Abstract
Results of a survey of veterinarians in British Columbia included 25 past cases of myiasis and 10 active cases. Most respondents received at least 5 to 10 cases per year, with some as high as 30 per year. This study revealed some advantages and disadvantages of using forensic entomology in living animals.
Abstract
Résumé — Myiase chez des animaux de compagnie en Colombie-Britannique : possibilité que l’utilisation de l’entomologie en médecine légale puisse servir à la détermination de la durée d’éventuels manque de soins. Les résultats d’une enquête chez des vétérinaires de Colombie-Britannique comprenaient 25 anciens cas de myiase et 10 cas actifs. La majorité des répondants recevaient au moins de 5 à 10 cas par année et certains jusqu’à 30 cas. Cette étude révèle certains avantages et inconvénients de l’utilisation de l’entomologie en médecine légale chez des animaux vivants.
Traduit par Docteur André Blouin
Introduction
Myiasis is the infestation of living vertebrate animals with dipteran larvae, which, at least for a period of time, feed on the host’s dead or living tissue, body fluids, or ingested food (1). This usually occurs in animals when an injury or the presence of excretory material makes the living animal attractive to insects. Adult flies are attracted to the wound or excrement and lay eggs on the animal, which develop in the tissue. Myiasis often occurs in wild animals, but it also occurs in domestic animals and even humans. In domestic animals, it results in a well-recognized and economically damaging problem; in sheep, for example, “blow fly strike” results in hundreds of thousands of dollars worth of damage to the industry every year (2–4).
In humans, myiasis is often the result of neglect and is most commonly found in the very old, the very young, or others who are unable or unwilling to ensure basic hygiene and wound cleanliness (5–18). In most cases, the insects are colonizing a living person and feeding on dead, rather than living, tissue. The ability of such insects to remove dead tissue and clean wounds, while leaving the living tissue intact, has been used in medicine for centuries (19, 20). The popularity of maggot debridement therapy declined with the discovery of antibiotics, but in the last 20 y, its use has been revived and it is now considered to be a viable alternative to surgery in certain cases (20–24).
In pet animals, myiasis can occur when a wound is left untreated or when neglect results in the accumulation of feces or urine, which then attracts flies. Flies attracted to the wound lay eggs. These eggs hatch after a predictable period of time into 1st instar larvae (maggots). These tiny maggots feed on liquid protein for a set time and then moult to form the 2nd instar larvae, which feed for a further period and then moult to the 3rd and final larval instar. The insect in this stage feeds voraciously for a set time and then leaves the food source in search of a safe and dry place in which to pupate. Away from the animal, the pupa forms an outer pupal case and metamorphosis occurs. After a few days, the adult fly emerges and the empty puparium is left behind as evidence that this cycle has occurred.
Forensic entomology is the application of the study of insects to law. It is usually used to determine elapsed time since death in a homicide victim (25), but it has also be used to determine time of death of illegally killed wildlife (26) and length of time of neglect in humans (18–27). Insects develop through a predictable life-cycle at a predictable rate, based primarily on temperature and species. Therefore, if the temperature, species, and stage of insect are known, an entomologist can determine how long the insects have been on the body and, therefore, the minimum time that has elapsed since death. This science is equally applicable to living humans or animals and can be used to determine the minimum time since abuse or the length of time of neglect or abuse. In a case in Hawaii, the age of the maggots in a diaper was used in the conviction of a mother for neglecting and abandoning her baby (27).
The objectives of this current study were to determine whether myiasis in pet animals was common in British Columbia and whether forensic entomology could be of value in determining length of time of neglect in such cases.
Materials and methods
A questionnaire was published in the spring newsletter of the British Columbia Veterinary Medical Association (2002), which is sent to all veterinarians in British Columbia. The questionnaire served 2 functions: 1) to determine the prevalence of larval infestations on animals previously brought to the attention of veterinarians, referred to as past cases, and 2) to solicit the submission of insects collected from active myiasis, subsequent cases.
