Abstract
Loss of glutamine synthetase (GS) in hippocampal astrocytes has been implicated in the causation of human mesial temporal lobe epilepsy (MTLE). However, the mechanism by which the deficiency in GS leads to epilepsy is incompletely understood. Here we ask how hippocampal GS inhibition affects seizure phenotype and neuronal activation during epilepsy development (epileptogenesis). Epileptogenesis was induced by infusing the irreversible GS blocker methionine sulfoximine (MSO) unilaterally into the hippocampal formation of rats. We then used continuous video-intracranial electroencephalogram (EEG) monitoring and c-Fos immunohistochemistry to determine the type of seizures and spatial distribution of neuronal activation early (1–5 days postinfusion) and late (16–43 days postinfusion) in epileptogenesis. Early in epileptogenesis, seizures were preferentially mild (stage 1–2), activating neurons in the entorhinal-hippocampal area, the basolateral amygdala, the piriform cortex, the midline thalamus, and the anterior olfactory area. Late in epileptogenesis, the seizures were generally more severe (stage 4–5) with neuronal activation extending to the neocortex, the bed nucleus of the stria terminalis, the mediodorsal thalamus, and the central nucleus of the amygdala. Our findings demonstrate that inhibition of GS focally in the hippocampal formation triggers a process of epileptogenesis characterized by gradual worsening of seizure severity and involvement of progressively larger neuronal populations over a period of several weeks. Knowledge about the underlying mechanism of epileptogenesis is important because such knowledge may result in more specific and efficacious treatments of MTLE by moving away from large and poorly specific surgical resections to highly targeted surgical or pharmacological interventions of the epileptogenic process.
Keywords: c-Fos, entorhinal cortex, epileptogenesis, hippocampus, limbic structures
Mesial temporal lobe epilepsy (MTLE) is one of the most common types of medication refractory epilepsies. For up to one-third of patients with MTLE, current antiepileptic drugs do not adequately control seizures, or cause side effects that limit their use. Because the seizures are thought to originate from mesial (i.e. limbic) structures within the medial temporal lobe–particularly the hippocampal formation, entorhinal cortex and amygdala–unilateral resection of these structures along with adjacent white matter tracts and neocortical areas has remained the standard of care for medication refractory MTLE for over half a century (Falconer and Taylor, 1968; Spencer et al., 1984). While such surgery leads to short-term cessation of seizures in more than 75% of cases, the procedure can result in complications and adverse outcomes (Hader et al., 2013). Moreover, the seizures will eventually recur in approximately 50% of patients who become seizure free after surgery (Foldvary et al., 2000; Jeha et al., 2006; Yoon et al., 2003), suggesting that the causative mechanism of MTLE is not removed by current neurosurgical approaches, but remains latent elsewhere in the brain. Knowledge about the causative mechanism of epilepsy is important because such knowledge is expected to result in more specific and efficacious treatments of MTLE.
There is increasing evidence to suggest that the enzyme glutamine synthetase (GS) is implicated in the causation of epilepsy. GS is deficient in hippocampal astrocytes in patients with medication refractory MTLE (Eid et al., 2004; van der Hel et al., 2005) and in astrocytes in the amygdala in patients with neocortical epilepsies (Steffens et al., 2005). Because GS is necessary for the conversion of glutamate and ammonia to glutamine, its deficiency is expected to cause an increase in glutamate and ammonia in astrocytes lacking the enzyme. It is possible that the high levels of glutamate and ammonia in astrocytes can enter the extracellular compartment of the brain via several mechanisms and lead to seizures, oxidative/nitrosative stress, inflammation, and cellular injury (Bezzi et al., 1998; Danbolt, 2001; Olney et al., 1972; Robert et al., 2015; Tian et al., 2005).
