Abstract
Grip strength (GS) has an age- and gender-dependent decline with advancing age. One study comparing GS among extremely old show a North–South gradient with lowest GS in Italy compared to France (intermediary) and Denmark (highest) even after adjusting for confounders. As GS is associated with higher rates of functional decline and mortality, and thus may be used as a health indicator, it is of interest to examine whether the results on extremely old can be reproduced in a large-scale European survey. GS was measured in a cross-sectional population-based sample of 27,456 individuals aged 50+ in 11 European countries included in the SHARE survey. We made a cross-country comparison of the age trajectory of GS in both genders. Northern-continental European countries had higher GS than southern European countries even when stratifying by age and gender and controlling for height, weight, education, health and socioeconomic status. The relative excess was found to be 11% and the absolute difference 5.0 kg for 50- to 54-year-old men, increasing to 28% and 6.9 kg among 80+ year-old men. The corresponding figures for women were 16% and 4.3 kg, and 21% and 3.5 kg, respectively. Southern European countries have lower GS in the age range 50+ year. Gene–environment interactions may explain country-specific differences. The use of GS in cross-national surveys should control not only for age and gender, but also for nationality.
Keywords: Hand strength, Epidemiology, Grip strength, Life expectancy, Aged, Cross-sectional studies
Introduction
Grip strength (GS) is a strong predictor of disability (Ishizaki et al. 2000; Kuh et al. 2005; Nybo et al. 2001; Rantanen et al. 1994; Rantanen et al. 1999), morbidity (Alfaro-Acha et al. 2006; Albrand et al. 2003; Klidjian et al. 1980; Rantanen et al. 1998a, 2000a, 2003), frailty (Syddall et al. 2003), and mortality (Fujita et al. 1995; Metter et al. 2002; Milne and Maule 1984; Philips 1986; Sasaki et al. 2007), even in initially healthy middle-aged men followed for 30 years (Rantanen et al. 2000a, 2000b). GS can thus be seen as a proxy for health; additionally, the measurement of GS is cheap and easily carried out even by trained survey interviewers in non-clinical settings. Also, the well-known age-dependent decline (Frederiksen et al. 2006; Kallmann et al. 1990; Milne and Maule1984; Shechtman et al. 2004) with clear gender differences at all ages, adds to its suitability as a reliable general health indicator in population-based studies covering large age spectrums. In fact, Frederiksen et al. (2006) found in their longitudinal analyses on the 1905 cohort GS to be predictive of both dropping out of the survey and of dying, while those who remained in the study had only small declines when reassessing GS after 2 and 4 years.
The underlying mechanism of the ability of GS to predict disability, morbidity and mortality is not yet understood. The age-dependent decline in GS has hitherto mainly been explained by the age-related decline in muscle mass (Gallagher et al. 1997), and muscle strength (Visser et al. 2000a, 2000b. But as the strength decline is more rapid than the concomitant loss of muscle mass (Goodpaster et al. 2006), it is suggested that there is an ageing-related decline in muscle quality defined by strength per unit muscle size (Goodpaster et al. 2008). However, the age-related decline in muscle functioning may result from the presence of a chronic, low-level inflammation, as poor muscle strength, defined as poor GS, is associated with chronic low-level inflammation (Cesari et al. 2004). Also, both environmental and genetic factors may be involved in GS. Recently, Kuh et al. (2002) demonstrated that birth weight, prepubertal height gain, pubertal growth and early infant motor development were associated with midlife grip strength, indicating a beneficial role of early childhood growth. Nutritional factors in early life may thus be in involved, but also genetic factors may play a role as twin studies have shown a substantial genetic component to muscle strength (Frederiksen et al. 2002; Tiainen et al. 2004, 2009).
Three studies have compared GS measurements across countries. A Nordic study of 75-year-olds found significantly lower grip strength values among Finnish, compared to Swedes and Danes, but no difference between the latter two (Era et al. 1994). Albert et al. (2005) found significantly lower GS in Indians (India) compared to New York citizens age 60+ year-olds after matching for age, gender, medical condition and self-rated disability, but not adjusted for height or socioeconomic status (SES). Only one study (Jeune et al. 2006) has compared GS between three different European regions, i.e. northern Europe (Denmark), continental Europe (France), and southern Europe (Italy). Although this study was made on an extreme population-based sample of 98+ year olds, it showed a North–South gradient of mean GS, with substantially lower GS values in the southern part of Italy compared to southern France and Denmark. Additionally, the North–South gradient remained significant after adjustment for age, gender, height, housing condition, activity of daily living, cognitive function test, chair stand and comorbidity, although these factors explained two-thirds of the variation in GS.
However, studies of exceptional survivors are very vulnerable to participation rates and non-performance, as the weakest are most likely to either be non-participant or to be unable to perform the hand grip test.
As GS is predictive of mortality, morbidity and disability and is easily measured even in non-clinical settings, it has become suitable for use as a general objective health indicator in population-based surveys. In cross-national surveys it is, however, important to know whether age- and gender-specific GS is directly comparable across countries, or if adjustment for country is needed as well, as suggested by Jeune et al. (2006). The purpose of this paper is to investigate GS in a population-based sample of Europeans, aged 50 or older, in order to see whether GS shows age- and gender-specific cross-national differences.
