Abstract
Epidemiological studies have concluded that hyperlipidemia and atherosclerosis were related to intervertebral disc degeneration (IVDD). The presence of oxidized low density lipoprotein (ox-LDL) and the expression of lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) have not been explored in this tissue. In this study, we investigated the presence of ox-LDL and the expression of its receptor LOX-1 in non-degenerated, degenerated or herniated human intervertebral discs (IVDs). The expression of LOX-1 and matrix metalloproteinase 3 (MMP3) were studied after incubating nucleus pulposus cells (NPCs) with ox-LDL. The presence of ox-LDL and LOX-1 was positively related with the extent of IVDD in nucleus pulposus (NP), end-plate cartilage and outer annulus fibrous, but not with the extent of degeneration of inter annulus fibrous. Ox-LDL significantly reduced the viability of human NPCs in a dose and time-dependent manner, and increased the expression of MMP3 induced by LOX-1. Pretreatment with anti-human LOX-1 monoclonal antibody reversed these effects. Ox-LDL, principally mediated by LOX-1, enhanced MMP3 production in NPCs through the NF-κB signaling pathway. In conclusion, increased accumulation of ox-LDL and LOX-1 in IVDs indicates a specific role of the receptor-ligand interaction in degeneration or herniation of IVDs.
Introduction
Back pain is a leading cause of disability and job-related disability1, 2. Lumbar disc herniation (LDH) is a major cause of low back pain and sciatica3. Though the etiology and treatment of intervertebral disc degeneration (IVDD) has been extensively investigated, the underlying pathophysiologic mechanism remains unclear4–7. Abnormal lipid metabolism and atherosclerosis (AS) were implicated to be critical players in the development of age-related degenerative diseases8, 9.
IVDD is a common age-related disease. As in other age-related degenerative diseases, serum lipid levels and AS were positively correlated with LDH. There are two main hypotheses on how abnormal lipid metabolism and AS can cause LDH. Firstly, dyslipidemia can accelerate the AS process and its morbidity, which will destroy vascular supply to the already poorly vascularized human intervertebral discs (IVDs). Secondly, release of inflammatory cytokines caused by dyslipidemia and AS may be another potential pathogenetic mechanism. To some extent, the IVDD was regarded as one inflammatory-related joint disease. However, the precise pathophysiological mechanism underlying these associations remains unclear10–14.
Oxidized low density lipoprotein (Ox-LDL) accumulation under oxidative stress conditions plays a very important role in the development of AS15. Ox-LDL has many biological functions; it causes lipid accumulation, elicits pro-inflammatory responses, promotes apoptosis, and increases protease activity16. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) is a type II membrane protein that belongs to the C-type lectin family, and can act as a cell-surface receptor for ox-LDL17. LOX-1 is expressed in various cells, including endothelial cells, macrophages and chondrocytes, and its expression is enhanced by proinflammatory cytokines such as interleukin-1 (IL-1). Previous studies suggested that ox-LDL can decrease cell viability18, induce reactive oxygen species production19, reduce proteoglycan synthesis20, increase matrix metalloproteinase 3 (MMP3) production21 and monocyte chemoattractant protein-1 (MCP-1) expression22 in human or bovine chondrocytes through LOX-1. Supporting the possible involvement of lipid peroxidation in the pathogenesis of IVDD, the lipid peroxidation inhibitors such as vitamin C inhibited degradation of the extracellular matrix (ECM) in nucleus pulposus cell (NPC) monolayer cultures [3]. However, the specific mechanism remains largely unknown. Previous studies showed that vitamin C could prevent ox-LDL binding to LOX-1 in osteoarthritis (OA). However, whether vitamin C has similar effects on NPCs remains largely unknown.
Current evidence implicates major pathological changes in a degenerating disc to begin with proteoglycan breakdown, cell loss and diminished water-binding capacity of the nucleus pulposus (NP)23. The breakdown of proteoglycan can be due to the decreased ability of NPCs to synthesize ECM and increased activity of matrix metalloproteinases (MMPs). MMPs are critical enzymes involved in the destruction of ECM of IVDs. Among the MMPs, MMP3 is regarded as a critical enzyme, which can degrade proteoglycan and fibronectin, and activate proMMPs24. Although the correlation between ox-LDL, LOX-1 and MMP3 is implicated in rheumatoid arthritis (RA) and OA21, 25, its role in the pathophysiology of IVDD remains unknown.
In this study, we investigated whether ox-LDL/LOX-1 ligand-receptor system was involved in IVD degeneration or herniation, and the effects of ox-LDL on cell viability and MMP3 production in cultured human NPCs.
Results
Patients demographics
Twenty-four disc samples were obtained by lumbar surgery (lumbar fracture or lumbar disc herniation). Patients demographics (age, serum ox-LDL and LDL) in the study and control groups are shown in Table 1. The average age of the patients was 45.08 ± 15.76 years, range 25–73 years. The average serum ox-LDL was 480.43 ± 279.98 mU/ml for non-degenerated IVDs (histological degeneration scores 0 to 3), 728.67 ± 256.69 mU/ml for intermediate-degenerated IVDs (histological degenerative scores 4 to 8), and 705.69 ± 185.43 mU/ml for severely degenerated IVDs (histological degenerative scores 9 to 12). The average serum LDL was 3.24 ± 1.32 mmol/L for non-degenerated IVDs, 3.21 ± 0.49 mmol/L for intermediate-degenerated IVDs, and 3.19 ± 0.67 mmol/L for severely degenerated IVDs. Therefore, the serum ox-LDL and LDL levels were not positively correlated with the extent of degenerated IVDs.
Table 1.
Patient details and grades of tissues used for immunohistochemistry analysis.
