Abstract
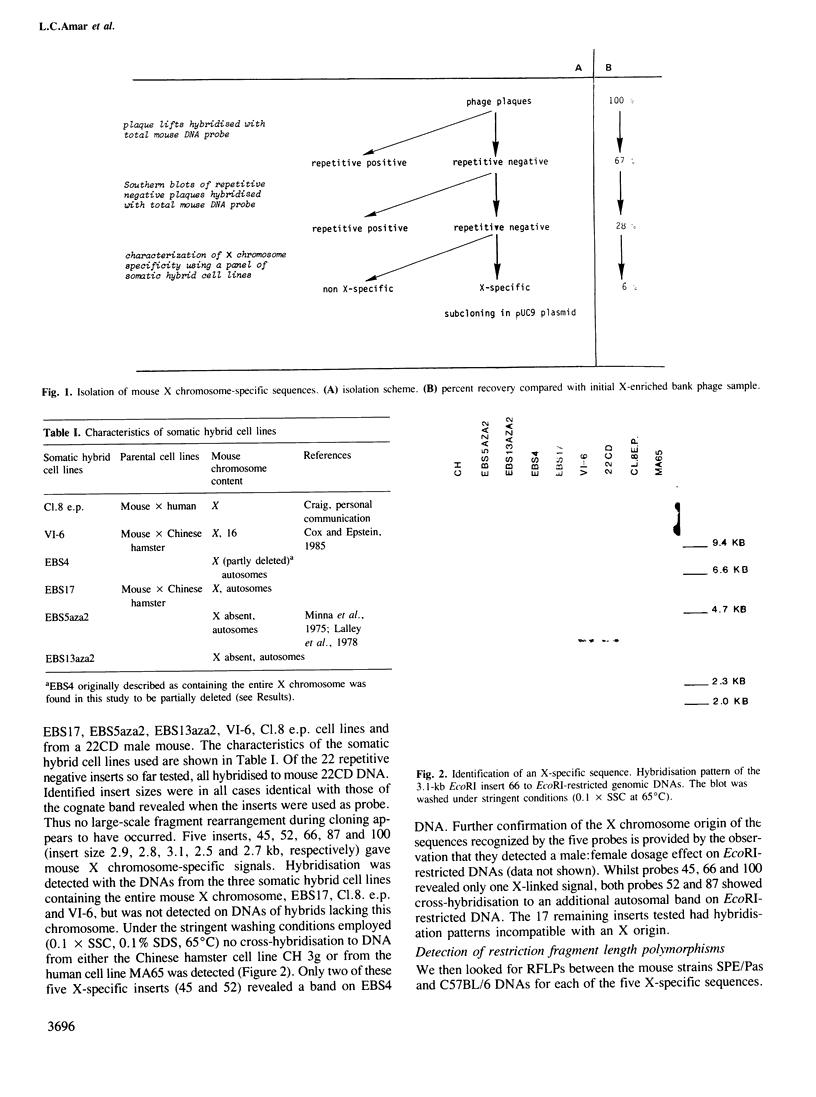
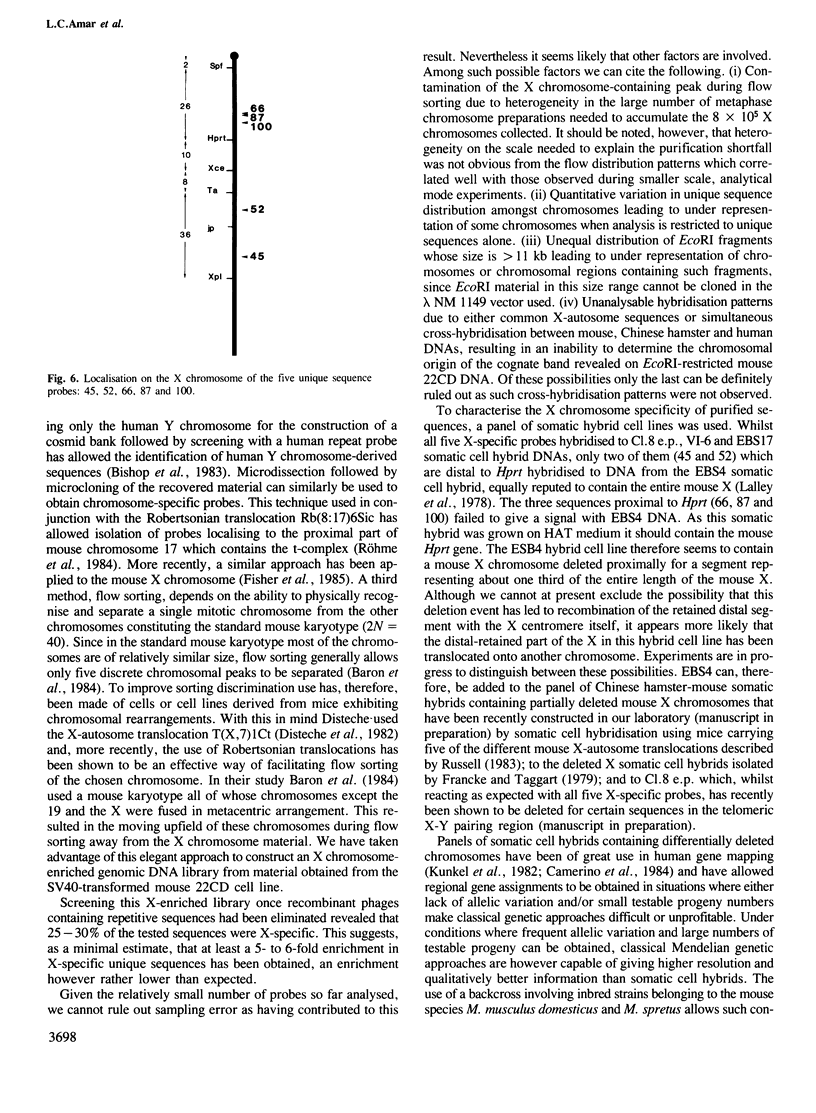
Two libraries enriched in murine X chromosome material have been constructed in the lambda vector NM 1149 from flow-sorted chromosomes. Inserts of unique genomic sequence DNA were purified and their X chromosome specificity characterised by hybridisation to a panel of somatic cell hybrid lines. Of the first five such X chromosome-specific probes characterised, all detect restriction fragment length polymorphisms (RFLPs) between inbred mouse laboratory strains such as C57BL/6 and BALB/c and the SPE/Pas mouse strain established from a wild Mus spretus mouse, when their DNAs are digested with the restriction enzyme TaqI. Taking advantage of these RFLPs, all five probes have been localised on the X chromosome using an interspecific backcross between the B6CBARI and SPE/Pas mouse strains segregating the X chromosome markers hypoxanthine phosphoribosyl transferase (Hprt) and Tabby (Ta). Three of the probes map to the region between the centromere and Hprt, and two distal to Ta. Since such X-specific sequence probes detect RFLPs between M. spretus and M. musculus domesticus DNAs with high frequency, a large panel of well localised probes should soon be available for studies of biological problems associated with the X chromosome which can best be approached using the murine species.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aldridge J., Kunkel L., Bruns G., Tantravahi U., Lalande M., Brewster T., Moreau E., Wilson M., Bromley W., Roderick T. A strategy to reveal high-frequency RFLPs along the human X chromosome. Am J Hum Genet. 1984 May;36(3):546–564. [PMC free article] [PubMed] [Google Scholar]
- Alwine J. C., Kemp D. J., Parker B. A., Reiser J., Renart J., Stark G. R., Wahl G. M. Detection of specific RNAs or specific fragments of DNA by fractionation in gels and transfer to diazobenzyloxymethyl paper. Methods Enzymol. 1979;68:220–242. doi: 10.1016/0076-6879(79)68017-5. [DOI] [PubMed] [Google Scholar]
- Baron B., Metezeau P., Kelly F., Bernheim A., Berger R., Guenet J. L., Goldberg M. E. Flow cytometry isolation and improved visualization of sorted mouse chromosomes. Purification of chromosomes X and ISO-1 from cell lines with Robertsonian translocations. Exp Cell Res. 1984 May;152(1):220–230. doi: 10.1016/0014-4827(84)90247-7. [DOI] [PubMed] [Google Scholar]