Methods
The data are derived from the SHARE baseline survey, which is a large-scale and longitudinally projected European study: The Survey of Health, Ageing and Retirement in Europe. (http://www.share-project.org) (Börsch-Supan et al. 2005a, b; Klevmarken et al. 2005). SHARE is the first cross-national study to include variables on work, retirement, health, health care, psychosocial factors, and socioeconomic position among people aged 50 years or more, in order to explore the various factors affecting health, ageing and retirement in an ageing Europe. Eleven countries, Sweden (SE), Denmark (DK), Germany (DE), Belgium (BE), the Netherlands (NL), France (FR), Switzerland (CH), Austria (AT), Spain (ES), Italy (IT) and Greece (GR), have contributed with data.
Population
The SHARE population sample was drawn as probability samples from each participating country. Due to institutional differences between SHARE countries, no uniform sample design could be used, and different sampling methods had to be applied, varying from simple random selection of households from national population registers to rather complicated multi-stage design using regional/local population registers. These differences are implemented in the design weights, which also take into account the background population in each country, thus adjusting for differences in non-participation. Comparison of data from the SHARE study with data from other prominent European studies, showed very similar distributions on employment, income, education and health (Klevmarken et al. 2005). Data were collected by trained interviewers using computer-assisted personal interviews (CAPI) conducted in the respondents’ home. Household response rates varied from 38.8% in Switzerland to 81.1% in France (Table 1), with a mean for all countries of 61.6%. Nine countries had rates above 50%. The present results are based on SHARE data “Release 2 Beta version” and contain 27,673 individuals in 19,498 households collected between April and October 2004. Eligible subjects were 50+ year-olds living in households including their spouses and children. Non-institutionalized persons were excluded. However, in Sweden, Denmark and the Netherlands they were included as eligible. As they comprised less than 100 persons their data were kept in the analysis.
Table 1.
Demographic variables of the SHARE sample (N of participants 27,456)
| DE | DK | AT | BE | SE | NL | CH | FR | GR | IT | ES | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Household resp. % | 63.4 | 63.2 | 55.6 | 39.2 | 46.9 | 61.6 | 38.8 | 81.0 | 63.1 | 54.5 | 53.0 |
| N (women %) | 2,941 (53) | 1,615 (53) | 1,849 (58) | 3,649 (53) | 2,995 (53) | 2,877 (53) | 962 (53) | 3,037 (55) | 2,669 (54) | 2,508 (55) | 2,354 (58) |
| Age group (%) | |||||||||||
| 50–54 | 18.7 | 19.9 | 13.4 | 16.3 | 15.9 | 19.4 | 19.7 | 19.3 | 20.7 | 13.0 | 15.8 |
| 55–59 | 15.2 | 20.0 | 16.8 | 21.0 | 19.6 | 22.3 | 17.0 | 20.0 | 18.2 | 20.1 | 15.3 |
| 60–64 | 19.4 | 16.8 | 21.1 | 14.6 | 17.6 | 17.3 | 15.8 | 13.8 | 15.0 | 20.4 | 14.8 |
| 65–69 | 19.1 | 11.6 | 17.6 | 14.4 | 15.4 | 13.8 | 14.1 | 12.7 | 13.6 | 17.3 | 14.9 |
| 70–74 | 11.1 | 11.3 | 11.8 | 12.8 | 11.8 | 11.1 | 12.1 | 12.6 | 13.2 | 14.0 | 14.8 |
| 75–79 | 9.5 | 9.4 | 9.7 | 10.4 | 9.3 | 8.1 | 10.4 | 10.1 | 8.3 | 8.1 | 11.7 |
| 80+ | 7.0 | 11.0 | 9.5 | 10.5 | 10.4 | 8.1 | 10.9 | 11.5 | 11.0 | 7.1 | 12.7 |
| Mean age (range) | 64.4 (50–97) | 64.6 (50–104) | 65.4 (50–100) | 65.2 (50–101) | 65.1 (50–102) | 63.7 (50–99) | 65.1 (50–96) | 65.0 (50–99) | 64.7 (50–97) | 64.7 (50–100) | 66.6 (50–103) |
| Education [mean years (SD)] | 13.5 (2.8) | 12.7 (3.4) | 11.4 (2.7) | 10.3 (3.8) | 10.3 (3.2) | 11.1 (3.3) | 12.2 (4.3) | 8.5 (5.4) | 8.7 (4.8) | 6.7 (4.3) | 5.5 (4.2) |
| Low/medium/high (%) | 18/55/27 | 25/44/31 | 32/45/23 | 51/26/23 | 53/18/29 | 57/23/20 | 52/22/26 | 54/28/18 | 62/21/17 | 78/15/7 | 86/7/7 |
| Forgo care due to costs (%) | 5.6 | 1.5 | 2.9 | 3.3 | 2.8 | 2.1 | 3.6 | 6.6 | 6.4 | 5.1 | 3.0 |
| Forgo care due to unavailable (%) | 1.5 | 2.0 | 0.7 | 0.9 | 3.4 | 0.6 | 0.7 | 2.2 | 5.1 | 3.5 | 1.9 |
| 1+ ADL limitation (%) | 8.7 | 10.3 | 9.2 | 11.6 | 8.8 | 7.3 | 6.9 | 12.0 | 8.7 | 10.5 | 14.1 |
| 2+ chron. cond. (%) | 40.0 | 43.4 | 33.4 | 46.2 | 41.4 | 34.0 | 28.1 | 42.8 | 40.1 | 44.9 | 51.4 |
| Depressive sympt. (%) | 18.6 | 17.9 | 19.9 | 24.6 | 19.3 | 19.4 | 18.8 | 33.5 | 24.9 | 33.8 | 36.8 |
| Seeing MD last 1 year 0/1–4/5+ visits (%) | 7/44/49 | 19/55/26 | 14/44/42 | 8/37/55 | 22/58/20 | 19/50/31 | 15/56/29 | 6/40/54 | 21/38/41 | 16/33/51 | 11/36/53 |
| Yearly income [€ ppp-adj, mean (SD)] | 48,131 (46,402) | 46,593 (39,358) | 40,581 (37,328) | 50,606 (72,758) | 47,389 (33,745) | 52,637 (46,104) | 56,844 (51,613) | 48,790 (58,472) | 22,673 (21,946) | 34,104 (34,719) | 29,624 (44,071) |
| Assets–net worth [€ ppp-adj, mean (SD)] | 377,144 (1,077,947) | 346,106 (716,401) | 215,493 (333,928) | 490,257 (927,337) | 289,004 (644,937) | 469,543 (1,778,525) | 557,832 (898,812) | 511,630 (1,205,365) | 222,667 (350,759) | 485,139 (1,974,600) | 469,080 (1,273,694) |
