Abstract
T-cell acute lymphoblastic leukaemia (T-ALL) is a challenging malignancy with a high relapse rate attributed to drug resistance. Tetrandrine (TET), a bisbenzylisoquinoline alkaloid extracted from a Chinese herb, is a potential anti-cancer and anti-leukaemic drug. In this study we investigated the mechanisms of TET resistance in T-ALL cells in vitro. Among the four T-ALL cell lines tested, Jurkat and CEM cells exhibited the lowest and highest resistance to TET with IC50 values at 24 h of 4.31±0.12 and 16.53±3.32 μmol/L, respectively. When treated with TET, the activity of transcription factor activator protein 1 (AP-1) was significantly decreased in Jurkat cells but nearly constant in CEM cells. To avoid cell-specific variation in drug resistance and transcription factor activities, we established a TET-R Jurkat subclone with the estimated IC50 value of 10.90±.92 μmol/L by exposing the cells to increasing concentrations of TET. Interestingly, when treated with TET, TET-R Jurkat cells exhibited enhanced AP-1 and NF-κB activity, along with upregulation of c-Jun N-terminal kinase (JNK) and extracellular signal-regulated kinase (ERK) signaling pathways, whereas the expression of P-gp was not altered. Selective inhibition of JNK but not ERK suppressed AP-1 activity and TET resistance in TET-R Jurkat cells and in CEM cells. These results demonstrate that Jurkat cells acquire TET resistance through activation of the JNK/AP-1 pathway but not through P-gp expression. The JNK/AP-1 pathway may be a potential therapeutic target in relapsed T-ALL.
Keywords: T-cell acute lymphoblastic leukaemia, Jurkat cells, tetrandrine, MAPK, JNK, activator protein 1, multidrug resistance, P-gp
Introduction
T-cell acute lymphoblastic leukaemia (T-ALL), a neoplastic monoclonal proliferation of abnormal immature T lymphocytes in bone marrow, is an aggressive malignancy that frequently manifests with multiple organ involvement and is associated with poor treatment outcomes1,2,3. Drug resistance, either intrinsic or acquired, is important in treatment failure of T-ALL chemotherapy4. Unravelling the mechanisms of drug resistance could help overcome treatment failure and improve prognosis.
Tetrandrine (TET), a bisbenzylisoquinoline alkaloid extracted from Han-Fang-Chi (Stephania tetrandra S Moore), is a well-known calcium channel blocker commonly used as an anti-hypertensive and anti-arrhythmic drug in modern China5. Several lines of evidence demonstrate the pleiotropic properties of TET, such as anti-inflammatory, immunosuppressive and anti-rheumatic effects6,7. Furthermore, growing evidence has revealed the anti-cancer effects of TET against diverse cancer cell lines, including leukaemia, lymphoma, neuroblastoma and glioma cell lines, as well as breast, lung, liver and colon cancer cells8,9,10,11. In a clinical trial, TET, in combination with traditional chemotherapy drugs, had encouraging anti-leukaemic effects and was well tolerated12. These findings suggest that TET is a potentially novel anti-leukaemic agent and adjuvant therapy for T-ALL. However, the mechanism of drug resistance to TET in T-ALL has not been investigated.
The activator protein-1 (AP-1) and nuclear factor-kappa B (NF-κB) families of transcription factors are now understood to be important in human cancer and are validated targets in cancer drug discovery13. Our previous study demonstrated that TET cytotoxicity (at therapeutic concentrations) is mediated through an apoptotic mechanism in leukaemia/lymphoma cell lines; this cytotoxic effect was relatively exclusive to T-ALL and peripheral blood T-cells, compared with B-cell lymphoma and monocytic leukaemia cell lines14. These findings suggest that differential activities of transcription factors, especially AP-1 and NF-κB, might be involved in the varying drug resistance/sensitivity to TET in T-ALL.
In this study, we examined the viability of four T-ALL cell lines treated with TET and compared AP-1 and NF-κB activities between cell lines with distinct TET resistance. A TET-resistant subclone of Jurkat cells was established to investigate the role of the MAPK/AP-1 pathway in T-ALL drug resistance against TET. Our results demonstrate that Jurkat cells might acquire resistance against TET through activation of the JNK/AP-1 pathway but not through modulation of P-glycoprotein (P-gp) expression, the most widely studied multidrug resistance (MDR) protein.
Materials and methods
Reagents and preparation of TET
Preparation of TET has been described previously15. Briefly, TET powder (purity >98%; Yichang Pharmaceutical Company, China) was dissolved in 0.1 mol/L HCl at a stock concentration of 50 mmol/L and stored at -20 °C until use. For each experiment, the stock solution was further diluted in culture medium to the desired concentrations. MAPK inhibitors, including extracellular signal-regulated kinase (ERK) and c-Jun N-terminal kinase (JNK) inhibitors (PD98059 and SP600125, respectively), and anti-P-gp mouse monoclonal antibody (mAb) were purchased from Darmstadt (Germany). Antibodies against phospho (p)-p44/42 ERK (Thr202/Tyr204), p-SAPK/JNK (Thr183/Thr185) kinase and p-p38 (Thr180/Tyr182) were obtained from Cell Signaling (Danvers, MA, USA). Total p44/42 ERK, SAPK/JNK, p38 and USF-2 were recognized by antibodies from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-actin mAb was obtained from Chemicon (Temecula, CA, USA). Unless otherwise specified, other reagents were purchased from Sigma-Aldrich Chemical Company (St Louis, MO, USA).
Cells and culture media
All cell lines were purchased from the Bioresource Collection and Research Center (BCRC, Taiwan, China). Human T-cell lines (Jurkat, CEM, MOLT-4 and SUP-T1) and the monocytic cell line THP-1 were grown in RPMI-1640 medium supplemented with 10% foetal bovine serum (FBS; Biological Industries), 2 mmol/L L-glutamine, 2 g/L sodium bicarbonate and antibiotics (100 unit/mL of penicillin and 100 μg/mL streptomycin). The culture media for Jurkat, THP-1 and SUP-T1 cells also contained 10 mmol/L HEPES and 1 mmol/L sodium pyruvate. In addition, the culture medium for SUP-T1 cells was supplemented with 4.5 g/L D-(+)-glucose. A549 cells (type II human lung alveolar epithelial cell carcinoma) were grown in Ham's F12K medium containing 2 mmol/L L-glutamine, 1.5 g/L sodium bicarbonate, 10% FBS and antibiotics. HepG2 cells (a human hepatocellular carcinoma cell line) were grown in minimum essential medium with Earle's salt, supplemented with 10% FBS, 2 mmol/L L-glutamine, 1.5 g/L sodium bicarbonate, 0.1 mmol/L non-essential amino acids and antibiotics. SW480 cells were grown in Leibovitz's L-15 medium supplemented with 10% FBS, 2 mmol/L L-glutamine and antibiotics. Jurkat, CEM, MOLT-4, SupT1, THP-1, A549 and HepG2 cells were cultured at 37 °C in a humidified incubator with 5% CO2 and maintained in log phase. SW480 cells were grown at 37 °C in a humidified incubator without CO2. Cells (3×105 cell/mL) were treated with different concentrations of TET for various incubation periods, as indicated in the figures and legends. All culture media and supplements were obtained from Invitrogen (Carlsbad, CA, USA), unless otherwise indicated.
Cell viability and cytotoxicity assays
A trypan blue exclusion assay was used to measure the effect of TET on the viability of T-ALL cells, as described previously14. Briefly, after treatment at the indicated dose and duration, the cells were harvested, stained with trypan blue (0.5% solution) and counted using a haemocytometer.
A 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to assess the cytotoxic effect of TET on cells. In brief, cells were seeded in a 96-well plate and treated with TET for 24 h. Then, the cells were washed once with phosphate-buffered saline (PBS; pH 7.4), and 200 μL of MTT (USB/Amersham Life Science, Cleveland, OH, USA) solution (1 mg/mL in PBS buffer) was added to each well. After incubation at 37 °C for 4–6 h, the supernatant was carefully removed, and the purple formazan precipitate that was converted from yellow MTT salt by mitochondrial dehydrogenases in viable cells was dissolved in 200 μL of dimethyl sulfoxide. Optical density was measured with an ELISA reader (Tecan, Grodig, Australia) at a wavelength of 590 nm. Cell viability is presented as the percentage of medium-treated cells, which was set as 100% viable. IC50 values (the compound concentration resulting in 50% inhibition of cell viability) after 24 h of TET treatment were calculated using Microsoft Excel software.
