Abstract
AIM
To evaluate the characteristics of the prescription of the proton pump inhibitor drugs (PPI) and the adherence to the indications of the guidelines regulating the reimbursement limitations set forth by the Italian Drug Agency.
METHODS
Thirty general practitioners (GP) participated in the study, providing data on more than 40000 patients in total. The population was divided into non occasional users of PPI drugs (PPI users) and non-users (PPI non-users) based on evidence of a prescription of at least 3 packs of PPIs in the last 90 d before analysis. The data provided allowed an assessment of compliance with the requirements of eligibility for PPI reimbursement according to the Italian Drug Agency rules, in order to obtain subpopulations which complied or not with the rules.
RESULTS
Six thousand three hundred and twenty-two patients were found to be PPI users, accounting for 14.9% of the patient population. PPI users were more frequently female, older and more frequently diagnosed with gastroesophageal reflux disease, gastric or duodenal ulcers, arthropathy, heart disease and cancer than the rest of the population. PPI users had more frequently received prescriptions for non-steroidal ant-inflammatory drugs (NSAIDS), acetylsalicylic acid (ASA), oral anticoagulant therapy (OAT) and systemic steroids. PPI reimbursement resulted applicable to 69.3% of the PPI users, but a potential for reimbursement of PPI prescriptions was identified in the non PPI users for the treatment of peptic or reflux disease (8.5%) and for the protection of gastric damage caused by NSAIDS (6.1%). Patients who are potentially eligible for reimbursement are older, diagnosed with arthropathy and heart disease more frequently and most commonly receive NSAID and ASA prescriptions compared with PPI users who do not satisfy eligibility requirements. Patients in whom it was not possible to identify conditions related to prescription suitability were more frequently associated with use of OAT.
CONCLUSION
A substantial number of patients who apparently do not meet prescription suitability conditions can be identified, but among non PPI users on the contrary, it is possible to identify an equal number of patients for whom prescription would be suitable. Poor suitability can be identified in the population receiving OAT. Thus, there is scope for decreasing inappropriate use of PPI drugs by adhering to certain criteria and by involving all interested parties.
Keywords: Proton pump inhibitors, Appropriateness, General practice, Gastroprotection, Peptic disease
Core tip: This study was carried out in a large unselected population to evaluate the characteristics of proton pump inhibitor (PPI) prescription and the adherence to the guidelines regulating the reimbursement limitations set forth by the Italian Drug Agency. A substantial number of patients who apparently do not meet prescription suitability conditions can be identified, but among non-PPI users on the contrary, it is possible to identify an equal number of patients for whom prescription would be suitable. According to our data the greatest problems in clinical decision originate in patients in antithrombotic therapy.
INTRODUCTION
Proton pump inhibitors (PPI) are among the most prescribed drugs in the world since their indications for use are manifold, including the treatment of gastro-esophageal reflux disease (GERD), peptic ulcer disease, the prevention of gastric damage by non-steroidal ant-inflammatory drugs (NSAIDS) and acetylsalicylic acid (ASA), dyspepsia and infection by Helicobacter pylori (H. pylori)[1-4]. There are five PPIs available in Italy (omeprazole, pantoprazole, lansoprazole, rabeprazole and esomeprazole), representing between 5% and 10% of total pharmaceutical prescriptions, similar to other countries[5-7].
PPIs are generally well tolerated and have few side effects but their prolonged use has been associated with various problems due to mechanisms which are especially related to the extensive and persistent inhibition of gastric acid secretion and the competitive inhibition of hepatic cytochrome P450[8-11].
Due to the high efficacy of PPIs in controlling the symptoms of upper gastrointestinal diseases, treatment often becomes ongoing and difficult to suspend[12].. This often makes it difficult to determine the prescription suitability of PPIs[12-16].
For this reason, rules to limit the reimbursement of these drugs which are paid for by the Italian National Health Service were introduced by the Italian Drug Agency twenty years ago. These were draw up according to the conditions of proven effectiveness and following major international guidelines. Table 1 describes the eligibility requirements for reimbursement of PPI prescriptions according to the Italian Drug Agency rules.
Table 1.