| Subject | Age | Vertebral level | Histological grade | Clinical diagnosis | serum LDL (mmol/L) | serum oxLDL (mU/ml) |
|---|---|---|---|---|---|---|
| 1 | 28 | L1-L2 | 1 | lumbar fracture | 1.65 | 405.90 |
| 2 | 37 | L2-L3 | 1 | lumbar fracture | 2.88 | 266.10 |
| 3 | 29 | L3-L4 | 2 | lumbar fracture | 4.13 | 664.67 |
| 4 | 30 | L3-S4 | 2 | lumbar fracture | 3.83 | 867.83 |
| 5 | 26 | L2-S3 | 2 | lumbar fracture | 1.93 | 97.74 |
| 6 | 25 | L4-L5 | 3 | disc herniation | 5.02 | 580.34 |
| 7 | 39 | L3-L4 | 4 | disc herniation | 3.22 | 989.31 |
| 8 | 35 | L3-L4 | 4 | disc herniation | 3.51 | 804.80 |
| 9 | 27 | L5-S1 | 5 | disc herniation | 2.94 | 170.40 |
| 10 | 31 | L5-S1 | 5 | disc herniation | 3.84 | 488.35 |
| 11 | 36 | L4-L5 | 6 | disc herniation | 3.50 | 580.34 |
| 12 | 28 | L4-L5 | 6 | disc herniation | 3.20 | 804.80 |
| 13 | 47 | L4-L5 | 7 | disc herniation | 2.80 | 989.31 |
| 14 | 52 | L4-L5 | 7 | disc herniation | 3.23 | 937.99 |
| 15 | 54 | L5-S1 | 8 | disc herniation | 3.76 | 825.08 |
| 16 | 55 | L5-S1 | 8 | disc herniation | 2.15 | 696.39 |
| 17 | 56 | L3-L4 | 9 | disc herniation | 2.95 | 713.02 |
| 18 | 59 | L3-L4 | 9 | disc herniation | 2.87 | 555.38 |
| 19 | 55 | L5-S1 | 10 | disc herniation | 3.08 | 804.80 |
| 20 | 59 | L4-L5 | 10 | disc herniation | 4.13 | 664.67 |
| 21 | 65 | L4-L5 | 10 | disc herniation | 3.75 | 555.38 |
| 22 | 67 | L4-L5 | 11 | disc herniation | 3.10 | 890.37 |
| 23 | 69 | L5-S1 | 11 | disc herniation | 3.70 | 397.85 |
| 24 | 73 | L5-S1 | 11 | disc herniation | 1.97 | 913.74 |
Immunohistochemical localization
The LOX-1 and ox-LDL immunopositive cells were found in normal and degenerated discs, but the proportion was significantly higher in the degenerated discs (Fig. 1). When normal IVDs were examined, slight immunoreactive staining was found with anti-LOX-1 and anti-ox-LDL antibodies. Non-specific control rabbit IgG yielded no positive staining in the IVDs (data not shown).
Figure 1.
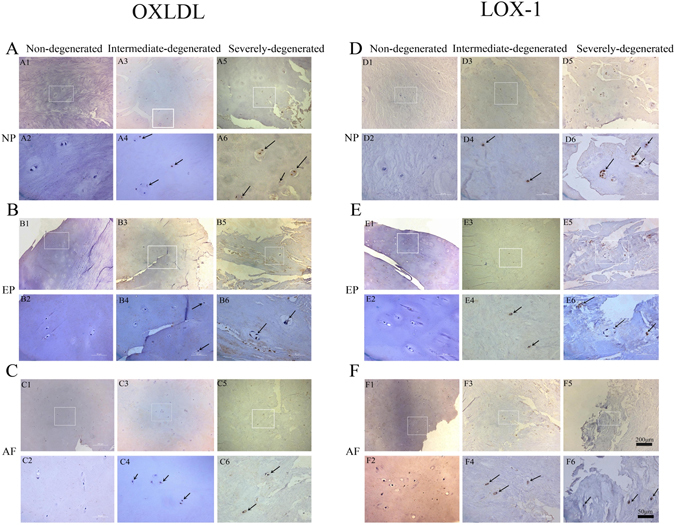
Examples of immunohistochemical staining for LOX-1 (1:100, Abcam, USA) and oxLDL (1:100, biorbyt, USA) in human intervertebral disc. LOX-1 (row A,B,C) and oxLDL (row D,E,F) in non-degenerate discs (A1,A2,B1,B2,C1,C2,D1,D2,E1,E2,F1,F2); intermediate degenerated disc: (A3,A4,B3,B4,C3,C4,D3,D4,E3,E4,F3,F4); Severe degenerated disc: (A5,A6,B5,B6,C5,C6,D5,D6,E5,E6,F5,F6). Immunopositivity is revealed by brown staining. In non-degenerate discs, no cell clusters were seen and little immunopositivity was observed in the single cells. In degenerated discs, a large number of cell clusters were observed, which were predominately immunopositive. Bars = 200 μm (row 1,3,5), Bars = 50 μm (row 2,4,6). A one-way ANOVA were used for statistical assessments. (*P < 0.05, **P < 0.01).
Immunohistochemical staining and quantification of immunopositive cells
The most prominent aspects of the immunophenotype of non-degenerated IVDs (histological degeneration scores 0 to 3) was slight immunoreactivity for ox-LDL and LOX-1 (Fig. 2). In the intermediate-degenerated IVDs (histological degenerative scores 4 to 8), the immunophenotype of cells differed in two ways from cells in non-degenerated IVDs (scores 0 to 3). Firstly, the proportion of cells immunopositive for LOX-1 and ox-LDL were higher than that of cells from non-degenerated IVDs, and this immunopositivity increased with the severity of degeneration in NPC, endplate cartilage (EPC), and outer annulus fibrosus (OAF). Secondly, the LOX-1 and ox-LDL immunopositive cells in the internal annulus fibrosus (IAF) showed no significant difference between the non-degenerated IVDs and intermediate-degenerated IVDs (histological degenerative scores 4 to 8). In the severely degenerated IVDs (histological degenerative scores 9 to 12), the proportion of cells immunopositive for LOX-1 and ox-LDL were significantly higher than that of non-degenerated IVDs.
Figure 2.

The percentage of cells with immunopositivity for (A) LOX-1, (B) oxLDL, according to location in the disc and grade of intervertebral disc degeneration. For oxLDL immunopositive cell. (C) The Correlations analysis between ox-LDL immunoreactivity and intervertebral disc degeneration score were performed.(R2 EP = 0.91, P < 0.0001; R2 NP = 0.83, P < 0.0001; R2 OAF = 0.85, P < 0.0001; R2 IAF = 0.75, P < 0.0001). (D) The Correlations analysis between LOX-1 and intervertebral disc degeneration score were performed (R2 EP = 0.83, P < 0.0001; R2 NP = 0.86, P < 0.0001; R2 OAF = 0.76, P < 0.0001; R2 IAF = 0.78, P < 0.0001). Data are presented as means ± SD. (*P < 0.05, **P < 0.01).
Notably, the mean percentage of ox-LDL-positive-immunostained NPCs was 0.41% in the non-degenerated IVDs, 2.23% in the intermediate degenerated IVDs, and 25.34% in the severely degenerated IVDs. The ox-LDL-positive-immunostaining for EPC was 2.23% in the non-degenerated IVDs, 23.8% in the intermediate degenerated IVDs, and 52.41% in the severely degenerated IVDs. For OAF, these percentages were 1.7%, 15.98% and 34.77%; while for IAF, these percentages changed to 0.3%, 3.29% and 10.7%.
The mean percentage of LOX-1-positive-immunostained NPCs was 7.98% in the non-degenerated IVDs, 51.73% in the intermediate degenerated IVDs and 81.64% in the severely degenerated IVDs. The LOX-1-positive-immunostaining for EPC was 5.2% in the non-degenerated IVDs, 33.97% in the intermediate degenerated IVDs and 59.42% in the severely degenerated IVDs. For OAF, these percentages were 2.73%, 20.28% and 41.77%; while for IAF, these percentages changed to 0.17%, 5.08% and 11.7%.
