Abstract
Sodium pumps are ubiquitously expressed membrane proteins that extrude three Na+ ions in exchange for two K+ ions, using ATP as an energy source. Recent studies have illuminated additional, dynamic roles for sodium pumps in regulating the excitability of neuronal networks in an activity-dependent fashion. We review their role in a novel form of short-term memory within rhythmic locomotor networks. The data we review derives mainly from recent studies on Xenopus tadpoles and neonatal mice. The role and underlying mechanisms of pump action broadly match previously published data from an invertebrate, the Drosophila larva. We therefore propose a highly conserved mechanism by which sodium pump activity increases following a bout of locomotion. This results in an ultraslow afterhyperpolarization (usAHP) of the membrane potential that lasts around 1 min, but which only occurs in around half the network neurons. This usAHP in turn alters network excitability so that network output is reduced in a locomotor interval-dependent manner. The pumps therefore confer on spinal locomotor networks a temporary memory trace of recent network performance.
Keywords: central pattern generator, locomotion, motor memory, sodium pumps, spinal cord
motor systems have evolved to meet the species-specific behavioral requirements on which animal survival and reproduction depend. To succeed, the underlying motor circuits must be adaptable in the face of the demands placed on individuals by prevailing external and internal conditions. Such circuit adaptations, which may relate to developmental stage and/or hormonal state, are mostly due to changes in the integrative electrical properties of, and synaptic weightings between, component neurons within motor circuits (Harris-Warrick and Marder 1991). Many of these changes are mediated by the opening of ion channels, and the consequent alterations to circuit function can involve both neuromodulation and activity-dependent neuronal plasticity. One disadvantage of this ion channel-based strategy is that the decrease in input resistance that accompanies channel opening could shunt incoming synaptic inputs and decrease the responsiveness of neurons and subsequent network output. This, in turn, could compromise the intended behavior, and if this involves the escape from a predator, for example, it could be potentially catastrophic for survival. An alternative strategy is for neuronal activity or neuromodulation to affect the function of ion pumps, which, since there is no change in input resistance, should not shunt the membrane response and hence preserve the responsiveness of the network to various inputs. Furthermore, changes in the activity of ion pumps can exert effects on the excitability of neurons on a much slower timescale, over many seconds and even minutes, leaving a prolonged memory trace of a neuron’s recent activity.
The Na+-K+-ATPase (the Na+ pump) is one of the most ubiquitously expressed proteins in the animal kingdom, which is most renowned for its role in establishing a gradient of high extracellular Na+ and high intracellular K+ ion concentrations across cell membranes. With each Na+ pump cycle, three Na+ ions are extruded and two K+ ions flow into the cell, utilizing ATP as an energy source. Because of this charge asymmetry, Na+ pump activity sets and homeostatically maintains the resting membrane potential on which neuronal firing relies, and in so doing accounts for more than half of all brain energy consumption (Engl and Attwell 2015).
Recently, a novel and dynamic role for the Na+ pump as an activity-dependent regulator of brain and spinal circuit function has been reported across a wide range of neurons, systems, behaviors, and species. Within motor systems, for example, seminal work on crawling in Drosophila larvae has demonstrated that high-frequency action potential firing of motoneurons causes a pump-mediated hyperpolarization lasting tens of seconds, which in turn influences future locomotory crawling behavior (Pulver and Griffith 2010). In the present article, we review and compare similar findings from spinal central pattern generator (CPG) circuits controlling rhythmic locomotion in two phylogenetically disparate vertebrate model systems: the Xenopus frog tadpole and the neonatal mouse. As in Drosophila, these circuits also possess an intrinsic pump-based mechanism that links future to past network activity. This suggests a highly conserved, pump-mediated dynamic regulation of motor circuit function. In spinal motor circuits, the duration of a bout of locomotion is influenced by previous network activity if two bouts occur within about a minute of each other; a form of short-term motor memory (Picton et al. 2017; Zhang and Sillar 2012; Zhang et al. 2015). This motor memory relies on the presence of a pump-mediated ultraslow afterhyperpolarization (usAHP) of up to 10 mV in spinal neurons, which lasts for the same duration of approximately a minute.
Na+ Pump Regulation in Three Locomotor Systems
Ultraslow afterhyperpolarization.
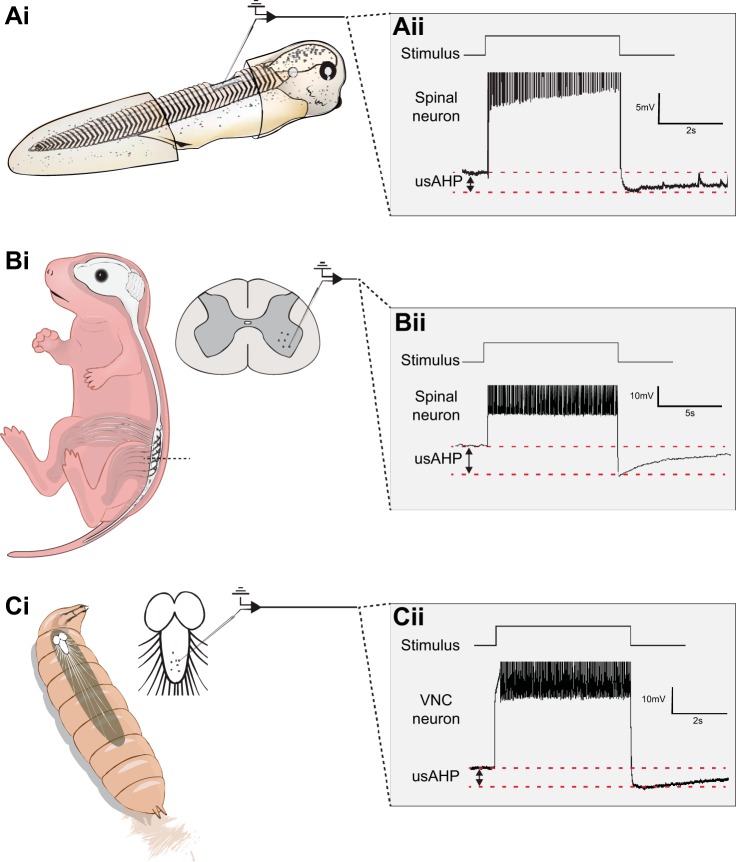
In both the tadpole (Fig. 1A) and neonatal mouse (Fig. 1B), high-frequency action potential firing drives the resting membrane potential to a more hyperpolarized level in a subset of motoneurons and interneurons in the spinal cord. A remarkably similar phenomenon has also been reported in Drosophila larva motoneurons (Pulver and Griffith 2010; Fig. 1C). This hyperpolarization is distinguished from other ion channel-mediated afterhyperpolarizations (AHPs; e.g., the “fast,” “medium,” or “slow” AHP; Storm 1987) largely by its duration, with neurons remaining hyperpolarized once activity has stopped for up to 1 min. Although the amplitude of an ultraslow AHP (usAHP) can vary quite considerably both within and between neuron types, our findings in Xenopus and mouse spinal neurons suggest that, on average, the pump AHP involves a hyperpolarization of ~5 mV (Fig. 1, Aii and Bii), remarkably similar to the equivalent event in Drosophila larvae (Fig. 1Cii).
Fig. 1.
The ultraslow afterhyperpolarization (usAHP) in CPG neurons of 3 species. Ai: experimental preparation for making patch-clamp recordings from an immobilized stage 37/8 Xenopus tadpole. Aii: following either swimming or, in this case, a long suprathreshold current pulse, the membrane potential is driven to a more hyperpolarized membrane potential (the usAHP). Bi: experimental preparation for making patch-clamp recordings from neonatal mice. Bii: following a long suprathreshold current pulse, a usAHP is observed in spinal motoneurons and interneurons in neonatal mice. Ci: schematic of a third-instar Drosophila larva. Cii: a usAHP observed in a Drosophila motoneuron. VNC, ventral nerve cord.