We have previously shown that inhibiting GS by chronic infusion of the irreversible enzyme blocker methionine sulfoximine (MSO) into the hippocampal formation or amygdala of rats, leads to a sequence of events similar to those experienced by some patients with MTLE. I.e. an initial episode of prolonged, low-grade seizures; a clinically silent latent period; and eventually, recurrent seizures that increase in severity as the disease evolves over time. The MSO-treated animals recapitulate many other features of human MTLE such as perturbations in the homeostasis of neurotransmitter glutamate, loss of hippocampal neurons, changes in monocarboxylate transporters, and comorbid depressive features (Eid et al., 2008; Gruenbaum et al., 2015; Lauritzen et al., 2012). We now use c-Fos staining in the MSO-model of MTLE to assess how hippocampal GS inhibition affects neuronal activation during epileptogenesis. Our working hypothesis is that epileptogenesis caused by hippocampal GS inhibition is characterized by activation of progressively larger neuronal populations over a period of weeks, suggestive of a process of evolving neuronal plasticity.
Materials and Methods
Chemicals and animals
All chemicals were obtained from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise noted. Male Sprague Dawley rats were used in this study (200 to 250g; Charles River Laboratories, Wilmington, Mass.). Rats had free access to food and water and were housed on a 12h/12h light/dark cycle, with lights on from 7 a.m. to 7 p.m. The animal care and use procedures were approved by the Institutional Animal Care and Use Committee of Yale University. All experiments were performed in accordance with current guidelines.
Intrahippocampal infusion of MSO or PBS
Nineteen rats were anesthetized with 1% to 2% Isoflurane (Baxter, Deerfield, Ill.) in O2 and placed in a stereotaxic frame (David Kopf Instruments, Tujunga, Calif.). A 30-gauge stainless steel cannula attached to a plastic pedestal (Plastics One, Roanoke, Va.) was introduced through a burr hole in the skull and into the right hippocampus, using the following coordinates with bregma as the reference: AP= −5.6 mm, ML=5.3 mm, DV= −6.5 mm. The cannula was cemented to the skull using cyanoacrylate and connected via plastic tubing to a subcutaneously implanted Alzet osmotic pump (Model 2004, Durect Corp., Cupertino, Calif.). This pump holds a total volume of 200 μL and delivers a continuous flow of 0.25 μL/h for 28 days (as per manufacturer’s specifications). One set of pumps was filled with MSO (2.5 mg/mL; dissolved in Dulbecco’s Phosphate Buffered Saline, PBS) to achieve a delivery of 0.625 μg of MSO per h (n = 10). Another set of pumps was filled with PBS and implanted in control animals (n = 9). Rats were operated on sequentially in 10 pairs of 2, each pair consisting of an MSO injected rat and a matched PBS control such that treatment and timing of fixation in relation to surgery could be maximally approximated in the control.
Four stainless steel epidural screw electrodes (Plastics One) were implanted to record cortical electroencephalogram (EEG) activity. Two electrodes (one in each hemisphere) were positioned in the epidural space overlying the parietal neocortex. A third electrode was positioned in the epidural space near lambda to serve as the reference. A fourth electrode was positioned over the occipital bone (not touching the dura) to serve as the ground. The female socket contacts on the end of each electrode were inserted into a plastic pedestal (Plastics One), and the entire implantation was secured by UV light cured acrylated urethane adhesive (Loctite 3106 Light Cure Adhesive, Henkel Corp., Rocky Hill, Conn.).
Video-intracranial EEG monitoring and seizure quantitation
The experimental setup for recording video-EEG was adapted from Bertram et al. (Bertram, 2006). The rats were placed individually in custom-made Plexiglas cages. A spring-covered, 6-channel cable was connected to the electrode pedestal on one end and to a commutator (Plastics One) on the other. A second cable connected the commutator to the digital EEG recording unit (CEEGraph Vision LTM, Natus Bio-logic Systems Corp., Mundelein, Ill.). Digital cameras with infrared light detection capacity were used to record animal behavior (two cages per camera). The digital video signal was encoded and synchronized to the digital EEG signals. Seizures were identified by visual inspection of the EEG record. As detailed in (Avoli and Gloor, 1994) seizures were defined by EEG characteristics and not by the duration of the discharge. Specifically, seizures displayed distinct signal changes from background (interictal) activity. Such signal changes included sustained rhythmic or spiking EEG patters and a clear evolution of signal characteristics from onset to termination.
