Abstract
C-X-C chemokine receptor 4 (CXCR4) stimulates cancer metastasis. NF-κB regulates CXCR4 expression in cancer cells, and O-GlcNAc modification of NF-κB promotes its transcriptional activity. Here, we determined whether CXCR4 expression is affected by O-GlcNAcylation of NF-κB in lung metastasis of cervical cancer. We found elevated levels of O-linked-N-actylglucosamine transferase (OGT) and O-GlcNAcylation in cervical cancer cells compared to those in non-malignant epithelial cells and detected increased expression of NF-κB p65 (p65) and CXCR4 in cervical cancer cells. Knockdown of OGT inhibited the O-GlcNAcylation of p65 and decreased CXCR4 expression levels in HeLa cells. Thiamet G treatment increased O-GlcNAcylated p65, which subsequently enhanced CXCR4 expression levels. Inhibition of O-GlcNAcylation by 6-Diazo-5-oxo-L-norleucine (DON) treatment decreased p65 activation, eventually inhibiting CXCR4 expression in HeLa cells. Lung tissues from mice engrafted with OGT-knockdown HeLa cells (shOGT) exhibited lower expression of Ki-67 and HPV E6 and E7 oncogenes compared to lung tissues from mice engrafted with control HeLa cells (shCTL). In addition, lung tissues from mice engrafted with shOGT cells exhibited lower p65 and CXCR4 immunoreactivity compared to tissues from mice engrafted with shCTL cells. Taken together, our data suggest that p65 O-GlcNAcylation promotes lung metastasis of cervical cancer cells by activating CXCR4 expression.
Keywords: cervical cancer, CXCR4, lung metastasis, NF-κB p65, O-GlcNAcylation
INTRODUCTION
Lung cancer is the leading cause of cancer related death worldwide (Siegel et al., 2015). Metastasis plays a major role in lung cancer progression and mortality (Wang and Adjei, 2015). Human cervical cancer infiltrates neighbouring tissues and metastasizes frequently to the lymph nodes, bones and lungs (Anderson et al., 2001; Reinhardt et al., 2001; Sironi et al., 2006; Tellis et al., 1982; Thanapprapasr et al., 2010). Metastasis of cervical cancer to lung tissue is recently described issue in the development of lung cancer and is therefore still in the initial stages of study.
C-X-C chemokine receptor type 4 (CXCR4) is the receptor for the small chemokine stromal cell-derived factor-1 alpha (SDF1α). CXCR4 is overexpressed in carcinomas of the breast, brain, cervix, colon, lung, ovaries, prostate and thyroid and has an important role in organ-specific metastasis (Kato et al., 2003; Kucia et al., 2004; Sekula et al., 2014; Taichman et al., 2002; Zlotnik, 2006a). It has been reported that CXCR4 inhibition decreases the growth of cervical carcinoma and inhibits lung and spleen metastasis of cervical cancer in mice (Sekula et al., 2014). Although previous studies have shown that increased levels of CXCR4 are linked to the incidence of metastasis, the molecular basis of this association is not fully understood (Zlotnik, 2008).
NF-κB is the key transcription factor involved in CXCR4 regulation and is constitutively activated in many cancers, including cervical cancer (Dolcet et al., 2005; Esencay et al., 2010; Zhi et al., 2015). It has been shown that the NF-κB transcriptional activity is regulated by O-GlcNAcylation, which is a post-translational modification in which β-D-N-acetylglucosamine (GlcNAc) is attached to the serine or threonine residue of nuclear and cytoplasmic proteins that are involved in biological processes. Increased O-GlcNAcylation levels are observed in several cancers and are associated with tumour metastasis. A series of studies have reported that NF-κB p65 (p65) is O-GlcNAcylated on Thr-352 and that this modification is required for its transcriptional activity. Furthermore, mutation at O-GlcNAc sites in p65 inhibits the development of cancer (Fardini et al., 2013; Jozwiak et al., 2014; Ma et al., 2013; Yang et al., 2008). In the current study, we found that O-GlcNAcylation of p65 regulates CXCR4 expression and that CXCR4 expression enhanced by O-GlcNAcylated p65 is involved in metastasis of cervical cancer.
MATERIALS AND METHODS
Dulbecco’s modified Eagle medium (DMEM), Minimum Essential Medium (MEM), Roswell Park Memorial Institute medium (RPMI), penicillin-streptomycin, and foetal bovine serum (FBS) were obtained from Gibco (Invitrogen, USA). Dimethyl sulfoxide (DMSO), lentiviral pLKO.1-puro vector, DON (6-diazo-5-oxo-norleucineis), and thiamet G were purchased from Sigma (USA). Mouse anti-O-GlcNAc (MA1-072-RL2) was purchased from Thermo Fisher Scientific (USA). Rabbit anti-CXCR4 (sc-9046), mouse anti-p65 (sc-8008), mouse anti-OGT (sc-74546), mouse anti-HPV18 E6 (sc-365089), mouse anti-HPV18 E7 (sc-365035), and mouse anti-Ki67 (sc-23900) were obtained from Santa Cruz Biotechnology (USA). Rabbit anti-OGT was purchased from Abcam (ab96718; USA). Rabbit anti-p65 (ADI-KAS-TF110-F) was obtained from Enzo Life Sciences (USA).
