Abstract
Objective
Research supports the efficacy and safety of Restrictive Transfusion Protocols (RTP) to reduce avoidable red blood cell (RBC) transfusions, but evidence of their effectiveness in practice is limited. This study assessed whether admission to an intensive care unit (ICU) with an RTP reduces the likelihood of transfusion for adult patients.
Design
Observational study using data from the multi-center, cohort Critical Illness Outcomes Study. Patient-level analyses were conducted with RBC transfusion on day of enrollment as the outcome and admission to an ICU with an RTP as the exposure of interest. Covariates included demographics, hospital course (e.g. lowest hematocrit, blood loss), severity of illness (e.g. SOFA score), interventions (e.g. sedation/analgesia), and ICU characteristics (e.g. size). Multivariable logistic regression modeling assessed the independent effects of RTPs on transfusions.
Setting
59 US ICUs.
Patients
6,027 adult ICU patients.
Interventions
None.
Measurements and Main Results
Of the 59 study ICUs, 24 had an RTP; 2,510 (41.6%) patients were in an ICU with an RTP. The incidence of RBC transfusion among patients with severe (Hct<21%), moderate (Hct: 21–30%), and mild (Hct>30%) anemia in RTP ICUs were 67%, 19%, and 4%, respectively, compared to 60%, 14%, and 2% for those in ICUs without an RTP. Only 27% of transfusions were associated with a hematocrit less than 21%. Adjusting for confounding factors, RTPs independently reduced the odds of transfusion in moderate anemia with an odds ratio of 0.59 (95%CI: 0.36–0.96) while demonstrating no effect in mild (p=0.93) or severe (p=0.52) anemia.
Conclusions
In this sample of ICU patients, transfusions often occurred outside evidence-based guidelines, but admission to an ICU with an RTP did reduce the risk of transfusion in moderately anemic patients controlling for patient and ICU factors. This study supports the effectiveness of RTPs for influencing transfusions in clinical practice.
Keywords: Intensive Care Unit/organization and administration, Critical Care/therapy, Erythrocyte Transfusion, Evidence-Based Medicine, Clinical Protocols
INTRODUCTION
Anemia is a common, life-threatening condition among critically ill patients, and almost 40% of intensive care unit (ICU) patients receive a red blood cell (RBC) transfusion during their stay[1–3]. However, high rates of transfusions are associated with increased cost, infection rate, multi-organ failure, and mortality[1, 4, 5]. Unnecessary transfusions should be avoided to reduce the risk of harm and excess costs.
Clinical studies have demonstrated the efficacy and safety of restrictive transfusion protocols targeting thresholds of hemoglobin less than 7g/dL or hematocrit (Hct) less than 21% for most ICU patients[6–10]. Spurred by this evidence, medical professional organizations have issued evidence-based practice guidelines that reflect these findings, including the Society of Critical Care Medicine in 2009, the Society of Thoracic Surgeons in 2011, and the American Association of Blood Banks in 2012[11–13]. Nevertheless, a significant proportion of RBC transfusions in the ICU continue to occur above recommended thresholds, with adoption varying between hospitals[14, 15].
Evidence suggests that organizational interventions such as clinical protocols are important drivers of optimal ICU care[16–19]. Significant opportunities remain to better characterize the adoption of these strategies and describe their effectiveness on improving of ICU care [20, 21]. RBC transfusion protocols exemplify this with a survey in 2000 reporting that less than 20% of ICUs had transfusion protocols, with no effect on practice detected[2]. Findings from single-center studies of such interventions may be limited in their ability to generalize results to other sites[22–24].
Given the limited understanding of transfusion protocols in routine clinical practice, we assessed whether the presence of a Restrictive Transfusion Protocol (RTP) is independently associated with a lower risk of transfusion for patients in the range of moderate anemia where new evidence discourages transfusion as a default.
MATERIALS AND METHODS
Study Design and Population
We conducted a planned secondary analysis of data accrued by the US Critical Illness and Injury Trials Group – Critical Illness Outcomes Study (CIOS), a multi-center, prospective, observational study. CIOS collected data on structural ICU characteristics and the health status and management of individual patients in 2010 and 2011 to assess the effect of ICU process factors on mortality[25–27]. Our analysis utilized data on ICU and patient factors to evaluate the effectiveness of RTPs in usual care.
All adult patients in one of 59 study ICUs at 8am on survey days were eligible for enrollment, excluding those already enrolled. Patient data were extracted from the medical record regarding baseline characteristics from admission and hospital course during the prior 24 hours. We excluded patients without transfusion status or hematocrit recorded.
Study Variables
The primary outcome was whether a patient had an RBC transfusion during the 24 hours preceding enrollment. The primary exposure was the presence of any RTP in each ICU, defined using the Medline MeSH subject heading of “a precise and detailed plan for a regimen of therapy,” which includes guiding rules initiated by a provider order or included as part of standing orders during admission[25]. RTPs are expected to influence transfusion practice most in the range between customary restrictive (Hct=21%) and liberal (Hct=30%) transfusion thresholds, so RTPs were assessed in three categories of mild (Hct≥30%), moderate (21%≤Hct<30%), and severe (Hct<21%) anemia.
Patient characteristics from demographics, comorbidities, admission diagnoses, operative status, and hospital course were selected based on biological plausibility and previous research. Patient demographics included age, sex, and race (white vs. other). Comorbid illnesses included any history of chronic diseases or specifically, chronic kidney disease or cancer. Admission diagnoses were captured as independent, dichotomous variables for any diagnoses in the central nervous system, circulatory system, respiratory system, or trauma. Patient operative status was classified as post-operative from elective surgery, from emergent surgery, or non-operative.
Hospital course variables from the prior 24 hours included clinical indications of anemia, shock, and factors previously reported to influence transfusion decisions. Severity of illness was described using the Acute Physiology and Chronic Health Evaluation (APACHE) II score[28] and the Sequential Organ Failure Assessment (SOFA) score[29]. Shock was defined for clinical interpretation by a lowest mean arterial blood pressure less than 65mmHg or vasopressor administration. A diagnosis of sepsis or acute kidney injury and use of renal replacement therapy or continuous sedation and analgesia were also included as covariates. Degree of anemia was defined by the lowest hematocrit during the study period as a surrogate for the pre-transfusion hemoglobin[30]. Blood loss was categorized as gastrointestinal bleeding and bleeding from any other source (e.g. procedure, operation, or other).