Rules of the Italian Drug Agency for the refund of proton pump inhibitor drugs
| The prescription of PPI refundable by the National Health Service is limited to |
| The prevention of serious complications of the upper gastrointestinal tract in patients in chronic treatment with NSAIDS or in antiaggregant therapy with low doses of ASA, provided there is one of the following conditions of risk: (1) history of past digestive hemorrhage or peptic ulcer not healed with Helicobacter pylori treatment; (2) concomitant therapy with anticoagulants or cortisone; and (3) advanced age |
| Duration of treatment 4 wk (occasionally 6 wk): Duodenal or gastric ulcer, in association with drugs eradicating the infection; GERD with or without esophagitis (first episode) |
| Duration of treatment extended to reevaluate after one year: Zollinger-Ellison syndrome; relapsing duodenal or gastric ulcer; GERD with and without esophagitis (relapsing) |
PPI: Proton pump inhibitor; GERD: Gastroesophageal reflux disease; ASA: Acetylsalicylic acid; NSAIDS: Non-steroidal ant-inflammatory drugs.
The aim of the study was to retrospectively evaluate, using the patient files provided by a large group of General Practitioners (GPs), the characteristics of PPI prescription and their adherence to the indications of the guidelines regulating the reimbursement limitations set forth by the Italian Drug Agency.
MATERIALS AND METHODS
Forty of the 400 GPs of the Health Agency of Bologna (Northern Italy) were requested to participate in the study. GPs were asked to submit a file containing anonymous data of all adult patients at 1 June 2015. This was obtained using an automated procedure available in the software which is used to manage clinical data. Demographic variables, presence of clinical diseases and drug use were reported in the file. A single database to obtain general population data was then created. The population was divided into non occasional users of PPI drugs (PPI users) and non-users (PPI non-users) based on evidence of a prescription of at least 3 packs of PPIs in the last 90 d before analysis (1 pack = 14 tablets). The data provided allowed an assessment of compliance with the requirements of eligibility for PPI reimbursement according to the Italian Drug Agency rules, in order to obtain subpopulations which complied or not with the rules. Table 1 describes the eligibility requirements for reimbursement of PPI prescriptions according to the Italian Drug Agency rules.
Differences between populations were evaluated using analysis of variance and the chi-squared test. P < 0.05 values were selected as the statistical significance limit. The statistical review of the study was performed by a biomedical statistician. The study did not need to be submitted to the Ethics Committee as retrospectively conducted on anonymous database.
RESULTS
Thirty GPs participated in the project and provided anonymous data files for 42548 patients. The study population was made up of 19632 males (46.1%) and 22916 females (53.9%) with a mean age 53 years (28.4% over 64 years old). This study population did not differ from the whole population on record at Health Agency of Bologna, which comprehends about 750000 adults (44% male and 56% female), of whom about 210000 (28%) are over 64 years old.
Six thousand three hundred and twenty-two patients were found to be PPI users, accounting for 14.9% of the patient population. Table 2 summarizes the characteristics of PPI users compared to non-PPI users.
Table 2.
Characteristics of proton pump inhibitor-users (at least 3 packs in 90 d) and non-proton pump inhibitor-users n (%)
| All | PPI-users | Non PPI users | |
| Patients | 42548 | 6322 | 36226 |
| Males | 19632 (46.1) | 2520 (39.9) | 17112 (47.2) |
| Aged over 64 yr | 12084 (28.4) | 3902 (61.7) | 8182 (22.6) |
| GERD | 5769 (13.6) | 2980 (47.1) | 2789 (7.7) |
| Peptic ulcer | 689 (1.6) | 375 (5.9) | 314 (0.9) |
| Arthropathy | 15661 (36.8) | 3786 (59.9) | 11875 (32.8) |
| Heart disease | 3932 (9.2) | 1674 (26.5) | 2258 (6.2) |
| Neoplasms | 3384 (8.0) | 1076 (17.0) | 2308 (6.4) |
| Use of NSAIDS | 1131 (2.7) | 416 (4.6) | 715 (2) |
| Use of ASA | 4522 (10.6) | 2017 (31.7) | 2505 (6.9) |
| Use of OAT | 1127 (2.6) | 500 (7.9) | 627 (1.7) |
| Use of systemic steroids | 547 (1.3) | 306 (4.8) | 241 (0.7) |
| EGD scopy | 5772 (13.6) | 2626 (41.5) | 3146 (8.7) |
| Test per H. pylori | 4761 (11.2) | 1641 (26.0) | 3120 (8.6) |
| PPI refundable for prevention of gastric damage by NSAIDS | 4105 (9.6) | 1896 (30.0) | 2209 (6.1) |
| PPI refundable for peptic ulcer or GERD | 6340 (14.9) | 3265 (51.6) | 3075 (8.5) |
| PPI refundable for prevention of gastric damage by NSAIDS or peptic ulcer or GERD | 9368 (22.0) | 4383 (69.3) | 4985 (13.8) |
All the features differ significantly (P < 0.01) between the two groups. PPI: Proton pump inhibitors; GERD: Gastroesophageal reflux disease; Heart disease: Heart failure, coronary ischemic disease, major heart valves disease; NSAIDS: Non steroid inflammatory drugs; ASA: Acetylsalicylic acid; OAT: Oral anticoagulant therapy; EGDscopy: Esophageal-gastro-duodenal endoscopy; H. pylori: Helicobacter pylori.