As timing of fixation following seizures was critical to the c-Fos staining method described below, the rats were followed in real time by a researcher blinded to their condition (MSO or PBS). Eight rats were monitored at a time, always consisting of 4 MSO rats paired with their matched PBS control. One set of MSO and PBS rats was monitored during the first five days of MSO infusion (early epileptogenesis group), and another set was monitored during days 15 to 43 after MSO infusion (late epileptogenesis group). When seizure activity was observed on the EEG and by monitoring rat behavior by the blinded researcher in real time, the time of the seizure was noted, and a second researcher immediately reviewed the video-EEG recordings to independently confirm the occurrence of, and stage of the seizure. The seizures were staged by behavioral analysis using a modification of Racine’s criteria (Racine et al., 1973), as follows: stage I, immobilization, eye blinking, twitching of vibrissae and mouth movements; stage II, head nodding, often accompanied by facial clonus; stage III, forelimb clonus; stage IV, rearing; stage V, rearing, falling and generalized convulsions. The MSO seizure rat and its matched PBS control were transported together from the monitoring room to the main lab 45 minutes following the seizure for perfusion fixation, which occurred 1 h after the onset of the seizure.
Pilocarpine control
In order to confirm the efficacy of the c-Fos staining protocol, 1 rat was injected subcutaneously with 325 mg/kg pilocarpine dissolved in PBS. Within a few hours of injection several stage V seizures occurred. One hour after the onset of the first stage V seizure the rat was perfusion fixed and processed for c-Fos staining following the same protocol as for MSO/PBS infused rats.
Tissue preparation, immunohistochemistry and nomenclature
At 1 hour after the observed seizure, the MSO seizure rat and its matched PBS control were anesthetized with Isoflurane and perfused transcardially with saline followed by 4% formaldehyde in phosphate buffer (PB; 0.1M, pH 7.4). The brains were removed and left in the same fixative overnight at 4°C and then transferred to 20% sucrose (dissolved in PB) the next day. After at least 24 hours in 20% sucrose, the brains were sectioned horizontally on a sliding microtome at 50-μm thickness. Every fifth section was mounted on gelatin-coated slides and stained with cresyl violet. The remaining four sets of sections were stored in FD Tissue Cryoprotection Solution (FD Neurotechnologies, Columbia, Md.) at −20 °C prior to staining. These sectioned brains were logged and randomly coded by one researcher in order to blind a second researcher to the condition of the rats. Blinding the primary researcher to both the condition (MSO, PBS, pilocarpine) and to the stage of seizure (stage 1 or stage 5) was critical due to the descriptive and subjective nature of the analysis.
