Abstract
Prevention has been identified as an effective strategy to lead healthy, active and independent lives in old age. Developing effective prevention programs requires understanding the influence of both individual and health system level factors on utilisation of specific services. This study examines the variations in utilisation of preventive services by the population aged 50 and over in 14 European countries, pooling data from the two waves of Survey of Health Ageing and Retirement in Europe and the British Household Panel Survey. The models used allow for the impact of individual level demand-side characteristics and supply-side health systems features to be separately identified. The analysis shows significant variations in preventive care utilisation both within and across European countries. In all countries, controlling for individual health status and country-level systemic differences, higher educated and higher income groups use more preventive services. At the health system level, high public health expenditures and high GP density is associated with a high level of preventive care use, but specialist density does not appear to have any effect. Moreover, payment schemes for GPs and specialists appear to significantly affect the incentives to provide preventive health care. In systems where doctors are paid by fee-for-service the utilisation of all health services, including cancer screening, are higher.
Keywords: Prevention, Health systems, Multilevel modelling, SHARE, BHPS
Introduction
Prevention has been identified as an effective strategy for enabling EU citizens to lead healthy, active and independent lives in old age (Oxley 2009). Yet, there are those who claim that health care systems do not currently make the best use of available resources to support prevention strategies (WHO 2008). Rather, priority is still being given to diagnosis and to the provision of treatments that are increasingly demanded by the members of ageing populations. In fact, a majority of health care costs in all European countries can be attributed to the diagnosis and treatment of chronic diseases and conditions which can be prevented. Greater prevention of disease and more efforts to improve the preventive capacity of health systems are essential for assuring healthy ageing populations.
There are different notions of prevention but generally, three main categories are distinguished in the literature. Primary prevention corresponds to activities reducing the occurrence of the disease, while secondary prevention aims to reduce the health consequences of diseases and tertiary prevention consists of activities for reducing the disabilities linked to chronic illnesses (Kenkel 2000). Many European countries have implemented programs in order to promote primary prevention, through vaccination campaigns, and secondary prevention, through cancer screening (European Commission 2006, 2008; European Union 2003). For example, EU guidelines set a target breast screening rate of at least 75% of eligible women in European countries. But the pace of implementation of prevention strategies appears to vary across countries.
Little systematic comparative evidence is available as to the way preventive services are provided and consumed across countries. The few studies available suggest that there are large variations across Europe in the utilisation of preventive services, such as influenza vaccination, breast and cervical cancer screenings (Blank and Szucs 2009; Borella 2008; OECD 2009). There is also evidence of disparities in prevention use within European countries, in favour of the highest socioeconomic groups (Lorant et al. 2002; Duport and Ancelle-Park 2006; Palencia et al. 2010; Patel et al. 2007; Stirbu et al. 2007).
Variations in prevention use across and within countries may arise from various sources (Van Doorslaer and Koolman 2004; Bago d’Uva and Jones 2009). First are the characteristics of individual demand, such as the ability to pay for a service, informational barriers and differences in health seeking behaviours (perceived utility of a service). However, variations may also stem from the design of the service supply, such as the level or availability of resources in the health system, the financial incentives for healthcare providers to promote specific services and the overall organisation of health care (Or et al. 2009). The way in which health services are provided and financed actually varies quite widely in Europe. How health care is financed (public, private insurance, etc.) may affect prevention in several respects, particularly regarding the emphasis put on prevention, the interaction between patients and health care professionals, and the capacity/willingness of physicians to inform patients about health promotion strategies. The design of the healthcare system (public/private mix), the level of healthcare resources available, and the nature of financial incentives for health care providers also influence the success or failure of preventive programs. Moreover, all of these factors may vary for different health services within a given system. For example, breast cancer screening might be free of charge for a specific target group while colonoscopy may be considered as specialist care and covered only partially. Therefore, we might observe different patterns of use for different prevention services even within the same system. Better understanding of the influence of both individual level factors and health system level factors on access to specific preventive health services is, thus, essential.
The study reported here provides new evidence on the variations in utilisation of preventive services among the older population in Europe separating the influence of individual level demand-side characteristics from supply-side health systems features. Pooling data from two waves of the Survey of Health Aging and Retirement in Europe (SHARE) and the British Household Panel Survey (BHPS), we compare the utilisation patterns of four preventive services (flu vaccination, eye examination, mammography and colonoscopy) for which there are clear recommendations of use after 50 years old. We also analyse visits to generalists and specialists for purposes of comparing the similarities and the differences in utilisation patterns.
Data and methods
Data
The data used in this analysis were taken selectively from the two waves (2004, 2006) of the Survey on Health Ageing and Retirement in Europe (SHARE), which provides comparable data on the individual life circumstances of more than 30,000 persons aged 50 and over in 13 European countries (Börsch-Supan and Jürges 2005). For 11 of these countries (Austria, Belgium, Denmark, France, Germany, Greece, Italy, the Netherlands, Spain, Sweden and Switzerland) the data come from the first wave (2004), and for the two newer participants (Czech Republic and Poland), from the second wave (2006). For the United Kingdom, we use the 2004 wave of the British Household Panel Survey (BHPS) (Taylor et al. 2010), which collects comparable data and can be matched with SHARE. While the English Longitudinal Study on Ageing would be the first candidate for pooling comparable data with SHARE, that survey does not include any variable on preventive care use. Therefore, the BHPS data were used in this study adjusting the age groups. In this analysis, we focus on the population aged 50 and over, representing 38,908 observations (Table 1).
Table 1.