Hospital and ICU structural characteristics were expected to be significant determinants of transfusion practice. Hospital characteristics of total beds and use of computerized physician order entry were included. The ICU type (medical, surgical, or mixed), bed count, annual admissions, staffing model (open, semi-open, or closed), and total number of protocols used were also considered as potential confounding exposures.
Statistical Methods
Descriptive analyses were performed stratifying by exposure to an RTP and by outcome of transfusion. Missing dichotomous variables were presumed not present in the chart and considered normal per study protocol. Covariates were modeled based on published literature, and when such information was not available, we examined a scatter plot of the covariate and outcome using locally weighted regression to determine appropriate modeling[31]. Unadjusted, bivariate regression analyses were conducted with the odds of transfusion on the patient level for both patient and unit characteristics.
Hospital-level variation in transfusion practice was expected for factors not captured in this study (e.g. regional differences, local initiatives). A random-effects term for hospital was used to account for clustering while optimizing generalizability of an RTP to an ICU. After checking for collinearity, we created a mixed-effect logistic regression model to evaluate the likelihood of transfusion among those in a unit with an RTP vs. one without, independent of patient and organizational factors. We used backward elimination for parsimony considering a 10% change in the RTP parameter as criteria for eliminating a covariate as a confounder from the final model. Surgical and mixed medical-surgical ICUs were forced into the model, demonstrated the same effect, and were collapsed. In this modeling, hematocrit was a continuous covariate using 21 and 30% as knots in a linear spline function, and the resulting three categories were used for the effect of an RTP. A second model with the same covariates, using five indicator variables for categories of hematocrit and RTP effects within each was created to assess the appropriateness of modeling the hematocrit range of 21–30% as continuous.
All participating CIOS sites received IRB approval with a waiver of informed consent[32]. SAS 9.3 software (SAS Institute, Cary, NC) was used for all statistical analyses. Two-sided p-values <0.05 were considered significant.
RESULTS
Participants
Data were collected from 6,179 patients at 36 hospitals in 59 ICUs, of which 23 were medical, 22 surgical, and 14 mixed ICUs (Figure 1). We excluded 25 patients without a documented transfusion status and 127 without a hematocrit or hemoglobin recorded. Of the remaining 6,027 patients, 42% were enrolled at ICUs with an RTP, while the remaining 58% were not exposed to an RTP.
Figure 1.
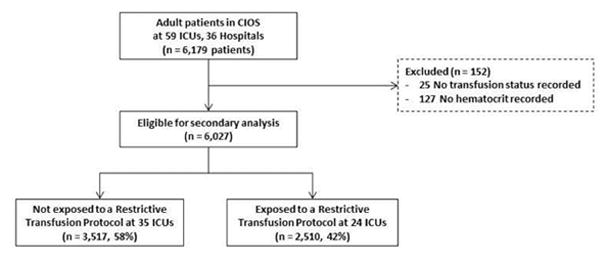
Study flow chart.
In this sample, participants in ICUs with RTPs were older (mean age 61.9 vs. 57.9 years, p<0.0001) and more often white (76 vs. 62%, p<0.0001), with similar APACHE II scores (mean 16.5 vs. 16.7, p=0.40) compared to those in non-RTP ICUs (Table 1). No significant difference existed in the average severity of anemia or frequency of bleeding between the groups. Participants exposed to RTPs were more often post-operative, hypotensive, with acute kidney injury, and on continuous infusion of sedatives or analgesics; those in non-RTP units more often had respiratory diagnoses. Patients in units with RTPs were more often in surgical, smaller, and closed ICUs, and those units had more protocols.
Table 1.
Patient cohort characteristics by exposure to Restrictive Transfusion Protocol (RTP)
| No RTP | RTP | p-value | |
|---|---|---|---|
| No. of patients | 3,517 | 2,510 | |
| No. of ICUs | 35 | 24 | |
| Patient characteristics | |||
| Demographics | |||
| Age, years, mean (SD) | 57.9 (16.7) | 61.9 (17.5) | <0.0001 |
| Male, n (%) | 1,939 (55) | 1,425 (57) | 0.18 |
| White, n (%) | 2,175 (62) | 1,917 (76) | <0.0001 |
| Chronic disease, n (%) | 2,192 (62) | 1,541 (61) | 0.35 |
| Cancer, n (%) | 800 (23) | 600 (24) | 0.29 |
| Chronic kidney disease, n (%) | 481 (14) | 372 (15) | 0.21 |
| Admission diagnoses, n (%) | |||
| Central nervous system | 782 (22) | 451 (18) | <0.0001 |
| Circulatory system | 1,093 (31) | 665 (26) | 0.0002 |
| Respiratory system | 1,419 (40) | 789 (31) | <0.0001 |
| Trauma | 202 (6) | 223 (9) | <0.0001 |
| Operative status, n (%) | |||
| Post-operative, elective | 511 (15) | 447 (18) | 0.0006 |
| Post-operative, emergent | 305 (9) | 277 (11) | <0.0001 |
| Hospital course in prior 24 hours | |||
| Severity of illness, mean (SD) | |||
| APACHE II | 16.7 (7.6) | 16.5 (7.0) | 0.40 |
| SOFA | 5.0 (3.8) | 4.7 (3.6) | 0.010 |
| Lowest Hct (%), mean (SD) | 29.9 (6.6) | 29.6 (6.2) | 0.11 |
| < 21%, n (%) | 205 (6) | 126 (5) | 0.13 |
| ≥ 21% and < 30%, n (%) | 1,674 (47) | 1,252 (50) | |
| ≥ 30%, n (%) | 1,638 (46) | 1,132 (44) | |
| Blood loss, n (%) | |||
| GI bleed | 151 (4) | 101 (4) | 0.62 |
| Other source | 281 (8) | 204 (8) | 0.70 |
| RBC transfusion, n (%) | 393 (11) | 378 (15) | <0.0001 |
| Shock, n (%) | 1697 (48) | 1456 (58) | <0.0001 |
| Sepsis, n (%) | 899 (26) | 509 (20) | <0.0001 |
| Acute kidney injury, n (%) | 628 (18) | 620 (25) | <0.0001 |
| Renal replacement therapy, n (%) | 279 (8) | 173 (7) | 0.13 |
| Continuous infusion of sedative/analgesic, n (%) | 1,045 (30) | 884 (35) | <0.0001 |
| Hospital characteristics | |||
| Hospital beds, mean (SD) | 620 (294) | 705 (272) | <0.0001 |
| CPOE present, n (%) | 2,536 (71) | 2,317 (91) | <0.0001 |
| Study ICU characteristics | |||
| Medical, n (%) | 1,696 (48) | 893 (36) | <0.0001 |
| Surgical or mixed, n (%) | 1,821 (52) | 1,617 (64) | <0.0001 |
| Beds in ICU, mean (SD) | 21.0 (8.9) | 16.3 (6.8) | <0.0001 |
| Annual ICU admissions, mean (SD) | 1,373 (627) | 1,424 (739) | 0.0002 |
| ICU organization, n (%) | |||
| Open units | 491 (14) | 200 (8) | <0.0001 |
| Semi-open units | 891 (25) | 332 (13) | <0.0001 |
| Closed units | 2,135 (61) | 1,978 (79) | <0.0001 |
| Number of protocols in ICU, mean (SD) | 15.2 (5.1) | 21.3 (3.6) | <0.0001 |
APACHE II = Acute Physiology and Chronic Health Evaluation II score; SOFA = Sequential Organ Failure Assessment score; “Blood loss, Other source” includes bleeding during surgery, procedures, or any otherwise documented; CPOE = Computerized Physician Order Entry
Transfusion Outcomes
A total of 771 patients (12.8%) were transfused in the 24-hour study period (Table 2). Of those transfused, the average lowest hematocrit was 23.6%(SD 6.4%). Only 27% were more anemic than the restrictive transfusion threshold (Hct<21%), while 63% had a hematocrit of 21 to 30% (Figure 4 in Supplementary Materials). Non-bleeding patients were transfused with a similar distribution of anemia, 24% having a hematocrit less than 21%. In unadjusted analyses, patients in RTP ICUs received RBCs more often with average transfusion frequencies of 67%, 19%, and 4% among severe (Hct<21%), moderate (Hct: 21–30%), and mild (>30%) anemia, respectively, compared to 60%, 14%, and 2% for those in ICUs without an RTP (Figure 2). Similarly, the presence of an RTP was associated with a higher average nadir hematocrit among those transfused (24.1 vs. 23.0%, p=0.002).