Cell lines
Cervical cancer cell lines HeLa (HPV-18-positive), SiHa (HPV-16-positive), and C-33A (HPV-negative) and a human keratinocyte cell line (HaCaT) were obtained from American Type Culture Collection (USA). HeLa and HaCaT cells were maintained in DMEM, and C33A cells were maintained in MEM. CaSki were maintained in RPMI. Cells were maintained in 5% CO2 at 37°C. All media were supplemented with 10% FBS, 100 μg/mL streptomycin, and 100 units/ml penicillin (Invitrogen, USA). Cells were grown in high-glucose media.
Lentiviral shRNA production and infection
Lentivirus expressing the shRNA against scramble (control) or OGT was produced as follows. The human OGT shRNA sequence (TRCN0000035064) was 5-GCCCTAAGTTTGAG TCCAAAT-CTCGAG-ATTTGGACTCAAACTTAGGGA-TTTTTG-3. The scramble RNA sequence (product no. SHC002V) was 5-CCGG-CAACAAGATGAAGAGCACCAA-CTCGAG-TTGGT GCTCTTCATCTTGTTGTTTTT-3. For lentivirus production, HEK293T cells were transfected with the pLKO.1-puro vector along with packaging plasmid encoding Gag/Pol, Rev, and VSV-G using the Lipofectamine 2000 reagent (Invitrogen) according to manufacturer’s instructions. Culture media containing lentiviral particles were collected 48 h and 72 h after transfection and filtered. Viral supernatants were pooled and stored at -80°C. HeLa cells were infected with virus in medium and selected for stable expression of shRNA in culture with puromycin at 10 μg/ml for 2 weeks.
Western blotting
Cells or tissues were homogenised in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, and 1% Nonidet P-40) with protease inhibitor cocktail (Sigma Aldrich). Protein concentration was determined with the BCA (bicinchoninic acid) protein assay kit (Thermo Scientific). Total protein lysates were separated by SDS-PAGE and transferred onto nitrocellulose membranes (Millipore, USA). The target proteins were detected by western blotting with antibodies against O-GlcNAc, p65, CXCR4, and β-actin. Immunoreactive antigens were detected with the enhanced chemiluminescence detection kit (Amersham Bioscience, USA). The specific protein bands were analysed by densitometry, and the density values were normalised to that of the β-actin control and then further analysed using the Image J software (USA).
Immunoprecipitation
Protein extracts were mixed with protein A/G agarose beads (Santa Cruz Biotechnology) and then incubated overnight with rotation at 4°C. The following day, the sample was centrifuged at 12,000 × g and then incubated with 2 μg of antibodies overnight at 4°C. The protein-bead complex was then washed and collected by centrifugation, samples were boiled in loading buffer to remove the agarose beads, and the protein was then separated by SDS-PAGE on 10% acrylamide gels. Proteins were then transferred to membranes, probed with antibodies against the interacting protein of interest, and processed for western blotting as described above.
Succinylated wheat germ agglutinin (sWGA) affinity purification
Cells or tissues were homogenised in lysis buffer (50 mM Tris [pH 7.5], 150 mM NaCl, 5 mM EDTA, and 1% Nonidet P-40) with protease inhibitor cocktail. Lysates containing 100 μg of protein were incubated with agarose-conjugated sWGA (Vector Laboratories, USA) overnight at 4°C. On the following day, the samples were centrifuged at 2000 × g for 2 min. Precipitates were washed four times and were eluted by boiling in SDS sample buffer. For the control, the inhibitory monosaccharide GlcNAc was added during sWGA lectinaffinity purification to demonstrate that all true carbohydrate-modified proteins disappeared in the assay.
Transfection of HeLa cells with Plasmids containing His-NFκ B p65
The pcDNA3.1-His mammalian expression vector containing the human NFκB p65 wild-type and point mutated p65 either at Thr-322 or Thr-352 sites was kindly provided by Dr. Jin Won Cho, Yonsei University, Korea (Yang et al., 2008). HeLa cells were plated in 6-well plates at a density of 2 × 105 cells per well and incubate for twenty four hours. Then, cells were transfected with plasmids by lipofectamine 3000 according to the protocols provided by the manufacturer (Invitrogen, USA). The cells were harvested and cell lysates were analyzes by western blotting against p65 and CXCR4.
Hematoxylin and Eosin (H&E) Staining
Lung tissues isolated from mice were fixed in a solution of formaldehyde and embedded into paraffin. Tissue sections on gelatin-coated slides were deparaffinized in xylene and rehydrated in graded alcohol solutions. The slides were then stained with H&E, and examined under light microscope.
Immunofluorescence analysis
Tissue sections that were fixed on gelatin-coated slides were deparaffinized in xylene and then rehydrated in graded alcohol solutions. The endogenous peroxidase activity was inhibited by incubation of the slide for 30 min in 0.3% H202 in 0.01 M Tris, and the non-specific binding was reduced by blocking in 5% serum. The samples were incubated overnight at 4°C with the desired primary antibody (1:200), washed with PBS, and then incubated with a specific fluorescence-conjugated secondary IgG for 1 h in a light-protected chamber at room temperature. Subsequently, the sections were counterstained with DAPI and mounted. Immunofluorescence signals were detected using fluorescence microscopy.