Staining with c-Fos was performed after completion of all seizure monitoring and brain sectioning on a total of 20 rats (10 MSO, 9 PBS, 1 pilocarpine). All of the following steps were performed at room temperature using a shaker on a low setting for continuous, but gentle mixing. Brain sections were removed from −20°C and washed in fresh PBS solution for 5 minutes, 3 times. Membranes were then permeabilized with a wash in 0.3% Triton-X-100 in 0.1 M PB for 30 minutes, followed by 3 washes in PBS of 5 minutes each. Brain sections were then transferred to 1 M ethanolamine in 0.1 M PB for 30 minutes to quench aldehydes, followed again by 3 washes in PBS of 5 minutes each. The sections were next transferred to 4% dry milk in PBS for blocking. After 1 hour in the dry milk, the primary antibody, rabbit anti-c-Fos IgG was added at a dilution of 1: 10,000, the dish was sealed with parafilm, and left overnight on the shaker. The following day, the sections were first washed in fresh PBS for 1 minute, followed by 3 consecutive 20 minute washes. Next, they were moved to secondary biotinylated anti-rabbit IgG antibody from a Vectastain Elite kit (Vector Laboratories, Burlingame, Calif.) diluted as instructed to 0.05% in PBS. The sections were then washed in fresh PBS for 1 minute, followed by 3 consecutive 15 minute washes. Next, the sections were transferred to the Vectastain A+B solution mixed at a ratio of 1 drop per 5 mL PBS, which was allowed to stand for at least 30 minutes prior to use. The sections were then washed again in fresh PBS for 1 minute followed by 3 consecutive 10 minute washes. Finally, the staining reaction was developed by transferring the sections to 0.05% DAB, 0.009% H2O2 in PBS. Sections were initially monitored carefully for development, periodically checking the progress under the microscope. Ultimately it was decided that 15 minutes was the optimal time. After 15 minutes in this solution, the sections were washed in 0.1 M PB for 10 minutes, 3 consecutive times. Sections were then carefully mounted on gelatin-coated slides using 0.1 M PB and allowed to dry overnight before they were covered and logged for analysis under the microscope.
The slides were covered and examined under a light microscope. The subdivisions of the hippocampus are in accordance with the work of Lorente de Nó (Lorente de Nó, 1934) with the modifications suggested by Amaral and Insausti (Amaral and Insausti, 1990). In brief, the hippocampus is divided into (a) the subiculum, (b) the Ammon’s horn (hippocampus proper), which comprises fields CA1-3, and (c) the dentate gyrus, which includes the molecular layer, granule cell layer, and polymorphic layer of the hilus.
Analysis and grading protocol
Four treatment groups were analyzed (Table 1): MSO injection Racine stage 1 seizure/early epileptogenesis (n = 7), MSO injection Racine stage 5 seizure/late epileptogenesis (n = 3), PBS injection negative control (n = 9), and pilocarpine Racine stage 5 seizure positive staining control (n = 1). The researcher performing the analysis remained blinded to the condition of the rats. First the sections stained at 1:10,000 primary antibody dilutions were observed generally. Of the 20 rats under analysis, 11 were found to have grossly apparent staining of varying extent at 4x magnification, and 9 were found to have nearly absent staining at 4x magnification. These were then re-examined at 10x magnification and it was determined that these 9 rats had no staining of significance. It was also determined at this time that the staining of the 11 rats, while grossly apparent, was suboptimal in terms of intensity. It was decided to repeat the above staining procedure with two minor changes as described. This was done on the 11 rats with apparent staining and 1 selected rat of the 9 without apparent staining from the original protocol. The researcher performing the staining and analysis remained blinded to the condition of the selected rats.
Table 1.
Log of animals and observed seizures under study. MSO-infused rats and matched PBS-infused controls were perfusion fixed with paraformaldehyde about 1 h after the observed seizure. The brains were then serial sectioned into 5 sets of evenly spaced 50 μm sections. One set was stained with cresyl violet for anatomic reference. All brains were stained with c-Fos.
