Abstract
There are no detailed, representative, horse-level data about equine management practices in different parts of Canada. To help address this, the demographics, management, and welfare of 312 nonracing horses in Prince Edward Island were examined in a randomized, horse-level survey during summer 2002. Owners completed a pretested questionnaire, and a veterinarian examined each horse. Owners were experienced caregivers and the horses were generally in good condition. Areas for improvement included parasite control, dental and hoof care, and tail docking. The mean fecal egg count was 428 eggs per gram; 76% of owners never removed manure from the pasture. Sixty-two percent of horses had never had a veterinary dental examination. Many horses had hoof defects (excessively long hooves, 26.8%; hoof wall breaks, 32.0%; and white line disease, 8.5%). Many (54.9%) draft horses had docked tails. These results suggest owners might benefit their horses by receiving education in aspects of equine care.
Abstract
Résumé — Démographie, gestion et bien-être des chevaux ne coursant pas à l’Île-du-Prince-Édouard. Il n’existe pas de données détaillées et représentatives se rapportant directement au cheval sur les pratiques de gestions équine dans les différentes régions du Canada. Afin d’aider à corriger cette situation, la démographie, la gestion et le bien-être de 312 chevaux qui ne coursent pas de l’Île-du-Prince-Édouard ont été examinés dans une étude au hasard centrée directement sur le cheval au cours de l’été 2002. Les propriétaires ont complété un questionnaire validé et un vétérinaire a examiné chacun des chevaux. Les propriétaires avaient de l’expérience dans le soin des chevaux et ceux-ci étaient généralement en bonne condition. Les points où une amélioration était souhaitable comprenaient le contrôle des parasites, le soin des dents et des sabots et le coupage de la queue. Le compte moyen des œufs fécaux était de 428 par gramme; 76 % des propriétaires n’enlevait jamais le fumier des pâturages. Soixante-deux pour cent des chevaux n’ avait jamais subit d’examen dentaire par un vétérinaire. Plusieurs chevaux présentaient des anomalies du sabot (sabots excessivement longs, 26,8 %; brisure de la muraille du sabot : 32 %, et maladie de la ligne blanche, 8,5 %). Plusieurs chevaux d’exposition (54,9 %) avaient la queue coupée. Ces résultats permettent de présumer que les propriétaires pourraient améliorer le bien-être de leurs animaux en suivant une formation sur les soins à donner aux chevaux.
Traduit par Docteur André Blouin
Introduction
There is a wide variety of equine management systems in Prince Edward Island (PEI), ranging from intensive boarding facilities with large numbers of horses to backyard establishments that, typically, house fewer horses. Each management system may have positive as well as negative effects on a horse’s welfare. Animal welfare encompasses the state of the animal’s body (physical health) and mind (mental health), and the extent to which its nature (genetically encoded traits reflected in breed and temperament [1]) is satisfied (2). An assessment of welfare must involve a broad spectrum of measurements, because each animal has a multitude of coping mechanisms that may or may not be sufficient for the environment or other influences to which it is subjected (3). Physical welfare may be assessed by physical examination, including body condition score (BCS). One index of potential mental welfare concern is the occurrence of stereotypic behavior (stereotypies). Stereotypies are behavioral patterns that are repetitive, invariant, and apparently functionless (4). They can serve as a marker for reduced equine welfare, because the behaviors indicate frustration (5).
Surveys of equine management have been conducted in the United States (USA) (6) and the United Kingdom (UK) (7,8), but not in Canada. In 1998, a national randomized survey was conducted in the USA to describe equine health and management (6). This survey was done at the barn level and there were no health assessments of individual horses, no data on behavior, and no links were made to welfare. In the UK, a survey was conducted to identify the distribution, management, and level of activity of horses kept in riding stables (8). This study was representative only of horse owners affiliated with veterinary practices, and provided horse-level data on management, but there were no veterinary assessments of health or data on behavior. In Canada, the Canadian Agri-Food Research Council (CARC) has produced national guidelines for the care and handling of horses (9), but it is not known if these guidelines are being followed, because there are no data on how horses are being managed in different parts of Canada. Market research has provided a nonrepresentative profile of the horse industry across the country, but it did not assess welfare directly (10).
The objectives of the present study were (i) to describe the demographics, management, and welfare (physical health, BCS, and occurrence of stereotypies) of a representative sample of nonracing horses (miniature horses, light horses and ponies not used for racing purposes, and draft horses) in PEI, and (ii) to examine factors affecting stereotypic behavior and BCS. This paper presents the descriptive data; a 2nd paper will present the factors that were found to influence 2 welfare endpoints.
Materials and methods
The following protocol was approved by the Research Ethics Board and the Animal Care Committee of the University of Prince Edward Island.
Owners were recruited by a random phone book search. Pages were selected through a computer-generated list of page numbers from the 2001 provincial phone book; all residential phone numbers from the first 2 of the 4 columns on each page were called, from February to August 2002. Informed consent was obtained by mailing interested owners a leaflet describing the study, a consent form, and a stamped return envelope. To encourage participation, the study was publicized on local radio and television stations, and in local newspapers. In addition, interested owners were sent the information in envelopes addressed by hand and stamped rather than franked (11).
The mail questionnaire was designed with 2 sections, each in a booklet format. Section I addressed general information about the owner and stable, including experience with horses, manure disposal, and access to a trailer. Section II pertained to each individual nonracing horse and included questions about its work and exercise, stabling and pasture, dental care, hoof care, deworming, stereotypic behavior, transportation, and feeding. Questions were developed after reviewing comparable surveys (6–8,10) and the CARC guidelines (9). Questions were open-ended, closed-ended, or partially closed-ended, depending on the type of information desired. The questionnaire was pretested with 11 horse owners who had not been recruited for the study. It took approximately 5 min to complete Section I and 10 to 15 min to complete Section II (a copy of the questionnaire is available on request).
