Abstract
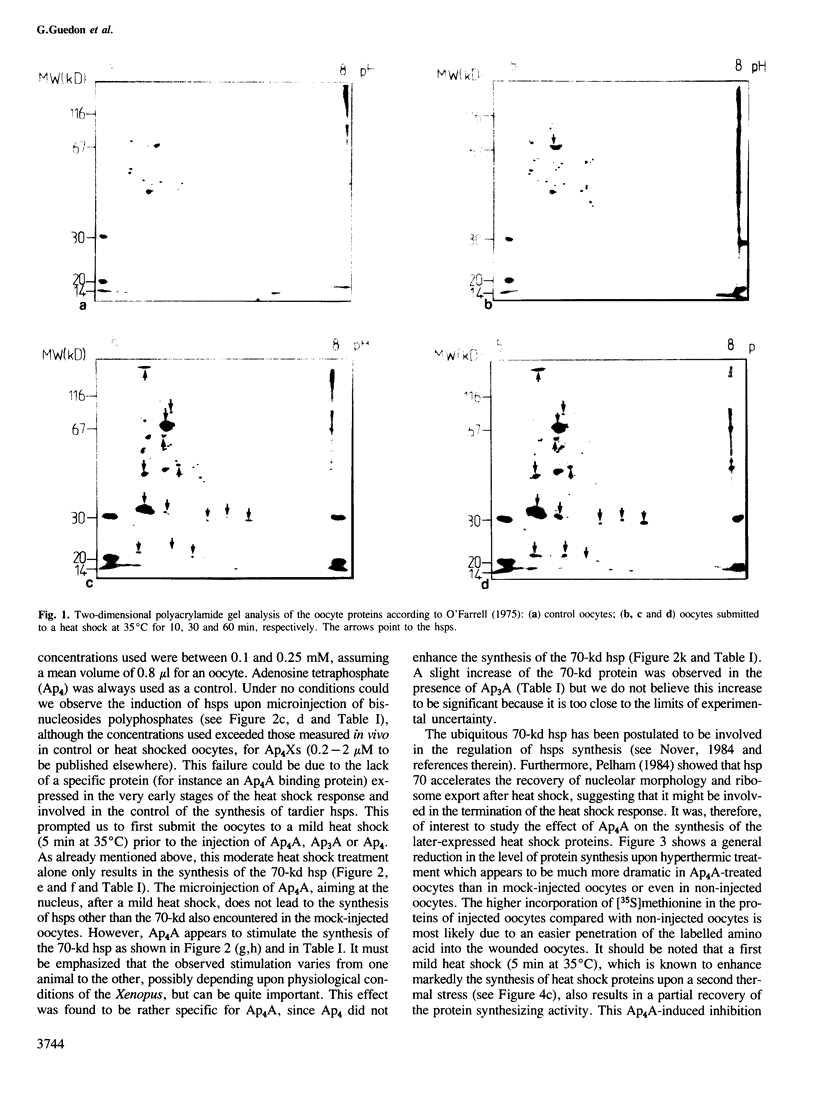
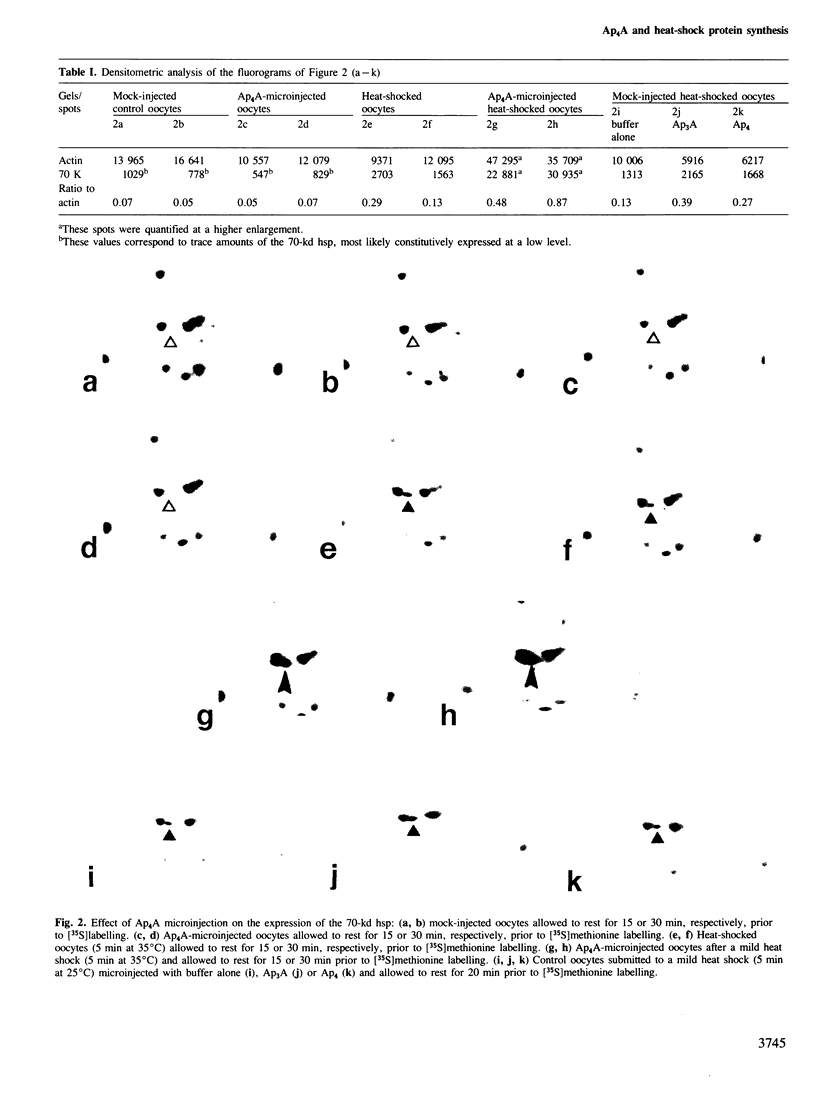
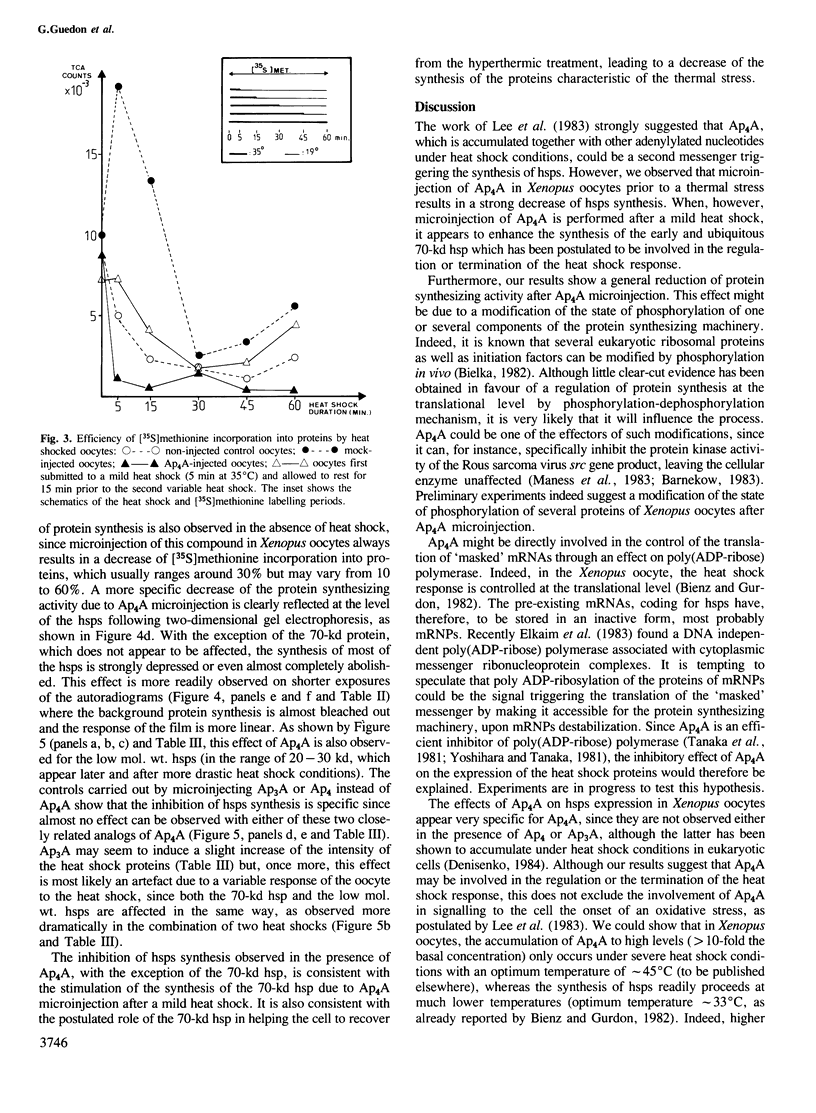
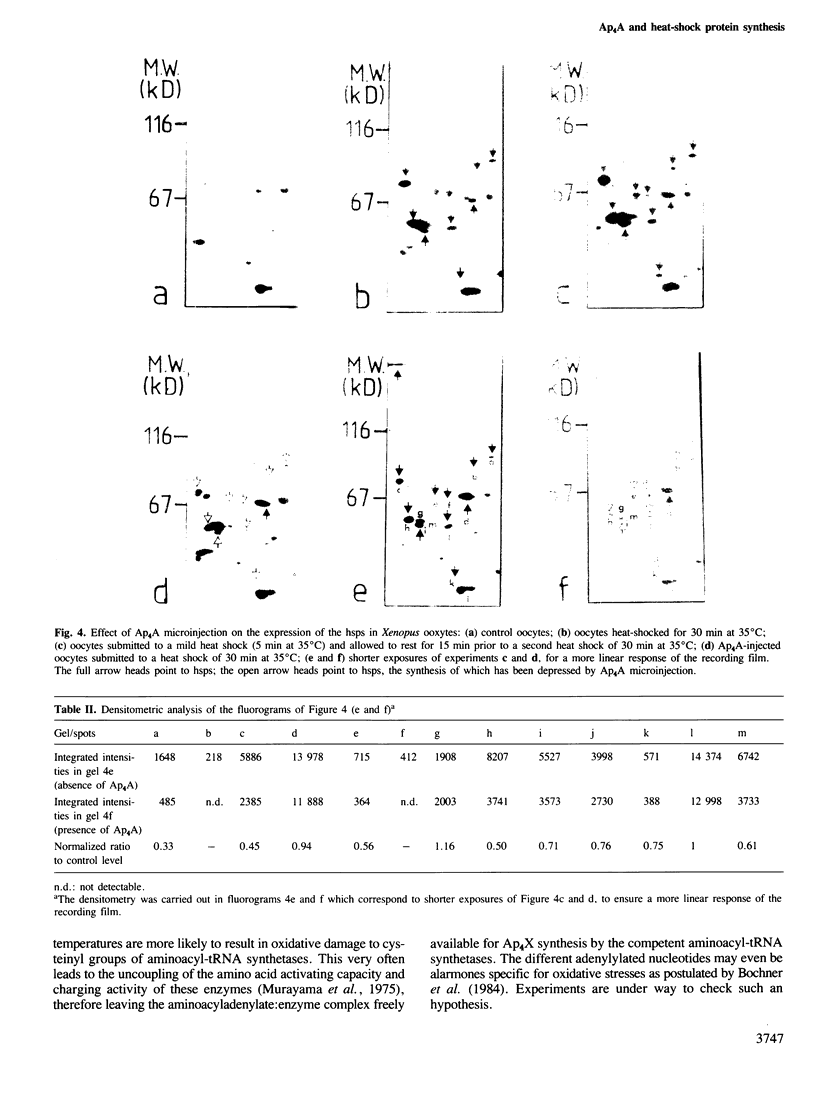
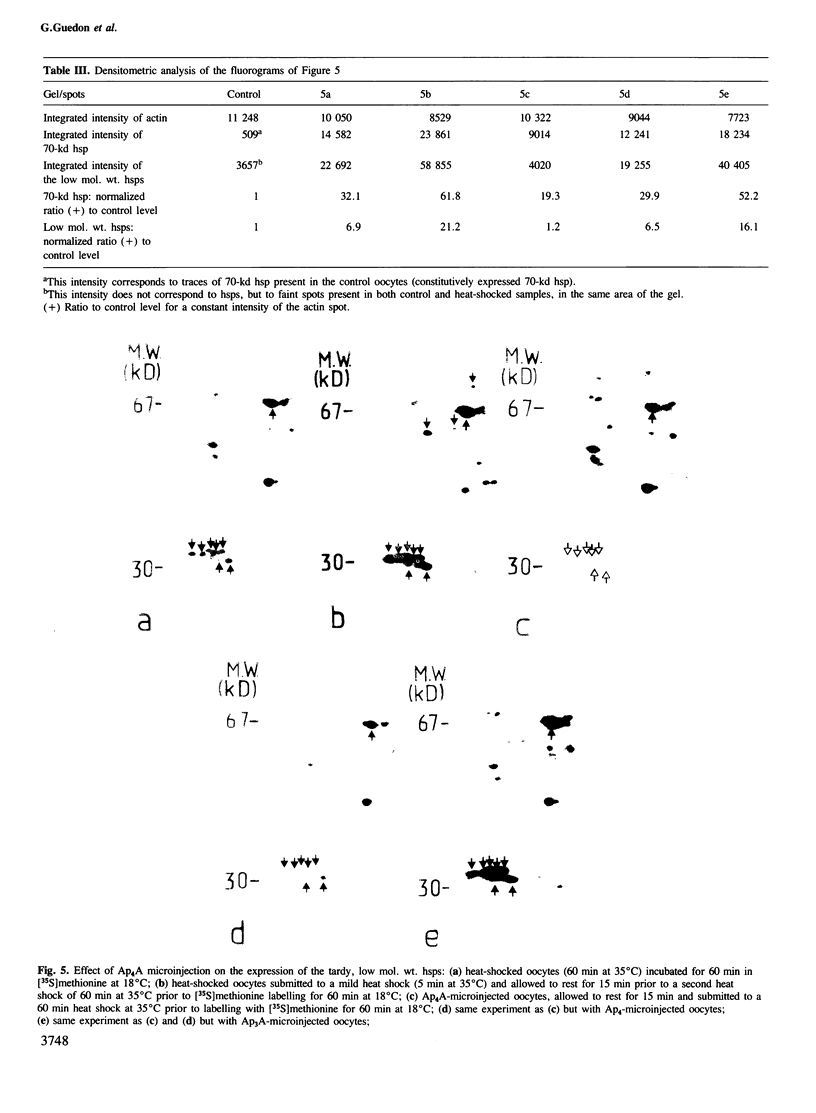
Bisnucleosides polyphosphates are thought to be chemical messengers signalling to the cell the onset of various stresses. Diadenosine tri- and tetraphosphates (respectively, Ap3A and Ap4A) accumulate in prokaryotic and eukaryotic cells under heat shock conditions, suggesting they could trigger the synthesis of heat shock proteins (hsps). In this study, Ap4A, Ap3A and, as a control, Ap4 (adenosine tetraphosphate) were injected into Xenopus oocytes. Whereas none of these compounds is able to trigger the synthesis of hsps in the absence of hyperthermic treatment, nuclear microinjection of Ap4A after a mild heat shock specifically enhances the synthesis of the 70-kd hsp, which is involved in the regulation and possibly the termination of the heat shock