The cytotoxicity of TET was estimated using a cytotoxicity detection kit (LDH; Roche, Indianapolis, IN, USA) according to the manufacturer's protocol. After treatment as indicated, 100 μL of supernatant from each well was collected, transferred into a new 96-well plate, and mixed with the kit reagents. Optical density at 490 nm, indicating lactate dehydrogenase activity, was measured with an ELISA reader (Tecan). Percent cytotoxicity was calculated as follows: (sample value−medium control)/(high control−medium control)×100. An equal number of cells treated with 1% Triton X-100 was used as the high control.
Development of a TET-resistant Jurkat cell line
The Jurkat cell line was maintained by serial passage in the presence of incrementally increasing doses of TET, starting from 0.5 μmol/L and finally reaching 5 μmol/L. Dead cells were removed by Ficoll gradient centrifugation, and the TET concentration in the medium was increased as cell lines achieved 80% viability. Over a period of 6–9 months, a Jurkat subclone resistant to 5 μmol/L TET was established and labelled the TET-resistant (TET-R) Jurkat cell line. The parental Jurkat cells, which were more sensitive to TET cytotoxicity, were labelled TET-sensitive (TET-S) Jurkat cells. Cells were counted for 3 consecutive days using a trypan blue assay, and cell doubling time was calculated using the equation Td (h)=T×[log 2/(log N−log N0)], where Td is the doubling time, T is the time in the logarithmic proliferative phase (h), N is the cell number at the end of the logarithmic proliferative phase and N0 is the cell number at the beginning of the logarithmic proliferative phase.
Flow cytometry
Flow cytometry was performed to evaluate apoptotic cell death and investigate the cell cycle distribution, as described in our previous report14. In brief, after treatment with 5 μmol/L TET for 24 h, TET-S and TET-R Jurkat cells were washed twice with PBS and fixed in ice-cold 70% ethanol for 2 h. Then, the cells were stained with a solution containing 20 μg/mL propidium iodide (PI), 0.2 mg/mL DNase-free RNase A and 0.1% Tritox-100 for 30 min at room temperature. Subsequently, cells were analysed by flow cytometry using a FACScan system (BD Biosciences, San Jose, CA, USA). The proportions of cells in all phases (G0/G1, S and G2) were quantified. Cell cycle was analysed with ModFit LT™ software (Verity Software House Inc, Topsham, ME, USA), and the percentage of cells in each phase was determined.
Annexin-V and 7-amino-actinomycin D (7-AAD) staining assay
The Annexin-V and 7-AAD staining assay was performed according to the manufacturer's protocol (PE Annexin-V Apoptosis Detection Kit I, BD PharmingenTM). Cells were washed twice with cold PBS and then phycoerythrin (PE) Annexin-V and 7-AAD were added to the binding buffer. The reaction proceeded in the dark for 15 min at room temperature. The cells were then analysed and quantified using flow cytometry.
Nuclear extract preparation
Nuclear extracts were prepared and quantified using a Bradford assay (Biorad), as previously described16. In brief, after collection and a single wash with PBS solution, cells (3×106 cells in 10-cm dishes in each treatment condition) were left at 4 °C in 100 μL of buffer A (10 mmol/L HEPES [pH 7.9], 1.5 mmol/L MgCl2, 10 mmol/L KCl, 1 mmol/L dithiothreitol [DTT], 1 mmol/L phenylmethylsulfonyl fluoride [PMSF] and 1 μg/mL aprotinin) for 50 min with occasional gentle vortexing. The swollen cells were subjected to centrifugation at 14 000 revolutions per minute for 3 min. After removal of the supernatants (cytoplasmic extracts), the pelleted nuclei were washed with 50 μL of buffer A, and the cell pellets were resuspended in 40 μL of buffer C (20 mmol/L HEPES [pH 7.9], 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 25% glycerol, 1 mmol/L DTT, 0.5 mmol/L PMSF and 1 μg/mL aprotinin) and incubated at 4 °C for 30 min with occasional vigorous vortexing, followed by centrifugation of the mixtures at 15 000 revolutions per minute for 20 min. The supernatants were used as nuclear extracts.
Electrophoretic mobility shift assay (EMSA)
EMSAs were performed as detailed in our previous report17. Oligonucleotides with NF-κB or AP-1 response elements (NF-κB binding site: 5′-AGTTGAGGGGACTTTCCCAGGC-3′ AP-1 binding site: 5′-CGCTTGATGAGTCAGCCGGAA-3′) were radiolabelled with [γ-32P]-ATP using T4 kinase (Promega, Madison, WI, USA) and used as DNA probes. The NF-κB DNA probe consisted of synthetic polynucleotides with a sequence equivalent to the IL-2 promoter NF-κB response element. Nuclear extract (4 μg) was incubated with radiolabelled AP-1 or NF-κB probes in EMSA-binding buffer containing 10 mmol/L Tris-HCl (pH 7.5), 50 mmol/L NaCl, 0.5 mmol/L EDTA, 1 mmol/L DTT, 1 mmol/L MgCl2, 4% glycerol and 0.5 μg poly(dI-dC). The binding reaction proceeded at room temperature for 20 min. DNA-binding specificity was determined by a competing assay in which the nuclear extract was incubated with a 100-fold molar excess of unlabelled AP-1 or NF-κB oligonucleotide for 10 min before the addition of the radiolabelled probes. Unlabelled non-specific oligonucleotides from Santa Cruz Biotechnology (Santa Cruz, CA, USA) were also included as a negative control. The final reaction mixtures were separated by electrophoresis on a 6.6% non-denaturing polyacrylamide gel at 250 V for 70 min. After electrophoresis, gels were dried and subjected to radiography.
Transient transfection and luciferase reporter assays
Transfections were performed using TransFast (Promega, Madison, WI, USA) as a transfection reagent according to the manufacturer's recommendations and our previous report, with some modification17. In brief, TET-S and TET-R Jurkat cells were seeded overnight at a concentration of 1×106 cells/mL onto 6-well culture plates and then transfected with 2 μg of the reporter plasmid pAP-1-luciferase (TGACTAA)7 or pNF-κB-luciferase (TGGGGACTTTCCGC)5 (Stratagene, La Jolla, CA, USA) in the presence of 1 ng of the internal control plasmid pTK-Renilla-luciferase (Promega, Madison, WI, USA) in 500 μL of serum- and antibiotic-free transfection medium with 6 μL of TransFast. The subunit of NF-κB studied in the luciferase experiment was the p65/p50 heterodimer. Cells in each well were refreshed with 5 mL of RPMI-1640 medium supplemented with 10% FBS 1 h after transfection and then further incubated for another 48 h. After treatment with 5 μmol/L TET, total cell lysates were collected and firefly luciferase activities were analysed using a Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA), with Renilla luciferase activity as an internal control. All experiments were performed at least 3 times.
Western blotting analysis
Western blotting was performed using an enhanced chemiluminescence kit (Amersham Bioscience, UK), as described previously14,17. Briefly, after treatment at the indicated concentration and duration of TET, the cells were washed once with PBS, pelleted and resuspended in 100 μL of RIPA buffer [150 mmol/L NaCl, 50 mmol/L Tris-HCL (pH 7.5), 1 mmol/L EDTA, 1% (v/v) NP-40, 0.25% deoxycholate containing proteinase inhibitors (1 mmol/L PMSF, 1 μg/mL aprotinin, 1 μg/mL leupeptin and 1 μg/mL pepstatin) and phosphatase inhibitor (1 mmol/L sodium orthovanadate; Sigma; 1 mmol/L sodium fluoride; Merck)]. After periodic vortexing, the mixtures were subjected to centrifugation at 14 000 revolutions per minute for 20 min at 4 °C, and the supernatants containing the total cell lysates were collected. Equal amounts of protein samples were resolved by 8% or 10% SDS-PAGE at 120 V and transferred to nitrocellulose membranes. For immunoblotting, the nitrocellulose membrane was incubated with 5% non-fat milk dissolved in TBS-T [10 mmol/L Tris-HCl (pH 8.0), 150 mmol/L NaCl and 0.05% Tween-20] for 2 h and then blotted with primary antibodies against individual proteins for 2 h at room temperature. After triple washing with TBS-T, the membranes were incubated with secondary antibody conjugated with horseradish peroxidase (HRP; Chemicon, Temecula, CA, USA) at a dilution of 1:3000 for 1 h at room temperature. The membranes were then incubated with enhanced chemiluminescence substrate (Amersham Bioscience, UK) and exposed to BioMax Light Film (Kodak).