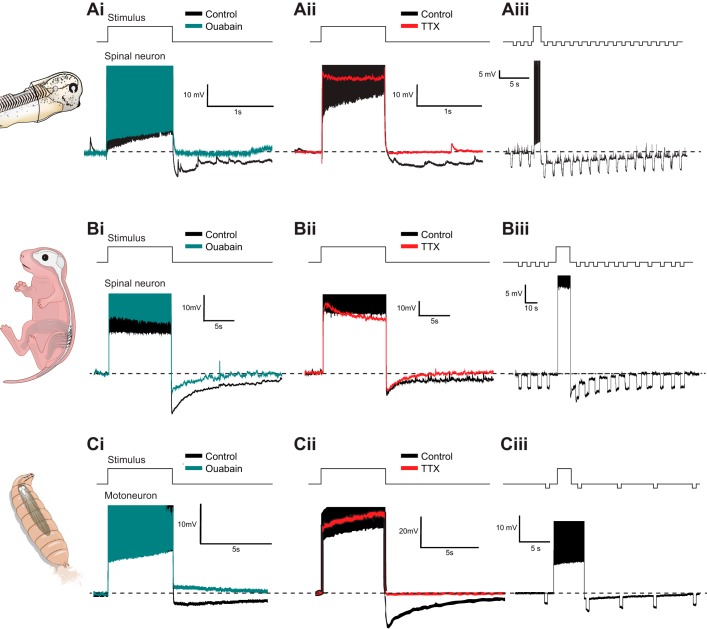
In addition to its long duration, several other features of the usAHP distinguish it from ion channel-mediated AHP mechanisms. For example, because it is mediated by the Na+ pump, it is selectively blocked by a low concentration of the cardiac glycoside ouabain (Fig. 2, Ai, Bi, and Ci). The usAHP is also highly dependent on the accumulation of intracellular Na+ that accompanies repetitive action potential firing. Therefore, blocking fast Na+ channels with tetrodotoxin (TTX), to prevent action potential generation, also effectively abolishes the usAHP (Fig. 2, Aii, Bii, and Cii). Finally, because the usAHP occurs upon the increased activation of ion pumps, rather than ion channel opening or closing, there are no detectable changes in conductance, and this can be observed by measuring a consistent membrane response to small injections of hyperpolarizing current throughout the usAHP (Fig. 2, Aiii, Biii, and Ciii). Perhaps not surprisingly, there are a number of differences in the features of the usAHP in tadpoles and mice at the single-cell level. For example, whereas ouabain and TTX completely abolish the usAHP in tadpoles, a shorter duration AHP often persists in many motoneurons and interneurons in mice (Fig. 2, Bi and Bii), presumably due to the presence of additional, voltage-dependent AHP mechanisms such as the medium and/or slow AHP, which can persist in the absence of spiking (Rekling et al. 2000).
Fig. 2.
A cross-species comparison of the basic features of the usAHP. Ai: the usAHP is abolished by the Na+ pump blocker ouabain. Aii: the usAHP is also abolished when fast Na+ channels are blocked using TTX. Aiii: by measuring the membrane response to a small hyperpolarizing current pulse, we found no changes in conductance before, during, or after the induction of a usAHP, suggesting the involvement of a Na+ pump [adapted from Zhang and Sillar (2012).] The experimental manipulations outlined in A have similar results in neonatal mouse CPG neurons [B; adapted from Picton et al. (2017)] and Drosophila motoneurons [C; adapted from Pulver and Griffith (2010)].
Physiological roles for the Na+ pump.
By its very nature, the usAHP is ideally positioned to function as a spike rate monitor whose duration and amplitude reflect the integration of spike frequency over time. Furthermore, the usAHP is not only generated in response to artificial current injection protocols used to evoke spikes, but by any stimulus that produces trains of action potentials sufficient to generate a buildup of intracellular Na+ (e.g., locomotion; Fig. 3Aii). Importantly, because the usAHP recovers over a period of around a minute, it acts as a transient engram of how recently and how intensely locomotor activity occurred.
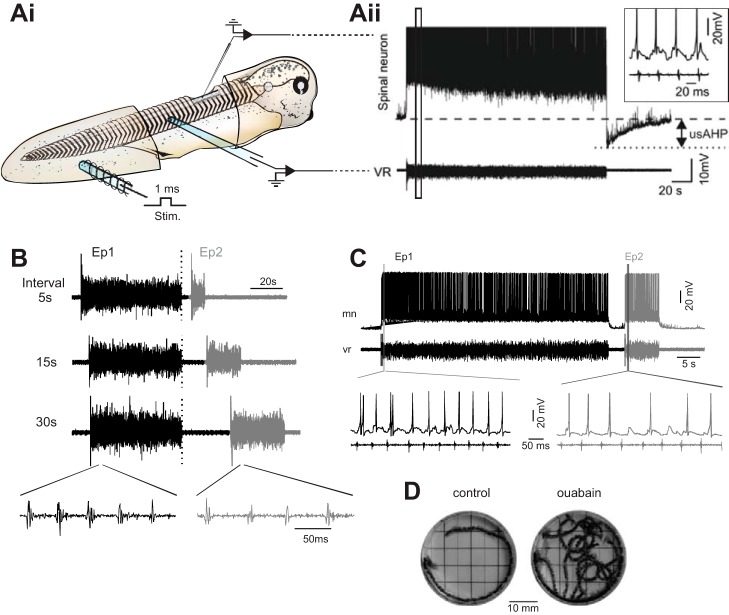
Fig. 3.
The usAHP as a short-term memory mechanism in Xenopus tadpoles. Ai: schematic showing the experimental setup. Aii: a brief (1 ms) current pulse to the tail (Stim.) initiates an episode of swimming that is recorded at both the single-cell level (top) and at the level of overall network output using ventral root recording (bottom). Note the prolonged membrane hyperpolarization (usAHP) in the intracellular trace at the end of the swim episode. Inset shows an expansion of the recording indicated by the black box showing the intracellular and ventral root traces during swimming. B: ventral root recordings showing that an evoked swim episode (Ep 2) is shorter and slower when it follows a previous episode (Ep 1) after a 5-, 15-, or 30-s interval. C: the interval relationship is apparent when activity is evoked within the 1-min usAHP that follows swimming, which reduces the spike probability of CPG neurons. D: real swimming behavior in a Xenopus tadpole with multiple consecutive video frames overlapped to show swim path in response to touch. When the Na+ pumps are blocked using ouabain, the tadpole is unable to regulate its activity and swims continuously. [Adapted from Zhang and Sillar (2012); Zhang et al. (2015).]
In Xenopus tadpoles, we have explored how this short-term memory of recent activity acts to regulate the interval relationship between evoked episodes of “fictive swimming” (motor output without muscle contraction). When the interval between swim episodes is set to longer than the duration of a usAHP (longer than 1 min), episodes of evoked swimming in a “well-rested” tadpole are statistically identical, in both the duration of a swim episode and all other parameters of swimming (swim frequency, burst durations, etc.). However, when this interval is reduced to 30, 15, or 5 s, the second episode is progressively shorter, slower, and weaker, in an interval-dependent manner (Fig. 3B; note spike failures in episode 2, Fig. 3C). The importance of the Na+ pump for this self-regulation of network output becomes clear when the pumps are blocked by ouabain; the animal becomes completely unable to regulate its own locomotor activity, causing it to swim almost indefinitely (Fig. 2D).
The swim durations and inter-episode intervals involved may seem short anthropomorphically (tens of seconds) but need to be scaled to be appreciated from a human perspective, and in the broader context of locomotion. If we treat a single tail undulation as equivalent to one human stride, then a typical 2-min episode of 20-Hz swimming (~2,400 swim cycles) could be considered broadly equivalent to a 5-km sprint for a human (assuming a typical stride length of ~2 m). This distance could comfortably be covered in around 30 min, but imagine resting only for a minute before being stimulated to sprint again while still fatigued; the runner is unlikely to get as far, or locomote at the same speed, as it could from a well-rested start. Whether Na+ pumps play a direct role in human fatigue is not yet completely clear, but certainly the evidence for central mechanisms of fatigue is extremely compelling (reviewed in Gandevia 2001). More specifically, there is strong evidence that central fatigue involves an activity-dependent reduction in motoneuron drive (Ranieri and Di Lazzaro 2012; Rossi et al. 2012). Furthermore, it has been shown that human motor axons display an activity-dependent hyperpolarization following natural activity, which is due to an enhancement of Na+ pump activity and whose duration and amplitude depend on the axonal discharge rate (Kiernan et al. 2004; Vagg et al. 1998). This raises the fascinating possibility that an activity-dependent enhancement of Na+ pump activity in spinal neurons may contribute to fatigue during human locomotion. Given the ubiquity of pumps throughout the nervous system, they have enormous potential as drug targets, with important implications not only for endurance athletes but also in the context of diseases associated with fatigue symptoms such as diabetes (Krishnan et al. 2008) and amyotrophic lateral sclerosis (ALS; Ellis et al. 2003), in which Na+ pump dysfunction has been implicated.
It has long been known that one way to experimentally “fatigue” a neuron is to raise the levels of intracellular Na+. These experiments were first conducted on the squid giant axon in the mid 1950s and, quite unexpectedly, high Na+ levels resulted in a tonic membrane hyperpolarization (Hodgkin and Keynes 1956) that turned out to be mediated by enhanced Na+ pump activity. In our experiments, we have used a drug called monensin, a sodium ionophore, to raise the level of intracellular Na+ in spinal CPG neurons. This not only enhances the usAHP by increasing Na+ pump activity, but in effect it causes the locomotor network to become chronically fatigued. Under these conditions, the swim network acts as if it is being activated from an unrested starting point, resulting in weaker, slower, and shorter locomotion.
