Abstract
Newborns affected by congenital diaphragmatic hernia (CDH) need cardio-respiratory stabilization before undergoing surgical repair. Open lung strategy is a well-established approach to optimize lung volume in preterm infants with Respiratory Distress Syndrome (RDS), using both High Frequency Oscillatory Ventilation (HFOV) and Conventional Mechanical Ventilation (CMV).
We report a case of left CDH with severe lung hypoplasia, managed applying open lung strategy in HFOV (pre-surgery period) and in Assist-Control with Volume Guarantee (post-surgery period), guided by SpO2 changes, TcPO2 and TcPCO2 monitoring. Opto-electronic plethysmography was used to measure end-expiratory chest wall volume changes (ΔEEcw) related to lung volume variations occurring during pressure changes. OEP confirmed the efficacy of using SpO2 and transcutaneous gas monitoring during this recruitment maneuver.
Keywords: CDH, Lung volume, Ventilation strategy, Newborn, Opto-electronic plethysmography
Abbreviations
- BW
Birth Weight
- CDH
Congenital Diaphragmatic Hernia
- CMV
Conventional Mechanical Ventilation
- ΔEEcw
end-expiratory chest wall volume variations
- FiO2
Fraction of inspired Oxygen
- GA
Gestational Age
- HFOV
High Frequency Oscillatory Ventilation
- iNO
inhaled Nitric Oxide
- LHR
lung area to head circumference ratio
- NICU
neonatal intensive care unit
- PEEP
positive end expiratory pressure
- PIP
peak inspiratory pressure
- P-V curve
Pressure-Volume curve
- RDS
respiratory distress syndrome
- SpO2
pulse oxygen saturation
- TcPO2
transcutaneous oxygen pressure
- TcPCO2
transcutaneous carbon dioxide pressure
- VG
volume guarantee
1. Introduction
Survival in patients affected by Congenital Diaphragmatic Hernia (CDH) ranges from 60 to 70% [1], and the main causes of death are related to lung hypoplasia and pulmonary hypertension [2].
Cardio-respiratory stabilization before surgical repair, in fact, is a crucial step for newborns affected by CDH [3], [4]. During the first hours of life all infants with severe disease require mechanical ventilation to establish normal gas-exchange. Moreover, the achievement of an adequate lung volume is crucial for these neonates' survival. Hence, the aim of the first hours' management is to optimize blood oxygenation and recruit lung volume, avoiding volu-barotrauma related injuries [4].
The “open lung strategy” has been demonstrated to be an effective procedure to recruit the lung in preterm infants with Respiratory Distress Syndrome (RDS) [5]. This technique can be performed while on High Frequency Oscillatory Ventilation (HFOV) or Conventional Mechanical Ventilation (CMV) and it consists of setting the lung volume on the deflation limb of the P-V curve.
The successful use of HFOV before surgery in CDH affected patients has been well described by Reye et al. and Miguet et al. [6], [7]. However, the efficacy of this recruitment maneuver in both HFOV and CMV during the stabilization phase and after surgical repair hasn't been previously monitored in these patients.
We describe a case of left CDH with severe lung hypoplasia which has been managed applying a high lung volume strategy under the guidance of SpO2 changes and TcPO2/TcPCO2 monitoring.
These changes were recorded by Opto-electronic plethysmography (OEP), which measures the end-expiratory chest wall volume variations (ΔEEcw) on a breath-by-breath basis [8]. These changes have shown to be correlated with lung volume variations in mechanically ventilated adult patients with RDS [9] and in the post-surgery follow-up of children affected by CDH at birth [10].
2. Case report
We describe the case of a female infant, born at 37 weeks of gestational age (GA) with antenatal diagnosis of CDH at 20+4 weeks' gestation. Fetal ultrasound and magnetic resonance imaging revealed left-sided CDH with severe lung hypoplasia (stomach, intestine, left lobe of liver in the thorax with mediastinal shift). Lung area to head circumference ratio (LHR) = 1.3 with observed/expected LHR = 0.7. No other congenital anomalies were detected.
Birth weight (BW) was 2645 g. She was intubated immediately after birth and positive pressure ventilation was given by T-piece resuscitator (Neo-puff, Fisher & Paykel) set on PIP = 25 cmH2O, PEEP = 5 cmH2O with FiO2 = 1.0. APGAR score at 1-5-10 minutes = 5-8-9.