The 1st part of the questionnaire was used to determine factors related to previous cases of myiasis that had been brought to a clinic and to provide an estimate of the number of previous cases for which details were no longer available. Questions included location of the clinic, the number of annual myiasis cases, the species of animal affected, the condition of the animal, the circumstances of the case, whether the animal was kept primarily indoors or outdoors, and any other pertinent details of the case.
The 2nd part of the questionnaire requested that any new cases of myiasis recorded over the subsequent season be submitted for analysis; insects from the animal were to be collected, preserved in ethanol, and sent to the Forensic Entomology Laboratory (FEL) at Simon Fraser University, together with details of the case.
All insects received at the FEL were examined, catalogued, and identified as to species and developmental stage, in a manner similar to that in a legal investigation (18). The 10 cases in which actual insects were sent by the veterinarian were referred to as “active cases.” The number of insects submitted for each case varied, as did whether the sample had been preserved in alcohol or kept alive. The age of the oldest maggots was then determined by using published developmental records for Phaenicia sericata and Lucilia illustris and the surface body temperature of the species (28,29).
Results
The details of 25 past cases were received from 9 small animal clinics and 1 government veterinarian. Most clinics reported having received 5 to 10 cases of myiasis per year, with some reporting as many as 30 cases per year. Over the following insect season, details of the case history and preserved or live larvae removed from the animal in 10 active cases of myiasis from 6 different clinics were referred to the FEL. Cases were reported from all areas of southern British Columbia, with the majority of past cases being reported from the Victoria area of Vancouver Island. Active cases were received from southern and central British Columbia. Figure 1 indicates the geographic location of veterinary clinics that reported both past and active cases of myiasis in pet animals.
Figure 1.
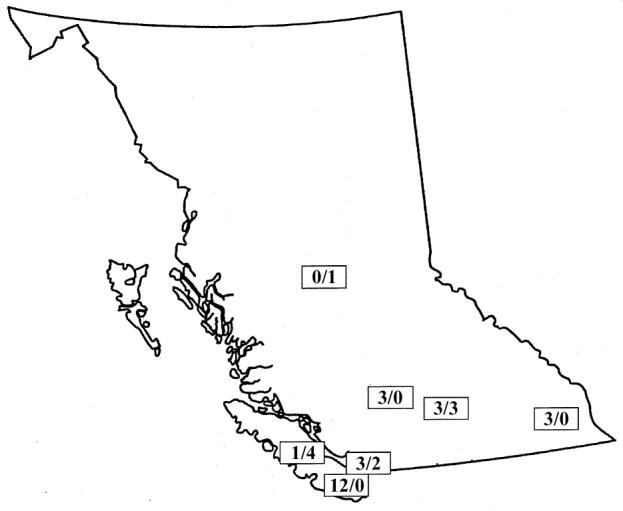
Map of British Columbia, indicating site of veterinary clinics reporting cases, numbers of cases submitted from past records (first number), and number of active cases submitted to the Forensic Entomology Laboratory at Simon Fraser University (second number).
Figure 2 indicates the species and percentages of cases of animals that were presented with myiasis to veterinary clinics. The majority of animals presented with myiasis were dogs, followed by cats, rabbits, and horses. In 1 case, an otter with myiasis was brought to the veterinarian. Dog breeds affected included spaniels (cocker and springer), German shepherds, a golden retriever, collie and collie crosses, an Old English sheepdog, a Bouvier des Flanders, and several mixed breeds. Cats that were presented with myiasis were usually domestic longhair. Veterinarians also reported seeing cases of myiasis in cattle, swallows, and small wild animals, such as voles, that were brought into the clinic.
Figure 2.