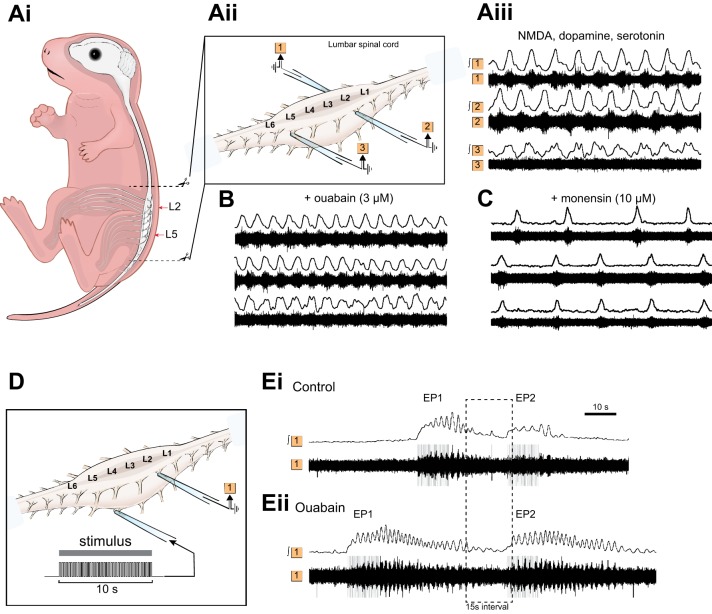
We have also explored the effects of Na+ pump manipulation in the lumbar spinal cord of neonatal mice, using two methods for evoking locomotor activity. Traditionally, a combination of drugs (dopamine, NMDA, serotonin) is applied to induce a continuous locomotor rhythm (Fig. 4A). Under these conditions, blockade of Na+ pumps using ouabain causes the rhythm frequency to increase (Fig. 4B). Conversely, raising the levels of intracellular Na+ using monensin, which indirectly activates the Na+ pump, causes the opposite effect (Fig. 4C). Although this reveals the importance of the Na+ pump for frequency control, it obviously cannot address the role of Na+ pumps in regulating intervals between locomotor episodes.
Fig. 4.
Na+ pump manipulation in the neonatal mouse preparation. Ai: schematic depicting neonatal mouse spinal cord preparation. Aii: glass suction electrodes are attached to the first or second lumbar ventral roots (L1, L2) on the left and right sides of an isolated spinal cord to record flexor-related activity, and a third electrode is attached to the fifth ventral root (L5) to record extensor-related activity. Aiii: raw and rectified/integrated traces showing drug-induced activity on the left and right L2 roots and the right L5 root. B: Na+ pump blockade increases the frequency of locomotor bursting. C: activation of the Na+ pump has the opposite effect of slowing locomotor burst frequency. D: for sensory stimulation, an electrode was attached to the fourth or fifth dorsal root (L4 or L5) to deliver current pulses to initiate locomotion. Ei: when 2 episodes of locomotor output are evoked with a short interval (15 s), the second episode is both shorter and slower compared with this first episode. Eii: following blockade of the Na+ pump, not only are episodes longer and faster compared with control, but the interval relationship is abolished. [Adapted from Picton et al. (2017).]
To address this question in a similar way to our earlier tadpole experiments, we switched to using dorsal root sensory stimulation to evoke individual, more natural bouts of locomotor activity (Fig. 4, D and E). In much the same way as in tadpoles, episode 2 is clearly influenced by episode 1 so long as the interval is shorter than 1 min (Fig. 4Ei). This relationship breaks down in the presence of ouabain such that episode 2 is now similar to episode 1 in duration, frequency, and amplitude (Fig. 4Eii).
Mechanism linking usAHP and A-current.
The Na+ pump-mediated usAHP clearly plays an important role in allowing locomotor networks to regulate their output in relation to past activity. However, it is not immediately obvious how the relatively modest membrane hyperpolarization (~5 mV) caused by increased activation of the Na+ pump can cause dramatic changes in neuronal excitability, especially since there is no obvious change in conductance. A likely possibility is that in different systems, different voltage-dependent currents are affected by the change in membrane potential. Two currents that appear to have important interactions with Na+ pump currents in CPG networks are Ih and IA (Kueh et al. 2016; Pulver and Griffith 2010; Zhang et al. 2015).
Pulver and Griffith (2010) showed in Drosophila larva motoneurons that the pump-mediated AHP brought the membrane potential into a range that caused the de-inactivation of an A-type K+ current, Ishal, which in turn introduced a delay to the first spike when activity resumed. Classically, channels mediating IA are largely inactivated at the resting membrane potential but are de-inactivated by hyperpolarization, so that when the neuron is next excited by a depolarizing input, the rate of depolarization is slowed by IA. We found precisely this mechanism at play in tadpole spinal neurons (Zhang et al. 2015). When a usAHP was induced by a high-frequency train of action potentials (Fig. 5A2), the delay to firing in response to a brief current pulse was longer compared with that before the induction of a usAHP (Fig. 5, A3 vs. A1). The presence of a 4-AP-sensitive A-type K+ current was confirmed using voltage-clamp recordings (Fig. 5B). Whether a similar mechanism involving an A-type K+ current contributes to the role of the usAHP in neonatal mice is yet to be confirmed, but this possibility seems likely.
Fig. 5.
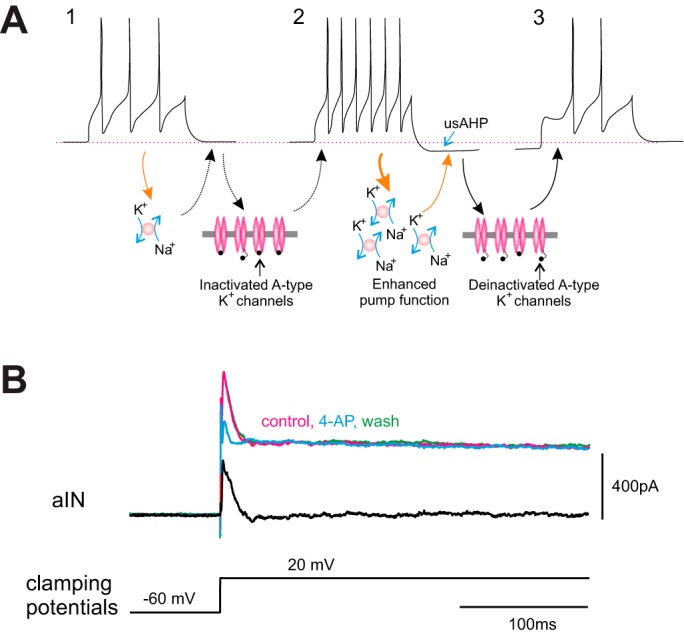
An A-type K+ current links the usAHP to inhibition of firing in Xenopus spinal neurons. A: summary of the mechanism illustrating how the Na+ pump and A-type K+ current are involved in the short-term memory of motor network output. At rest, most A-type K+ channels are inactivated. Weak activity (1) does not increase Na+ pump current sufficiently to hyperpolarize the membrane potential, so when the membrane potential is subsequently depolarized above threshold (2), most A-type K+ channels cannot be activated, and thus the first spike delay is unaffected. Stronger activity (2) can potentiate Na+ pump function and induce a larger pump current, which hyperpolarizes the membrane potential (usAHP). This hyperpolarization removes the inactivation of A-type K+ channels so that when depolarized above threshold (3), the A-type current is large enough to impede membrane depolarization, prolonging first spike delay and reducing the total number of spikes to a given depolarizing input. B: voltage-clamp evidence for a 4-AP-sensitive A current in a spinal ascending interneuron (aIN). 4-AP preferentially blocks transient K+ currents. Red current trace is control, blue is in 4-AP, and green is wash. Black trace is the difference in currents between control and 4-AP. [Adapted from Zhang et al. (2015).]
Heterogenous distribution.
The functional anatomy of the tadpole spinal network is known in considerable detail (Roberts et al. 2010) such that the presence or absence of a usAHP can be ascribed to each class of spinal neuron that participates in locomotory swimming. In three of the four main CPG classes [motoneurons (MNs), commissural interneurons (cINs), and ascending interneurons (aINs)], we found that approximately half of neurons display a usAHP, whereas in the other half of each subtype it is absent (Fig. 6; Zhang and Sillar 2012). Furthermore, in one entire class, the excitatory rhythm-generating descending interneurons (dINs), the usAHP is absent altogether. The fact that dINs appear to be spared the influence of a usAHP presumably explains why some residual rhythm-generating capability remains regardless of how short the interswim interval is (e.g., Fig. 3B, 5-s interval). However, the firing of dINs relies on rebound from mid-cycle inhibition coming from cINs on the contralateral side of the spinal cord, and therefore the impact of IA on cIN firing will indirectly compromise dIN firing, and in turn the maintenance of the swim rhythm. The explanation for a lack of a pump current in dINs is yet to be determined, but one possibility is that they do not possess specific Na+ pump isoforms responsible for mediating the usAHP (see discussion). Alternatively, the usAHP may be masked in this cell type by an equal but opposite depolarizing current, such as a persistent Na+ current, or an Ih current, which may also become activated during intense spiking protocols (Darbon et al. 2004; Gulledge et al. 2013; Wang et al. 2012). This possibility is currently under investigation, with preliminary evidence suggesting that this may be the case.
Fig. 6.