For immunocytochemistry, HeLa cells were grown on glass coverslips in 24-well culture plates, and after 24 h, the cells were treated with or without Thiamet G. After 24 h, cells were washed with PBS, fixed for 10 min at room temperature with 4% paraformaldehyde, and permeabilised for 15 min with 0.5% Triton X-100 in PBS. Then cells were blocked with 5% normal serum in PBS for 1 h. Next, the cells were incubated at 4°C with the desired primary antibody overnight, followed by incubation with a specific fluorescence-conjugated secondary IgG for 1 h in the dark. Cells were mounted using prolong gold antifade reagent containing the nuclear staining 4′,6-diamidino -2-phenylindole,dicloride (DAPI). Images were analysed using fluorescence microscopy.
In vivo tumour xenograft studies
Tumour xenograft athymic female nude mice (BALB/c-nu/nu, 5–6 weeks old; Orientbio, Korea; n = 10) were maintained on a 12-h light/dark cycle with food and water supplied ad libitum. HeLa cells treated with non-targeting (shCTL) or OGT-specific shRNAs (5 × 106 HeLa/shCTL or HeLa/shOGT cells in 100 μl PBS) were injected subcutaneously to the right flank of the animal. After 12 weeks, the mice were sacrificed, and lung tissue was isolated. All animal work was performed in accordance with the Gyeongsang National University Institutional Animal Care and Use Committee (Approval No. GNU-131121-M0069).
Statistical analysis
Data are representative of three independent experiments and presented as mean ± S.E.M. Statistical analyses were performed using Student t-test or ANOVA. P < 0.05 was considered statistically significant.
RESULTS
Expression of NF-κB and CXCR4 is elevated in hyper-OGlcNAcylated cervical cancer cell lines
First, we examined O-GlcNAcylation and OGT expression in human cervical cell lines (HeLa, CaSki, and C3-AA) compared to normal epithelial cells (HaCaT). O-GlcNAcylation was elevated in cervical cancer cells, and higher OGT expression was found in cervical cancer cells compared to normal epithelial cells (Fig. 1). Then, we examined p65 and CXCR4 expression in cervical cancer cells compared to normal epithelial cells and found that expression of both p65 and CXCR4 was enhanced in cervical cancer cells compared to normal epithelial cells (Fig. 1).
Fig. 1. Expression of NFκB p65 and CXCR4 is elevated in hyper-O-GlcNAcylated cervical cancer cell lines.
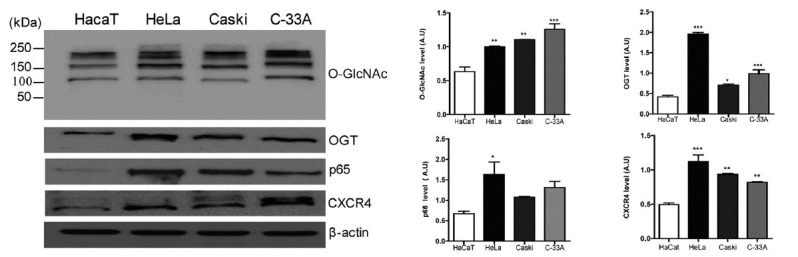
An immortalised human epithelial cell line (HaCaT) and human cervical cancer cells (HeLa, Caski, and C33-A) were lysed and analysed by western blotting for O-GlcNAc, OGT, NFκB p65, and CXCR4. β-actin was used as a loading control. Data represent the mean ± SEM of three independent experiments. *** P < 0.001, **P < 0.005, * P < 0.05.
O-GlcNAcylation regulates NF-κB and CXCR4 expression
To study the role of O-GlcNAcylation in cancer metastasis, we constructed stable knockdown HeLa cells using a lentiviral delivery system with either the scramble shRNA (shCTL) or shRNA against OGT (shOGT). Decreased O-GlcNAcylation and downregulated OGT expression by OGT shRNA were confirmed by western blotting (Fig. 2A). Then, we found that expression of p65 and CXCR4 was decreased in shOGT cells compared to that in shCTL cells (Fig. 2A). Next, we employed Thiamet G, which is an O-GlcNAcase (OGA) inhibitor, and DON (6-diazo-5-oxo-norleucineis), which is a glutamine fructose-6-phosphate aminotransferase (GFAT) inhibitor, to examine the effect of O-GlcNAcylation on p65 and CXCR4 expression. We found that hyper-O-GlcNAcylation induced by thiamet G increased p65 and CXCR4 expression and that hypo-O-GlcNAcylation induced by DON decreased p65 and CXCR4 expression (Fig. 2B). To investigate the interaction between p65 and OGT, immunoprecipitation assays were applied to shCTL and shOGT cells using antibodies against p65 and OGT. The interaction between p65 and OGT was decreased in shOGT cells compared to shCTL cells (Figs. 3A and 3B). Then, we checked the content of O-GlcNAcylated p65 using sWGA affinity purification assay in shCTL and shOGT cells. The addition of inhibitory monosaccharide Glc-NAc during the sWGA-lectin-affinity purification step diminished or completely blocked the O-GlcNAc signal. Our result showed decreased O-GlcNAcylated p65 in shOGT cells compared to levels in shCTL cells (Fig. 3C). Next, we examined whether site specific p65 O-GlcNAcylation contributes to the regulation of CXCR4 expression. As a result, transfection with point mutated p65 at Thr-322 or Thr-352 in HeLa cells decreased CXCR4 expression compared to transfection with wild-type p65 (Fig. 3D). Together, these results suggest that increased expression of OGT promotes the O-GlcNAcylation of p65, which enhances CXCR4 expression in cervical cancer cells.