The two groups were statistically different when all the evaluated conditions were compared. PPI users were more frequently female, older and more frequently diagnosed with gastroesophageal reflux disease, gastric or duodenal ulcers, arthropathy, heart disease and cancer than the rest of the population. PPI users had more frequently received prescriptions for NSAIDS, ASA, oral anticoagulant therapy (OAT) and systemic steroids. In addition, PPI users had been more frequently prescribed an esophagogastroduodenoscopy (EGDscopy) and tests for the diagnosis of H. pylori infection.
Based on the clinical characteristics of the patients, it was possible to determine the prevalence of patients in the two groups who satisfy requirements set forth by Italian Drug Agency rules and who may be eligible for PPI reimbursement. Based on the data available, PPI reimbursement for the protection of gastric damage caused by NSAIDs is applicable to 30% of PPI users, for ulcers or GERD disease it is applicable to 51.6%, for at least one of the two cases it is applicable to 69.3% of the group. One thousand nine hundred and thirty-nine out of 6322 (30.7%) patients do therefore not comply with PPI prescription suitability according to the Italian Drug Agency.
However potential conditions which are eligible for PPI reimbursement are identifiable for peptic or GERD disease in 8.5% of the non-PPI users (equal to 3075 out of 36226 patients) and for the protection of gastric damage caused by NSAIDS in 6.1% of patients (in 2209 out of 36226 patients).
Figure 1 shows, relative to the total study population, the PPI users who comply with PPI prescription suitability for the protection of gastric damage caused by NSAIDS (1896), the PPI users who are not suitable for prescription (1939) and the non-PPI users who are suitable for prescription for the protection of the gastric damage from NSAIDS (2209), relative to the total study population.
Figure 1.
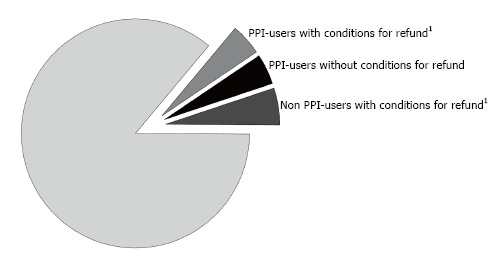
Subgroups of the population of the study divided according the use of proton pump inhibitors and the conditions for refund established by the Italian Drug Agency. 1For the prevention of gastric damage of NSAIDS. Grey: PPI-users with refundable drug according the Italian Drug Agency because of protection of gastric damage by NSAIDS (4.5%); Black: PPI-users without identifiable refundable drug (4.6%); Dark Grey: Non PPI-users with identifiable need of the protection of gastric damage by NSAIDS (5.2%); White: The remaining population (85.7%); PPI: Proton pump inhibitors; NSAIDS: Non steroid inflammatory drugs.
Table 3 shows the characteristics of PPI users who do or do not comply with reimbursement eligibility conditions. The two groups were statistically different in relation to some characteristics. Patients who were potentially eligible for reimbursement were older, were more frequently diagnosed with arthropathy and heart disease and more frequently received NSAID and ASA prescriptions compared with PPI users who do not satisfy eligibility requirements. Also PPI users who comply with reimbursement characteristics were most frequently associated with prescriptions of NSAIDS and ASA. Patients in whom it was not possible to identify conditions related to prescription suitability were more frequently associated with OAT prescriptions.
Table 3.
