Abstract
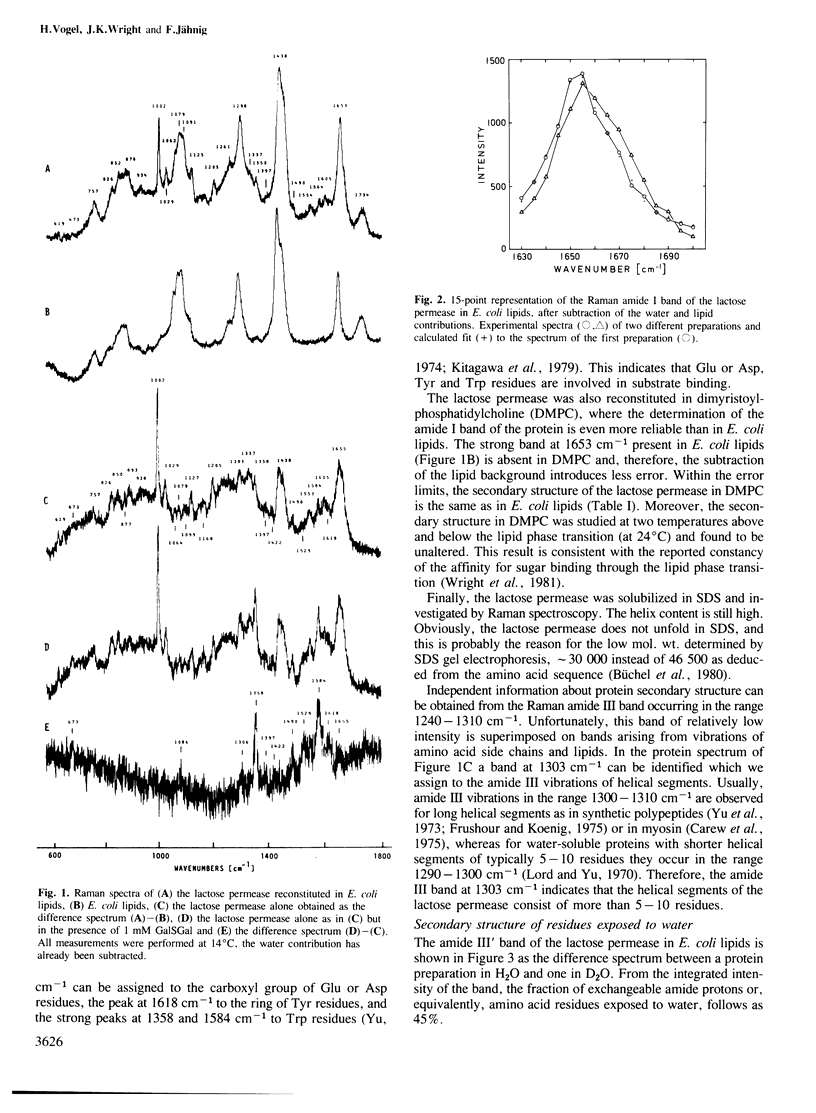
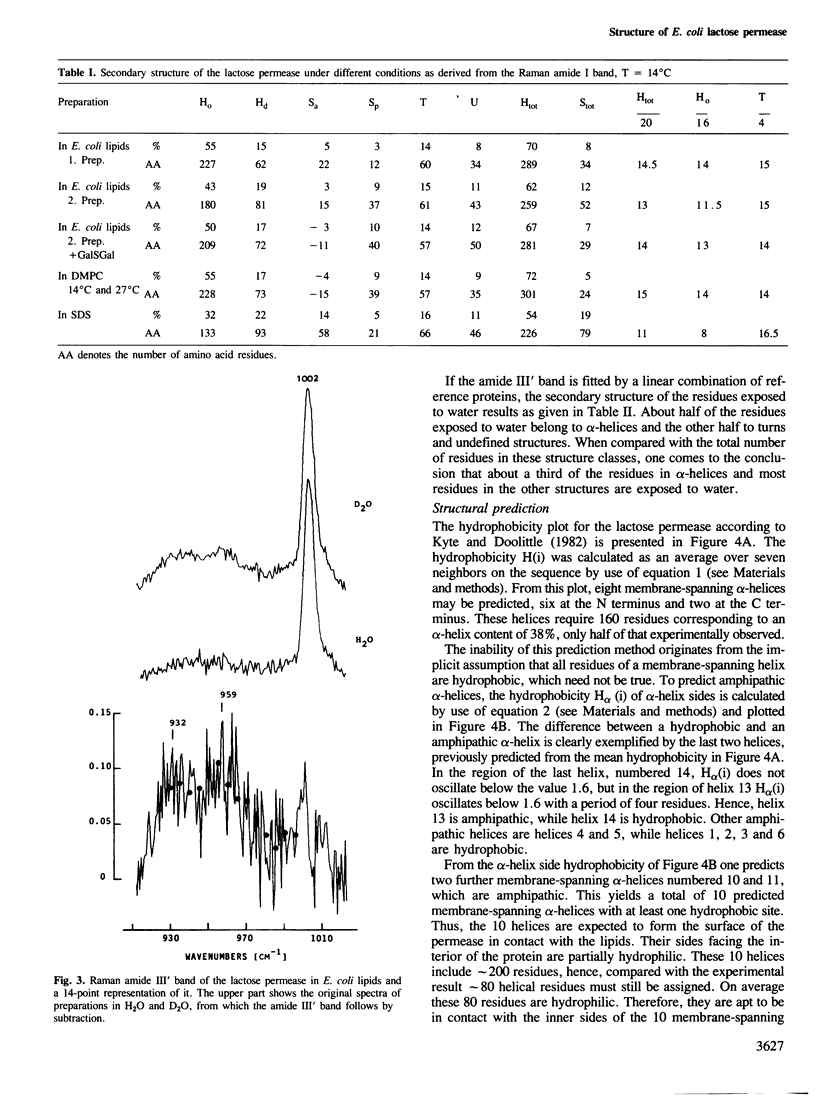
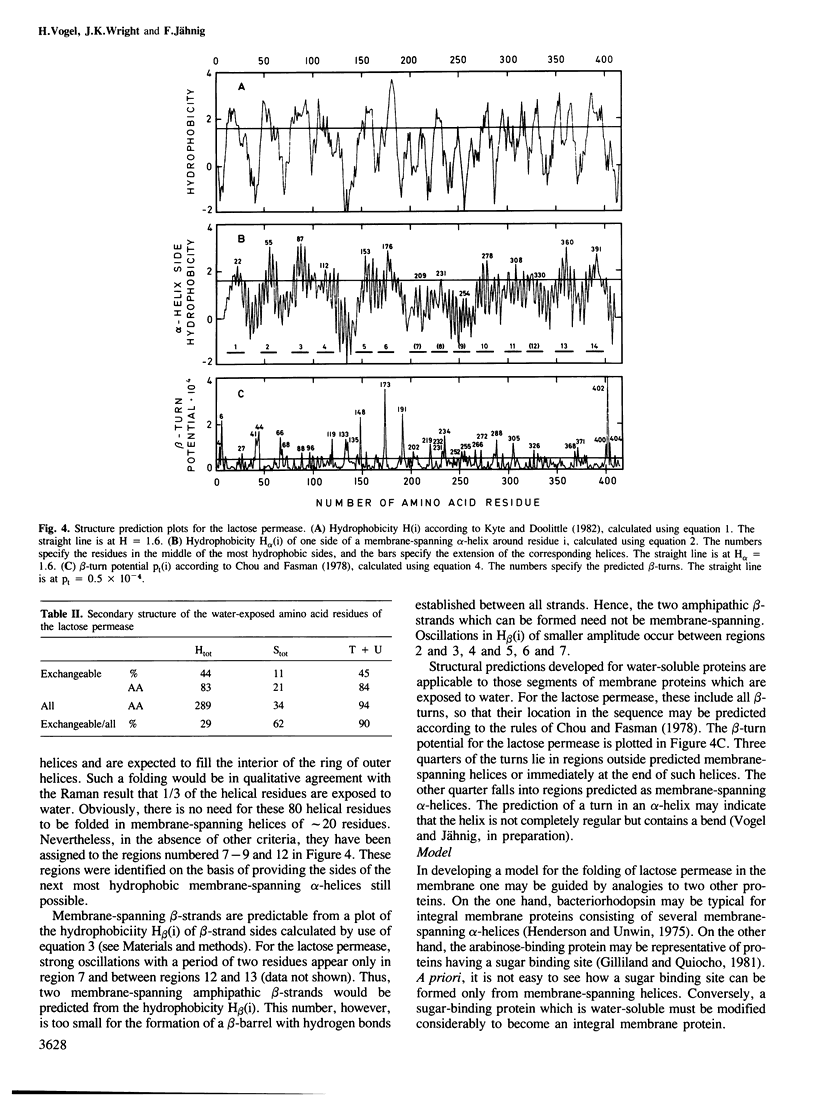
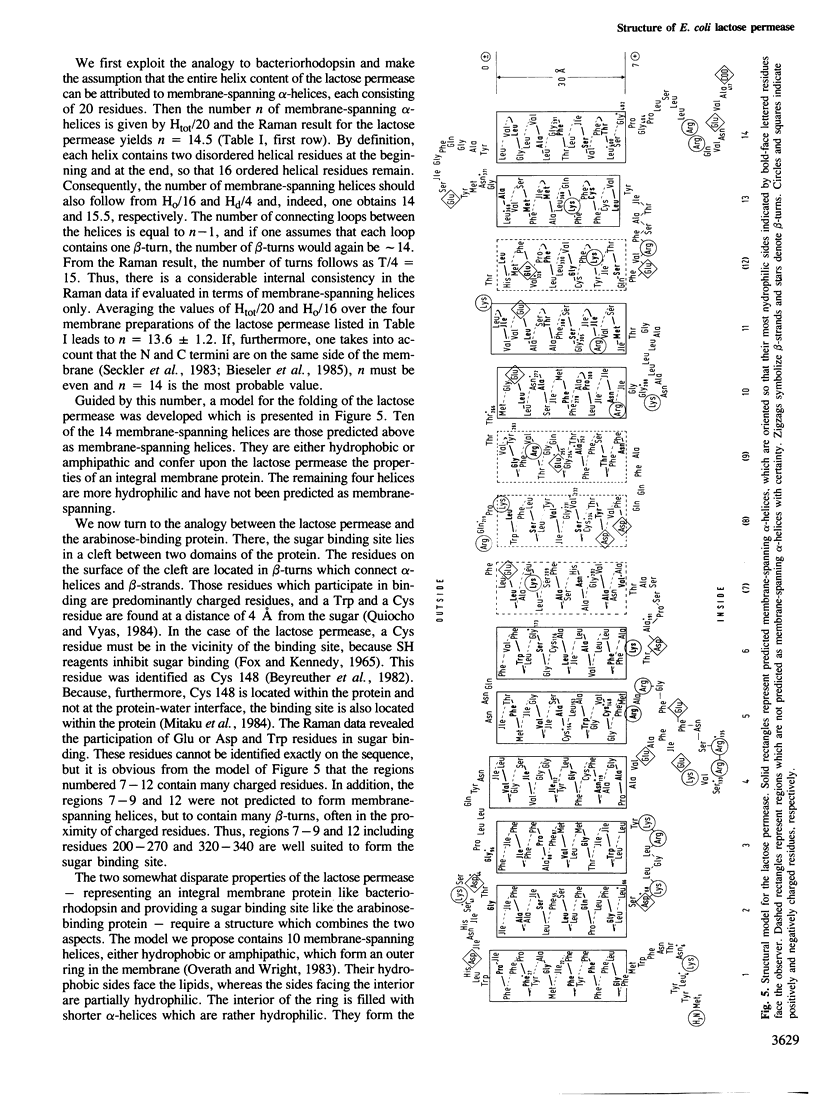
The secondary structure of the lactose permease of Escherichia coli reconstituted in lipid membranes was determined by Raman spectroscopy. The alpha-helix content is approximately 70%, the beta-strand content below 10% and beta-turns contribute 15%. About 1/3 of the residues in alpha-helices and most other residues are exposed to water. Employing a method for structural prediction which accounts for amphipathic helices, 10 membrane-spanning helices are predicted which are either hydrophobic or amphipathic. They are expected to form an outer ring of helices in the membrane. The interior of the ring would be made of residues which are predominantly hydrophilic and, evoking the analogy to sugar-binding proteins, suited to provide the sugar binding site.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Büchel D. E., Gronenborn B., Müller-Hill B. Sequence of the lactose permease gene. Nature. 1980 Feb 7;283(5747):541–545. doi: 10.1038/283541a0. [DOI] [PubMed] [Google Scholar]
- Carew E. B., Asher I. M., Stanley H. E. Laser raman spectroscopy--new probe of myosin substructure. Science. 1975 May 30;188(4191):933–936. doi: 10.1126/science.1138362. [DOI] [PubMed] [Google Scholar]
- Chen C. C., Wilson T. H. The phospholipid requirement for activity of the lactose carrier of Escherichia coli. J Biol Chem. 1984 Aug 25;259(16):10150–10158. [PubMed] [Google Scholar]