Table 2.
Logistic regression analysis of transfusion risk in relation to independent risk factors of Restrictive Transfusion Protocol and covariates in the CIOS population.
| Odds Ratio (95% CI) | ||||
|---|---|---|---|---|
| Transfused | Not Transfused | Unadjusted | Adjusted | |
| Patient characteristics | n=771 | n=5,256 | ||
| Demographics | ||||
| Age (yr), mean (SD) | 59.7 (16.7) | 59.6 (17.2) | 1.00 (0.96–1.05) | |
| Male, n (%) | 326 (42) | 2,337 (44) | 0.92 (0.81–1.06) | |
| White, n (%) | 558 (72) | 3,534 (67) | 1.24 (1.07–1.43) | |
| Chronic disease, n (%) | 541 (70) | 3,262 (61) | 1.40 (1.22–1.61) | |
| Cancer, n (%) | 231 (30) | 1,169 (22) | 1.50 (1.27–1.78) | |
| Chronic kidney disease, n (%) | 134 (17) | 719 (14) | 1.22 (1.02–1.45) | |
| Admission diagnoses | ||||
| Central nervous system, n (%) | 54 (7) | 1,179 (22) | 0.29 (0.22–0.38) | 0.55 (0.38–0.78) |
| Circulatory system, n (%) | 239 (31) | 1,519 (29) | 1.09 (0.95–1.26) | |
| Respiratory system, n (%) | 223 (29) | 1,985 (38) | 0.70 (0.60–0.81) | 0.77 (0.61–0.96) |
| Trauma, n (%) | 59 (8) | 366 (7) | 1.10 (0.86–1.41) | |
| Operative status, 1 n (%) | ||||
| Post-operative, elective | 145 (19) | 813 (15) | 1.33 (1.10–1.63) | |
| Post-operative, emergent | 94 (12) | 488 (9) | 1.37 (1.09–1.73) | |
| Hospital course in prior 24 hours | ||||
| Severity of illness | ||||
| APACHE II, mean (SD) | 19.4 (7.7) | 16.3 (7.2) | 1.06 (1.05–1.07) | |
| SOFA, mean (SD) | 6.5 (4.3) | 4.7 (3.6) | 1.12 (1.11–1.15) | |
| Lowest Hct (%), mean (SD) | ||||
| < 21% | 18.4 (2.5) | 18.2 (2.8) | 1.03 (0.95–1.13) | 0.98 (0.90–1.06) |
| ≥ 21% and < 30% | 24.4 (2.4) | 26.1 (2.3) | 0.72 (0.69–0.75) | 0.71 (0.64–0.78) |
| ≥ 30% | 33.0 (2.9) | 35.4 (4.5) | 0.83 (0.77–0.90) | 0.89 (0.81–0.97) |
| Blood loss | ||||
| GI bleed, n (%) | 159 (21) | 93 (2) | 20.5 (15.6–27.0) | 15.0 (10.5–21.3) |
| Other, n (%) | 205 (27) | 280 (5) | 8.78 (7.14–10.8) | 6.95 (5.30–9.11) |
| Shock, n (%) | 522 (68) | 2,631 (50) | 2.09 (1.78–2.46) | 1.34 (1.09–1.65) |
| Sepsis, n (%) | 207 (27) | 1,201 (23) | 1.24 (1.04–1.47) | |
| Acute kidney injury, n (%) | 232 (30) | 1,016 (19) | 1.69 (1.46–1.95) | 1.47 (1.17–1.84) |
| Renal replacement therapy, n (%) | 92 (12) | 360 (7) | 1.69 (1.39–2.06) | |
| Continuous infusion of sedative/analgesic, n (%) | 330 (43) | 1,599 (30) | 1.62 (1.42–1.85) | |
| Hospital characteristics | ||||
| Hospital beds, mean (SD) 2 | 696 (283) | 650 (288) | 1.06 (1.03–1.09) | |
| CPOE present, n (%) | 659 (85) | 4,098 (78) | 1.58 (1.30–1.91) | |
| Study ICU characteristics | ||||
| Surgical or mixed, n (%) 3 | 478 (62) | 2,960 (56) | 1.27 (1.08–1.48) | 1.18 (0.87–1.61) |
| Beds in ICU, mean (SD) 4 | 19.1 (8.5) | 18.6 (7.8) | 0.96 (0.92–1.01) | 0.91 (0.79–1.05) |
| Annual ICU admissions, mean (SD) 5 | 1,397 (677) | 1,387 (695) | 0.99 (0.95–1.04) | 1.07 (0.93–1.24) |
| ICU organization 6 | ||||
| Semi-open units, n (%) | 148 (19) | 1,188 (22) | 0.69 (0.53–0.90) | 1.64 (0.91–2.94) |
| Closed units, n (%) | 529 (69) | 3,794 (70) | 0.78 (0.62–0.97) | 1.07 (0.68–1.68) |
| Number of protocols in ICU, mean (SD) | 17.5 (5.4) | 19.0 (5.1) | 1.06 (1.04–1.07) | 1.07 (1.03–1.12) |
| Restrictive transfusion protocol, n (%) | ||||
| For Hct < 21% | 84 (40) | 91 (37) | 1.25 (0.79–2.00) | 1.03 (0.54–1.96) |
| For 21% ≤ Hct < 30% | 250 (51) | 1,002 (41) | 1.52 (1.25–1.85) | 0.59 (0.36–0.96) |
| For Hct ≥ 30% | 44 (59) | 1,088 (40) | 2.17 (1.35–3.47) | 0.86 (0.54–1.36) |
Reference group is non-operative
Odds ratio reported per 100 adult hospital beds
Reference group is Medical ICUs
Odds ratio reported per 5 beds in study ICU
Odds ratio reported per 400 annual ICU admissions
Reference group is Open ICUs
CI = Confidence Interval; APACHE II = Acute Physiology and Chronic Health Evaluation II score; SOFA = Sequential Organ Failure Assessment score; ”Other blood loss” includes bleeding during surgery, procedures, or otherwise documented; CPOE = Computerized Physician Order Entry
Figure 2.