| Animal | Treatment | Injection Site | Seizure Stage | Post-Op Day of seizure | Time Seizure to Perfusion (h:min) |
|---|---|---|---|---|---|
|
Early Epileptogenesis Rats (Days 1–5; Stage 1 Seizure) and Paired Controls
| |||||
| A | MSO | Angular Bundle | 1 | 1 | 1:15 |
| B | PBS | Angular Bundle | |||
| J | MSO | Deep Entorhinal Cortex | 1 | 4 | 1:00 |
| I | PBS | Deep Entorhinal Cortex | |||
| N | MSO | Deep Entorhinal Cortex | 1 | 3 | 1:20 |
| M | PBS | Deep Entorhinal Cortex | |||
| D | MSO | Deep Entorhinal Cortex | 1 | 1 | 1:00 |
| C | PBS | Angular Bundle | |||
| E | MSO | Deep Entorhinal Cortex | 1 | 1 | 1:00 |
| F | PBS | Angular Bundle | |||
| K | MSO | Deep Entorhinal Cortex | 1 | 3 | 1:05 |
| L | PBS | Subiculum | |||
| R | MSO | Angular Bundle | 1 | 5 | 1:00 |
| Q | PBS | Angular Bundle | |||
|
| |||||
|
Late Epileptogenesis Rats (Days 16–43; Stage 5 Seizure) and Paired Controls
| |||||
| O | MSO | Subiculum | 5 | 16 | 1:03 |
| P | PBS | Subiculum | |||
| H | MSO | Subiculum | 5 | 18 | 1:00 |
| G | PBS | Subiculum | |||
| S | MSO | Subiculum | 5 | 43 | 1:00 |
| T | Pilocarpine | N/N(Subcutaneous) | 5 | - | 1:00 |
The 1:2,500 dilution c-Fos stained sections from the 12 rats were generally observed and a semi-quantitative grading scale was devised to summarize the findings. The sections were analyzed sequentially, using the Paxinos Rat Brain Atlas as a reference (Paxinos and Watson, 2006), first to identify the approximate dorso-ventral level of the horizontal section, then to grade nuclear c-Fos staining in the various regions/nuclei. One hundred and nine different regions/nuclei were considered independently, including 27 regions of the hippocampus and cortex, which were considered separately on the ipsilateral and contralateral hemispheres relative to the injection site. Fifty-five subcortical regions were considered bilaterally, but differences in staining of between ipsilateral and contralateral side were noted. It was attempted to consider as much detail as possible, but some nuclei had to be grouped (for example the subnuclei related to the stria terminalis) due to an inability to resolve any finer detail after considering all available anatomical clues to orientation.
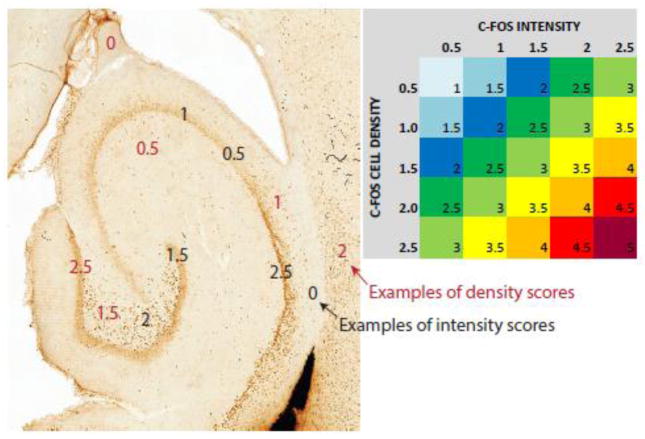
Staining in each region was graded in terms of both density (number of cells per unit area) and intensity (darkness of the coloring within each cell; Figure 1). Density was graded on a stepwise scale from 0.5 to 2.5, with 0.5 representing the highest density and 2.5 the highest density staining observed. Intensity was graded on a similar scale from 0.5 to 2.5, with 0.5 representing c-fos visible just above the background staining and 2.5 representing extremely intense staining. Finally, the scores for intensity and density were added to a single score, ranging from 1 (lowest density and intensity observed) to 5 (highest density and intensity observed). The scores are listed in Table 2 and color coded according to their magnitude. It was recognized that this method of analysis is subjective and would likely not be perfectly replicable with a different researcher doing the analysis. The goal was not to rigorously quantitate the level of c-Fos staining, but rather to assess the relative activation of various areas of the brain during seizures, and how these networks of activation change and develop with development from stage 1 to stage 5 seizures. The combined density and intensity scores for the 7 early and 3 late stage animals were compared using a two-sided Mann-Whitney U test, separately for each of the areas studied. The areas which were significantly different (p <0.05) were reported. A correction for multiple comparisons was not performed for this exploratory data analysis.