Immunoprecipitation kinase assay
Cells were lysed in 100 μL of buffer C (20 mmol/L HEPES, 420 mmol/L NaCl, 1.5 mmol/L MgCl2, 0.2 mmol/L EDTA, 25% glycerol, 1 mmol/L DTT, 1 mmol/L PMSF and 1 μg/mL aprotinin), and 50 μg of protein was incubated with 2 μL of anti-phospho-p44/p42 MAPK antibody in 1 mL of incubation buffer for 4–6 h, mixed with 40 μL of protein-A Sepharose CL-4B beads (GE Health Care, Montreal, Canada) at 4 °C, and rotated overnight. After a few washes, the immunoprecipitates were collected for further kinase assays. Kinase reactions were performed in a solution containing 10.5 μL ddH20, 20 μL of 2× kinase buffer, 3 μL of 2 mg/mL ERK substrate (myelin basic protein, MBP), 6 μL of unlabelled ATP and 0.5 μL of [γ-32P]-ATP at room temperature for 30 min. The proteins were then separated by SDS-PAGE (15% acrylamide gel), followed by autoradiography to visualize MBP phosphorylation.
Statistical analysis
All experiments were performed independently at least 3 times, except where otherwise stated in the figure legends. IC50 values represent the mean±SD (n=3). Paired conditions were compared using Student's t-test, and differences were considered significant at a P value of <0.05.
Results
TET cytotoxicity varied in diverse cancer, T-ALL cell lines and a TET-R Jurkat subclone
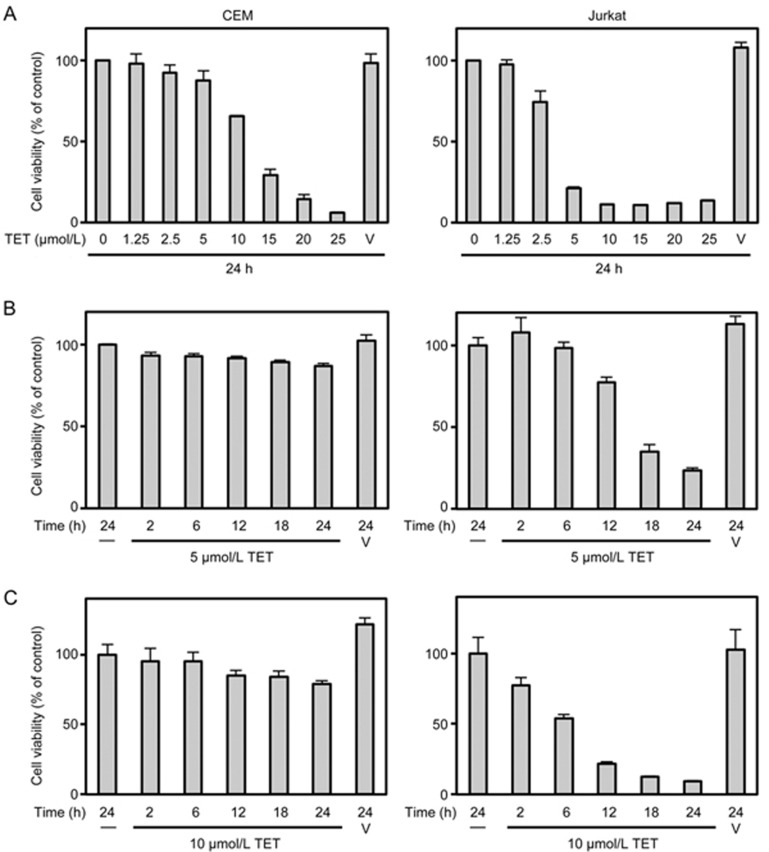
The viability of various human cancer cell lines against different concentrations of TET (0–100 μmol/L) were determined using MTT assays, and the IC50 of TET at 24 h was measured accordingly (Table 1). We found that cell lines derived from haematologic malignancies, including CEM, MOLT-4, SUP-T1 and Jurkat cells (ie, the 4 T-ALL cell lines), as well as THP-1 cells (an acute monocytic leukaemia cell line), were more sensitive to TET cytotoxicity than were other carcinoma cell lines (namely, A549, HepG2 and SW480 cells). Among the 4 T-ALL cell lines, the IC50 of TET was highest against CEM cells (16.53±3.32 μmol/L) and lowest against Jurkat cells (4.31±0.12 μmol/L), as was the case in our previous study14. After treatment with TET for 24 h, the cell viability of CEM and Jurkat cells diverged greatly at both 5 μmol/L and 10 μmol/L (Figure 1A). The time course for the cytotoxic effect of TET on both cell lines was further studied at these two concentrations. After treatment with 5 μmol/L TET, almost 90% of the CEM cells survived at 24 h, but less than 40% of the Jurkat cells survived at 18 h (Figure 1B). After exposure to 10 μmol/L TET, nearly 80% of the CEM cells survived at 24 h, but less than 40% of the Jurkat cells survived at 12 h (Figure 1C). Because the sensitivity to TET cytotoxicity between CEM and Jurkat cells differed the most, these two cell lines were used in subsequent experiments to examine the mechanism of resistance to TET in T-ALL cells.
Table 1. IC50 values of tetrandrine (TET) for various cancer cell lines.
| Source and disease | Cell line designation | Tetrandrine IC50 (μmol/L) |
|---|---|---|
| T-acute lymphoblastic leukaemia (T-ALL) | CEM | 16.53±3.32 |
| MOLT-4 | 11.07±0.10 | |
| SUP-T1 | 9.88±1.03 | |
| Jurkat | 4.31±0.12 | |
| Monocytic leukaemia | THP-1 | 13.43±1.61 |
| Lung carcinoma | A549 | 45.17±17.47 |
| Hepatocellular carcinoma | HepG2 | 30.15±7.06 |
| Colorectal adenocarcinoma | SW480 | 46.67±15.87 |
Cells were incubated in 96-well plates for 24 h with various concentrations of TET (0–100 μmol/L in cell growth media). Cell proliferation was evaluated as the ability of cells to reduce MTT to blue formazan crystals. The 50% inhibitory concentration (IC50) was defined as the TET concentration that reduced cell proliferation by 50% in 24 h. The results are presented as the mean±SD of 3–4 independent experiments.
Figure 1.
Differential cytotoxicity of tetrandrine (TET) in CEM and Jurkat cells. The viability of CEM and Jurkat cells seeded in 96-well plates (3×105 cell/mL) was measured by performing an MTT assay before and after treatment with various concentrations of TET for 24 h (A) or 5 μmol/L (B) or 10 μmol/L (C) TET for 2, 6, 12, 18 and 24 h. V, vehicle control. Data were obtained from at least 3 independent studies and are expressed as the mean±the standard deviation (SD).
