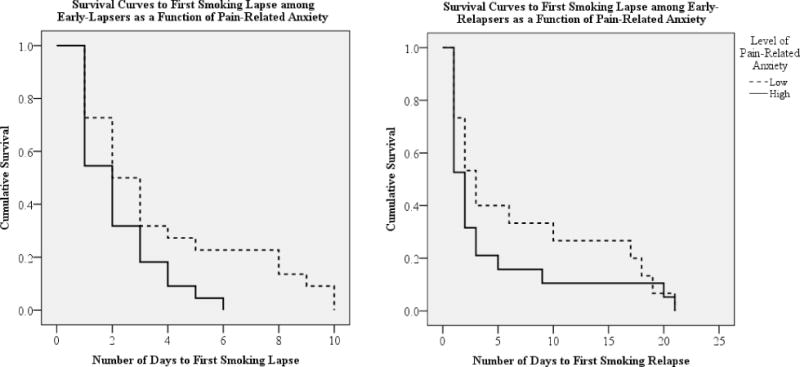
Kaplan Meier survival curves.
Pain-Related Anxiety and Early Lapse/Relapse among Smokers with Pain
To further explore relations between pain-related anxiety and early lapse/relapse, we replicated our analyses among the subsample of participants who endorsed past four-week pain. Similar to the primary findings, Cox regression analysis revealed that pain-related anxiety was a significant predictor of early smoking lapse among smokers who endorsed past four-week pain (HR = 1.050, p = .009; shown in Table 2), such that every one-point increase on the PASS-20 was associated with a 5.0% increase in the risk of early lapse. Although 78.3% of smokers with high levels of pain-related anxiety endorsed an early relapse to smoking (vs. 47.1% of smokers with low levels of pain-related anxiety), pain-related anxiety was not found to be a significant predictor of early relapse among smokers with past four-week pain (HR = 1.036, p = .075; see Table 2). Kaplan Meier survival analyses revealed no differences in trajectory to lapse among early-lapsers (p = .098) or to relapse among early-relapsers (p = .958) as a function of pain-related anxiety among participants with co-occurring pain.
Discussion
The current study is the first to examine pain-related anxiety as a predictor of lapse and relapse to smoking. As hypothesized, pain-related anxiety was observed to be a significant predictor of both early lapse and early relapse among a sample of smokers who were recruited from the local community to participate in an unaided quit attempt. Importantly, these effects were evident above and beyond the variance accounted for by tobacco dependence, past four-week pain severity, anxiety sensitivity, and the presence of current Axis I psychopathology. Among early-lapsers, greater pain-related anxiety was also associated with a more rapid trajectory to lapse. No differences in trajectory to early relapse were observed as a function of pain-related anxiety. Pain and anxiety have both been shown to motivate smoking behavior, and it is possible that these factors may be more likely to interfere with quit attempts among smokers who endorse higher levels of pain-related anxiety (e.g., Ditre & Brandon, 2008; Ditre et al., 2010; Kimbrel, Morissette, Gulliver, Langdon, & Zvolensky, 2014). Only recently have researchers begun to evaluate pain and anxiety-related cognitive-affective processes as potential mechanisms in the maintenance of tobacco dependence (e.g., Ditre et al., 2011; Hooten, Shi, Gazelka, & Warner, 2011), and these findings are consistent with demonstrated positive associations between pain-related anxiety, smoking dependence motives (Ditre, Langdon, et al., 2015; Ditre et al., 2013), and perceived barriers to cessation (Ditre, Langdon, et al., 2015).
Analyses conducted among the subsample of participants who endorsed past four-week pain largely corroborated the primary findings, and suggest that pain-related anxiety may be associated with an even greater risk of early lapse among smokers with co-occurring pain (every one-point increase on the PASS-20 was associated with a 5% increased risk in early lapse among smokers with co-occurring pain, compared to a 3.7% increased risk among the total sample). Pain-related anxiety was not associated with risk of early relapse when analyses were limited to smokers who endorsed past four-week pain, though this may have been a function of reduced statistical power (n = 40 smokers endorsed past four-week pain). Interestingly, we observed a wide range of PASS-20 scores among the subsample of smokers who reported no past four-week pain (M = 19.60, SD = 14.68, range = 55), demonstrating that smokers may endorse varying levels of pain-related anxiety, even in the absence of co-occurring pain. Additionally, we noted that anxiety sensitivity was associated with a decreased likelihood of early lapse among both the entire sample and the subsample of smokers with co-occurring pain (ps < .05). In contrast, previous work has documented an increased risk of early lapse among smokers with greater anxiety sensitivity (e.g., Assayag et al., 2012; Zvolensky, Stewart, et al., 2009), however, these studies did not account for the influence of pain-related anxiety. Future work should attempt to explicate the relative contributions of pain-related anxiety and anxiety sensitivity to smoking cessation outcomes.