- Chou P. Y., Fasman G. D. Empirical predictions of protein conformation. Annu Rev Biochem. 1978;47:251–276. doi: 10.1146/annurev.bi.47.070178.001343. [DOI] [PubMed] [Google Scholar]
- Costello M. J., Viitanen P., Carrasco N., Foster D. L., Kaback H. R. Morphology of proteoliposomes reconstituted with purified lac carrier protein from Escherichia coli. J Biol Chem. 1984 Dec 25;259(24):15579–15586. [PubMed] [Google Scholar]
- Ehring R., Beyreuther K., Wright J. K., Overath P. In vitro and in vivo products of E. coli lactose permease gene are identical. Nature. 1980 Feb 7;283(5747):537–540. doi: 10.1038/283537a0. [DOI] [PubMed] [Google Scholar]
- Eisenberg D., Weiss R. M., Terwilliger T. C. The hydrophobic moment detects periodicity in protein hydrophobicity. Proc Natl Acad Sci U S A. 1984 Jan;81(1):140–144. doi: 10.1073/pnas.81.1.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster D. L., Boublik M., Kaback H. R. Structure of the lac carrier protein of Escherichia coli. J Biol Chem. 1983 Jan 10;258(1):31–34. [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliland G. L., Quiocho F. A. Structure of the L-arabinose-binding protein from Escherichia coli at 2.4 A resolution. J Mol Biol. 1981 Mar 5;146(3):341–362. doi: 10.1016/0022-2836(81)90392-2. [DOI] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kaback H. R. Topology of the lac carrier protein in the membrane of Escherichia coli. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3322–3326. doi: 10.1073/pnas.80.11.3322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldkorn T., Rimon G., Kempner E. S., Kaback H. R. Functional molecular weight of the lac carrier protein from Escherichia coli as studied by radiation inactivation analysis. Proc Natl Acad Sci U S A. 1984 Feb;81(4):1021–1025. doi: 10.1073/pnas.81.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Herzlinger D., Viitanen P., Carrasco N., Kaback H. R. Monoclonal antibodies against the lac carrier protein from Escherichia coli. 2. Binding studies with membrane vesicles and proteoliposomes reconstituted with purified lac carrier protein. Biochemistry. 1984 Jul 31;23(16):3688–3693. doi: 10.1021/bi00311a018. [DOI] [PubMed] [Google Scholar]
- Kaback H. R. The lac carrier protein in Escherichia coli. J Membr Biol. 1983;76(2):95–112. doi: 10.1007/BF02000610. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Lord R. C., Yu N. T. Laser-excited Raman spectroscopy of biomolecules. I. Native lysozyme and its constituent amino acids. J Mol Biol. 1970 Jun 14;50(2):509–524. doi: 10.1016/0022-2836(70)90208-1. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Wright J. K., Best L., Jähnig F. Localization of the galactoside binding site in the lactose carrier of Escherichia coli. Biochim Biophys Acta. 1984 Oct 3;776(2):247–258. doi: 10.1016/0005-2736(84)90214-1. [DOI] [PubMed] [Google Scholar]