The sample size was estimated assuming that the prevalences of stereotypies (12–14) and low BCS were 10% and that the data would provide prevalence estimates within 4% of the true prevalence, 95% of the time. The sample size estimate also allowed for clustering within stables (the “barn effect”; correlation coefficient of r = 0.3) (15) and for a 30% nonresponse rate after enrollment into the study. The calculation indicated that 450 nonracing horses would be required. A previous study had indicated that there are 4.43 nonracing horses per owner in PEI (10), but this appeared a priori to be an overestimate of the current horse population. Consequently, 3 horses per horse owner were assumed and 150 owners were sought.
From June to August 2002, consenting owners were contacted by telephone to arrange a site visit; the questionnaire was mailed to them at least 1 wk before the visit. During the visit, a veterinarian, a technician, and the principal researcher (JC) met the owner at the location where the horse was kept in order to examine the horse and obtain feed and fecal samples. The questionnaire was collected at this time. If the owner had not yet completed the questionnaire, a self-addressed, stamped envelope was provided.
The veterinarian completed a physical examination of the horse(s), which included dental examination, gait evaluation, and BCS; the researcher (JC) also assigned a BCS to each horse. The dental examination involved a visual inspection of the teeth without any devices to hold open the horse’s mouth, but with a flashlight. The gait evaluation consisted of watching the horse walk and trot in 2 directions. Body condition was evaluated on a scale of 1 to 9, with 1 being emaciated and 9 being extremely fat (16). Each horse’s height, body weight, and rectal temperature were recorded. The body weight of light horses was measured by using a Horse and Pony height-weight tape (The Coburn Company, Whitewater, Wisconsin, USA). The girth of draft and miniature horses was measured with a sewing tape and converted into body weight by using a conversion table (17,18). Height was measured by using an aluminum height stick with a liquid level. Rectal temperature was taken by using a digital thermometer (model #5531; Life Brand, Toronto, Ontario). If data could not be obtained (the horse was fractious), they were indicated in the relevant section of the report as missing. The report was signed and dated by the veterinarian and a carbon copy was given to the horse’s owner.
If the horse did not defecate during the visit, manure was taken from the stall or pasture and it was noted where the sample had been taken and if it was fresh. The samples were kept in a cooler containing ice and then in a refrigerator at 5°C until fecal egg counts (FECs) could be performed. The number of strongyle-type eggs was counted by using the Cornell-McMaster dilution egg counting technique (19).
Information about each horse’s feed and the amount of hay or grain fed was obtained. If hay was fed, the amount fed per meal was weighed by using a hanging scale and a sample was taken for analysis by using a uni-forage sampler (Star Quality Samplers, Edmonton, Alberta). Hay samples were obtained from at least 3 different bales. If grain was fed, the amount was weighed by using a digital hand-held hanging scale (Model #160393; Extech Instruments, Waltham, Massachusetts, USA) with a graduation of 28 g (1 oz) and capacity of 15 kg (33 lbs). If the grain was not a commercial preparation, a sample of approximately 0.45 kg (1 lb) was taken for analysis of energy content. An analysis of each horse’s energy requirements was performed by using a specialized equine nutrition program (PC-Horse, Version 1.24; Knut Hove, Agricultural University of Norway, Aas, Norway). Information about the horse’s age, breed, amount of work done, hours spent at grass, body weight, and the amounts and types of feed given were entered into the program, producing a summary of the amount of energy required and the amount provided. The results of the fecal egg count, feed analysis, and nutritional analysis were mailed to each owner with a cover letter in which it was indicated whether the owner should seek veterinary advice because the results of the analysis were outside the normal range.
An abuse policy was formulated in the event that, during site visits, study personnel encountered a horse that appeared to be at risk of physical abuse. The policy was designed to help study personnel to decide whether or not to report the horse’s owner to the PEI Department of Agriculture and Forestry for further investigation. The policy was developed based on advice from a lawyer and 3 ethicists, and on a similar policy for dogs (20). The policy was compiled to reduce the risk of false positives, so that any owners who did provide their animal with adequate care had a minimal chance of being reported.
All questionnaires, veterinary reports, and additional site visit information were coded according to the owner and horse. Data were entered into a computer program (EpiData; The EpiData Association, Odense, Denmark [21]) by 2 people and, after checks for consistency, a single, accurate file was kept. Descriptive statistics were generated from the questionnaire and data from the site visit, using a statistics program (Stata 7; Stata Corporation, College Station, Texas, USA). Clustering of observations (the “farm effect”) was assessed by using a generalized estimating equation. The mean, standard deviation (s), and 95% confidence interval (CI) were calculated, as appropriate. Categorical and dichotomous variables were examined by using frequencies in each group. Pearson’s χ2 was calculated to determine if there was a difference in the prevalence of hoof wall abnormalities and dental abnormalities between the 3 types of horses. The inter-rater (veterinarian, researcher) agreement of BCSs was estimated by using the concordance correlation coefficient (CCC) (22) and Bland and Altman’s limit of agreement plot (23).
Results
Of the 86 643 residential numbers listed in the 2001 PEI telephone book, approximately 10700 (12.3%) numbers were phoned. Respondents in 171 households reported having a nonracing horse, but 54 of these respondents did not participate for the following reasons: 5 owners did not want to learn more about the study; 36 chose not to participate on receipt of the information about the study, because they did not want to commit time to the survey (33/36), they believed that surveys are a waste of time (2/36), or they were not interested in the study (1/36). A further 11 owners did not answer their telephone after being mailed the information, and 2 owners sold their horses after completing the consent form. Thus, the participation rate was 68.4% (117/171) and 312 horses were recruited for the study. All data could not be obtained from some horses for practical reasons: the horses were difficult to handle, feed or fecal samples were not available, or the owners did not provide information. Such data were recorded as missing. The average cluster size was 2.7 and clustering of observations was not a concern (24).