Methods and measures
GS was measured using a handheld dynamometer (Smedley, S Dynamometer, TTM, Tokyo, 100 kg). Respondents were instructed to stand (preferably) or sit, with the elbow at a 90° angle, the wrist in neutral position, keeping the upper arm tight against the trunk, and the inner lever of the dynamometer adjusted to suit the hand (the second phalanxes against the lever). Before the study period, interviewers participated in uniformly and centrally developed training sessions using a protocol for measuring GS. Also, the interviewers were instructed to verbally encourage the participant to squeeze the handles as hard as possible. Two values were recorded for each hand alternating between left and right hand. Valid measurements were defined as the values of two measurements in one hand that differed by less than 20 kg. GS measurements with values =0 kg or ≥100 kg were excluded as well as if GS was only measured once in one hand. The maximum value (MaxGS) was defined as the maximum GS measurement of both hands (2 × 2) or of one hand (2 × 1). Self-reported height (centimeter) and weight (kilograms) were used in the analyses.
Level of education was measured using the seven levels of International Standard Classification of Educational Degrees (ISCED-97). These levels were recoded into three groups: low, medium and high education. Various indicators of bad health were used in a dichotomous way: (1) having 0 or 1 or more (1+) limitation in activities of daily living (ADL), (2) reporting 0–1 or 2 or more (2+) chronic conditions, (3) reporting to forgo health care due to financial problems (no/yes; 0–1), (4) reporting to forgo health care due to unavailability or inaccessibility (no/yes; 0–1), and (5) having depression [no =(EURO-D score 3 or less)/yes=(EURO-D score of 4 or more) (33)]. Additionally, in three categories (0; 1–4; 5+), the number of visits per year to a medical doctor.
Total annual household income (the incomes of all household members in the participant’s house hold) consists of wages, self-employment income, capital income, pensions and other payments, rental income, and long-term insurance payment. The income variable was adjusted for purchasing power parity (ppp). Financial assets were calculated from the sum of seven broad categories (bank accounts, securities, mutual funds, individual retirement’s accounts, contractual savings for housing, and life-insurance policies) minus liabilities rendering a ppp adjusted ‘total household net worth’ (Christelis et al. 2005).
Statistics
Data were analysed using STATA SE version 9 (Stata Corp, College Station, TX). Mean of unadjusted MaxGS (mean MaxGS) measures and 95% confidence intervals in 5-year age groups stratified by gender and country were calculated. Based on the country-specific GS measurements by age, a similar pattern with a northern-continental to south difference was found in both males and females with the highest mean GS measurement in the northernmost and continental countries and the lowest in the southernmost countries. Subsequently, the countries were regrouped into two groups, a northern-continental (SE, DK, DE, NL, FR, CH, AT) and a southern (ES, IT, GR).
Separately for each gender a linear regression on MaxGS (dependent variable) was performed with height, weight, age and indicator variables for countries (independent variables). For both genders Austria was chosen as reference group as this country had the highest GS measurements in women, and the third highest in men. To control for potential confounders we ran additional linear regression including the following variables: reporting 1+ ADL limitation, reporting 2+ chronic conditions, reporting 4 or more depressive symptoms, having low education, reporting to forgo contact with a medical professional because of (1) costs, and because (2) unavailability or inaccessibility, rare or frequent contact with a medical doctor within last 12 months, total ppp adjusted household income, and total household net worth.
The average excess strength in the northern-continental region was calculated using the formula:
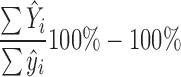 |
where the i runs through all individuals in the population with a valid MaxGS measure, Ŷ i is individual i’s expected score in the northern-continental region, while ŷ i is individual i’s expected score in the southern region. For the present analysis we used calibrated individual design weights, which were obtained by adjusting the design weights to the total population number in each country by age group and gender. (Klevmarken et al. 2005).
Results
A total of 27,456 persons participated in the survey. Descriptive data are shown in Table 1. A valid GS measurement was performed in 25,198 individuals (91.1%; age range, 50–104 years). Including valid measurements of height and weight the number decreased to 24,806 (89.6%).