Fig. 2. O-GlcNAcylation regulates NF-κB and CXCR4 expression.
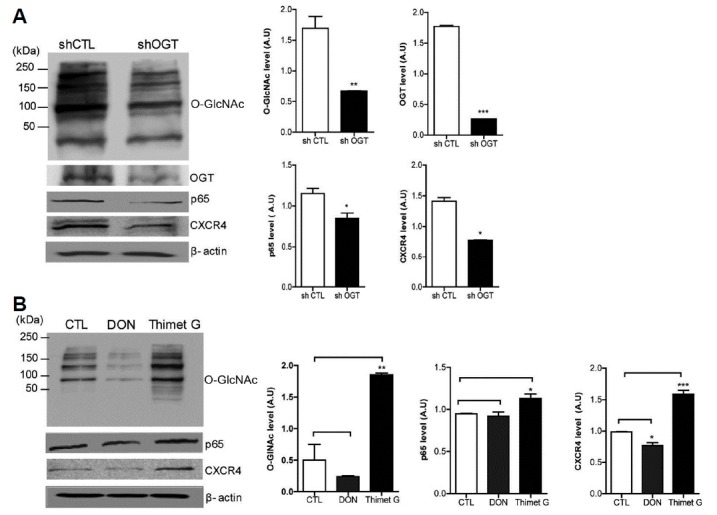
(A) HeLa cells infected with either scramble shRNA (shCTL) or shRNA against OGT (shOGT) lentivirus were lysed and subjected to immunoblotting for O-GlcNAc, OGT, NFκB p65, and CXCR4. (B) HeLa cells were treated with or without DON or thiamet G for 24 h. Cells were lysed and analysed by western blotting for O-GlcNAc, NFκB p65, and CXCR4. Data represent mean ± SEM of three independent experiments. ***P < 0.001, **P < 0.005, *P < 0.05.
Fig. 3. OGT knockdown decreases the interaction of NFκB p65 with OGT and reduces O-GlcNAcylation of NFκB p65.
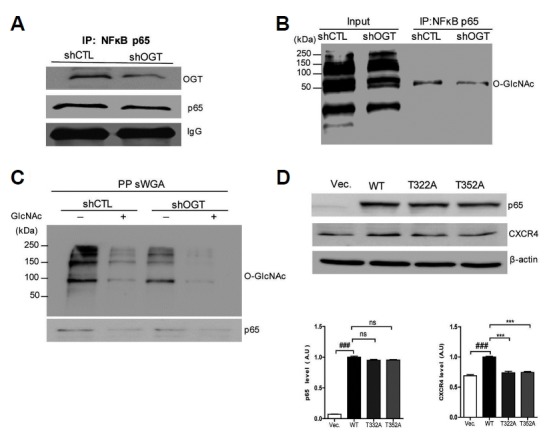
(A, B) HeLa cells infected with either scramble shRNA (shCTL) or shRNA against OGT (shOGT) lentivirus were lysed, and cell lysates were immunoprecipitated with an anti-p65 antibody. Immunoprecipitates were then analysed by western blotting for OGT and O-GlcNAc. (C) HeLa cells infected with shCTL or shOGT lentivirus were lysed, and their cell lysates were subjected to sWGAlectin-affinity purification. The precipitates were analysed with western blotting for O-GlcNAc and p65. The inhibitory monosaccharide GlcNAc was added during sWGA-lectinaffinity purification. (D) HeLa cells transfected with plasmids encoding His- NFκB p65 or point mutated NFκB p65 were lysed and subjected to western blot analysis using p65 and CXCR4 antibodies. Data represent the mean ± SEM of three independent experiments. ***P < 0.001, **P < 0.005, *P < 0.05.
O-GlcNAcylation increases the co-localization of OGT, p65, and O-GlcNAc and induces CXCR4 expression
Immunofluorescence staining showed increased OGT and p65 expression in HeLa cells after treatment with Thiamet G, indicating increased expression of p65 and OGT under hyper-O-GlcNAcylation conditions (Fig. 4A). Thiamet G treatment also resulted in increased O-GlcNAc and its interaction with p65 in HeLa cells (Fig. 4B). Immunoreactive CXCR4 was detected in the cytoplasm of p65-positive cells, and the double-positive cells were increased, suggesting that O-GlcNAcylation of p65 is related to the upregulation of CXCR4 expression (Fig. 4C).
Fig. 4. O-GlcNAcylation increases the co-localization of OGT and p65, and O-GlcNAc regulates CXCR4 expression.
