Abstract
AIM
To determine the prevalence of Chlamydia trachomatis (CT) and Neisseria gonorrhea (GC) in young men seeking care in the emergency department (ED) for non-sexually transmitted infection (STI) related symptoms.
METHODS
This was a prospective, cross-sectional study in an urban ED. The main outcome was the rate of positive CT and GC on urine nucleic acid amplification testing in males aged 16-21 presenting with non-STI related complaints.
RESULTS
Two hundred and eighty-four patients were enrolled, 271 were included in the final data analysis [age range 16-21, median: 18 (quartiles 16-18, 19-21)]. Overall, 17 (6.3%, 95%CI: 4%-10%) tested positive for CT and 0% (95%CI: 0%-2%) were found to have GC. The proportion of sexually active subjects was 71% (95%CI: 65%-76%) and 2% (95%CI: 0.6%-4%) reported sex with men. Previous STI testing was reported in 46% (95%CI: 43%-54%) and 13% (95%CI: 8%-20%) of those patients previously tested had a history of STI. Of the patients who tested positive for CT in the ED, 88% (95%CI: 64%-98%) were successfully followed up.
CONCLUSION
The prevalence of CT infection found by screening was 6.3%. Screening and follow-up from the ED was successful. The findings justify routine STI screening in male adolescents presenting to the ED with non-STI related complaints.
Keywords: Chlamydia, Gonorrhea, Adolescent, Public health, Emergency department, Pediatric
Core tip: Chlamydia trachomatis and Neisseria gonorrhea are the most common bacterial sexually transmitted infections (STIs), the sequelae of which are among the most costly of any STI except human immunodeficiency virus infection and acquired immune deficiency syndrome. Disease is often asymptomatic in young males, for whom there is a lack of consensus on screening recommendations and who are screened less often than women. Most studies on emergency department screening focus on young females, or group both asymptomatic and symptomatic patients together. We found 6.3% prevalence of asymptomatic Chlamydia by screening adolescent males who were not seeking screening and would likely not otherwise have been tested.
INTRODUCTION
Chlamydia trachomatis (CT) and Neisseria gonorrhea (GC) are the most common bacterial sexually transmitted infections (STIs) among sexually active adolescents[1]. The sequelae of untreated disease such as ectopic pregnancy and pelvic inflammatory disease are among the most costly of any STI except human immunodeficiency virus infection and acquired immune deficiency syndrome (HIV/AIDS)[2,3]. Risk factors for these diseases include age less than 25, low socioeconomic status, lack of health insurance, African American, high-risk behaviors such as multiple partners, older sex partners for females, alcohol use and lack of condom use[4-7].
Current CDC recommendations do not include screening asymptomatic adolescent males for STIs, focusing instead on sexually active women, men who have sex with men, drug users and prisoners[8]. Their rationale for not regularly screening asymptomatic males is that there are little to no reproductive consequences for patients with asymptomatic disease and therefore no substantial secondary prevention is gained. However, many infectious disease specialists argue that by screening young men the burden of disease among young women (and subsequent reproductive and infectious consequences) can be significantly decreased.
Studies on the prevalence of Chlamydia and Gonorrhea among adolescents largely include males and females, with emergency department (ED) screening studies reporting rates from 4%-14%[1,2,9,10]. Rates are generally reported as higher in females. The lower rate of both Chlamydia and Gonorrhea reported in males is likely due to lower testing rates[11]. Males are tested less often than women in part because they are less likely to have symptoms and also because until recently, the only available method of testing was a painful and invasive urethral swab. With the advent of urine RNA amplification tests, there now exists a non-invasive, inexpensive, highly sensitive and specific screening method[12,13]. While many studies focus on screening females and symptomatic patients in the ED setting, and some focus on screening asymptomatic adult males and females, none that we found focused solely on males seeking care for non-genitourinary (GU) complaints[14,15].
MATERIALS AND METHODS
We performed a prospective, cross-sectional study of adolescent males age 16-21 who presented to either the pediatric ED or the adult fast track area at Kings County Hospital Center, Brooklyn, NY from October 2013 to May of 2015. The State University of New York Downstate institutional review board approved the study protocol.