Several study limitations should be noted, including the modest sample size. It is important that future work replicate these findings among a larger and more diverse sample of tobacco smokers (though it can be beneficial to study smaller samples during the early stages of hypothesis testing, and we took steps to ensure that the Cox proportional hazards models were adequately powered). It is also important to consider that participants volunteered to engage in an unaided quit attempt. Although an unaided quit approach confers several benefits (e.g., provides knowledge about self-quitters, limits interpretive problems related to delivering an intervention, provides a natural history of smoking cessation that can yield normative data against which treatment outcomes can be compared; e.g., Marlatt, Curry, & Gordon, 1988; Zvolensky et al., 2008), the extent to which these results may generalize to smokers receiving cessation treatment remains unclear. Additionally, we did not assess chronic pain status or use of pain medications. Future work would benefit from examining these factors in relation to pain-related anxiety and smoking cessation trajectories. It is also possible that the four-week timeframe may have been too brief to observe a relationship between pain-related anxiety and trajectory to relapse. Indeed, previous work has suggested that anxiety-related constructs (e.g., anxiety sensitivity) may place smokers at increased risk for relapse in the longer term due to repeated lapses over time (Zvolensky, Stewart, et al., 2009). Future research should examine the effects of pain-related anxiety on trajectory to relapse beyond the first four weeks of a quit attempt. Finally, although duration of abstinence prior to first lapse and relapse has been shown to predict longer-term cessation outcomes (e.g., Gilpin, Pierce, Farkas, & Farkas, 1997), early lapse and relapse cannot adequately capture the dynamic process of quitting (e.g., Velicer, Prochaska, Rossi, & Snow, 1992), and future research should examine pain-related anxiety as a predictor of both prolonged and point prevalence cessation outcomes to better account for smokers who regain abstinence following an early lapse or relapse.
Clinical research has implicated pain-related anxiety in the onset and exacerbation of pain (e.g., Boersma & Linton, 2006), and these data contribute to an emerging literature which suggests that pain-related anxiety may also play a role in the maintenance of cigarette smoking. Indeed, smokers with high pain-related anxiety may face unique cessation challenges that warrant tailored intervention, and pain-related anxiety may function as a transdiagnostic factor in the co-occurrence of pain and tobacco addiction. Treatments that incorporate psychoeducation, cognitive restructuring, and interoceptive exposure have been shown to decrease pain-related anxiety among persons with chronic pain (e.g., Watt, Stewart, Lefaivre, & Uman, 2006; Wetherell et al., 2011), and improve cessation outcomes among tobacco smokers (Fiore et al., 2008; Gifford et al., 2004). Future research should examine the efficacy of tailored treatments for smokers who present with high pain-related anxiety. Such treatments could provide psychoeducation regarding pain-smoking interrelations to aid in the development of discrepancy between continued tobacco smoking and stated goals for reducing pain and pain-related anxiety. Tailored pre-cessation coping skills training could also help smokers to better manage their pain and pain-related anxiety during the early stages of quitting. Finally, future research would benefit from testing the utility of sequential versus integrated treatments (e.g., Ries, 1996) for pain-related anxiety and smoking cessation among smokers with co-occurring pain.
In summary, results of the current study represent an initial, yet important step towards better understanding the role of pain-related anxiety in the maintenance of tobacco dependence. No previous research has examined associations between pain-related anxiety and attempts to abstain from smoking, and these findings provide the first evidence that high levels of pain-related anxiety may be associated with early lapse and relapse to smoking tobacco cigarettes. This and future work has the potential to inform the development of tailored cessation interventions for smokers who experience varying levels of pain intensity and pain-related anxiety.
Public Significance Statement.
Pain-related anxiety was shown to predict early lapse and relapse to cigarette smoking. This study provides additional evidence that pain-related anxiety may contribute to the maintenance of tobacco dependence among smokers who experience varying levels of pain intensity.
