Abstract
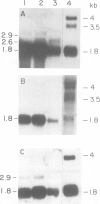
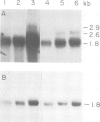
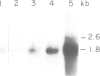
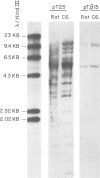
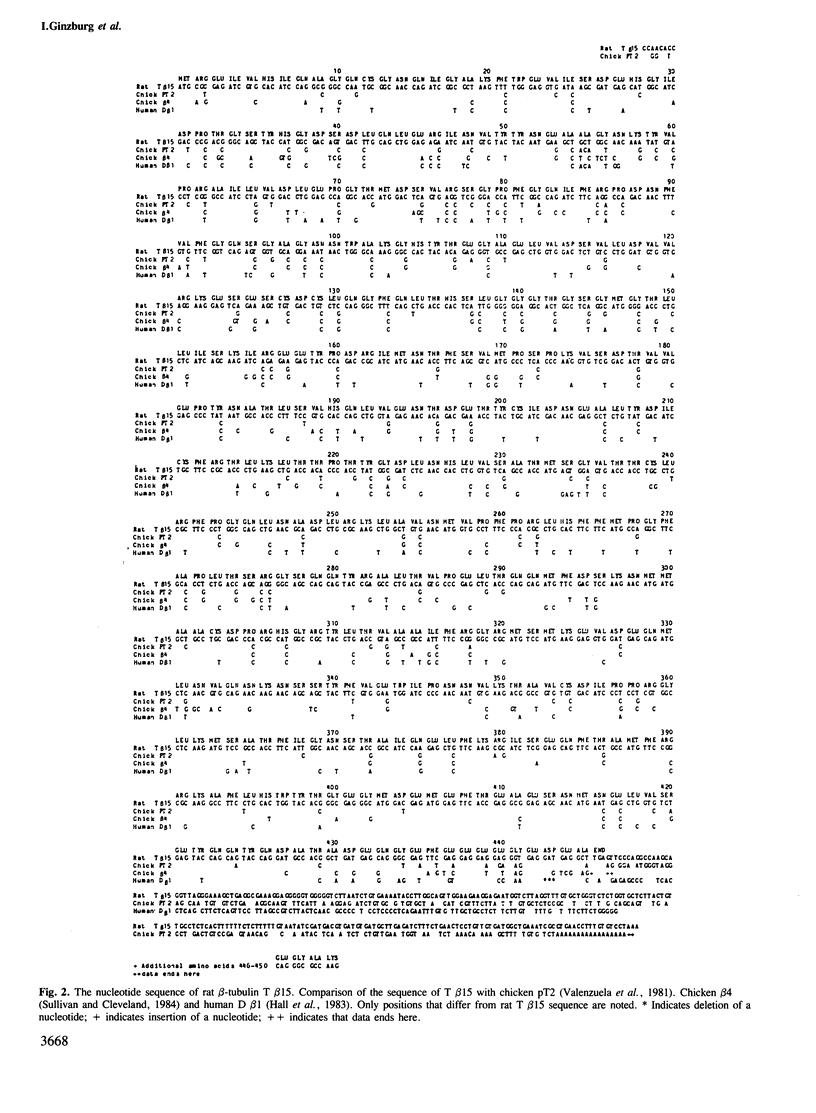
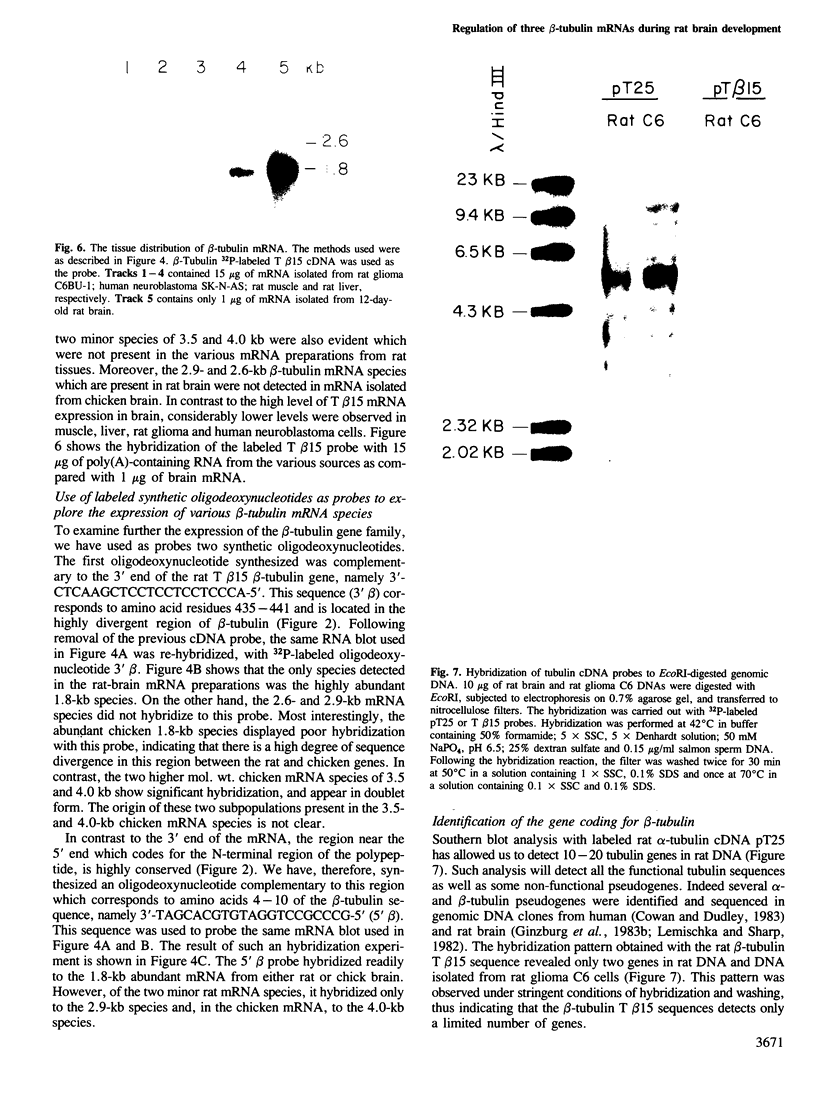
The nucleotide sequence of a complete rat brain beta-tubulin T beta 15 has been determined from three overlapping cDNA clones. The overall length of the T beta 15 sequence is 1589 bp and shows between 84.5% and 88.6% homology within the coding region as compared with chick and human beta-tubulin sequences. On the other hand, the 3'-non-coding region is highly divergent. Comparison of the derived amino acid sequences from different species demonstrates that the amino acid changes are not randomly distributed, but rather there are several conserved and two highly variable regions common to beta-tubulin polypeptides from various sources. The T beta 15 sequence encodes a dominant neuronal 1.8-kb beta-tubulin mRNA species. Two other minor beta-tubulin mRNA species of 2.6 and 2.9 kb are present in rat brain. By using two synthetic oligonucleotide probes complementary to the carboxyl-terminal divergent region and to the amino-terminal conserved region, we have shown that the three mRNAs are distinct species, which are developmentally regulated. The level of the 1.8-kb mRNA species increases till the age of 12 days thereafter its level decreases. The 2.9-kb mRNA is an early neuronal mRNA species, while the 2.6-kb mRNA is a late neuronal species which is detected at 30 days of rat brain development. The data illustrate that there is a differential expression of the beta-tubulin multigene family during rat brain development which may suggest different functions for the various beta-tubulin isotopes.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond J. F., Robinson G. S., Farmer S. R. Differential expression of two neural cell-specific beta-tubulin mRNAs during rat brain development. Mol Cell Biol. 1984 Jul;4(7):1313–1319. doi: 10.1128/mcb.4.7.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bray D., Gilbert D. Cytoskeletal elements in neurons. Annu Rev Neurosci. 1981;4:505–523. doi: 10.1146/annurev.ne.04.030181.002445. [DOI] [PubMed] [Google Scholar]
- Bray D. Model for membrane movements in the neural growth cone. Nature. 1973 Jul 13;244(5411):93–96. doi: 10.1038/244093a0. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Lopata M. A., MacDonald R. J., Cowan N. J., Rutter W. J., Kirschner M. W. Number and evolutionary conservation of alpha- and beta-tubulin and cytoplasmic beta- and gamma-actin genes using specific cloned cDNA probes. Cell. 1980 May;20(1):95–105. doi: 10.1016/0092-8674(80)90238-x. [DOI] [PubMed] [Google Scholar]