Enrollment
Only male patients were enrolled by a health care provider or research assistant (to be referred to as “recruiter”). All recruiters were trained in enrollment, informed consent and urine Nucleic Acid Amplification Testing (NAAT) specimen collection.
A recruiter other than the physician primarily responsible for the patient approached subjects during their evaluation and invited them to participate in the study. Patients under 16 or over 21 years of age, those with a chief complaint involving GU symptoms, or Emergency Severity Index score 1-3 (higher acuity) were excluded. While we considered including younger teenagers (age 13 and above) this was not allowed by our institutional review board (IRB) due to concerns that parents of these patients would find the suggestion of sexual activity in this age inappropriate. If a patient agreed to participate, written informed consent was obtained from the subject and their parent or guardian if under 18. In accordance with our IRB’s requirements and New York State law, parents were informed of the nature of the study, but not involved in follow up or informed of results. In other words, they consented to allow their son to receive information about test results and follow up for treatment independently. Results were part of the permanent medical record, but patients were not billed for the testing.
Data collection
Once enrolled and consented to participate in the study, parents/guardians or others present were asked to leave the room. A verbal survey was administered by the recruiter collecting data on age, race, chief reason for visit, follow up contact information, current GU symptoms, previous sexual activity, previous STI testing results and treatment, and history of primary care visits. All questions were asked in laymen’s terms and the answers were recorded in writing by the recruiter. Next, an early stream “dirty catch” specimen was collected and sent for CT and GC testing using urine NAAT testing (gen-probe®). The test is 98.9% sensitive and and 97.4% specific for CT in male urine samples. The coefficient of variation is 7.8%[16].
The ED staff reviewed urine NAAT results as per hospital protocol as soon as results became available (usually within 48-72 h) and patients were contacted at confidential phone numbers provided and treatment arranged if the results were positive. As per hospital protocol, if patients were not contacted successfully by phone after 3 attempts, a telegram was sent to the patient’s address, prompting them to contact the ED for test results.
Statistical analysis
Sample size estimate: Based on previous studies and available public health data we anticipated a positive STI rate of 7%. We calculated a sample size goal of 300 to obtain a prevalence of 7% with a 95%CI between 4% and 10%.
Data analysis
The main outcome was the proportion of positive tests for either CT or GC. Data are presented as medians with quartiles for continuous variables and as percentages with 95%CIs for proportions. Descriptive statistics are used to report additional variables. When applicable, a Fisher exact test was used to report associations. Data analysis was performed using IBM SPSS Statistics for Windows, Version 20.0.
RESULTS
During our study recruitment period a total of 3494 males between age 16 and 21 with ESI levels 4 and 5 presented to the pediatric ED and fast track areas combined. One hundred and nineteen of these patients presented with chief complaints of urinary symptoms, penile discharge, or need for STI testing. We recruited a total of 284 subjects, of whom 13 were not included in the final data analysis due to un-resulted samples (Figure 1). The vast majority of subjects were black (81%), and the majority (73%) were age 16-18 (Table 1 and Figure 2, Figure 3, Figure 4). Due to limitations in staffing and inconsistencies with initial data collection, we were unable to collect data on rates of declination for the entire study period of 19 mo. Over a sample period of 5 mo, 61% (95%CI: 51%-69%) of patients approached agreed to participate.
Figure 1.
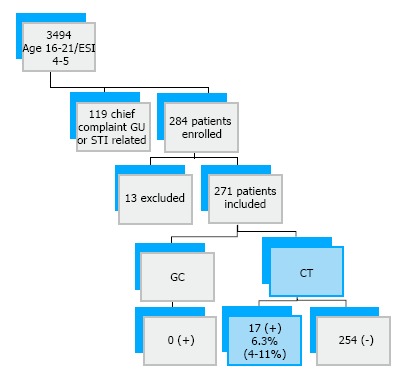
Patient recruitment and results summary. GC: Neisseria gonorrhea; CT: Chlamydia trachomatis; STI: Sexually transmitted infection; GU: Genitourinary.
Table 1.