response. The microinjection of Ap4A prior to the hyperthermic treatment results in a strong inhibition of hsps synthesis (with the exception of the 70-kd hsp) suggesting that Ap4A is involved in the regulation and/or termination of the heat shock response. Ap3A and Ap4 do not induce any detectable modification of hsps expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnekow A. Effect of several nucleotides on the phosphorylating activities of the Rous-sarcoma-virus transforming protein pp60v-src and its cellular homologue, pp60c-src. Biosci Rep. 1983 Feb;3(2):153–162. doi: 10.1007/BF01121946. [DOI] [PubMed] [Google Scholar]
- Bienz M. Developmental control of the heat shock response in Xenopus. Proc Natl Acad Sci U S A. 1984 May;81(10):3138–3142. doi: 10.1073/pnas.81.10.3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienz M., Gurdon J. B. The heat-shock response in Xenopus oocytes is controlled at the translational level. Cell. 1982 Jul;29(3):811–819. doi: 10.1016/0092-8674(82)90443-3. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Dumont J. N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J Morphol. 1972 Feb;136(2):153–179. doi: 10.1002/jmor.1051360203. [DOI] [PubMed] [Google Scholar]
- Elkaim R., Thomassin H., Niedergang C., Egly J. M., Kempf J., Mandel P. Adenosine diphosphate ribosyltransferase and protein acceptors associated with cytoplasmic free messenger ribonucleoprotein particles. Biochimie. 1983 Nov-Dec;65(11-12):653–659. doi: 10.1016/s0300-9084(84)80029-2. [DOI] [PubMed] [Google Scholar]
- Lee P. C., Bochner B. R., Ames B. N. AppppA, heat-shock stress, and cell oxidation. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7496–7500. doi: 10.1073/pnas.80.24.7496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness P. F., Perry M. E., Levy B. T. P1,P4-Di(adenosine-5')tetraphosphate inhibits phosphorylation of immunoglobulin G by Rous sarcoma virus pp60src. J Biol Chem. 1983 Apr 10;258(7):4055–4058. [PubMed] [Google Scholar]