- Cowan N. J., Dobner P. R., Fuchs E. V., Cleveland D. W. Expression of human alpha-tubulin genes: interspecies conservation of 3' untranslated regions. Mol Cell Biol. 1983 Oct;3(10):1738–1745. doi: 10.1128/mcb.3.10.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan N. J., Dudley L. Tubulin isotypes and the multigene tubulin families. Int Rev Cytol. 1983;85:147–173. doi: 10.1016/s0074-7696(08)62372-4. [DOI] [PubMed] [Google Scholar]
- Dalbadie-McFarland G., Cohen L. W., Riggs A. D., Morin C., Itakura K., Richards J. H. Oligonucleotide-directed mutagenesis as a general and powerful method for studies of protein function. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6409–6413. doi: 10.1073/pnas.79.21.6409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels M. P. Colchicine inhibition of nerve fiber formation in vitro. J Cell Biol. 1972 Apr;53(1):164–176. doi: 10.1083/jcb.53.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Camilli P., Miller P. E., Navone F., Theurkauf W. E., Vallee R. B. Distribution of microtubule-associated protein 2 in the nervous system of the rat studied by immunofluorescence. Neuroscience. 1984 Apr;11(4):817–846. [PubMed] [Google Scholar]
- Efstratiadis A., Posakony J. W., Maniatis T., Lawn R. M., O'Connell C., Spritz R. A., DeRiel J. K., Forget B. G., Weissman S. M., Slightom J. L. The structure and evolution of the human beta-globin gene family. Cell. 1980 Oct;21(3):653–668. doi: 10.1016/0092-8674(80)90429-8. [DOI] [PubMed] [Google Scholar]
- Elliott E. M., Okayama H., Sarangi F., Henderson G., Ling V. Differential expression of three alpha-tubulin genes in Chinese hamster ovary cells. Mol Cell Biol. 1985 Jan;5(1):236–241. doi: 10.1128/mcb.5.1.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellous A., Ginzburg I., Littauer U. Z. Modulation of tubulin mRNA levels by interferon in human lymphoblastoid cells. EMBO J. 1982;1(7):835–839. doi: 10.1002/j.1460-2075.1982.tb01256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Behar L., Givol D., Littauer U. Z. The nucleotide sequence of rat alpha-tubulin: 3'-end characteristics, and evolutionary conservation. Nucleic Acids Res. 1981 Jun 25;9(12):2691–2697. doi: 10.1093/nar/9.12.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Scherson T., Giveon D., Behar L., Littauer U. Z. Modulation of mRNA for microtubule-associated proteins during brain development. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4892–4896. doi: 10.1073/pnas.79.16.4892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg I., Scherson T., Rybak S., Kimhi Y., Neuman D., Schwartz M., Littauer U. Z. Expression of mRNA for microtubule proteins in the developing nervous system. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):783–790. doi: 10.1101/sqb.1983.048.01.080. [DOI] [PubMed] [Google Scholar]