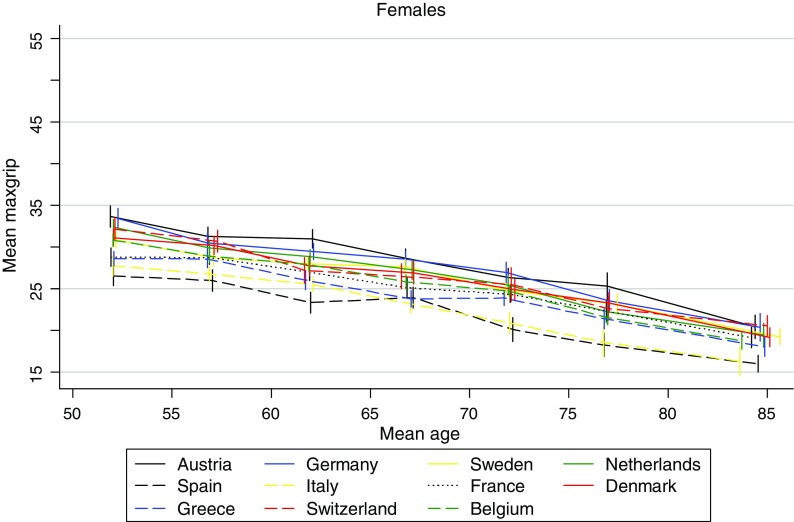
The unadjusted mean MaxGS show an age-dependent decline with each 5-year age group over the entire age range and in both genders (Figs. 1, 2). In all countries females have lower age-specific mean MaxGS compared to men. The highest gender-specific mean MaxGS is seen in the age group 50–55 years, being 33.8 kg (95%CI: 32.4; 35.1) among Austrian women, and 53.7 kg (95%CI: 52.1; 55.2) among German men. In the same age group the lowest gender-specific mean MaxGS is seen among Spanish women and men being 27.2 kg (95%CI: 26.1; 28.2) and 45.1 kg (95%CI:42.8; 47.3), respectively. Among participants aged 80 and over the highest gender-specific mean MaxGS is observed among German women and men being 20.5 kg (95%CI:18.8; 22.1) and 33.4 kg (95%CI: 31.0; 35.9), respectively, while the Spanish women and men perform the lowest 16.3 kg (95%CI: 15.2; 17.4) and 23.3 kg (95%CI: 21.4; 25.3), respectively. Interestingly, at a country level the best performing oldest old men have about the same GS as the best performing and youngest women (Figs. 1, 2).
Fig. 1.
Unadjusted mean maximum grip strength by 5-year age groups and country (men)
Fig. 2.
Unadjusted mean maximum grip strength by 5-year age groups and country (women)
The pattern of decline is similar in all SHARE countries. However, the data also show geographical differences in GS in both genders, with the highest scores in northern and continental (SE, DK, NL, DE, AT, CH, FR) countries and the lowest in southern countries (ES, IT and GR). Using Austrians as reference in both genders, the regression analysis controlling for age, weight and height confirms this pattern by yielding the lowest coefficients in the southernmost SHARE countries (Table 2), even after adjusting not only for weight and height but also for potential confounders.
Table 2.
Regression coefficients with 95% confidence intervals by countries
| Men | Women | ||||
|---|---|---|---|---|---|
| Countrya | Coefficient | (95% CI) | Countrya | Coefficient | (95% CI) |
| DE | 0.28 | (−0.53;1.10) | AT | Reference group | |
| DK | 0.26 | (−0.60;1.11) | DE | −0.16 | (−0.71;0.40) |
| AT | Reference group | BE | −1.11 | (−1.62;−0.60) | |
| BE | −0.25 | (−1.01;0.51) | CH | −1.14 | (−1.81;−0.47) |
| SE | −0.98 | (−1.77;−0.20) | FR | −1.58 | (−2.12;−1.04) |
| NL | −1.00 | (−1.79;−0.20) | NL | −1.75 | (−2.30;−1.19) |
| CH | −1.22 | (−2.16;−0.27) | DK | −1.98 | (−2.58;−1.39) |
| FR | −1.24 | (−2.06;−0.43) | SE | −2.29 | (−2.83;−1.74) |
| GR | −3.45 | (−4.31;−2.59) | GR | −3.12 | (−3.69;−2.56) |
| IT | −3.47 | (−4.33;−2.62) | IT | −3.50 | (−4.05;−2.93) |
| ES | −5.25 | (−6.12;−4.37) | ES | −4.05 | (−4.63;−3.46) |
GS is the dependent variable, and age, weight, height, depression, number of visits to general practitioner, education, forgo visits to a medical doctor because of unavailability or cost, income, assets, ADL limitations, chronic diseases as independent variables
aReference group in both genders is Austria. Ranking order is not similar in men and women
The unadjusted gender-specific average relative excesses of GS in 5-year age groups of the northern-continental countries compared to the southern countries are in the range of 11% among 50- to 54-year-old men to 28% among 80+ year-old men (Table 3). For women the range is 16–21% in the corresponding age groups. The unadjusted absolute differences were likewise significantly lower in the southern countries in all age groups. Adjusting for confounders attenuated both the relative excess (19–54% in men, 38–65% in women) and absolute difference (1.02–2.49 kg in men, 1.23–2.45 in women) between northern-continental and southern countries, but they were still significantly lower in southern countries. The overall average excess GS of the northern-continental countries adjusted for age, gender, weight and height was 11.1%. The absolute difference in GS (kg) tended to be larger in men than in women in each 5-year age group (Table 3).
Table 3.