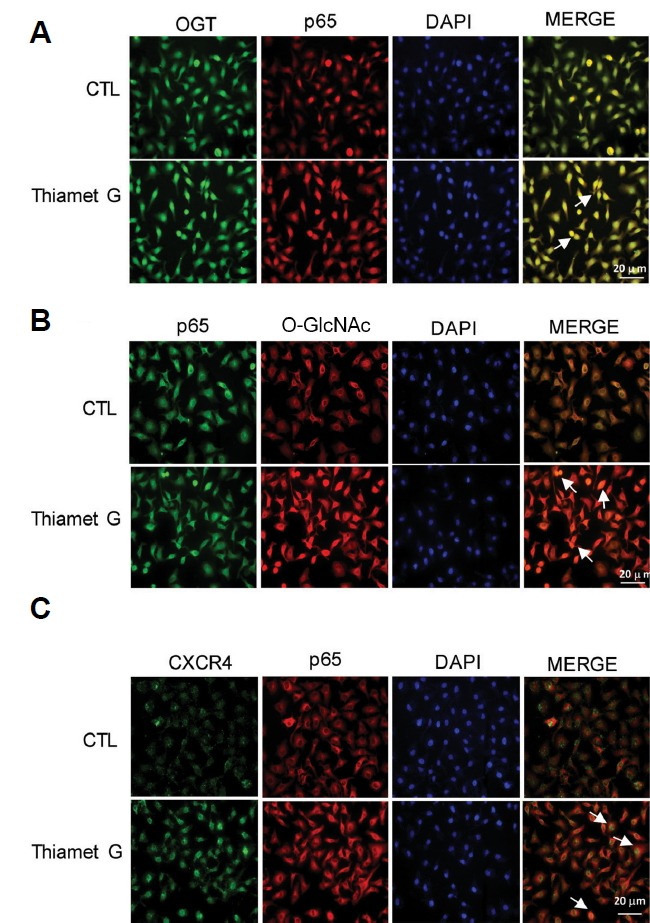
HeLa cells were treated with or without thiamet G and subjected to double immunostaining. (A) Double immunofluorescence analysis was performed for OGT and p65. DAPI fluorescence is shown as blue, OGT immunofluorescence is shown as green, and p65 immunofluorescence is shown as red. Merged images show the co-localization of OGT and p65. (B) Double immunofluorescence analysis of p65 and O-GlcNAc is shown. DAPI fluorescence is shown in blue, p65 immunofluorescence is shown in green, and O-GlcNAc immunofluorescence is shown as red. Merged images show the co-localization of p65 and O-GlcNAc. (C) Double immunofluorescence analysis of CXCR4 and p65 is shown. DAPI fluorescence is shown as blue, CXCR4 immunofluorescence is shown as green, and p65 immunofluorescence is shown as red. Merged images show the co-localization of CXCR4 and p65. Cells were treated with 10 μM of thiamet G for 24 h. Scale bar, 20 μm. Cells were analysed under a fluorescence microscope.
OGT depletion inhibits lung metastasis of cervical cancer through decreased CXCR4 expression via reduction of O-GlcNAcylation of p65 in vivo
To determine the influence of O-GlcNAcylation on lung metastasis of cervical cancer in vivo, we performed xenograft studies with shCTL and shOGT HeLa cells in nude mice. Either shCTL or shOGT HeLa cells were injected subcutaneously into the right flank of the nude mice, lung tissues were isolated after three months. In result of H&E staining, histology of mice injected with shCTL cells showed infiltration of some unknown cell, morphologically similar to tumor cells, having large abundant cytoplasm and large nuclei. Next, we examined Ki67 as a cell proliferation marker in lung tissue using immunostaining. We found decreased expression of Ki67 in lungs from animals injected with shOGT cells compared to those injected with shCTL cells (Fig. 5A). We also found decreased expression of HPV 18 E6/ E7 in lung tissues of mice injected with shOGT cells compared to those injected with shCTL cells (Fig. 5A). The colocalization of p65 and OGT was confirmed by immunofluorescence staining (Fig. 5B). Immunoreactivities of p65 and OGT were increased in lungs tissues of nude mice implanted with shCTL cells compared to those implanted with shOGT cells, supporting the increased interaction between p65 and OGT in nude mice injected with shCTL cells (Fig. 5B). Immunoreactive CXCR4 was detected in the cytoplasm of OG-positive cells (Fig. 5C, indicated by arrows). Double-positive cells were also increased in mice implanted with shCTL cells compared to mice implanted with shOGT cells, suggesting that hyper-O-GlcNAcylation is related to upregulation of CXCR4 expression via increased p65 (Fig. 5C).
Fig. 5. Immunofluorescent staining of lung tissue in nude mice.