Characteristics of proton pump inhibitors-users with or without refundable conditions according to the Italian Drug Agency n (%)
| PPI refundable | PPI not refundable | P value | |
| Patients | 4383 | 1939 | |
| Males | 1762 (40.2) | 758 (39.1) | ns |
| Aged over 64 yr | 2967 (67.7) | 935 (48.4) | 0.01 |
| Arthropathy | 2717 (62.0) | 1069 (55.1) | 0.01 |
| Heart disease | 1263 (28.8) | 411 (21.9) | 0.01 |
| Neoplasms | 764 (17.4) | 312 (16.1) | ns |
| Use of NSAIDS | 333 (7.6) | 83 (4.3) | 0.01 |
| Use of ASA | 1857 (42.4) | 150 (7.7) | 0.01 |
| Use of OAT | 255 (5.8) | 245 (12.6) | 0.01 |
| Use of systemic steroids | 201 (4.6) | 105 (5.4) | ns |
| EGDscopy | 1984 (45.3) | 642 (33.1) | 0.01 |
| H. pylori test | 1232 (28.1) | 409 (21.1) | 0.01 |
All the features differ significantly (P < 0.01) between the two groups, except for gender, frequency of neoplasms and use of systemic steroids. PPI: Proton pump inhibitors; GERD: Gastroesophageal reflux disease; Heart disease: Heart failure, coronary ischemic disease, major heart valves disease; NSAIDS: Non steroid inflammatory drugs; ASA: Acetylsalicylic acid; OAR: Oral anticoagulant therapy; ns: Not significant; EGDscopy: Esophageal-gastro-duodenal endoscopy; H. pylori: Helicobacter pylori.
PPI users considered suitable for prescription were more frequently subjected to EGDscopy and tests for the diagnosis of H. pylori infection. No differences were found between the two groups with regard to gender, frequency of malignancies or prescription of systemic steroids.
DISCUSSION
The results of this survey describe the actual prescribing behaviour of a large group of GPs related to the use of PPI drugs.
Unlike other analyses based purely on the assessment of administrative databases, this study allows a connection to be made between the prescription data and the records of clinical diagnoses. The analysis of a database of over 40000 patients allows us to highlight the fact that long-term prescription of PPI drugs is found in almost 15% of the population. These data are not dissimilar to those available on the whole Italian population[5], and show how PPI users present a number of clinical conditions (heart disease, cancer, arthropathy, use of ASA, OAT, systemic steroids, NSAIDS) which characterises them as a potentially fragile population. Epidemiological data showing an association between PPI and clinically dangerous conditions (e.g., ischemic heart disease, renal failure, pulmonary disease) must therefore be interpreted with caution since PPIs could actually be used as markers of fragility (probably not always properly) in populations with a high prevalence of serious diseases[17-19]. It is very difficult to compare the prevalence of PPI users obtained in our work with that of other studies carried out on selected populations and in Countries with different health system, as highlighted by a recent study conducted in Sweden[20].
In any case, the main interest of this study concerns prescription suitability based on the requirements for reimbursement eligibility drawn up by the Italian Drug Agency. The methodology of the study has allowed the identification of potential reimbursement eligibility for 69% of PPI users. This is therefore a significant proportion of patients in an area of apparently poor suitability.
On the other hand over 2000 patients can be identified in the population of non-users who comply with reimbursement eligibility criteria for the prevention of damage caused by NSAIDs and over 3000 patients diagnosed with GERD or gastric/duodenal ulcers in whom the use of PPIs could be appropriate. There is therefore a need to rebalance PPI prescription patterns by reassessing patient characteristics according to overall suitability criteria[21].
The study data have made it possible to better define the characteristics of PPI users who are not suitable for PPI prescription. This population, as well as being comprised of younger patients and a lower prevalence of joint disease, heart disease and NSAIDS use, shows an extremely interesting higher prevalence of OAT use and no differences in the prevalence of cancer or use of systemic steroids.
This could indicate that the most effort to modify treatment in order to promote proper use of PPIs could be made among younger patients using OAT in the absence of further gastric hemorrhagic risk factors.
It is important to note that the current reimbursement eligibility criteria were drafted more than 10 years ago. Without an updated version it is difficult and disadvantageous to use PPIs in clinical conditions which are known to potentially cause serious gastrointestinal bleeding, such as the use of new strategies in antiplatelet and anticoagulation therapy[22] and the use of reuptake inhibitors of serotonin especially in conjunction with ASA and NSAIDS[23].
