Abstract
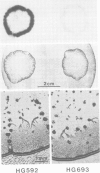
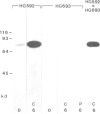
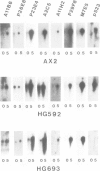
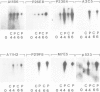
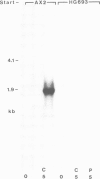
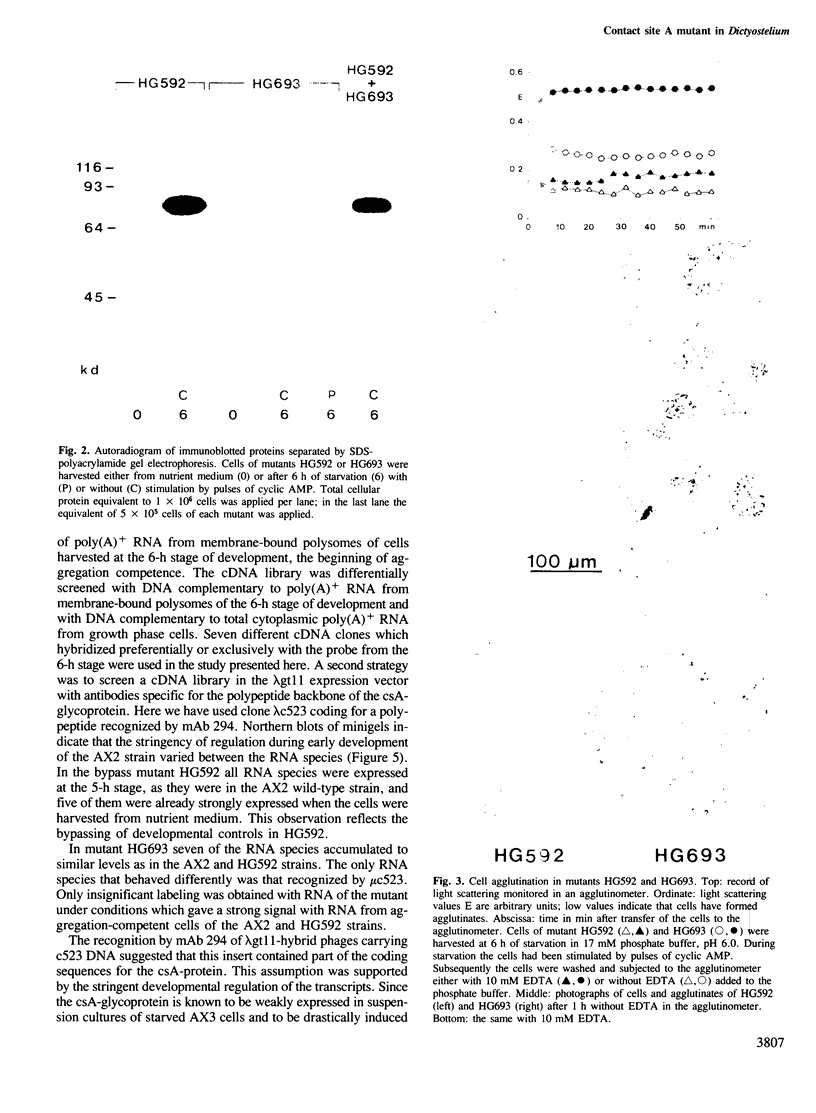
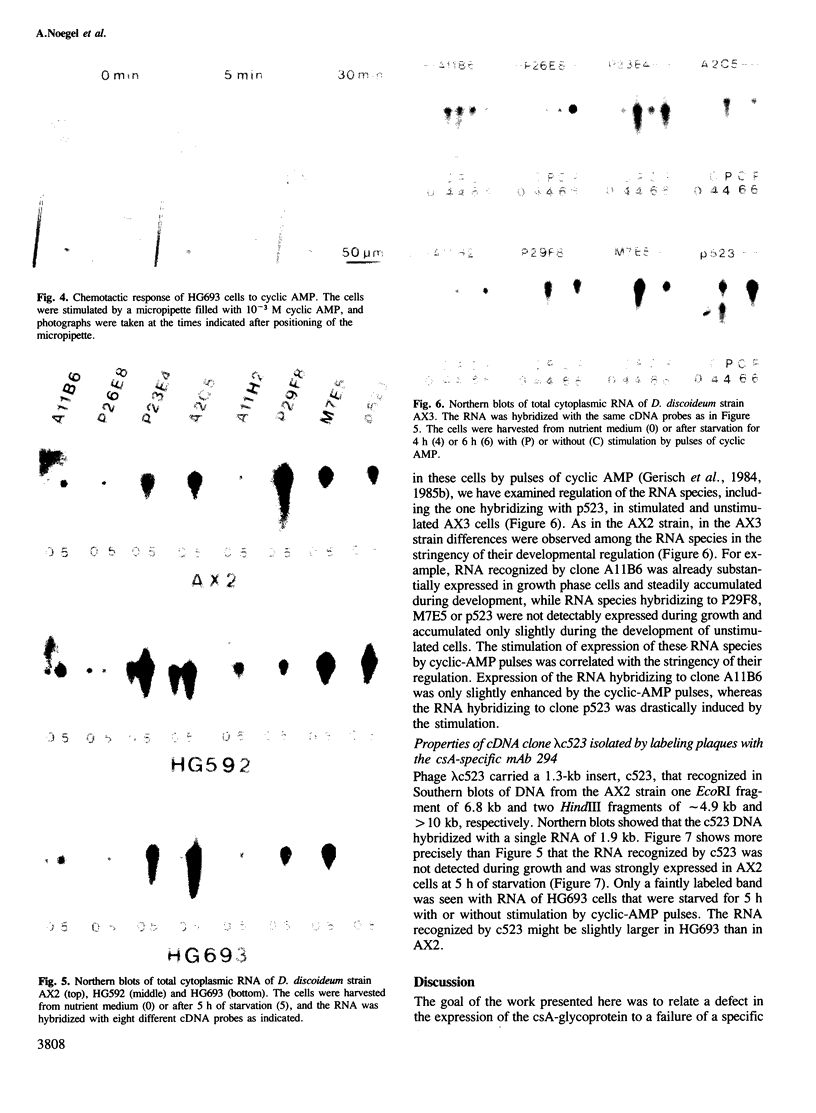
Expression of developmentally regulated membrane proteins of aggregating cells of Dictyostelium discoideum is subject to several control mechanisms. One of them involves periodic cyclic-AMP pulses as signals for gene expression. To increase the probability of selecting mutants specifically defective in the contact site A (csA) glycoprotein, one of the characteristic proteins of aggregating cells, we have bypassed the requirement for both cyclic-AMP pulses and another control element by two runs of mutagenesis. A `double bypass' mutant, HG592, was obtained which aggregated in nutrient medium where wild-type did not develop. Mutants defective in expression of the csA-glycoprotein were selected from HG592 by fluorescence-activated cell sorting and colony immunoblotting using a monoclonal antibody specific for that protein. One among 51 csA-negative mutants, HG693, specifically lacked the capability of forming EDTA-stable intercellular contacts. It acquired chemotactic responsiveness and developed into fruiting bodies. Expression of the transcripts for eight developmentally regulated proteins was determined in HG693. Seven of the RNA species were normally expressed; they were recognized by cDNA clones which had been produced from poly(A)+ RNA isolated from membrane-bound polysomes. The single RNA species which was not substantially expressed in HG693 was recognized by a cDNA clone that was obtained by screening a λgt11 library with an antibody specific for the csA-glycoprotein. When probing RNA from wild-type cells, this clone hybridized with a single developmentally regulated RNA species of 1.9 kb whose expression was strongly enhanced by cyclic-AMP pulses. Appearance of this RNA coincided with the expression of the csA-glycoprotein.