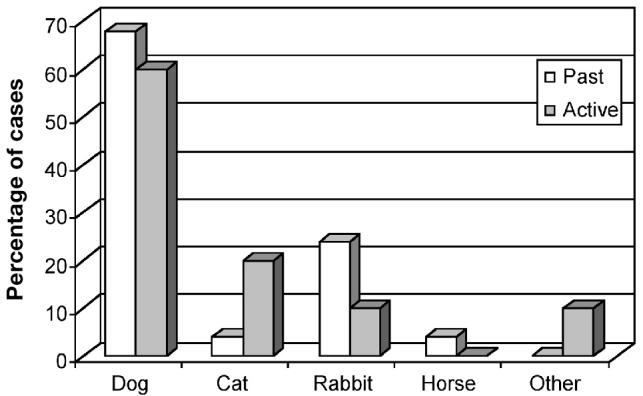
Species of animals presented with myiasis at veterinary clinics in British Columbia.
Figure 3 indicates conditions reported by the veterinarian that were considered to be related to the initiation of myiasis. In most cases, matted hair, together with the consequent buildup of urine and feces in the coat, initiated the 1st insect strike (1). In many cases, wounds were present; in some cases, a bacterial skin infection or estrus probably initiated the strike. Sometimes, the veterinarian indicated specifically that an animal was elderly and that its advanced age had led to a debilitated state that had promoted the onset of myiasis.
Figure 3.
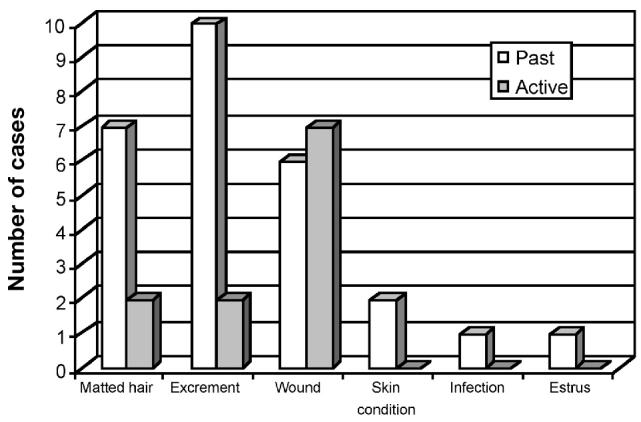
Conditions reported by veterinarians as contributory factors in myiasis in past and active cases of myiasis in pet animals. Some animals had several conditions.
Of the 10 active cases presented to the FEL, 8 animals were kept outside and 2 were primarily or wholly kept indoors. In 9 of the 10 cases, the insects colonizing the animals were the metallic blue-green blow fly (Lucilia illustris, Meigen) or the green bottle fly (Phaenicia sericata, Meigen) (Diptera: Calliphoridae). In 1 case, the larvae of a bot fly (Cuterebra jellisoni, Curran) (Diptera: Oestridae) colonized a pet rabbit.
Data indicated that the oldest maggots collected from the active cases were between 2 and 3 d old. In most cases, the scientific evidence corroborated the estimate presented by the veterinarian. In 1 case, the veterinarian had estimated a slightly longer period of 4 d. This is entirely possible, as insect evidence is usually used to indicate a minimum elapsed time since death.
Some cases were of particular interest. One veterinarian reported that a dog was presented with a severe edema of the muzzle and several maxillary fractures of unknown cause. The maxilla was wired and the dog sent home. Four days later the owner noticed that skin and subcutaneous tissue appeared to be sloughing, so the veterinarian was again consulted. On closer examination, some of the teeth were found to be fractured and rotting; therefore, they were extracted. At this point, maggots were observed throughout the injured area, and the veterinarian took several radiographs. The radiographs indicated that almost 100 pieces of lead buckshot were present throughout the area. In this case, the presence of the maggots alerted the veterinarian to the more serious nature of the injuries.
In another case, a pregnant mare, close to term, was struck by a train. Five days after the accident, she was presented to the veterinarian with a very large open laceration on the left buttock, “large enough to insert the human hand and forearm up to the elbow.” The animal had a bad odor and was heavily infested with maggots. The wound was cleansed and flushed continuously, but it took 2 to 3 d to remove all the maggots. The mare gave birth to a healthy foal 3 d after she arrived at the clinic.