Horses could be broadly classified as miniature, light, and draft types (n = 34, 227, and 51, respectively). The most common breeds are summarized in Figure 1. Many horses were a cross between at least 2 breeds (18.6%, 58/284). In some cases, the breed was unknown (9.9%, 31/284). The mean number of horses owned per person was 2.93, s = 4.1 (95% CI, 2 to 4). Owners reported using the horses for a variety of purposes; the most common uses are summarized in Figure 2 (these data were not mutually exclusive).
Figure 1.
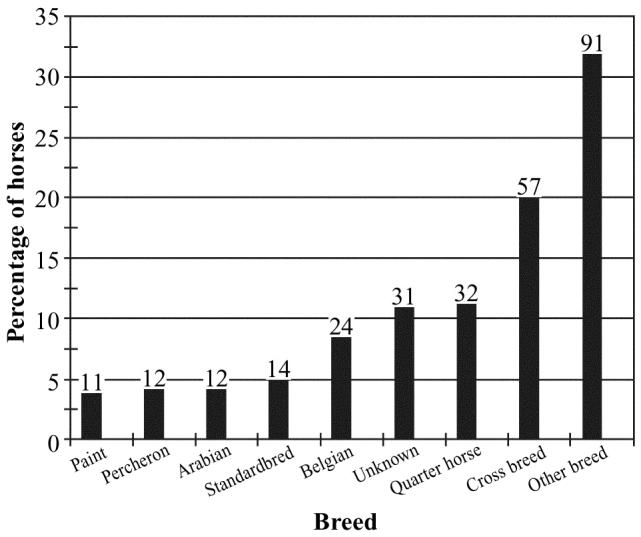
Common breeds represented among 284 nonracing horses in Prince Edward Island. The numbers of horses in each category are shown above the bars.
Figure 2.
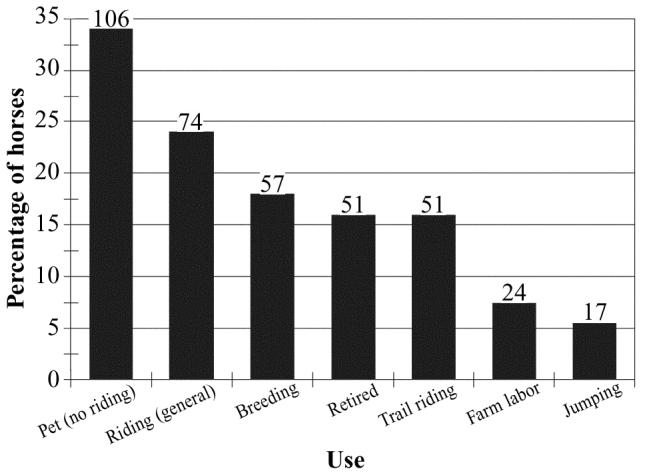
Common uses of 312 nonracing horses in Prince Edward Island (data not mutually exclusive). The numbers of horses in each category are shown above the bars.
Thirty percent (35/110) of owners who completed Section I were members of a horse-related organization. The mean years of experience that owners had of caring for horses, owning horses, and riding or driving horses were 19.1, s = 15.1 (95% CI, 16 to 22), 17.1, s = 13.9 (95% CI, 14 to 20) and 18.0, s = 14.9 (95% CI, 15 to 21), respectively.
The mean BCS as assigned by the veterinarian was 5.7, s = 1.08 (95% CI, 5.6 to 5.9). The inter-rater agreement of BCS between the veterinarian and researcher was 0.85 and the 95% limits of agreement were −1.1 and 1.1; 95% of observation pairs were within approximately 1 point of each other. The mean heart rate, respiratory rate, and rectal temperature were 47.6, s = 11.6 (95% CI, 46 to 49) beats/min, 26.4, s = 12.1 (95% CI, 25 to 28) breaths/min, and 37.6°C, s = 0.41 (95% CI, 37.5°C to 37.6°C), respectively. Eighteen percent (57/311) of horses showed abdominal breathing and 1.3% (4/311) had prominent lower abdominal musculature, commonly called a “heaves line.” Other results from the veterinary examination are summarized in Table 1.
Table 1.
General veterinary findings on physical examination of 312 nonracing horses in Prince Edward Island
| Description | n/total | % | Mean, standard deviation | 95% confidence interval |
|---|---|---|---|---|
| Loose feces | 21/298 | 7.0 | ||
| Dry feces | 6/298 | 2.0 | ||
| FEC (strongyle eggs per gram) | 428, 861 | 332, 524 | ||
| 0 | 104/225 | 46.2 | ||
| 1–300 | 55/225 | 24.5 | ||
| 300 | 66/225 | 29.3 | ||
| Tail docked | ||||
| Draft | 28/51 | 54.9 | ||
| Light | 0/227 | 0 | ||
| Miniature | 0/34 | 0 | ||
| Vibrissae (whiskers) removed | ||||
| Draft | 5/46 | 9.8 | ||
| Light | 20/206 | 8.8 | ||
| Miniature | 4/30 | 11.8 | ||
| Gait irregularity | 54/290 | 18.6 | ||
| Musculoskeletal disease | 26/312 | 8.3 | ||
| BCS | 5.7, 1.1 | 5.6, 5.8 | ||
| BCS equal to or over 6.5 | 89/311 | 28.6 | ||
| BCS equal to or under 3.5 | 6/311 | 1.9 |
FEC — fecal egg count; BCS — body condition score
Eleven percent (32/292) of horses had never had their hooves trimmed or shod by a farrier. Thirteen percent (39/297) of horses wore shoes during the summer. The most common foot problems reported by owners were thrush (8.4%, 25/299), laminitis (5.0%, 15/299), and abscesses (3.0%, 9/299). Navicular syndrome was reported to have occurred in 2.3% (7/299) of horses. Forty-five percent (138/306) of horses had a hoof wall abnormality. Most of these abnormalities were more prevalent in draft horses than in miniature or light horses, with the exception of excessive length of toe (Table 2). Nineteen percent (54/290) of horses had an abnormal gait.