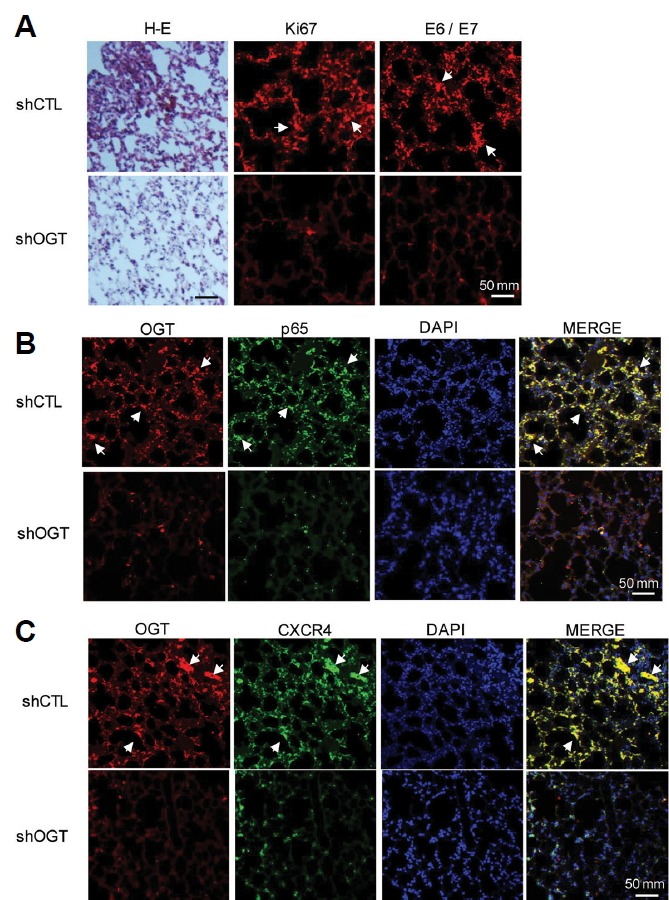
(A) HeLa cells infected with shCTL or shOGT lentivirus were injected into nude mice. After 3 months, lung tissue samples from control subjects and OGT-knockdown mice were embedded in paraffin blocks and sectioned for staining. Left panel shows H&E staining, middle panel shows immunofluorescence analysis of KI67 and right panel shows HPV 18 E6/E7. (B) To confirm the relationship between OGT and p65, double immunofluorescence staining was performed, and merged images were obtained .DAPI fluorescence is shown as blue, OGT immunofluorescence is shown as red, and p65 immunofluorescence is shown as green. Merged images show the co-localization of NFκB p65 and OGT. (C) To confirm the relationship between OGT and CXCR4, double immunofluorescence staining was performed, and merged images were obtained. DAPI fluorescence is shown as blue, OGT immunofluorescence is shown as red, and CXCR4 immunofluorescence is shown as green. Merged images show the co-localization of p65 and OGT. Upper images are from lung tissues of control mice, and lower images are from lung tissue of OGT knockdown mice. Scale bar, 50 μm. Samples were analysed under a fluorescence microscope.
To confirm the immunofluorescence studies, we performed Western blot analysis of lung tissues from nude mice injected with shCTL and shOGT cells. O-GlcNAcylation and OGT expression were decreased in the mice implanted with shOGT cells (Fig. 6A). In addition, p65 and CXCR4 expression was also decreased in mice implanted with shOGT cells. Next, we investigated O-GlcNAcylation of p65 using sWGA affinity purification assay. The addition of inhibitory monosaccharide GlcNAc during sWGA-lectin-affinity purification diminished or completely blocked the O-GlcNAc signals, and we observed decreased O-GlcNAcylation of p65 in nude mice implanted with shOGT cells compared to animals implanted with shCTL cells (Fig. 6B). Increased expression of CXCR4 in lung tissue of nude mice implanted with shCTL cells suggests enhanced metastasis of cervical cancer to the lung. Thus, elevated O-GlcNAcylation shows that higher CXCR4 expression has a higher potential for metastasis.
Fig. 6. O-GlcNAcylation of NFκB p65 regulates CXCR4 expression in vivo.

HeLa cells infected with shCTL or shOGT lentivirus were injected into nude mice. After 3 months, assessments were performed, and protein was extracted from lung tissue. (A) Lysates were analysed by western blotting for O-GlcNAc, OGT, p65, and CXCR4. (B) Lysates were subjected to sWGA-lectin-affinity purification, and the precipitates were analysed by western blot for O-GlcNAc and p65. The inhibitory monosaccharide GlcNAc was added during sWGA-lectin-affinity purification. Data represent the mean ± SEM of three independent experiments. ***P < 0.001, *P < 0.05.
DISCUSSION
Cancer metastasis is the main cause of cancer-related deaths (Chambers et al., 2002; Spano et al., 2012); however, the mechanisms of metastasis are largely unknown. Chemokines and their receptors play significant roles in cancer metastasis. CXCR4 is one of the important chemokines that affects primary tumour metastasis to central organs (Zlotink et al., 2006b). As the key modulator of CXCR4, NF-κB has been shown to be constitutively activated in a variety of cancers, including cervical and lung cancer (Cai et al., 2011; Nair et al., 2003). Enhancing the expression of CXCR4 through NF-κB activity may contribute to the metastasis of cancer; however, the mechanism underlying constitutive activation of NF-κB is not fully understood.
The present study results are in agreement with previous studies of the molecular mechanism of CXCR4 regulation and its metastatic potential. Here, we demonstrated a correlation between O-GlcNAcylation of p65 and CXCR4 expression in cervical cancer and its potential for metastasis to the lungs. Hyper-O-GlcNAcylation is linked to the incidence of metastasis in cancer, but until now, no studies had been reported about this link in cervical cancer. Several studies have reported regulation of NF-κB by O-GlcNAcylation. The CXCR4 promoter region contains NF-κB binding elements, and expression of CXCR4 can be enhanced by NF-κB signalling. A study in breast cancer cells showed that constitutively active NF-κB upregulates CXCR4 expression and promotes tumour cell migration and metastasis. O-linked GlcNAc modification of NF-κB inhibit its interaction with the inhibitor IκB, causing nuclear translocation of NF-κB and activation of its targets gene. CXCR4 is one of the target genes of NF-κB. NF-κB subunits p65 bind to the CXCR4 promoter and activate transcription (Helbig et al., 2003; Yang et al., 2008). Therefore elevated O-GlcNAcylation may promote CXCR4 regulation by activating NF-κB signaling. Our study identified increased OGT levels and increased O-GlcNAcylation in cervical cancer cells compared to normal epithelial cells. Hyper-O-GlcNAcylated cancer cells also exhibited higher p65 and CXCR4 expression. Depletion of O-GlcNAcylation decreased p65 and CXCR4 expression, reduced O-GlcNAcylation of p65, and decreased the interaction of p65 with OGT. Furthermore, we increased O-GlcNAcylation by inhibiting OGA with Thiamet G or decreased O-GlcNAcylation by inhibiting GFAT with DON. Notably, raising O-GlcNAcylation enhanced the expression of p65 and CXCR4, and conversely, decreasing O-GlcNAcylation reduced p65 and CXCR4. As previously reported, hyper-O-GlcNAcylation contributes to the oncogenic activation of pancreatic cancer cells (Ma et al., 2013). In addition, high concentrations of glucose and glucosamine increase the extent of O-GlcNAcylated p65 and enhance the NF-κB-dependent transcription of target genes (Yang et al., 2008). In our study, an immunofluorescence assay was performed to confirm the effect of O-GlcNAcylation on CXCR4-mediated p65 expression. Immunoreactivities of p65 and OGT were increased under hyper-O-GlcNAcylation conditions.