Participant characteristics
| Characteristic | n (%) |
| Chief complaint | |
| Minor trauma | 129 (48) |
| Acute illness | 100 (35) |
| Other | 40 (14) |
| Age | |
| 16-18 | 208 (73) |
| 19-21 | 62 (22) |
| Ethnicity | |
| Black | 230 (81) |
| Latino | 21 (7) |
| Asian/Indian | 5 (2) |
| White | 3 (1) |
| Other | 11 (4) |
| Sexual activity | 202 (71) |
| Sex with men | 5 (2) |
| Previous STI test | 131 (46) |
| Previous STI | 17 (6) |
| Regular PMD | 201 (7) |
STI: Sexually transmitted infection.
Figure 2.

Chief complaint.
Figure 3.

Ethnicity.
Figure 4.
Age.
Prevalence
Six point three percent (95%CI: 4%-10%) of patients tested positive for CT and 0% tested positive for GC. Seventy-one percent (95%CI: 65%-76%) of patients reported being sexually active. Forty-six percent (95%CI: 43%-54%) reported previous STI testing and 13% of those reported having had an STI in the past. Of those who tested positive, none denied sexual activity. Of those who tested positive for CT in the ED, 88% (95%CI: 64%-98%) were successfully followed up. As expected, the prevalence of chlamydia was significantly higher at 9% (95%CI: 5%-14%) in the subgroup of sexually active patients than in the overall group.
The only patient characteristics with statistically significant associations with positive Chlamydia NAAT were sexual activity and lack of regular primary care (Table 2). All of the patients who tested positive were either Black or Latino, therefore other races were not included in the results table. The proportion of sexually active subjects was significantly higher in the 19-21 year age group than the 16-18 year age group (Table 3 and Figure 5).
Table 2.
Results and significant associations with patient characteristics
| Chlamydia positive n = 17, n (%) | Chlamydia negative n = 271, n (%) | P values Fisher’s exact test | |
| Chief complaint | 0.514 | ||
| Minor trauma | 9 (53) | 120 (44) | |
| Acute Illness | 5 (29) | 90 (35) | |
| Other | 3 (15) | 37 (14) | |
| Age | |||
| 16-18 | 12 (71) | 208 (77) | 0.346 |
| 19-21 | 5 (29) | 57 (21) | |
| Ethnicity | |||
| Black | 16 (94) | 230 (85) | 1.00 |
| Latino | 1 (6) | 20 (7) | |
| Sexual activity (y) | 17 (100) | 172 (68) | 0.008+ |
| Sex with men (y) | 0 (0) | 5 (2) | 1.00 |
| Previous STI test (y) | 11 (65) | 114 (42) | 0.467 |
| Previous STI (y) | 3 (18) | 14 (5) | 0.099 |
| Regular PMD (y) | 9 (53) | 192 (70) | 0.044+ |
STI: Sexually transmitted infection.
Table 3.
Differences in rates of sexual activity by age
| Sexual activity | n (%) |
| 16-18 | 148 (71) |
| 19-21 | 54 (87) |
Sixteen percent of difference in sexual activity rates between 16-18 and 19-21 years old age groups (95%CI: 10%-25%), P = 0.012.
Figure 5.

Differences in rates of sexual activity by age.
DISCUSSION
We found a 6.3% prevalence of Chlamydia and 0% prevalence of Gonorrhea in male patients 16-21 years presenting to the ED for non-STI related complaints. Our study confirms that the burden of disease among sexually active male adolescents is high when compared with public health department estimates. The New York City (NYC) public health department estimates a CT rate of 1343 cases per 100000 men (1.34%) between age 15-19 and 1847 per 100000 in men (1.85%) between age 20 and 24. Gonorrhea rates are estimated to be lower at 319 per 100000 (0.3%) in the 15-19 age group and 567 per 100000 (0.6%) in the 20-24 age group. These are less than half the rates reported in females[8,10,17]. The NYC Department of Health and Mental Hygiene (DOHMH) collects data on all positive tests and relies on reported cases. Additionally, they exclude cases where age or gender information is missing - which could account for the relatively low rates in comparison with our findings.
Our numbers are comparable to studies that focus on targeted screening of undifferentiated symptomatic and asymptomatic male and female ED patients, which cite CT and GC rates in the young adult age group ranging from 4%-14%[1,2,9,14,18].