Acknowledgments
This work was supported in part by NRSA grant F31-DA026634 awarded to Kirsten J. Langdon and an endowment awarded to Michael. J. Zvolensky.
Footnotes
All authors contributed in a significant way to the manuscript and all authors have read and approved the final manuscript.
We have no conflicts of interest to declare.
There has been no prior dissemination of the ideas and data appearing in this manuscript.
References
- Abrams MP, Carleton RN, Asmundson GJ. An exploration of the psychometric properties of the PASS-20 with a nonclinical sample. The Journal of Pain. 2007;8(11):879–886. doi: 10.1016/j.jpain.2007.06.004. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hatsukami D, Davis GL. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology (Berl) 2005;181(1):107–117. doi: 10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Nakajima M, Allen S, Lemieux A, Hatsukami D. Sex differences in hormonal responses to stress and smoking relapse: a prospective examination. Nicotine & Tobacco Research. 2015;17(4):382–389. doi: 10.1093/ntr/ntu340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assayag Y, Bernstein A, Zvolensky M, Steeves D, Stewart S. Nature and role of change in anxiety sensitivity during NRT-aided cognitive-behavioral smoking cessation treatment. Cognitive Behaviour Therapy. 2012;41(1):51–62. doi: 10.1080/16506073.2011.632437. [DOI] [PubMed] [Google Scholar]
- Benowitz NL, Jacob P, III, Ahijevych K, Jarvis MJ, Hall S, LeHouezec J, Tsoh J. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4(2) [Google Scholar]
- Bittoun R. Carbon monoxide meter: the essential clinical tool—the ‘stethoscope’—of smoking cessation. Journal of Smoking Cessation. 2008;3(02):69–70. [Google Scholar]
- Boersma K, Linton SJ. Expectancy, fear and pain in the prediction of chronic pain and disability: a prospective analysis. European Journal of Pain. 2006;10(6):551–557. doi: 10.1016/j.ejpain.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Brown RA, Burgess ES, Sales SD, Whiteley JA, Evans DM, Miller IW. Reliability and validity of a smoking timeline follow-back interview. Psychology of Addictive Behaviors. 1998;12(2):101–112. doi: 10.1037//0893-164X.12.2.101. [DOI] [Google Scholar]
- Brown RA, Kahler CW, Niaura R, Abrams DB, Sales SD, Ramsey SE, Miller IW. Cognitive–behavioral treatment for depression in smoking cessation. Journal of Consulting and Clinical Psychology. 2001;69(3):471. doi: 10.1037//0022-006x.69.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Lejuez C, Strong DR, Kahler CW, Zvolensky MJ, Carpenter LL, Price LH. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11(5):493–502. doi: 10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RA, Palm KM, Strong DR, Lejuez CW, Kahler CW, Zvolensky MJ, Gifford EV. Distress tolerance treatment for early-lapse smokers: rationale, program description, and preliminary findings. Behavior Modification. 2008;32(3):302–332. doi: 10.1177/0145445507309024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC. Quitting smoking among adults–United States, 2001–2010. MMWR. Morbidity and Mortality Weekly Report. 2011;60(44):1513. [PubMed] [Google Scholar]
- Chaiton MO, Cohen JE, McDonald PW, Bondy SJ. The Heaviness of Smoking Index as a predictor of smoking cessation in Canada. Addictive Behaviors. 2007;32(5):1031–1042. doi: 10.1016/j.addbeh.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Courvoisier DS, Etter JF. Comparing the predictive validity of five cigarette dependence questionnaires. Drug and Alcohol Dependence. 2010;107(2):128–133. doi: 10.1016/j.drugalcdep.2009.09.011. [DOI] [PubMed] [Google Scholar]
- Cox DR, Oakes D. Analysis of Survival Data. Vol. 21. CRC Press; 1984. [Google Scholar]