- Bishop C. E., Guellaen G., Geldwerth D., Voss R., Fellous M., Weissenbach J. Single-copy DNA sequences specific for the human Y chromosome. Nature. 1983 Jun 30;303(5920):831–832. doi: 10.1038/303831a0. [DOI] [PubMed] [Google Scholar]
- Bonhomme F., Catalan J., Britton-Davidian J., Chapman V. M., Moriwaki K., Nevo E., Thaler L. Biochemical diversity and evolution in the genus Mus. Biochem Genet. 1984 Apr;22(3-4):275–303. doi: 10.1007/BF00484229. [DOI] [PubMed] [Google Scholar]
- Brennand J., Chinault A. C., Konecki D. S., Melton D. W., Caskey C. T. Cloned cDNA sequences of the hypoxanthine/guanine phosphoribosyltransferase gene from a mouse neuroblastoma cell line found to have amplified genomic sequences. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1950–1954. doi: 10.1073/pnas.79.6.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerino G., Grzeschik K. H., Jaye M., De La Salle H., Tolstoshev P., Lecocq J. P., Heilig R., Mandel J. L. Regional localization on the human X chromosome and polymorphism of the coagulation factor IX gene (hemophilia B locus). Proc Natl Acad Sci U S A. 1984 Jan;81(2):498–502. doi: 10.1073/pnas.81.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattanach B. M. Control of chromosome inactivation. Annu Rev Genet. 1975;9:1–18. doi: 10.1146/annurev.ge.09.120175.000245. [DOI] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Quarantillo B. A. Electrophoretic variation for X chromosome-linked hypoxanthine phosphoribosyl transferase (HPRT) in wild-derived mice. Genetics. 1983 Apr;103(4):785–795. doi: 10.1093/genetics/103.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman V. M., Kratzer P. G., Siracusa L. D., Quarantillo B. A., Evans R., Liskay R. M. Evidence for DNA modification in the maintenance of X-chromosome inactivation of adult mouse tissues. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5357–5361. doi: 10.1073/pnas.79.17.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chasin L. A., Urlaub G. Mutant alleles for hypoxanthine phosphoriboxyltransferase: codominant expression, complementation, and segregation in hybrid Chinese hamster cells. Somatic Cell Genet. 1976 Sep;2(5):453–467. doi: 10.1007/BF01542725. [DOI] [PubMed] [Google Scholar]
