Pregnant mothers are advised to avoid alcohol. This is because even small amounts of alcohol can alter fetal brain development and increase the risk of adolescent alcohol abuse. We asked how fetal alcohol exposure (FAE) produces the latter effect in adolescent rats by measuring responsiveness of taste nerves and trigeminal chemosensory neurons. We found that FAE substantially reduced taste and trigeminal responsiveness to ethanol and its flavor components.
Keywords: taste, chemesthesis, development, fetal alcohol exposure, electrophysiology
Abstract
Fetal alcohol exposure (FAE) leads to increased intake of ethanol in adolescent rats and humans. We asked whether these behavioral changes may be mediated in part by changes in responsiveness of the peripheral taste and oral trigeminal systems. We exposed the experimental rats to ethanol in utero by administering ethanol to dams through a liquid diet; we exposed the control rats to an isocaloric and isonutritive liquid diet. To assess taste responsiveness, we recorded responses of the chorda tympani (CT) and glossopharyngeal (GL) nerves to lingual stimulation with ethanol, quinine, sucrose, and NaCl. To assess trigeminal responsiveness, we measured changes in calcium levels of isolated trigeminal ganglion (TG) neurons during stimulation with ethanol, capsaicin, mustard oil, and KCl. Compared with adolescent control rats, the adolescent experimental rats exhibited diminished CT nerve responses to ethanol, quinine, and sucrose and GL nerve responses to quinine and sucrose. The reductions in taste responsiveness persisted into adulthood for quinine but not for any of the other stimuli. Adolescent experimental rats also exhibited reduced TG neuron responses to ethanol, capsaicin, and mustard oil. The lack of change in responsiveness of the taste nerves to NaCl and the TG neurons to KCl indicates that FAE altered only a subset of the response pathways within each chemosensory system. We propose that FAE reprograms development of the peripheral taste and trigeminal systems in ways that reduce their responsiveness to ethanol and surrogates for its pleasant (i.e., sweet) and unpleasant (i.e., bitterness, oral burning) flavor attributes.
NEW & NOTEWORTHY Pregnant mothers are advised to avoid alcohol. This is because even small amounts of alcohol can alter fetal brain development and increase the risk of adolescent alcohol abuse. We asked how fetal alcohol exposure (FAE) produces the latter effect in adolescent rats by measuring responsiveness of taste nerves and trigeminal chemosensory neurons. We found that FAE substantially reduced taste and trigeminal responsiveness to ethanol and its flavor components.
fetal alcohol exposure (FAE) increases the risk of alcohol abuse in adolescents (Alati et al. 2006; Baer et al. 2003; Olson et al. 1997; Yates et al. 1998). Little is known, however, about how FAE produces these effects. Given that the flavor attributes of alcohol (e.g., bitter taste and burning sensation) are important determinants of consumption (Intranuovo and Powers 1998; Lanier et al. 2005; Moore and Weiss 1995; Settle 1979), it is possible that FAE makes the flavor of alcohol less aversive. This could increase the risk of adolescents experimenting with alcohol and developing a pattern of abuse.
The rat is an established model system for studying how maternal diet alters the flavor attributes of foods, including alcohol (Ventura and Worobey 2013). When pregnant dams ingest ethanol, it enters the bloodstream and amniotic fluid and is detected by the fetus (Chotro and Molina 1990; Szeto 1989). Protracted FAE increases the oral acceptability of ethanol to adolescent rats, in part, by decreasing its quinine-like bitter taste (Youngentob and Glendinning 2009), aversive odor (Youngentob and Glendinning 2009), and capsaicin-like burning sensations (Glendinning et al. 2012). These effects ameliorate by adulthood, however.
Ethanol is detected orally by the taste and trigeminal systems. The taste system consists of four different taste bud fields. The chorda tympani (CT) nerve innervates taste buds in the fungiform papillae on the anterior two-thirds of the tongue; the glossopharyngeal (GL) nerve innervates taste buds in the foliate and circumvallate papillae on the back of the tongue; the greater superficial petrosal (GSP) nerve innervates taste buds on the palate; and the superior laryngeal branch of the vagus nerve innervates taste buds in the larynx. Lingual stimulation with ethanol has been found to generate excitatory responses in both the CT and GL nerves of adult rats (Coleman et al. 2011; Sako and Yamamoto 1999), although the GL nerve appears to respond more robustly (Sako and Yamamoto 1999). The response of taste cells to ethanol has been found to be mediated by several classes of taste receptor (T1r3 and T2rs) and transduction protein (gustducin and Trpm5) (Blednov et al. 2008; Nolden et al. 2016).
Free nerve endings from the trigeminal branch of the somatosensory system innervate the tongue and cheeks. A subset of these neurons (C and Aδ neurons) respond to irritant compounds such as ethanol, capsaicin, and mustard oil (allyl isothiocyanate, AITC) (Gerhold and Bautista 2009; Green 1987; Simon and Sostman 1991). The chemosensory response to ethanol and capsaicin is mediated by transient receptor-potential vanilloid receptor-1 (TrpV1) (Ellingson et al. 2009; Trevisani et al. 2002), whereas that to AITC is mediated by TrpV1 and TrpA1 (named for its amino-terminal ankyrin repeat domain) (Bang et al. 2007; Everaerts et al. 2011). All of the C and Aδ neurons that express TrpA1 also express TrpV1; there is also a smaller population of trigeminal ganglion (TG) neurons that only express TrpV1 (Kobayashi et al. 2005).
We know that FAE reduces responsiveness of the peripheral olfactory epithelium to alcohol (Youngentob et al. 2007a). There is reason to expect that FAE could also reduce responsiveness of the peripheral taste and trigeminal systems of offspring. For instance, when developing rats are subjected to prenatal and continued postnatal sodium restriction, their CT nerve displays diminished responsiveness to sodium salts (Hill 1987; Hill and Przekop 1988). Prenatal dietary sodium restriction does not alter responsiveness of the GSP nerve, however (Sollars and Hill 2000). Furthermore, when dams are maintained on a high-NaCl diet (4%) throughout pregnancy, there are no associated changes in CT nerve responsiveness to NaCl in their offspring (Bird and Contreras 1987).
Here we examined the impact of FAE on responsiveness of the peripheral taste and trigeminal systems. For the taste studies, we measured responsiveness of the CT and GL nerves to lingual stimulation with ethanol and two surrogates for ethanol’s bitter-sweet taste (i.e., quinine and sucrose) (Di Lorenzo et al. 1986). We included both taste nerves to determine whether the FAE-induced changes (if any) were apparent in both the anterior and posterior lingual taste fields. We tested adolescent and adult rats to determine whether the FAE-induced changes (if any) persisted into adulthood. For the trigeminal studies, we measured responsiveness of TG neurons to ethanol, capsaicin, AITC, or KCl in adolescent rats. To this end, we collected neurons from the TG ganglion and recorded their responses to chemical stimulation in vitro. Despite the prevalence of C and Aδ neurons in the trigeminal nerve, their contribution to trigeminal nerve recordings is paradoxically weak (B. Bryant, unpublished observations). This likely reflects technical difficulties associated with obtaining quantifiable recordings from C and Aδ TG neurons using extracellular recording methods (Okuni 1978; Wang et al. 1993). For this reason, we used the somatic membrane model of trigeminal reception (Bautista et al. 2005; Inoue and Bryant 2010; Klein et al. 2011) to assess the impact of FAE on an unbiased population of TG neurons.
METHODS
Experimental treatment of pregnant dams.
On gestational day (G)5, female Long-Evans rats (Harlan-Sprague Dawley, Indianapolis, IN) were divided into blocks of two weight-matched dams and randomly assigned to either the ethanol or control group. Following established protocols, ethanol dams were administered an ad libitum liquid diet (L10251; Research Diets, New Brunswick, NJ) in which 35% of their daily calories was derived from ethanol during G11–G20 (Youngentob et al. 2007a, 2007b). Dams were transitioned to the diet from G6 to G10. Peak blood alcohol levels reach ~150 mg/dl (Miller 1992; Youngentob et al. 2007a). This approach yields a relatively consistent level of exposure and should simulate moderate ethanol intake during the development of the taste and trigeminal sensory systems. Immediately after birth, the offspring (henceforth “ethanol” rats) were transferred to a foster mother who did not have any prior (or current) access to ethanol.
Dams in the control group (henceforth “control” rats) were provided ad libitum access to an isonutritive liquid diet supplemented with maltose-dextrin, which provided caloric content equivalent to the ethanol diet (L10252; Research Diets). We limited our control treatment to ad libitum-fed dams, since previous work has shown no difference in the olfactory, taste-mediated licking, or ethanol intake responses of progeny derived from pair-fed and free-choice access animals (Youngentob et al. 2007a; Youngentob and Glendinning 2009). Rat pups were weaned on postnatal day (P)21 and transported on P24 from SUNY Upstate to Barnard College or Monell Chemical Senses Center for testing.
The number of animals used in each treatment group is indicated in the figures. All animal procedures were approved by the Institutional Animal Care and Use Committees at SUNY Upstate Medical University, Columbia University, and Monell Chemical Senses Center. The procedures were conducted in accordance with the National Institutes of Health guidelines for the care and use of laboratory animals.
Taste nerve recordings.
We tested the following chemical solutions in the indicated order: NaCl (0.03, 0.1, 0.3, and 1 M), quinine (1, 3, 10, and 20 mM), sucrose (0.03, 0.1, 0.3, and 1 M), and ethanol (1%, 3%, 6%, and 9%). We used the indicated concentrations of each stimulus because they matched concentrations used in prior behavioral studies (Youngentob and Glendinning 2009). To provide a reference stimulus and control for time-dependent changes in responsiveness, we recorded responses to NH4Cl (0.1 M for CT and 0.5 M for GL) both before and after each concentration series with a given taste stimulus. We paused at least 40 s between each stimulation. We used a higher NH4Cl concentration for the GL nerve recordings because the posterior taste field appears to be less responsive to this salt. All chemicals were dissolved in an artificial saliva solution (Ogawa et al. 1972).