Unadjusted (unadj.) and adjusted (adj.) excess grip strength of northern-continental European countries to southern European countries by gender
| Age group | Men | Women | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Excess (%) | Δ (%) | Difference (kg) [95% CI] | Δ (kg) | Excess (%) | Δ (%) | Difference (kg) [95% CI] | Δ (kg) | |||||
| Unadj. | Adj.a | Unadj. | Adj.a | Unadj. | Adj.a | Unadj. | Adj.a | |||||
| 50–54 | 10.8 | 6.6 | 39 | 5.03 [3.50; 6.56] | 2.88 [2.08;3.68] | 2.15 | 15.9 | 9.0 | 43 | 4.34 [3.34; 5.34] | 2.33 [1.88; 2.78] | 2.01 |
| 55–59 | 11.3 | 9.2 | 19 | 4.94 [3.45; 6.43] | 3.86 [3.11;4.61] | 1.08 | 11.6 | 7.2 | 38 | 3.08 [2.24; 3.92] | 1.85 [1.42; 2.27] | 1.23 |
| 60–64 | 11.8 | 8.6 | 27 | 4.86 [3.56; 6.16] | 3.52 [2.83; 4.20] | 1.34 | 15.2 | 8.9 | 41 | 3.76 [2.91; 4.62] | 2.23 [1.76; 2.71] | 1.53 |
| 65–69 | 18.4 | 11.3 | 39 | 6.95 [5.54; 8.36] | 4.46 [3.69; 5.22] | 2.49 | 16.1 | 9.3 | 42 | 3.79 [2.81; 4.76] | 2.27 [1.76; 2.78] | 1.52 |
| 70–74 | 17.5 | 11.0 | 37 | 6.03 [4.54; 7.52] | 4.22 [3.39; 5.05] | 1.81 | 22.9 | 12.1 | 47 | 4.78 [3.73; 5.83] | 2.80 [2.23; 3.37] | 1.98 |
| 75–79 | 13.2 | 8.6 | 35 | 4.27 [2.66; 5.87] | 3.25 [2.28; 4.23] | 1.02 | 23.2 | 8.2 | 65 | 4.32 [3.06; 5.58] | 1.87 [1.01; 2.73] | 2.45 |
| 80+ | 27.6 | 12.7 | 54 | 6.88 [4.92; 8.84] | 4.48 [3.15; 5.82] | 2.40 | 21.4 | 10.2 | 52 | 3.48 [2.20; 4.75] | 2.13 [1.32; 2.93] | 1.35 |
Relative (%) and absolute differences (kg), and relative (Δ %) and absolute (Δ kg) changes
aAdjusted for depression (dichotomized), number of visits to general practitioner (3 categories), education (3 categories), forgo doctor visits due to unavailability or costs, chronic diseases (dichotomized), ADL limitations (dichotomized), income, assets, weight, and height
Discussion
The result of the present study not only confirms the age-dependent decline in GS in both genders aged 50 and over, but it has also led to the novel finding that GS is significantly lower in the southernmost and Mediterranean countries compared to northern and continental countries, even when controlling for height and weight and potential confounders. Nevertheless, the pattern of decline in GS with advancing age is the same regardless of the latitude of the participating country.
The SHARE data set is, to our knowledge, the largest cross-national sample ever collected on GS using the same method and type of dynamometer. For validation we compared the Danish SHARE GS measurements with those of a large Danish population-based cross-sectional and longitudinal study of 8,342 males and females aged 50 years and over, using the same method and dynamometer (Frederiksen et al. 2006), and we obtained virtually the same results when comparing within each gender, age and height strata (data not shown).
Due to different methodologies throughout the 11 participating countries for creating a population-based sample, design weights based on age and gender were implemented (Klevmarken et al. 2005). However, as both age and gender are used in the regression analyses, the results based on a weighted population sample could be affected. The same calculations were therefore made using unweighted data, but the results did not differ from the results based on the weighted sample, with the exception that Danish males were found to be slightly stronger than German males in the youngest age groups (data not shown).
A limitation to this study is the use of self-reported weight and height. According to a recent review on height and weight validation studies in the general population, there is an under-reporting of overweight, while height is over-reported (Gorber et al. 2007). Of the included studies few were from Europe: United Kingdom, Sweden and Spain and showed a tendency towards Spanish people over-reporting higher heights than Swedes, i.e. 2.2 cm and 0.7 cm mean difference in self-report minus direct measure, respectively. No similar pattern was identified in differences in under-reporting weight. But all in all, the differences are small and seem to be more of a systematic bias, than a bias between countries. However, if there is a clear tendency of the more southern countries over-reporting higher heights than their more northern peers this could hamper our cross-national results.
A common training using a standardized protocol and the same dynamometers can minimize a possible bias in the measurements, but not exclude the possibility of measurement errors. While others have found a 3–8% measurement error (personal communication), we believe such an error has very little influence on the uncertainty of the estimated mean GS value due to the large sample size in each country. Additionally, in the pretest we did three measurements on each hand and found the correlations between the country-specific measurements to be extremely high (r > 0.9), which is why the number of GS measurements was limited to only two measurements on each hand.
Poorer GS in the more southern countries could be explained by a generally lower stature of southern Europeans compared to more northern Europeans. However, compared to the unadjusted mean MaxGS, the northern/continental to south pattern of decline is attenuated, but still significant when controlling for both height and weight, thus indicating that other factors related to nationality are influencing GS.
Other body dimensions, e.g. hand size could affect the GS, even though, and according to the protocol, this was to be accounted for by adjusting the dynamometer to the individual hand. But of course it cannot be excluded that a slender hand may result in a different measurement than a broad hand in otherwise matched participants.
The relative excess in GS among northern-continental men shows a marked augmentation, almost a doubling, from age group 75–79 years to age 80+. A parallel augmentation among women is seen from age group 65–69 years to age group 70–74. These marked changes may be explained by a selection bias. Southern European countries have fewer nursing homes and a cultural tradition of old parents living in a household with a child. With the exception of Sweden, Denmark and the Netherlands, which all together included less than 100 institutionalized participants, SHARE only included non-institutionalized persons. Thus a higher density of frail older people living in the southern European households is to be expected and may explain this marked difference in GS.