Sample size by country and indicator
| Total | Flu vaccination | Eye exam | Breast cancer screening | Colon cancer screening | GP | Specialist | |
|---|---|---|---|---|---|---|---|
| Austria | 1849 | 1665 | 1643 | 966 | 1664 | 1835 | 1847 |
| Belgium | 3699 | 2609 | 2560 | 1381 | 2607 | 3686 | 3698 |
| Czech Rep. | 2749 | 1658 | 1658 | 937 | – | 2682 | 2734 |
| Denmark | 1615 | 1198 | 1198 | 642 | 1197 | 1598 | 1610 |
| France | 3052 | 1183 | 1183 | 643 | 1183 | 2964 | 3048 |
| Germany | 2943 | 1889 | 1889 | 1000 | 1884 | 2925 | 2941 |
| Greece | 2680 | 1833 | 1832 | 995 | 1948 | 2667 | 2678 |
| Italy | 2508 | 1536 | 1536 | 846 | 1531 | 2487 | 2505 |
| Netherlands | 2877 | 2025 | 2025 | 1098 | 2025 | 2852 | 2877 |
| Poland | 2425 | 1566 | 1566 | 876 | – | 2402 | 2423 |
| Spain | 2354 | 1540 | 1540 | 901 | 1535 | 2329 | 2354 |
| Sweden | 2997 | 2130 | 2130 | 1124 | 2121 | 2979 | 2989 |
| Switzerland | 962 | 712 | 712 | 369 | 726 | 957 | 962 |
| UK | 6198 | – | 6076 | 3371 | – | 6068 | 6075 |
| Total | 38908 | 21544 | 27548 | 15149 | 16757 | 38431 | 38741 |
The SHARE questionnaire consists of a number of modules that cover several aspects relevant to the present inquiry: health status, risk factors, socio-economic characteristics and health care utilisation. Data for GP and specialist use as well as socio-demographic and health status variables come from the main SHARE questionnaire, while data for preventive care utilisation were collected through a self-administered drop-off questionnaire. The average household response rate to the general questionnaire was about 63%, but it varied across countries from a low of about 40% in Belgium to a high of some 81% in France and drop-off response rates range between 70% in Sweden and 93% in Greece. The corresponding response rate for BHPS, where data was collected by interview only, was relatively high (93%). For additional information on the respective survey methodologies, see Börsch-Supan and Jürges (2005) for SHARE and Taylor et al. (2010) for BHPS.
Variables
Measures of prevention use
The prevention use outcome variables included four common preventive services: flu vaccination, eye examination, breast cancer screening and colon cancer screening. In addition, visits to a general practitioner and to medical specialists were queried. Respondents were asked whether they had received a flu vaccination over the past year, an eye examination by a specialist or a mammography over the past 2 years, and a colonoscopy or blood stool test over the past 10 years. These periods correspond to the recommended period for receipt of each service. The use of GP and specialist services was indicated for the previous year.
The number of visits to the GP were initially measured as a count with a minimum value of 0 (no visit) and a maximum value that varied across country. We recoded this variable as a binary indicator to make it comparable to specialist and prevention use: those who had at least one visit to the GP during the last 12 months (1) and those who had none (0). Specialist use, as measured in SHARE, refers to services received from any of the following: cardiologists, specialists for pulmonary, gastroenterology or endocrine diseases, dermatologist, neurologist, ophthalmologist, ear, nose and throat specialist, rheumatologist, orthopedist, surgeon, psychiatrist, gynecologist, urologist, oncologist or geriatrician. The corresponding question asked in BHPS differs somewhat. It asked “Have you yourself made use of hospital consultant/outpatient services? (in the past year)”. Separate questions inquired about the receipt of psychiatric care. BHPS respondents who declared that they had made use of a hospital consultant/outpatient services and/or had a visit to a psychiatrist were coded as 1 against those who had none (0).
It should be pointed out that the sample size for each prevention use indicator differed because not every respondent received every probe. For example, the information on colonoscopy or blood stool test was not available for respondents from the Czech Republic and Poland, as it was not asked in SHARE wave 2, and it was not asked in the BHPS (the United Kingdom). Information on flu vaccination was also not asked of the United Kingdom respondents (Table 1). Thus, the sample size for the respective indicators ranged from 15,149 for mammography to 27,548 for eye exam and about 38,000 for GP and specialist visits.
In order to validate the prevalence rates obtained in the SHARE and BHPS surveys, we compared them to the available published corresponding data for most countries (OECD 2010a). The comparison revealed that the prevalence rates and country rankings for the different measures in both sources were consistent with the published data. We took this precaution because some of the data on prevention use in the current analysis came from the self-administered drop-off questionnaire in SHARE, and the response to this type of questionnaire may not always be representative of the population (the least educated tend not to respond). However, as we noted above, the rates were comparable.
Table 2 presents the age-standardised prevalence rates across the countries in the present study. The table shows that there were wide differences across European countries in the utilisation of both preventive services and doctor visits. For example, flu vaccination rates among older people varied from 10% in Poland to close to 60% in Belgium. Differences in breast and colon cancer screening rates were also evident. Although the Netherlands reported the highest breast cancer preventive screening (mammography), colon cancer screening rates in that country were quite low (but still above the rates for Greece and Spain). In comparison, in Austria and Germany where mammography rates were relatively modest, colon cancer screening rates were more than 60%. The variations in GP visits across countries were much less pronounced. These data thus attest to differential patterns of preventive service use in different countries.
Table 2.