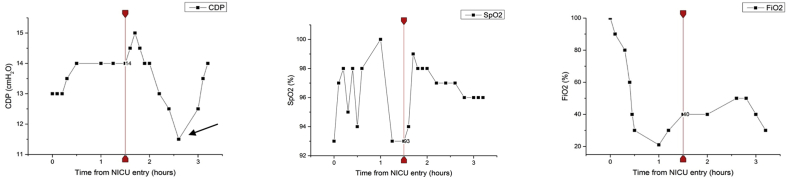
At 10 minutes of life SpO2 was 88–90% with FiO2 = 0.5. At 25 minutes of life the infant was transferred to NICU and HFOV was immediately started (Sensor Medics 3100 A, Care Fusion) with a high lung volume strategy using incremental and decreasing Continuous Distending Pressure (CDP) maneuver [11]. HFOV was started with CDP = 13 cmH20, FiO2 = 1.0, amplitude (ΔP) = 30 cmH20, frequency = 10 Hz. Targets for SpO2 and TcPCO2 were ≥95% and 40–65 mmHg respectively.
During the “first phase” of the maneuver, CDP was stepwise increased by 1 cmH20 (on average every 5 minutes) and FiO2 progressively decreased until FiO2 requirement was 0.3, maintaining SpO2 and TcPCO2 targets. After echocardiographic assessment of pulmonary hypertension, inhaled Nitric Oxide (iNO) administration was started at 20 ppm. During the “second phase”, CDP was progressively decreased by 0.5 cmH2O (on average every 5 minutes) until FiO2 had to be increased to maintain SpO2 and TcPCO2 targets. This moment was considered the ‘critical closing pressure point’.
During the “third phase”, the lung was recruited again and stabilized with stepwise increments of CDP by 0.5–1 cmH2O above the critical closing pressure point, decreasing FiO2 until 0.3. SpO2 and TcPCO2 targets were maintained in range during this phase, as well (Fig. 1).
Fig. 1.
Continuous distending pressure (CDP), fraction of inspired oxygen (FiO2) and pulse oxygen saturation (SpO2) during the three phases of the high lung volume strategy maneuver in HFOV. The vertical line indicates the start of inhaled nitric oxide (iNO). The black arrow points out the “critical closing pressure point”.
Throughout the whole maneuver, mean non-invasive blood pressure was stable above 50 mmHg. The complete maneuver lasted 3 hours and 20 min and the optimal CDP was established at 14 cmH2O. During the whole maneuver the infant was monitored with OEP. Through the analysis of ΔEEcw measurements, this device provided estimated variations of end-expiratory lung volume at different CDP levels.
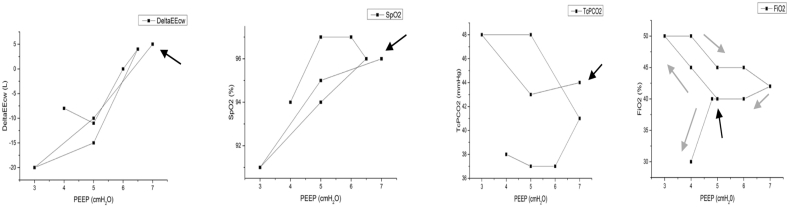
Surgery was performed on day 3 via conventional open abdominal approach. A large diaphragmatic defect was repaired with a prosthetic patch. On day 13 the infant was switched from HFOV to CMV, in Assist-Control with volume guarantee (VG) (Vt = 4.5 ml/Kg) (VN 500, Draeger) with initial PEEP = 5 cmH2O. The optimal PEEP was found through stepwise incremental and decreasing changes of 0.2 cmH2O on average every 5 minutes [10], using the same strategy describe above for HFOV, always guided by SpO2 changes and TcPO2/TcPCO2 monitoring. The complete maneuver lasted 1 hour and the optimal PEEP was equal to 4 cmH2O. OEP was used to measure ΔEEcw at different PEEP levels (Fig. 2).
Fig. 2.