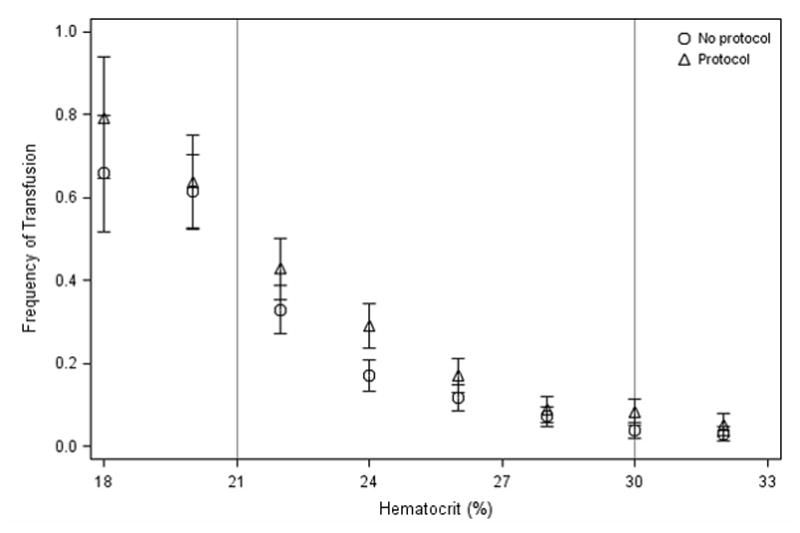
Unadjusted frequency of RBC transfusion by hematocrit, comparing subjects in ICUs with Restrictive Transfusion Protocols vs. ICUs without. Hematocrits (Hct) of subjects are rounded to nearest 2%. Error bars represent the Standard Error of Proportions. Vertical reference lines mark standard hematocrit transfusion thresholds of 21 and 30%, demarcating three categories of mild (Hct≥30%, n=2,770) moderate (21%≤Hct<30%, n=3,053) and severe (Hct<21%, n=331) anemia.
After adjusting for confounding covariates, however, the presence of an RTP was associated with a reduction in the probability of transfusion across the spectrum of anemia (Figure 3A). Further, with hematocrit modeled as a continuous variable, the presence of an RTP was independently associated with a 31% reduction in the odds of transfusion in the intended range (21%≤Hct<30%), where restrictive guidelines recommend against transfusion (AOR=0.59 [95% CI, 0.36–0.96]). Outside this range, where restrictive and liberal strategies agree, there was no association in more anemic or less anemic patients (Figure 3B). Sensitivity analyses excluding bleeding patients did not alter these findings.
Figure 3.
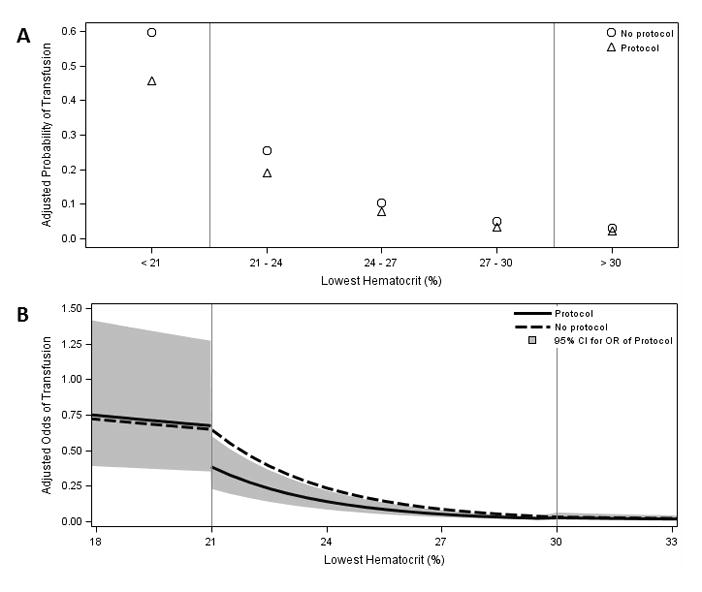
Final adjusted model for RBC transfusion by hematocrit comparing exposure to a Restrictive Transfusion Protocol(RTP) vs. not exposed, adjusting for covariates with (A) hematocrit as categorical variable and the effect of an RTP as independent in each category and (B) hematocrit as a continuous variable with spline knots at hematocrits of 21 and 30% and RTP effects assessed in each range relative to spline knots. Predicted adjusted outcomes were calculated for a patient with mean values for all other covariates. Gray band indicates 95% confidence interval for adjusted odds ratio of transfusion for an RTP. On the study day, 228 (63%) patients with a Hct <21% were transfused, 239 (32%) with Hct 21–23.9%, 152 (13.9%) with Hct 24–26.9%, 82 (7.4%) with Hct 27–29.9%, and 70 (2.6%) with Hct >30%.