Use of preventive services across countries (age-standardised rates)
| Flu vaccinationa | Eye exam | Breast cancer screeningb | Colon cancer screening | GP | Specialist | |
|---|---|---|---|---|---|---|
| Austria | 38.4 | 62.7 | 72.4 | 67.3 | 81.1 | 36.9 |
| Belgium | 57.1 | 62.0 | 75.4 | 23.6 | 88.8 | 48.0 |
| Czech Rep. | 16.7 | 55.3 | 59.6 | – | 87.2 | 51.9 |
| Denmark | 44.0 | 58.2 | 24.7 | 25.6 | 78.4 | 17.6 |
| France | 53.2 | 72.0 | 83.2 | 44.1 | 88.8 | 44.7 |
| Germany | 39.4 | 72.4 | 49.1 | 61.9 | 86.1 | 55.0 |
| Greece | 22.5 | 56.2 | 44.4 | 10.8 | 65.0 | 35.2 |
| Italy | 48.9 | 54.1 | 64.9 | 24.4 | 77.8 | 39.7 |
| Netherlands | 61.5 | 41.6 | 84.5 | 14.8 | 75.9 | 37.1 |
| Poland | 10.6 | 41.0 | 43.7 | – | 77.3 | 28.5 |
| Spain | 56.1 | 49.7 | 70.9 | 11.9 | 83.2 | 40.8 |
| Sweden | 42.7 | 52.8 | 83.9 | 23.8 | 65.7 | 32.4 |
| Switzerland | 41.2 | 68.8 | 49.8 | 42.5 | 76.6 | 32.9 |
| UK | – | 51.2 | 29.1 | – | 80.8 | 39.5 |
| Average | 41.0 | 57.0 | 59.7 | 31.9 | 79.5 | 38.6 |
aPeople over 60 years old who have had a flu vaccination in the past year
bWomen aged 50–69 years who have had a mammography in the past 2 years
Control of individual characteristics
Among the individual characteristics taken into account, we looked first at the effect of age on preventive care utilisation within the target population, given the demonstrated impact of this variable on the need for other services. Gender was also introduced as a control variable in all equations, except for breast cancer screening. In addition, we controlled for income and for the education level of respondents. Previous studies have shown that both income level and education are significant determinants of health care use (Van Doorslaer and Koolman 2004; Bago d’Uva and Jones 2009; Or et al., 2009). The education variable gives the highest level of education that was completed by the respondent. It was introduced in three categories: no or only primary education, secondary education and tertiary education. The income variable corresponds to total net annual household income in Euros weighted by household size and composition and it was introduced in four categories, the first one corresponding to the lowest income quartile of each country. Conversions rates and weights used can be found at the SHARE website (http://www.share-project.org/documentation).
The presence of health conditions was also taken into account. Utilisation of preventive services should be independent of the health status of individuals, but some health conditions might require carrying out more prevention, and in certain conditions, those services could be part of a treatment. Therefore, following prevention guidelines, we introduced dummy variables for controlling the existence of specific conditions in the population for each of these services, as follows.
For flu vaccination, we controlled for the following conditions: chronic lung disease (such as chronic bronchitis or emphysema), asthma, cardiovascular disorder including congestive heart failure and diabetes. Subjective health (grouped in two categories “very bad to fair = 1” against “good to very good = 0”) and the limitations in daily activities (are you limited in your activities of daily living?) were also included to control for unmeasured health conditions which may require vaccination. The need for mammography is mainly defined by age (50–70 years). Similarly, recommendations for colonoscopy or blood stool test vary, but it is generally recommended that average-risk adults should begin colorectal cancer screening at age 50, every 10 years. However, both mammography and colonoscopy can also be provided for therapeutic purposes. Therefore, for these two services we controlled for the existence of cancer as one of the declared chronic conditions. Concerning eye exams, two risk factors were controlled: high blood pressure and diabetes. Finally, GP and specialist visits are generally conditioned by the health status of the individual. Therefore, in these cases we controlled for all the available health measures: self perceived health (good/very good against others), limitations in daily activities (yes/no) and declarations of major chronic conditions (high blood pressure, diabetes, chronic lung disease, asthma, cardiovascular disorder and cancer).
Health system variables
Key health system characteristics which can influence service use were also taken into account. They included: doctor availability, the methods of physician remuneration, the referral system (gatekeeping or not) and public–private mixture of health care financing (Or et al. 2009). First, high physician availability should facilitate access to care and is expected to lead to high levels of use. Accordingly, the number of physicians per 1000 habitants was the variable used to measure physician availability. We further distinguished the availability of specialists versus generalists as they play different roles with respect to cure and prevention. Second, physician remuneration methods may impact services utilisation since they determine the incentives for doctors. In theory, doctors have financial incentives to increase the volume of services they provide under fee-for-service (FFS). Capitation is often introduced as an effort to control the cost of health care, but it also raises concerns about care appropriateness. In contrast, doctors under salary have little financial incentive to compete for patients and to provide appropriate care. In some countries GP remuneration is a mixture of different schemes. For example, in Denmark on average a third of GP remuneration comes from capitation while the remainder comes from fees for consultation and individual procedures. Therefore differences in payment schemes for ambulatory physicians (fee-for-service, capitation or salary) were measured by variables which took values between 0 and 1 in each country which corresponds to the relative share of doctors paid by each payment scheme (Appendix 1). Separate sets of variables were developed for GPs and specialists as they may be paid under different schemes. For example, in the Netherlands, GPs are mostly under capitation while specialists are paid by fee-for-service.
Third, gatekeeping is expected to lead to lower levels of specialist services and to higher levels of GP use. The impact on prevention use might differ as some preventive services are provided by specialists but others are provided by GPs. A dummy variable was constructed to distinguish countries with a “gatekeeper”, corresponding to 1 for countries where GPs control patient access to specialist care and zero, otherwise. Finally, the public share of health expenditure may influence health care demand by reducing the apparent cost for patients (out-of-pocket). Two variables were introduced to define the public–private mixture of health care financing: share of public health expenditure in GDP, and share of out-of-pocket expenditure in total health expenditure.