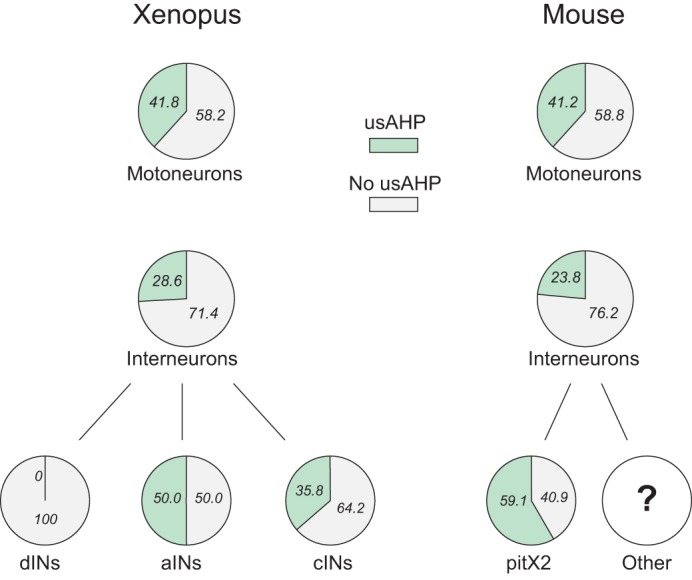
Heterogenous distribution (%) of the usAHP among neuron types in Xenopus tadpoles (Zhang and Sillar 2012) and neonatal mice (Picton et al. 2017).
A similar heterogenous usAHP distribution is present in the neonatal mouse CPG (Fig. 6; Picton et al. 2017). For MNs, a very similar proportion to that in the tadpole (~40%) display the usAHP. For interneurons, there are many more classes in the mouse compared with the tadpole (Kiehn 2016), but around one-quarter of unidentified interneurons that were recorded displayed a usAHP. This proportion is similar to that in tadpole interneurons when cINs, aINs, and dINs are pooled. Although the identity of all the specific interneuron classes displaying a usAHP in neonatal mice is not yet known, one type of modulatory neurons, the cholinergic pitx2 class, was found to display a usAHP in around 60% of the population (Picton et al. 2017).
Phylogenetically Conserved Functions of Dynamic Na+ Pumps
Intrinsic memory through a spike-rate monitor.
Networks of neurons require the intrinsic capacity to monitor their own activity, allowing for the initiation of important homeostatic control mechanisms that adjust their output in light of past activity. Changes in neuronal and synaptic function often begin with changes in ionic conductances. The activity of a neuron may be reflected in changes in intracellular calcium concentration, leading to the activation of a range of downstream signaling pathways including protein phosphorylation and ion channel modulation. However, the clearance of calcium itself, mediated primarily by the calcium pump, is often relatively rapid (Benham et al. 1992), and therefore calcium influx is usually not considered to be responsible for electrical changes in the timescale of tens of seconds. Another ion intrinsically linked to neuronal activity is Na+, whose intracellular levels also rise rapidly during spiking before decaying slowly over tens of seconds after activity has ceased (Rose 2002). The Na+ pump is the primary means of restoring intracellular Na+ concentrations. It is therefore strategically positioned both to homeostatically control changes in intracellular Na+ levels resulting from neuronal firing and to link neuronal activity to intrinsic excitability. It was shown as early as the 1950s that rises in intracellular Na+ level can cause a prolonged membrane hyperpolarization (Coombs et al. 1955) and that this effect is mediated by the activation of the Na+ pump (Connelly 1959; Ritchie and Straub 1957).
This phenomenon has since been reported in a range of neuronal types at every level of the motor pathway. For example, pump-mediated AHPs have been reported in the sensory neurons of a range of species including insects (French 1989), lamprey (Parker et al. 1996), leech (Arganda et al. 2007; Baylor and Nicholls 1969; Scuri et al. 2002), crayfish (Nakajima and Takahashi 1966; Sokolove and Cooke 1971), frogs (Davidoff and Hackman 1980; Kobayashi et al. 1997), horseshoe crabs (Smith et al. 1968), and rats (Gordon et al. 1990). Similar posttetanic AHP mechanisms mediated by the Na+ pump have also been found in the interneurons of numerous species including the leech (Tobin and Calabrese 2005), Aplysia (Carpenter and Alving 1968; Pinsker and Kandel 1969), and rats (Darbon et al. 2002, 2003; Krey et al. 2010; Tsuzawa et al. 2015). Finally, the motoneurons of diverse species have also been shown to display a spike-dependent, pump-mediated hyperpolarization, including in the motor axons of lizards (Morita et al.1993), guinea pigs (del Negro et al. 1999), rats (Ballerini et al. 1997; Gage and Hubbard 1966), and humans (Kiernan et al. 2004; Vagg et al. 1998). In several networks, these activity-dependent hyperpolarizations have been shown to perform important roles in shaping the rhythmic output of the network itself; from neurosecretory networks in the snail brain (Nikolić et al. 2008, 2012; Tsai and Chen 1995) to rhythmic networks in the rat brain, including the suprachiasmatic nucleus (Wang and Huang 2004, 2006; Wang et al. 2012) and midbrain dopaminergic neurons (Johnson et al. 1992). More recently, Na+ pumps have also been found to play an important role in shaping the output of hippocampal neurons (Azarias et al. 2013; Gulledge et al. 2013; Gustafsson and Wigström, 1983), striatal neurons (Azarias et al. 2013), cerebellar Purkinje fibers (Forrest et al. 2012), and neurons in the auditory pathway (Kim et al. 2007; Kim and von Gersdorff 2012). Sodium pumps thus play important roles throughout the nervous system and across diverse species, and participate at every level of the motor pathway, from modifying sensory information, to the integration and relay of this information by interneuronal networks, and right through to the regulation of the final motor output by motoneurons. However, only recently has the functional importance of the Na+ pump as a spike-rate monitor been explored in depth in the spinal CPG networks controlling vertebrate locomotion.
Because of the close link between intracellular Na+ levels and Na+ pump activity, pharmacological tools that raise the levels of Na+ in a neuron can be useful for studying the effects of increased Na+ pump activity. Hence monensin, a sodium ionophore that exchanges one Na+ ion intracellularly for one proton extracellularly, has been used extensively in studying Na+ pumps (e.g., Kueh et al. 2016; Wang et al. 2012; Zhang et al. 2015). Monensin essentially acts as a proxy for intense spiking, imposing on neurons the pharmacological equivalent of a long train of high-frequency action potentials. In both Xenopus and mouse spinal neurons, monensin increases Na+ pump activity, hyperpolarizing the membrane potential to the level attained by the usAHP. Locomotor activity, again in both species, becomes shorter and slower under monensin, as if the network has been intensely active for a long period of time. Thus monensin appears to chronically fatigue spinal networks by maximally activating the Na+ pump autoregulation mechanism. Monensin has also recently been used to study the role of Na+ pumps in the heartbeat network of the leech, where a fascinating interaction between a pump current and a depolarizing Ih current was revealed (Kueh et al. 2016). Directly increasing intracellular Na+ concentration with the use of a modified intracellular solution could be done in future studies to confirm these findings.
Molecular and cellular basis for activity-dependent pump activation.
The pump-based mechanisms that link future to past network activity transcend major phylogenetic boundaries and occur on multiple levels, from the molecular to the cellular and circuit levels. At the molecular level, there is an emerging hypothesis that there exist both tonic and dynamic contributions of the Na+ pump to membrane potential, and that these contributions rely partly on the heterogeneity of subunit composition of the pumps. In neurons in general, the α-subunit of the Na+ pump takes one of two forms with different affinities for intracellular Na+: α1 (high affinity) or α3 (low affinity). Thus, at typical resting intracellular Na+ levels, the α1 is maximally active, whereas the α3 remains inactive, or submaximally active, allowing it to act as a sensor for activity-dependent rises in Na+ (Azarias et al. 2013; Dobretsov and Stimers, 2005). The subsequent increase in the activity of α3-containing Na+ pumps is thought to be responsible for generating the transient membrane hyperpolarization that reduces the excitability of the neuron for tens of seconds. The different isoforms also have differential sensitivity to ouabain such that low concentrations of ouabain, including those used in our experiments (1–3 µM), selectively block the α3 isoform (Blanco and Mercer 1998; Dobretsov and Stimers 2005). Our pharmacological experiments showing that the usAHP is blocked by these low concentrations of ouabain are therefore in support of the above hypothesis.
In mice, both α1 and α3 expression are found throughout the ventral and dorsal horns of the spinal cord, although α3 expression is more widespread (Edwards et al. 2013; Hieber et al. 1991; Watts et al. 1991). However, both α1 and α3 expression appear to be restricted to some neurons and not others. For instance, α-motoneurons predominantly express α3, whereas γ-motoneurons predominantly express α1 (Edwards et al. 2013). The functional importance of this difference is not yet clear. Expression of α3 is also found in interneurons, and in our experiments, we specifically focused on α3 expression in one interneuron type, the cholinergic pitx2 cells (Zagoraiou et al. 2009). We found α3 expression in around half of this population, which broadly matches the number of pitx2 neurons found to display the usAHP (Picton et al. 2017). This is also similar to findings of previous studies in rats, which documented an activity-dependent, pump-mediated hyperpolarization in around half of cultured spinal interneurons (Darbon et al. 2002, 2003). It will be important in future studies to further characterize α3 expression in other interneuron types. It will also be important to characterize developmental changes in α3 expression. For example, calyx of Held neurons in young rats have lower expression of α3 compared with those in adults, and this is accompanied by a significantly smaller and shorter duration usAHP (Kim et al. 2007).