More typical cases included elderly, long-haired animals with coats matted and soaked with urine and feces. Usually, removing the matted hair and cleaning the area was all that was required to remove the insects.
Discussion
When a survey is conducted, only a small percentage of those surveyed will respond. Since it was published in a newsletter, this survey was considered to be a mail-out questionnaire, where it is typical to generate a 10% to 40% response rate (30). However, only 6 different clinics (out of the 450 veterinary clinics in British Columbia) participated in the survey, giving a 1.3% response rate. It is, therefore, unlikely that the cases reported here are a true reflection of cases of myiasis seen in veterinary clinics, as it is quite probable that some veterinarians did have cases but did not respond to the survey. Similarly, the active cases received by the FEL are probably related more to individual veterinarian’s and veterinary staff’s interest in the subject, rather than a reflection of true distribution, as some veterinarians sent us several cases and were most interested in following up the results, whereas others reported past cases but did not submit active cases. As well, only the cases in which a veterinarian was consulted were surveyed, and it is very probable that larval infestations on pets were missed by owners, or owners were reluctant to consult a veterinarian for fear of censure. In cases of deliberate abuse or neglect, it is unlikely that owners would consult a veterinarian. Nevertheless, the numbers of cases reported here suggest that myiasis in pet animals is probably common, with some clinics seeing many cases every year.
Dogs were the species most commonly presented with myiasis. The breed of dog varied considerably and included pure bred and mixed breed dogs, long- and short-haired dogs, and small to large breed dogs. Several veterinarians reported myiasis in rabbits; in fact, overall, more cases were reported in rabbits than in cats. This may be a reflection of a cat’s normal scrupulous attention to grooming and personal hygiene; however, some cases occurred on injured or long-haired cats, suggesting a lack of complete grooming.
Most cases of myiasis appeared to be the result of an untended wound or matted hair, and the presence of excrement, which indicates that owner neglect has a major role in the occurrence of myiasis. In many cases, this is probably the result of ignorance rather than deliberate neglect or abuse, and owner education may go a long way to alleviating the problem. As a majority of people find maggots particularly repugnant, it is probable that explaining to the owner how the maggots came to be present on the animal, and how such infestations can be prevented in future, may result in more diligence in care and grooming on the part of the owner.
Lucilia illustris and Phaenicia sericata are common and ubiquitous blow fly species that are frequently reported in forensic cases involving human homicide (31–34) and wildlife crime (26). They have also been reported to commonly colonize live humans and animals (1,8,35–37).
Lucilia illustris is considered a more rural species and is one of the primary insects involved in sheep blow fly strike (4), although it can be found in urban areas as well (33), whereas Phaenicia sericata is considered to be primarily an urban species (38). Many species of blow fly, particularly P. sericata, enter residential buildings and are commonly found on homicide victims inside houses (33). Therefore, although the majority of active cases were kept outdoors, animals kept as house pets are not immune to colonization. Both P. sericata and L. illustris are used medicinally in human maggot debridement therapy (39).
Cuterebra jellisoni is a myiasis species commonly found on wildlife, particularly rabbits (40). It feeds on both living and dead flesh (1).
In the 10 active cases, the estimate that the larvae were 2 to 3 d old is likely an underestimate, as the oldest insects may not have been collected, having dropped off the animal and moved away to a safe, dry place in which to pupate or undergo metamorphosis to the adult fly. Usually, in human forensic entomology cases, this is not a problem, as the area surrounding a homicide victim is carefully searched for other evidence, so prepupal larvae, pupae, and teneral adults will be discovered and seized. However such evidence drops off when the animal is alive and can be lost anywhere, although it is probable that many maggots leave the animal when it is resting. Evidence of these pupal cases can still aid in determining a minimum time of abuse or neglect. Therefore, if the animal has a favourite sleeping spot, or kennel, this should be searched to see whether older specimens can be found, in which case the estimate of elapsed time since colonization would be extended.