- Ditre JW, Brandon TH. Pain as a motivator of smoking: Effects of pain induction on smoking urge and behavior. Journal of Abnormal Psychology. 2008;117(2):467–472. doi: 10.1037/0021-843X.117.2.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Brandon TH, Zale EL, Meagher MM. Pain, nicotine, and smoking: Research findings and mechanistic considerations. Psychological Bulletin. 2011;137(6):1065–1093. doi: 10.1037/a0025544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Heckman BW, Butts EA, Brandon TH. Effects of expectancies and coping on pain-induced motivation to smoke. Journal of Abnormal Psychology. 2010;119(3):524–533. doi: 10.1037/a0019568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Langdon KJ, Kosiba JD, Zale EL, Zvolensky MJ. Relations between pain-related anxiety, tobacco dependence, and barriers to quitting among a community-based sample of daily smokers. Addictive Behaviors. 2015;42:130–135. doi: 10.1016/j.addbeh.2014.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ditre JW, Zale EL, Brandon TH. The Tobacco Epidemic. Vol. 42. Karger Publishers; 2015. Patterns and predictors of smoking cessation; pp. 210–218. [Google Scholar]
- Ditre JW, Zale EL, Kosiba JD, Zvolensky MJ. A pilot study of pain-related anxiety and smoking-dependence motives among persons with chronic pain. Experimental and Clinical Psychopharmacology. 2013;21(6):443–449. doi: 10.1037/a0034174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans S, Seidman LC, Lung KC, Zeltzer LK, Tsao JC. Sex differences in the relationship between maternal fear of pain and children’s conditioned pain modulation. Journal of Pain Research. 2013;6:231. doi: 10.2147/JPR.S43172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris SG, Leventhal AM, Schmidt NB, Zvolensky MJ. Anxiety sensitivity and pre-cessation smoking processes: testing the independent and combined mediating effects of negative affect-reduction expectancies and motives. Journal of Studies on Alcohol and Drugs. 2015;76(2):317–325. doi: 10.15288/jsad.2015.76.317. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25785807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore MC, Jaen CR, Baker TB, Bailey WC, Benowitz NL, Curry SJ, Dorfman SF. Treating Tobacco Use and Dependence: 2008 Update Clinical Practice Guideline. Rockville, MD: U.S. Department of Health and Human Services. Public Health Service; 2008. May 2008. [Google Scholar]
- Gifford EV, Kohlenberg BS, Hayes SC, Antonuccio DO, Piasecki MM, Rasmussen-Hall ML, Palm KM. Acceptance-based treatment for smoking cessation. Behavior Therapy. 2004;35(4):689–705. doi: 10.1016/j.beth.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Gilpin EA, Pierce JP, Farkas AJ, Farkas AJ. Duration of Smoking Abstinence and Success in Quitting. Journal of the National Cancer Institute. 1997;89(8):572. doi: 10.1093/jnci/89.8.572. [DOI] [PubMed] [Google Scholar]
- Goel M, Khanna P, Kishore J. Understanding survival analysis: Kaplan-Meier estimate. International Journal of Ayurveda Research. 2010;1(4):274. doi: 10.4103/0974-7788.76794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez A, Hogan J, McLeish AC, Zvolensky MJ. An evaluation of pain-related anxiety among daily cigarette smokers in terms of negative and positive reinforcement smoking outcome expectancies. Addictive Behaviors. 2010;35(6):553–557. doi: 10.1016/j.addbeh.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Rickert W, Robinson J. Measuring the heaviness of smoking: using self-reported time to the first cigarette of the day and number of cigarettes smoked per day. British Journal of Addiction. 1989;84(7):791–799. doi: 10.1111/j.1360-0443.1989.tb03059.x. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/2758152. [DOI] [PubMed] [Google Scholar]
- Hogan J, Gonzalez A, Howell A, Bonn-Miller MO, Zvolensky MJ. Pain-related anxiety and marijuana use motives: a pilot test among active marijuana-using young adults. Cognitive Behaviour Therapy. 2010;39(4):283–292. doi: 10.1080/16506073.2010.505247. [DOI] [PubMed] [Google Scholar]