These numbers indicate that the prevalence of disease in patients seeking care for reasons entirely unrelated to STI screening is significantly high. A sizeable number of patients with disease are likely being missed by not being screened. None of the patients in our study reported GU symptoms on initial presentation, and likely none would have been screened during their ED visit.
Many adolescents have no centralized primary care[19]. They commonly seek health care only for non-preventive reasons, often in the ED setting, and males are even less likely to seek regular primary care then females[20]. The acute care setting is ideal for screening adolescents who do not have regular primary care. Adolescents have been found to be responsive and agreeable to screening for STIs in the ED setting[2,9,21-25]. However, in the current state there is a lack of consensus on whether the ED should provide routine preventive services[26].
One of the challenges to stopping spread of CT is that infections are often asymptomatic in both males and females[8,27,28]. Some studies report as many as 90% of infections in males being asymptomatic[5,21]. Routine screening of all patients is therefore a crucial step in decreasing the burden of disease. The CDC currently recommends annual screening for all females, and biannual screening if high risk[17]. Concise recommendations for males focus mostly on those who are incarcerated and those who have sex with men[17]. We believe these recommendations are insufficient and lead to missed screening opportunities, contributing to spread of an easily treatable disease.
In addition to being feasible, screening efforts in the ED setting have been shown to be cost effective and to decrease the burden of disease[29,30]. Opt-out screening in the ED setting using non-rapid technology in the setting of HIV has been shown to be feasible and effective[31]. Given its success, we view opt-out screening as a potential model for how screening for other STIs such as CT and GC may be implemented.
There are several limitations to our study. Firstly, our sample size is relatively small due to lack of around the clock patient recruitment staff and relatively inconsistent recruitment efforts and training. Early in the study, the training of the enrollers was not as rigorous, and this likely lead to incomplete data collection (missing variables on several patients in Tables 1 and 2). We also did not collect comprehensive data on patients who declined. Over a 5 mo period, when data was collected on enrollment, we found that 39% of patients approached declined screening. We can only speculate that the IRB’s requirement for parental consent contributed since it is higher than other similar studies on STD screening. We presume that younger patients may have not wanted to participate with their parents’ knowledge and likewise some of these declinations could have been due to parental refusal to consent. Conversely, there could have been a selection bias; patients who agreed to participate could have been more likely to engage in risk-taking behavior or had unreported symptoms causing them to choose testing. Our patient population and thus our study sample was largely black and urban, which while consistent with studies of similar microorganisms in similar populations may not be generalizable to rural and more diverse populations in which other STIs we did not test for may be more prevalent[1,2,9,14,18]. Lastly, the study was not powered to assess demographic and behavioral factors associated with having an STI, nor was it powered to assess feasibility, both of which are important ideas for future studies.
To our knowledge, our study is one of the only to date that focuses on targeting asymptomatic males for screening. Although the general prevalence was 6.3%, if the study were translated into an opt-out model for asymptomatic males, it would likely be closer to the 9% found in sexually active patients.
In conclusion, our results support the need for increased STI screening efforts in the acute care setting, particularly among asymptomatic sexually active young men. Increased screening and effective treatment has been shown to reduce both risk-taking behaviors[32,33] and sequelae of disease[2,34]. Young men are well established as a vulnerable population who are likely to miss screening opportunities[35]. Further studies should focus on expanded screening efforts including implementation of opt-out policies. Additionally, more comprehensive and demographic data on declination rates could help develop a targeted screening approach to maximize acceptance rates. Future studies should assess disease reduction as a result of new and improved screening interventions.
COMMENTS
Background
Chlamydia and Gonorrhea are the most common bacterial sexually transmitted infections. The sequelae of untreated disease include abscesses, chronic pelvic inflammation and infertility. Testing and treatment is inexpensive and easy. While screening practices in young women are well established, there is a paucity of concise screening recommendations in young men, in whom infection is often asymptomatic. The study investigates the prevalence of disease in asymptomatic young men being seen in the emergency department (ED) who would otherwise not have been screened.