At the cellular level, we have partially characterized the details of the cascade of events in Xenopus tadpoles that link spinal neuron firing to network regulation. This cascade involves the spike-dependent accumulation of Na+ ions, which in turn triggers an increase in ion exchange by the Na+ pump, hyperpolarizing the neuron. This hyperpolarization de-inactivates an A-type K+ channel, and enhanced A-current delays spiking in a subset of spinal motor and interneurons when activity resumes, causing a collapse of swim network activity. Thus swimming activity evoked within a minute after the end of previous swimming is both shorter in duration and slower in frequency, in a time-dependent manner. In mice, a similar physiological mechanism appears to be at play, but unsurprisingly, additional mechanisms of locomotor bout termination are likely to be involved. For example, unlike in tadpoles, blockade of the Na+ pump does not produce continuous locomotion, but merely extends the duration of evoked locomotor bouts (Picton et al. 2017). It is likely that synaptic depression plays a role in locomotor bout termination, a possibility that has been explored previously in rat spinal neurons in the context of the Na+ pumps (Darbon et al. 2002, 2003; Rozzo et al. 2002). We also do not yet know whether A-currents play a role in neonatal mice. As we come to understand more about Na+ pump currents, we will likely uncover species-specific mechanisms involving a range of other currents, such as Ih, which has been shown to have important interactions with pump currents in a number of different brain areas (Gulledge et al. 2013; Kim and von Gersdorff 2012; Rozzo et al. 2002; Trotier and Døving 1996).
Heterogeneity allied to circuit role.
The usAHP is a powerful way of reducing network excitability. However, if it were to be homogenously expressed in all CPG neurons, then there would be a distinct possibility that the network could render itself completely unresponsive. This, in turn, could be catastrophic because of the requirement to retain a residual capacity to respond to potentially life-threatening stimuli such as an approaching predator. In both tadpole and neonatal mouse spinal locomotor networks, there is strong evidence for a heterogenous distribution of the usAHP among spinal CPG network components.
There are a number of possible explanations for the heterogenous distribution of the usAHP among neuron subtypes in the spinal cord. One possibility, for which we have preliminary evidence in the mouse (described above), is that the ability of the pump to respond dynamically to intense activity requires the presence of an α3-containing Na+ pump, which is only recruited by high intracellular Na+ concentrations achieved following intense neuronal firing. Alternatively, the α-subunit may also be subject to direct phosphorylation in some neurons, but not others (Therien and Blostein 2000), which can tune the affinity of the subunit for Na+. A similar mechanism could also involve a set of accessory proteins, known as FYXD proteins, which are also subject to phosphorylation (Geering 2006). Thus it will be important in future studies to establish not only the distribution of α1- and α3-subunit isoforms but also the expression of FXYD proteins in the spinal cord.
The importance of the Na+ pump as an intrinsic locomotor memory mechanism, and its high conservation through evolution, makes it a useful target for a range of neuromodulators, and this could also explain differences in usAHP expression. The range of neuromodulators known to impinge on the Na+ pump is extensive (Therien and Blostein 2000), but dopamine, serotonin, and nitric oxide seem particularly important, especially in the spinal cord. Indeed, in mice we have shown that the effects of Na+ pump manipulation are dopamine dependent and that dopamine extends the duration of the usAHP (Picton et al. 2017). Whether this involves direct phosphorylation of Na+ pumps or via FXYD accessory proteins, or both, is a topic for future experiments.
Phylogenetic conservation.
In this article, we have reviewed the evidence that the activity-dependent increase in Na+ pump activity, manifest as the usAHP, functions as a simple form of short-term motor memory in animals as diverse as fruit flies, frog tadpoles, and neonatal mice. Modern amphibians and mammals diverged from a common ancestor that existed around 360 million years ago. The nervous system underwent dramatic changes to accommodate changes in lifestyle, morphology, and behavioral repertoire, with the number of neurons increasing from around 16 million in adult frogs to around 70 million in adult mice. However, many components of the nervous system are known to be highly conserved (Katz 2016; Katz and Harris-Warrick 1999; Keifer and Summers 2016). The basic architecture of many neural circuits appears to have been retained through evolutionary time, with extant species displaying variations on a theme rather than completely new circuit architecture. Thus we can often identify conserved principles of circuit function, and this often appears to be true for the circuits controlling locomotor behaviors, including at the cellular and molecular levels (Goulding and Pfaff 2005). The neuronal Na+ pump is especially highly conserved between vertebrates in terms of its structure and function, with around 96% cross-species similarity (Dobretsov and Stimers 2005; Takeyasu et al. 1990). This implies that the Na+ pump plays an important and conserved neuronal function. Our own mammalian lineage diverged from the common ancestor with mice around 65 million years ago (O’Leary et al. 2013), and so it will be interesting in future studies, especially with a rise in the use of human induced pluripotent stem cells (iPSCs), to study whether the Na+ pumps embedded in human spinal motoneurons and interneurons also play a similar role in neuronal self-regulation.
Dysfunction of the Na+ pump.
Na+ pumps are receiving increasing attention in mammalian systems not only for their importance for normal network function but also for their relevance to both the aging process and a range of debilitating diseases of the nervous system (de Lores Arnaiz and Ordieres 2014; Holm and Lykke-Hartmann 2016). The α3 Na+ pump isoform is highly expressed in the human brain and spinal cord (Peng et al. 1992), and several mutations in the gene encoding this subunit (ATP1A3) are known to cause at least three neurological disorders: alternating hemiplegia of childhood (AHC; Heinzen et al. 2012; Rosewich et al. 2012); rapid-onset dystonia parkinsonism (RDP; de Carvalho Aguiar et al. 2004; Rodacker et al. 2006); and cerebellar ataxia, areflexia, pes cavus, optic atrophy, and sensorineural hearing loss (CAPOS) syndrome (Demos et al. 2014). Furthermore, a wide range of other disorders are also known to involve changes in the activity of the α3 Na+ pump isoform. In recent studies, the α3 isoform has been shown to directly interact with both superoxide dismutase 1 (SOD1; Martin et al. 2007; Ruegsegger et al. 2016) and α-synuclein (Shrivastava et al. 2015) in ALS and Parkinson’s disease mouse models, respectively. This aggregation leads to reduced α3 activity and a general inability to respond to rises in intracellular Na+ level (Ellis et al. 2003; Shrivastava et al. 2015). Given that dysfunction of α3 also contributes to epilepsy (Krishnan et al. 2015) and bipolar disorder (Kirshenbaum et al. 2011a), it is possible that the inability to respond dynamically and homeostatically to activity-induced rises in intracellular Na+ level may be a general feature of pump disorders involving the α3 isoform (Azarias et al. 2013; Benarroch 2011).
Genetically modified zebrafish and rodent disease models have been used to explore the underlying mechanisms of Na+ pump deficiency. ATP1A3 knockdown zebrafish display abnormal motor activity accompanied by depolarization of spinal sensory neurons (Doğanli et al. 2013). Homozygous knockout mice for α1 are embryonic lethal (James et al. 1999), whereas homozygous α3 knockout mice die shortly after birth (Moseley et al. 2007). However, a number of α3 knockin mouse lines have been developed, and heterozygote mice all show severe motor deficits (DeAndrade et al. 2011; Hunanyan et al. 2015; Ikeda et al. 2013; Kirshenbaum et al. 2011b; Moseley et al. 2007; Sugimoto et al. 2014). The hyperactivity phenotype in these mice is especially pronounced, with mutant mice showing almost continuous, high-frequency locomotor activity compared with control mice. The α3 mutation affects Na+ pumps throughout the nervous system, including presumably the spinal cord, and therefore this phenotype may relate to the role of the α3 Na+ pumps explored in this review. Indeed, this behavioral phenotype would be predicted by the effects covered in this review using low concentrations of ouabain, namely, longer duration bouts of locomotion with a higher frequency of limb movements, and a general inability to regulate locomotion.
Summary
Na+/K+ exchange pumps are ubiquitously distributed, abundantly expressed, and phylogenetically conserved proteins that are often viewed as molecular automata engaged exclusively in the maintenance of ionic distributions across cell membranes. In this review, we have discussed recent data in Xenopus tadpoles, neonatal mice, and also Drosophila, showing that Na+ pumps respond dynamically to changes in intracellular Na+ concentration that accompany intense neuronal firing. This capacity endows networks of the spinal cord with a homeostatic control mechanism to shape motor output in an activity-dependent manner. Moreover, despite the ubiquity of Na+ pump distribution among network neurons, their ability to respond homeostatically to the changes in intracellular Na+ level triggered by activity may result from the highly targeted insertion of α3-containing pumps in selected neurons and neuronal subtypes. The possibility that the balance of α1 to α3 expression is a mutable entity that can change during development, or with circuit use, is an exciting idea that should be pursued in the future.