- Ginzburg I., de Baetselier A., Walker M. D., Behar L., Lehrach H., Frischauf A. M., Littauer U. Z. Brain tubulin and actin cDNA sequences: isolation of recombinant plasmids. Nucleic Acids Res. 1980 Aug 25;8(16):3553–3564. doi: 10.1093/nar/8.16.3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gozes I., Littauer U. Z. Tubulin microheterogeneity increases with rat brain maturation. Nature. 1978 Nov 23;276(5686):411–413. doi: 10.1038/276411a0. [DOI] [PubMed] [Google Scholar]
- Gozes I., Walker M. D., Kaye A. M., Littauer U. Z. Synthesis of tubulin and actin by neuronal and glial nuclear preparations from devloping rat brain. J Biol Chem. 1977 Mar 10;252(5):1819–1825. [PubMed] [Google Scholar]
- Gozes I., de Baetselier A., Littauer U. Z. Translation in vitro of rat brain mRNA coding for a variety of tubulin forms. Eur J Biochem. 1980 Jan;103(1):13–20. doi: 10.1111/j.1432-1033.1980.tb04283.x. [DOI] [PubMed] [Google Scholar]
- Hall J. L., Dudley L., Dobner P. R., Lewis S. A., Cowan N. J. Identification of two human beta-tubulin isotypes. Mol Cell Biol. 1983 May;3(5):854–862. doi: 10.1128/mcb.3.5.854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P. C. Developmental regulation of Drosophila alpha-tubulin genes. Cell. 1982 May;29(1):91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Krauhs E., Little M., Kempf T., Hofer-Warbinek R., Ade W., Ponstingl H. Complete amino acid sequence of beta-tubulin from porcine brain. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4156–4160. doi: 10.1073/pnas.78.7.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M. G., Lewis S. A., Wilde C. D., Cowan N. J. Evolutionary history of a multigene family: an expressed human beta-tubulin gene and three processed pseudogenes. Cell. 1983 Jun;33(2):477–487. doi: 10.1016/0092-8674(83)90429-4. [DOI] [PubMed] [Google Scholar]
- Lemischka I., Sharp P. A. The sequences of an expressed rat alpha-tubulin gene and a pseudogene with an inserted repetitive element. Nature. 1982 Nov 25;300(5890):330–335. doi: 10.1038/300330a0. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Gilmartin M. E., Hall J. L., Cowan N. J. Three expressed sequences within the human beta-tubulin multigene family each define a distinct isotype. J Mol Biol. 1985 Mar 5;182(1):11–20. doi: 10.1016/0022-2836(85)90023-3. [DOI] [PubMed] [Google Scholar]
- Lopata M. A., Havercroft J. C., Chow L. T., Cleveland D. W. Four unique genes required for beta tubulin expression in vertebrates. Cell. 1983 Mar;32(3):713–724. doi: 10.1016/0092-8674(83)90057-0. [DOI] [PubMed] [Google Scholar]