In many cases of myiasis, forensic entomology is able to indicate only that the insects were oviposited a minimum of 3 d prior to examination; therefore, the wound occurred a minimum of at least 3 d prior, or, in the case of neglect, the duration was a minimum of 3 d. It is probable that the veterinarian will be able to make a similar estimate, based on wound characteristics, but although it is often very accurate, it is usually based only on personal experience and therefore difficult to defend in court. Forensic entomology, on the other hand, provides straightforward scientific evidence, which has been successfully defended in court many times. As such, entomological evidence can be very helpful in corroborating the veterinarian’s estimate, if required, in court.
The following factors must be present for myiasis to occur in a pet animal. There must be abuse or neglect that results in a wound with blood, necrotic tissue, feces, or urine attracting flies. The animal must be somewhat helpless or unable to clean itself, in which case, the rapid cleansing of any wound or ‘hot spot’ and appropriate veterinary attention will greatly reduce the risks of infestation, particularly in summer. Matts and burrs in long-coated animals can easily cause irritation, leading to hot spots, scratching, open lesions, and infestation. Animals with long coats and mats are particularly prone to the accumulation of excrement near the genitalia. This situation becomes exacerbated when the animal is elderly or debilitated and can no longer clean itself. The risk is further increased if the animal spends the majority of its life outdoors. Regular grooming and examination of the animal can greatly reduce these risks. Also, removing other fly attractants, such as uneaten animal food, fecal material, etc., will reduce risk. Obviously, the summer season, when insects are common, is when the highest risk occurs, so owners should be extra vigilant at this time.
In certain situations, the root cause of the myiasis may be deliberate neglect or cruelty. Animal neglect and animal cruelty cases are being reported more frequently in the media, and more attention is being given to such cases. Laws to protect animals exist and the rights of animals are more protected than in the past (Canadian Criminal Code s. 446 — 2003). However, in many cases of animal neglect or cruelty, it is difficult to prosecute the offender, due to lack of conclusive evidence (41,42). An indication of the length of time of neglect, or the time elapsed since the abuse occurred, may strengthen a prosecutor’s case. Also, the actual presence of the insects on a living animal is very upsetting to most people, despite the fact that, in most cases, they are feeding on dead tissue and are doing no direct harm. Therefore, when insects are presented as physical evidence of the crime, they provide very powerful and graphic verification of the offence for judge and jury.
Animal cruelty is not only repugnant but also may have even more serious and far reaching consequences than might originally be considered. Although many acts of serious neglect result from overbreeding large numbers of animals in cramped and poor conditions for reasons of greed, some acts of cruelty are deliberate and malicious. It is well known in the criminology field that people who perpetrate acts of cruelty on animals, frequently escalate to torturing humans, usually the young and helpless (43). Many known serial killers began their careers by hurting pet animals. Identifying such offenders early may result in a greater chance of preventing future atrocities.
As with any forensic evidence, entomology is only of value if the evidence is collected correctly. Collection of insects from a living victim is similar to that in any forensic entomology case (18,25,44). However, it is worth describing here, as most veterinarians and animal cruelty investigatory personnel are probably not familiar with this type of evidence.
There are 2 areas that should be examined for insect evidence. The first is the animal itself. Eggs, 1st, 2nd, and 3rd instar larvae or maggots may be found on and in the wound. Maggots should be very carefully removed, without damaging them. Breaking a maggot within the animal releases a tremendous amount of foreign protein, which can result in shock, anaphylaxis and even death (39,45). Therefore, maggots should only be removed manually and not killed with a chemical treatment, as the death of maggots in the wound can also cause anaphylaxis. If only a few maggots are present, they can be removed by hand. Several veterinarians in this survey referred to flushing the area with water to remove the maggots. For the health of the animal, all maggots should be removed, if possible. Although the maggots are often those that feed only on dead tissue and are, consequently, probably not harming the animal, many species will feed on living tissue; the species cannot be determined until it has been examined under a microscope.