The correlation analysis between ox-LDL immunoreactivity and IVD degeneration score was performed (R2 EP = 0.91, P < 0.0001; R2 NP = 0.83, p < 0.0001; R2 OAF = 0.85, p < 0.0001; R2 IAF = 0.75, p < 0.0001). The correlation analysis between LOX-1 and IVD degeneration score was performed (R2 EP = 0.83, p < 0.0001; R2 NP = 0.86, p < 0.0001; R2 OAF = 0.76, p < 0.0001; R2 IAF = 0.78, p < 0.0001). Data are presented as means ± SD (*p < 0.05, **p < 0.01).
Effect of ox-LDL on cell viability
We investigated the effect of ox-LDL on the viability of NPCs (The result in relation to the identification of NPCs could be found in the supplement files). Increasing concentrations of ox-LDL (10 μg/ml, 20 μg/ml, 30 μg/ml and 40 μg/ml) were added to human NPCs and cell viability was assessed by the CCK8 assay after 24 h, 48 h and 72 h (p < 0.05). ox-LDL dramatically reduced the viability of NPCs in a time and dose-dependent manner (Fig. 3). The cell number decreased to 99.1% for 10 μg/ml, 89.1% for 20 μg/ml, 75.12% for 30 μg/ml and 65.6% for 40 μg/ml after incubating with ox-LDL for 24 h. The cell number decreased to 90.39% for 10 μg/ml, 68.17% for 20 μg/ml, 55.28% for 30 μg/ml and 47.12% for 40 μg/ml after incubating with ox-LDL for 48 h, and into 73.1% for 10 μg/ml, 46.1% for 20 μg/ml, 35.12% for 30 μg/ml and 25.6% for 40 μg/ml after incubating with ox-LDL for 72 h. We repeated the experiments with cells from three patients and obtained similar results.
Figure 3.
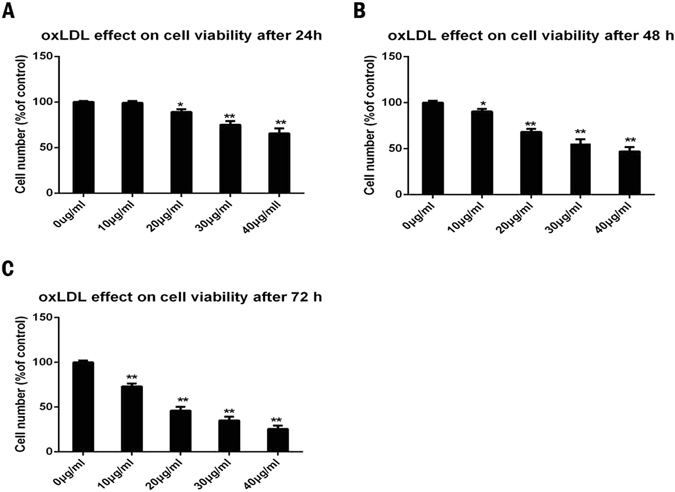
OxLDL induced apoptotic cell death in nucleus pulposus with CCK8 assay. (A,B,C) Effect of increasing concentrations of ox-LDL (0–40 μg/ml) on nucleus pulposus cells viability after 24 h (A), 48 h (B) and 72 h (C). The cells without ox-LDL treatment were served as control. The cell number were decreased into 99.1% for 10 μg/ml, 89.1% for 20 μg/ml, 75.12% for 30 μg/ml, 65.6% for 40 μg/ml after incubating with oxLDL for 24 h. The cell number were decreased into 90.39% for 10 μg/ml, 68.17% for 20 μg/ml, 55.28% for 30 μg/ml, 47.12% for 40 μg/ml after incubating with oxLDL for 48 h and into 73.1% for 10 μg/ml, 46.1% for 20 μg/ml, 35.12% for 30 μg/ml, 25.6% for 40 μg/ml after incubating with oxLDL for 72 h. Cell numbers are expressed as a percentage of the controls. Data are shown as the mean ± SD of three independent experiments. A one-way ANOVA were used for statistical assessments. (*P < 0.05, **P < 0.01).
Enhanced MMP3 expression induced by LOX-1 in ox-LDL-stimulated NPCs
We used ox-LDL (10 μg/ml, 20 μg/ml, 30 μg/ml and 40 μg/ml) to stimulate NPCs for 48 h, and the levels of MMP3 and LOX-1 were determined by western blotting and immunofluorescence. The expression of LOX-1 and MMP3 increased in NPCs in a dose-dependent manner as detected by western blotting and immunofluorescence (p < 0.05). Increased LOX-1 and MMP3 expression was evident after stimulation with 10 μg/ml ox-LDL and persisted at 40 μg/ml (Fig. 4).
Figure 4.

OxLDL can increase the expression of LOX-1 and MMP3. (A,C) Western blotting analysis for the protein expressions of LOX-1 and MMP3. Nucleus pulposus cells were treated with 48 h for different concentration periods (0–40 μg/ml). The LOX-1 and MMP3 was quantified. Data were presented as mean ± SD from three independent experiments. The cells without oxLDL treatment were served as control. (B) The Correlations analysis between LOX-1 and intervertebral disc degeneration score were performed (R2 LOX-1 = 0.92, P < 0.0001; R2 MMP3 = 0.80, P < 0.0001). Data are presented as means ± SD. (D) Representative fluorescent microscopic images of Hoechst/PI double stained nucleus pulposus cells showing that the increasing concentration of oxLDL (only show 0 μg/ml, 20 μg/ml and 40 μg/ml) can promote the expression of LOX-1 and MMP3. Bars = 200μm. (*P < 0.05, **P < 0.01).
To examine whether LOX-1-induced MMP3 expression was stimulated by ox-LDL in NPCs, the expression of MMP3 and LOX-1 in NPCs was investigated by western blotting after stimulation with 40 μg/ml n-LDL, 2 ng/ml IL-1β (Proteintech, USA), 10 uM ascorbic acid (Sigma, USA), 40 μg/ml ox-LDL and pretreatment with 15 μg/ml anti-human LOX-1 monoclonal antibody (TS92) for 24 h followed by stimulation with 40 μg/ml ox-LDL for 48 h. As shown in Fig. 5, IL-1β and ox-LDL induced the expression of LOX-1 and MMP3 (p < 0.05); n-LDL did not affect LOX-1 and MMP3 expression (p > 0.05); LOX-1 and MMP3 expression decreased after addition of ascorbic acid (p < 0.05); and pretreatment of NPCs with TS92 significantly suppressed the increase in expression of LOX-1 and MMP3 induced by ox-LDL.
Figure 5.