GRANTS
We are grateful for the financial support of Biotechnology and Biological Sciences Research Council Grants BB/M024946/1 and BB/JO1446X/1, the Carnegie Trust, and the University of St Andrews.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.D.P. and H.Z. prepared figures; L.D.P., H.Z., and K.T.S. drafted manuscript; L.D.P., H.Z., and K.T.S. edited and revised manuscript; K.T.S. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Stefan Pulver (School of Psychology and Neuroscience, University of St Andrews) for providing images and data on Drosophila third-instar larval crawling.
REFERENCES
- Arganda S, Guantes R, de Polavieja GG. Sodium pumps adapt spike bursting to stimulus statistics. Nat Neurosci 10: 1467–1473, 2007. doi: 10.1038/nn1982. [DOI] [PubMed] [Google Scholar]
- Azarias G, Kruusmägi M, Connor S, Akkuratov EE, Liu XL, Lyons D, Brismar H, Broberger C, Aperia A. A specific and essential role for Na,K-ATPase α3 in neurons co-expressing α1 and α3. J Biol Chem 288: 2734–2743, 2013. doi: 10.1074/jbc.M112.425785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballerini L, Bracci E, Nistri A. Pharmacological block of the electrogenic sodium pump disrupts rhythmic bursting induced by strychnine and bicuculline in the neonatal rat spinal cord. J Neurophysiol 77: 17–23, 1997. [DOI] [PubMed] [Google Scholar]
- Baylor DA, Nicholls JG. After-effects of nerve impulses on signalling in the central nervous system of the leech. J Physiol 203: 571–589, 1969. doi: 10.1113/jphysiol.1969.sp008880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Na+, K+-ATPase: functions in the nervous system and involvement in neurologic disease. Neurology 76: 287–293, 2011. doi: 10.1212/WNL.0b013e3182074c2f. [DOI] [PubMed] [Google Scholar]
- Benham CD, Evans ML, McBain CJ. Ca2+ efflux mechanisms following depolarization evoked calcium transients in cultured rat sensory neurones. J Physiol 455: 567–583, 1992. doi: 10.1113/jphysiol.1992.sp019316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998. [DOI] [PubMed] [Google Scholar]
- Carpenter DO, Alving BO. A contribution of an electrogenic Na+ pump to membrane potential in Aplysia neurons. J Gen Physiol 52: 1–21, 1968. doi: 10.1085/jgp.52.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connelly CM. Recovery processes and metabolism of nerve. Rev Mod Phys 31: 475–484, 1959. doi: 10.1103/RevModPhys.31.475. [DOI] [Google Scholar]
- Coombs JS, Eccles JC, Fatt P. The electrical properties of the motoneurone membrane. J Physiol 130: 291–325, 1955. doi: 10.1113/jphysiol.1955.sp005411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darbon P, Scicluna L, Tscherter A, Streit J. Mechanisms controlling bursting activity induced by disinhibition in spinal cord networks. Eur J Neurosci 15: 671–683, 2002. doi: 10.1046/j.1460-9568.2002.01904.x. [DOI] [PubMed] [Google Scholar]
- Darbon P, Tscherter A, Yvon C, Streit J. Role of the electrogenic Na/K pump in disinhibition-induced bursting in cultured spinal networks. J Neurophysiol 90: 3119–3129, 2003. doi: 10.1152/jn.00579.2003. [DOI] [PubMed] [Google Scholar]
- Darbon P, Yvon C, Legrand JC, Streit J. INaP underlies intrinsic spiking and rhythm generation in networks of cultured rat spinal cord neurons. Eur J Neurosci 20: 976–988, 2004. doi: 10.1111/j.1460-9568.2004.03565.x. [DOI] [PubMed] [Google Scholar]
- Davidoff RA, Hackman JC. Hyperpolarization of frog primary afferent fibres caused by activation of a sodium pump. J Physiol 302: 297–309, 1980. doi: 10.1113/jphysiol.1980.sp013243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carvalho Aguiar P, Sweadner KJ, Penniston JT, Zaremba J, Liu L, Caton M, Linazasoro G, Borg M, Tijssen MA, Bressman SB, Dobyns WB, Brashear A, Ozelius LJ. Mutations in the Na+/K+-ATPase α3 gene ATP1A3 are associated with rapid-onset dystonia parkinsonism. Neuron 43: 169–175, 2004. doi: 10.1016/j.neuron.2004.06.028. [DOI] [PubMed] [Google Scholar]
- de Lores Arnaiz GR, Ordieres MG. Brain Na+, K+-ATPase activity in aging and disease. Int J Biomed Sci 10: 85–102, 2014. [PMC free article] [PubMed] [Google Scholar]
- DeAndrade MP, Yokoi F, van Groen T, Lingrel JB, Li Y. Characterization of Atp1a3 mutant mice as a model of rapid-onset dystonia with parkinsonism. Behav Brain Res 216: 659–665, 2011. doi: 10.1016/j.bbr.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Negro CA, Hsiao CF, Chandler SH. Outward currents influencing bursting dynamics in guinea pig trigeminal motoneurons. J Neurophysiol 81: 1478–1485, 1999. [DOI] [PubMed] [Google Scholar]
- Demos MK, van Karnebeek CD, Ross CJ, Adam S, Shen Y, Zhan SH, Shyr C, Horvath G, Suri M, Fryer A, Jones SJ, Friedman JM; FORGE Canada Consortium . A novel recurrent mutation in ATP1A3 causes CAPOS syndrome. Orphanet J Rare Dis 9: 15, 2014. doi: 10.1186/1750-1172-9-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobretsov M, Stimers JR. Neuronal function and alpha3 isoform of the Na/K-ATPase. Front Biosci 10: 2373–2396, 2005. doi: 10.2741/1704. [DOI] [PubMed] [Google Scholar]
- Doğanli C, Beck HC, Ribera AB, Oxvig C, Lykke-Hartmann K. α3Na+/K+-ATPase deficiency causes brain ventricle dilation and abrupt embryonic motility in zebrafish. J Biol Chem 288: 8862–8874, 2013. doi: 10.1074/jbc.M112.421529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards IJ, Bruce G, Lawrenson C, Howe L, Clapcote SJ, Deuchars SA, Deuchars J. Na+/K+ ATPase α1 and α3 isoforms are differentially expressed in α- and γ-motoneurons. J Neurosci 33: 9913–9919, 2013. doi: 10.1523/JNEUROSCI.5584-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis DZ, Rabe J, Sweadner KJ. Global loss of Na,K-ATPase and its nitric oxide-mediated regulation in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci 23: 43–51, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engl E, Attwell D. Non-signalling energy use in the brain. J Physiol 593: 3417–3429, 2015. doi: 10.1113/jphysiol.2014.282517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forrest MD, Wall MJ, Press DA, Feng J. The sodium-potassium pump controls the intrinsic firing of the cerebellar Purkinje neuron. PLoS One 7: e51169, 2012. doi: 10.1371/journal.pone.0051169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French AS. Ouabain selectively affects the slow component of sensory adaptation in an insect mechanoreceptor. Brain Res 504: 112–114, 1989. doi: 10.1016/0006-8993(89)91604-1. [DOI] [PubMed] [Google Scholar]
- Gage PW, Hubbard JI. The origin of the post-tetanic hyperpolarization of mammalian motor nerve terminals. J Physiol 184: 335–352, 1966. doi: 10.1113/jphysiol.1966.sp007918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev 81: 1725–1789, 2001. [DOI] [PubMed] [Google Scholar]
- Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241–F250, 2006. doi: 10.1152/ajprenal.00126.2005. [DOI] [PubMed] [Google Scholar]
- Gordon TR, Kocsis JD, Waxman SG. Electrogenic pump (Na+/K+-ATPase) activity in rat optic nerve. Neuroscience 37: 829–837, 1990. doi: 10.1016/0306-4522(90)90112-H. [DOI] [PubMed] [Google Scholar]
- Goulding M, Pfaff SL. Development of circuits that generate simple rhythmic behaviors in vertebrates. Curr Opin Neurobiol 15: 14–20, 2005. doi: 10.1016/j.conb.2005.01.017. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Dasari S, Onoue K, Stephens EK, Hasse JM, Avesar D. A sodium-pump-mediated afterhyperpolarization in pyramidal neurons. J Neurosci 33: 13025–13041, 2013. doi: 10.1523/JNEUROSCI.0220-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigström H. Hyperpolarization following long-lasting tetanic activation of hippocampal pyramidal cells. Brain Res 275: 159–163, 1983. doi: 10.1016/0006-8993(83)90429-8. [DOI] [PubMed] [Google Scholar]
- Harris-Warrick RM, Marder E. Modulation of neural networks for behavior. Annu Rev Neurosci 14: 39–57, 1991. doi: 10.1146/annurev.ne.14.030191.000351. [DOI] [PubMed] [Google Scholar]