Anesthesia.
The rats were anesthetized with 2–5% isoflurane (Baxter, Deerfield, IL) throughout the recordings. The isoflurane was delivered initially in an induction chamber and subsequently via a nose cone or tracheal tube, depending on the stage in the surgery. The isoflurane provided a consistent and controlled anesthetic dose; however, it also tended to cause the rats to breathe deeply, resulting in breathing artifacts in some but not all CT and GL recordings (e.g., see Fig. 3). Throughout the surgery and nerve recordings, the rat was maintained on a thermostat-controlled circulating-water heating pad set at 37°C (HTP-1500; Adroit Medical Systems, Loudon, TN).
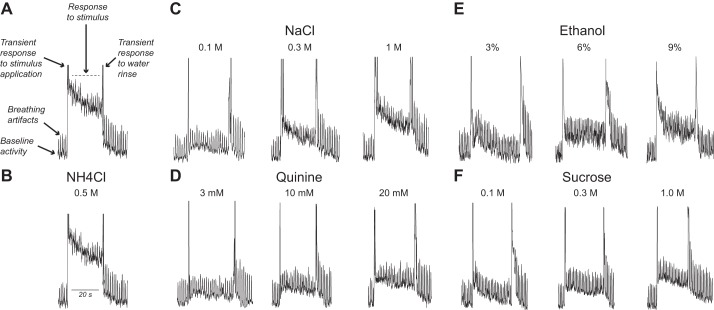
Fig. 3.
Typical whole nerve integrated responses of the glossopharyngeal (GL) nerve (from an adolescent control rat) to lingual stimulation with chemical stimuli. A: components of the integrated response: baseline activity (i); breathing artifacts (ii), transient responses to the infusion of the chemical stimulus or water rinse into the oral cavity(iii); and the actual taste response to the chemical stimulus (iv) (dashed line indicates the time period over which the taste response was quantified). B–F: GL nerve responses to 1 concentration of NH4Cl (B) and 3 concentrations of NaCl (C), quinine (D), ethanol (E), and sucrose (F).
Surgery and stimulation procedures.
To access the CT nerve, we secured the tracheotomized rat in a nontraumatic head holder and used the mandibular approach (Kawai et al. 2000) to expose the CT nerve. The CT nerve passed underneath the auriculo-temporal, mylohyoid, and inferior alveolar nerves before connecting with the lingual nerve. The CT nerve was cut near the auditory bulla. To elicit responses in the CT nerve, we delivered the taste solutions to the anterior tongue (flow rate: 10 ml/min) with a continuous-flow system (VC-6 Perfusion Valve Control System; Warner Instruments, Hamden, CT). The tongue was rinsed continuously with artificial saliva, both before and after each 20-s stimulation trial. All taste solutions were maintained at 35°C with an automatic temperature controller (Warner Instruments).
To access the GL nerve, we used a ventral approach, similar to that described by Martin and Sollars (2015). We removed the posterior digastricus muscle and posterior horn of the hyoid bone, dissected the fascia, and then used the hypoglossal nerve to locate the GL nerve where it exits the skull. Next, we inserted a 10-cm polyethylene tube (with a flange on its back end) though the mouth and into the esophagus; it exited the esophagus through a small incision about halfway to the stomach. The esophagus was sutured anterior to this incision point to prevent solutions from flowing beyond that point. The flange on the back end of the polyethylene tube 1) prevented the back end of the tube from entering the esophagus; 2) ensured that the back end of the polyethylene tube rested at the entrance to the pharynx, and thus permitted taste solutions to access the circumvallate and foliate papillae; and 3) prevented taste stimuli from entering the pharynx. To elicit responses, we infused 5 ml of taste stimulus into the back of the mouth (at 35°C). The taste stimulus was left in the mouth for 20 s, after which it was flushed out with artificial saliva solution until nerve activity returned to baseline.
One of the challenges of stimulating taste buds in the posterior tongue is that they are recessed within the circumvallate and foliate trenches. To open the entrance of the trenches, we applied traction to the tongue via a suture in the median eminence. We also rapidly infused the 5 ml of taste stimulus into the back of the mouth (i.e., within 3–5 s) during each stimulation; this increased the volumetric pressure to a degree that the taste stimulus solutions were effectively forced into the trenches. While the infusion produced a mechanical artifact (e.g., see Fig. 3), we focused on the neural response that occurred 5 s after the infusion was completed.
Electrophysiological recordings.
Because the CT nerve sheath was thin, we could record strong responses by simply placing the nerve on a platinum wire (0.005 in.; A-M Systems, Sequim, WA) electrode. For the GL recordings, we had to desheath the nerve before placing it on the platinum wire. We placed the ground electrode in nearby tissue. Electrophysiological responses were amplified 10,000× with an optically coupled isolated bioamplifier (ISO-80; World Precision Instruments), passed through a band-pass filter (300–3,000 Hz), and then digitized (sampling rate: 1,000 samples/s), transformed (root mean square), and integrated (time constant: 1 s) (Biopac Software, Goleta, CA). All electrical activity was monitored via a computer screen and speaker.
Trigeminal ganglion calcium imaging.
We recorded responses of TG neurons to ethanol (1% and 3%), AITC (100 µM), capsaicin (0.3 µM), and KCl (40 mM). These concentrations were chosen because they were suprathreshold but not so high that TG neurons would fatigue or remain adapted to subsequent stimuli. We included KCl to obtain a measure of neuronal health and excitability. Importantly, both chemesthetic and nonchemesthetic TG neurons should respond to KCl.
All of the stimuli were dissolved in a Ringer solution (for details, see Inoue and Bryant 2005). Capsaicin and AITC stock solutions were first dissolved in 95% ethanol and then diluted with the Ringer solution so that final ethanol concentrations were <0.1% ethanol, which elicited no responses from TG neurons.
Trigeminal ganglia were obtained from five control and five ethanol rats (all adolescents), and neuron populations were processed according to the methods described by Inoue and Bryant (2005). In brief, the TG neurons (in growth medium) were added to cloning cylinders (ID = 3 mm) set with silicone grease on poly-l-lysine-laminin-coated glass coverslips. The cells were placed in an incubator (37°C, air atmosphere) and allowed to settle overnight. Subsequently, the medium in each well was replaced with 1 ml of fresh medium. All experiments were conducted within 16–48 h of neuronal dissociation and plating. Because the TG neurons fell into many size classes, including those associated with Aβ fibers (Djouhri and Lawson 2004), we did not discriminate with respect to size class. We recorded from 20 to 40 TG neurons from each of the rats in the control and ethanol maternal treatment groups, respectively.
Changes in intraneuronal calcium levels ([Ca2+]i) were measured with ratiometric digital fluorescence calcium imaging (Grynkiewicz et al. 1985). Cultured neurons were loaded with fura-2 by incubation in 10 µM fura-2 AM, 0.5% DMSO, and 80 µg/ml Pluronic-127 in Ringer solution (at 37°C) for 30 min. Images of fluorescing neurons were captured every 5 s with a cooled charge-coupled device camera (Photometrics CoolSnap HQ2), using 150-ms exposures. Autofluorescence was negligible, and no appreciable photobleaching was observed.
Coverslips with attached neurons were placed in a flow chamber through which Ringer solution (30–32°C) flowed constantly. Chemical stimuli in Ringer solution were applied to the flow with manually controlled valves, and pairs of images (excitation at 340 and 380 nm) were acquired every 5 s. Chemical stimuli were applied to the neurons for 15 s, and then the chambers were rinsed for at least 2–3 min between each stimulus. The average fluorescence ratio, F340/F380 (an index of [Ca2+]i), was calculated for each neuron with “regions of interest” drawn automatically for each neuron with Metafluor software (Universal Imaging).
Data analysis.
For taste responses, the dependent variable was relative response of the CT or GL nerve to each taste solution. To calculate the relative response, we 1) calculated the mean integrated response (in mV/s) during the 20 s immediately before stimulation (= baseline response) and that during the initial 20 s of the taste response; 2) determined the absolute response by subtracting the baseline response from the taste response; and then 3) divided the absolute response to the taste stimulus by the mean of the absolute response to NH4Cl before and after each taste stimulus concentration series. We excluded any breathing artifacts from our measurements of the baseline and taste responses.
For CT recordings, there was no transient response associated with stimulus application because we used a continuous-flow system. Thus the 20-s taste response was considered to have begun immediately after the chemical stimulus reached the lingual surface. For the GL recordings, a transient mechanical response was elicited during the 3–5 s injection of the taste stimulus into the back of the oral cavity. For this reason, we considered the 20-s taste response to have begun once the transient mechanical response ended.
We used mixed-model ANOVAs to test for effects of maternal diet, tastant concentration, and age on the relative response. Stimulus concentration was treated as a within factor and maternal diet and age as between factors. In this and all subsequent statistical comparisons, we set the α level at 0.05. We used IBM SPSS Statistics 23 (https://www.ibm.com/us-en/marketplace/analytics) to conduct all statistical analyses.