For doctor density, public share of health expenditures, and out-of-pocket expenditures, we used data from the World Health Organisation database and the OECD Health data. Variables on gatekeeping and doctors payment schemes were built by the authors on the basis of national reports and “Health Systems in Transition” publications (HiTs) of the European Observatory on Health Systems and Policies at the World Health Organisation. HiTs are country-based reports that provide a detailed description of each health care system and of reform and policy initiatives in progress or under development.
Analysis
Pooling data across countries, we adopted a multilevel logistic regression approach to establish the determinants of the probability of using several heath services (GP/specialist visits, flu vaccination, eye exam, mammography, and colonoscopy). This method allows controlling simultaneously the variations in individual level determinants of use, health system supply/organisational characteristics and other country-level (unobserved) factors which could influence service utilisation (Leyland and Goldstein 2001) as follows:
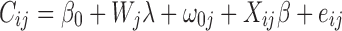 |
The first stage of our analysis consisted of estimating the association between the probability of using each given service (C ij) and several individual explanatory variables (X ij: age, sex, education income, health status). The slope coefficients β of the individual explanatory variables were treated as fixed across countries. However, a random country intercept β0j was introduced in the model in order to take into account the differences between country in the average level of use a given health service. Across countries, the country intercepts β0j were assumed to be normally distributed with a mean β0 and a variance σ2. The estimate of the variance σ2 provided then a measure of the differences in health care use across countries after controlling for compositional effect due to individual characteristics. If σ2 was significantly different from zero, it indicated that the level of health services use significantly varies from one country to another.
The second stage of the analysis aimed to establish the association between various health system features and the probability of service utilisation at the country level. In order to explain the variation in health care use across countries shown by the first stage of this analysis, health systems variables were introduced, in addition to individual characteristics. A random country intercept ω0j (normally distributed) was also introduced in the model in order to control for unobserved country-level factors influencing preventive care use independently of health system characteristics.
While it was desirable to control the impact of several health system characteristics simultaneously, these variables W j were introduced one by one in the equations because of the low degrees of freedom at country level. The equations for GP/specialist care, eye exams and mammography were based on 14 countries, those for flu vaccination on 13 and the colonoscopy/blood stool test on 11. We note that this should not bias the results. The introduction of “residual country effect” (ω0j) ensures to have unbiased measures of the parameters of the health system variables.
Results
Utilisation of preventive services and individual factors
Table 3 presents the individual determinants of the probability of using different health services across countries, controlling country specific unmeasured factors on the probability of health service utilisation. Estimated coefficients were translated into odds ratios for facilitating the interpretation.
Table 3.
Individual determinants and cross country variation of prevention use
| Independent variable | Dependant variables | |||||
|---|---|---|---|---|---|---|
| GP visit | Specialist visit | Flu vaccination | Eye exam | Breast cancer screening | Colon cancer screening | |
| Gender | ||||||
| Female | 1.271*** | 1.311*** | 0.983 | 1.388*** | – | 1.027 |
| Male (Ref.) | Ref. | Ref. | Ref. | Ref. | – | Ref. |
| Age | ||||||
| 50–54 | 0.655*** | 1.301*** | 0.127*** | 0.658*** | 14.578*** | 0.460*** |
| 55–59 | 0.690*** | 1.331*** | 0.159*** | 0.665*** | 16.024*** | 0.620*** |
| 60–64 | 0.775*** | 1.349*** | 0.237*** | 0.669*** | 13.903*** | 0.719*** |
| 65–69 | 0.842** | 1.378*** | 0.563*** | 0.751*** | 8.718*** | 0.845** |
| 70–74 | 0.966 | 1.280*** | 0.768*** | 0.799*** | 4.398*** | 0.907 |
| 75–79 | 0.998 | 1.382*** | 0.896 | 0.989 | 2.083*** | 0.981 |
| 80+ | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Income | ||||||
| Quartile 1 | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Quartile 2 | 1.140*** | 1.137*** | 1.044 | 1.127*** | 1.212*** | 1.049 |
| Quartile 3 | 1.163*** | 1.243*** | 1.078 | 1.258*** | 1.287*** | 1.084 |
| Quartile 4 | 1.115** | 1.329*** | 1.179*** | 1.388*** | 1.547*** | 1.161** |
| Education | ||||||
| Primary | Ref. | Ref. | Ref. | Ref. | Ref. | Ref. |
| Secondary | 1.102** | 1.370*** | 1.177*** | 1.384*** | 1.272*** | 1.178*** |
| Tertiary | 1.007 | 1.547*** | 1.148*** | 1.532*** | 1.352*** | 1.335*** |
| Self-assessed health | ||||||
| Good, very good, excellent | 1.610*** | 1.540*** | 1.216*** | – | – | – |
| Less than good | Ref. | Ref. | Ref. | – | – | – |
| Limitations in daily activities | ||||||
| Limited or severely limited | 1.868*** | 2.009*** | 1.162*** | – | – | – |
| Not limited | Ref. | Ref. | Ref. | – | – | – |
| Reported chronic conditions | ||||||
| High blood pressure (Ref. = No) | 2.636*** | 1.228*** | – | 1.224*** | – | – |
| Diabetes (Ref. = No) | 1.799*** | 1.401*** | 1.475*** | 2.086*** | – | – |
| Chronic lung disease (Ref. = No) | 1.851*** | 1.307*** | 1.769*** | – | – | – |
| Cardiovascular disease (Ref. = No) | 1.099 | 1.311*** | 1.105 | – | – | – |
| Cancer (Ref. = No) | 1.304*** | 3.421*** | – | – | 3.324*** | 2.613*** |
| Inter-country variance | 0.226*** | 0.246*** | 0.531*** | 0.172*** | 0.850*** | 0.339*** |
| Log-likelihood | −48524.45 | −48164.74 | −27299.99 | −35074.76 | −19499.4 | −21925.77 |
| Obs. | 33772 | 33901 | 18692 | 24669 | 13604 | 15455 |
Note Odds ratios. * Significant at 10%, ** significant at 5%, *** significant at 1%
The analysis provided several results concerning the determinants of health services use at the individual level. First, age is an important predictor of most of the health services considered but some services were used more by older individuals than others. The odds of visiting a generalist increased with age while all age groups have higher odds of visiting a specialist than those 80 years and over. As expected, flu vaccination significantly increased with age: the odds of having a vaccination at the age of 50–55 was eight times lower than at age 80 years and over. For colonoscopy or blood stool test, all the age groups had lower odds of being advised than the age group 80 and over. On the other hand, the probability of having breast cancer screening strongly decreased with age. The odds of having a mammography screening are the highest until 60 years old and women between 65 and 69 years old had half the propensity to have a screening than those 50–55 years (OR 0.74). Across these countries on average, women are more likely to have GP and specialists services, as well as eye exams. There was no gender effect concerning flu vaccination and colon cancer screening.