Once all maggots have been removed, they should be collected for forensic examination. Preferably, half should be placed for a few minutes in very hot (not boiling) water to destroy the internal enzymes and improve preservation (18,44). They should then be placed in a vial with 75% to 90% ethanol and labelled. The other half should be kept alive in a small vial, with food and air. A small piece of crumpled paper towel should be placed at the bottom of a vial or jar, to which a small cube of meat, preferably beef liver, is placed. The collected live maggots should then be placed on the top of the liver. A double layer of paper towels should then be placed on top of the vial and secured in place with an elastic band. The vial should also be labelled with the date, time of collection, and location of wound; details of animal, clinic, veterinarian; and case number of client, etc., as these vials may become police exhibits if the case goes to court. Photographs of the animal and the wound site before and after maggot removal will also be valuable.
If the maggots have only reached the early stages of the 3rd instar, all the insects will be on the animal and no further collection will be required. However, if the animal has been infested for some time, the older maggots will have already left the animal. So, if entomology is required to accurately determine length of time of neglect, bedding and the surrounding area should be searched for postfeeding maggots and pupae. Pupae are dark brown, approximately 10 to 14 mm long, and oval or football shaped. Maggots should be collected and preserved, as described before, and pupae should be placed in a vial with a crumpled paper towel to prevent damage, with another paper towel on top, as described before, to allow air to circulate. They should not be preserved. Some of the pupae may actually be empty pupal cases, in which case they indicate that an entire life cycle has taken place. These look identical to the pupae but have the tip broken off. They should also be collected and placed in a separate vial.
All the forensic evidence, together with details of the case, should be forwarded to a forensic entomologist for analysis.
Myiasis is a relatively common and entirely preventable veterinary problem in pet animals. Education of owners concerning risk factors may greatly reduce the prevalence of myiasis. However, in cases of deliberate neglect or maltreatment, entomological evidence may become powerful evidence for the length of time of abuse or neglect, in court.
Acknowledgments
The authors thank the British Columbia Veterinary Medical Association for publishing the questionnaire in its newsletter and the veterinarians and their veterinary staff for responding to the questionnaire and submitting cases to the Forensic Entomology Laboratory. CVJ
Footnotes
Funding for this work was provided by The Vancouver Foundation and The Animal Welfare Foundation.
This report has been peer reviewed.
References
- 1.James MT. The Flies that cause Myiasis in Man. Miscellaneous Publication #631, Washington, D.C.: US Department of Agriculture, 1947.
- 2.Wall R, French N, Morgan K. Blowfly species composition in sheep myiasis in Britain. Med Vet Entomol. 1992;6:177–178. doi: 10.1111/j.1365-2915.1992.tb00601.x. [DOI] [PubMed] [Google Scholar]
- 3.Wall R, French NP, Morgan KL. Population suppression for control of the blowfly Lucilia sericata and sheep blowfly strike. Ecol Entomol. 1995;20:91–97. [Google Scholar]
- 4.Hall MJ. Traumatic myiasis of sheep in Europe: a review. Parasitologia. 1997;139:409–413. [PubMed] [Google Scholar]
- 5.Erzinclioglu YZ, Whitmore RP. Chrysomya albiceps (Wiedemann) (Diptera: Calliphoridae) in dung and causing myiasis in Oman. Entomologist’s Monthly Magazine. 1983;119:51–52. [Google Scholar]