We limited analysis of the TG neurons to morphologically identifiable cells, which exhibited a peak response (i.e., fluorescence ratio) > 0.02 to at least one of the chemical stimuli. The response properties of individual TG neurons fell into four groups, based on the stimuli to which they responded: KCl alone, ethanol+KCl, capsaicin+AITC+KCl, or capsaicin+AITC+ethanol+KCl. We tested for an association between maternal diet treatment and the number of neurons in each response grouping, using the χ2-test. We also compared the fluorescence ratios across maternal diet treatments, separately for each chemical stimulus, with unpaired t-tests (with Welch’s correction for unequal variances).
RESULTS
Taste nerve recordings.
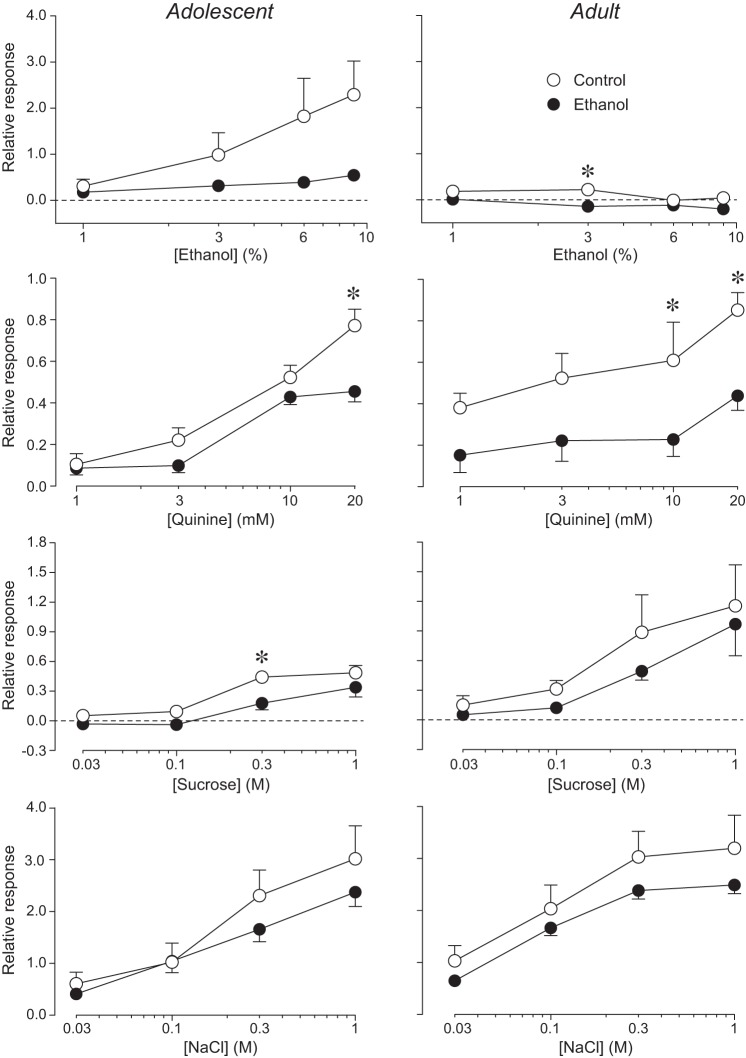
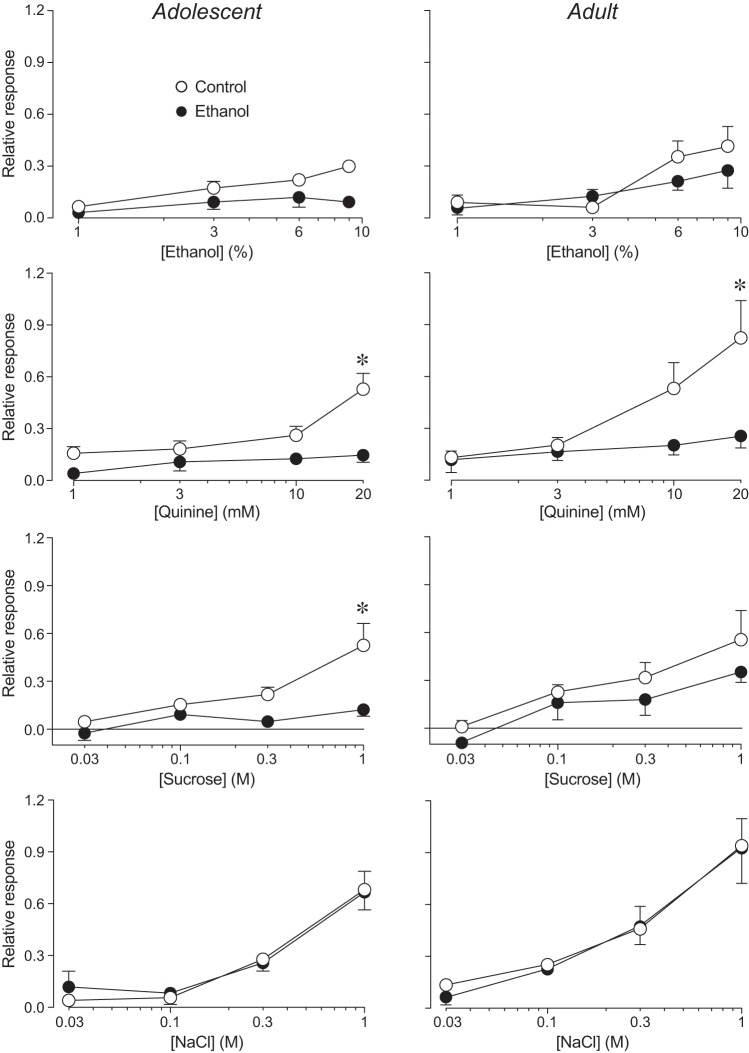
In adolescent rats, there were significant concentration-dependent increases in responsiveness to ethanol, quinine, sucrose, and NaCl for both the CT and GL nerves (Table 1). For the CT nerve, the concentration-dependent increases in responsiveness (Fig. 1) were attenuated in the ethanol rats (compared with the control rats) for ethanol, quinine, and sucrose but not NaCl (Fig. 2, left). For the GL nerve, the concentration-dependent increases in responsiveness (Fig. 3) were attenuated in the ethanol rats for quinine and sucrose but not ethanol and NaCl (Fig. 4, left). When the main effect of maternal diet was significant, the interaction of maternal diet × concentration was often significant, illustrating that the slope of the concentration-response curve was shallower in ethanol rats. Overall, these results show that FAE weakened responses of the CT and GL nerves to lingual stimulation with quinine, sucrose, and ethanol (CT nerve only), particularly at the higher concentrations of each stimulus.
Table 1.
Results of mixed-model ANOVAs performed on CT or GL nerve responses in adolescent rats, separately for each chemical stimulus
| Taste Nerve | Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|---|
| CT | Ethanol | Maternal diet | 1,12 | 7.4 | <0.02 | Yes |
| Concentration | 3,36 | 3.8 | <0.05 | Yes | ||
| Interaction | 3,36 | 1.9 | 0.17 | |||
| Quinine | Maternal diet | 1,12 | 5.3 | 0.035 | Yes | |
| Concentration | 3,36 | 69.5 | <0.0001 | Yes | ||
| Interaction | 3,36 | 4.5 | 0.01 | Yes | ||
| Sucrose | Maternal diet | 1,12 | 7.9 | 0.012 | Yes | |
| Concentration | 3,36 | 30.4 | <0.0001 | Yes | ||
| Interaction | 3,36 | 1.1 | 0.35 | |||
| NaCl | Maternal diet | 1,12 | 1.2 | 0.29 | ||
| Concentration | 3,36 | 28.9 | <0.0001 | Yes | ||
| Interaction | 3,36 | 0.9 | 0.45 | |||
| GL | Ethanol | Maternal diet | 1,14 | 1.0 | 0.33 | |
| Concentration | 3,42 | 9.7 | <0.001 | Yes | ||
| Interaction | 3,42 | 0.7 | 0.52 | |||
| Quinine | Maternal diet | 1,14 | 12.3 | 0.003 | Yes | |
| Concentration | 3,42 | 11.9 | 0.001 | Yes | ||
| Interaction | 3,42 | 5.4 | 0.017 | Yes | ||
| Sucrose | Maternal diet | 1,14 | 12.5 | 0.003 | Yes | |
| Concentration | 3,42 | 11.3 | 0.001 | Yes | ||
| Interaction | 3,42 | 4.3 | 0.037 | Yes | ||
| NaCl | Maternal diet | 1,14 | <0.1 | 0.77 | ||
| Concentration | 3,42 | 32.0 | <0.001 | Yes | ||
| Interaction | 3,42 | 0.2 | 0.77 |
Values are results of mixed-model ANOVAs performed on CT or GL nerve responses in adolescent rats, separately for each chemical stimulus (data in Figs. 2 and 4). We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure; for clarity, we present uncorrected degrees of freedom (df).
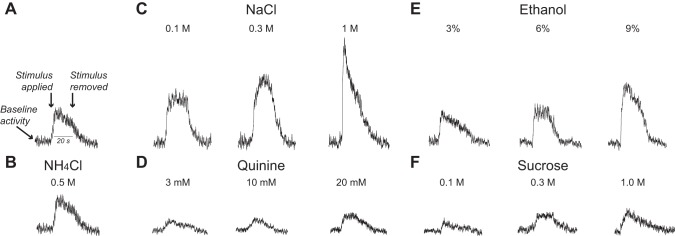
Fig. 1.
Typical whole nerve integrated responses of the chorda tympani (CT) nerve (from an adolescent control rat) to lingual stimulation with chemical stimuli. A: the salient components of the integrated response: baseline activity, the moments when the chemical stimulus was applied and then removed, and a timescale bar. B–F: CT nerve responses of the same rat to 1 concentration of NH4Cl (B) and 3 concentrations of NaCl (C), quinine (D), ethanol (E), and sucrose (F).