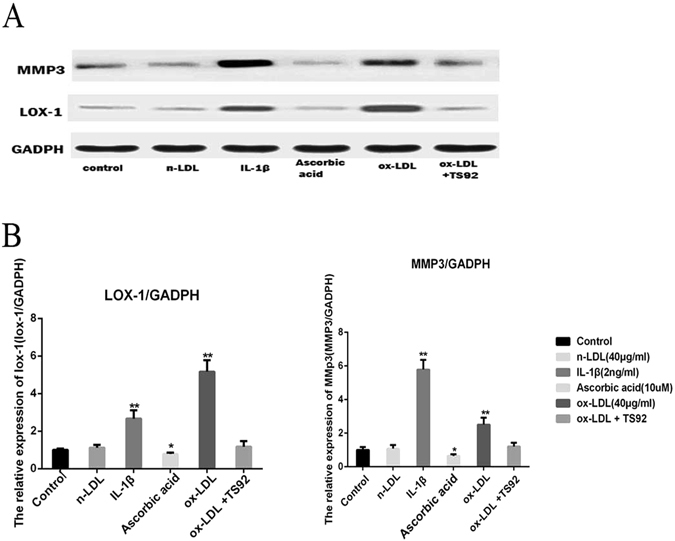
(A) Western blotting analysis for the protein expressions of LOX-1 and MMP3. NPC were respectively incubated with 40 μg/ml n-LDL, 2ng/ml IL-1β, 10 μM ascorbic acid, 40 mg/ml ox-LDL, or preincubated with 15 mg/ml anti-human LOX-1 mAb (TS92) for 24 h than stimulated with ox-LDL for 48 h. (B) The expression of LOX-1 and MMP3 was detected by western blotting. Data were presented as mean ± SD from three independent experiments. The cells without oxLDL treatment were served as control. A one-way ANOVA were used for statistical assessments. (*P < 0.05, **P < 0.01).
Ox-LDL increased the expression of MMP3 by activating the NF-κB signaling pathway
NPCs were treated with 40 μg/ml ox-LDL for different time periods (0, 15, 30 and 45 min). The expression levels of NF-κB-p65 (P65) and phospho-NF-κB-p65 (PP65) were quantified. The expression of PP65/actin increased in NPCs in a time-dependent manner as detected by western blotting. The correlation analyses between the stimulation duration and the expression of P65, PP65 were performed (R2 PP65 = 0.00128, p < 0.9121; R2 P65 = 0.952, p < 0.0001).
NPCs were incubated with 40 μg/ml n-LDL, 2 ng/ml IL-1β, 10 μM ascorbic acid, 40 mg/ml ox-LDL, or preincubated with 15 mg/ml anti-human LOX-1 mAb (TS92) for 24 h and then stimulated with ox-LDL for 48 h. The cells without ox-LDL treatment served as controls. As shown in Fig. 6, IL-1β and ox-LDL induced the expression of PP65 (p < 0.01); n-LDL did not affect PP65 expression (p > 0.05) and pretreatment with TS92 significantly suppressed the increase in expression of PP65 induced by ox-LDL.
Figure 6.
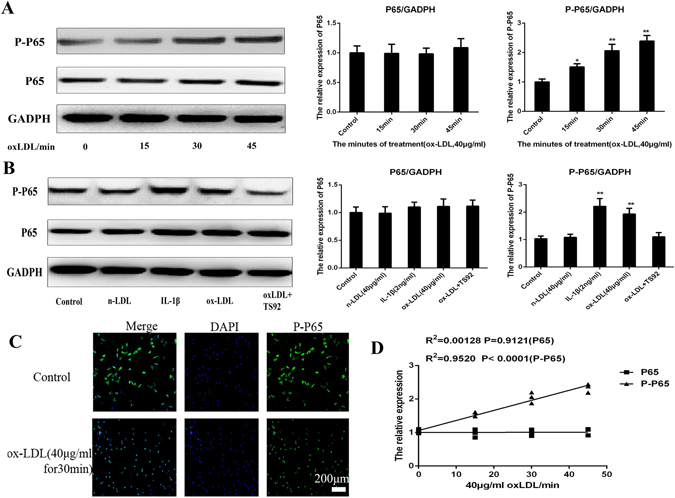
OxLDL can increase the expression of MMP3 by activating the NF-κB signal pathway. (A) Western blotting analysis for the protein expressions of P65 and PP65. Nucleus pulposus cells were treated with 40 μg/ml oxLDL for different periods (0, 15, 30, 45 min). The expressions of P65 and PP65 was quantified. Data were presented as mean ± SD from three independent experiments. (B) Western blotting analysis for the protein expressions of P65 and PP65. NPC were respectively incubated with 40 μg/ml n-LDL, 2 ng/ml IL-1β, 10 μM ascorbic acid, 40 mg/ml ox-LDL, or preincubated with 15 mg/ml anti-human LOX-1 mAb (TS92) for 24 h than stimulated with ox-LDL for 48 h. The cells without oxLDL treatment were served as control. It shows that IL-1β and ox-LDL could improve the expression of PP65 (P < 0.01); n-LDL did not affect PP65 expression (P > 0.05) and pretreatment of NPCs with TS92 significantly suppressed the increase in expression of PP65 by ox-LDL. A one-way ANOVA were used for statistical assessments. (C) Representative fluorescent microscopic images of Hoechst/PI double stained nucleus pulposus cells showing that the 40 μg/ml oxLDL can promote the PP65 enter into the cell nuclear from cytoplasm from 0–45 min (only show 30 min). Bars = 200 μm. (D) The Correlations analysis between the stimulate period and the expression of P65, PP65 were performed (R2PP65 = 0.00128, P < 0.9121; R2P65 = 0.952, P < 0.0001). Data are presented as means ± SD. (*P < 0.05, **P < 0.01).
Immunofluorescence of Hoechst/PI double-stained NPCs showed that 40 μg/ml ox-LDL could promote PP65 entry into the nucleus from the cytoplasm in 0–45 min.
Discussion
The progression of IVDs by serum lipids and atherosclerosis is thought to be associated with the decrease in nutrition caused by the narrowing of arteries, which indirectly leads to IVDD14, 26. The IVD is located at the end of the nutrient chain, which makes it the first structure to suffer from insufficient nutrient supply26–29. However, whether lipids directly affect the structural destabilization of IVD matrix remains unclear. Among the abnormally metabolized lipids, ox-LDL is considered to be the most harmful, since its oxidative modification represents the initial event in atherogenesis15, 30.
Previous studies suggested that ox-LDL/LOX-1 system played an important role in the development of RA and OA18–22, 31–35. Knocking down the LOX-1 gene relieved the progression of OA in mice36. In our study, the mean percentages of LOX-1 and ox-LDL immunopositive cells in IVD samples increased with the IVDD grades, as measured by the degree of morphological degeneration. These results suggested that the ox-LDL/LOX-1 ligand-receptor system may be involved in the pathogenesis and progression of IVDD. Interestingly, we found that the number of LOX-1 and ox-LDL immunopositive cells in IAF showed no significant difference between the non-degenerated and intermediate-degenerated IVDs, which could be due to the small sample size.