Research frontiers
Adolescents are a generally healthy population and typically do not have regular primary care where most screening occurs. Particularly in poor communities, the ED is often the only place they are seen and evaluated by physicians and thus is an ideal setting for addressing public health issues.
Innovations and breakthroughs
Screening adolescents for sexually transmitted infections (STIs) in the ED setting has been well studied, however to our knowledge this is the only study focusing solely on young men who are both asymptomatic and presenting for low acuity issues, who might not otherwise have been screened.
Applications
Opt-out testing has been shown to be effective at achieving early diagnosis and treatment of HIV in sexually active adolescents and adults. By showing a significant burden of disease in asymptomatic patients who are not regularly screened in any other setting, they support the use of opt-out testing in the context of bacterial STIs as well as human immunodeficiency virus which could potentially significantly reduce spread of disease.
Terminology
The authors use the word screening to describe testing for a disease as a matter of routine rather than in response to symptomatology. The authors use the term adolescent to describe patients aged 16-21.
Peer-review
This is a very interesting paper.
Footnotes
Institutional review board statement: The State University of New York Downstate institutional review board approved the study protocol.
Informed consent statement: All participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: We have no conflicts of interest to disclose.
Data sharing statement: There is no additional data available.
Manuscript source: Invited manuscript
Specialty type: Pediatrics
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: January 19, 2017
First decision: April 19, 2017
Article in press: June 13, 2017
P- Reviewer: Sergi CM S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Embling ML, Monroe KW, Oh MK, Hook EW 3rd. Opportunistic urine ligase chain reaction screening for sexually transmitted diseases in adolescents seeking care in an urban emergency department. Ann Emerg Med. 2000;36:28–32. doi: 10.1067/mem.2000.105930. [DOI] [PubMed] [Google Scholar]
- 2.Aldeen T, Haghdoost A, Hay P. Urine based screening for asymptomatic/undiagnosed genital chlamydial infection in young people visiting the accident and emergency department is feasible, acceptable, and can be epidemiologically helpful. Sex Transm Infect. 2003;79:229–233. doi: 10.1136/sti.79.3.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levitt MA, Johnson S, Engelstad L, Montana R, Stewart S. Clinical management of chlamydia and gonorrhea infection in a county teaching emergency department--concerns in overtreatment, undertreatment, and follow-up treatment success. J Emerg Med. 2003;25:7–11. doi: 10.1016/s0736-4679(03)00131-8. [DOI] [PubMed] [Google Scholar]
- 4.Gavin L, MacKay AP, Brown K, Harrier S, Ventura SJ, Kann L, Rangel M, Berman S, Dittus P, Liddon N, Markowitz L, Sternberg M, Weinstock H, David-Ferdon C, Ryan G; Centers for Disease Control and Prevention (CDC) Sexual and reproductive health of persons aged 10-24 years - United States, 2002-2007. MMWR Surveill Summ. 2009;58:1–58. [PubMed] [Google Scholar]
- 5.Al-Tayyib AA, Miller WC, Rogers SM, Leone PA, Gesink Law DC, Ford CA, Ellen JM. Health care access and follow-up of chlamydial and gonococcal infections identified in an emergency department. Sex Transm Dis. 2008;35:583–587. doi: 10.1097/OLQ.0b013e3181666ab7. [DOI] [PubMed] [Google Scholar]
- 6.Woodhead N, Chung SE, Joffe A. Protective and risk factors for sexually transmitted infections in middle school students. Sex Transm Dis. 2009;36:280–283. doi: 10.1097/OLQ.0b013e318195c2e3. [DOI] [PubMed] [Google Scholar]