Table 2.
Hoof wall abnormalities found on physical examination of 306 nonracing horses in Prince Edward Island. Categories are not mutually exclusive
| Horse type |
||||||||
|---|---|---|---|---|---|---|---|---|
| Miniature |
Light |
Draft |
||||||
| Hoof wall abnormality | n = 34 | % | n = 221 | % | n = 51 | % | χ2 | P |
| Cracks | 0 | 0 | 48 | 21.7 | 29 | 56.8 | 40.0 | <0.001 |
| Breaks | 3 | 8.8 | 69 | 31.2 | 26 | 51.0 | 16.9 | <0.001 |
| White line disease | 0 | 0 | 20 | 9.1 | 6 | 11.8 | 3.9 | 0.14 |
| Excessive length | 9 | 26.5 | 66 | 29.9 | 7 | 13.7 | 5.5 | 0.064 |
Sixty-three percent (187/298) of horses had never had their teeth examined by a veterinarian. Among the 111 horses whose teeth had been examined, 2.7% (3/111) had their teeth checked more than once per year, 37.8% (42/111) had their teeth checked annually, 33.3% (37/111) had their teeth checked once every 2 to 3 y, and 22.5% (25/111) had their teeth checked less often than once every 3 y. The prevalence of dental abnormalities in 298 horses is summarized in Table 3; the prevalence of wave mouth and molar hooks was significantly higher in draft horses than in the other types. The prevalence of stereotypies (crib-biting, wind-sucking, weaving, wood-chewing, and stall-digging) is summarized in Table 4; 3.4% (10/296) of horses wore a cribbing collar.
Table 3.
Dental abnormalities found on physical examination of 298 nonracing horses in Prince Edward Island. Categories are not mutually exclusive
| Horse type |
||||||||
|---|---|---|---|---|---|---|---|---|
| Miniature |
Light |
Draft |
||||||
| Abnormality | n = 34 | % | n = 213 | % | n = 51 | % | χ2 | P |
| Sharp enamel points | 3 | 8.8 | 19 | 8.9 | 5 | 10.0 | 0.06 | 0.97 |
| Molar hook | 4 | 11.8 | 21 | 9.9 | 15 | 30.0 | 14.1 | 0.001 |
| Wave mouth | 3 | 8.8 | 7a | 3.3 | 0 | 0.0 | 4.84 | 0.09 |
| Teeth missing | 0 | 0 | 4 | 1.9 | 1 | 2.0 | 0.66 | 0.72 |
| Malocclusion | 1 | 2.9 | 5 | 2.4 | 0 | 0.0 | 1.3 | 0.52 |
an = 205
Table 4.
Prevalence of stereotypies in 292 nonracing horses in Prince Edward Island, as indicated by the horse owner
| Behavior | n | % |
|---|---|---|
| Crib-biting | 11 | 3.8 |
| Wind-sucking | 11 | 3.8 |
| Weaving | 14 | 4.8 |
| Wood-chewing | 62 | 21.2 |
| Stall-digging | 13 | 4.5 |
Twenty-three percent (67/297) of horses were never kept in a stall. Among the remaining 77%, the most common bedding materials were straw (68.8%, 159/230) and shavings (26.5%, 61/230). General management practices are summarized in Table 5. Only 38.2% (42/110) of owners had any of their horses vaccinated. Among these horses, the most common vaccine was for prevention of tetanus (85.7%, 36/42). The annual frequency of deworming in 296 nonracing horses is summarized in Figure 3. Approximately 50% of horses were dewormed at least 3 times annually. The most commonly used deworming medications were ivermectin (Eqvalan; Merial Canada, Baie D’Urfe, Quebec) and pyrantel pamoate (Strongid; Pfizer Canada, London, Ontario), with 39.6% and 26.0% horses having had these respective medications in the year 2002, up to the time when the owners completed the questionnaire. Seventy-five percent (82/109) of owners never removed manure from the pasture in which their horses grazed.
Table 5.
Summary statistics of general management practices for 312 nonracing horses in Prince Edward Island. The mean, standard deviation, confidence intervals, and quartiles are presented
| Management practice | Mean | s | CI | 25th percentile | Median | 75th percentile |
|---|---|---|---|---|---|---|
| Number of hours spent in a stall per day (summer) | 5.1 | 7.4 | 4.3, 5.9 | 0 | 0 | 9 |
| Number of hours spent in a stall per day (winter) | 15.0 | 6.5 | 14.3, 16.0 | 11 | 14 | 21 |
| Frequency of farrier care (weeks) | 11.5 | 8.0 | 11.0, 13.0 | 7 | 9 | 13 |
| Number of hours worked per week | 1.9 | 4.6 | 1.4, 2.5 | 0 | 0 | 2 |
Figure 3.