It should also be noted that these problems are widespread when used by hospital doctors[24-28], but the prescribing behaviour of GPs greatly influences PPI use since they are the main prescribers of the drug[29].
A study of the Italian College of General Practitioners showed that almost half of PPIs are suggested or encouraged by specialists, with different degrees of agreement depending on the disease and the type of specialist[30].
This study has limitations due to the retrospective method and due to the potential of poor accuracy of data logging which is typical to databases. In particular, clinical conditions related to prescription suitability such as the diagnosis of GERD or the use of ASA may not be recorded correctly, as due to their very low cost, some patients prefer to buy them without an NHS-paid prescription.
It should be noted that this study takes into account only the use of PPI and does not take account of the use of other drugs such as receptor antagonists H2.
A substantial number of patients who apparently do not meet prescription suitability conditions can be identified, but among non-PPI users on the contrary, it is possible to identify an equal number of patients for whom prescription would be suitable. It is possible that a large proportion of poor suitability can be identified in the population receiving OAT.
Even taking into account that the current rules of reimbursement eligibility in Italy have not undergone an adequate update in response to changes in the use of potentially gastrolesive medications, there is no doubt scope for decreasing inappropriate use of PPI drugs by adhering to certain criteria.
ACKNOWLEDGMENTS
The authors thank their colleagues who participated in the study: Emanuela Aldrovandi, Loris Brini, Roberto Casadio, Roberto Cau, Corrado Cobianchi, Shirley Ehrlich, Giuliano Ermini, Franco Livio, Angela Inì, Vincenzo La Fratta, Marco Maccaferri, Mara Mori, Massimo Oggianu, Maria Palasciano, Marco Patierno, Anna Rosa Poli, Alberto Serio, Elisabetta Simoncini, Roberto Pierallini, Stefano Quadrelli, Marcello Salera, Anna Maria Savarino, Antonella Silletti, Pietro Speziali, Luigi Spinnato, Stefano Tovoli, Pietro Velonà, Andrea Verri, Donato Zocchi. The study has been carried out thanks to the collaboration of the Bologna Section of the Italian College of General Practitioners and Primary Care.
COMMENTS
Background
Proton pump inhibitors (PPI) are among the most prescribed drugs in the world but it is often difficult to determine the prescription suitability of PPIs. The Italian National Health Service introduced rules to limit the reimbursement of these drugs that were draw up according to the conditions of proven effectiveness and following major international guidelines.
Research frontiers
Most studies showing a wide use of PPIs suspected for an inadequate compliance with the available scientific evidences are based on the analysis of administrative data. These studies cannot fully understand the relationship between clinical characteristics of the patient and the relative drug prescription.
Innovations and breakthroughs
The study clearly shows that most patients who do not meet prescription suitability conditions can be identified in the population receiving anticoagulant treatments. On the contrary, among patients not receiving PPIs, it is possible to identify an equal number of patients for whom prescription would be suitable.
Applications
The findings of this study can help the drug prescribers and the integrated units formed by specialists and general practitioners to identify specific areas of intervention to improve the suitability of the use of this class of drugs.
Terminology
There are five PPIs available in Italy (omeprazole, pantoprazole, lansoprazole, rabeprazole and esomeprazole). This study does not take account of the use of other drugs such as receptor antagonists H2. The population was divided into non occasional users of PPI drugs (PPI users) and non-users (PPI non-users) based on evidence of a prescription of at least 3 packs of PPIs in the last 90 d before analysis (1 pack = 14 tablets).
Peer-review
This is an interesting retrospective analysis of PPI use in Italy assessing adherence to the indications of the guidelines issued by the Italian Drug Agency.
Footnotes
Conflict-of-interest statement: No financial relationships to disclose.
Data sharing statement: No additional data are available.