- 7.Suss AL, Homel P, Hammerschlag M, Bromberg K. Risk factors for pelvic inflammatory disease in inner-city adolescents. Sex Transm Dis. 2000;27:289–291. doi: 10.1097/00007435-200005000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Committee on Adolescence. Society for Adolescent Health and Medicine. Screening for nonviral sexually transmitted infections in adolescents and young adults. Pediatrics. 2014;134:e302–e311. doi: 10.1542/peds.2014-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baird A, Green T, King H, Kinghorn G, Kudesia G. Screening for genital Chlamydia trachomatis in teenagers attending a family planning youth clinic: a prevalence study using a strand displacement assay on urine samples. J Fam Plann Reprod Health Care. 2002;28:215–217. doi: 10.1783/147118902101196658. [DOI] [PubMed] [Google Scholar]
- 10.Monroe KW, Weiss HL, Jones M, Hook EW 3rd. Acceptability of urine screening for Neisseria gonorrheae and Chlamydia trachomatis in adolescents at an urban emergency department. Sex Transm Dis. 2003;30:850–853. doi: 10.1097/01.OLQ.0000086600.71690.14. [DOI] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention. Sexually Transmitted Disease Surveillance 2014. Atlanta: U.S. Department of Health and Human Services; 2015. pp. 65–70 [accessed 2017 May 23]. Available from: http://www.cdc.gov/std/stats. [Google Scholar]
- 12.Gaydos CA, Theodore M, Dalesio N, Wood BJ, Quinn TC. Comparison of three nucleic acid amplification tests for detection of Chlamydia trachomatis in urine specimens. J Clin Microbiol. 2004;42:3041–3045. doi: 10.1128/JCM.42.7.3041-3045.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho MK, Lo JY, Lo AC, Cheng FK, Chan FK. Evaluation of replacing the existing diagnostic strategy for Neisseria gonorrhoeae and Chlamydia trachomatis infections with sole molecular testing of urine specimens in a sexually transmitted infection clinic setting. Sex Transm Infect. 2009;85:322–325. doi: 10.1136/sti.2008.035220. [DOI] [PubMed] [Google Scholar]
- 14.Goyal M, Hayes K, Mollen C. Sexually transmitted infection prevalence in symptomatic adolescent emergency department patients. Pediatr Emerg Care. 2012;28:1277–1280. doi: 10.1097/PEC.0b013e3182767d7c. [DOI] [PubMed] [Google Scholar]
- 15.Todd CS, Haase C, Stoner BP. Emergency department screening for asymptomatic sexually transmitted infections. Am J Public Health. 2001;91:461–464. doi: 10.2105/ajph.91.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hologic® Aptima 2 combo test for CT/NG. [accessed 2015 May 4] Available from: http://www.hologic.com/products/clinical-diagnostics-blood screening/assays-and-tests/aptima-combo-2-ctng-assay.
- 17.Bureau of Sexually Transmitted Disease Control. USA: The New York City Department of Health and Mental Hygiene; 2015. 4th Quarter 2008 Quarterly Report; p. [accessed 2015 May 4]. Available from: http://www.nyc.gov/html/doh/downloads/pdf/std/std-quarterlyreport2008-4.pdf. [Google Scholar]
- 18.Monroe KW, Jones M, Desmond R, Hook EW. Health-seeking behaviors and sexually transmitted diseases among adolescents attending an urban pediatric emergency department. Compr Ther. 2007;33:120–126. doi: 10.1007/s12019-007-0011-3. [DOI] [PubMed] [Google Scholar]
- 19.Geisler WM, Chyu L, Kusunoki Y, Upchurch DM, Hook EW 3rd. Health insurance coverage, health care-seeking behaviors, and genital chlamydial infection prevalence in sexually active young adults. Sex Transm Dis. 2006;33:389–396. doi: 10.1097/01.olq.0000194584.80513.4a. [DOI] [PubMed] [Google Scholar]
- 20.Nordin JD, Solberg LI, Parker ED. Adolescent primary care visit patterns. Ann Fam Med. 2010;8:511–516. doi: 10.1370/afm.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marrazzo JM, Scholes D. Acceptability of urine-based screening for Chlamydia trachomatis in asymptomatic young men: a systematic review. Sex Transm Dis. 2008;35:S28–S33. doi: 10.1097/OLQ.0b013e31816938ca. [DOI] [PubMed] [Google Scholar]
- 22.Miller CA, Tebb KP, Williams JK, Neuhaus JM, Shafer MA. Chlamydial screening in urgent care visits: adolescent-reported acceptability associated with adolescent perception of clinician communication. Arch Pediatr Adolesc Med. 2007;161:777–782. doi: 10.1001/archpedi.161.8.777. [DOI] [PubMed] [Google Scholar]