Fig. 2.
Chorda tympani (CT) nerve responses of adolescent (left) and adult (right) rats to lingual stimulation with 4 concentrations of ethanol, quinine, sucrose, and NaCl. In each panel, we juxtapose relative responses of control and ethanol rats. Relative responses (mean ± SE) reflect the ratio of the integrated response to a taste stimulus divided by that to 0.1 M NH4Cl. We used different scales on the y-axes for each stimulus because the magnitude of the relative responses varied greatly. We obtained CT nerve responses from 14 adolescent (6 control and 8 ethanol) and 16 adult (7 control and 9 ethanol) rats. We present analyses of these responses in Table 1. When there was a significant main effect of maternal diet (or interaction of maternal diet × age class) for a given stimulus, we compared relative response to each concentration across control and ethanol rats (separately for each age class), using Sidak’s multiple comparison test (*P < 0.05).
Fig. 4.
Glossopharyngeal (GL) nerve responses of adolescent (left) and adult (right) rats to lingual stimulation with 4 concentrations of ethanol, quinine, sucrose, and NaCl. Within each panel, we juxtapose relative responses of control and ethanol rats. Relative responses (mean ± SE) reflect the ratio of the integrated response to a taste stimulus divided by that to 0.5 M NH4Cl. We obtained GL nerve responses from 16 adolescent (8 control and 8 ethanol) and 13 adult (6 control and 7 ethanol) rats. We show analyses of these responses in Table 2. When there was a significant main effect of maternal diet (or interaction of maternal diet × age class) for a given stimulus, we compared relative response to each concentration across control and ethanol rats (separately for each age class), using Sidak’s multiple comparison test (*P < 0.05).
In adult rats, there were significant concentration-dependent increases in responsiveness of the CT and GL nerves to quinine, sucrose, and NaCl (Table 2). While the response of the GL nerve to ethanol increased with concentration, that of the CT nerve unexpectedly decreased with concentration. FAE significantly attenuated responsiveness of the CT (Fig. 2, right) and GL (Fig. 4, right) nerves to quinine; it also attenuated responsiveness of the CT nerve to ethanol (albeit to a small degree). FAE had no apparent impact on responsiveness of the CT and GL nerves to sucrose and NaCl in adult rats.
Table 2.
Results of mixed-model ANOVAs performed on CT or GL nerve responses in adult rats, separately for each chemical stimulus
| Taste Nerve | Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|---|
| CT | Ethanol | Maternal diet | 1,14 | 22.5 | 0.001 | Yes |
| Concentration | 3,42 | 5.3 | 0.003 | Yes | ||
| Interaction | 3,42 | 2.1 | 0.11 | |||
| Quinine | Maternal diet | 1,14 | 12.1 | 0.004 | Yes | |
| Concentration | 3,42 | 9.0 | 0.001 | Yes | ||
| Interaction | 3,42 | 0.5 | 0.57 | |||
| Sucrose | Maternal diet | 1,14 | 1.0 | 0.33 | ||
| Concentration | 3,42 | 16.4 | 0.001 | Yes | ||
| Interaction | 3,42 | 0.3 | 0.62 | |||
| NaCl | Maternal diet | 1,14 | 1.8 | 0.20 | ||
| Concentration | 3,42 | 48.5 | <0.001 | Yes | ||
| Interaction | 3,42 | 0.4 | 0.63 | |||
| GL | Ethanol | Maternal diet | 1,11 | 0.9 | 0.37 | |
| Concentration | 3,33 | 8.8 | 0.02 | Yes | ||
| Interaction | 3,33 | 1.2 | 0.34 | |||
| Quinine | Maternal diet | 1,11 | 4.3 | 0.06 | ||
| Concentration | 3,33 | 8.3 | 0.005 | Yes | ||
| Interaction | 3,33 | 4.2 | 0.04 | Yes | ||
| Sucrose | Maternal diet | 1,11 | 1.7 | 0.22 | ||
| Concentration | 3,33 | 14.9 | <0.001 | Yes | ||
| Interaction | 3,33 | 0.3 | 0.72 | |||
| NaCl | Maternal diet | 1,11 | <0.1 | 0.84 | ||
| Concentration | 3,33 | 32.9 | <0.001 | Yes | ||
| Interaction | 3,33 | <0.1 | 0.83 |
Values are results of mixed-model ANOVAs performed on CT or GL nerve responses in adult rats, separately for each chemical stimulus (data in Figs. 2 and 4). We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure; for clarity, we present uncorrected df.
In separate ANOVAs, we tested for effects of sex and age on the relative response of the CT nerve to each chemical stimulus with a mixed-model ANOVA, separately for control (Table 3) and ethanol (Table 4) rats. While there were no effects of sex, there were a few notable effects of age. There was a significant age-dependent loss in responsiveness of the CT nerve to ethanol. The magnitude of the loss was substantially larger in the control rats. There was also a significant main effect of age on the response of the CT nerve to sucrose, revealing an age-dependent increase in responsiveness.
Table 3.
Analysis of how age, sex, and concentration alter CT nerve responses in control rats
| Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|
| Ethanol | Age (A) | 1,9 | 17.2 | 0.003 | Yes |
| Sex (S) | 1,9 | 0.6 | 0.46 | ||
| Concentration (C) | 3,27 | 1.9 | 0.15 | ||
| A × S | 1,9 | 0.12 | 0.74 | ||
| A × C | 3,27 | 2.8 | 0.10 | ||
| S × C | 3,27 | <0.1 | 0.89 | ||
| A × S × C | 3,27 | <0.1 | 0.90 | ||
| Quinine | Age (A) | 1,9 | 1.5 | 0.25 | |
| Sex (S) | 1,9 | 0.3 | 0.61 | ||
| Concentration (C) | 3,27 | 19.2 | <0.001 | Yes | |
| A × S | 1,9 | 0.8 | 0.41 | ||
| A × C | 3,27 | 1.3 | 0.29 | ||
| S × C | 3,27 | 0.3 | 0.84 | ||
| A × S × C | 3,27 | 0.6 | 0.65 | ||
| Sucrose | Age (A) | 1,9 | 2.2 | 0.17 | |
| Sex (S) | 1,9 | 0.2 | 0.68 | ||
| Concentration (C) | 3,27 | 10.4 | <0.0001 | Yes | |
| A × S | 1,9 | 0.2 | 0.70 | ||
| A × C | 3,27 | 1.6 | 0.23 | ||
| S × C | 3,27 | 0.3 | 0.63 | ||
| A × S × C | 3,27 | 0.4 | 0.55 | ||
| NaCl | Age (A) | 1,9 | 0.7 | 0.44 | |
| Sex (S) | 1,9 | 0.3 | 0.60 | ||
| Concentration (C) | 3,27 | 21.0 | <0.0001 | Yes | |
| A × S | 1,9 | 0.8 | 0.38 | ||
| A × C | 3,27 | 2.2 | 0.15 | ||
| S × C | 3,27 | 2.5 | 0.12 | ||
| A × S × C | 3,27 | 0.9 | 0.40 |
Values are results of analysis of how age, sex, and concentration alter CT nerve responses in control rats. We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure. For clarity, we present uncorrected df.
Table 4.
Analysis of how age, sex, and concentration alter CT nerve responses in ethanol rats
| Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|
| Ethanol | Age (A) | 1,13 | 6.1 | <0.03 | Yes |
| Sex (S) | 1,13 | 3.5 | 0.09 | ||
| Concentration (C) | 3,39 | 0.3 | 0.66 | ||
| A × S | 1,13 | 3.7 | 0.08 | ||
| A × C | 3,39 | 2.4 | 0.14 | ||
| S × C | 3,39 | 1.4 | 0.26 | ||
| A × S × C | 3,39 | 1.7 | 0.21 | ||
| Quinine | Age (A) | 1,13 | <0.1 | 0.77 | |
| Sex (S) | 1,13 | 2.1 | 0.17 | ||
| Concentration (C) | 3,39 | 12.1 | <0.001 | Yes | |
| A × S | 1,13 | 3.2 | 0.10 | ||
| A × C | 3,39 | 2.6 | 0.10 | ||
| S × C | 3,39 | 0.3 | 0.76 | ||
| A × S × C | 3,39 | 0.1 | 0.86 | ||
| Sucrose | Age (A) | 1,13 | 6.7 | <0.03 | Yes |
| Sex (S) | 1,13 | 0.5 | 0.49 | ||
| Concentration (C) | 3,39 | 15.7 | <0.001 | Yes | |
| A × S | 1,13 | 1.3 | 0.28 | ||
| A × C | 3,39 | 2.9 | 0.11 | ||
| S × C | 3,39 | 0.7 | 0.42 | ||
| A × S × C | 3,39 | 1.8 | 0.19 | ||
| NaCl | Age (A) | 1,13 | 4.2 | 0.063 | |
| Sex (S) | 1,13 | 1.6 | 0.23 | ||
| Concentration (C) | 3,39 | 95.6 | <0.001 | Yes | |
| A × S | 1,13 | 0.9 | 0.37 | ||
| A × C | 3,39 | 2.9 | 0.06 | ||
| S × C | 3,39 | 1.9 | 0.16 | ||
| A × S × C | 3,39 | 0.6 | 0.56 |
Values are results of analysis of how age, sex, and concentration alter CT nerve responses in ethanol rats. We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure. For clarity, we present uncorrected df.
We also tested for effects of sex and age on responsiveness of the GL nerve to each chemical stimulus with a mixed-model ANOVA, separately for control (Table 5) and ethanol (Table 6) rats. However, there were no significant effects of either variable on the relative response of the GL nerve.
Table 5.