Those reporting less-than-good health, having difficulties in activities of daily living and having chronic health conditions had a higher probability of visiting a generalist or specialist. Moreover, individuals with chronic pulmonary problems and diabetes and those with bad general health had a higher probability of getting a flu vaccination. Existence of previous cancer was associated with both mammography and colonoscopy/blood stool test use.
Adjusted for the need for care, the results also showed that education and income were significant determinants of access to all types of health services including prevention. The probability of using any health services was significantly lower for individuals with the lowest level of education. The association between education and health service use was the most pronounced for specialist care and eye exam, with odds ratios reaching 1.5 for more educated groups. It was somewhat less pronounced for GP use and flu vaccination. Controlling for education levels, health service utilisation was still significantly higher for the highest income group (fourth quartile). The influence of income was the most pronounced for mammography use, the odds of having a mammography test, on average, being 1.5 times higher for women in the highest income group compared with those in the lowest income groups. This was followed by eye exams and specialist consultations with odds ratios associated to the highest income quartile equal to 1.4 and 1.3, respectively, with comparison to the lowest income quartile. Income was also significantly associated with an increased probability of consulting a generalist, having a flu vaccination and colon cancer screening, but here the differences between income groups were relatively less pronounced.
At the country level, the variance statistics at the bottom of Table 3 confirmed that the inter-country variance was significantly different from 0 at 1% for all health services indicators. These results indicated that there were still significant differences across countries in the average level of preventive service use after controlling for individual determinants of utilisation.
Link between healthcare system and preventive services use
In order to explain these residual heterogeneities in health/prevention service utilisation across countries, we investigated the role of health system variables as introduced in “Data and methods” section. These variables are introduced one by one in the preventive use equations because of the low degrees of freedom at country level (13–10).
Table 4 summarises the results of the second stage analysis which explored successively the role of a range of health system variables on the probability of health services use. All the models control for the individual level variables presented in Table 3 and unobserved country-level factors, but only odds ratios associated to health systems variables are presented to keep the table to a manageable size. Therefore, in Table 4, each line corresponds to a different model.
Table 4.
Association between health system characteristics and cross country variation in health service utilisation
| Level-2 indep var. | Dependent variables | ||||||
|---|---|---|---|---|---|---|---|
| GP visit | Specialist visit | Flu vaccination | Eye exam | Breast cancer screening | Colon cancer screening | ||
| Model 1 | No. of GP/1000 hab | 1.983*** | 1.646* | 1.811 | 1.629** | 1.833 | 1.457 |
| Model 2 | No. of specialists/1000 hab | 0.871 | 1.135 | 0.646 | 1.213 | 0.606 | 0.903 |
| Model 3 | GP fee-for-service | 1.990** | 1.247 | 1.984 | 2.084*** | 1.425 | 3.038*** |
| Model 4 | SP fee-for-service | 1.614* | 1.361 | 1.020 | 1.309 | 1.939 | 2.342** |
| Model 5 | GP capitation | 0.769 | 0.758 | 0.415 | 0.493*** | 0.498 | 0.593 |
| Model 6 | GP wage-earner | 0.563* | 1.015 | 0.946 | 0.842 | 1.331 | 0.395** |
| Model 7 | SP wage-earner | 0.620* | 0.735 | 0.981 | 0.764 | 0.516 | 0.427** |
| Model 8 | Gatekeeper | 0.611* | 0.546** | 1.083 | 0.530*** | 0.850 | 0.634 |
| Model 9 | Public health exp. (% GDP) | 1.205* | 1.077 | 1.288 | 1.241** | 1.099 | 1.474*** |
| Model 10 | Out-of-pocket (%THE) | 0.978 | 0.992 | 0.973 | 1.000 | 0.972 | 0.974 |
Note Odds ratios. All models controlled for age, sex, education, income and residual country effects as in Table 3. Self-assessed health, LDA and chronic conditions are introduced as a function of each dependant variable studied (cf. Table 3). * Significant at 10%, ** significant at 5%, *** significant at 1%
On doctor availability, as expected, all else being equal, the number of generalists (Model 1) was positively correlated with GP use but also with specialist use and eye exams (ORs equal to 2, 1.6 and 1.6, respectively), but not with flu vaccination, breast or colon cancer screening. Interestingly, the number of specialists did not appear to have a significant association with the propensity to use any of the preventive services (Model 2).
Several significant associations were found between payment schemes for doctors and care utilisation. All else being equal, the propensity to use GP services and colonoscopy was significantly higher in health systems where generalists were paid by fee-for-service rather than by capitation or by salary (model 3) The propensity to have a colonoscopy was three times higher in countries where generalists were paid by fee-for-service (FFS). Results of model 4 are quite similar for payment of specialists under FFS. On the contrary, the propensity to have regular eye exams was lower in countries where GPs were paid by capitation (model 5, OR = 0.5). The propensity to use GP and preventive services were lower in countries where doctors are wage-earners (models 6 and 7).