- Heinzen EL, Swoboda KJ, Hitomi Y, Gurrieri F, Nicole S, de Vries B, Tiziano FD, Fontaine B, Walley NM, Heavin S, Panagiotakaki E; European Alternating Hemiplegia of Childhood (AHC) Genetics Consortium; Biobanca e Registro Clinico per l’Emiplegia Alternante (I.B.AHC) Consortium; European Network for Research on Alternating Hemiplegia (ENRAH) for Small and Medium-sized Enterpriese (SMEs) Consortium, Fiori S, Abiusi E, Di Pietro L, Sweney MT, Newcomb TM, Viollet L, Huff C, Jorde LB, Reyna SP, Murphy KJ, Shianna KV, Gumbs CE, Little L, Silver K, Ptáček LJ, Haan J, Ferrari MD, Bye AM, Herkes GK, Whitelaw CM, Webb D, Lynch BJ, Uldall P, King MD, Scheffer IE, Neri G, Arzimanoglou A, van den Maagdenberg AM, Sisodiya SM, Mikati MA, Goldstein DB. De novo mutations in ATP1A3 cause alternating hemiplegia of childhood. Nat Genet 44: 1030–1034, 2012. doi: 10.1038/ng.2358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hieber V, Siegel GJ, Fink DJ, Beaty MW, Mata M. Differential distribution of (Na, K)-ATPase α isoforms in the central nervous system. Cell Mol Neurobiol 11: 253–262, 1991. doi: 10.1007/BF00769038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Keynes RD. Experiments on the injection of substances into squid giant axons by means of a microsyringe. J Physiol 131: 592–616, 1956. doi: 10.1113/jphysiol.1956.sp005485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm TH, Lykke-Hartmann K. Insights into the pathology of the α3 Na+/K+-ATPase in neurological disorders; lessons from animal models. Front Physiol 7: 209, 2016. doi: 10.3389/fphys.2016.00209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunanyan AS, Fainberg NA, Linabarger M, Arehart E, Leonard AS, Adil SM, Helseth AR, Swearingen AK, Forbes SL, Rodriguiz RM, Rhodes T, Yao X, Kibbi N, Hochman DW, Wetsel WC, Hochgeschwender U, Mikati MA. Knock-in mouse model of alternating hemiplegia of childhood: behavioral and electrophysiologic characterization. Epilepsia 56: 82–93, 2015. doi: 10.1111/epi.12878. [DOI] [PubMed] [Google Scholar]
- Ikeda K, Satake S, Onaka T, Sugimoto H, Takeda N, Imoto K, Kawakami K. Enhanced inhibitory neurotransmission in the cerebellar cortex of Atp1a3-deficient heterozygous mice. J Physiol 591: 3433–3449, 2013. doi: 10.1113/jphysiol.2012.247817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James PF, Grupp IL, Grupp G, Woo AL, Askew GR, Croyle ML, Walsh RA, Lingrel JB. Identification of a specific role for the Na,K-ATPase α 2 isoform as a regulator of calcium in the heart. Mol Cell 3: 555–563, 1999. doi: 10.1016/S1097-2765(00)80349-4. [DOI] [PubMed] [Google Scholar]
- Johnson SW, Seutin V, North RA. Burst firing in dopamine neurons induced by N-methyl-d-aspartate: role of electrogenic sodium pump. Science 258: 665–667, 1992. doi: 10.1126/science.1329209. [DOI] [PubMed] [Google Scholar]
- Katz PS. Evolution of central pattern generators and rhythmic behaviours. Philos Trans R Soc Lond B Biol Sci 371: 20150057, 2016. doi: 10.1098/rstb.2015.0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz PS, Harris-Warrick RM. The evolution of neuronal circuits underlying species-specific behavior. Curr Opin Neurobiol 9: 628–633, 1999. doi: 10.1016/S0959-4388(99)00012-4. [DOI] [PubMed] [Google Scholar]
- Keifer J, Summers CH. Putting the “biology” back into “neurobiology”: the strength of diversity in animal model systems for neuroscience research. Front Syst Neurosci 10: 69, 2016. doi: 10.3389/fnsys.2016.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehn O. Decoding the organization of spinal circuits that control locomotion. Nat Rev Neurosci 17: 224–238, 2016. doi: 10.1038/nrn.2016.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Lin CS-Y, Burke D. Differences in activity-dependent hyperpolarization in human sensory and motor axons. J Physiol 558: 341–349, 2004. doi: 10.1113/jphysiol.2004.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Sizov I, Dobretsov M, von Gersdorff H. Presynaptic Ca2+ buffers control the strength of a fast post-tetanic hyperpolarization mediated by the α3 Na+/K+-ATPase. Nat Neurosci 10: 196–205, 2007. doi: 10.1038/nn1839. [DOI] [PubMed] [Google Scholar]
- Kim JH, von Gersdorff H. Suppression of spikes during posttetanic hyperpolarization in auditory neurons: the role of temperature, Ih currents, and the Na+-K+-ATPase pump. J Neurophysiol 108: 1924–1932, 2012. doi: 10.1152/jn.00103.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum GS, Clapcote SJ, Duffy S, Burgess CR, Petersen J, Jarowek KJ, Yücel YH, Cortez MA, Snead OC 3rd, Vilsen B, Peever JH, Ralph MR, Roder JC. Mania-like behavior induced by genetic dysfunction of the neuron-specific Na+,K+-ATPase α3 sodium pump. Proc Natl Acad Sci USA 108: 18144–18149, 2011a. doi: 10.1073/pnas.1108416108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirshenbaum GS, Saltzman K, Rose B, Petersen J, Vilsen B, Roder JC. Decreased neuronal Na+, K+-ATPase activity in Atp1a3 heterozygous mice increases susceptibility to depression-like endophenotypes by chronic variable stress. Genes Brain Behav 10: 542–550, 2011b. doi: 10.1111/j.1601-183X.2011.00691.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi J, Ohta M, Terada Y. Evidence for the involvement of Na+-K+ pump and K+ conductance in the post-tetanic hyperpolarization of the tetrodotoxin-resistant C-fibers in the isolated bullfrog sciatic nerve. Neurosci Lett 236: 171–174, 1997. doi: 10.1016/S0304-3940(97)00790-8. [DOI] [PubMed] [Google Scholar]
- Krey RA, Goodreau AM, Arnold TB, Del Negro CA. Outward currents contributing to inspiratory burst termination in preBötzinger complex neurons of neonatal mice studied in vitro. Front Neural Circuits 4: 124, 2010. doi: 10.3389/fncir.2010.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Kiernan MC. Activity-dependent excitability changes suggest Na+/K+ pump dysfunction in diabetic neuropathy. Brain 131: 1209–1216, 2008. doi: 10.1093/brain/awn052. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Filatov G, Shilnikov A, Bazhenov M. Electrogenic properties of the Na+/K+ ATPase control transitions between normal and pathological brain states. J Neurophysiol 113: 3356–3374, 2015. doi: 10.1152/jn.00460.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh D, Barnett WH, Cymbalyuk GS, Calabrese RL. Na+/K+ pump interacts with the h-current to control bursting activity in central pattern generator neurons of leeches. eLife 5: e19322, 2016. doi: 10.7554/eLife.19322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: mechanisms of mitochondriopathy and cell death. J Comp Neurol 500: 20–46, 2007. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- Morita K, David G, Barrett JN, Barrett EF. Posttetanic hyperpolarization produced by electrogenic Na+-K+ pump in lizard axons impaled near their motor terminals. J Neurophysiol 70: 1874–1884, 1993. [DOI] [PubMed] [Google Scholar]
- Moseley AE, Williams MT, Schaefer TL, Bohanan CS, Neumann JC, Behbehani MM, Vorhees CV, Lingrel JB. Deficiency in Na,K-ATPase α isoform genes alters spatial learning, motor activity, and anxiety in mice. J Neurosci 27: 616–626, 2007. doi: 10.1523/JNEUROSCI.4464-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima S, Takahashi K. Post-tetanic hyperpolarization and electrogenic Na pump in stretch receptor neurone of crayfish. J Physiol 187: 105–127, 1966. doi: 10.1113/jphysiol.1966.sp008078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolić L, Kartelija G, Nedeljković M. Effect of static magnetic fields on bioelectric properties of the Br and N1 neurons of snail Helix pomatia. Comp Biochem Physiol A Mol Integr Physiol 151: 657–663, 2008. doi: 10.1016/j.cbpa.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Nikolić L, Todorović N, Zakrzewska J, Stanić M, Rauš S, Kalauzi A, Janać B. Involvement of Na+/K+ pump in fine modulation of bursting activity of the snail Br neuron by 10 mT static magnetic field. J Comp Physiol A Neuroethol Sens Neural Behav Physiol 198: 525–540, 2012. doi: 10.1007/s00359-012-0727-0. [DOI] [PubMed] [Google Scholar]
- O’Leary MA, Bloch JI, Flynn JJ, Gaudin TJ, Giallombardo A, Giannini NP, Goldberg SL, Kraatz BP, Luo ZX, Meng J, Ni X, Novacek MJ, Perini FA, Randall ZS, Rougier GW, Sargis EJ, Silcox MT, Simmons NB, Spaulding M, Velazco PM, Weksler M, Wible JR, Cirranello AL. The placental mammal ancestor and the post-K-Pg radiation of placentals. Science 339: 662–667, 2013. doi: 10.1126/science.1229237. [DOI] [PubMed] [Google Scholar]