End-expiratory chest wall volume variation (ΔEEcw), pulse oxygen saturation (SpO2), trans-cutaneous partial pressure of carbon dioxide (TcPCO2) and fraction of inspired oxygen (FiO2) during high lung volume strategy after surgery in CMV. Driving pressure is positive end-expiratory pressure (PEEP) and it is reported on the x-axis. The black arrow indicates the starting point and the grey ones clarify the direction of the maneuver.
3. Discussion
CDH is a severe respiratory and cardiovascular disorder and the proper ventilation management is crucial to ensure normal gas-exchange avoiding additional lung injuries. The lung hypoplasia secondary to the viscera herniation in the thorax is associated with abnormal development of the pulmonary vascular bed, and both these anomalies cause pulmonary hypertension. Moreover, the risk of lung hyperinflation related to high volumes can worsen the cardiovascular function and lead to hypoxia and hypercapnia.
Surgical repair remains the life-saving intervention in CDH. However, it has been well described that a delayed surgical treatment after stabilization with HFOV is a safe and effective strategy for better outcomes [6], [7].
In fact, a “gentle” lung recruitment with oscillatory ventilation reduces the volume and barotrauma and minimize the effects of ventilator-induced lung injury (VILI), which are additional causes of morbidity and mortality in CDH infants [11]. Although the efficacy of HFOV in the pre-surgical phase has been widely established, to our knowledge the monitoring of the “recruitment maneuver” during lung volume optimization strategy has not been described in these patients, neither before nor after surgery.
The search for the “optimal and safe lung volume” with the minimal CDP which can provide adequate gas exchange with the lowest FiO2 could be, in fact, a useful method to avoid further lung injuries.
In the pre-surgery period, during HFOV, we have set the initial CDP close to 13 cmH2O in agreement with the most recent recommendations [12]. Then, according to our guidelines, as soon as the infant is stable after surgical repair, we switch from HFOV to VG-CMV.
In order to reach the optimal PEEP level and the minimal FiO2 to maintain the SpO2 target, we use SpO2 changes and TcPO2/TcPCO2 monitoring.
To date, it is impossible to establish at the bedside the adequate and safe lung volume to deliver. SpO2 changes and TCpO2/TcPCO2 monitoring are the only available parameters for choosing the lowest pressure to set the lung volume on the deflation limb of the P-V curve.
Opto-Electronic Plethysmography, which has been recently successfully applied in the follow up of children with previous surgical repair of CDH [10], gives an accurate estimation of lung volume variations from thoraco-abdominal motion analysis.
In this case report, we have confirmed with OEP that a high lung volume ventilation strategy can be guided by SpO2 and transcutaneous gas monitoring. However, OEP was not used to adjust ventilation in real time, since its data were retrospectively analyzed.
4. Conclusion
A recruitment maneuver with high lung volume strategy using HFOV guided by SpO2 and TcPCO2 monitoring seems to be a promising approach not only in the treatment of RDS in preterm babies, but also for the early respiratory stabilization of infants affected by CDH. Thanks to OEP measurements, this maneuver has shown to be effective both in the acute phase before surgery and in the recovery phase after surgical repair. Infact, OEP data have confirmed the efficacy of the recruitment maneuver and the reliability of the parameters we use in routine clinical practice to guide this kind of ventilator's adjustments.
Even if pulmonary hypertension caused by lung hypoplasia needs to be diagnosed and treated with specific medical interventions, this ventilation strategy is helpful in finding the correct CDP level to recruit the lung avoiding further injuries.
Even if not in real time, Opto-electronic plethysmography could be useful to confirm this strategy when only SpO2 and transcutaneous gas monitoring are available.
Funding source
No funding was secured for this study.
Financial disclosure
Authors declare no financial relationship relevant to this article.
Conflict of interest
Authors have no conflicts of interest to disclose.
Contributor's statement
Dr. Lista and Prof. Aliverti conceived the study and drafted the initial manuscript.
Dr. Bresesti critically reviewed the manuscript and revised the initial draft.
Dr. Cavigioli, Dr. Castoldi and Dr. Lupo helped with the coordination of the project and critically discussed the manuscript.
Dr. Lo Mauro e Prof. Aliverti were responsible for applying optoelectronic plethysmography, extracted the data and performed the data analysis.