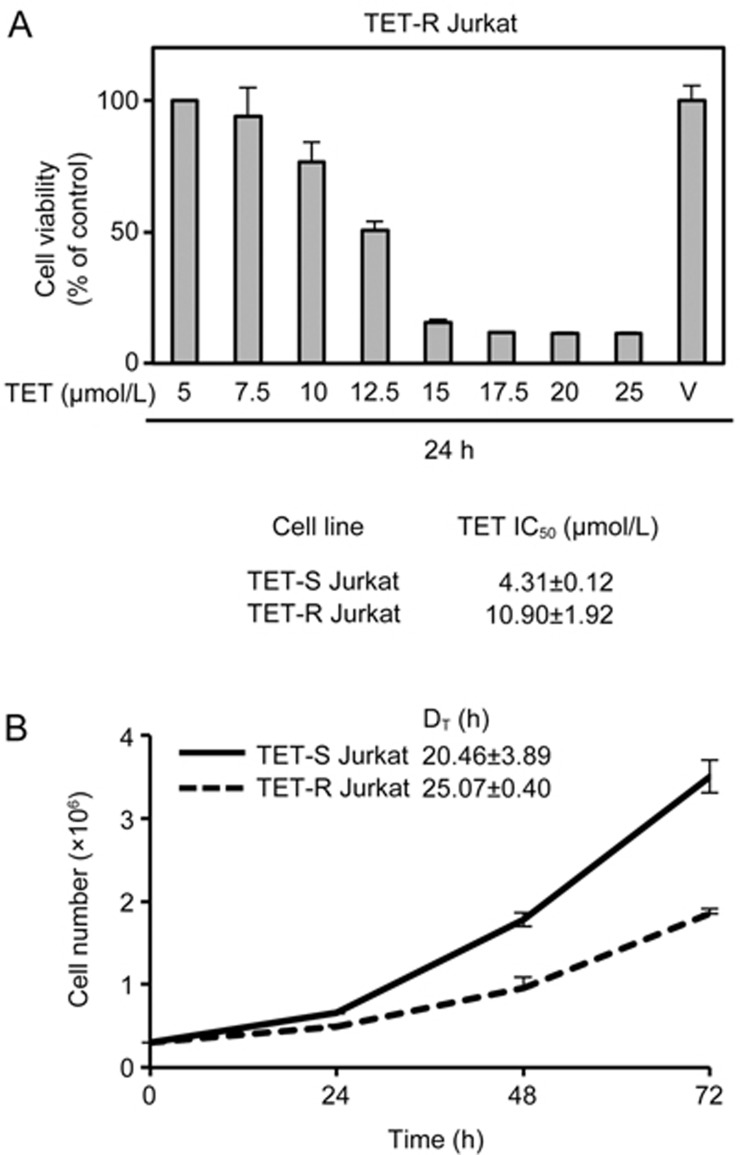
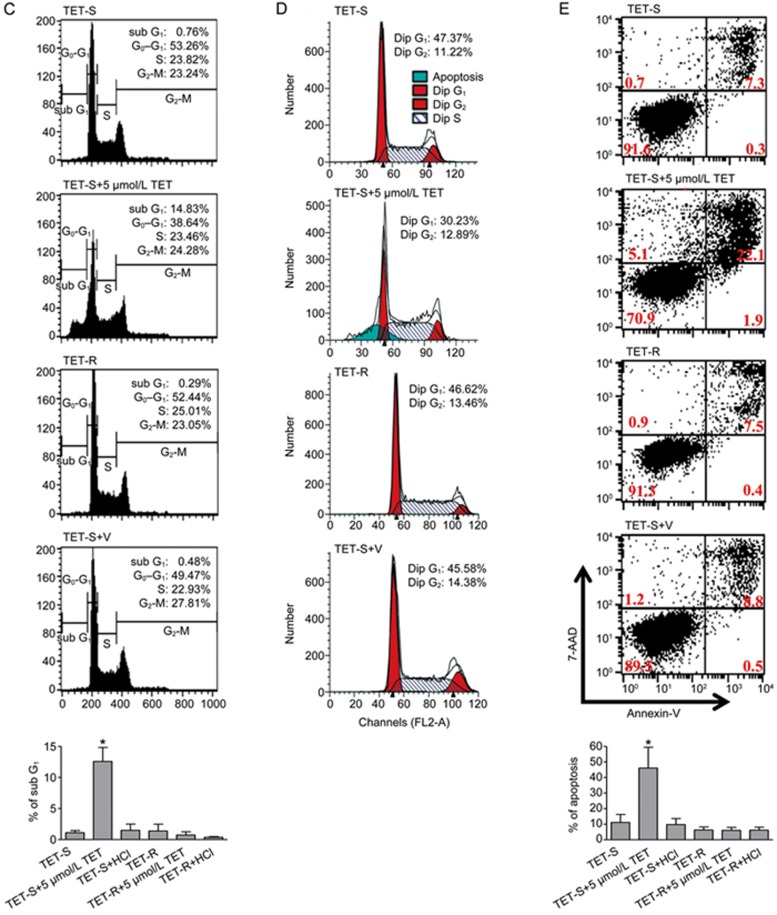
To avoid cell-specific variation in drug resistance and transcription factor activities, a TET-R Jurkat subclone was established as mentioned in the material and methods. The estimated IC50 of TET at 24 h in the TET-R Jurkat cells was 10.90±0.92 μmol/L, approximately 2.5 times that in the TET-S Jurkat cells (4.31±0.12 μmol/L) (Figure 2A). However, the proliferation rate of TET-R Jurkat cells was slower than that of TET-S Jurkat cells, and the population doubling time was approximately 25 h and 20 h, respectively (Figure 2B). After treatment with 5 μmol/L TET for 24 h, the percentage of sub-G1 cells in the population (indicating apoptotic cells) significantly increased to 14.8% in TET-S Jurkat cells, compared with vehicle and TET-R Jurkat cells (Figure 2C). Further analysis of the cell cycle distribution with ModFit software revealed an increase in apoptosis of TET-S Jurkat cells treated with 5 μmol/L TET (Figure 2D), which was also confirmed using another approach, ie, by staining cells with Annexin-V and 7-AAD (Figure 2E).
Figure 2A, 2B.
Resistance to TET was higher, but proliferation was slower, in TET-resistant (TET-R) Jurkat cells. TET-R Jurkat cells resistant to 5 μmol/L TET were established as a model for investigating drug resistance. TET-R Jurkat cells (3×105 cell/mL) incubated in a 96-well plate were subjected to higher concentrations of TET, as indicated, for 24 h. (A) Cell viability was determined by performing MTT assays, and the IC50 against TET in TET-R Jurkat cells was calculated. (B) Cell proliferation was measured by trypan blue exclusion, and the cell doubling time of TET-R Jurkat cells was calculated. TET-S and TET-R Jurkat cells (3×105 cell/mL) were incubated in 6-well plates overnight and treated with 5 μmol/L TET for 24 h, after which they were sorted and fixed with alcohol.
Figure 2C–2E.
Resistance to TET was higher, but proliferation was slower, in TET-resistant (TET-R) Jurkat cells. TET-R Jurkat cells resistant to 5 μmol/L TET were established as a model for investigating drug resistance. TET-R Jurkat cells (3×105 cell/mL) incubated in a 96-well plate were subjected to higher concentrations of TET, as indicated, for 24 h. (C) After stained with propidium iodide, cell cycle distribution of the cells was analyzed using flow cytometry. The bars in the bottom diagram show the percentage (mean±SD, n=3) of the apoptotic cells in the sub-G1 phase. *P<0.05 vs control. (D) Cell cycle profiles were further analyzed using ModFit software. The sub-G1 peak (blue peak) indicates apoptotic cells. (E) Representative flow cytometric dot plots of cells stained with Annexin V and 7-AAD are shown. The bars in the bottom diagram display the percentage (mean±SD, n=3) of apoptotic cells in different groups. *P<0.05 vs control. Representative results from at least three independent experiments are shown.
AP-1 activity was suppressed by TET in TET-S Jurkat cells
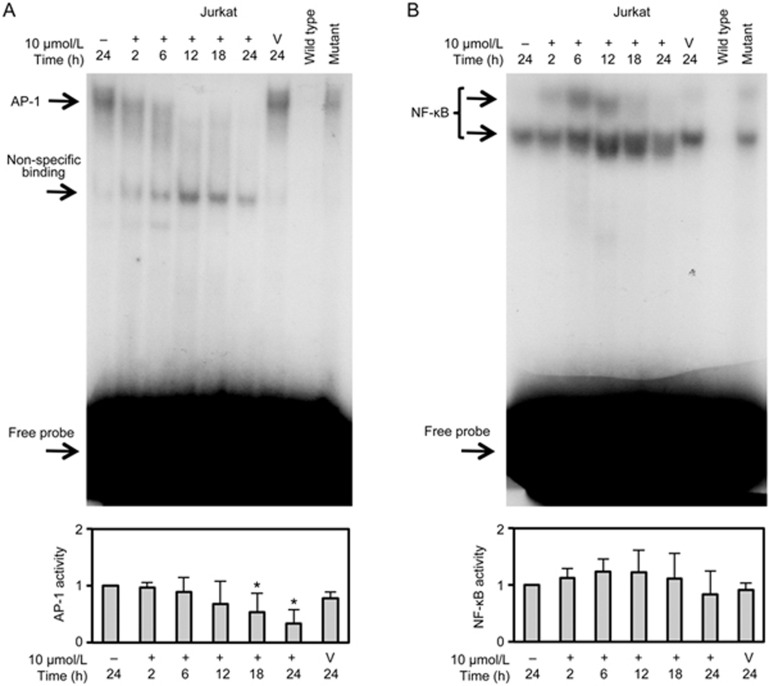
To investigate the roles of AP-1 and NF-κB in the mechanism of drug resistance to TET, the DNA-binding activity of AP-1 and NF-κB in the nuclear extracts of TET-S Jurkat cells treated with 10 μmol/L TET for 2, 6, 12, 18 and 24 h was evaluated using EMSAs. The results showed that AP-1 activity significantly decreased in Jurkat cells as the exposure time increased following treatment (Figure 3A), whereas NF-κB activity seemed to markedly increase at 12 h and then return to near-basal levels at 24 h (Figure 3B). These EMSA results suggested a correlation between AP-1 activity and resistance to TET in T-ALL cells.