- Holt LJ, Litt MD, Cooney NL. Prospective analysis of early lapse to drinking and smoking among individuals in concurrent alcohol and tobacco treatment. Psychology of Addictive Behaviors. 2012;26(3):561–572. doi: 10.1037/a0026039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Shi Y, Gazelka HM, Warner DO. The effects of depression and smoking on pain severity and opioid use in patients with chronic pain. Pain. 2011;152(1):223–229. doi: 10.1016/j.pain.2010.10.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM, Townsend CO, Bruce BK, Schmidt JE, Kerkvliet JL, Patten CA, Warner DO. Effects of smoking status on immediate treatment outcomes of multidisciplinary pain rehabilitation. Pain Medicine. 2009;10(2):347–355. doi: 10.1111/j.1526-4637.2008.00494.x. [DOI] [PubMed] [Google Scholar]
- Hsu C, Harden RN, Houle T. Nicotine and caffeine intake in complex regional pain syndrome. Journal of Back and Musculoskeletal Rehabilitation. 2002;16(1):33–38. doi: 10.3233/bmr-2002-16106. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/22387362. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction. 2004;99(1):29–38. doi: 10.1111/j.1360-0443.2004.00540.x. [DOI] [PubMed] [Google Scholar]
- IASP. Part III: Pain Terms, A Current List with Definitions and Notes on Usage. In: HB Merskey N, editor. Classification of Chronic Pain. Second. Seattle: IASP Press; 1994. [Google Scholar]
- IBM Corp. IBM SPSS Statistics Version 21. Boston, Mass: International Business Machines Corp; 2012. p. 126. [Google Scholar]
- Jamal A, Homa DM, O’Connor E, Babb SD, Caraballo RS, Singh T, King BA. Current Cigarette Smoking Among Adults-United States, 2005–2014. MMWR. Morbidity and Mortality Weekly Report. 2015;64(44):1233–1240. doi: 10.15585/mmwr.mm6444a2. [DOI] [PubMed] [Google Scholar]
- Johnson KA, Farris SG, Schmidt NB, Smits JA, Zvolensky MJ. Panic attack history and anxiety sensitivity in relation to cognitive-based smoking processes among treatment-seeking daily smokers. Nicotine & Tobacco Research. 2013;15(1):1–10. doi: 10.1093/ntr/ntr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Stewart S, Rosenfield D, Steeves D, Zvolensky MJ. Prospective evaluation of the effects of anxiety sensitivity and state anxiety in predicting acute nicotine withdrawal symptoms during smoking cessation. Psychology of Addictive Behaviors. 2012;26(2):289. doi: 10.1037/a0024133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53(282):457–481. [Google Scholar]
- Kimbrel NA, Morissette SB, Gulliver SB, Langdon KJ, Zvolensky MJ. The effect of social anxiety on urge and craving among, smokers with and without anxiety disorders. Drug and Alcohol Dependence. 2014;135:59–64. doi: 10.1016/j.drugalcdep.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon KJ, Farris SG, Øverup CS, Zvolensky MJ. Associations between anxiety sensitivity, negative affect, and smoking during a self-guided smoking cessation attempt. Nicotine & Tobacco Research. 2015;18(5):1188–1195. doi: 10.1093/ntr/ntv253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasser K, Boyd JW, Woolhandler S, Himmelstein DU, McCormick D, Bor DH. Smoking and mental illness: A population-based prevalence study. Journal of the American Medical Association. 2000;284(20):2606–2610. doi: 10.1001/jama.284.20.2606. [DOI] [PubMed] [Google Scholar]
- Marlatt GA, Curry S, Gordon J. A longitudinal analysis of unaided smoking cessation. Journal of Consulting and Clinical Psychology. 1988;56(5):715. doi: 10.1037//0022-006x.56.5.715. [DOI] [PubMed] [Google Scholar]
- Mayo Clinic. Managing pain: Attitude, medication and therapy are keys to control. Mayo Clinic Health Letter. 2001 Retrieved from http://www.mayoclinic.com.