- Cohen D. I., Hedrick S. M., Nielsen E. A., D'Eustachio P., Ruddle F., Steinberg A. D., Paul W. E., Davis M. M. Isolation of a cDNA clone corresponding to an X-linked gene family (XLR) closely linked to the murine immunodeficiency disorder xid. 1985 Mar 28-Apr 3Nature. 314(6009):369–372. doi: 10.1038/314369a0. [DOI] [PubMed] [Google Scholar]
- Cox D. R., Epstein C. J. Comparative gene mapping of human chromosome 21 and mouse chromosome 16. Ann N Y Acad Sci. 1985;450:169–177. doi: 10.1111/j.1749-6632.1985.tb21491.x. [DOI] [PubMed] [Google Scholar]
- Disteche C. M., Eicher E. M., Latt S. A. Late replication in an X-autosome translocation in the mouse: correlation with genetic inactivation and evidence for selective effects during embryogenesis. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5234–5238. doi: 10.1073/pnas.76.10.5234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disteche C. M., Kunkel L. M., Lojewski A., Orkin S. H., Eisenhard M., Sahar E., Travis B., Latt S. A. Isolation of mouse x-chromosome specific DNA from an x-enriched lambda phage library derived from flow sorted chromosomes. Cytometry. 1982 Mar;2(5):282–286. doi: 10.1002/cyto.990020503. [DOI] [PubMed] [Google Scholar]
- Fisher E. M., Cavanna J. S., Brown S. D. Microdissection and microcloning of the mouse X chromosome. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5846–5849. doi: 10.1073/pnas.82.17.5846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U., Taggart R. T. Assignment of the gene for cytoplasmic superoxide dismutase (Sod-1) to a region of chromosome 16 and of Hprt to a region of the X chromosome in the mouse. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5230–5233. doi: 10.1073/pnas.76.10.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Tantravahi U., Eisenhard M., Latt S. A. Regional localization on the human X of DNA segments cloned from flow sorted chromosomes. Nucleic Acids Res. 1982 Mar 11;10(5):1557–1578. doi: 10.1093/nar/10.5.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley P. A., Francke U., Minna J. D. Homologous genes for enolase, phosphogluconate dehydrogenase, phosphoglucomutase, and adenylate kinase are syntenic on mouse chromosome 4 and human chromosome 1p. Proc Natl Acad Sci U S A. 1978 May;75(5):2382–2386. doi: 10.1073/pnas.75.5.2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskay R. M., Evans R. J. Inactive X chromosome DNA does not function in DNA-mediated cell transformation for the hypoxanthine phosphoribosyltransferase gene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4895–4898. doi: 10.1073/pnas.77.8.4895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minna J. D., Marshall T. H., Shaffer-Berman P. V. Chinese hamster X mouse hybrid cells segregating mouse chromosomes and isozymes. Somatic Cell Genet. 