- Matus A., Huber G., Bernhardt R. Neuronal microdifferentiation. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 2):775–782. doi: 10.1101/sqb.1983.048.01.079. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J., Vieira J. A new pair of M13 vectors for selecting either DNA strand of double-digest restriction fragments. Gene. 1982 Oct;19(3):269–276. doi: 10.1016/0378-1119(82)90016-6. [DOI] [PubMed] [Google Scholar]
- Miyata T., Hayashida H., Kikuno R., Hasegawa M., Kobayashi M., Koike K. Molecular clock of silent substitution: at least six-fold preponderance of silent changes in mitochondrial genes over those in nuclear genes. J Mol Evol. 1982;19(1):28–35. doi: 10.1007/BF02100221. [DOI] [PubMed] [Google Scholar]
- Natzle J. E., McCarthy B. J. Regulation of Drosophila alpha- and beta-tubulin genes during development. Dev Biol. 1984 Jul;104(1):187–198. doi: 10.1016/0012-1606(84)90047-2. [DOI] [PubMed] [Google Scholar]
- Olmsted J. B., Borisy G. G. Microtubules. Annu Rev Biochem. 1973;42:507–540. doi: 10.1146/annurev.bi.42.070173.002451. [DOI] [PubMed] [Google Scholar]
- Ricciardi R. P., Miller J. S., Roberts B. E. Purification and mapping of specific mRNAs by hybridization-selection and cell-free translation. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4927–4931. doi: 10.1073/pnas.76.10.4927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson C. C. Phosphorylation of nucleic acid by an enzyme from T4 bacteriophage-infected Escherichia coli. Proc Natl Acad Sci U S A. 1965 Jul;54(1):158–165. doi: 10.1073/pnas.54.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt H., Gozes I., Littauer U. Z. Decrease in levels and rates of synthesis of tubulin and actin in developing rat brain. Brain Res. 1977 Feb;121(2):327–342. doi: 10.1016/0006-8993(77)90155-x. [DOI] [PubMed] [Google Scholar]
- Shen S. H., Slightom J. L., Smithies O. A history of the human fetal globin gene duplication. Cell. 1981 Oct;26(2 Pt 2):191–203. doi: 10.1016/0092-8674(81)90302-0. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F., Cleveland D. W. Sequence of a highly divergent beta tubulin gene reveals regional heterogeneity in the beta tubulin polypeptide. J Cell Biol. 1984 Nov;99(5):1754–1760. doi: 10.1083/jcb.99.5.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytell M., Brady S. T., Lasek R. J. Axonal transport of a subclass of tau proteins: evidence for the regional differentiation of microtubules in neurons. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1570–1574. doi: 10.1073/pnas.81.5.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Quiroga M., Zaldivar J., Rutter W. J., Kirschner M. W., Cleveland D. W. Nucleotide and corresponding amino acid sequences encoded by alpha and beta tubulin mRNAs. Nature. 1981 Feb 19;289(5799):650–655. doi: 10.1038/289650a0. [DOI] [PubMed] [Google Scholar]