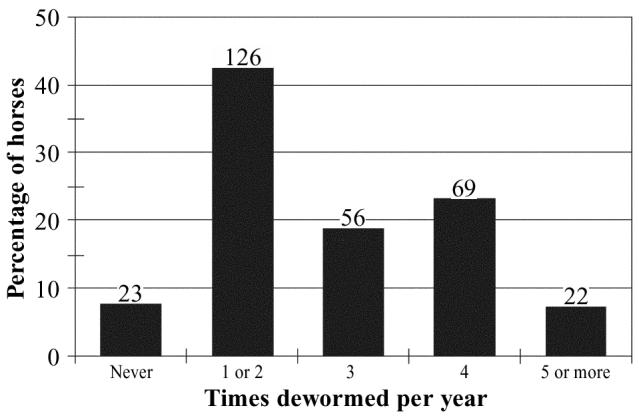
Typical annual frequency of deworming in 296 nonracing horses in Prince Edward Island. The numbers of horses in each category are shown above the bars.
Forty-seven percent (142/299) of horses were reported to have been transported in the previous year. Ten percent (14/142) of these horses were difficult to load (put on the trailer). Among the horses that had been transported at any time, the most common methods of enticing them onto the trailer were with food (28.5%, 35/123), with a lunge line (23.6%, 29/123), or by putting another horse on first (23.6%, 29/123).
Six percent (17/296) of horses were ridden in a martingale. Only 39.3% (117/298) of horses had been ridden or driven with a bit in the 4 wk preceding completion of the questionnaire. The most commonly used bits were snaffle (77.9%, 88/113) and curb (17.9%, 20/112). The others were pelhams and kimberwicks (8.9%, 10/112) and gags (3.6%, 4/112).
Horses were fed forage approximately 1.1, s = 1.3 (95% CI, 1 to 3) times per day in the summer and 2.7, s = 0.93 (95% CI, 2 to 3) times per day in the winter. Fifteen percent of horses had salt added to their feed (45/293), 72.7% (213/293) of horses had access to a salt lick or mineral block, and 17.2% (51/296) of horses were fed supplements. The mean percentage of daily recommended intake of energy that horses received was typically 160.1, s = 54.7 (95% CI, 153.6 to 166.6).
Discussion
To the knowledge of the authors, this is the 1st report of a horse-level study of equine welfare and management in North America and the 1st such study based on a true random sampling protocol. The study has generated new information about nonracing horses in PEI during the summer months, and indicates existing or potential welfare concerns in the following areas: BCS, tail docking, whisker removal, abdominal respiration, parasite control, dental care, hoof wall abnormalities, vaccination, and behavior.
The sample size was approximately 33% lower than estimated; this was because there were fewer horse owners in PEI than anticipated, and locating them took more time than expected. In addition, the average number of horses per owner (2.7) was lower than had been assumed, indicating that previous market research on PEI (10) was not accurate. The present estimate was based on a more representational sample of owners. The smaller sample size reduces the accuracy of the prevalence estimates. Given the high response rate, the results may be generalized to many nonracing horse owners in PEI who have a listed telephone number (11). However, no generalizations may be made about the nonresponders and about horse owners from households without a listed telephone number. Approximately 5% of all PEI households are not listed in the telephone book (M. MacLean, Aliant Telecom, personal communication).
Abuse was not suspected in any animal. The inter-rater agreement for BCS was good. Disagreement may have been because one rater was an experienced veterinarian and the other, while an experienced horse person, had no previous experience in health assessments. There were very few horses with a BCS below 3.5, which suggests that low BCSs were not prevalent in PEI during the summer of the study. The preponderance of high BCSs could have reflected the time of year that the population was sampled, lack of exercise, or overfeeding. The data indicate that horses were being fed more energy than is required, but it was beyond the scope of the study to do a detailed analysis of diet. Obesity may cause laminitis and problems with breeding; it may also affect longevity (25,26). However, a high BCS by itself does not necessarily indicate reduced welfare, unless physical functioning is affected or there are associated constraints on the behavior of the horse.
The CARC guidelines state that tail docking of horses for cosmetic reasons is unacceptable (9). Over half of the draft horses sampled had a docked tail, indicating the need for further research on tail docking procedures, the effects of docking on behavior, and better education of draft horse owners about the CARC guidelines. The present survey did not acquire data on who did the docking, but anecdotal information suggests that horse owners in PEI dock tails by applying an elastic band to the tail (elastration), which constricts blood flow, resulting in necrosis of the tail, which subsequently falls off. Unlike in lambs (27,28) and pigs (29), whose tails are docked to prevent flystrike or tail-biting, respectively, there are no obvious advantages of docking in horses and no studies have been conducted that examine potential benefits to the horse. Hypothetical benefits for owners are eligibility for draft horse competitions, increased cleanliness, and a reduced risk of the tail being caught in equipment. However, docking may involve serious disadvantages to the horse, such as pain associated with the procedure and lack of use of the full tail to dislodge flies. These concerns are comparable with those expressed for dairy cows that have had their tails docked (30).
Horses that are involved in competition frequently have their facial vibrissae clipped and hairs removed from their inner ears to give a neater appearance. In the present study, the practice of removing vibrissae was evident in all 3 types of horses. Effects of vibrissae removal have not been studied. The only available reference was from internet discussions among applied ethologists and others who have termed whisker removal as a cosmetic operation that may cause sensory deficit (31).
A large proportion of horses exhibited abdominal breathing. These data are difficult to interpret, because abdominal respiration may occur when there is an increased effort required for breathing, although such abdominal respiration does not necessarily indicate pulmonary disease. Effort is usually due to painful air movement in the thorax or to decreased pulmonary compliance in chronic respiratory disease (32). The high proportion of nonracing horses with abdominal breathing in PEI may reflect the high environmental temperatures during the months when data were collected. The data may also be associated with obesity, exposure to poor quality (moldy or dusty) hay or straw, or an underlying pathological condition (chronic obstructive pulmonary disease). It was outside the scope of the present study to determine the cause of the abdominal breathing.