- Parker D, Hill R, Grillner S. Electrogenic pump and a Ca2+-dependent K+ conductance contribute to a posttetanic hyperpolarization in lamprey sensory neurons. J Neurophysiol 76: 540–553, 1996. [DOI] [PubMed] [Google Scholar]
- Peng JHF, Zeng YC, Parker JC Jr. Highly ouabain-sensitive α3 isoform of Na+,K+-ATPase in human brain. J Neurochem 58: 1180–1183, 1992. doi: 10.1111/j.1471-4159.1992.tb09380.x. [DOI] [PubMed] [Google Scholar]
- Picton LD, Nascimento F, Broadhead MJ, Sillar KT, Miles GB. Sodium pumps mediate activity-dependent changes in mammalian motor networks. J Neurosci 37: 906–921, 2017. doi: 10.1523/JNEUROSCI.2005-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsker H, Kandel ER. Synaptic activation of an electrogenic sodium pump. Science 163: 931–935, 1969. doi: 10.1126/science.163.3870.931. [DOI] [PubMed] [Google Scholar]
- Pulver SR, Griffith LC. Spike integration and cellular memory in a rhythmic network from Na+/K+ pump current dynamics. Nat Neurosci 13: 53–59, 2010. doi: 10.1038/nn.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranieri F, Di Lazzaro V. The role of motor neuron drive in muscle fatigue. Neuromuscul Disord 22, Suppl 3: S157–S161, 2012. doi: 10.1016/j.nmd.2012.10.006. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev 80: 767–852, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM, Straub RW. The hyperpolarization which follows activity in mammalian non-medullated fibres. J Physiol 136: 80–97, 1957. doi: 10.1113/jphysiol.1957.sp005744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts A, Li WC, Soffe SR. How neurons generate behavior in a hatchling amphibian tadpole: an outline. Front Behav Neurosci 4: 16, 2010. doi: 10.3389/fnbeh.2010.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodacker V, Toustrup-Jensen M, Vilsen B. Mutations Phe785Leu and Thr618Met in Na+,K+-ATPase, associated with familial rapid-onset dystonia parkinsonism, interfere with Na+ interaction by distinct mechanisms. J Biol Chem 281: 18539–18548, 2006. doi: 10.1074/jbc.M601780200. [DOI] [PubMed] [Google Scholar]
- Rose CR. Na+ signals at central synapses. Neuroscientist 8: 532–539, 2002. doi: 10.1177/1073858402238512. [DOI] [PubMed] [Google Scholar]
- Rosewich H, Thiele H, Ohlenbusch A, Maschke U, Altmüller J, Frommolt P, Zirn B, Ebinger F, Siemes H, Nürnberg P, Brockmann K, Gärtner J. Heterozygous de-novo mutations in ATP1A3 in patients with alternating hemiplegia of childhood: a whole-exome sequencing gene-identification study. Lancet Neurol 11: 764–773, 2012. doi: 10.1016/S1474-4422(12)70182-5. [DOI] [PubMed] [Google Scholar]
- Rossi A, Rossi S, Ginanneschi F. Activity-dependent changes in intrinsic excitability of human spinal motoneurones produced by natural activity. J Neurophysiol 108: 2473–2480, 2012. doi: 10.1152/jn.00477.2012. [DOI] [PubMed] [Google Scholar]
- Rozzo A, Ballerini L, Abbate G, Nistri A. Experimental and modeling studies of novel bursts induced by blocking Na+ pump and synaptic inhibition in the rat spinal cord. J Neurophysiol 88: 676–691, 2002. [DOI] [PubMed] [Google Scholar]
- Ruegsegger C, Maharjan N, Goswami A, Filézac de L’Etang A, Weis J, Troost D, Heller M, Gut H, Saxena S. Aberrant association of misfolded SOD1 with Na+/K+ATPase-α3 impairs its activity and contributes to motor neuron vulnerability in ALS. Acta Neuropathol 131: 427–451, 2016. doi: 10.1007/s00401-015-1510-4. [DOI] [PubMed] [Google Scholar]
- Scuri R, Mozzachiodi R, Brunelli M. Activity-dependent increase of the AHP amplitude in T sensory neurons of the leech. J Neurophysiol 88: 2490–2500, 2002. doi: 10.1152/jn.01027.2001. [DOI] [PubMed] [Google Scholar]
- Shrivastava AN, Redeker V, Fritz N, Pieri L, Almeida LG, Spolidoro M, Liebmann T, Bousset L, Renner M, Léna C, Aperia A, Melki R, Triller A. α-Synuclein assemblies sequester neuronal α3-Na+/K+-ATPase and impair Na+ gradient. EMBO J 34: 2408–2423, 2015. doi: 10.15252/embj.201591397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TG, Stell WK, Brown JE, Freeman JA, Murray GC. A role for the sodium pump in photoreception in Limulus. Science 162: 456–458, 1968. doi: 10.1126/science.162.3852.456. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Cooke IM. Inhibition of impulse activity in a sensory neuron by an electrogenic pump. J Gen Physiol 57: 125–163, 1971. doi: 10.1085/jgp.57.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storm JF. Action potential repolarization and a fast after-hyperpolarization in rat hippocampal pyramidal cells. J Physiol 385: 733–759, 1987. doi: 10.1113/jphysiol.1987.sp016517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto H, Ikeda K, Kawakami K. Heterozygous mice deficient in Atp1a3 exhibit motor deficits by chronic restraint stress. Behav Brain Res 272: 100–110, 2014. doi: 10.1016/j.bbr.2014.06.048. [DOI] [PubMed] [Google Scholar]
- Takeyasu K, Lemas V, Fambrough DM. Stability of Na+-K+-ATPase α-subunit isoforms in evolution. Am J Physiol Cell Physiol 259: C619–C630, 1990. [DOI] [PubMed] [Google Scholar]
- Therien AG, Blostein R. Mechanisms of sodium pump regulation. Am J Physiol Cell Physiol 279: C541–C566, 2000. [DOI] [PubMed] [Google Scholar]
- Tobin A-E, Calabrese RL. Myomodulin increases Ih and inhibits the Na/K pump to modulate bursting in leech heart interneurons. J Neurophysiol 94: 3938–3950, 2005. doi: 10.1152/jn.00340.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trotier D, Døving KB. Direct influence of the sodium pump on the membrane potential of vomeronasal chemoreceptor neurones in frog. J Physiol 490: 611–621, 1996. doi: 10.1113/jphysiol.1996.sp021171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai MC, Chen YH. Bursting firing of action potentials in central snail neurons elicited by d-amphetamine: role of the electrogenic sodium pump. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 111: 131–141, 1995. doi: 10.1016/0742-8413(94)00097-D. [DOI] [PubMed] [Google Scholar]
- Tsuzawa K, Yazawa I, Shakuo T, Ikeda K, Kawakami K, Onimaru H. Effects of ouabain on respiratory rhythm generation in brainstem-spinal cord preparation from newborn rats and in decerebrate and arterially perfused in situ preparation from juvenile rats. Neuroscience 286: 404–411, 2015. doi: 10.1016/j.neuroscience.2014.12.006. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol 507: 919–925, 1998. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HY, Huang RC. Diurnal modulation of the Na+/K+-ATPase and spontaneous firing in the rat retinorecipient clock neurons. J Neurophysiol 92: 2295–2301, 2004. doi: 10.1152/jn.00061.2004. [DOI] [PubMed] [Google Scholar]
- Wang YC, Huang RC. Effects of sodium pump activity on spontaneous firing in neurons of the rat suprachiasmatic nucleus. J Neurophysiol 96: 109–118, 2006. doi: 10.1152/jn.01369.2005. [DOI] [PubMed] [Google Scholar]
- Wang YC, Yang JJ, Huang RC. Intracellular Na+ and metabolic modulation of Na/K pump and excitability in the rat suprachiasmatic nucleus neurons. J Neurophysiol 108: 2024–2032, 2012. doi: 10.1152/jn.00361.2012. [DOI] [PubMed] [Google Scholar]
- Watts AG, Sanchez-Watts G, Emanuel JR, Levenson R. Cell-specific expression of mRNAs encoding Na+,K+-ATPase α- and β-subunit isoforms within the rat central nervous system. Proc Natl Acad Sci USA 88: 7425–7429, 1991. doi: 10.1073/pnas.88.16.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoraiou L, Akay T, Martin JF, Brownstone RM, Jessell TM, Miles GB. A cluster of cholinergic premotor interneurons modulates mouse locomotor activity. Neuron 64: 645–662, 2009. doi: 10.1016/j.neuron.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Picton L, Li WC, Sillar KT. Mechanisms underlying the activity-dependent regulation of locomotor network performance by the Na+ pump. Sci Rep 5: 16188, 2015. doi: 10.1038/srep16188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang HY, Sillar KT. Short-term memory of motor network performance via activity-dependent potentiation of Na+/K+ pump function. Curr Biol 22: 526–531, 2012. doi: 10.1016/j.cub.2012.01.058. [DOI] [PubMed] [Google Scholar]