1975 Oct;1(4):355–369. doi: 10.1007/BF01538667. [DOI] [PubMed] [Google Scholar]
- Nesbitt M. N., Gartler S. M. Replication of the mouse sex chromosomes early in the S period. Cytogenetics. 1970;9(3):212–221. doi: 10.1159/000130091. [DOI] [PubMed] [Google Scholar]
- Rastan S. Primary non-random X-inactivation caused by controlling elements in the mouse demonstrated at the cellular level. Genet Res. 1982 Oct;40(2):139–147. doi: 10.1017/s0016672300019017. [DOI] [PubMed] [Google Scholar]
- Robert B., Barton P., Minty A., Daubas P., Weydert A., Bonhomme F., Catalan J., Chazottes D., Guénet J. L., Buckingham M. Investigation of genetic linkage between myosin and actin genes using an interspecific mouse back-cross. Nature. 1985 Mar 14;314(6007):181–183. doi: 10.1038/314181a0. [DOI] [PubMed] [Google Scholar]
- Röhme D., Fox H., Herrmann B., Frischauf A. M., Edström J. E., Mains P., Silver L. M., Lehrach H. Molecular clones of the mouse t complex derived from microdissected metaphase chromosomes. Cell. 1984 Mar;36(3):783–788. doi: 10.1016/0092-8674(84)90358-1. [DOI] [PubMed] [Google Scholar]
- Seed B. Diazotizable arylamine cellulose papers for the coupling and hybridization of nucleic acids. Nucleic Acids Res. 1982 Mar 11;10(5):1799–1810. doi: 10.1093/nar/10.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skolnick M. H., Willard H. F., Menlove L. A. Report of the Committee on Human Gene Mapping by Recombinant DNA Techniques. Cytogenet Cell Genet. 1984;37(1-4):210–273. doi: 10.1159/000132011. [DOI] [PubMed] [Google Scholar]
- Vandeberg J. L. Developmental aspects of X chromosome inactivation in eutherian and metatherian mammals. J Exp Zool. 1983 Nov;228(2):271–286. doi: 10.1002/jez.1402280211. [DOI] [PubMed] [Google Scholar]
- Venolia L., Gartler S. M., Wassman E. R., Yen P., Mohandas T., Shapiro L. J. Transformation with DNA from 5-azacytidine-reactivated X chromosomes. Proc Natl Acad Sci U S A. 1982 Apr;79(7):2352–2354. doi: 10.1073/pnas.79.7.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]