The results indicate that strongyles remain a common parasite in nonracing horses in PEI (33). Although strongyle infestation does not appear to reduce BCS, the risk of colic due to strongyles remains a concern (26). The high FECs were consistent with the lack of manure removal from the pasture and an inadequate use of anthelmintics. The CARC guidelines recommend that a parasite control program be established in consultation with a veterinarian and that the program should include pasture management and regular deworming (9). Many owners were not following the guideline on pasture management (9). Transmission of strongyles in PEI is greatest during the grazing season from July to September, and parasite control programs should focus on this period (33).
The average FEC for a herd should remain less than 200 eggs per gram (34). A high FEC in an otherwise healthy horse does not necessarily indicate reduced welfare, but the horse may have an increased risk of parasite-related colic (35). Conversely, a FEC of zero does not necessarily indicate that no intestinal parasites are present; it could indicate that no strongyles were shedding eggs at the time of sampling. The apparent prevalence of strongyle infestation in this study differs from previously reported prevalences (36–38); some of those studies utilized postmortem examinations (the gold standard) and not a FEC, and none was randomized (36–38). A postmortem survey in tropical Australia found that 89% (51/57) of horses were infected with strongyles, although not all of the horses had a sufficiently high number of strongyles to cause harm (36). In a Swedish study, 78% (923/1183) of horses shed strongyle eggs and the output was highest in horses aged 2 and 3 y (37). A necropsy survey of horses in Kentucky found only 2 of 52 horses had evidence of Strongylus vulgaris infestations, leading the authors to believe that the control of this parasite had greatly improved (38). In the present study, small strongyles (cyathostomes) were not distinguished from large strongyles (Strongylus spp.); cyathostomes typically account for 90% to 100% of strongyle-type eggs in equine feces (34,39).
Studies on parasite control methods are less common than studies on prevalence. In a nonrepresentative telephone survey of Thoroughbred trainers in England, 51% (54/106) of stables removed feces from the pasture, but not always with sufficient frequency to prevent pasture contamination by infective larvae (40). Those data suggest that the frequency of manure removal from the pasture may be higher in England than it is in PEI (40), which could indicate a difference in awareness between the 2 populations of caregivers. Regarding anthelmintic use, it was beyond the scope of the present study to examine the doses of anthelmintic given to each horse or the specific patterns of anthelmintic drug use. These would have been useful to determine if owners had been using anthelmintics effectively. Improper use of anthelmintics may lead to cyathostome resistance, an impending problem in horses (41,42).
The CARC guidelines recommend that horses’ teeth be examined at least annually (9); the guideline was not followed by the majority of owners in the study. This is similar to the finding of the American equine survey (6) in which over half of the establishments sampled did not provide any type of dental care. The present research and the American study (6) suggest that North American owners may not be well informed about equine dental care. Owner education is indicated, because dental abnormalities may cause pain when the horse is being ridden (due to interference with the bit) and may interfere with normal eating habits (43).
The frequency of hoof care in the present study was lower than recommended by veterinary guidelines (44,45), perhaps due to a shortage of farriers in PEI or to neglect by owners. However, while many horses had hoof wall abnormalities, few of the abnormalities appeared to cause lameness. In comparison, a nonrepresentative study of hoof wall abnormalities among riding horses in Texas indicated a lower prevalence of hoof wall abnormalities (46). That study had a low response rate (as low as 15% in 1 stable) and the data were obtained by owner reports, which may explain the lower prevalence. In the present study, hoof wall abnormalities were especially common in draft horses, but these horses had a lower frequency of excessive length of hoof than did other types of horses. This may indicate that the hooves of draft horses break off or wear more easily than do the hooves of light or miniature horses.
Vaccination frequency differed from the findings of the US Department of Agriculture National Health Monitoring System (NAHMS) (6) and from Mellor et al (8). The NAHMS (barn level) survey indicated that at least 1 resident equid was not vaccinated against any disease on nearly 40% of operations (6). Mellor et al (8) conducted a horse level study and found that 82% of all horses were vaccinated; this high prevalence of vaccination may have been a reflection of the study population, which did not include horses used for purposes other than riding. The consequence of not vaccinating horses is an increased risk of microbial diseases, but this risk is generally lower in horses that have reduced exposure to other horses.
It was beyond the scope of the study to thoroughly explain stereotypic behavior patterns to respondents, so the estimated prevalences of the 4 behaviors may be inaccurate. Some of the reported behaviors may have been (i) developing stereotypies (not completely invariant or repetitive behavior patterns); (ii) normal conflict behaviors (47); (iii) partly conditioned by the owners’ attention; (iv) in some cases of wood-chewing, the result of nutritional imbalances, such as lack of roughage in the diet and abnormal levels of lactate, propionate, and acetate in the cecum (48). However, with the exception of some cases of wood-chewing, the underlying cause of the above behaviors is thought to be past or present frustration, or motivational conflict; therefore, the behaviors may be said to be valid markers of potential mental welfare concern (49). The prevalences of crib-biting, wind-sucking, and weaving in PEI fell within the range of reported values (12–14). The prevalence of wood-chewing (21.2%) was slightly higher than that of a previous estimate (5.1% to 20%) (50), perhaps because wood-chewing is not always stereotypic.
Even though owners reported many years of experience caring for, riding or driving, and owning horses, there was still evidence of poor management. This is consistent with lack of familiarity with the CARC guidelines. In the authors’ experience, these guidelines are not very accessible and are not well known to many owners; those owners who do have them do not report consulting them often. Representative data on owner usage of the CARC guidelines would clarify this. An alternative to the guidelines, in light of the survey findings, is an educational brochure addressing management factors of concern for nonracing horses in PEI (feeding, deworming, vaccinating, hoof care, and behavior). A brochure has been produced and distributed to the owners who participated, to veterinary clinics across Canada, and to equine interest groups (Caring for your Horse, Animal Welfare Series Brochure #5, Sir James Dunn Animal Welfare Centre, Atlantic Veterinary College, Prince Edward Island).