- 23.Sood T, Sally D, Spencer N, Banerjee A, Hinchley G. Feasibility of screening for Chlamydia trachomatis in young men attending an emergency department. Emerg Med J. 2008;25:428–430. doi: 10.1136/emj.2007.054155. [DOI] [PubMed] [Google Scholar]
- 24.Merchant RC, DePalo DM, Liu T, Rich JD, Stein MD. Developing a system to predict laboratory-confirmed chlamydial and/or gonococcal urethritis in adult male emergency department patients. Postgrad Med. 2010;122:52–60. doi: 10.3810/pgm.2010.01.2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marrazzo JM, Ellen JM, Kent C, Gaydos C, Chapin J, Dunne EF, Rietmeijer CA. Acceptability of urine-based screening for Chlamydia trachomatis to asymptomatic young men and their providers. Sex Transm Dis. 2007;34:147–153. doi: 10.1097/01.olq.0000230438.12636.eb. [DOI] [PubMed] [Google Scholar]
- 26.Musacchio NS, Gehani S, Garofalo R. Emergency department management of adolescents with urinary complaints: missed opportunities. J Adolesc Health. 2009;44:81–83. doi: 10.1016/j.jadohealth.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 27.Mehta SD, Rothman RE, Kelen GD, Quinn TC, Zenilman JM. Clinical aspects of diagnosis of gonorrhea and Chlamydia infection in an acute care setting. Clin Infect Dis. 2001;32:655–659. doi: 10.1086/318711. [DOI] [PubMed] [Google Scholar]
- 28.Al-Tayyib AA, Miller WC, Rogers SM, Leone PA, Law DC, Ford CA, Rothman RE. Evaluation of risk score algorithms for detection of chlamydial and gonococcal infections in an emergency department setting. Acad Emerg Med. 2008;15:126–135. doi: 10.1111/j.1553-2712.2008.00027.x. [DOI] [PubMed] [Google Scholar]
- 29.Irvin CB, Nowak B, Moore M, Flynn K, Vretta C. Emergency department Chlamydia screening through partnership with the public health department. Acad Emerg Med. 2009;16:1217–1220. doi: 10.1111/j.1553-2712.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 30.Mehta SD, Bishai D, Howell MR, Rothman RE, Quinn TC, Zenilman JM. Cost-effectiveness of five strategies for gonorrhea and chlamydia control among female and male emergency department patients. Sex Transm Dis. 2002;29:83–91. doi: 10.1097/00007435-200202000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Hoxhaj S, Davila JA, Modi P, Kachalia N, Malone K, Ruggerio MC, Miertschin N, Brock P, Fisher A, Mitts B, et al. Using nonrapid HIV technology for routine, opt-out HIV screening in a high-volume urban emergency department. Ann Emerg Med. 2011;58:S79–S84. doi: 10.1016/j.annemergmed.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 32.Huppert JS, Reed JL, Munafo JK, Ekstrand R, Gillespie G, Holland C, Britto MT. Improving notification of sexually transmitted infections: a quality improvement project and planned experiment. Pediatrics. 2012;130:e415–e422. doi: 10.1542/peds.2011-3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sznitman SR, Carey MP, Vanable PA, DiClemente RJ, Brown LK, Valois RF, Hennessy M, Farber N, Rizzo C, Caliendo A, et al. The impact of community-based sexually transmitted infection screening results on sexual risk behaviors of African American adolescents. J Adolesc Health. 2010;47:12–19. doi: 10.1016/j.jadohealth.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anschuetz GL, Asbel L, Spain CV, Salmon M, Lewis F, Newbern EC, Goldberg M, Johnson CC. Association between enhanced screening for Chlamydia trachomatis and Neisseria gonorrhoeae and reductions in sequelae among women. J Adolesc Health. 2012;51:80–85. doi: 10.1016/j.jadohealth.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Cuffe KM, Newton-Levinson A, Gift TL, McFarlane M, Leichliter JS. Sexually Transmitted Infection Testing Among Adolescents and Young Adults in the United States. J Adolesc Health. 2016;58:512–519. doi: 10.1016/j.jadohealth.2016.01.002. [DOI] [PubMed] [Google Scholar]