To investigate the relationship between circulating LDL, ox-LDL and the presence of ox-LDL in IVDs with the state of degeneration, the serum LDL and ox-LDL levels in the study participants were measured. The serum LDL and ox-LDL was not associated with the state of degeneration and the presence of ox-LDL in IVDs. The small sample size may contribute to these vague results. In fact, ox-LDL is usually found in the blood as lipoproteins or bound to carrier plasma proteins in order to increase their solubility. In this way, lipids become part of a large complex, which restricts their transport through cartilage since size is a determining factor for molecule diffusion30. The large complex of ox-LDL may access the IVDD by the following three ways. Firstly, it is likely that ox-LDL in the systemic circulating blood37 enters the IVDs through the EP and OAF vasculature. Furthermore, with the degeneration of IVDs, numerous large molecules such as hyaluronan and ox-LDL can penetrate the cartilage matrix after IL-1 and other inflammatory mediator treatment38. Secondly, LDL in the systemic circulating blood might enter and get oxidized in the inflamed IVD20. The resultant ox-LDL might then penetrate the IVDs matrix and cells. Thirdly, LDL penetrating the degraded cartilage matrix might be oxidatively modified by ROS produced by IVD cells39, 40 and the resultant ox-LDL could associate with LOX-1 in IVD cells.
Interestingly, we found that LOX-1-positive IVD cells were more widely distributed than ox-LDL-positive cells, which indicates that LOX-1 may be induced by other ligands such as proinflammatory cytokines (TNFα, IL-1β, IFN-γ and CRP), modified lipoproteins (ox-LDL and LPC), high glucose, advanced glycation end-products, growth factors, mechanical stimuli such as cyclic tensile stress, etc15, 18, 31. In the present study, IL-1β and ox-LDL could upregulate the expression of LOX-1. Other related factors that induce the expression of LOX-1 in IVDs are being currently investigated.
Having established a basis for a functional excess of ox-LDL/LOX-1 ligand-receptor system in degenerated and herniated IVDs, we then investigated the role of ox-LDL/LOX-1 ligand-receptor system in the processes that characterize disc degeneration, namely, increased apoptosis and production of MMPs.
We observed that 10–40 μg/ml ox-LDL can reduce the viability of NPCs. Previous studies have shown that ox-LDL induces apoptosis of endothelial cells through the NF-κB pathway, and non-apoptotic cell death in association with Akt activation in articular chondrocytes of rats41. In addition, ox-LDL and cyclic tensile stretch load can induce LOX-1 expression in human chondrocytes, resulting in decreased cell viability and proteoglycan synthesis18, 20, 31.
MMP3 has been suggested to play a very important role in IVDD. Studies on RA and endothelial cells have demonstrated that ox-LDL can enhance the expression of MMP321, 42. A recent study conducted in IVDs of an ApoE knockout mice model also suggested that hyperlipidemia can increase the expression of MMP3 and accelerate IVDD43. This is the first study to show that ox-LDL can enhance the expression of MMP3 in human NPCs through LOX-1 in a dose-dependent manner. To further confirm the inter-relationship between MMP3, ox-LDL, and LOX-1, we incubated NPCs with IL-1β, nLDL, anti-human LOX-1 monoclonal antibody TS92, and ascorbic acid.
The addition of vitamin C to NPCs resulted in a decrease in expression of LOX-1 suggesting that this anti-oxidative nutrient has the ability to stop the cycle of ox-LDL binding to LOX-1. Vitamin C has been shown to play a role in ECM production44, as it is required for the synthesis of type II collagen, the most abundant protein in IVD, which moderately stimulates the synthesis of aggrecan. Its absence has been associated with reduced mechanical resilience of collagen fibrils and increased turnover rates45. Although vitamin C is known to facilitate repair of IVDD, the specific mechanism remains unclear. Suppressing the production of LOX-1 and MMP3 induced by ox-LDL may be an important mechanism.
In this study, IL-1β and ox-LDL could improve the expression of PP65, an important transcription factor in the NF-κB signaling pathway (p < 0.01). Meanwhile, immunofluorescence study showed that ox-LDL can promote PP65 entry into the nucleus from cytoplasm in 0–45 min. These results suggested that NF-κB signaling pathway plays a critical role in the expression of MMP3 induced by ox-LDL in NPCs. The effect of pretreatment with anti-human LOX-1 monoclonal antibody TS92 on NPCs also confirmed this result. NF-κB controls the expression of numerous proinflammatory molecules, including cytokines tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), chemokines (interleukin-8, IL-8), and adhesion molecules (intercellular adhesion molecule-1 and vascular cell adhesion molecule-1)19. In chronic cartilage-related degenerative diseases such as OA, the negative regulatory loop with its inhibitor, IkB-α is overwhelmed by the positive loop involving NF-κB activation by TNF-α, IL-1β, and NF-κB-dependent expression of these two major proinflammatory cytokines. Indeed, IVDD is associated with persistent in situ NF-κB activity46. In the context of chronic cartilage-related degenerative disease, induction of NF-κB activity in NPCs would facilitate degradation of the ECM of IVDs. In conclusion, ox-LDL binding to LOX-1 in human NPCs activated NF-κB and increased MMP3 expression.
These observations support the hypothesis that hypercholesterolemia is a risk factor of arthritis, and lipid peroxidation products such as ox-LDL are involved in cartilage matrix degradation in OA and IVDD.
However, there were some limitations in this study. Firstly, the sample size was relatively small. Secondly, some tissues were collected from herniated discs. The changes and cellular processes associated with degeneration in early stages or within the intact disc are somewhat distinct from those in herniated discs because the herniated disc tissues have been exposed to numerous factors outside the IVD environment (i.e., vascularity, inflammation) for variable periods of time.
Conclusion
This is the first study to show that colocalization of IVDs is associated with the presence of ox-LDL and LOX-1 expression. In addition, ox-LDL can up-regulate MMP3 production induced by LOX-1 in human NPCs. The in vivo and in vitro studies indicated that the ox-LDL/LOX-1 ligand-receptor system may be involved in the pathogenesis and progression of IVD degeneration or herniation. LOX-1 could be a promising target in the treatment of IVD degeneration or herniation.
Methods
Tissue samples
Specimens were derived from surgical disc procedures performed on patients with fresh herniated discs, degenerative disc disease, and lumbar burst fracture. All the methods were performed in accordance with relevant guidelines and regulations. All the participants consented to participate in this study. All IVDs consisted of full-thickness wedges of 120° of anteriorly removed arc. Surgical specimens were immediately transported to the laboratory in a sterile tissue culture medium (for cell cultures) or placed in 10% neutral buffered formalin (for histological studies).