Keywords: cell adhesion, contact sites A, bypass mutants, cyclic-AMP signals, Dictyostelium discoideum
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bertholdt G., Stadler J., Bozzaro S., Fichtner B., Gerisch G. Carbohydrate and other epitopes of the contact site A glycoprotein of Dictyostelium discoideum as characterized by monoclonal antibodies. Cell Differ. 1985 May;16(3):187–202. doi: 10.1016/0045-6039(85)90516-0. [DOI] [PubMed] [Google Scholar]
- Beug H., Gerisch G. A micromethod for routine measurement of cell agglutination and dissociation. J Immunol Methods. 1972 Nov;2(1):49–57. doi: 10.1016/0022-1759(72)90017-8. [DOI] [PubMed] [Google Scholar]
- Beug H., Katz F. E., Gerisch G. Dynamics of antigenic membrane sites relating to cell aggregation in Dictyostelium discoideum. J Cell Biol. 1973 Mar;56(3):647–658. doi: 10.1083/jcb.56.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner J. T., Barkley D. S., Hall E. M., Konijn T. M., Mason J. W., O'Keefe G., 3rd, Wolfe P. B. Acrasin, Acrasinase, and the sensitivity to acrasin in Dictyostelium discoideum. Dev Biol. 1969 Jul;20(1):72–87. doi: 10.1016/0012-1606(69)90005-0. [DOI] [PubMed] [Google Scholar]
- Brachet P., Dicou E. L., Klein C. Inhibition of cell differentiation in a phosphodiesterase defective mutant of Dictyostelium discoideum. Cell Differ. 1979 Aug;8(4):255–265. doi: 10.1016/0045-6039(79)90001-0. [DOI] [PubMed] [Google Scholar]
- Chisholm R. L., Barklis E., Lodish H. F. Mechanism of sequential induction of cell-type specific mRNAs in Dictyostelium differentiation. Nature. 1984 Jul 5;310(5972):67–69. doi: 10.1038/310067a0. [DOI] [PubMed] [Google Scholar]
- Devreotes P. N., Sherring J. A. Kinetics and concentration dependence of reversible cAMP-induced modification of the surface cAMP receptor in Dictyostelium. J Biol Chem. 1985 May 25;260(10):6378–6384. [PubMed] [Google Scholar]
- Francis D., Toda K., Merkl R., Hatfield T., Gerisch G. Mutants of Polysphondylium pallidum altered in cell aggregation and in the expression of a carbohydrate epitope on cell surface glycoproteins. EMBO J. 1985 Oct;4(10):2525–2532. doi: 10.1002/j.1460-2075.1985.tb03966.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerisch G., Keller H. U. Chemotactic reorientation of granulocytes stimulated with micropipettes containing fMet-Leu-Phe. J Cell Sci. 1981 Dec;52:1–10. doi: 10.1242/jcs.52.1.1. [DOI] [PubMed] [Google Scholar]
- Gerisch G., Weinhart U., Bertholdt G., Claviez M., Stadler J. Incomplete contact site A glycoprotein in HL220, a modB mutant of Dictyostelium discoideum. J Cell Sci. 1985 Feb;73:49–68. doi: 10.1242/jcs.73.1.49. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hohmann H. P., Gerisch G., Lee R. W., Huttner W. B. Cell-free sulfation of the contact site A glycoprotein of Dictyostelium discoideum and of a partially glycosylated precursor. J Biol Chem. 1985 Nov 5;260(25):13869–13878. [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Klein C., Lubs-Haukeness J., Simons S. cAMP induces a rapid and reversible modification of the chemotactic receptor in Dictyostelium discoideum. J Cell Biol. 1985 Mar;100(3):715–720. doi: 10.1083/jcb.100.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein P., Theibert A., Fontana D., Devreotes P. N. Identification and cyclic AMP-induced modification of the cyclic AMP receptor in Dictyostelium discoideum. J Biol Chem. 1985 Feb 10;260(3):1757–1764. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Murray B. A., Wheeler S., Jongens T., Loomis W. F. Mutations affecting a surface glycoprotein, gp80, of Dictyostelium discoideum. Mol Cell Biol. 1984 Mar;4(3):514–519. doi: 10.1128/mcb.4.3.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K., Gerisch G. A specific glycoprotein as the target site of adhesion blocking Fab in aggregating Dictyostelium cells. Nature. 1978 Aug 3;274(5670):445–449. doi: 10.1038/274445a0. [DOI] [PubMed] [Google Scholar]
- Noegel A., Welker D. L., Metz B. A., Williams K. L. Presence of nuclear associated plasmids in the lower eukaryote Dictyostelium discoideum. J Mol Biol. 1985 Sep 20;185(2):447–450. doi: 10.1016/0022-2836(85)90416-4. [DOI] [PubMed] [Google Scholar]
- Roos W., Malchow D., Gerisch G. Adenylyl cyclase and the control of cell differentiation in Dictyostelium dicoideum. Cell Differ. 1977 Oct;6(3-4):229–239. doi: 10.1016/0045-6039(77)90018-5. [DOI] [PubMed] [Google Scholar]
- Rossier C., Gerisch G., Malchow D. Action of a slowly hydrolysable cyclic AMP analogue on developing cells of Dictyostelium discoideum. J Cell Sci. 1979 Feb;35:321–338. doi: 10.1242/jcs.35.1.321. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts D. J., Ashworth J. M. Growth of myxameobae of the cellular slime mould Dictyostelium discoideum in axenic culture. Biochem J. 1970 Sep;119(2):171–174. doi: 10.1042/bj1190171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]