Manuscript source: Invited manuscript
Specialty Type: Gastroenterology and hepatology
Country of Origin: Italy
Peer-Review Report Classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: December 29, 2016
First decision: March 13, 2017
Article in press: July 17, 2017
P- Reviewer: Blonski W, Castro LA, Koch TR S- Editor: Gong ZM L- Editor: A E- Editor: Lu YJ
References
- 1.Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol. 2013;108:308–328; quiz 329. doi: 10.1038/ajg.2012.444. [DOI] [PubMed] [Google Scholar]
- 2.Hunt RH, Lanas A, Stichtenoth DO, Scarpignato C. Myths and facts in the use of anti-inflammatory drugs. Ann Med. 2009;41:423–437. doi: 10.1080/07853890902887295. [DOI] [PubMed] [Google Scholar]
- 3.Malfertheiner P, Megraud F, O’Morain CA, Atherton J, Axon AT, Bazzoli F, Gensini GF, Gisbert JP, Graham DY, Rokkas T, et al. Management of Helicobacter pylori infection--the Maastricht IV/ Florence Consensus Report. Gut. 2012;61:646–664. doi: 10.1136/gutjnl-2012-302084. [DOI] [PubMed] [Google Scholar]
- 4.Johnson DA, Chilton R, Liker HR. Proton-pump inhibitors in patients requiring antiplatelet therapy: new FDA labeling. Postgrad Med. 2014;126:239–245. doi: 10.3810/pgm.2014.05.2772. [DOI] [PubMed] [Google Scholar]
- 5.Osservatorio Nazionale sull’impiego dei Medicinali. Roma: Agenzia Italiana del Farmaco; 2015. L’uso dei farmaci in Italia. Rapporto Nazionale 2014. [Google Scholar]
- 6.Rotman SR, Bishop TF. Proton pump inhibitor use in the U.S. ambulatory setting, 2002-2009. PLoS One. 2013;8:e56060. doi: 10.1371/journal.pone.0056060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Johansen ME, Huerta TR, Richardson CR. National use of proton pump inhibitors from 2007 to 2011. JAMA Intern Med. 2014;174:1856–1858. doi: 10.1001/jamainternmed.2014.2900. [DOI] [PubMed] [Google Scholar]
- 8.Lam JR, Schneider JL, Zhao W, Corley DA. Proton pump inhibitor and histamine 2 receptor antagonist use and vitamin B12 deficiency. JAMA. 2013;310:2435–2442. doi: 10.1001/jama.2013.280490. [DOI] [PubMed] [Google Scholar]
- 9.Sarkar M, Hennessy S, Yang YX. Proton-pump inhibitor use and the risk for community-acquired pneumonia. Ann Intern Med. 2008;149:391–398. doi: 10.7326/0003-4819-149-6-200809160-00005. [DOI] [PubMed] [Google Scholar]
- 10.Howell MD, Novack V, Grgurich P, Soulliard D, Novack L, Pencina M, Talmor D. Iatrogenic gastric acid suppression and the risk of nosocomial Clostridium difficile infection. Arch Intern Med. 2010;170:784–790. doi: 10.1001/archinternmed.2010.89. [DOI] [PubMed] [Google Scholar]
- 11.Bavishi C, Dupont HL. Systematic review: the use of proton pump inhibitors and increased susceptibility to enteric infection. Aliment Pharmacol Ther. 2011;34:1269–1281. doi: 10.1111/j.1365-2036.2011.04874.x. [DOI] [PubMed] [Google Scholar]
- 12.Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Therap Adv Gastroenterol. 2012;5:219–232. doi: 10.1177/1756283X12437358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ladd AM, Panagopoulos G, Cohen J, Mar N, Graham R. Potential costs of inappropriate use of proton pump inhibitors. Am J Med Sci. 2014;347:446–451. doi: 10.1097/MAJ.0b013e31829f87d5. [DOI] [PubMed] [Google Scholar]
- 14.Haastrup P, Paulsen MS, Zwisler JE, Begtrup LM, Hansen JM, Rasmussen S, Jarbøl DE. Rapidly increasing prescribing of proton pump inhibitors in primary care despite interventions: a nationwide observational study. Eur J Gen Pract. 2014;20:290–293. doi: 10.3109/13814788.2014.905535. [DOI] [PubMed] [Google Scholar]