CXCR4 was detected in the cytoplasm of p65-positive cells, and double-positive cells were also increased under hyper-O-GlcNAcylation conditions, suggesting that hyper-O-GlcNAcylation is related to upregulation of CXCR4 expression via increased p65.
To elucidate the role of hyper-GlcNAcylation of p65 on CXCR4 expression and its effects on the potential of the cervical cancer cells for metastasis to the lung in vivo, nude mice were transplanted with HeLa cells treated with shCTL or shOGT. Interestingly, we found the same results in vivo as those found in vitro. Most importantly, nude mice treated with shOGT HeLa cells exhibited reduced p65 and CXR4 expression as well as decreased O-GlcNAcylated p65 compared to those transplanted with shCTL cells. Our results coincide with results of a previous study that showed that CXCR4 disruption retards the growth of a cervical cell line and represses lung and spleen invasion in mice (Sekula et al., 2014). It is therefore possible that increased CXCR4 expression by O-GlcNAcylated p65 enhances metastasis of cervical cancer to lung. In addition, Ki-67 staining was performed to check the proliferation activity of these cells in lung tissue. We found increased levels of Ki-67 in lungs of nude mice with implanted shCTL cells than in lungs of mice implanted with shOGT cells. Our data also demonstrate high levels of HPV oncogenes E6 / E7 and elevated O-GlcNAcylation levels in lungs from animals transplanted with shCTL cells. HPV E6 / E7 genes, which initiate carcinogenesis, are required in malignant cervical carcinoma cells and maintain proliferation of HeLa cells (DeFilippis et al., 2003). Our results suggest that upregulation of Ki-67, E6, and E7 upregulation is related to CXCR4 regulation, providing an increased risk of metastasis.
In conclusion, we demonstrate that hyper-O-GlcNAcylation affects the regulation of p65, resulting in upregulation of CXCR4 expression. Our data also show that overexpression of CXCR4 by O-GlcNAcylated p65 in lung tissue of nude mice may have a role in facilitating cervical cancer metastasis to lungs. Thus, it appears that cancer cells that express high levels of O-GlcNAcylation help cancer cells to respond to CXCR4-mediated migration. In addition, hyper-O-GlcNAcylation increases the potential of cervical cancer cells to metastasize to the lung.
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program (NRF-2014049413 and NRF-2015R1A5A200883) through the National Research Foundation of Korea funded by the Ministry of Science, ICT and Future Planning
REFERENCES
- Anderson T.M., McMahon J.J., Nwogu C.E., Pombo M.W., Urschel J.D., Driscoll D.L., Lele S.B. Pulmonary resection in metastatic uterine and cervical malignancies. Gynecol Oncol. 2001;83:472–476. doi: 10.1006/gyno.2001.6427. [DOI] [PubMed] [Google Scholar]
- Cai Z., Tchou-Wong K.M., Rom W.N. NF-kappaB in lung tumourigenesis. Cancers. 2011;3:4258–4268. doi: 10.3390/cancers3044258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers A.F., Groom A.C., MacDonald I.C. Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- DeFilippis R.A., Goodwin E.C., Wu L., DiMaio D. Endogenous human papillomavirus E6 and E7 proteins differentially regulate proliferation, senescence, and apoptosis in HeLa cervical carcinoma cells. J Viol. 2003;77:1551–1563. doi: 10.1128/JVI.77.2.1551-1563.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolcet X., Llobet D., Pallares J., Matias-Guiu X. NF-kB in development and progression of human cancer. Virchows Arch. 2005;446:475–482. doi: 10.1007/s00428-005-1264-9. [DOI] [PubMed] [Google Scholar]
- Esencay M., Newcomb E.W., Zagzag D. HGF upregulates CXCR4 expression in gliomas via NF-kappaB. Implications for glioma cell migration. J Neurooncol. 2010;99:33–40. doi: 10.1007/s11060-010-0111-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardini Y., Dehennaut V., Lefebvre T., Issad T. O-GlcNAcylation. A new cancer hallmark? Front Endocrinol. 2013;4:1–14. doi: 10.3389/fendo.2013.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helbig G., Christopherson K.W., Bhat-Nakshatri P., Kumar S., Kishimoto H., Miller K.D., Broxmeyer H.E., Nakshatri H. NF-kappaB promotes breast cancer cell migration and metastasis by inducing the expression of the chemokine receptor CXCR4. J Biol Chem. 2003;278:21631–21638. doi: 10.1074/jbc.M300609200. [DOI] [PubMed] [Google Scholar]