Figure 3.
Suppression of AP-1 activity by TET in Jurkat cells. Electrophoretic mobility shift assay of DNA-binding activity of AP-1 (A) and NF-κB (B) in nuclear extracts from Jurkat cells treated with 10 μmol/L TET for 2, 6, 12, 18 and 24 h is shown. Some reactions included an unlabelled competitor (wild-type oligonucleotide probe) and an unlabelled mutant (non-specific) probe. Quantitative densitometry data are expressed as the mean±SD (n=2 in CEM groups, n=3 in Jurkat groups). *P<0.05 vs control.
AP-1 activity increased in TET-R Jurkat cells
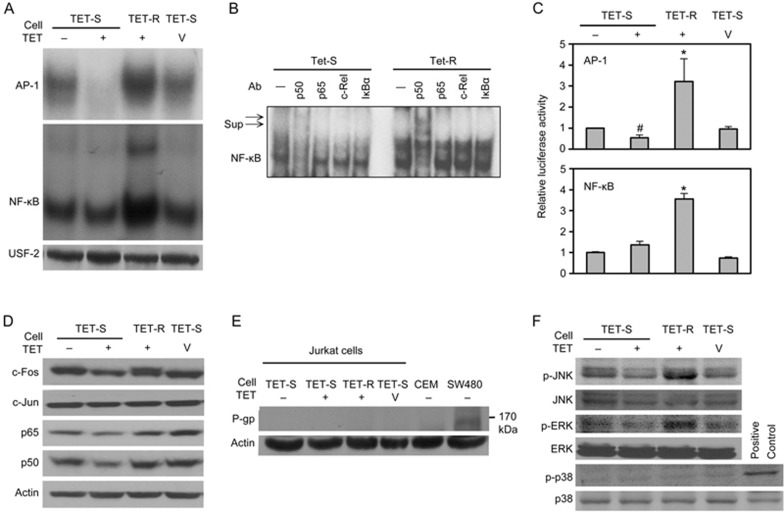
EMSA analyses showed higher DNA-binding activities of AP-1 and NF-κB in TET-R Jurkat cells (Figure 4A, lane 3) than that in control (TET-S) Jurkat cells (Figure 4A, lane 1). A supershift assay using specific NF-κB subunits showed that pre-incubation with anti-p50 monoclonal antibodies resulted in two supershifted bands, clearly demonstrating the involvement of the NF-κB p50 subunit (Figure 4B, lane 7). We also treated TET-S Jurkat cells with 5 μmol/L TET, the same concentration as that in the TET-R Jurkat culture medium, to verify the difference between TET-S and TET-R Jurkat cells with regard to AP-1 activity in response to TET. The results showed that although the AP-1 activity was markedly suppressed, NF-κB activity remained unchanged in TET-S Jurkat cells treated with 5 μmol/L TET for 24 h (Figure 4A, lane 2), in comparison with control (Figure 4A, lane 1). These findings further highlighted the significance of AP-1 activation in drug resistance against TET (Figure 4A, lanes 2 and 4). In addition, both AP-1 and NF-κB activities constitutively increased in TET-R Jurkat cells (Figure 4A, lane 3).
Figure 4.
MAPK/AP-1 and possible NF-κB activation, but not P-glycoprotein, contributes to TET resistance. TET-S and TET-R Jurkat cells (3×105 cells/mL) were seeded into 10-cm cell culture dishes and treated with 5 μmol/L TET or 0.1 mol/L HCL, as a vehicle control, for 24 h. (A) The DNA-binding activities of AP-1 and NF-κB in nuclear proteins were detected with an EMSA; USF-2 served as a loading control. (B) A supershift assay was used to determine the components in the NF-κB oligonucleotide-binding complex. The nuclear extracts were incubated with different mAbs for 30 min before incubation with radiolabelled NF-κB oligonucleotides. The mixtures were analysed with an EMSA. Sup indicates supershifts. c-Rel and IκBα were used as negative controls. (C) Cells (1×106 cells/mL) were transiently transfected with pNF-κB-luciferase or pAP-1-luciferase as well as pTK-Renilla-luciferase reporter plasmid. Eight hours after transfection, and 24 h after treatment with 5 μmol/L TET, cells were collected and analysed for luciferase activity in total lysates; untreated cells were used as a control. Activation of the reporter gene was expressed as fold-change relative to untreated Jurkat cells. *P<0.05 vs control. Cells (3×105 cells/mL) were incubated in 10-cm cell culture dishes in the presence of 5 μmol/L TET for 24 h; then, (D) p65, p50, c-Jun and c-Fos, and (F) total and phosphorylated JNK, ERK and p38 in equal amounts of total cell lysate protein (50 μg) for each sample were analysed by Western blotting. The results from a representative experiment are shown. Similar results were obtained in 2 other independent trials. (E) The expression of P-glycoprotein (P-gp) in cells with different levels of resistance to TET after treatment with or without TET for 24 h was evaluated by Western blotting. β-Actin was used as an internal control for equal loading. SW480 cells, which constitutively express P-gp, served as a positive control.
The transcriptional activities of AP-1 and NF-κB analysed using dual-luciferase reporter assays showed results similar to those of the EMSA analyses (Figure 4C). These results suggested that enhanced AP-1 DNA-binding activity and subsequent transcriptional activity contributed to drug resistance and protected TET-R Jurkat cells against TET toxicity. However, treatment with TET reduced AP-1 reporter activities but had no effects on NF-κB reporter activities in TET-S Jurkat cells (Figure 4C, lane 2). Total protein levels of p65, p50 and c-Fos, but not c-Jun, decreased in TET-S Jurkat cells treated with TET (Figure 4D). The discrepancies between p65/p50 protein expression and NF-κB reporter activities might be due to compensatory mechanisms from the activation of other alternative NF-κB pathways, such as those associated with the p52 and RelB subunits18. The results from reporter assays suggested that AP-1, but not NF-κB signalling, may be involved in the mechanism of resistance to TET in T-ALL cells.
P-gp was not expressed in TET-R Jurkat and CEM cells
The expression of P-gp, an important cell-membrane-associated protein mediating multidrug resistance in cancer, was analysed using Western blotting. As shown in Figure 4E, neither CEM nor TET-R Jurkat cells expressed P-gp in comparison to SW480 cells that constitutively express P-gp. This finding implied that another mechanism rather than P-gp overexpression contributed to the higher TET resistance of CEM and TET-R Jurkat cells.
JNK and ERK, but not p38, MAPKs were activated in TET-R Jurkat cells
To investigate the upstream mechanism of AP-1 activation, MAPKs were analysed by Western blotting of cell lysate. As shown in Figure 4F, after treatment with 5 μmol/L TET for 24 h, the expression of phosphorylated JNK (p-JNK) and phosphorylated ERK (p-ERK) increased in TET-R Jurkat cells but was suppressed in TET-S Jurkat cells. Expression of phosphorylated p38 (p-p38) was not detected in either cell type. These results suggested that the signal for AP-1 activation in TET-R Jurkat cells might be mediated by overexpression of p-JNK and p-ERK.