In the adjusted model, blood loss was highly associated with an increased odds of transfusion (gastrointestinal bleed AOR=15.0; 95% CI, 10.5–21.3). Differences in hematocrit affected odds of transfusion significantly in the range of 21 to 30% (for 1% change, AOR=0.71; 95% CI, 0.64–0.78). Differences above 30% had a smaller effect, and those less than 21% had no effect. Shock and acute kidney injury were also significant risk factors in the adjusted model, while diagnoses of central nervous or respiratory systems decreased the risk (Table 2).
For ICU covariates in the final model, higher ICU bed-count was associated with a reduced likelihood of transfusion, while annual volume had the opposite association. The number of protocols overall in each ICU was independently associated with transfusion as well (AOR=1.07; 95% CI, 1.03–1.12).
DISCUSSION
This investigation utilized data from a large, multi-center, prospective cohort study to assess the effects of RTPs on the likelihood of transfusion controlling for other patient and ICU factors. Our analysis identified two important findings. First, RBC transfusions remain common in ICUs and continue to occur outside of evidence-based guidelines. Second, the presence of an RTP reduces the odds of transfusion by more than 40% for ICU patients with anemia in the range between the restrictive and liberal guidelines (Hct=21–30%), sparing many avoidable transfusions.
Our study extends existing literature describing the prevalence of anemia and RBC transfusions in critical illness. We demonstrate that RBC transfusions remain common for ICU patients with a daily incidence of nearly 13%, a lower average nadir hematocrit in this study of 23.6% (comparable to Hgb of 7.9 g/dL), and 73% of transfused patients had a nadir hemoglobin greater than 7 g/dL (Figure 4 Supplementary Materials). In two large studies describing transfusion practices in European and US ICUs, Vincent and Corwin reported mean pre-transfusion hemoglobins of 8.4 and 8.6 g/dL, respectively[1, 2]. While direct comparisons are difficult, RBC utilization may be moving closer to evidence-based guidelines.
This study also contributes to research on the effect of ICU characteristics and clinical protocols on transfusions[24]. Practice variation attributable to clinician factors can be seen in differences among institutions adopting new restrictive transfusion evidence[15], and in variations among specialties and individuals[33, 34]. For interventions intended to affect clinicians’ practice, however, prior studies implementing transfusion guidelines or protocols are frequently single center “before-after” studies[23, 35, 36]. Many studies also included other initiatives, like education[37], targeted provider feedback[22, 38, 39], communication with blood bank staff[40], or a combination of these[41–43], reflecting local barriers and solutions.
In unadjusted analyses, ICUs with RTPs provided more transfusions and transfused patients with less severe anemia than ICUs without RTPs, but patients in these ICUs with protocols also had a higher proportion of risk factors for transfusion both in their physiology (e.g. shock) and care setting (e.g. surgical, smaller ICUs). Although clinical indications for each transfusion were not recorded, in the final model, anemia, bleeding, shock, and acute kidney injury are each risk factors for transfusion consistent with clinical practice. Higher volume ICUs have been associated with adoption of restrictive transfusion practices, and we demonstrate a similar effect[15]. However, controlling for volume, larger ICU size is also associated with a decreased likelihood of transfusion, possibly as an adjustment for patient turnover. Total protocols in each ICU was an independent risk factor as well, suggesting that this structural attribute may influence transfusions even when these protocols are not strictly related to blood product use.
In the range between restrictive and liberal thresholds for transfusion, RBC use has been associated with equivalent or worse outcomes in large clinical trials, and of all patients in this sample, 49% were in this range of moderate anemia.{Hebert, 1999 #11} This study quantifies the effect of an RTP for decreasing the daily likelihood of transfusions in this group, where reducing avoidable transfusions represents an improvement toward better, more evidence-based care. Our findings suggest that of the 237 patients transfused with moderate anemia at ICUs without a protocol, an RTP could have reduced that number by 88. Further, we find no effect of RTPs outside this range, supporting the conclusion that clinical protocols effectively change provider behavior toward more restrictive practice. Finally, we also demonstrate that most transfusions still occur above the restrictive threshold, even in the setting of an RTP, suggesting additional opportunities to reduce avoidable risk in current transfusion practice.
Replicable mechanisms, like protocols, to bring new evidence into clinical practice are important to high quality care for ICU patients. In particular, when evidence shows that withholding an intervention reduces the risk of future harm, full adoption faces a unique challenge: the decision not to intervene goes against physiologic reasoning of clinicians. CIOS provides patient and ICU data across many sites allowing for generalizable conclusions about how deliberately “precise and detailed plan(s)” for transfusion decisions affect practice, and in this analysis, RTPs show effectiveness in reducing avoidable transfusions, translating evidence from clinical trials into routine care.
Despite the abundance of information captured, several limitations to this observational study exist. First, these data were collected in 2010 and 2011, and since that time several studies have demonstrated the safety of restrictive transfusion strategies in additional patient populations, which may further drive adoption of restrictive transfusion practice[8, 44–46]. Still, this study demonstrates a delay in translating evidence into clinical practice eleven years after the landmark Transfusion Requirements in Critical Care (TRICC) trial was published, and it investigates the mechanism by which RTPs affect routine clinical practice[6].
Second, unmeasured ICU structure or provider practice patterns may confound the observed association between a transfusion protocol and transfusion decisions. This study utilized a robust dataset with rich organizational data that reduces, but does not eliminate this risk. Future studies should continue to consider these factors and better define those relevant to replicable strategies.
Third, the definition of a protocol used provides one measure for existing interventions but does not capture features within each RTP nor whether protocols were actually used for individual patients[47]. Additionally, other interventions to address appropriate RBC use, such as education and feedback, are frequently implemented in combination with protocols which were not captured for this analysis. Subsequent studies should examine the specific attributes of protocols and their relationship to improving evidence based practice.
Finally, this study was not designed to assess the effect of an RTP on patient outcomes like mortality, but rather on RBC transfusion practice in a single day. The dataset did not capture the number of units given per transfusion or the number of transfusions per admission. Assessing variables in a 24-hour period allows for analysis of clinical decisions but reveals less about total exposure to blood products. Additional studies should consider assessing these outcomes as potential consequences of protocolized practice.
Conclusions
In this sample of critically ill patients, anemia and therapeutic RBC transfusions were very common. These transfusions often occurred above evidence-based thresholds, yet RTPs were associated with an independent reduction in the risk of transfusion for patients with moderate anemia when other patient and ICU factors were taken into consideration. Transfusion protocols may have a significant role in reducing avoidable transfusions, and methods to drive and assess behavior change in transfusion practice deserve continued examination to improve evidence-based care.
Supplementary Material
Distribution of anemia among patients that received an RBC transfusion (n=771).
Acknowledgments
Grant support: Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456.