- 6.Deroo H, Jongbloet L, Aelbrecht M, Van Hecke E, Naeyaert JM. Human cutaneous parasitosis: two cases of furuncular and creeping myiasis. Dermatologica. 1990;180:199–200. [PubMed] [Google Scholar]
- 7.Bauch R, Ziesenhenn K, Groskoppf C. Lucilia sericata myiasis (Diptera: Calliphoridae) on a gangrene of the foot. Angew Parasitol. 1984;25:67–169. [PubMed] [Google Scholar]
- 8.Fotedar R, Banerjee U, Verma AK. Human cutaneous myiasis due to mixed infestation in a drug addict. Ann Trop Med Parasitol. 1991;85:339–340. doi: 10.1080/00034983.1991.11812570. [DOI] [PubMed] [Google Scholar]
- 9.Hall RD, Anderson PC, Clark DP. A case of human myiasis caused by Phormia regina (Diptera: Calliphoridae) in Missouri, USA. J Med Entomol. 1986;23:578–579. doi: 10.1093/jmedent/23.5.578. [DOI] [PubMed] [Google Scholar]
- 10.Morsy TA, Farrag AM. Two cases of human ophthalmomyiasis. J Egypt Soc Parasitol. 1991;21:853–855. [PubMed] [Google Scholar]
- 11.Miller KB, Hribar LJ, Sanders LJ. Human myiasis caused by Phormia regina in Pennsylvania. J Am Podiatr Med Assoc. 1990;80:600–602. doi: 10.7547/87507315-80-11-600. [DOI] [PubMed] [Google Scholar]
- 12.Fawzy AF. Otitis media and aural myiasis. J Egypt Soc Parasitol. 1991;21:883–885. [PubMed] [Google Scholar]
- 13.Cilla G, Pico F, Peris A, Idigoras P, Urbieta M, Perez Trallero E. Human genital myiasis due to Sarcophaga. Rev Clin Esp. 1992;190:189–190. [PubMed] [Google Scholar]
- 14.Lee HL, Yong YK. Human aural myiasis. Southeast Asian J Trop Med Public Health. 1991;22:274–275. [PubMed] [Google Scholar]
- 15.Carpenter TL, Chastain DO. Facultative myiasis by Megaselia sp. (Diptera: Phoridae) in Texas: a case report. J Med Entomol. 1992;29:561–563. doi: 10.1093/jmedent/29.3.561. [DOI] [PubMed] [Google Scholar]
- 16.Nielsen BO. Cases of human myiasis from Denmark. Entomologiske Meddelelser. 1993;61:81–82. [Google Scholar]
- 17.Kpea N, Zywocinski C. “Flies in the flesh”: a case report and review of cutaneous myiasis. Cutis. 1995;55:47–48. [PubMed] [Google Scholar]
- 18.Anderson GS, Cervenka VJ. Insects associated with the body: their use and analyses. In: Haglund WD, Sorg M, eds. Advances in Forensic Taphonomy Method, Theory and Archeological Perspectives, 2002. Boca Raton: CRC Pr: 174–200.
- 19.Baer WS. The treatment of chronic osteomyelitis with the maggot (larva of the blow fly) J Bone Joint Surg. 1931;13:438–475. doi: 10.1007/s11999-010-1416-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sherman RA, Pechter EA. Maggot therapy: a review of the therapeutic applications of fly larvae in human medicine, especially for treating osteomyelitis. Med Vet Entomol. 1988;2:225–230. doi: 10.1111/j.1365-2915.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 21.Pechter EA, Sherman RA. Maggot therapy: Surgical metamorphosis. Plast Reconstr Surg. 1983;72:567–570. doi: 10.1097/00006534-198310000-00032. [DOI] [PubMed] [Google Scholar]
- 22.Thomas S, Jones M, Shutler S, Jones S. Using larvae in modern wound management. J Wound Care. 1996;5:60–69. doi: 10.12968/jowc.1996.5.2.60. [DOI] [PubMed] [Google Scholar]
- 23.King AB, Flynn KJ. Maggot therapy revisited: a case study. Dermatol Nurs. 1991;3:100–102. [PubMed] [Google Scholar]
- 24.Sherman RA, Pechter EA. Maggot therapy: A review of the therapeutic applications of fly larvae in human medicine, especially for treating osteomyelitis. Med Vet Entomol. 1988;2:225–230. doi: 10.1111/j.1365-2915.1988.tb00188.x. [DOI] [PubMed] [Google Scholar]
- 25.Anderson GS. Forensic entomology: the use of insects in death investigations. In: Fairgreave S, ed. Case Studies in Forensic Anthropology, Toronto: Charles C. Thomas: 1999:303–326.