Furthermore, in health systems where generalists work as gatekeepers, not only the propensity to visit GPs and specialists but also having an eye exam was significantly lower (model 8, OR = 0.6, 0.5, and 0.5, respectively). The coefficients were in the same direction for mammography and colon cancer screenings, even if they were not statistically significant. Concerning the role of public/private funding, the results of the model 9 suggested that in countries where public health expenditure was higher (calculated as the share of GDP) individuals had higher rates of GP visits, eye exams and colon cancer screening (ORs equal to 1.2, 1.2 and 1.5, respectively). Finally, no significant association was found between the share of out-of-pocket payment in total health expenditure and health service use.
Discussion
This study offers some new comparative evidence on variations in preventive care utilisation among older adults in Europe, using data from the Survey on Health Ageing and Retirement in Europe (SHARE) and the British Household Panel Survey. These surveys provide some unique comparable data on health and preventive care utilisation patterns of the population aged 50 and over in 13 European countries. Currently, there are no other comparable data (including in international databases of the OECD and WHO) for analysing prevention and healthcare use across countries. Utilisation of this unique cross-country data set allowed us to explore, via multilevel regressions, the association between preventive care use and major health systems features, controlling for the influence of individual level population characteristics and unobserved country factors that may influence prevention use.
Our study suggests that there are significant associations between some health system features and the propensity to use different preventive services across countries. The results point to the significant role played by the generalists (their density and position in the health system) and the importance of financial incentives for providers via their payment schemes. Generalists are key actors for assuring both appropriate primary prevention (such as flu vaccination after a certain age) and adequate referral for secondary prevention (such as cancer screening). Clearly, it is difficult to characterise precisely relevant dimensions of the health care systems and to provide prescriptive solutions. Health systems were categorised in this study by four key characteristics: number of available doctors, methods of remunerating physicians, referral system (gatekeeping or not) and public–private mixture of health care financing. These measures may be crude, and distinguish only some broad system features, but they vary significantly across the European countries studied. While we could not test the influence of several characteristics simultaneously, pooling the results from different models enabled us to get an insight into systemic factors which influence provision and utilisation of different health services.
First, the level of public investment in the health system (measured by the share of public health expenditure in GDP) appears to have a direct influence on the use of preventive services. In countries where the level of public investment is low, such as Greece and Poland (about 4% of GDP against the average of 7%), the use of most preventive services (including GP visits) are significantly lower. However, the link between the level of preventive care and the share of out-of-pocket expenditure in total health expenditure, which is an indicator of the direct cost of care for patients, does not appear to be significant. This could be due to the fact that co-payment arrangements for patients vary widely across different services. The services examined in this study, especially preventive care, are often among those for which cost-sharing is very low. As expected, the number of GPs is positively correlated with doctor consultations including specialist visits for eye exams. In countries with high GP density, the opportunity cost of care (travel cost and waiting time) for patients would be lower. But also generalists may have more time to spend with patients which in turn might give more opportunity for prevention. It is interesting to note that high numbers of specialists do not appear to lead automatically to higher prevention rates. This may reflect the fact that in most health systems generalists are given the responsibility for health promotion via primary prevention but also via referral to secondary and tertiary prevention which are provided by specialists. Therefore, the specialist’s role in disseminating prevention is rather limited.
Second, payment schemes for providers appear to have a significant influence on the provision and utilisation of preventive and health services. In systems where doctors are paid by fee-for-service, the utilisation of all health services, including secondary prevention are higher. This is coherent with economic theory that under fee-for-service doctors have incentives to increase the volume of their services (OECD 1994). Moreover, in some countries like France, FFS payments are complemented with a P4P (Pay for Performance) scheme where specific incentives are given to GPs to provide more prevention. Capitation is often used as an alternative to provide focus on primary care without dropping cost control, but there seems to be a risk in these schemes of under-referring for secondary prevention by specialists (such as eye exams and colonoscopy). Several studies, all from the United States, suggested that in the context of managed care, capitation payment reduces service use in general (Gosden et al. 2000). But capitation can also be designed to increase the access to preventive care (Zukevas and Hill 2004) and to provide a better case management and coordination of services, especially for people with chronic diseases (Mitchell and Gaskin 2007). Our results point to a lower propensity to use eye exams only in countries where GPs are paid by capitation. In countries where doctors are paid by salary, the probability to visit a GP is relatively lower as well as colon cancer screening. This result is consistent with the fact that under salary, general practitioners have little financial incentive to compete for patients who may then suffer from inappropriate referral to specialists who provide secondary prevention (Gosden et al. 1999).
Gatekeeping is considered as a mechanism of cost containment in part because of the evidence that specialists can induce demand for costly and sometimes unnecessary procedures. Therefore, it is not surprising that in countries with gatekeeping, the propensity to visit specialists is lower. However, the impact of gatekeeping on other health services has not been widely investigated. Our results suggest that, all else being equal, the probability of visiting a GP in countries where there is gatekeeping might also be lower. This is somewhat counter-intuitive, as we might expect that in countries where GPs are given the role to orient patients in the system and where direct access to specialists is not allowed, the demand for GP services would be higher. The fact that the level of healthcare resources are often limited by central budgets in strict gatekeeping systems, and that the number of GPs is relatively low may explain this result.
Our results also confirm the existence of significant variation in preventive care utilisation within the European countries. Age is an important determinant of health services use. While the use of most health services is positively correlated with age, the probability of visiting a specialist decreases with age. Our result also highlighted that age could be a barrier to access to certain preventive services. Concerning breast cancer screening, despite the recommendations for regular check-ups until the age of 70 (European Commission 2006), woman aged 65–69 years old have half the probability to have a screening than those in their 50s. On average in these countries, woman have a higher propensity to use doctor services, except flu vaccination and colon cancer screening where there is no gender difference.