This study investigated the demographics, management, physical health status, and prevalences of stereotypic behaviors in nonracing horses in PEI. The results indicated that vaccination frequency and the prevalences of wood-chewing and strongyle infection differ from those in previous reports. The results also indicate that the standard of management of nonracing horses in PEI did not meet national guidelines in 4 of the areas examined (parasite control, hoof care, dental care, and tail-docking). Further research is indicated on the effects of whisker removal and tail docking, and the significance of hoof wall diseases in draft horses. CVJ
Footnotes
This research was funded by a grant from the Sir James Dunn Animal Welfare Centre, Atlantic Veterinary College, University of Prince Edward Island.
References
- 1.Rollin B. Animal welfare, science, and value. J Agric Environ Ethics. 1993;6:44–50. [Google Scholar]
- 2.Duncan IJH, Fraser D. Understanding animal welfare. In: Appleby M, Hughes B, eds. Animal Welfare. Wallingford, Oxfordshire, UK: CAB Int, 1997:19–32.
- 3.Broom DM. Physiological indicators of stress and welfare. In: Ethics, Welfare, Law and Market Forces: The Veterinary Interface. Proc Royal Coll Vet Surg & Univ Fed Anim Welfare Symp 1998:167–176.
- 4.Mason GJ. Stereotypies: a critical review. Anim Behav. 1991;41:1015–1037. [Google Scholar]
- 5.Mills DS, Nankervis K. Equine Behavior: Principles & Practice. Oxford, UK: Blackwell Scientific, 1999:134.
- 6.NAHMS. Part I: Baseline Reference of 1998 Equine Health and Management. National Animal Health Monitoring System. Fort Collins, Colorado. United States Department of Agriculture. 1998.
- 7.Mellor DJ, Love S, Gettinby G, Reid SW. Demographic characteristics of the equine population of northern Britain. Vet Rec. 1999;145:299–304. doi: 10.1136/vr.145.11.299. [DOI] [PubMed] [Google Scholar]
- 8.Mellor DJ, Love S, Walker R, Gettinby G, Reid SW. Sentinel practice-based survey of the management and health of horses in northern Britain. Vet Rec. 2001;149:417–423. doi: 10.1136/vr.149.14.417. [DOI] [PubMed] [Google Scholar]
- 9.Canadian Agri-Food Research Council. Recommended Code of Practice for the Care and Handling of Farm Animals: Horses. Ottawa: Canadian Agri-Food Research Council, 1998:6–7.
- 10.Evans V. Canadian Horse Industry Research Study. Ottawa, Canada: Canadian Farm Business Management Council, 1998.
- 11.Salant P, Dillman DA. How to conduct your own survey. New York: John Wiley, 1994.
- 12.Luescher UA, McKeown DB, Dean H. A cross-sectional study on compulsive behaviour (stable vices) in horses. Equine Vet J Suppl. 1998;27:14–18. doi: 10.1111/j.2042-3306.1998.tb05138.x. [DOI] [PubMed] [Google Scholar]
- 13.McGreevy PD, French NP, Nicol CJ. The prevalence of abnormal behaviours in dressage, eventing and endurance horses in relation to stabling. Vet Rec. 1995;137:36–37. doi: 10.1136/vr.137.2.36. [DOI] [PubMed] [Google Scholar]
- 14.McBride SD, Long L. Management of horses showing stereotypic behaviour, owner perception and the implications for welfare. Vet Rec. 2001;148:799–802. doi: 10.1136/vr.148.26.799. [DOI] [PubMed] [Google Scholar]
- 15.Shoukri MM, Martin SW. Estimating the number of clusters for the analysis of correlated binary response variables from unbalanced data. Stat Med. 1992;11:751–760. doi: 10.1002/sim.4780110606. [DOI] [PubMed] [Google Scholar]
- 16.Henneke DR, Potter GD, Kreider JL, Yeates BF. Relationship between condition score, physical measurements and body fat percentage in mares. Equine Vet J. 1983;15:371–372. doi: 10.1111/j.2042-3306.1983.tb01826.x. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy MA, Hoekstra KE. Field method for determining the weight of the miniature horse. World Equine Vet Rev. 1998;3:14–18. [Google Scholar]
- 18.Draft Horse Resource [homepage on the Internet]. Ponca City, Oklahoma; c1997–2001 [updated 2003 Apr 26; cited 2004 Jun 10]. My weight tape; [about 6 screens]. Available from http://www.draftresource.com/Draft_Wt_Tape.html. Last accessed June 10,2004.
- 19.Bowman D. Georgis’ Parasitology for Veterinarians. Philadelphia: WB Saunders, 1999:124,170,172,290,344.
- 20.Patronek G. Tuft’s animal care condition (TACC) scales for assesseing body condition, weather and environmental safety, and physical care in dogs. A manual to aid veterinarians in preventing, recognizing, and verifying animal abuse. Denver, Colorado. Am Humane Assoc, 1997.
- 21.EpiData.dk [homepage on the Internet]. Odense, Denmark: the EpiData Association; c2002–2003 [updated 2004 May 2; cited 2004 Jun 10]. Available from: http://www.epidata.dk. Last accessed June 10, 2004.
- 22.Lin L. A concordance correlation coefficient to evaluate reproductivity. Biometrics. 1989;45:255–268. [PubMed] [Google Scholar]
- 23.Bland J, Altman D. Statistical method for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 24.Hosmer D, Lemeshow S. Applied Logistic Regression. 2nd ed. Toronto: John Wiley, 2000:164–167.
- 25.Bray G. Complications of obesity. Ann Intern Med. 1985;103:1052. doi: 10.7326/0003-4819-103-6-1052. [DOI] [PubMed] [Google Scholar]
- 26.Reed SM, Bayly WM. Equine Internal Medicine. Philadelphia: WB Saunders, 1998:139,230,529.