- 26.Anderson GS. Wildlife forensic entomology: determining time of death in two illegally killed black bear cubs, a case report. J Forensic Sci. 1999;44:856–859. [PubMed] [Google Scholar]
- 27.Goff ML, Charbonneau S, Sullivan W. Presence of fecal matter in diapers as potential source of error in estimations of postmortem intervals using arthropod development patterns. J Forensic Sci. 1991;36:1603–1606. [PubMed] [Google Scholar]
- 28.Greenberg B. Flies as forensic indicators. J Med Entomol. 1991;28:565–577. doi: 10.1093/jmedent/28.5.565. [DOI] [PubMed] [Google Scholar]
- 29.Anderson GS. Minimum and maximum developmental rates of some forensically significant Calliphoridae (Diptera) J Forensic Sci. 2000;45:824–832. [PubMed] [Google Scholar]
- 30.Palys T. Research Decisions: Quantitative and Qualitative Perspectives. 2nd ed. Toronto: Harcourt Brace. 1997:144–150.
- 31.Greenberg B. Forensic entomology: case studies. Bull Entomol Soc Am. 1985;31:25–28. [Google Scholar]
- 32.Lord WD, Catts EP, Scarboro DA, Hadfield DB. The green blowfly, Lucilia illustris (Meigen), as an indicator of human post-mortem interval: a case of homicide from Fort Lewis, Washington. Bull Soc Vector Ecol. 1986;11:271–275. [Google Scholar]
- 33.Anderson GS. The use of insects in death investigations: an analysis of forensic entomology cases in British Columbia over a five year period. Can Soc Forensic Sci J. 1995;28:277–292. [Google Scholar]
- 34.Anderson GS. Insect succession on carrion and its relationship to determining time of death. In: Byrd JH, Castner JL, eds. Forensic Entomology. The Utility of Arthropods in Legal Investigations. Boca Raton: CRC Pr 2001:143–175.
- 35.Hudson HF. Lucilia sericata attacking a live calf. Can Entomol. 1914;46:416. [Google Scholar]
- 36.Bishopp FC. Flies which cause myiasis in man and animals — some aspects of the problem. J Econ Entomol. 1915;8:317–329. [Google Scholar]
- 37.Khan MAJ, Khan RJ. Hematoma of scalp in a baby caused by the common green bottle — Lucilia sericata (Meigen) (Diptera: Calliphoridae) in Karachi, Pakistan. Jap J Sanit Zool. 1987;38:103–105. [Google Scholar]
- 38.Smith KGV. A Manual of Forensic Entomology. London: Trustees of The British Museum (Nat. Hist.) and Cornell University Pr. 1986.
- 39.Sherman RA, Hall MJR, Thomas S. Medicinal maggots: an ancient remedy for some contemporary afflictions. Annu Rev Entomol. 2000;45:55–81. doi: 10.1146/annurev.ento.45.1.55. [DOI] [PubMed] [Google Scholar]
- 40.Stehr FW, ed. Immature Insects. vol. 2. Duboque, Iowa: Kendall/Hunt Publ. 1991.
- 41.Miller L, Zawistowski, SA. A call for veterinary forensics: The preparation and interpretation of physical evidence for cruelty investigation and prosecution. In: Recognizing and Reporting Animal Abuse: A Veterinarian’s Guide. Englewood, Colorado: American Humane 1998:63–67.
- 42.Ascione FR, Arkow P, eds. Child Abuse, Domestic Abuse, and Animal Abuse: Linking the Circles of Compassion for Prevention and Intervention. West Lafayette, Indiana: Purdue Univ Pr, 1999.
- 43.MacDonald JM. The threat to kill. Am J Psychiatry. 1963;120:125–130. [Google Scholar]
- 44.Catts EP, Haskell NHE. Entomology and Death — A Procedural Guide. Clemson, South Carolina: Joyce’s Print Shop. 1990.
- 45.Guerrini VH. Ammonia toxicity and alkalosis in sheep infested by Lucilia cuprina larvae. Int J Parasitol. 1988;18:79–81. doi: 10.1016/0020-7519(88)90040-9. [DOI] [PubMed] [Google Scholar]