JNK/AP-1 activation contributed to drug resistance in TET-R Jurkat cells
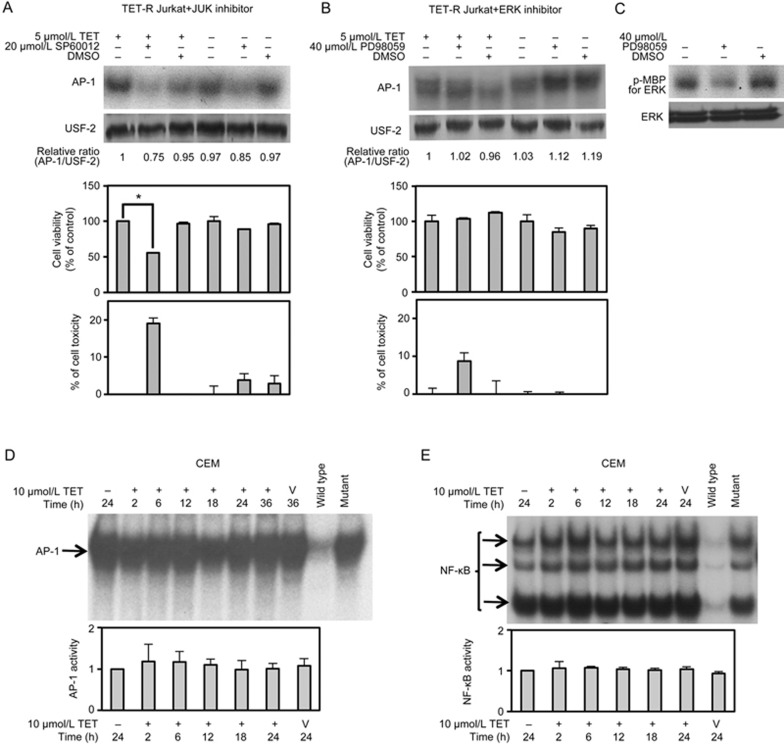
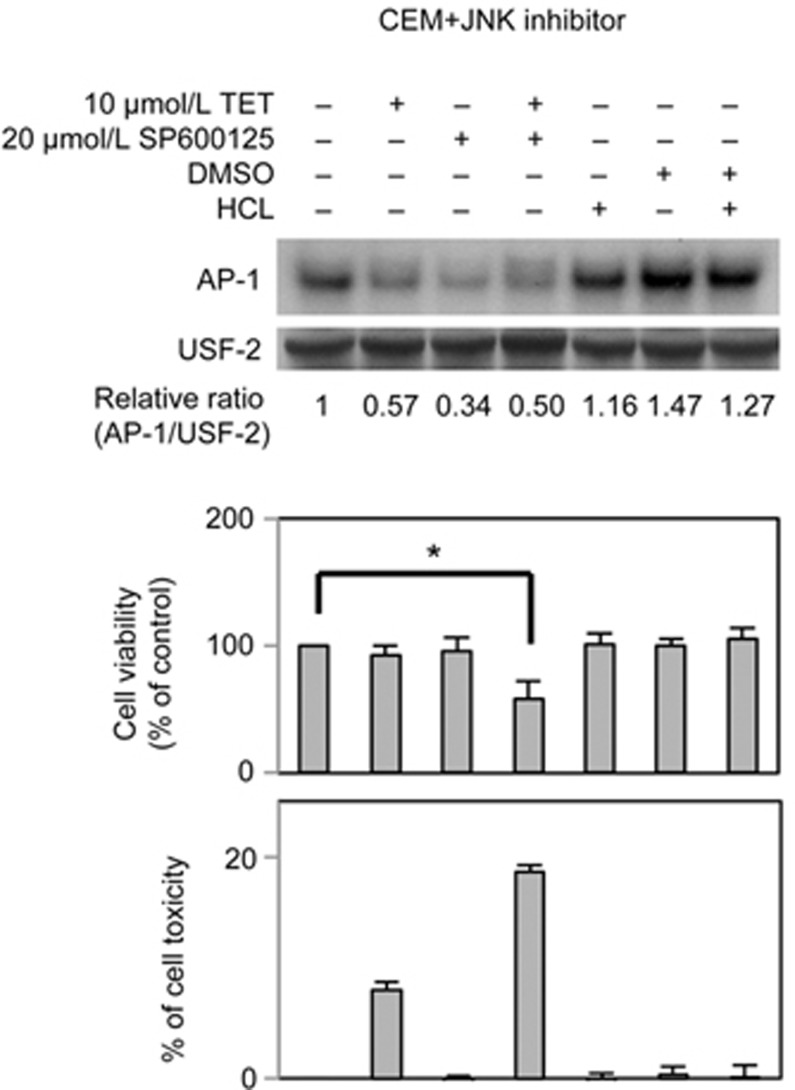
To verify the contribution of the MAPK pathway to AP-1 activation and consequent resistance to TET, cells were also treated with specific MAPK inhibitors for 24 h, after which AP-1 DNA-binding activity, cell viability and cytotoxicity were measured using EMSA, MTT and LDH assays, respectively. As shown in Figure 5A, selective inhibition of JNK by 20 μmol/L SP600125 markedly suppressed the downstream AP-1 activity and cell viability (to approximately 60% of control levels) of TET-R Jurkat cells treated with 5 μmol/L TET for 24 h (Figure 5A, lane 2). Moreover, 20 μmol/L SP600125 also noticeably suppressed AP-1 activity in TET-R Jurkat cells in the absence of TET treatment, but cell viability and LDH release were not significantly affected (Figure 5A, lane 5). Nevertheless, the AP-1 activity and cell viability of TET-R Jurkat cells were not significantly affected by selective ERK inhibition with PD98059 for 24 h, although LDH release was moderately increased (Figure 5B, lane 2). The effect of 40 μmol/L PD98059 on selective ERK inhibition was confirmed by an immunoprecipitation kinase assay (Figure 5C). To further confirm the role of AP-1 in the mechanism of drug resistance to TET in CEM cells, equal amounts of protein from nuclear extracts of CEM cells sorted at 2, 6, 12, 18, 24 and 36 h post-treatment with 10 μmol/L TET were subjected to an EMSA. The results showed that the DNA-binding activities of AP-1 and NF-κB were well maintained in CEM cells (Figure 5D and 5E). Consistent with the results from TET-R Jurkat cells, treatment with 20 μmol/L SP600125 markedly suppressed the downstream AP-1 activity and cell viability of CEM cells treated with 10 μmol/L TET for 24 h (Figure 6, lane 4). Taken together, the results indicated a substantial role of the JNK/AP-1 pathway activation in drug resistance against TET in T-ALL cells.
Figure 5.
Effects of specific MAPK inhibitors on AP-1 activity and viability of TET-R Jurkat and CEM cells treated with TET. (A) TET-R Jurkat cells (3×105 cells/mL) were incubated in 96-well plates or 10-cm cell culture dishes and treated with or without 20 μmol/L SP600125 (a specific JNK inhibitor) for 24 h in the presence or absence of 5 μmol/L TET in the medium. The DNA-binding activity of AP-1 was detected with an EMSA; USF-2 served as a loading control. Cell viability and TET toxicity were analysed by performing MTT and LDH assays, respectively. (B) TET-R Jurkat cells were treated with or without 40 μmol/L PD98059 (a specific ERK inhibitor) for 24 h in the presence or absence of 5 μMmol/L TET in the medium. AP-1 activity was detected with an EMSA. The data represent the mean±SD of 3 separate experiments. *P<0.05 vs control; paired Student's t-test. (C) The effect of ERK inhibition by 40 μmol/L PD98059 was verified by performing an immunoprecipitation kinase assay. (D, E) An EMSA of DNA-binding activity of AP-1 and NF-κB in nuclear extracts from CEM cells treated with 10 μmol/L TET for 2, 6, 12, 18 and 24 h is shown. Quantitative densitometry data are expressed as the mean±SD (n=2). *P<0.05 vs control.
Figure 6.
Effects of specific MAPK inhibitors on AP-1 activity and viability of TET-R Jurkat and CEM cells treated with TET. CEM cells (3×105 cells/mL) were incubated in 96-well plates or 10-cm cell culture dishes and treated with or without 20 μmol/L SP600125 (a specific JNK inhibitor) for 24 h in the presence or absence of 10 μmol/L TET in the medium. The DNA-binding activity of AP-1 was detected with an EMSA. Cell viability and TET toxicity were analysed using MTT and LDH assays, respectively.
Discussion
Aberrant cellular signalling pathways and dysregulated signalling networks are important in tumourigenesis and drug resistance of T-cell malignancies and hence serve as good targets for leukaemia therapy19. In this study, we analysed T-ALL cell lines with varying resistances to TET and experimentally induced TET resistance in a Jurkat subclone. We found that TET resistance in TET-R Jurkat cells was attributable to activation of AP-1 activity but not to expression of the multidrug transporter P-gp. AP-1 activation and TET resistance were apparently mediated by JNK activation, as both AP-1 activity and cell survival were suppressed by selective inhibition of JNK (but not ERK) in TET-R cells.
T-cell lymphoblastic leukaemias usually originate from T-cell progenitors and are characterized by limited differentiation and incessant proliferation. These leukaemic cells are regarded as the malignant equivalents of their normal counterparts but have stopped at specific maturation stages during differentiation20. CEM, MOLT-4, SUP-T1 and Jurkat cells are T-ALL cell lines frequently used in immunologic and molecular studies, either as a model of T-cell malignancy or as a substitute for normal T-cells. However, immunophenotyping and T-cell receptor studies have shown that these cell lines correlate with various differentiation stages of normal human thymocytes21. CEM belongs to the pre-T-cell stage, MOLT-4 and SUP-T1 to the cortical T-cell stage, and Jurkat cells to the mature T-cell stage. Unexpectedly, the cytotoxicity of TET positively correlated with the cell maturation stage of different T-ALL cells (Table 1): T-ALL cells with a more immature phenotype were more resistant to TET cytotoxicity. The MOLT-4 T-ALL cell line is derived from the same patient as the MOLT-3 cell line but was established from cells taken from this patient during relapse after multidrug chemotherapy22. The SUP-T1 T-lymphoblastic lymphoma cell line was originally derived from the pleural effusion of an 8-year-old boy during relapse23. In the present study, TET displayed marked cytotoxicity in the MOLT-4 and SUP-T1 cell lines, even more so than in the CEM cells. These findings further demonstrated the potential of TET, both as an anti-cancer agent and against relapsed or refractory T-cell malignancy. Nevertheless, resistance to TET may eventually evolve by certain mechanism(s) in T-ALL cells after exposure to TET in clinical chemotherapy, given the properties of evolution and clonal expansion of cancers24.