USCIITG-CIOS investigators:
ARIZONA: University of Arizona Medical Center, Tucson, AZ, Terence O’Keeffe (PI), Coy Collins; Laurel Rokowski; CALIFORNIA: LA County-University of South California Hospital, Los Angeles, CA, Janice Liebler (PI), Ali Ahoui, Anahita Nersiseyan, Usman Shah, Hidenobu Shigemitsu, Nanditha Thaiyananthan; Stanford University Medical Center, Stanford, CA, Joe Hsu (PI), Lawrence Ho; VA Palo Alto Health Care System, Juliana Barr (PI); CONNECTICUT: Bridgeport Hospital, Bridgeport, CT; David Kaufman (PI) Yale University Hospital, New Haven, CT, Jonathan M. Siner (PI), Mark D. Siegel; GEORGIA: Emory University Hospital, Atlanta, GA, Greg S. Martin (PI), Craig Coopersmith, Micah Fisher, David Gutteridge, Mona Brown, Sang Lee, Apryl Smith; Emory University Midtown Hospital, Atlanta, GA, Greg S. Martin (PI), Kenneth Leeper, Mona Brown; Grady Memorial Hospital, Atlanta, GA, Greg S. Martin (PI), Sushma Cribbs, Annette Esper, Mona Brown, David Gutteridge, Olufunmilayo Dosunmu; KANSAS: VA Medical Center, Wichita, KS, Zubair Hassan (PI), Jing Liu, Bart Ridder; ILLINOIS: Northwest Community Hospital, Arlington Heights, IL, Melanie Atkinson (PI), Aimee Draftz, Jackie Durgin, Yelena Rikhman, Jessica Scheckel, Mary Walthers; Saint Francis Hospital, Evanston, IL, Gerald Luger (PI), Carol Downer; University of Illinois Medical Center, Chicago, IL, Ruxana T. Sadikot (PI), Kamran Javaid, Daniel Rodgers, Vibhu Sharma; MARYLAND: Johns Hopkins University, Baltimore, MD, Jon Sevransky (PI), William Checkley, Romer Geocadin, David J. Murphy, Dale Needham, Adam Sapirstein, Steven Schwartz, Glenn Whitman, Brad Winters, Addisu Workneh, Sammy Zakaria; St. Agnes Hospital, Baltimore, MD, Anthony Martinez (PI), Fran Keith; University of Maryland Medical Center, Baltimore, MD, Steven Johnson (PI), Dan Herr, Giora Netzer, Carl Shanholtz, Arabela Sampaio, Jennifer Titus; NIH Clinical Center, Bethesda, MD; Michael Eberlein Suburban Hospital Bethesda, Bethesda, MD, Leo Rotello (PI), Jennifer Anderson; MASSACHUSETTS: Beth Israel Deaconess Medical Center, Boston, MA, Sajid Shahul (PI), Valerie Banner-Goodspeed, Michael Howell, Sabina Hunziker, Victoria Nielsen, Jennifer Stevens, Daniel Talmor; Brigham and Women’s Hospital, Boston, MA, Namrata Patil (PI), Lisa Chin, Michael Myers, Stanthia Ryan; MICHIGAN: St Joseph Mercy Health System, Ann Arbor, MI, Joseph Bander, (PI) University of Michigan Health Systems, Ann Arbor, MI, Pauline K. Park (PI), James M. Blum, Vivek Arora, Kristin Brierley, Jessica DeVito, Julie Harris, Elizabeth Jewell, Deborah Rohner; Kathleen B. To, Sharon Dickinson; MINNESOTA: Mayo Clinic, Rochester, MN, Brian W. Pickering (PI), Jyothsna Giru, Rahul Kashyap, Naman Trivedi; MISSOURI: University of Missouri-Columbia Hospital, Columbia, Missouri; University of Kansas, Kansas City, MO, Timothy Dwyer (PI), Kyle Brownback; NEW JERSEY: University of Medicine and Dentistry of New Jersey, Newark, NJ, Steven Chang (PI), Zaza Cohen, Frank Italiano, Zeeshan Kahn, Amee Patrawalla; NEW MEXICO: Presbyterian Healthcare Services, Albuquerque, NM, Denise Gonzales (PI), Paul Campbell; NEW YORK: Columbia University Medical Center, New York, NY, David Chong (PI), Matthew Baldwin, Luke Benvenuto, Natalie Yip; Memorial Sloan Kettering Cancer Center, New York, NY; Steven M Pastores; University of Rochester Medical Center, Rochester, NY, Anthony Pietropaoli (PI), Kathleen Falkner, Timothy Bouck, Ann Marie Mattingly; NORTH CAROLINA: Wake Forest University Health Science, Winston-Salem, NC, Peter E. Morris (PI), Lori S. Flores; East Carolina University, Greenville, NC, Abid Butt (PI), Mark Mazer, Kelly Jernigan; Moses Cone Health, Greensboro, NC, Patrick Wright (PI), Sarah Groce, Jeanette McLean, Arshena Overton; OHIO: Cleveland Clinic, Cleveland, OH, Jorge A. Guzman (PI), Mohammed Abou El Fadl, Tonya Frederick, Gustavo Cumbo-Nacheli, John Komara; The Ohio State Wexner University Medical Center, Columbus, OH, James M. O’Brien (PI), Naeem Ali, Matthew Exline; PENNSYLVANIA: Eastern Regional Medical Center Cancer Treatment Centers of America, Philadelphia, PA, Jeffrey Hoag (PI), Daniela Albu, Pat McLaughlin; Hahnemann University Hospital, Philadelphia, PA Jeffrey Hoag (PI); Emil Abramian, John Zeibeq; Hospital of the University of Pennsylvania, Philadelphia, PA, Meeta Prasad (PI), Scott Zuick; TENNESSEE: Meharry Medical College Hospital, Nashville, TN, Richard D. Fremont (PI), Chinenye O. Emuwa, Victor C. Nwazue, Olufemi S. Owolabi; Vanderbilt University Medical Center, Nashville, TN, Bryan Cotton (PI), George Hart, Judy Jenkins; Vanderbilt University Medical Center, Nashville, TN, Todd W. Rice (PI), Timothy D. Girard, Margaret Hays, Susan Mogan; TEXAS: University of Texas-Houston Medical Center, Houston, TX; Imo P. Aisiku (PI); UTAH: Intermountain Medical Center, Murray, UT, Samuel Brown (PI), Colin Grissom, Russ Miller III, Anita Austin, Heather Gallo, Naresh Kumar; VIRGINIA: Inova Health Systems, Falls Church, VA, Maryann Putman (PI), Joanne Ondrush.