In all countries, controlling for individual characteristics and country-level systemic differences, better educated and individuals with higher incomes have a higher probability to use all types of health services including GP visits and preventive care. These results differ from previous studies based on the European Household Panel data suggesting that there is pro-poor inequality in Europe concerning GP visits (Van Doorslaer and Koolman 2004; Bago d’Uva and Jones 2009), but are consistent with the results based on national surveys (Or et al. 2009). Nevertheless, we also find that the differences between education and income groups are more pronounced for specialist visits, eye exams and breast and colon cancer screening which are often provided by specialists. These results suggest that more attention needs to be paid to low income groups of older adults in designing preventive programs. Previous studies have shown that public programs offering free care are not always sufficient for achieving full coverage of the population (Duport and Ancelle-Park 2006; Palencia et al. 2010; Spadea et al. 2010).
In conclusion, prevention has been identified as an effective strategy to lead healthy, active and independent lives in old age. Developing effective prevention programs requires understanding the determinants of utilisation of specific services both at the individual and systemic level. This study adds to the literature on the determinants of access to health services in Europe by investigating the specific role of health care system design (beyond health care insurance) on service utilisation by the older population. It is important to understand the variables involved in healthcare use overall and the determinants of physician–patient interaction that predispose prevention in order to prevent diseases and promote a healthy older population. It appears that the position of GPs in the health system and the financial incentives for providers are essential elements for promoting both primary and secondary prevention.
Acknowledgments
This article uses data from SHARE release 2.3.0, as of November 13th 2009. SHARE data collection in 2004-2007 was primarily funded by the European Commission through its 5th and 6th framework programmes (project numbers QLK6-CT-2001- 00360; RII-CT-2006-062193; CIT5-CT-2005-028857). Additional funding by the US National Institute on Aging (grant numbers U01 AG09740-13S2; P01 AG005842; P01 AG08291; P30 AG12815; Y1-AG-4553-01; OGHA 04-064; R21 AG025169) as well as by various national sources is gratefully acknowledged (see http://www.share-project.org for a full list of funding institutions). This article also uses data from the BHPS study which is funded by the Economic and Social Research Council (ESRC). The data was originally collected by the ESRC Research Centre on Micro-Social Change at the University of Essex. Over time, additional funding for the British Household Panel Survey (BHPS) has been provided by the Health Education Authority (HEA), Office for National Statistics (ONS) and Eurostat. The Northern Ireland sample, included from Wave 11, is jointly funded by the Economic and Social Research Council (ESRC) and various Northern Ireland government departments. Our research benefited from the jointed financial support of the French Institute for Health Promotion and Health Education (INPES), the National Authority for Health (HAS), the National Institute for Health and Medical Research (INSERM), the National Health Insurance Fund for Self-employed (RSI) as part of the research program “Prevention” supported by the French Institute for Public Health Research (IRESP) in 2007.
Appendix
See Table 5.
Table 5.
Health system features in Europe, 2004
| Country | Public health exp. (% GDP) | Out-of-pocket (%THE) | No. of GP/1000 hab | No. of specialists/1000 hab | Gatekeeper | GP capitation | GP fee-for-service | GP wage-earner | SP fee-for-service | SP wage-earner |
|---|---|---|---|---|---|---|---|---|---|---|
| Austria | 7.67 | 16.56 | 1.40 | 1.95 | 0.5 | 0 | 1 | 0 | 1 | 0 |
| Belgium | 7.02 | 23.20 | 2.10 | 1.85 | 0 | 0 | 1 | 0 | 1 | 0 |
| Czech Rep. | 6.32 | 10.28 | 0.70 | 2.75 | 0 | 0.8 | 0.2 | 0 | 1 | 0 |
| Germany | 8.28 | 12.16 | 1.05 | 2.30 | 0 | 0 | 1 | 0 | 1 | 0 |
| Denmark | 7.40 | 15.17 | 0.72 | 2.18 | 1 | 0.3 | 0.7 | 0 | 0.3 | 0.7 |
| Spain | 5.45 | 22.25 | 0.87 | 1.84 | 1 | 0.1 | 0 | 0.9 | 0 | 1 |
| France | 8.20 | 7.07 | 1.62 | 1.70 | 0 | 0 | 1 | 0 | 1 | 0 |
| Greece | 4.45 | 36.37 | 1.00 | 3.24 | 0 | 0 | 0 | 1 | 0 | 1 |
| Italy | 6.28 | 22.38 | 0.90 | 3.30 | 1 | 0.8 | 0.2 | 0 | 1 | 0 |
| Netherlands | 5.23 | 8.33 | 0.50 | 0.95 | 1 | 0.7 | 0.3 | 0 | 1 | 0 |
| Poland | 4.27 | 28.06 | 0.50 | 1.85 | 1 | 1 | 0 | 0 | 1 | 0 |
| Sweden | 7.60 | 14.98 | 0.56 | 1.78 | 1 | 0 | 0 | 1 | 0 | 1 |
| Switzerland | 6.48 | 31.70 | 0.45 | 2.33 | 0 | 0 | 1 | 0 | 1 | 0 |
| UK | 6.90 | 11.79 | 0.70 | 1.60 | 1 | 1 | 0 | 0 | 0 | 1 |
| Total | 6.54 | 18.59 | 0.93 | 2.12 | 0.54 | 0.34 | 0.46 | 0.21 | 0.66 | 0.34 |
Source OECD Health data (2008), Authors’ calculations
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s10433-011-0211-7.