- Maytin E. V., Colbert R. A., Young D. A. Early heat shock proteins in primary thymocytes. Evidence for transcriptional and translational regulation. J Biol Chem. 1985 Feb 25;260(4):2384–2392. [PubMed] [Google Scholar]
- Mitchell H. K., Petersen N. S., Buzin C. H. Self-degradation of heat shock proteins. Proc Natl Acad Sci U S A. 1985 Aug;82(15):4969–4973. doi: 10.1073/pnas.82.15.4969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama A., Raffin J. P., Remy P., Ebel J. P. Yeast phenylalanyl-tRNA synthetase: properties of the sulfhydryl groups; evidence for -SH requirement in tRNA acylation. FEBS Lett. 1975 Apr 15;53(1):15–22. doi: 10.1016/0014-5793(75)80671-5. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., VanBogelen R. A. Positive regulatory gene for temperature-controlled proteins in Escherichia coli. Biochem Biophys Res Commun. 1981 May 29;100(2):894–900. doi: 10.1016/s0006-291x(81)80257-4. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Hsp70 accelerates the recovery of nucleolar morphology after heat shock. EMBO J. 1984 Dec 20;3(13):3095–3100. doi: 10.1002/j.1460-2075.1984.tb02264.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Matsunami N., Yoshihara K. Inhibition of ADP-ribosylation of histone by diadenosine 5', 5"' -p (1), p(4)-tetraphosphate. Biochem Biophys Res Commun. 1981 Apr 15;99(3):837–843. doi: 10.1016/0006-291x(81)91240-7. [DOI] [PubMed] [Google Scholar]
- Yamamori T., Yura T. Genetic control of heat-shock protein synthesis and its bearing on growth and thermal resistance in Escherichia coli K-12. Proc Natl Acad Sci U S A. 1982 Feb;79(3):860–864. doi: 10.1073/pnas.79.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshihara K., Tanaka Y. ADP-ribosylation of diadenosine 5',5"-P1,P4-tetraphosphate by poly(ADP-ribose) polymerase in vitro. J Biol Chem. 1981 Jul 10;256(13):6756–6761. [PubMed] [Google Scholar]