Analysis of how age, sex, and concentration alter GL nerve responses in control rats
| Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|
| Ethanol | Age (A) | 1,10 | 0.8 | 0.38 | |
| Sex (S) | 1,10 | 2.6 | 0.14 | ||
| Concentration (C) | 3,30 | 12.1 | <0.001 | Yes | |
| A × S | 1,10 | 0.1 | 0.78 | ||
| A × C | 3,30 | 2.1 | 0.15 | ||
| S × C | 3,30 | 1.0 | 0.39 | ||
| A × S × C | 3,30 | 1.5 | 0.25 | ||
| Quinine | Age (A) | 1,10 | 1.4 | 0.26 | |
| Sex (S) | 1,10 | <0.1 | 0.84 | ||
| Concentration (C) | 3,30 | 13.3 | <0.001 | Yes | |
| A × S | 1,10 | 0.3 | 0.58 | ||
| A × C | 3,30 | 1.3 | 0.29 | ||
| S × C | 3,30 | 1.0 | 0.36 | ||
| A × S × C | 3,30 | 0.3 | 0.72 | ||
| Sucrose | Age (A) | 1,10 | 0.1 | 0.75 | |
| Sex (S) | 1,10 | 2.5 | 0.14 | ||
| Concentration (C) | 3,30 | 19.2 | <0.001 | Yes | |
| A × S | 1,10 | <0.1 | 0.95 | ||
| A × C | 3,30 | 0.54 | 0.55 | ||
| S × C | 3,30 | 4.0 | 0.06 | ||
| A × S × C | 3,30 | 0.2 | 0.73 | ||
| NaCl | Age (A) | 1,10 | 3.7 | 0.09 | |
| Sex (S) | 1,10 | 0.6 | 0.46 | ||
| Concentration (C) | 3,30 | 30.3 | <0.001 | Yes | |
| A × S | 1,10 | 0.5 | 0.51 | ||
| A × C | 3,30 | 0.3 | 0.69 | ||
| S × C | 3,30 | 0.9 | 0.38 | ||
| A × S × C | 3,30 | 0.1 | 0.79 |
Values are results of analysis of how age, sex, and concentration alter GL nerve responses in control rats. We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure. For clarity, we present uncorrected df.
Table 6.
Analysis of how age, sex, and concentration alter GL nerve responses in ethanol rats
| Chemical Stimulus | Source of Variation | df | F Ratio | P Value | Significant? |
|---|---|---|---|---|---|
| Ethanol | Age (A) | 1,11 | 0.9 | 0.38 | |
| Sex (S) | 1,11 | 0.4 | 0.54 | ||
| Concentration (C) | 3,33 | 7.2 | 0.001 | Yes | |
| A × S | 1,11 | 1.0 | 0.35 | ||
| A × C | 3,33 | 1.2 | 0.33 | ||
| S × C | 3,33 | 0.3 | 0.76 | ||
| A × S × C | 3,33 | 2.0 | 0.16 | ||
| Quinine | Age (A) | 1,11 | 2.1 | 0.18 | |
| Sex (S) | 1,11 | 0.2 | 0.65 | ||
| Concentration (C) | 3,33 | 4.3 | <0.02 | Yes | |
| A × S | 1,11 | 0.9 | 0.37 | ||
| A × C | 3,33 | 0.3 | 0.76 | ||
| S × C | 3,33 | 1.2 | 0.33 | ||
| A × S × C | 3,33 | 3.0 | 0.06 | ||
| Sucrose | Age (A) | 1,11 | 1.6 | 0.23 | |
| Sex (S) | 1,11 | 1.4 | 0.27 | ||
| Concentration (C) | 3,33 | 11.5 | <0.001 | Yes | |
| A × S | 1,11 | <0.1 | 0.85 | ||
| A × C | 3,33 | 3.0 | 0.07 | ||
| S × C | 3,33 | 0.1 | 0.88 | ||
| A × S × C | 3,33 | 0.5 | 0.65 | ||
| NaCl | Age (A) | 1,11 | 3.6 | 0.08 | |
| Sex (S) | 1,11 | 1.3 | 0.28 | ||
| Concentration (C) | 3,33 | 29.8 | <0.001 | Yes | |
| A × S | 1,11 | 0.7 | 0.43 | ||
| A × C | 3,33 | 1.4 | 0.27 | ||
| S × C | 3,33 | 2.0 | 0.17 | ||
| A × S × C | 3,33 | 0.5 | 0.46 |
Values are results of analysis of how age, sex, and concentration alter GL nerve responses in ethanol rats. We did not assume sphericity for the repeated measure (i.e., concentration), and we present corrected P values according to the Greenhouse-Geisser procedure. For clarity, we present uncorrected df.
Finally, we compared the absolute responses of the CT nerve to 0.1 M NH4Cl (or the GL nerve to 0.5 M NH4Cl) across maternal treatments, separately for each age class, using unpaired t-tests. The fact that the P value for all t-tests was >0.18 establishes that the taste response of the CT nerve to 0.1 NH4Cl and the GL nerve to 0.5 M NH4Cl was not altered by FAE. This justifies our use of NH4Cl as the reference stimulus in calculating relative responses to each stimulus.
Trigeminal ganglion neuron responses.
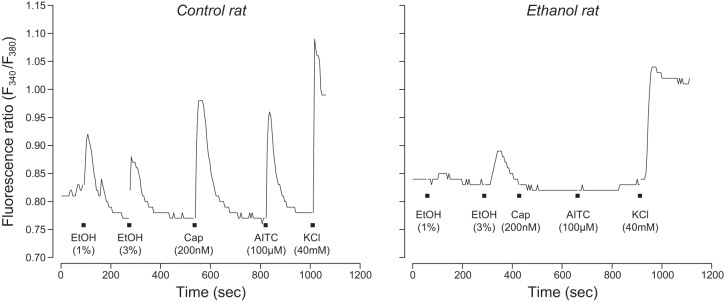
In Fig. 5, we show typical calcium responses of TG neurons from control and ethanol rats to the battery of chemical stimuli. These representative responses illustrate that the responses had a high signal-to-noise ratio and that FAE profoundly altered the response properties of TG neurons. In Fig. 6, we show the percentage of TG neurons that responded to capsaicin+AITC+KCl, capsaicin+AITC+ethanol+KCl, ethanol+KCl, or KCl alone. There was a significant association between maternal diet and number of TG neurons in each response grouping (χ2 = 38.1, df = 3, P < 0.0001). This result reflects the fact that FAE 1) had no impact on the relative number of TG neurons responsive to capsaicin+AITC+KCl and 2) reduced by half the relative number of TG neurons responsive to capsaicin+AITC+ethanol+KCl and ethanol+KCl. More generally, it is apparent the FAE selectively reduced the abundance of TG neurons that respond to ethanol.
Fig. 5.
Typical intracellular calcium responses (as indicated by fluorescence ratio, F340/F380) of a TG neuron from a control (left) vs. an ethanol (right) rat to 4 chemical stimuli: ethanol (1% or 3%), capsaicin (Cap; 200 nM), AITC (100 µM), and KCl (40 mM). Black squares indicate the timing of the 15-s application of each chemical stimulus.
Fig. 6.
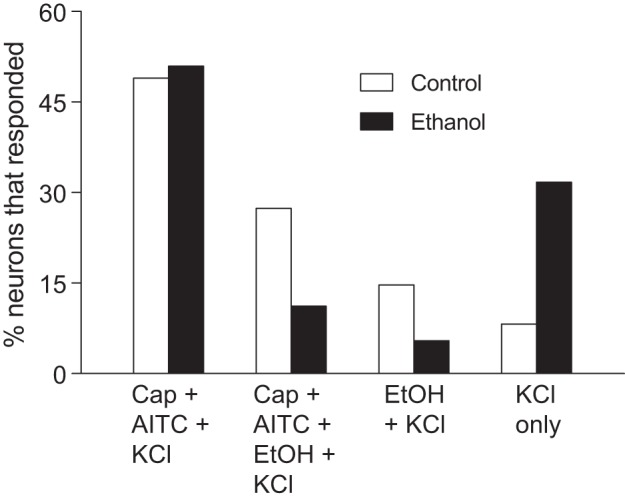
Percentage of TG neurons from control (n = 134 cells) vs. ethanol (n = 178 cells) rats that responded to 1) capsaicin (Cap) + AITC + KCl, 2) Cap + AITC + ethanol (EtOH) + KCl, 3) EtOH + KCl, or 4) KCl only. There was a significant association between the number of neurons in each response category and maternal diet (χ2 value = 38.1, df = 3, P < 0.0001).
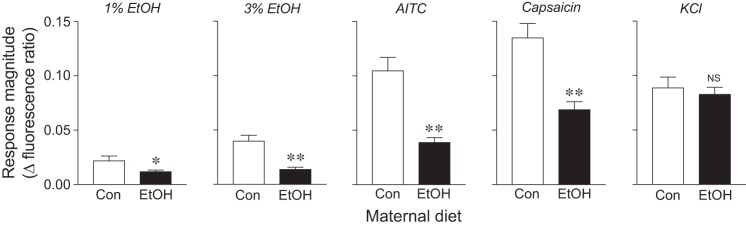
We also compared the impact of FAE on the magnitude of the calcium response (as indicated by the fluorescent ratio), separately for each chemical stimulus (Fig. 7). We found that (relative to control rats) the ethanol rats exhibited significantly attenuated calcium responses to ethanol (1% and 3%), AITC, and capsaicin. However, the response magnitude to KCl did not vary with maternal diet. These findings demonstrate that FAE selectively diminished peripheral responsiveness of TG neurons to ethanol, AITC, and capsaicin.