Genetic influence on GS even within homogeneous populations, e.g. Danes (Frederiksen et al. 2002) and Finns (Tiainen et al. 2004), is well documented, and could explain some of the northern/continental–south discrepancy. In muscle physiological studies on separate ethnic groups others have shown differences in muscle composition, muscle anatomy, and muscle metabolism (Saltin et al. 1995). Rantanen et al. (1998b)) found racial differences with black Americans having better GS than white Americans at equal levels of disability or physical activity and suggested the difference to be explained by the black Americans’ greater muscle mass, as shown by Gallagher et al. (1997). Gender differences in GS may be explained by gender genetic differences in muscle mass. Likewise, it could be hypothesised that the variation in country-specific GS could partly be explained by genetic determinants of muscle mass and strength.
The finding of lower level of GS in the southern countries is intriguing as it is not reflected in a correspondingly lower life expectancy as could have been expected based on the well-known association between poor GS and higher risk of mortality (Frederiksen et al. 2006; Visser et al. 2000a). In fact, based on life expectancy at birth, Italian and Spanish women are among the longest surviving Europeans, although Swiss and French women come very close (Lanzieri 2008). Several factors may be in play. One argument could be that southern Europeans may be weaker due to population background differences [e.g. genetics, low birth weight (Kuh et al. 2002)] but due to more favourable environment (e.g. warmer climate, better food, and fewer health hazards) they survive to higher ages. Likewise, the more continental and northern countries may be genetically stronger, but living in a less favourable environment (e.g. colder climate, higher humidity, lower quality of food, more health hazards) leading to a lower life expectancy at birth. However, although Italy and Spain are among the top five of the highest life expectancies in the SHARE countries (Lanzieri 2008), France, Switzerland and Sweden are the other three. Specifically looking at the affluent and well-developed welfare state Sweden, it is interesting to compare with its neighbouring country, Denmark, which holds an almost similar welfare system. They have very similar GS and yet they differ in life expectancy at birth by about 2.5 years in year 2006 (Lanzieri 2008). Cause-specific mortality and morbidity data suggest that the main reason for this discrepancy is due to life style factors, especially smoking (Juel et al. 2008; Juel 2008). Thus, the difference in life expectancy may be explained by Danes living in a more hazardous environment than the Swedes, even though they share genetic determinants for high grip strength. In parallel to this, Greeks may be genetically weaker (low GS), but also have lower life expectancy than their Italian and Spanish peers, due to more hazardous environmental factors with a strong effect on mortality, not counterbalanced by life enhancing environmental factors, such as diet or a warm climate. On the other hand, a higher mortality in earlier ages among, e.g. Greeks, compared to Italians and Spanish people may have lead to a selection of the stronger ones, which could explain the relatively higher age- and gender-specific GS in Greeks compared to their Mediterranean peers.
Finally, it could be hypothesised that differences in current medical care could influence the GS; e.g. countries with relatively better health and medical care would have stronger GS. However, we did adjust for this by using the variables (a) to forgo care due to unavailability, (b) to forgo care due to cost, (c) having depressive symptoms, (d) having 1+ ADL limitation, (e) 2+ chronic conditions, and (f) seeing an MD within last 1 year, and it did not change the results. Thus, we find our results to be in accordance with the findings by Jeune et al. (2006) of lower GS in the south compared to two more northern European regions in an unselected population (i.e. including institutionalized) of extremely old persons (median age 98 years), although they cannot either differentiate between population background differences (e.g. genetic variations, childhood conditions) and environmental factors (e.g. lifestyle factors, health care).
Although both genetic and environmental factors may influence the country-specific differences in GS, it is interesting to observe that the pattern of decline is similar in all countries. Longitudinal data from the ongoing SHARE study may in the future help us understand what the predictors of future health are, whether measured by mortality, morbidity or disability levels, and irrespective of country.
Meanwhile, when using GS as a health indicator not only age, gender and height, but also geographical region should be accounted for in the analyses.
Acknowledgments
This paper uses data from Release 2 beta SHARE 2004. The SHARE data collection has been primarily funded by the European Commission through the 5th framework programme (project QLK6-CT-2001-00360 in the thematic programme Quality of Life). Additional funding came from the US National Institute on Aging (U01 AG09740-13S2, P01 AG005842, P01 AG08291, P30 AG12815, Y1-AG-4553-01 and OGHA 04-064). Data collection in Austria (through the Austrian Science Foundation, FWF), Belgium (through the Belgian Science Policy Office) and Switzerland (through BBW/OFES/UFES) were nationally funded.