Primary cultures of human nucleus pulposus cells
The human NPCs were dissected from surgical disc procedures performed on individuals with lumbar burst fracture (without degenerated disc, patient No. 1, 3 and 4 in Table 1). NPCs were isolated and cultured as previously described47. After isolation, NPCs were re-suspended in Dulbecco’s modified Eagle’s medium/F-12 (HyClone, USA) containing 10% fetal bovine serum (Gibco, USA) and 50 units/ml of penicillin and streptomycin (HyClone, USA), and then incubated at 37 °C in a humidified atmosphere with 95% air and 5% CO2. The confluent cells were detached by trypsinization, seeded into 25 cm2 cell culture box in complete culture medium (DMEM supplemented with 10% FBS, 100 mg/ml streptomycin and 50 U/ml penicillin) in a 37 °C and 5% CO2 environment. The medium was changed every three days. At 90% confluency, the second passage human NPCs were cultured under serum-free conditions for 24 h for subsequent experiments.
Treatment of tissue specimens
Specimens were fixed in 10% neutral-buffered formalin for 24 hours. As some specimens contained bone, all the samples were decalcified in ethylenediaminetetraacetic acid (EDTA) (Beyotime) for 14 days48. The specimens were embedded in paraffin. The slides were deparaffinized in xylene and hydrated through graded alcohols to distilled water. Slices from the tissue blocks were subjected to H&E staining to score the degree of morphological degeneration according to previously published criteria49. Based on this scoring, discs were selected to represent a range of scores from non-degenerated (0 to 3) to the most severe level of degeneration (12). A score of 0–3 represents a histologically normal disc, 4–8 indicates intermediate degeneration and 9–12 suggests severe degeneration.
Detection of ox-LDL and LOX-1 proteins by immunohistochemistry
In the quantitative determination of serum ox-LDL and LDL levels, the samples were analyzed using a specific enzyme-linked immunosorbent assay (Huamei, Wuhan, China) according to the manufacturer’s instructions. Ox-LDL and LDL concentrations were determined in duplicate.
Immunohistochemistry (IHC) was performed as previously described50. Endogenous peroxidase was blocked using 3% H2O2 in methanol. Slides were incubated in 10% serum (of the species in which the secondary antibody was generated) and then incubated with anti human ox-LDL (1:100, Biorbyt, USA) and anti human LOX-1 (1:100, Abcam, USA) at 4 °C overnight. Negative controls were treated with normal rabbit IgG (Sigma, USA) under the same conditions. After washing, the slides were incubated with the secondary antibody for 40 minutes at room temperature followed by addition of avidin–biotin–peroxidase complex. The samples were visualized with 0.025% diaminobenzidine solution and counterstained with Mayer’s hematoxylin solution.
Image analysis
All slides were visualized using a Leica (Leica, Cambridge, UK) RMDB research microscope. The images were captured using a digital camera and analyzed using the Bioquant Nova image analysis system (Bioquant, Nashville, TN, USA). Within each area, 200 cells were counted and the number of immunopositive cells (brown-stained cells), expressed as a proportion of total cells, were calculated for disc sections grouped with the scores 0–3, 4–8 and 9–12. Data was presented on graphs as means ± SD.
Preparation of native LDL (nLDL) and ox-LDL
Human nLDL was isolated from freshly donated plasma by sequential ultracentrifugation, as previously described17.
Preparation of ox-LDL
Native LDL (200 μg/ml) was oxidized by exposure to CuSO4 (5 μmol/l free Cu2+) in phosphate-buffered saline at 37 °C for 20 h. Oxidation was terminated by refrigeration. The oxidative state was confirmed by measuring the quantity of thiobarbituric acid-reactive substances (TBARS) (nmol/mg protein). Agarose gel electrophoresis showed increased electrophoretic mobility and minimal aggregation of ox-LDL particles. The fluorescent carbocyanine dye DiI was conjugated with ox-LDL according to the manufacturer’s instructions17.
Cell counting kit assay (CCK-8)
Human NPCs were seeded in 96-well plates and incubated in the presence of increasing concentrations of ox-LDL (10–40 μg/ml) for 24 h, 48 h, and 72 h. Cell survival rate was determined using the established cell counting kit (CCK-8) assay. Each well was incubated with the CCK-8 solution (Sigma) for 4 h, and the absorbance was measured at 590 nm using a spectrophotometer. The number of viable cells per well in the presence of ox-LDL was compared with untreated control cells. Wells containing only medium served as blank controls.
Western Blot
The expression levels of proteins were determined by western blot analysis of total protein extracts from NPCs. Cell samples were lysed in RIPA buffer, sonicated, and protein concentrations were calculated using the BCA protein assay kit. Proteins were loaded onto 8% SDS-PAGE gels and transferred to PVDF membranes (Millipore, Billerica, MA, USA). After blocking for 1 hour, the membranes were incubated with primary antibodies overnight at 4 °C. Primary antibodies specific to LOX-1 (1:1000, Abcam), MMP3 (1:1000, Cell Signaling Technology) and β-actin (1:1000, Cell Signaling Technology) were used. Negative controls were performed with normal rabbit IgG (Sigma) under the same conditions. After washing with Tris Buffered Saline with Tween (TBST) for three times, the membranes were incubated with the respective secondary antibodies. Then the bands were detected with ECL plus reagent (Millipore) by the ChemiDoc™ XRS + System (BIO-RAD, USA). Relative expression levels of proteins were determined by quantitative densitometric analysis using image analysis software (Image lab, Bio-Rad, USA).
Immunofluorescence
The cells were fixed in 10% neutral-buffered formalin for 15 min. After perforation and sealing, the cells were incubated with anti-LOX-1 antibody (1:200, Abcam, USA) and anti-MMP3 antibody (1:200, Cell Signaling Technology), overnight at 4 °C, washed three times for 5 min each, and then incubated with secondary anti-rabbit antibody conjugated with Alexa Fluor 488 for 1 h at room temperature. Negative controls were treated with normal rabbit IgG (Sigma, USA) under the same conditions. Images were obtained with a Fluo view confocal microscope and prepared with Photoshop software.
Statistical analysis
Results are presented as means ± SD. GraphPad Prism 6.02 software was used to perform the statistical analysis. The combined results of the individual experiments were used to prepare the Figures, and data were presented as mean ± 95% CI (lower limit, upper limit) for the three experiments. Analyses of variance, Scheffe’s test, and one-way ANOVA were used for statistical assessments. The correlation analyses were performed. p < 0.05 was considered to be statistically significant, p < 0.01 was considered to be extremely statistically significant.
Ethical Approval
Experimental study of disc specimens were approved prospectively by the authors’ human subjects Institutional Review Board. (Institutional Review Board of shanghai east hospital).
Informed consent: All of the participants consented to participate in this study.