Fig. 7.
Response magnitude (as indicated by fluorescence ratio) of TG neurons from control (n = 134 cells) vs. ethanol (n = 178 cells) rats to 1% or 3% ethanol, AITC, capsaicin, or KCl. We compare fluorescence ratios (mean ± SE) across maternal diet treatments within each panel, using the unpaired t-test with Welch’s correction for unequal variances (*P ≤ 0.03, **P < 0.0001; NS, not significant).
DISCUSSION
We hypothesized that FAE would reduce peripheral taste and trigeminal responses to ethanol and surrogates for its sweet (sucrose), bitter (quinine), and burning (capsaicin) flavor components. We obtained several lines of support for this hypothesis.
With respect to taste, we found that FAE altered responsiveness of the CT and GL nerves during adolescence, but in different ways. It attenuated responsiveness of the CT nerve to ethanol, quinine, and sucrose and that of the GL nerve to quinine and sucrose. The mechanisms underlying the FAE-induced loss in peripheral taste responsiveness are unclear, but it is notable that (at least in humans) separate bitter T2R receptors appear to interact with quinine [i.e., T2R4, T2R7, T2R10, T2R39, T2R40, T2R43, T2R44, and T2R46 (Meyerhof et al. 2010)] vs. ethanol [i.e., T2R13 and T2R38 (Allen et al. 2014; Nolden et al. 2016)]. This raises the possibility that quinine and ethanol each activate distinct bitter taste pathways. Future studies should examine whether FAE diminishes responsiveness of both taste nerves to a broader range of bitter tastants and reduces T2R expression in taste cells. The fact that FAE produced such a complex pattern of age-related changes in nerve responsiveness indicates that the taste pathways for quinine, sucrose, and ethanol were each modified by independent developmental mechanisms.
It is also notable that FAE reduced ethanol responsiveness of taste cells innervated by the CT but not the GL nerve. More work is needed to explain how taste cells in the posterior tongue were spared the attenuating effects of FAE on ethanol responsiveness. This observation is not without precedent, however. When rats are subjected to fetal sodium restriction, they exhibit decreased taste responses to sodium salts in the CT but not the GSP nerve (Hill 2004; Sollars and Hill 2000).
Age-related changes in taste nerve responsiveness.
To determine whether the effects of FAE persist into adulthood, we maintained some of the ethanol and control rats on a diet of chow and water until adulthood (i.e., P90). We found that the attenuating effect of FAE on taste nerve responsiveness became less pronounced with age for ethanol (CT only) and sucrose (CT and GL) but appeared to become more pronounced with age for quinine (CT and GL). For instance, FAE decreased responsiveness of the CT nerve to 20 mM quinine in adolescents but to 10 and 20 mM quinine in adults.
It is important to emphasize that once the ethanol and control rats were born they were transferred to a foster mother who did not have any prior (or current) access to ethanol. Accordingly, the persistent loss of taste responsiveness in rats with FAE stemmed entirely from in utero ethanol exposure. For comparison, if prenatal sodium restriction is not continued over postnatal development in rats, then responsiveness of the CT nerve to sodium salts recovers rapidly (Hill 1987). Indeed, a single drink of 30 ml of isotonic saline is sufficient to trigger full recovery of the CT-innervated taste cells (Przekop et al. 1990).
One unexpected finding was that the CT and GL nerves in control rats exhibited divergent developmental changes in responsiveness to ethanol. On one hand, the response of the CT nerve to ethanol was extremely robust (i.e., similar in magnitude to that of NaCl) during adolescence but virtually disappeared by adulthood. On the other hand, ethanol elicited a moderate response in the GL nerve during both adolescence and adulthood. This result has been partially replicated elsewhere in a study that examined adult rats raised on a standard chow diet. The authors found that 6% ethanol elicited a moderate response in the GL nerve and a weak-to-nonexistent response in the CT nerve (Sako and Yamamoto 1999). The precipitous age-related loss in responsiveness of the CT nerve to ethanol is remarkable because all prior studies of the ontogeny of the CT nerve in rat reported that responsiveness to prototypical taste stimuli (e.g., NaCl, sucrose, quinine, and citric acid) either increased or remained stable from the weanling stage through early adulthood (Harada and Maeda 2004; Hill and Almli 1980; Hill and Przekop 1988; Miura et al. 2014; Schafe and Bernstein 1997).
Effect of FAE on TG neurons.
FAE decreased responsiveness of the TG neurons to ethanol, capsaicin, and AITC, as indicated by a reduced magnitude of response to each stimulus (Fig. 7). Notably, FAE did not impact responsiveness to KCl, which provided a measure of overall neural excitability. This latter observation indicates that FAE did not alter expression of voltage-gated calcium channels or other general excitability mechanisms in the TG neurons. Instead, FAE may have altered the expression of transduction mechanisms that are upstream of the depolarization mechanisms (e.g., TrpV1 and TrpA1). Alternatively, FAE may have increased expression of another ethanol transduction mechanism in the ethanol-responsive neurons, which is capable of hyperpolarizing the membrane—namely, the family of G protein-coupled inwardly rectifying potassium (GIRK) channels. Because GIRK channels are expressed in TG neurons (Chung et al. 2014) and are activated by ethanol (Bodhinathan and Slesinger 2014), they should reduce responsiveness of TG neurons to ethanol if overexpressed. This explanation would appear to be inconsistent with the observation that GIRK-knockout mice consume more ethanol than wild-type control mice in long-term intake tests (Blednov et al. 2001). However, the latter study did not examine orosensory-mediated feeding responses in the mice. GIRK channels are expressed throughout the central nervous system (Mayfield et al. 2015), and thus it is possible that factors other than trigeminal sensation mediated the elevated intake in the GIRK-knockout mice.
Our analysis indicated that the control rats have four distinct subpopulations of TG neurons. The most abundant class of TG neuron was responsive to capsaicin+AITC+KCl but not ethanol. FAE did not appear to impact this subpopulation of cells. However, FAE caused an ~50% reduction in the number of TG neurons that respond to capsaicin+AITC+ethanol+KCl or ethanol+KCl. Accordingly, FAE appears to have selectively targeted the two subpopulations of TG neurons that respond to ethanol and either impeded their development or rendered many of them unresponsive to ethanol, capsaicin, and AITC. The latter inference is supported by the roughly fourfold increase in percentage of TG neurons that responded exclusively to KCl in the ethanol rats.
Taken together, the results indicate that FAE reduced responsiveness to ethanol, capsaicin, and AITC in two ways. It reduced both the number of TG neurons that respond to these chemicals and the responsiveness of individual TG neurons to each chemical. It is likely that these effects of FAE are not limited to TG neurons in the oral cavity. Indeed, there is evidence that FAE elevates pain thresholds in human infants (Oberlander et al. 2010) and children (Gardner 2000) in body regions outside of the oral cavity. Given that the somatosensory neurons that mediate pain also respond to compounds like capsaicin (Julius and Basbaum 2001), it is possible that FAE elevates pain thresholds in humans by lowering both the number and responsiveness of these neurons.
As a caveat, it should be emphasized that our trigeminal recordings were generated by adding chemical stimuli to a bath containing isolated TG neurons. A potential limitation of this approach is that the stimulus concentrations that each nerve ending encountered in the bath could be higher than what it would have encountered in situ (below the permeability barrier of the oral epithelium). To minimize this concern, we selected concentrations of each stimulus that were less than saturating for the relevant target receptor mechanisms—i.e., TrpV1 and TrpA1 (Bandell et al. 2004; Trevisani et al. 2002). In so doing, we kept the chemical stimuli at physiologically relevant concentrations.
Do the changes in taste nerve responsiveness complement prior behavioral studies?
Previously, we used brief-access lick tests to evaluate the impact of FAE on the oral acceptability of ethanol and its flavor components in adolescent rats (Glendinning et al. 2012; Youngentob and Glendinning 2009). Because these lick tests measured immediate responses of rats to chemical stimuli during 10-s trials, they minimized the potential contribution of postoral feedback. We found that FAE increased the oral acceptability of ethanol (3–9%), quinine (0.01–0.3 mM), and capsaicin (1 µM) but had no impact on oral acceptability of sucrose or AITC in adolescent rats (Glendinning et al. 2012; Youngentob and Glendinning 2009). If these behavioral changes were mediated (at least in part) by changes in peripheral taste and trigeminal input, then we predicted that the ethanol rats should exhibit decreased taste and trigeminal responses to ethanol, quinine, and capsaicin but not sucrose or AITC.
Our results were partially consistent with this prediction. We found that peripheral taste and trigeminal responses of ethanol rats to ethanol, quinine, and capsaicin were diminished relative to those of control rats. Accordingly, the FAE-induced changes in taste and trigeminal input could have contributed to the increased oral acceptability of ethanol, quinine, and capsaicin. The fact that FAE does not alter the oral acceptability of sucrose and AITC (Glendinning et al. 2012; Youngentob and Glendinning 2009) indicates that the observed reductions in peripheral responsiveness to these latter chemical stimuli were not sufficient to alter their oral acceptability.
The lack of a simple predictive relationship between FAE-induced changes in peripheral chemosensory inputs and lick responses could stem from several complicating factors. One is that lick responses of rats are mediated by a diverse array of inputs from the taste, trigeminal (oral and nasal), and olfactory systems. We measured only a subset of these inputs. Second, it is likely that inputs from the different chemosensory systems summate in nonlinear ways in the nucleus of the solitary tract and higher brain regions (Shepard 2012). Indeed, an examination of the literature reveals a complex relationship between peripheral chemosensory inputs and ingestive behavior. For example, there is a report describing profound ontogenetic changes in attractiveness of umami taste stimuli to rats without any associated changes in peripheral taste responsiveness (of the CT and GSP nerves) to the same stimuli (Miura et al. 2014). Likewise, there are reports that bilateral transection of a particular taste nerve (e.g., the CT) has limited or no impact on taste-mediated licking for a broad range of sucrose concentrations in rats (Krimm et al. 1987; Spector et al. 1993).