Footnotes
Conflicts of interest: None
Copyright form disclosures:
Dr. Murphy received support for article research from the National Institutes of Health (NIH). His institution received funding (Supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and TL1TR000456). Dr. Seitz received support for article research from the National Institutes of Health (NIH) and disclosed other (He was supported by a grant from the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000454). Dr. Sevransky received funding from the Food and Drug Administration and received support for article research from the FDA. His institution received funding from the Society for Critical Care Medicine. Dr. Martin received support for article research from the NIH and received funding from CR Bard, Cumberland Pharmaceuticals, and Grifols. His institution received funding from the NIH & FDA, Baxter Healthcare. Dr. Roback disclosed other relationships/activities (unrelated to manuscript): 1) Member, Clinical Transfusion Medicine Committee, American Association of Blood Banks (2014 - present) 2) Chair and Member, NIH/NHLBI Review Group ZRG1 VH-F (55)R, Selected Topics in Transfusion Medicine (2015) 3) Standing Member, NHLBI Program Projects Review Committee (2015 – 2019) 4) Consultant, UnitedPharma, Duluth, GA (MacoPharma US; CLIA 11D1052845) (2006 – 2016) 5) Consultant, Transfusion & Transplantation Technologies LLC (3Ti), Atlanta, GA (CLIA 11D1054838) (2008 – 2016) 6) Consultant, Castle Medical, Inc., Smyrna, GA (CLIA 11D2017949) (2010 – present) 7) Consultant, CSL Plasma, Inc., Decatur, GA (CLIA 11D2064762) (2013 – present) 8) Consultant, BioMet, Warsaw Indiana (2014 – present) 9) Editorial Board: Transfusion (Journal of the American Association of Blood Banks; AABB); 2004 – present).
References
- 1.Vincent JL, et al. Anemia and blood transfusion in critically ill patients. JAMA. 2002;288(12):1499–507. doi: 10.1001/jama.288.12.1499. [DOI] [PubMed] [Google Scholar]
- 2.Corwin HL, et al. The CRIT Study: Anemia and blood transfusion in the critically ill--current clinical practice in the United States. Crit Care Med. 2004;32(1):39–52. doi: 10.1097/01.CCM.0000104112.34142.79. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen BV, et al. Time course of hemoglobin concentrations in nonbleeding intensive care unit patients. Crit Care Med. 2003;31(2):406–10. doi: 10.1097/01.CCM.0000048623.00778.3F. [DOI] [PubMed] [Google Scholar]
- 4.Shander A, et al. Activity-based costs of blood transfusions in surgical patients at four hospitals. Transfusion. 2010;50(4):753–65. doi: 10.1111/j.1537-2995.2009.02518.x. [DOI] [PubMed] [Google Scholar]
- 5.Marik PE, Corwin HL. Efficacy of red blood cell transfusion in the critically ill: a systematic review of the literature. Crit Care Med. 2008;36(9):2667–74. doi: 10.1097/CCM.0b013e3181844677. [DOI] [PubMed] [Google Scholar]
- 6.Hebert PC, et al. A multicenter, randomized, controlled clinical trial of transfusion requirements in critical care. Transfusion Requirements in Critical Care Investigators, Canadian Critical Care Trials Group. N Engl J Med. 1999;340(6):409–17. doi: 10.1056/NEJM199902113400601. [DOI] [PubMed] [Google Scholar]
- 7.Carson JL, et al. Liberal or restrictive transfusion in high-risk patients after hip surgery. N Engl J Med. 2011;365(26):2453–62. doi: 10.1056/NEJMoa1012452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanueva C, et al. Transfusion strategies for acute upper gastrointestinal bleeding. N Engl J Med. 2013;368(1):11–21. doi: 10.1056/NEJMoa1211801. [DOI] [PubMed] [Google Scholar]
- 9.Carson JL, Carless PA, Hebert PC. Transfusion thresholds and other strategies for guiding allogeneic red blood cell transfusion. Cochrane Database Syst Rev. 2012;4:CD002042. doi: 10.1002/14651858.CD002042.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holst LB, et al. Restrictive versus liberal transfusion strategy for red blood cell transfusion: systematic review of randomised trials with meta-analysis and trial sequential analysis. BMJ. 2015;350:h1354. doi: 10.1136/bmj.h1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Napolitano LM, et al. Clinical practice guideline: red blood cell transfusion in adult trauma and critical care. Crit Care Med. 2009;37(12):3124–57. doi: 10.1097/CCM.0b013e3181b39f1b. [DOI] [PubMed] [Google Scholar]
- 12.Society of Thoracic Surgeons Blood Conservation Guideline Task, F., et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–82. doi: 10.1016/j.athoracsur.2010.11.078. [DOI] [PubMed] [Google Scholar]
- 13.Carson JL, et al. Red blood cell transfusion: a clinical practice guideline from the AABB*. Ann Intern Med. 2012;157(1):49–58. doi: 10.7326/0003-4819-157-1-201206190-00429. [DOI] [PubMed] [Google Scholar]
- 14.Netzer G, et al. Transfusion practice in the intensive care unit: a 10-year analysis. Transfusion. 2010;50(10):2125–34. doi: 10.1111/j.1537-2995.2010.02721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy DJ, et al. RBC transfusion practices among critically ill patients: has evidence changed practice? Crit Care Med. 2013;41(10):2344–53. doi: 10.1097/CCM.0b013e31828e9a49. [DOI] [PubMed] [Google Scholar]
- 16.Chang SY, Multz AS, Hall JB. Critical care organization. Crit Care Clin. 2005;21(1):43–53. viii. doi: 10.1016/j.ccc.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 17.Treggiari MM, et al. Effect of intensive care unit organizational model and structure on outcomes in patients with acute lung injury. Am J Respir Crit Care Med. 2007;176(7):685–90. doi: 10.1164/rccm.200701-165OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang SY, Sevransky J, Martin GS. Protocols in the management of critical illness. Crit Care. 2012;16(2):306. doi: 10.1186/cc10578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weled BJ, et al. Critical Care Delivery: The Importance of Process of Care and ICU Structure to Improved Outcomes: An Update From the American College of Critical Care Medicine Task Force on Models of Critical Care. Crit Care Med. 2015;43(7):1520–5. doi: 10.1097/CCM.0000000000000978. [DOI] [PubMed] [Google Scholar]
- 20.Sinuff T, et al. Knowledge translation interventions for critically ill patients: a systematic review*. Crit Care Med. 2013;41(11):2627–40. doi: 10.1097/CCM.0b013e3182982b03. [DOI] [PubMed] [Google Scholar]