- Nelson S. O., Wright J. K., Postma P. W. The mechanism of inducer exclusion. Direct interaction between purified III of the phosphoenolpyruvate:sugar phosphotransferase system and the lactose carrier of Escherichia coli. EMBO J. 1983;2(5):715–720. doi: 10.1002/j.1460-2075.1983.tb01490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiocho F. A., Vyas N. K. Novel stereospecificity of the L-arabinose-binding protein. Nature. 1984 Aug 2;310(5976):381–386. doi: 10.1038/310381a0. [DOI] [PubMed] [Google Scholar]
- Seckler R., Wright J. K., Overath P. Peptide-specific antibody locates the COOH terminus of the lactose carrier of Escherichia coli on the cytoplasmic side of the plasma membrane. J Biol Chem. 1983 Sep 25;258(18):10817–10820. [PubMed] [Google Scholar]
- Thomas G. J., Jr, Prescott B., Day L. A. Structure similarity, difference and variability in the filamentous viruses fd, If1, IKe, Pf1 and Xf. Investigation by laser Raman spectroscopy. J Mol Biol. 1983 Apr 5;165(2):321–356. doi: 10.1016/s0022-2836(83)80260-5. [DOI] [PubMed] [Google Scholar]
- Williams R. W., Dunker A. K. Determination of the secondary structure of proteins from the amide I band of the laser Raman spectrum. J Mol Biol. 1981 Nov 15;152(4):783–813. doi: 10.1016/0022-2836(81)90127-3. [DOI] [PubMed] [Google Scholar]