Perspectives and significance.
Our results demonstrate that FAE reprograms development of the rat’s peripheral taste and trigeminal systems. There are two nonmutually exclusive ways that FAE could have caused this reprogramming—humoral and intraoral. According to the humoral route, ethanol entered the fetal bloodstream, diffused out of capillaries, and acted directly on cells in the developing chemosensory systems throughout gestation (Szeto 1989). According to the intraoral route, ingested alcohol accumulated in the amniotic fluid and was ingested and detected by rats in the womb (Chotro and Molina 1990; Szeto 1989). This latter type of stimulation could only have occurred after the taste and oral trigeminal systems had innervated their target fields—i.e., after embryonic day (E)16 for the taste system and E18 for the oral trigeminal system (Dillon et al. 2004; Mbiene and Mistretta 1997).
More work is needed to determine whether FAE reprogrammed development of each of the orosensory systems independently. It is possible that FAE triggered release of paracrine signals from one system, which altered development of the other system. This cross-modal hypothesis stems from recent evidence of interactions between the developing trigeminal and taste systems in the tongue (Omelian et al. 2016).
GRANTS
This research was supported in part by grants from the Howard Hughes Medical Institute, Merck Foundation, Sherman Fairchild Foundation to Barnard College, and National Institute on Alcohol Abuse and Alcoholism Grant AA-017823.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.I.G., B.P.B., and S.L.Y. conceived and designed research; J.I.G., J.T., A.P.M.A., B.P.B., and L.Y. performed experiments; J.I.G., J.T., A.P.M.A., and B.P.B. analyzed data; J.I.G., J.T., A.P.M.A., B.P.B., and S.L.Y. interpreted results of experiments; J.I.G. and B.P.B. prepared figures; J.I.G. drafted manuscript; J.I.G., J.T., A.P.M.A., B.P.B., L.Y., and S.L.Y. edited and revised manuscript; J.I.G., J.T., A.P.M.A., B.P.B., L.Y., and S.L.Y. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Louis J. Martin for showing us the glossopharyngeal nerve recording procedure and Jiang Xu for preparation of trigeminal neurons.
REFERENCES
- Alati R, Al Mamun A, Williams GM, O’Callaghan M, Najman JM, Bor W. In utero alcohol exposure and prediction of alcohol disorders in early adulthood: a birth cohort study. Arch Gen Psychiatry 63: 1009–1016, 2006. doi: 10.1001/archpsyc.63.9.1009. [DOI] [PubMed] [Google Scholar]
- Allen AL, McGeary JE, Hayes JE. Polymorphisms in TRPV1 and TAS2Rs associate with sensations from sampled ethanol. Alcohol Clin Exp Res 38: 2550–2560, 2014. doi: 10.1111/acer.12527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baer JS, Sampson PD, Barr HM, Connor PD, Streissguth AP. A 21-year longitudinal analysis of the effects of prenatal alcohol exposure on young adult drinking. Arch Gen Psychiatry 60: 377–385, 2003. doi: 10.1001/archpsyc.60.4.377. [DOI] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron 41: 849–857, 2004. doi: 10.1016/S0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bang S, Kim KY, Yoo S, Kim YG, Hwang SW. Transient receptor potential A1 mediates acetaldehyde-evoked pain sensation. Eur J Neurosci 26: 2516–2523, 2007. doi: 10.1111/j.1460-9568.2007.05882.x. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Movahed P, Hinman A, Axelsson HE, Sterner O, Högestätt ED, Julius D, Jordt SE, Zygmunt PM. Pungent products from garlic activate the sensory ion channel TRPA1. Proc Natl Acad Sci USA 102: 12248–12252, 2005. doi: 10.1073/pnas.0505356102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird E, Contreras RJ. Maternal dietary NaCl intake influences weanling rats’ salt preferences without affecting taste nerve responsiveness. Dev Psychobiol 20: 111–130, 1987. doi: 10.1002/dev.420200203. [DOI] [PubMed] [Google Scholar]
- Blednov YA, Stoffel M, Chang SR, Harris RA. Potassium channels as targets for ethanol: studies of G-protein-coupled inwardly rectifying potassium channel 2 (GIRK2) null mutant mice. J Pharmacol Exp Ther 298: 521–530, 2001. [PubMed] [Google Scholar]
- Blednov YA, Walker D, Martinez M, Levine M, Damak S, Margolskee RF. Perception of sweet taste is important for voluntary alcohol consumption in mice. Genes Brain Behav 7: 1–13, 2008. doi: 10.1111/j.1601-183X.2007.00309.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodhinathan K, Slesinger PA. Alcohol modulation of G-protein-gated inwardly rectifying potassium channels: from binding to therapeutics. Front Physiol 5: 76, 2014. doi: 10.3389/fphys.2014.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotro MG, Molina JC. Acute ethanol contamination of the amniotic fluid during gestational day 21: postnatal changes in alcohol responsiveness in rats. Dev Psychobiol 23: 535–547, 1990. doi: 10.1002/dev.420230608. [DOI] [PubMed] [Google Scholar]
- Chung MK, Cho YS, Bae YC, Lee J, Zhang X, Ro JY. Peripheral G protein-coupled inwardly rectifying potassium channels are involved in δ-opioid receptor-mediated anti-hyperalgesia in rat masseter muscle. Eur J Pain 18: 29–38, 2014. doi: 10.1002/j.1532-2149.2013.00343.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J, Williams A, Phan T-HT, Mummalaneni S, Melone P, Ren Z, Zhou H, Mahavadi S, Murthy KS, Katsumata T, DeSimone JA, Lyall V. Strain differences in the neural, behavioral, and molecular correlates of sweet and salty taste in naive, ethanol- and sucrose-exposed P and NP rats. J Neurophysiol 106: 2606–2621, 2011. doi: 10.1152/jn.00196.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lorenzo PM, Kiefer SW, Rice AG, Garcia J. Neural and behavioral responsivity to ethyl alcohol as a tastant. Alcohol 3: 55–61, 1986. doi: 10.1016/0741-8329(86)90071-6. [DOI] [PubMed] [Google Scholar]
- Dillon TE, Saldanha J, Giger R, Verhaagen J, Rochlin MW. Sema3A regulates the timing of target contact by cranial sensory axons. J Comp Neurol 470: 13–24, 2004. doi: 10.1002/cne.11029. [DOI] [PubMed] [Google Scholar]
- Djouhri L, Lawson SN. Abeta-fiber nociceptive primary afferent neurons: a review of incidence and properties in relation to other afferent A-fiber neurons in mammals. Brain Res Brain Res Rev 46: 131–145, 2004. doi: 10.1016/j.brainresrev.2004.07.015. [DOI] [PubMed] [Google Scholar]
- Ellingson JM, Silbaugh BC, Brasser SM. Reduced oral ethanol avoidance in mice lacking transient receptor potential channel vanilloid receptor 1. Behav Genet 39: 62–72, 2009. doi: 10.1007/s10519-008-9232-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaerts W, Gees M, Alpizar YA, Farre R, Leten C, Apetrei A, Dewachter I, van Leuven F, Vennekens R, De Ridder D, Nilius B, Voets T, Talavera K. The capsaicin receptor TRPV1 is a crucial mediator of the noxious effects of mustard oil. Curr Biol 21: 316–321, 2011. doi: 10.1016/j.cub.2011.01.031. [DOI] [PubMed] [Google Scholar]
- Gardner J. Living with a child with fetal alcohol syndrome. MCN Am J Matern Child Nurs 25: 252–257, 2000. doi: 10.1097/00005721-200009000-00007. [DOI] [PubMed] [Google Scholar]
- Gerhold KA, Bautista DM. Molecular and cellular mechanisms of trigeminal chemosensation. Ann NY Acad Sci 1170: 184–189, 2009. doi: 10.1111/j.1749-6632.2009.03895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glendinning JI, Simons YM, Youngentob L, Youngentob SL. Fetal ethanol exposure attenuates aversive oral effects of TrpV1, but not TrpA1 agonists in rats. Exp Biol Med (Maywood) 237: 236–240, 2012. doi: 10.1258/ebm.2011.011345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green BG. The sensitivity of the tongue to ethanol. Ann NY Acad Sci 510: 315–317, 1987. doi: 10.1111/j.1749-6632.1987.tb43541.x. [DOI] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260: 3440–3450, 1985. [PubMed] [Google Scholar]
- Harada S, Maeda S. Developmental changes in sugar responses of the chorda tympani nerve in preweanling rats. Chem Senses 29: 209–215, 2004. doi: 10.1093/chemse/bjh024. [DOI] [PubMed] [Google Scholar]
- Hill DL. Susceptibility of the developing rat gustatory system to the physiological effects of dietary sodium deprivation. J Physiol 393: 413–424, 1987. doi: 10.1113/jphysiol.1987.sp016830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL. Neural plasticity in the gustatory system. Nutr Rev 62: S208–S217, 2004. doi: 10.1111/j.1753-4887.2004.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Almli CR. Ontogeny of chorda tympani nerve responses to gustatory stimuli in the rat. Brain Res 197: 27–38, 1980. doi: 10.1016/0006-8993(80)90432-1. [DOI] [PubMed] [Google Scholar]
- Hill DL, Przekop PR Jr. Influences of dietary sodium on functional taste receptor development: a sensitive period. Science 241: 1826–1828, 1988. doi: 10.1126/science.3175625. [DOI] [PubMed] [Google Scholar]