References
- Bago d’Uva T, Jones AM. Health care utilisation in Europe: new evidence from the ECHP. J Health Econ. 2009;28:265–279. doi: 10.1016/j.jhealeco.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Blank PR, Szucs TD. Vaccination coverage rates in eleven European countries during two consecutive influenza seasons. J Infect. 2009;58(6):446–458. doi: 10.1016/j.jinf.2009.04.001. [DOI] [PubMed] [Google Scholar]
- Borella L. Les pays européens face au cancer: étude d’un ensemble restreint d’indicateurs de santé publique. Bull Cancer. 2008;95(11):1053–1062. doi: 10.1684/bdc.2008.0737. [DOI] [PubMed] [Google Scholar]
- Börsch-Supan, A, Jürges H (eds) (2005) The survey of health, ageing and retirement in Europe—methodology. Mannheim: Mannheim Research Institute for the Economics of Aging (MEA)
- Duport N, Ancelle-Park R. Do socio-demographic factors influence mammography use of French women? Analysis of a French cross-sectional survey. Eur J Cancer Prev. 2006;15(3):219–224. doi: 10.1097/01.cej.0000198902.78420.de. [DOI] [PubMed] [Google Scholar]
- European Commission (2006) European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th edn. Luxembourg
- European Commission (2008) European Guidelines for Quality Assurance in Cervical Cancer Screening, 2nd edn. Luxembourg
- European Union /879/EC) Official Journal of the European Union, L327. 2003;16:34–38. [Google Scholar]
- Gosden T, Pedersen L, Torgerson D. How should we pay doctors? A systematic review of salary payments and their effect on doctor behaviour. Q J Med. 1999;92:47–55. doi: 10.1093/qjmed/92.1.47. [DOI] [PubMed] [Google Scholar]
- Gosden T, Forland F, Kristiansen IS, et al (2000) Capitation, salary, fee-for-service and mixed systems of payment: effects on the behaviour of primary care physicians. Cochrane Database Syst Rev 3, CD002215 [DOI] [PMC free article] [PubMed]
- HiTs. http://www.euro.who.int/en/home/projects/observatory/publications/health-system-profiles-hits
- Kenkel DS (2000) Prevention. In: Culyer AJ, Newhouse JP (eds) Handbook of health economics, 1st edn, vol 1, chapter 3. Elsevier, pp 1675–1720
- Leyland AH, Goldstein H, editors. Multilevel modelling of health statistics. New York: Wiley; 2001. [Google Scholar]
- Lorant V, Boland B, Humblet P, Deliège D. Equity in prevention and health care. J Epidemiol Community Health. 2002;56:510–516. doi: 10.1136/jech.56.7.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell J, Gaskin D. Caregivers’ rating of access: do children with special health care needs fare better under fee-for-service or partially capitated managed care? Med Care. 2007;45:146–153. doi: 10.1097/01.mlr.0000241047.99214.ed. [DOI] [PubMed] [Google Scholar]
- OECD (1994) Health care reform controlling spending and increasing efficiency. Economics Department Working Papers, 149, Paris
- OECD . Health at a glance 2009: OECD indicators. Paris: OECD Publishing; 2009. [Google Scholar]
- OECD . Health at a glance: Europe 2010. Paris: OECD Publishing; 2010. [Google Scholar]
- OECD . Value for money in health spending, OECD health policy studies. Paris: OECD Publishing; 2010. [Google Scholar]
- Or Z, Jusot F, Yilmaz E, The European Union Working Group on Socioeconomic Inequalities in Health Inégalités sociales de recours aux soins en Europe: Quel rôle pour le système de soins? Revue Economique. 2009;60(2):521–543. doi: 10.3917/reco.602.0521. [DOI] [Google Scholar]
- Oxley H (2009) Policies for healthy ageing: an overview. OECD Health Working Papers, 42
- Palencia L, Espelt A, Rodriguez-Sanz M, Puigpinos R, Pons-Viques M, Pasarin IM, Spadea T, Kunst AE, Borell C. Socio-economic inequalities in breast and cervical cancer screening practices in Europe: influence of the type of screening program. Int J Epidemiol. 2010;39(3):757–765. doi: 10.1093/ije/dyq003. [DOI] [PubMed] [Google Scholar]
- Patel R, Lawlor DA, Ebrahim S. Socio-economic position and the use of preventive health care in older British women: a cross-sectional study using data from the British Women’s Heart and Health Study cohort. Fam Pract. 2007;24(1):7–10. doi: 10.1093/fampra/cml064. [DOI] [PubMed] [Google Scholar]
- Spadea T, Bellini S, Kunst A, Stirbu I, Costa G. The impact of interventions to improve attendance in female cancer screening among lower socioeconomic groups: a review. Prev Med. 2010;50(4):159–164. doi: 10.1016/j.ypmed.2010.01.007. [DOI] [PubMed] [Google Scholar]
- Stirbu I, Kunst AE, Mielck A, Mackenbach JP (2007) Educational inequalities in preventives services among elderly in Europe. In: Tackling health inequalities in Europe: an integrated approach EUROTHINE, Final Report. Rotterdam: Department of Public Health, University Medical Centre Rotterdam, pp 483–499
- Taylor MF, Brice J, Buck N, Prentice-Lane E (2010) In: Taylor MF (ed) British household panel survey user manual. Vol A: introduction, technical report and appendices. University of Essex, Colchester
- Van Doorslaer E, Koolman X. Explaining income-related inequalities in doctor utilisation in Europe. Health Econ. 2004;13(7):629–647. doi: 10.1002/hec.919. [DOI] [PubMed] [Google Scholar]
- WHO (2008) The World Health Report 2008—Primary Health Care (Now More Than Ever)
- Zukevas S, Hill S. Does capitation matter? Impacts on access, use and quality. Inquiry. 2004;41:316–335. doi: 10.5034/inquiryjrnl_41.3.316. [DOI] [PubMed] [Google Scholar]