Consent for publication
Not applicable Availability of supporting data
Electronic supplementary material
Acknowledgements
This paper was supported by grants from National Natural Science Foundation of China (81672199), National Natural Science Foundation of China (81371994) Desheng Wu; National Science Foundation of Jiangxi Province, China (20171BAB205034), National Health and Family Planning Commission Foundation of Shanghai, China (201640063) Li-Jun Li; National Natural Science Foundation of China (81572181), Young Excellent Talents in Pudong New Area health system (PWRq2016-27), Shanjing-Wang.
Author Contributions
Xinhua Li contributed to made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data; Xuejun Wang, Jun Tan, Zhouyang Hu, Yingchao Han, ZhanYing Wei, Xiao bo He, Zhaoxiong Chen, Haoxi Li, Xiaoming Liu, ZhiYao Yong, Shanjing Wang and Cong Li involved in drafting the manuscript or revising it critically for important intellectual content; Desheng Wu, Lijun Li, Guixin Sun given final approval of the version to be published. Each author should have participated sufficiently in the work to take public responsibility for appropriate portions of the content and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All authors read and approved the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-07780-x
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Guixin Sun, Email: sunguixing@sina.com.
Desheng Wu, Email: eastspinesci@163.com.
Lijun Li, Email: liliju@163.com.
References
- 1.Andersson GB. Epidemiological features of chronic low-back pain. Lancet. 1999;354:581–5. doi: 10.1016/S0140-6736(99)01312-4. [DOI] [PubMed] [Google Scholar]
- 2.Luoma K, et al. Low back pain in relation to lumbar disc degeneration. Spine (Phila Pa 1976). 2000;25:487–92. doi: 10.1097/00007632-200002150-00016. [DOI] [PubMed] [Google Scholar]
- 3.Juniper M, Le TK, Mladsi D. The epidemiology, economic burden and pharmacological treatment of chronic low back pain in France, Germany, Italy, Spain and the UK: a literature-based review. Expert Opin Pharmacother. 2009;10:2581–92. doi: 10.1517/14656560903304063. [DOI] [PubMed] [Google Scholar]
- 4.Wade KR, Robertson PA, Thambyah A, Broom ND. How healthy discs herniate: a biomechanical and microstructural study investigating the combined effects of compression rate and flexion. Spine (Phila Pa 1976). 2014;39:1018–28. doi: 10.1097/BRS.0000000000000262. [DOI] [PubMed] [Google Scholar]
- 5.Hussein AI, Jackman TM, Morgan SR, Barest GD, Morgan EF. The intravertebral distribution of bone density: correspondence to intervertebral disc health and implications for vertebral strength. Osteoporos Int. 2013;24:3021–30. doi: 10.1007/s00198-013-2417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Modic MT, Ross JS. Lumbar degenerative disk disease. Radiology. 2007;245:43–61. doi: 10.1148/radiol.2451051706. [DOI] [PubMed] [Google Scholar]
- 7.Hirayama J, et al. Relationship between low-back pain, muscle spasm and pressure pain thresholds in patients with lumbar disc herniation. European spine journal: official publication of the European Spine Society, the European Spinal Deformity Society & the European Section of the Cervical Spine Research Society. 2006;15:41–7. doi: 10.1007/s00586-004-0813-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rutsch F, Terkeltaub R. Deficiencies of physiologic calcification inhibitors and low-grade inflammation in arterial calcification: lessons for cartilage calcification. Joint, bone, spine: revue du rhumatisme. 2005;72:110–8. doi: 10.1016/j.jbspin.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 9.Mehta JL, Chen J, Hermonat PL, Romeo F, Novelli G. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): a critical player in the development of atherosclerosis and related disorders. Cardiovascular research. 2006;69:36–45. doi: 10.1016/j.cardiores.2005.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Kauppila LI, Penttila A, Karhunen PJ, Lalu K, Hannikainen P. Lumbar disc degeneration and atherosclerosis of the abdominal aorta. Spine (Phila Pa 1976). 1994;19:923–9. doi: 10.1097/00007632-199404150-00010. [DOI] [PubMed] [Google Scholar]
- 11.Kauppila, L et al. Disc degeneration/back pain and calcification of the abdominal aorta. A 25-year follow-up study in Framingham. Spine (Phila Pa 1976). 22, 1642–7; discussion 8–9 (1997). [DOI] [PubMed]
- 12.Kurunlahti M, Tervonen O, Vanharanta H, Ilkko E, Suramo I. Association of atherosclerosis with low back pain and the degree of disc degeneration. Spine (Phila Pa 1976). 1999;24:2080–4. doi: 10.1097/00007632-199910150-00003. [DOI] [PubMed] [Google Scholar]
- 13.Turgut AT, Sonmez I, Cakit BD, Kosar P, Kosar U. Pineal gland calcification, lumbar intervertebral disc degeneration and abdominal aorta calcifying atherosclerosis correlate in low back pain subjects: A cross-sectional observational CT study. Pathophysiology. 2008;15:31–9. doi: 10.1016/j.pathophys.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Kauppila LI. Atherosclerosis and disc degeneration/low-back pain–a systematic review. European journal of vascular and endovascular surgery: the official journal of the European Society for Vascular Surgery. 2009;37:661–70. doi: 10.1016/j.ejvs.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 15.Pirillo A, Norata, G. D. & Catapano, A. LOX-1, OxLDL and atherosclerosis. Mediators Inflamm. 152786 (2013). [DOI] [PMC free article] [PubMed]
- 16.Kataoka H, et al. Expression of lectinlike oxidized low-density lipoprotein receptor-1 in human atherosclerotic lesions. Circulation. 1999;99:3110–7. doi: 10.1161/01.CIR.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 17.Sawamura T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386:73–7. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 18.Simopoulou T, Malizos KN, Tsezou A. Lectin-like oxidized low density lipoprotein receptor 1 (LOX-1) expression in human articular chondrocytes. Clin Exp Rheumatol. 2007;25:605–12. [PubMed] [Google Scholar]
- 19.Nishimura S, et al. Oxidized low-density lipoprotein (ox-LDL) binding to lectin-like ox-LDL receptor-1 (LOX-1) in cultured bovine articular chondrocytes increases production of intracellular reactive oxygen species (ROS) resulting in the activation of NF-kappaB. Osteoarthritis Cartilage. 2004;12:568–76. doi: 10.1016/j.joca.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 20.Akagi M, et al. Possible involvement of the oxidized low-density lipoprotein/lectin-like oxidized low-density lipoprotein receptor-1 system in pathogenesis and progression of human osteoarthritis. Osteoarthritis Cartilage. 2007;15:281–90. doi: 10.1016/j.joca.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 21.Kakinuma T, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates matrix metalloproteinase 3 synthesis enhanced by oxidized low-density lipoprotein in rheumatoid arthritis cartilage. Arthritis and rheumatism. 2004;50:3495–503. doi: 10.1002/art.20581. [DOI] [PubMed] [Google Scholar]