Many studies have found that transcription factors are important in drug resistance and are good targets for cancer therapy, but these investigations mostly focused on NF-κB25. The roles of AP-1 in oncogenesis and tumour suppression are contradictory because the actions of AP-1 are complex and highly context-specific26. Limited evidence suggests an association of AP-1 activity with resistance of leukaemia to some chemotherapeutic agents, such as etoposide27, glucocorticoid28 and vitamin D29, not involving a multidrug resistance mechanism. In the present study, a Jurkat subclone with resistance to 5 μmol/L TET was established to investigate the possible mechanism of TET resistance at the transcriptional level. Western blotting, EMSA and luciferase reporter assays were used to evaluate total protein expression of specific subunits of AP-1 and NF-κB, DNA-binding activities and DNA-transcriptional activities, respectively, in nuclear extracts of cells with different TET resistance. Our results revealed the contribution of AP-1 and possible NF-κB activation to TET resistance in T-ALL cells. There might be some concern about whether the EMSA results in Figure 2B are relevant if most Jurkat cells were killed soon after exposure to 10 μmol/L TET (Figure 1C). However, when using a nuclear extraction protocol, it is likely that nuclear proteins are extracted from residual viable cells. In addition, equal amounts of nuclear extracts quantified by the Bradford assay were subjected to EMSA. Moreover, the NF-κB activity was not reduced, in contrast to AP-1 activities. Instead, it increased at 6 to 12 h after exposure to 10 μmol/L TET. This suggests that AP-1 and NF-κB have different roles in the mechanisms of cell survival and TET resistance in T-ALL. As shown in Figure 4C, treatment with TET had no effects on luciferase reporter activities in TET-S Jurkat cells, suggesting that NF-κB signalling might not be involved in the mechanism regarding resistance against TET in T-ALL cells.
AP-1 activation is mainly mediated by upstream MAPK intracellular signalling cascades consisting of 3 major MAPKs, namely, JNK, ERK and p3830,31. The MAPK pathway is important in controlling cell differentiation, proliferation and survival, and its mutation is associated with drug resistance in some malignancies32, but its role in T-ALL is unclear. Phosphorylation of AP-1 components by kinases in the MAPK pathway, such as JNK, ERK and p38 MAPKs, correlates closely with functional activation of AP-1 complexes. Interestingly, as Figure 4D shows, not only AP-1 but also upstream p-JNK and p-ERK were clearly activated in TET-R Jurkat cells treated with TET. AP-1 activity and drug resistance to TET in TET-R Jurkat and CEM cells were markedly attenuated by the specific JNK inhibitor SP600125 (Figure 5A and 6) but were unaffected by the specific ERK inhibitor PD98059 (Figure 5B). These results demonstrate a correlation between the JNK/AP-1 pathway and TET resistance in T-ALL cells. Activation of the JNK pathway in T-ALL indirectly reduced the sensitivity of T-ALL to other chemotherapeutic agents. In SUP-T1 cells, lack of expression of the Bcl-2-interacting mediator of cell death (Bim) protein, a BH3-only member of the Bcl-2 family of proteins, confers resistance to etoposide-induced apoptosis. Constitutive activation of the JNK pathway in SUP-T1 cells promotes phosphorylation and degradation of Bim via the proteasome, thereby contributing to etoposide resistance. Suppression of JNK by SP600125 restores the Bim level and abolishes resistance of SUP-T1 cells to etoposide33. Another study of human T-ALL cell lines showed that JNK inhibition by SP600125 and small interfering RNA led to cell cycle arrest and apoptosis and increased sensitivity to Fas-mediated apoptosis, whereas constitutive JNK activity in T-ALL cells resulted in accelerated cell cycle progression and resistance to Fas-mediated apoptosis. These past and present results shed light on the mechanism of TET resistance and further suggest the potential of JNK as a therapeutic target in the treatment of T-ALL.
P-gp is encoded by the multidrug resistance protein-1 (MDR1) gene, which is also known as the ATP-binding cassette transporter B1 (ABCB1) gene. P-gp is a key efflux transporter leading to multidrug resistance and chemotherapy failure. Experimentally inducing drug resistance in cultured leukaemic cell lines by gradually increasing drug concentration yielded insights into the underlying mechanisms of treatment failure. A previous study showed that Jurkat cells express very little MDR1 and that the intrinsic drug resistance of Jurkat cells does not appear to involve MDR134. However, MDR1/P-gp expression was induced and MDR was acquired in several experimental drug-resistant T-ALL cell lines, including Jurkat cells, after exposure to incremental concentrations of various chemotherapeutic agents35,36,37. Unlike other chemotherapeutic agents, TET and its derivatives clearly show the effects of reversing MDR in a human T-ALL cell line and carcinoma cell lines mediated by P-gp38,39. A TET-R Jurkat subclone obtained by serial passage in the presence of increasing TET concentrations did not express P-gp in our studyg (Figure 4C). This suggests that chemoresistance is both cell- and drug-specific. We hypothesize that the anti-MDR activity of TET remains even after drug resistance against the anti-cancer effect of TET develops.
This study has some limitations. Although TET-R Jurkat cells were successfully selected and clearly had enhanced AP-1 activity, the IC50 of TET was only a few times greater than that of TET-S Jurkat cells at 24 h. However, the IC50 of TET against TET-S Jurkat cells would have been much lower if cell viability had been determined after 5 d of culture, as in some previous studies35. Apart from AP-1 expression, a difference in proliferation rate between TET-R and TET-S Jurkat cells was unexpectedly discovered. The proliferation rate of TET-R Jurkat cells with higher AP-1 activity was slower than that of the TET-S clone. It remains to be determined whether specific activation of the JNK/AP-1 pathway or merely AP-1 overexpression reduces the proliferation rate and confers TET resistance in Jurkat T-ALL cells.
In conclusion, to our knowledge this is the first study to examine the mechanism of TET resistance in T-ALL cells at the transcriptional level. The findings confirmed that TET resistance in T-ALL cells is P-gp-independent and suggest that this drug resistance is associated with activation of the JNK/AP-1 pathway. Our study provides a comprehensive mechanistic approach for cancer chemotherapeutic resistance, which could be applied to the treatment of patients with refractory T-ALL in the near feature.
Acknowledgments
We thank Dr Cha L, Lee S, Sytwu K, and Yang F for their kind gifts and assistance. This work was supported by grants from the National Science Council (NSC 100-2314-B-016-014 and NSC 98-2314-B-400-002-MY3), Taiwan, China.