- Jozwiak P., Forma E., Brys M., Krzeslak A. O-GlcNAcylation and metabolic reprograming in cancer. Front Endocrinol. 2014;5:1–13. doi: 10.3389/fendo.2014.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Kitayama J., Kazama S., Nagawa H. Expression pattern of CXC chemokine receptor-4 is correlated with lymph node metastasis in human invasive ductal carcinoma. Breast Cancer Res. 2003;5:144–150. doi: 10.1186/bcr627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M., Jankowski K., Reca R., Wysoczynski M., Bandura L., Allendorf D.J., Zhang J., Ratajczak J., Ratajczak M.Z. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. J Mol Histol. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Ma Z., Vocadlo D.J., Vosseller K. Hyper-O-GlcNAcylation is anti-apoptotic and maintains constitutive NF-kappaB activity in pancreatic cancer cells. J Biol Chem. 2013;288:15121–15130. doi: 10.1074/jbc.M113.470047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nair A., Venkatraman M., Maliekal T.T., Nair B., Karunagaran D. NF-kappaB is constitutively activated in high grade squamous intraepithelial lesions and squamous cell carcinomas of the human uterine cervix. Oncogene. 2003;22:50–58. doi: 10.1038/sj.onc.1206043. [DOI] [PubMed] [Google Scholar]
- Reinhardt M.J., Ehritt-Braun C., Vogelgesang D., Ihling C., Hogerle S., Mix M., Moser E., Krause T.M. Metastatic lymph nodes in patients with cervical cancer: detection with MR imaging and FDFG PET. Radiology. 2001;218:776–782. doi: 10.1148/radiology.218.3.r01mr19776. [DOI] [PubMed] [Google Scholar]
- Sekula M., Miekus K., Majka M. Downregulation of the CXCR4 receptor inhibits cervical carcinoma metastatic behavior in vitro and in vivo. Int J Oncol. 2014;44:1853–1860. doi: 10.3892/ijo.2014.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65:5–29. doi: 10.3322/caac.21254. [DOI] [PubMed] [Google Scholar]
- Sironi S., Buda A., Picchio M., Perego P., Moreni R., Pellegrino A., Colombo M., Mangioni C., Messa C., Fazio F. Lymph node metastasis in patients with clinical early-stage cervical cancer. Detection with integrated FDG PET/CT. Radiology. 2006;238:272–279. doi: 10.1148/radiol.2381041799. [DOI] [PubMed] [Google Scholar]
- Spano D., Heck C., De Antonellis P., Christofori G., Zollo M. Molecular networks that regulate cancer metastasis. Semin Cancer Biol. 2012;22:234–249. doi: 10.1016/j.semcancer.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Taichman R.S., Cooper C., Keller E.T., Pienta K.J., Taichman N.S., McCauley L.K. Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res. 2002;62:1832–1837. [PubMed] [Google Scholar]
- Tellis C.J., Beechler C.R. Pulmonary metastasis of carcinoma of the cervix: a retrospective study. Cancer. 1982;49:705–1709. doi: 10.1002/1097-0142(19820415)49:8<1705::aid-cncr2820490828>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Thanapprapasr D., Nartthanarung A., Likittanasombut P., Na Ayudhya N.I., Charakorn C., Udomsubpayakul U., Subhadarbandhu T., Wilailak S. Bone metastasis in cervical cancer patients over a 10-year period. Int J Gynecol Cancer. 2010;20:373–378. doi: 10.1111/IGC.0b013e3181d4a0a1. [DOI] [PubMed] [Google Scholar]
- Wang X., Adjei A.A. Lung cancer and metastasis:New opportunities and challenges. Cancer Metastasis Rev. 2015;34:169–171. doi: 10.1007/s10555-015-9562-4. [DOI] [PubMed] [Google Scholar]
- Yang W.H., Park S.Y., Nam H.W., Kim D.H., Kang J.G., Kang E.S., Kim Y.S., Lee H.C., Kim K.S., Cho J.W. NF-kappaB activation is associated with its O-GlcNAcylation state under hyperglycemic conditions. Proc Natl Acad Sci USA. 2008;105:17345–17350. doi: 10.1073/pnas.0806198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhi Y., Lu H., Duan Y., Sun W., Guan G., Dong Q., Yang C. Involvement of the Nuclear Factor-KappaB signaling pathway in the regulation of CXC chemokine receptor-4 expression in neuroblastoma cells induced by tumor necrosis factor-alpha. Int J Mol Med. 2015;35:349–357. doi: 10.3892/ijmm.2014.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A. Chemokines and cancer: Int. J Cancer. 2006a;119:2026–2029. doi: 10.1002/ijc.22024. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. Involvement of chemokine receptors in organ-specific metastasis. Contrib Microbiol. 2006b;13:191–199. doi: 10.1159/000092973. [DOI] [PubMed] [Google Scholar]
- Zlotnik A. New insights on the role of CXCR4 in cancer metastasis. J Pathol. 2008;215:211–213. doi: 10.1002/path.2350. [DOI] [PubMed] [Google Scholar]