- 22.Akagi M, et al. Oxidized LDL binding to LOX-1 enhances MCP-1 expression in cultured human articular chondrocytes. Osteoarthritis Cartilage. 2009;17:271–5. doi: 10.1016/j.joca.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 23.Song YQ, et al. Lumbar disc degeneration is linked to a carbohydrate sulfotransferase 3 variant. The Journal of clinical investigation. 2013;123:4909–17. doi: 10.1172/JCI69277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jing W, Jiang W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells. Cell Prolif. 2015;48:284–92. doi: 10.1111/cpr.12176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aktas E, Sener E, Gocun PU. Mechanically induced experimental knee osteoarthritis benefits from anti-inflammatory and immunomodulatory properties of simvastatin via inhibition of matrix metalloproteinase-3. Journal of orthopaedics and traumatology: official journal of the Italian Society of Orthopaedics and Traumatology. 2011;121:45–51. doi: 10.1007/s10195-011-0154-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, et al. Serum lipid levels are positively correlated with lumbar disc herniation-a retrospective study of 790 Chinese patients. Lipids in health and disease. 2016;15:80. doi: 10.1186/s12944-016-0248-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Urban JP, Roberts S. Degeneration of the intervertebral disc. Arthritis Res Ther. 2003;5:120–30. doi: 10.1186/ar629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heuch I, Hagen K, Zwart JA. Do abnormal serum lipid levels increase the risk of chronic low back pain? The Nord-Trondelag Health Study. PloS one. 2014;9:e108227. doi: 10.1371/journal.pone.0108227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ha IH, Lee J, Kim MR, Kim H, Shin JS. The association between the history of cardiovascular diseases and chronic low back pain in South Koreans: a cross-sectional study. PloS one. 2014;9:e93671. doi: 10.1371/journal.pone.0093671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Villalvilla A, Gomez R, Largo R, Herrero-Beaumont G. Lipid transport and metabolism in healthy and osteoarthritic cartilage. Int J Mol Sci. 2013;14:20793–808. doi: 10.3390/ijms141020793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akagi M, et al. Cyclic tensile stretch load and oxidized low density lipoprotein synergistically induce lectin-like oxidized ldl receptor-1 in cultured bovine chondrocytes, resulting in decreased cell viability and proteoglycan synthesis. J Orthop Res. 2006;24:1782–90. doi: 10.1002/jor.20211. [DOI] [PubMed] [Google Scholar]
- 32.Nakagawa T, et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates leukocyte infiltration and articular cartilage destruction in rat zymosan-induced arthritis. Arthritis and rheumatism. 2002;46:2486–94. doi: 10.1002/art.10504. [DOI] [PubMed] [Google Scholar]
- 33.Kanata S, et al. Oxidized LDL binding to LOX-1 upregulates VEGF expression in cultured bovine chondrocytes through activation of PPAR-gamma. Biochemical and biophysical research communications. 2006;348:1003–10. doi: 10.1016/j.bbrc.2006.07.133. [DOI] [PubMed] [Google Scholar]
- 34.Kishimoto H, et al. Induction of hypertrophic chondrocyte-like phenotypes by oxidized LDL in cultured bovine articular chondrocytes through increase in oxidative stress. Osteoarthritis Cartilage. 2010;18:1284–90. doi: 10.1016/j.joca.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 35.Ishikawa M, et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis and rheumatism. 2012;64:1024–34. doi: 10.1002/art.33452. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto, K. et al. Lectin-like oxidized low density lipoprotein receptor 1-deficient mice show resistance to instability-induced osteoarthritis. Scandinavian journal of rheumatology. 1–11 (2016). [DOI] [PubMed]
- 37.Ehara S, et al. Elevated levels of oxidized low density lipoprotein show a positive relationship with the severity of acute coronary syndromes. Circulation. 2001;103:1955–60. doi: 10.1161/01.CIR.103.15.1955. [DOI] [PubMed] [Google Scholar]
- 38.Hadjipavlou AG, Tzermiadianos MN, Bogduk N, Zindrick MR. The pathophysiology of disc degeneration: a critical review. J Bone Joint Surg Br. 2008;90:261–70. doi: 10.1302/0301-620X.90B10.20910. [DOI] [PubMed] [Google Scholar]
- 39.Suzuki S, et al. Excessive reactive oxygen species are therapeutic targets for intervertebral disc degeneration. Arthritis Res Ther. 2015;17:316. doi: 10.1186/s13075-015-0834-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen JW, et al. The responses of autophagy and apoptosis to oxidative stress in nucleus pulposus cells: implications for disc degeneration. Cell Physiol Biochem. 2014;34:1175–89. doi: 10.1159/000366330. [DOI] [PubMed] [Google Scholar]
- 41.Nakagawa T, et al. LOX-1 expressed in cultured rat chondrocytes mediates oxidized LDL-induced cell death-possible role of dephosphorylation of Akt. Biochemical and biophysical research communications. 2002;299:91–7. doi: 10.1016/S0006-291X(02)02597-4. [DOI] [PubMed] [Google Scholar]
- 42.Li D, et al. LOX-1 mediates oxidized low-density lipoprotein-induced expression of matrix metalloproteinases in human coronary artery endothelial cells. Circulation. 2003;107:612–7. doi: 10.1161/01.CIR.0000047276.52039.FB. [DOI] [PubMed] [Google Scholar]
- 43.Zhang D, et al. Intervertebral disc degeneration and ectopic bone formation in apolipoprotein E knockout mice. J Orthop Res. 2013;31:210–7. doi: 10.1002/jor.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chothe PP, et al. Sodium-coupled vitamin C transporter (SVCT2): expression, function & regulation in intervertebral disc cells. Spine J. 2013;13:549–57. doi: 10.1016/j.spinee.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 45.Smith VH. Vitamin C deficiency is an under-diagnosed contributor to degenerative disc disease in the elderly. Med Hypotheses. 2010;74:695–7. doi: 10.1016/j.mehy.2009.10.041. [DOI] [PubMed] [Google Scholar]
- 46.Liu, Z. et al. SDF-1/CXCR4 axis induces apoptosis of human degenerative nucleus pulposus cells via the NF-κB pathway. Mol Med Rep. 14, 783–9 (2016). [DOI] [PMC free article] [PubMed]
- 47.Li Z, et al. Leptin induces cyclin D1 expression and proliferation of human nucleus pulposus cells via JAK/STAT, PI3K/Akt and MEK/ERK pathways. PloS one. 2012;7:e53176. doi: 10.1371/journal.pone.0053176. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 48.Walsh L, Freemont AJ, Hoyland JA. The effect of tissue decalcification on mRNA retention within bone for in-situ hybridization studies. International journal of experimental pathology. 1993;74:237–41. [PMC free article] [PubMed] [Google Scholar]
- 49.Sive JI, et al. Expression of chondrocyte markers by cells of normal and degenerate intervertebral discs. Mol Pathol. 2002;55:91–7. doi: 10.1136/mp.55.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. The Journal of pathology. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