All authors have read and approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
References
- 1.Kotecha S., Barbato A., Bush A., Claus F., Davenport M., Delacourt C., Deprest J., Eber E., Frenckner B., Greenough A., Nicholson A.G., Anton-Pacheco J.L., Midulla F. Congenital diaphragmatic hernia. Eur. Respir. J. 2012;39:820–829. doi: 10.1183/09031936.00066511. [DOI] [PubMed] [Google Scholar]
- 2.Stege G., Fenton A., Jaffray B. Nihilism in the 1990s: the true mortality of congenital diaphragmatic hernia. Pediatrics. 2003;112:532–535. doi: 10.1542/peds.112.3.532. [DOI] [PubMed] [Google Scholar]
- 3.Logan J.W., Rice H.E., Goldberg R.N., Cotten C.M. Congenital diaphragmatic hernia: a systematic review and summary of best-evidence practice strategies. J. Perinatol. 2007;27:535–549. doi: 10.1038/sj.jp.7211794. [DOI] [PubMed] [Google Scholar]
- 4.Logan J.W., Cotten C.M., Goldberg R.N., Clark R.H. Mechanical ventilation strategies in the management of congenital diaphragmatic hernia. Semin. Pediatr. Surg. 2007;16:115–125. doi: 10.1053/j.sempedsurg.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 5.Dargaville P.A., Tingay D.G. Lung protective ventilation in extremely preterm infants. J. Paediatr. Child. Health. 2012;48:740–746. doi: 10.1111/j.1440-1754.2012.02532.x. [DOI] [PubMed] [Google Scholar]
- 6.Miguet D., Claris O., Lapillonne A., Bakr A., Chappuis J.P., Salle B.L. Preoperative stabilization using high-frequency oscillatory ventilation in the management of congenital diaphragmatic hernia. Crit. Care Med. 1994;22:S77–S82. doi: 10.1097/00003246-199422091-00008. [DOI] [PubMed] [Google Scholar]
- 7.Reyes C., Chang L.K., Waffarn F., Mir H., Warden M.J., Sills J. Delayed repair of congenital diaphragmatic hernia with early high-frequency oscillatory ventilation during preoperative stabilization. J. Pediatr. Surg. 1998;33 doi: 10.1016/s0022-3468(98)90523-1. 1010-1014; discussion 1014–1016. [DOI] [PubMed] [Google Scholar]
- 8.Massaroni C., Carraro E., Vianello A., Miccinilli S., Morrone M., Levai I.K., Schena E., Saccomandi P., Sterzi S., Dickinson J.W., Winter S., Silvestri S. Optoelectronic plethysmography in clinical practice and research: a review. Respiration. 2017;93:339–354. doi: 10.1159/000462916. [DOI] [PubMed] [Google Scholar]
- 9.Dellaca R.L., Aliverti A., Pelosi P., Carlesso E., Chiumello D., Pedotti A., Gattinoni L. Estimation of end-expiratory lung volume variations by optoelectronic plethysmography. Crit. Care Med. 2001;29:1807–1811. doi: 10.1097/00003246-200109000-00026. [DOI] [PubMed] [Google Scholar]
- 10.Laviola M., Zanini A., Priori R., Macchini F., Leva E., Torricelli M., Ceruti C., Aliverti A. Thoraco-abdominal asymmetry and asynchrony in congenital diaphragmatic hernia. Pediatr. Pulmonol. 2015;50:915–924. doi: 10.1002/ppul.23081. [DOI] [PubMed] [Google Scholar]
- 11.De Jaegere A., van Veenendaal M.B., Michiels A., van Kaam A.H. Lung recruitment using oxygenation during open lung high-frequency ventilation in preterm infants. Am. J. Respir. Crit. Care Med. 2006;174:639–645. doi: 10.1164/rccm.200603-351OC. [DOI] [PubMed] [Google Scholar]
- 12.Snoek K.G., Reiss I.K., Greenough A., Capolupo I., Urlesberger B., Wessel L., Storme L., Deprest J., Schaible T., van Heijst A., Tibboel D. Standardized postnatal management of infants with congenital diaphragmatic hernia in Europe: the CDH EURO consortium consensus - 2015 update. Neonatology. 2016;110:66–74. doi: 10.1159/000444210. [DOI] [PubMed] [Google Scholar]