- McCracken LM, Dhingra L. A short version of the Pain Anxiety Symptoms Scale (PASS–20): Preliminary development and validity. Pain Research and Management. 2002 doi: 10.1155/2002/517163. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Gross RT, Sorg PJ, Edmands TA. Prediction of pain in patients with chronic low back pain: effects of inaccurate prediction and pain-related anxiety. Behaviour Research and Therapy. 1993;31(7):647–652. doi: 10.1016/0005-7967(93)90117-D. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Keogh E. Acceptance, mindfulness, and values-based action may counteract fear and avoidance of emotions in chronic pain: an analysis of anxiety sensitivity. The Journal of Pain. 2009;10(4):408–415. doi: 10.1016/j.jpain.2008.09.015. [DOI] [PubMed] [Google Scholar]
- McCracken LM, Zayfert C, Gross RT. The Pain Anxiety Symptoms Scale: development and validation of a scale to measure fear of pain. Pain. 1992;50(1):67–73. doi: 10.1016/0304-3959(92)90113-P. [DOI] [PubMed] [Google Scholar]
- Michna E, Ross EL, Hynes WL, Nedeljkovic SS, Soumekh S, Janfaza D, Jamison RN. Predicting aberrant drug behavior in patients treated for chronic pain: importance of abuse history. Journal of Pain and Symptom Management. 2004;28(3):250–258. doi: 10.1016/j.jpainsymman.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Nakajima M, al’Absi M. Enhanced pain perception prior to smoking cessation is associated with early relapse. Biological Psychology. 2011 doi: 10.1016/j.biopsycho.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson AL, Gritzner S, Resnick MP, Dobscha SK, Turk DC, Morasco BJ. Smoking cigarettes as a coping strategy for chronic pain is associated with greater pain intensity and poorer pain-related function. The Journal of Pain. 2012;13(3):285–292. doi: 10.1016/j.jpain.2011.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries R. Assessment and treatment of patients with coexisting mental illness and alcohol and other drug abuse. DIANE Publishing; 1996. [PubMed] [Google Scholar]
- Shiri R, Karppinen J, Leino-Arjas P, Solovieva S, Viikari-Juntura E. The association between smoking and low back pain: a meta-analysis. American Journal of Medicine. 2010;123(1):87 e87–35. doi: 10.1016/j.amjmed.2009.05.028. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. Measuring alcohol consumption. Humana Press; 1992. Timeline follow-back; pp. 41–72. [Google Scholar]
- Sugiyama D, Nishimura K, Tamaki K, Tsuji G, Nakazawa T, Morinobu A, Kumagai S. Impact of smoking as a risk factor for developing rheumatoid arthritis: a meta-analysis of observational studies. Annals of the Rheumatic Diseases. 2010;69(1):70–81. doi: 10.1136/ard.2008.096487. [DOI] [PubMed] [Google Scholar]
- Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Cardenas SJ. Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity Index-3. Psychological Assessment. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- US Department of Health & Human Services. The health consequences of smoking—50 years of progress. A Report of the Surgeon General 2014 [Google Scholar]
- Velicer WF, Prochaska JO, Rossi JS, Snow MG. Assessing outcome in smoking cessation studies. Psychological Bulletin. 1992;111(1):23. doi: 10.1037/0033-2909.111.1.23. [DOI] [PubMed] [Google Scholar]
- Vittinghoff E, McCulloch CE. Relaxing the rule of ten events per variable in logistic and Cox regression. American Journal of Epidemiology. 2007;165(6):710–718. doi: 10.1093/aje/kwk052. [DOI] [PubMed] [Google Scholar]
- Ware JE, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Medical Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- Watt MC, Stewart SH, Lefaivre MJ, Uman LS. A brief cognitive-behavioral approach to reducing anxiety sensitivity decreases pain-related anxiety. Cognitive Behavior Therapy. 2006;35(4):248–256. doi: 10.1080/16506070600898553. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, Lang AJ. A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain. 2011;152(9):2098–2107. doi: 10.1016/j.pain.2011.05.016. [DOI] [PubMed] [Google Scholar]
- Wong DC, Chan SS, Lam T-h. Depressive symptoms delayed quit attempts and shortened abstinence in young smokers of the Hong Kong Youth Quitline. Nicotine & Tobacco Research. 2016;18(3):251–258. doi: 10.1093/ntr/ntv065. [DOI] [PubMed] [Google Scholar]
- World Health Organization. WHO report on the global tobacco epidemic, 2008: the MPOWER package. 2008. [Google Scholar]
- Yong HH, Borland R, Balmford J, Hyland A, O’Connor RJ, Thompson ME, Spittal MJ. Heaviness of smoking predicts smoking relapse only in the first weeks of a quit attempt: Findings from the international tobacco control four-country survey. Nicotine & Tobacco Research. 2014;16(4):423–429. doi: 10.1093/ntr/ntt165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zale EL, Maisto SA, Ditre JW. Anxiety and depression in bidirectional relations between pain and smoking: implications for smoking cessation. Behavior Modification. 2016;40(1–2):7–28. doi: 10.1177/0145445515610744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Gibson LE, Vujanovic AA, Gregor K, Bernstein A, Kahler C, Feldner MT. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008;10(8):1415–1427. doi: 10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, McMillan K, Gonzalez A, Asmundson GJ. Chronic pain and cigarette smoking and nicotine dependence among a representative sample of adults. Nicotine & Tobacco Research. 2009;11(12):1407–1414. doi: 10.1093/ntr/ntp153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & Tobacco Research. 2009;11(3):323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]