References
- Hunger SP, Mullighan CG. Acute lymphoblastic leukemia in children. N Engl J Med 2015; 373: 1541–52. [DOI] [PubMed] [Google Scholar]
- Bhojwani D, Pui CH. Relapsed childhood acute lymphoblastic leukaemia. Lancet Oncol 2013; 14: e205–17. [DOI] [PubMed] [Google Scholar]
- Passaro D, Quang CT, Ghysdael J. Microenvironmental cues for T-cell acute lymphoblastic leukemia development. Immunol Rev 2016; 271: 156–72. [DOI] [PubMed] [Google Scholar]
- Swerts K, De Moerloose B, Dhooge C, Laureys G, Benoit Y, Philippe J. Prognostic significance of multidrug resistance-related proteins in childhood acute lymphoblastic leukaemia. Eur J Cancer 2006; 42: 295–309. [DOI] [PubMed] [Google Scholar]
- Kwan CY, Achike FI. Tetrandrine and related bis-benzylisoquinoline alkaloids from medicinal herbs: cardiovascular effects and mechanisms of action. Acta Pharmacol Sin 2002; 23: 1057–68. [PubMed] [Google Scholar]
- Lai JH. Immunomodulatory effects and mechanisms of plant alkaloid tetrandrine in autoimmune diseases. Acta Pharmacol Sin 2002; 23: 1093–101. [PubMed] [Google Scholar]
- Ho LJ, Lai JH. Chinese herbs as immunomodulators and potential disease-modifying antirheumatic drugs in autoimmune disorders. Curr Drug Metab 2004; 5: 181–92. [DOI] [PubMed] [Google Scholar]
- Liu T, Liu X, Li W. Tetrandrine, a Chinese plant-derived alkaloid, is a potential candidate for cancer chemotherapy. Oncotarget 2016; 7: 40800–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei L, Chen Y, Wang Z, Wang J, Wan J, Yu C, et al. Synergistic anti-tumour effects of tetrandrine and chloroquine combination therapy in human cancer: a potential antagonistic role for p21. Br J Pharmacol 2015; 172: 2232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, Wang T, Qian X, Liu G, Yu L, Ding Y. Anticancer effect of tetrandrine on primary cancer cells isolated from ascites and pleural fluids. Cancer Lett 2008; 268: 166–75. [DOI] [PubMed] [Google Scholar]
- Wu JM, Chen Y, Chen JC, Lin TY, Tseng SH. Tetrandrine induces apoptosis and growth suppression of colon cancer cells in mice. Cancer Lett 2010; 287: 187–95. [DOI] [PubMed] [Google Scholar]
- Xu WL, Shen HL, Ao ZF, Chen BA, Xia W, Gao F, et al. Combination of tetrandrine as a potential-reversing agent with daunorubicin, etoposide and cytarabine for the treatment of refractory and relapsed acute myelogenous leukemia. Leuk Res 2006; 30: 407–13. [DOI] [PubMed] [Google Scholar]
- Libermann TA, Zerbini LF. Targeting transcription factors for cancer gene therapy. Curr Gene Ther 2006; 6: 17–33. [DOI] [PubMed] [Google Scholar]
- Lai JH, Ho LJ, Lu KC, Chang DM, Shaio MF, Han SH. Western and Chinese antirheumatic drug-induced T cell apoptotic DNA damage uses different caspase cascades and is independent of Fas/Fas ligand interaction. J Immunol 2001; 166: 6914–24. [DOI] [PubMed] [Google Scholar]
- Lai JH, Ho LJ, Kwan CY, Chang DM, Lee TC. Plant alkaloid tetrandrine and its analog block CD28-costimulated activities of human peripheral blood T cells: potential immunosuppressants in transplantation immunology. Transplantation 1999; 68: 1383–92. [DOI] [PubMed] [Google Scholar]
- Yang SP, Ho LJ, Lin YL, Cheng SM, Tsao TP, Chang DM, et al. Carvedilol, a new antioxidative beta-blocker, blocks in vitro human peripheral blood T cell activation by downregulating NF-kappaB activity. Cardiovasc Res 2003; 59: 776–87. [DOI] [PubMed] [Google Scholar]
- Liou JT, Chen ZY, Ho LJ, Yang SP, Chang DM, Liang CC, et al. Differential effects of triptolide and tetrandrine on activation of COX-2, NF-kappaB, and AP-1 and virus production in dengue virus-infected human lung cells. Eur J Pharmacol 2008; 589: 288–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS. A less-canonical, canonical NF-kappaB pathway in DCs. Nat Immunol 2012; 13: 1139–41. [DOI] [PubMed] [Google Scholar]
- Zhao WL. Targeted therapy in T-cell malignancies: dysregulation of the cellular signaling pathways. Leukemia 2010; 24: 13–21. [DOI] [PubMed] [Google Scholar]
- Greaves MF. Differentiation-linked leukemogenesis in lymphocytes. Science 1986; 234: 697–704. [DOI] [PubMed] [Google Scholar]
- Burger R, Hansen-Hagge TE, Drexler HG, Gramatzki M. Heterogeneity of T-acute lymphoblastic leukemia (T-ALL) cell lines: suggestion for classification by immunophenotype and T-cell receptor studies. Leuk Res 1999; 23: 19–27. [DOI] [PubMed] [Google Scholar]
- Minowada J, Onuma T, Moore GE. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst 1972; 49: 891–5. [PubMed] [Google Scholar]
- Smith SD, Shatsky M, Cohen PS, Warnke R, Link MP, Glader BE. Monoclonal antibody and enzymatic profiles of human malignant T-lymphoid cells and derived cell lines. Cancer Res 1984; 44: 5657–60. [PubMed] [Google Scholar]
- Merlo LM, Pepper JW, Reid BJ, Maley CC. Cancer as an evolutionary and ecological process. Nat Rev Cancer 2006; 6: 924–35. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov 2009; 8: 33–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaulian E. AP-1 — The Jun proteins: Oncogenes or tumor suppressors in disguise? Cell Signal 2010; 22: 894–9. [DOI] [PubMed] [Google Scholar]
- Ritke MK, Bergoltz VV, Allan WP, Yalowich JC. Increased c-jun/AP-1 levels in etoposide-resistant human leukemia K562 cells. Biochem Pharmacol 1994; 48: 525–33. [DOI] [PubMed] [Google Scholar]
- Chen DW, Saha V, Liu JZ, Schwartz JM, Krstic-Demonacos M. Erg and AP-1 as determinants of glucocorticoid response in acute lymphoblastic leukemia. Oncogene 2013; 32: 3039–48. [DOI] [PubMed] [Google Scholar]
- Chen-Deutsch X, Garay E, Zhang J, Harrison JS, Studzinski GP. c-Jun N-terminal kinase 2 (JNK2) antagonizes the signaling of differentiation by JNK1 in human myeloid leukemia cells resistant to vitamin D. Leuk Res 2009; 33: 1372–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr I, Debatin KM. Cellular stress response and apoptosis in cancer therapy. Blood 2001; 98: 2603–14. [DOI] [PubMed] [Google Scholar]
- Xie JY, Chen N, Ren H, Wang WM. Angiotensin II-mediated activation of fibrotic pathways through ERK1/2 in rat peritoneal mesothelial cells. Ren Fail 2010; 32: 871–9. [DOI] [PubMed] [Google Scholar]
- Pritchard AL, Hayward NK. Molecular pathways: mitogen-activated protein kinase pathway mutations and drug resistance. Clin Cancer Res 2013; 19: 2301–9. [DOI] [PubMed] [Google Scholar]
- Leung KT, Li KK, Sun SS, Chan PK, Ooi VE, Chiu LC. Activation of the JNK pathway promotes phosphorylation and degradation of BimEL — a novel mechanism of chemoresistance in T-cell acute lymphoblastic leukemia. Carcinogenesis 2008; 29: 544–51. [DOI] [PubMed] [Google Scholar]
- Martel J, Payet MD, Dupuis G. The MDR1 (P-glycoprotein) and MRP (P-190) transporters do not play a major role in the intrinsic multiple drug resistance of Jurkat T lymphocytes. Leuk Res 1997; 21: 1077–86. [DOI] [PubMed] [Google Scholar]
- Estes DA, Lovato DM, Khawaja HM, Winter SS, Larson RS. Genetic alterations determine chemotherapy resistance in childhood T-ALL: modelling in stage-specific cell lines and correlation with diagnostic patient samples. Br J Haematol 2007; 139: 20–30. [DOI] [PubMed] [Google Scholar]
- Onda K, Suzuki R, Tanaka S, Oga H, Oka K, Hirano T. Decitabine, a DNA methyltransferase inhibitor, reduces P-glycoprotein mRNA and protein expressions and increases drug sensitivity in drug-resistant MOLT4 and Jurkat cell lines. Anticancer Res 2012; 32: 4439–44. [PubMed] [Google Scholar]
- Labroille G, Dumain P, Lacombe F, Belloc F. Flow cytometric evaluation of fas expression in relation to response and resistance to anthracyclines in leukemic cells. Cytometry 2000; 39: 195–202. [PubMed] [Google Scholar]
- Liu ZL, Hirano T, Tanaka S, Onda K, Oka K. Persistent reversal of P-glycoprotein-mediated daunorubicin resistance by tetrandrine in multidrug-resistant human T lymphoblastoid leukemia MOLT-4 cells. J Pharm Pharmacol 2003; 55: 1531–7. [DOI] [PubMed] [Google Scholar]
- Wei N, Sun H, Wang F, Liu G. H1, a novel derivative of tetrandrine reverse P-glycoprotein-mediated multidrug resistance by inhibiting transport function and expression of P-glycoprotein. Cancer Chemother Pharmacol 2011; 67: 1017–25. [DOI] [PubMed] [Google Scholar]