- Inoue T, Bryant BP. Multiple types of sensory neurons respond to irritating volatile organic compounds (VOCs): calcium fluorimetry of trigeminal ganglion neurons. Pain 117: 193–203, 2005. doi: 10.1016/j.pain.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Inoue T, Bryant BP. Multiple cation channels mediate increases in intracellular calcium induced by the volatile irritant, trans-2-pentenal in rat trigeminal neurons. Cell Mol Neurobiol 30: 35–41, 2010. doi: 10.1007/s10571-009-9428-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Intranuovo LR, Powers AS. The perceived bitterness of beer and 6-n-propylthiouracil (PROP) taste sensitivity. Ann NY Acad Sci 855: 813–815, 1998. doi: 10.1111/j.1749-6632.1998.tb10665.x. [DOI] [PubMed] [Google Scholar]
- Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature 413: 203–210, 2001. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- Kawai K, Sugimoto K, Nakashima K, Miura H, Ninomiya Y. Leptin as a modulator of sweet taste sensitivities in mice. Proc Natl Acad Sci USA 97: 11044–11049, 2000. doi: 10.1073/pnas.190066697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein AH, Carstens MI, Zanotto KL, Sawyer CM, Ivanov M, Cheung S, Carstens E. Self- and cross-desensitization of oral irritation by menthol and cinnamaldehyde (CA) via peripheral interactions at trigeminal sensory neurons. Chem Senses 36: 199–208, 2011. doi: 10.1093/chemse/bjq115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J Comp Neurol 493: 596–606, 2005. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Nejad MS, Smith JC, Miller IJ Jr, Beidler LM. The effect of bilateral sectioning of the chorda tympani and the greater superficial petrosal nerves on the sweet taste in the rat. Physiol Behav 41: 495–501, 1987. doi: 10.1016/0031-9384(87)90086-2. [DOI] [PubMed] [Google Scholar]
- Lanier SA, Hayes JE, Duffy VB. Sweet and bitter tastes of alcoholic beverages mediate alcohol intake in of-age undergraduates. Physiol Behav 83: 821–831, 2005. doi: 10.1016/j.physbeh.2004.10.004. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Sollars SI. Long-term alterations in peripheral taste responses to NaCl in adult rats following neonatal chorda tympani transection. Chem Senses 40: 97–108, 2015. doi: 10.1093/chemse/bju063. [DOI] [PubMed] [Google Scholar]
- Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int Rev Neurobiol 123: 279–313, 2015. doi: 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbiene JP, Mistretta CM. Initial innervation of embryonic rat tongue and developing taste papillae: nerves follow distinctive and spatially restricted pathways. Acta Anat (Basel) 160: 139–158, 1997. doi: 10.1159/000148006. [DOI] [PubMed] [Google Scholar]
- Meyerhof W, Batram C, Kuhn C, Brockhoff A, Chudoba E, Bufe B, Appendino G, Behrens M. The molecular receptive ranges of human TAS2R bitter taste receptors. Chem Senses 35: 157–170, 2010. doi: 10.1093/chemse/bjp092. [DOI] [PubMed] [Google Scholar]
- Miller MW. Development of the Central Nervous System. Effects of Alcohol and Opiates. New York: Wiley-Liss, 1992. [Google Scholar]
- Miura H, Ooki M, Kanemaru N, Harada S. Decline of umami preference in aged rats. Neurosci Lett 577: 56–60, 2014. doi: 10.1016/j.neulet.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Moore M, Weiss S. Reasons for non-drinking among Israeli adolescents of four religions. Drug Alcohol Depend 38: 45–50, 1995. doi: 10.1016/0376-8716(95)01104-7. [DOI] [PubMed] [Google Scholar]
- Nolden AA, McGeary JE, Hayes JE. Differential bitterness in capsaicin, piperine, and ethanol associates with polymorphisms in multiple bitter taste receptor genes. Physiol Behav 156: 117–127, 2016. doi: 10.1016/j.physbeh.2016.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberlander TF, Jacobson SW, Weinberg J, Grunau RE, Molteno CD, Jacobson JL. Prenatal alcohol exposure alters biobehavioral reactivity to pain in newborns. Alcohol Clin Exp Res 34: 681–692, 2010. doi: 10.1111/j.1530-0277.2009.01137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Yamashita S, Noma A, Sato M. Taste responses in the macaque monkey chorda tympani. Physiol Behav 9: 325–331, 1972. doi: 10.1016/0031-9384(72)90153-9. [DOI] [PubMed] [Google Scholar]
- Okuni Y. Response of lingual nerve fibers of the rat to pungent spices and irritants in pungent spices (author’s transl). Shikwa Gakuho 78: 325–339, 1978. [PubMed] [Google Scholar]
- Olson HC, Streissguth AP, Sampson PD, Barr HM, Bookstein FL, Thiede K. Association of prenatal alcohol exposure with behavioral and learning problems in early adolescence. J Am Acad Child Adolesc Psychiatry 36: 1187–1194, 1997. doi: 10.1097/00004583-199709000-00010. [DOI] [PubMed] [Google Scholar]
- Omelian JM, Berry MJ, Gomez AM, Apa KL, Sollars SI. Developmental time course of peripheral cross-modal sensory interaction of the trigeminal and gustatory systems. Dev Neurobiol 76: 626–641, 2016. doi: 10.1002/dneu.22349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przekop P Jr, Mook DG, Hill DL. Functional recovery of the gustatory system after sodium deprivation during development: how much sodium and where. Am J Physiol Regul Integr Comp Physiol 259: R786–R791, 1990. [DOI] [PubMed] [Google Scholar]
- Sako N, Yamamoto T. Electrophysiological and behavioral studies on taste effectiveness of alcohols in rats. Am J Physiol Regul Integr Comp Physiol 276: R388–R396, 1999. [DOI] [PubMed] [Google Scholar]
- Schafe GE, Bernstein IL. Development of the enhanced neural response to NaCl in Fischer 344 rats. Physiol Behav 61: 775–778, 1997. doi: 10.1016/S0031-9384(96)00566-5. [DOI] [PubMed] [Google Scholar]
- Settle RG. The alcoholic’s taste perception of alcohol: preliminary findings. Curr Alcohol 5: 257–267, 1979. [PubMed] [Google Scholar]
- Shepard GM. Neurogastronomy: How the Brain Creates Flavor and Why It Matters. New York: Columbia Univ. Press, 2012. [Google Scholar]
- Simon SA, Sostman AL. Electrophysiological responses to non-electrolytes in lingual nerve of rat and in lingual epithelia of dog. Arch Oral Biol 36: 805–813, 1991. doi: 10.1016/0003-9969(91)90030-X. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Lack of functional and morphological susceptibility of the greater superficial petrosal nerve to developmental dietary sodium restriction. Chem Senses 25: 719–727, 2000. doi: 10.1093/chemse/25.6.719. [DOI] [PubMed] [Google Scholar]
- Spector AC, Travers SP, Norgren R. Taste receptors on the anterior tongue and nasoincisor ducts of rats contribute synergistically to behavioral responses to sucrose. Behav Neurosci 107: 694–702, 1993. doi: 10.1037/0735-7044.107.4.694. [DOI] [PubMed] [Google Scholar]
- Szeto HH. Maternal-fetal pharmacokinetics and fetal dose-response relationships. Ann NY Acad Sci 562: 42–55, 1989. doi: 10.1111/j.1749-6632.1989.tb21006.x. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Smart D, Gunthorpe MJ, Tognetto M, Barbieri M, Campi B, Amadesi S, Gray J, Jerman JC, Brough SJ, Owen D, Smith GD, Randall AD, Harrison S, Bianchi A, Davis JB, Geppetti P. Ethanol elicits and potentiates nociceptor responses via the vanilloid receptor-1. Nat Neurosci 5: 546–551, 2002. doi: 10.1038/nn0602-852. [DOI] [PubMed] [Google Scholar]
- Ventura AK, Worobey J. Early influences on the development of food preferences. Curr Biol 23: R401–R408, 2013. doi: 10.1016/j.cub.2013.02.037. [DOI] [PubMed] [Google Scholar]
- Wang Y, Erickson RP, Simon SA. Selectivity of lingual nerve fibers to chemical stimuli. J Gen Physiol 101: 843–866, 1993. doi: 10.1085/jgp.101.6.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates WR, Cadoret RJ, Troughton EP, Stewart M, Giunta TS. Effect of fetal alcohol exposure on adult symptoms of nicotine, alcohol, and drug dependence. Alcohol Clin Exp Res 22: 914–920, 1998. doi: 10.1111/j.1530-0277.1998.tb03889.x. [DOI] [PubMed] [Google Scholar]
- Youngentob SL, Glendinning JI. Fetal ethanol exposure increases ethanol intake by making it smell and taste better. Proc Natl Acad Sci USA 106: 5359–5364, 2009. doi: 10.1073/pnas.0809804106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Kent PF, Sheehe PR, Molina JC, Spear NE, Youngentob LM. Experience-induced fetal plasticity: the effect of gestational ethanol exposure on the behavioral and neurophysiologic olfactory response to ethanol odor in early postnatal and adult rats. Behav Neurosci 121: 1293–1305, 2007a. doi: 10.1037/0735-7044.121.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngentob SL, Molina JC, Spear NE, Youngentob LM. The effect of gestational ethanol exposure on voluntary ethanol intake in early postnatal and adult rats. Behav Neurosci 121: 1306–1315, 2007b. doi: 10.1037/0735-7044.121.6.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]