- Williams R. W. Estimation of protein secondary structure from the laser Raman amide I spectrum. J Mol Biol. 1983 Jun 5;166(4):581–603. doi: 10.1016/s0022-2836(83)80285-x. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Overath P. Purification of the lactose:H+ carrier of Escherichia coli and characterization of galactoside binding and transport. Eur J Biochem. 1984 Feb 1;138(3):497–508. doi: 10.1111/j.1432-1033.1984.tb07944.x. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Riede I., Overath P. Lactose carrier protein of Escherichia coli: interaction with galactosides and protons. Biochemistry. 1981 Oct 27;20(22):6404–6415. doi: 10.1021/bi00525a019. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Teather R. M., Overath P. Lactose permease of Escherichia coli. Methods Enzymol. 1983;97:158–175. doi: 10.1016/0076-6879(83)97130-6. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Weigel U., Lustig A., Bocklage H., Mieschendahl M., Müller-Hill B., Overath P. Does the lactose carrier of Escherichia coli function as a monomer? FEBS Lett. 1983 Oct 3;162(1):11–15. doi: 10.1016/0014-5793(83)81039-4. [DOI] [PubMed] [Google Scholar]
- Yu N. T. Comparison of protein structure in crystals, in lyophilized state, and in solution by laser Raman scattering. 3. Alpha-Lactalbumin. J Am Chem Soc. 1974 Jul 10;96(14):4664–4668. doi: 10.1021/ja00821a049. [DOI] [PubMed] [Google Scholar]
- Yu T. J., Lippert J. L., Peticolas W. L. Laser Raman studies of conformational variations of poly-L-lysine. Biopolymers. 1973;12(9):2161–2175. doi: 10.1002/bip.1973.360120919. [DOI] [PubMed] [Google Scholar]