- 15.Bianco MA, Rotondano G, Buri L, Tessari F, Cipolletta L; Gas. Pro. Italian Group. Gastro-protective strategies in primary care in Italy: the “Gas.Pro.” survey. Dig Liver Dis. 2010;42:359–364. doi: 10.1016/j.dld.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 16.Cahir C, Fahey T, Tilson L, Teljeur C, Bennett K. Proton pump inhibitors: potential cost reductions by applying prescribing guidelines. BMC Health Serv Res. 2012;12:408. doi: 10.1186/1472-6963-12-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomson AB, Sauve MD, Kassam N, Kamitakahara H. Safety of the long-term use of proton pump inhibitors. World J Gastroenterol. 2010;16:2323–2330. doi: 10.3748/wjg.v16.i19.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Savarino V, Dulbecco P, Savarino E. Are proton pump inhibitors really so dangerous? Dig Liver Dis. 2016;48:851–859. doi: 10.1016/j.dld.2016.05.018. [DOI] [PubMed] [Google Scholar]
- 19.Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases - A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14:179. doi: 10.1186/s12916-016-0718-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wallerstedt SM, Fastbom J, Linke J, Vitols S. Long-term use of proton pump inhibitors and prevalence of disease- and drug-related reasons for gastroprotection-a cross-sectional population-based study. Pharmacoepidemiol Drug Saf. 2017;26:9–16. doi: 10.1002/pds.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thiéfin G, Schwalm MS. Underutilization of gastroprotective drugs in patients receiving non-steroidal anti-inflammatory drugs. Dig Liver Dis. 2011;43:209–214. doi: 10.1016/j.dld.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 22.Chan EW, Lau WC, Leung WK, Mok MT, He Y, Tong TS, Wong IC. Prevention of Dabigatran-Related Gastrointestinal Bleeding With Gastroprotective Agents: A Population-Based Study. Gastroenterology. 2015;149:586–595.e3. doi: 10.1053/j.gastro.2015.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Jiang HY, Chen HZ, Hu XJ, Yu ZH, Yang W, Deng M, Zhang YH, Ruan B. Use of selective serotonin reuptake inhibitors and risk of upper gastrointestinal bleeding: a systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2015;13:42–50.e3. doi: 10.1016/j.cgh.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Parente F, Cucino C, Gallus S, Bargiggia S, Greco S, Pastore L, Bianchi Porro G. Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther. 2003;17:1503–1506. doi: 10.1046/j.1365-2036.2003.01600.x. [DOI] [PubMed] [Google Scholar]
- 25.Kelly OB, Dillane C, Patchett SE, Harewood GC, Murray FE. The Inappropriate Prescription of Oral Proton Pump Inhibitors in the Hospital Setting: A Prospective Cross-Sectional Study. Dig Dis Sci. 2015;60:2280–2286. doi: 10.1007/s10620-015-3642-8. [DOI] [PubMed] [Google Scholar]
- 26.Lodato F, Poluzzi E, Raschi E, Piccinni C, Koci A, Olivelli V, Napoli C, Corvalli G, Nalon E, De Ponti F, et al. Appropriateness of Proton Pump Inhibitor (PPI) prescription in patients admitted to hospital: Attitudes of general practitioners and hospital physicians in Italy. Eur J Intern Med. 2016;30:31–36. doi: 10.1016/j.ejim.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 27.van den Bemt PM, Chaaouit N, van Lieshout EM, Verhofstad MH. Noncompliance with guidelines on proton pump inhibitor prescription as gastroprotection in hospitalized surgical patients who are prescribed NSAIDs. Eur J Gastroenterol Hepatol. 2016;28:857–862. doi: 10.1097/MEG.0000000000000634. [DOI] [PubMed] [Google Scholar]
- 28.van Vliet EP, Steyerberg EW, Otten HJ, Rudolphus A, Knoester PD, Hoogsteden HC, van Gelder T, Kuijpers PM, Siersema PD. The effects of guideline implementation for proton pump inhibitor prescription on two pulmonary medicine wards. Aliment Pharmacol Ther. 2009;29:213–221. doi: 10.1111/j.1365-2036.2008.03875.x. [DOI] [PubMed] [Google Scholar]
- 29.Wermeling M, Himmel W, Behrens G, Ahrens D. Why do GPs continue inappropriate hospital prescriptions of proton pump inhibitors? A qualitative study. Eur J Gen Pract. 2014;20:174–180. doi: 10.3109/13814788.2013.844787. [DOI] [PubMed] [Google Scholar]
- 30.Ubaldi E, Tosetti C, Benedetto E, Disclafani G, De Bastiani. Dinamiche prescrittive degli inibitori di pompa protonica. Rivista SIMG. 2009;2:6–8. [Google Scholar]