References
- Albert SM, Alam M, Nizamuddin M. Comparative study of functional limitation and disability in old age: Delhi and New York City. J Cross Cult Gerontol. 2005;20:231–241. doi: 10.1007/s10823-006-9014-2. [DOI] [PubMed] [Google Scholar]
- Albrand G, Munoz F, Sornay-Rendu E, DuBoeuf F, Delmas PD. Independent predictors of all osteoporosis-related fractures in healthy postmenopausal women: the OFELY Study. Bone. 2003;32:78–85. doi: 10.1016/S8756-3282(02)00919-5. [DOI] [PubMed] [Google Scholar]
- Alfaro-Acha A, Snih SA, Raji MA, Kuo YF, Markides KS, Ottenbacher KJ. Hand grip strength and cognitive decline in older Mexican Americans. J Gerontol Med Sci. 2006;61A:859–865. doi: 10.1093/gerona/61.8.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börsch-Supan A, Brugiavini A, Jürges H, Mackenbach J, Siegrist J, Weber G. Health, ageing and retirement in Europe. First results from the survey of health, ageing and retirement in Europe. Mannheim: Mannheim Research Institute for the Economics of Ageing; 2005. [Google Scholar]
- Börsch-Supan A, Hank K, Jürges H. A new comprehensive and international view on aging: Introducing the survey of ‘Health, Ageing and Retirement in Europe’. Eur J Ageing. 2005;2:245–253. doi: 10.1007/s10433-005-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Williams GR, Guralnik JM, Ferruci L. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol Med Sci. 2004;59A:242–248. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- Christelis D, Jappelli T, Padula M. Wealth and portofolio composition. In: Börsch-Supan A, Brugiavini A, Jürges H, Mackenbach J, Siegrist J, Weber G, editors. Health, ageing and retirement in Europe. First results from the survey of health, ageing and retirement in Europe. Mannheim: Mannheim Research Institute for the Economics of Ageing; 2005. pp. 310–317. [Google Scholar]
- Era P, Rantanen T, Avlund K, Gause-Nilsson I, Heikkinen E, Schroll M, Steen B, Suominen H. Maximal isometric muscle strength and anthropometry in 75-year-old men and women in three Nordic localities. Scand J Med Sci Sports. 1994;4:26–31. [Google Scholar]
- Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K. Hand grip strength: a phenotype suitable for identifying genetic variants affecting mid- and late-life physical functioning. Genet Epidemiol. 2002;23:110–122. doi: 10.1002/gepi.1127. [DOI] [PubMed] [Google Scholar]
- Frederiksen H, Hjelmborg J, Mortensen J, McGue M, Vaupel JW, Christensen K. Age trajectories of grip strength: cross-sectional and longitudinal data among 8, 342 Danes aged 46 to 102. Ann Epidemiol. 2006;16:554–562. doi: 10.1016/j.annepidem.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nakamura Y, Hiraoka J, Kobayashi K, Sakata K, Nagai M, Hiroshi Y. Physical-strength tests and mortality among visitors to health-promotion centers in Japan. J Clin Epidemiol. 1995;48:1349–1359. doi: 10.1016/0895-4356(95)00533-1. [DOI] [PubMed] [Google Scholar]
- Gallagher D, Visser M, de Meersman RE, Sepulveda D, Baumgartner RN, Pierson RN, Harris T, Heymsfield SB. Appendicular skeletal muscle mass: effects of age, gender, and ethnicity. J Appl Physiol. 1997;83:229–239. doi: 10.1152/jappl.1997.83.1.229. [DOI] [PubMed] [Google Scholar]
- Goodpaster B, Won Park S, Harris T, Kritchevsky SB, Nevitt M, Schwartz AV, Simonsick EM, Tylavsky FA, Visser M, Newmann AB. The loss of skeletal muscle strength, mass and quality in older adults: the health, aging and body composition study. J Gerontol A Biol Sci Med Sci. 2006;61:1059–1064. doi: 10.1093/gerona/61.10.1059. [DOI] [PubMed] [Google Scholar]
- Goodpaster BH, Chomentowski P, Ward BK, Rossi A, Glynn NW, Delmonico MJ, Kritchevsky SB, Pahor M, Newmann AB. Effects of physical activity on strength and skeletal muscle fat infiltration in older adults: a randomized controlled trial. J Appl Physiol. 2008;105:1498–1503. doi: 10.1152/japplphysiol.90425.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorber SC, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- Ishizaki T, Watanabe S, Suzuki T, Shibata H, Haga H. Predictors for functional decline among nondisabled older Japanese living in a community during a 3-year follow-up. J Am Geriatr Soc. 2000;48:1527–1528. doi: 10.1111/j.1532-5415.2000.tb02632.x. [DOI] [PubMed] [Google Scholar]
- Jeune B, Skytthe A, Cournil A, Greco V, Gampe J, Berardelli M, Andersen-Ranberg K, DeBenedictis G, Robine JM. Handgrip strength among nonagenarians and centenarians in three European regions. J Gerontol Med Sci. 2006;61A:707–712. doi: 10.1093/gerona/61.7.707. [DOI] [PubMed] [Google Scholar]
- Juel K. Life expectancy and mortality in Denmark compared to Sweden. What is the effect of smoking and alcohol? Ugeskr Laeger. 2008;170:2423–2427. [PubMed] [Google Scholar]
- Juel K, Sørensen J, Brønnum-Hansen H. Risk factors and public health in Denmark. Scand J Public Health. 2008;36(Suppl 1):11–227. doi: 10.1177/1403494808097590. [DOI] [PubMed] [Google Scholar]
- Kallmann DA, Plato CC, Tobin JD. The role of muscle loss in the age-related decline of grip strength: cross-sectional and longitudinal perspectives. J Gerontol Med Sci. 1990;45:M82–M88. doi: 10.1093/geronj/45.3.m82. [DOI] [PubMed] [Google Scholar]
- Klevmarken NA, Swensson B, Hesselius P (2005) The SHARE sampling procedures and calibrated design weights. In: Börsch-Supan A, Jürges A (eds) The Survey of Health, Ageing and Retirement in Europe. Methodology. Mannheim Research Institute for the economics of ageing, Mannheim, Germany, L13, 17, pp 28–70