- 21.Hart J, Halpern SD. Default options in the ICU: widely used but insufficiently understood. Curr Opin Crit Care. 2014;20(6):662–7. doi: 10.1097/MCC.0000000000000146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohn CS, et al. A data-driven approach to patient blood management. Transfusion. 2014;54(2):316–22. doi: 10.1111/trf.12276. [DOI] [PubMed] [Google Scholar]
- 23.Rana R, AB, Keegan MT. Evidence-based red cell transfusion in the critically ill: quality improvement using computerized physician order entry. Crit Care Med. 2006;34(7):1892–7. doi: 10.1097/01.CCM.0000220766.13623.FE. [DOI] [PubMed] [Google Scholar]
- 24.Tinmouth A, et al. Reducing the amount of blood transfused: a systematic review of behavioral interventions to change physicians’ transfusion practices. Arch Intern Med. 2005;165(8):845–52. doi: 10.1001/archinte.165.8.845. [DOI] [PubMed] [Google Scholar]
- 25.NAA, et al. Critical illness outcome study: an observational study of protocols and mortality in intensive care units. Open Access Journal of Clinical Trials, 2011. 2011;3:55–65. doi: 10.2147/OAJCT.S24223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Checkley W, et al. Structure, process, and annual ICU mortality across 69 centers: United States critical illness and injury trials group critical illness outcomes study. Crit Care Med. 2014;42(2):344–56. doi: 10.1097/CCM.0b013e3182a275d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sevransky JE, et al. Protocols and Hospital Mortality in Critically Ill Patients: The United States Critical Illness and Injury Trials Group Critical Illness Outcomes Study. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000001157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knaus WA, et al. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–29. [PubMed] [Google Scholar]
- 29.Vincent JL, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996;22(7):707–10. doi: 10.1007/BF01709751. [DOI] [PubMed] [Google Scholar]
- 30.Murphy DJ, et al. Red blood cell transfusion practices in acute lung injury: what do patient factors contribute? Crit Care Med. 2009;37(6):1935–40. doi: 10.1097/CCM.0b013e3181a0022d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diggle P, Liang K-Y, Zeger SL. Oxford statistical science series. Oxford New York: Clarendon Press; Oxford University Press; 1994. Analysis of longitudinal data; p. xi.p. 253. [Google Scholar]
- 32.Polito CC, et al. Navigating the institutional review board approval process in a multicenter observational critical care study. Crit Care Med. 2014;42(5):1105–9. doi: 10.1097/CCM.0000000000000133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Frank SM, et al. Variability in blood and blood component utilization as assessed by an anesthesia information management system. Anesthesiology. 2012;117(1):99–106. doi: 10.1097/ALN.0b013e318255e550. [DOI] [PubMed] [Google Scholar]
- 34.Salem-Schatz SR, Avorn J, Soumerai SB. Influence of clinical knowledge, organizational context, and practice style on transfusion decision making. Implications for practice change strategies. JAMA. 1990;264(4):476–83. [PubMed] [Google Scholar]
- 35.McWilliams B, et al. Trends in RBC ordering and use after implementing adaptive alerts in the electronic computerized physician order entry system. Am J Clin Pathol. 2014;141(4):534–41. doi: 10.1309/AJCPEN6VHT0ECAFI. [DOI] [PubMed] [Google Scholar]
- 36.Rothschild JM, et al. Assessment of education and computerized decision support interventions for improving transfusion practice. Transfusion. 2007;47(2):228–39. doi: 10.1111/j.1537-2995.2007.01093.x. [DOI] [PubMed] [Google Scholar]
- 37.Brandt MM, et al. Transfusion insurgency: practice change through education and evidence-based recommendations. American Journal of Surgery. 2009;197(3):279–83. doi: 10.1016/j.amjsurg.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 38.Ansari S, Szallasi A. Blood management by transfusion triggers: when less is more. Blood Transfus. 2012;10(1):28–33. doi: 10.2450/2011.0108-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baer VL, et al. Implementing a program to improve compliance with neonatal intensive care unit transfusion guidelines was accompanied by a reduction in transfusion rate: a pre-post analysis within a multihospital health care system. Transfusion. 2011;51(2):264–9. doi: 10.1111/j.1537-2995.2010.02823.x. [DOI] [PubMed] [Google Scholar]
- 40.Brown RE, et al. Algorithmic and consultative integration of transfusion medicine and coagulation: a personalized medicine approach with reduced blood component utilization. Annals of Clinical & Laboratory Science. 2011;41(3):211–6. [PubMed] [Google Scholar]
- 41.Arnold DM, et al. A multifaceted strategy to reduce inappropriate use of frozen plasma transfusions in the intensive care unit. Journal of Critical Care. 2011;26(6):636.e7–636.e13. doi: 10.1016/j.jcrc.2011.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Sarode R, et al. Prospective monitoring of plasma and platelet transfusions in a large teaching hospital results in significant cost reduction. Transfusion. 2010;50(2):487–92. doi: 10.1111/j.1537-2995.2009.02413.x. [DOI] [PubMed] [Google Scholar]
- 43.Roubinian NH, et al. Trends in red blood cell transfusion and 30-day mortality among hospitalized patients. Transfusion. 2014;54(10 Pt 2):2678–86. doi: 10.1111/trf.12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hajjar LA, et al. Transfusion requirements after cardiac surgery: the TRACS randomized controlled trial. JAMA. 2010;304(14):1559–67. doi: 10.1001/jama.2010.1446. [DOI] [PubMed] [Google Scholar]
- 45.Holst LB, et al. Lower versus higher hemoglobin threshold for transfusion in septic shock. N Engl J Med. 2014;371(15):1381–91. doi: 10.1056/NEJMoa1406617. [DOI] [PubMed] [Google Scholar]
- 46.Robertson CH, JH, Yamal J-M, Gopinath S, Goodman JC, Tilley BC Epo Severe TBI Trial Investigators. Effect of Erythropoietin and Transfusion Threshold on Neurological Recovery After Traumatic Brain Injury: A Randomized Clinical Trial. JAMA. 2014;312(1):36–47. doi: 10.1001/jama.2014.6490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prasad M, et al. The availability of clinical protocols in US teaching intensive care units. J Crit Care. 2010;25(4):610–9. doi: 10.1016/j.jcrc.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Distribution of anemia among patients that received an RBC transfusion (n=771).