- 27.French NP, Wall R, Morgan KL. Lamb tail docking: a controlled field study of the effects of tail amputation on health and productivity. Vet Rec. 1994;134:463–467. doi: 10.1136/vr.134.18.463. [DOI] [PubMed] [Google Scholar]
- 28.Molony VJ, Kent JE, Robertson LS. Behavioural responses of lambs of three ages in the first three hours after three methods of castration and tail docking. Res Vet Sci. 1993;55:236–254. doi: 10.1016/0034-5288(93)90087-v. [DOI] [PubMed] [Google Scholar]
- 29.Schrøder-Petersen D, Simonsen H. Tail biting in pigs. Vet J. 2001;162:196–210. doi: 10.1053/tvjl.2001.0605. [DOI] [PubMed] [Google Scholar]
- 30.Tucker C, Fraser D, Weary D. Tail docking in dairy cattle: Effects on cow cleanliness and udder health. J Dairy Sci. 2001;84:84–87. doi: 10.3168/jds.S0022-0302(01)74455-4. [DOI] [PubMed] [Google Scholar]
- 31.Burchard J. Removing of whiskers in horses, International Society of Applied Ethology. 2002. http://www.usask.ca/wcvm/ae/archives/jan01-15.02. 2002. Last accessed Feb 3, 2004.
- 32.Speirs V. Clinical Examination of Horses. Philadelphia: WB Saunders, 1997:27.
- 33.M’Aburi KM. The epidemiology of equine strongylosis in the maritime region of eastern Canada. (MSc thesis) University of Prince Edward Island, 1995.
- 34.Uhlinger CA. Equine small strongyles: epidemiology, pathology and control. Compend Contin Educ Pract Vet. 1991;13:863–869. [Google Scholar]
- 35.Jones SL, Snyder JR, Spier SJ. Obstructive conditions of the large intestine. In: Reed SM, Bayly WM, eds. Equine Internal Medicine. Philadelphia: WB Saunders, 1998:682–694.
- 36.Mfitilodze M, Hutchinson G. Prevalence and abundance of equine strongyles (Nematoda: Strongyloidea) in tropical Australia. J Parasitol. 1990;76:487–494. [PubMed] [Google Scholar]
- 37.Osterman L, Hoglund J, Ljungsrom B, Nilsson O, Uggla A. A field study on the distribution of strongyle infections of horses in Sweden and factors affecting faecal egg counts. Equine Vet J. 1999;31:68–72. doi: 10.1111/j.2042-3306.1999.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 38.Lyons ET, Swerczek TW, Tolliver SC, Bair HD, Drudge JH, Ennis LE. Prevalence of selected species of internal parasites in equids at necropsy in central Kentucky (1995–1999) Vet Parasitol. 2000;92:51–62. doi: 10.1016/s0304-4017(00)00266-1. [DOI] [PubMed] [Google Scholar]
- 39.Love S, Murphy D, Mellor D. Pathogenicity of cyathostome infection. Vet Parasitol. 1999;85:97–122. doi: 10.1016/s0304-4017(99)00092-8. [DOI] [PubMed] [Google Scholar]
- 40.Earle C, Kington H, Coles G. Helminth control used by trainers of thoroughbreds in England. Vet Rec. 2002;150:405–408. doi: 10.1136/vr.150.13.405. [DOI] [PubMed] [Google Scholar]
- 41.Proudman C, Matthews J. Control of intestinal parasites in horses. In Pract 2000:90–97.
- 42.Tarigo-Martinie JL, Wyatt AR, Kaplan RM. Prevalence and clinical implications of anthelmintic resistance in cyathostomes of horses. J Am Vet Med Assoc. 2001;218:1957–1960. doi: 10.2460/javma.2001.218.1957. [DOI] [PubMed] [Google Scholar]
- 43.Baker G. Problems involving the mouth. In: Reed SM, Bayly WM, eds. Equine Internal Medicine. Philadelphia: WB Saunders, 1998:602–608.
- 44.Chase BE. Hoof care review: shoeing and trimming the equine foot. Large Anim Vet 2000:30–34.
- 45.Stashak TS, Hill CH, Klimesh R, Ovnicek G. Trimming and shoeing for balance and soundness. In: Stashak TS, ed. Adams’ Lameness in Horses. Baltimore: Lippincott Williams & Williams, 2002:1081–1143.
- 46.Slater MR, Hood DM. A cross-sectional epidemiological study of equine hoof wall problems and associated factors. Equine Vet J. 1997;29:67–69. doi: 10.1111/j.2042-3306.1997.tb01640.x. [DOI] [PubMed] [Google Scholar]
- 47.Mench JA, Mason GJ. Behaviour. In: Appleby MC, Hughes BO, eds. Animal Welfare. Wallingford, Oxfordshire, UK: CAB Int, 1997:127–142.
- 48.Willard J, Wolfram S, Baker J. Effect of diet on cecal pH and feeding behaviour of horses. J Anim Sci. 1977;45:87. doi: 10.2527/jas1977.45187x. [DOI] [PubMed] [Google Scholar]
- 49.Duncan IJH, Rushen J, Lawrence AB. Conclusions and implications for animal welfare. In: Lawrence A, Rushen J, eds. Stereotypic Animal Behaviour: Fundamentals and Applications to Welfare. Wallingford, Oxfordshire, UK: CAB Int, 2003:193–206.
- 50.Pell SM, McGreevy PD. Prevalence of stereotypic and other problem behaviours in thoroughbred horses. Aust Vet J. 1999;77:678–679. doi: 10.1111/j.1751-0813.1999.tb13166.x. [DOI] [PubMed] [Google Scholar]