- Klidjian AM, Foster KJ, Kammerling RM, Cooper A, Karran SJ. Relation of anthropometric and dynamometric variables to serious postoperative complications. BMJ. 1980;281:899–901. doi: 10.1136/bmj.281.6245.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuh D, Bassey J, Hardy R, Sayer AA, Wadsworth M, Cooper C. Birth weight, childhood size and muscle strength in adult life: evidence from a birth cohort study. Am J Epidemiol. 2002;156:627–633. doi: 10.1093/aje/kwf099. [DOI] [PubMed] [Google Scholar]
- Kuh D, Bassey EJ, Butterworth S, Hardy R, Wadsworth MEJ, the Musculoskeletal Study Team Grip strength, postural control, and functional leg power in a representative cohort of British men and women: associations with physical activity, health status, and socioeconomic conditions. J Gerontol Med Sci. 2005;60A:224–231. doi: 10.1093/gerona/60.2.224. [DOI] [PubMed] [Google Scholar]
- Lanzieri G (2008) Population in Europe 2007: first results. Eurostat. Statistics in focus. No. 81/2008
- Metter EJ, Talbot LA, Schrager M, Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol Med Sci. 2002;57A:B359–B365. doi: 10.1093/gerona/57.10.b359. [DOI] [PubMed] [Google Scholar]
- Milne JS, Maule MM. A longitudinal study of handgrip and dementia in older people. Age Ageing. 1984;13:42–48. doi: 10.1093/ageing/13.1.42. [DOI] [PubMed] [Google Scholar]
- Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Chrisensen K. Functional status and self-rated health in 2, 262 nonagenarians: the Danish 1905 cohort survey. J Am Geriatr Soc. 2001;49:601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Philips P. Grip strength, mental performance and nutritional status as indicators of mortality risk among female geriatric patients. Age Ageing. 1986;15:53–56. doi: 10.1093/ageing/15.1.53. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Era P, Kauppinen M, Heikkinen E. Maximal isometric muscle strength and socioeconomic status, health, and physical activity in 75-year-old persons. J Aging Phys Act. 1994;2:206–220. [Google Scholar]
- Rantanen T, Masaki K, Foley D, Izmirlian G, White L, Guralnik JM. Grip strength changes over 27 yr in Japanese-American men. J Appl Physiol. 1998;85:2047–2053. doi: 10.1152/jappl.1998.85.6.2047. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Leveille S, Izmirlian G, Hirsch R, Simonsick E, Ling S, Fried LP. Racial differences in muscle strength in disabled older women. J Gerontol Biol Sci. 1998;53A:B355–B361. doi: 10.1093/gerona/53a.5.b355. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Guralnik JM, Sakari-Rantala R, Leveille S, Simonsick E, Ling S, Fried LP. Disability, physical activity, and muscle strength in older women: the women’s health and aging study. Arch Phys Med Rehab. 1999;80:130–135. doi: 10.1016/S0003-9993(99)90109-0. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Penninx BWJH, Masaki K, Lintunen T, Foley D, Guralnik JM. Depressed mood and body mass index as predictors of muscle strength decline in old men. J Am Geriatr Soc. 2000;48:613–617. doi: 10.1111/j.1532-5415.2000.tb04717.x. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, Masaki K, Guralnik JM. Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol Med Sci. 2000;55:M168–M173. doi: 10.1093/gerona/55.3.m168. [DOI] [PubMed] [Google Scholar]
- Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried L, Guralnik JM. Handgrip strength and cause-specific and total mortality in older disabled women: exploring the mechanism. J Am Geriatr Soc. 2003;51:636–641. doi: 10.1034/j.1600-0579.2003.00207.x. [DOI] [PubMed] [Google Scholar]
- Saltin B, Larsen H, Terrados N, Bangsbo J, Bak T, Kim CK, Svedenhag J, Rolf CJ. Aerobic exercise capacity at sea level and at altitude in Kenyan boys, junior and senior runners compared with Scandinavian runners. Scand J Med Sci Sports. 1995;5:209–221. doi: 10.1111/j.1600-0838.1995.tb00037.x. [DOI] [PubMed] [Google Scholar]
- Sasaki H, Kasagi F, Yamada M, Fukita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120:337–342. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Shechtman O, Mann WC, Justiss MD, Tomita M. Grip strength in the frail elderly. Am J Phys Med Rehabil. 2004;83:819–826. doi: 10.1097/01.PHM.0000143398.00788.4E. [DOI] [PubMed] [Google Scholar]
- Syddall H, Cooper C, Martin F, Briggs R, Sayer AA. Is grip strength a useful single marker of frailty? Age Ageing. 2003;32:650–656. doi: 10.1093/ageing/afg111. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Sipilä S, Alen M, Heikkinen E, Kaprio J, Koskenvuo M, Tolvanen A, Pajala S, Rantanen T. Heritability of maximal isometric muscle strength in older female twins. J Appl Physiol. 2004;96:173–180. doi: 10.1152/japplphysiol.00200.2003. [DOI] [PubMed] [Google Scholar]
- Tiainen K, Sipilä S, Kauppinen M, Kaprio J, Rantanen T (2009) Genetic and environmental effects on isometric muscle strength and leg extensor power followed up for three years among older female twins. J Appl Physiol (in press). doi:10.1152/japplphysiol.91056.2008 [DOI] [PubMed]
- Visser M, Deeg DJH, Lips P, Harris TB, Bouter LM. Skeletal muscle mass and muscle strength in relation to lower-extremity performance in older men and women. J Am Geriatr Soc. 2000;48:381–386. doi: 10.1111/j.1532-5415.2000.tb04694.x. [DOI] [PubMed] [Google Scholar]
- Visser M, Harris TB, Fox KM, Hawkes W, Hebel JR, Yu Yahiro J, Michael R, Zimmerman SI, Magaziner J. Change in muscle mass and muscle strength after a hip fracture: relationship to mobility recovery. J Gerontol Med Sci. 2000;55A:M434–M440. doi: 10.1093/gerona/55.8.m434. [DOI] [PubMed] [Google Scholar]