Figure 1. Grading and color coding.
C-Fos staining was graded on two independent scales, 0.5 to 2.5 for the density (number of stained cells/unit area) and 0.5 to 2.5 for intensity (darkness of staining/cell) in all analyzed brain regions. Examples of density scoring are shown in red font and of intensity scoring in black font. The numbers for density and intensity were added to provide single scores, which were plotted in Table 2.
Table 2.
C-Fos staining in the hippocampal formation and cortical regions (Table 2A), and in subcortical regions and the brainstem (Table 2B). Seven rats from early epileptogenesis and three rats from late epileptogenesis are shown in columns A and B followed in column C by one representative PBS-treated negative control and one pilocarpine-treated positive control. Statistical comparisons between early epileptogenesis (A) and late epileptogenesis (B) are provided in the rightmost column. Only p-values <0.05, with their corresponding brain areas highlighted in gray, are shown. Each region was independently graded for staining density (number of stained cells per unit area, scored 0.5–2.5) and intensity (darkness of stain within each cell, scored 0.5–2.5), and the grades for density and intensity were added to a single numerical score and color coded according to its magnitude (see also Figure 1). For regions not explicitly shown bilaterally, differences in staining ipsilateral and contralateral to the injection are noted: * decreased staining contralateral vs. ipsilateral; ** staining only observed ipsilateral to injection.
| Brain Regions | (A) MSO Early Epileptogenesis | (B) MSO Late Epileptogenesis | (C) Controls | Statistics Early vs Late (P) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Rat A (m) | Rat D (m) | Rat E (m) | RatJ (m) | Rat K (m) | Rat N (m) | Rat R (m) | Rat H (m) | Rat O (m) | Rat S (m) | Rat C (pbs) | RatT (pilo) | |||
| Molecular | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ipsilateral Dentate | Granular | 3 | 3 | 3 | 3 | 3 | 4 | 4.5 | 3 | 3.5 | 4.5 | 0 | 3.5 | |
| Polymorphic | 2.5 | 2.5 | 2.5 | 3.5 | 3 | 3 | 3 | 2 | 3.5 | 2.5 | 0 | 0 | ||
| Stratum Oriens | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ipsilateral CA3 | Pyramidal Cell | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | |
| SR/SL | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1.5 | ||
| Ipsilateral SLM | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | ||
| Stratum Oriens | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Ipsilateral CA2 | Pyramidal Cell | 2 | 2 | 2.5 | 2.5 | 2 | 2.5 | 2.5 | 3 | 2.5 | 2.5 | 0 | 3 | |
| SR/SL | 1 | 1 | 0 | 1 | 0 | 1 | 1 | 0 | 1 | 0 | 0 | 1.5 | ||
| Stratum Oriens | 1 | 1 | 1 | 1.5 | 1 | 1 | 1 | 0 | 1.5 | 1 | 0 | 0 | ||
| Ipsilateral CA1 | Pyramidal Cell | 4 | 4 | 3.5 | 3.5 | 3 | 3.5 | 3.5 | 3 | 3.5 | 3.5 | 0 | 3.5 | |
| SR/SL | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1.5 | ||
| Ipsilateral Subiculum | Superficial | 2.5 | 3 | 3 | 3 | 2.5 | 3 | 3 | 2.5 | 3.5 | 3.5 | 0 | 2.5 | |
| Deep | 3 | 2.5 | 2 | 2 | 2 | 2 | 2 | 2 | 3.5 | 2 | 0 | 3 | ||
| Ipsilateral Presubiculum | 3.5 | 3.5 | 0 | 0 | 0 | 2 | 0 | 0 | 5 | 0 | 0 | 3 | ||
| Ipsilateral Parasubiculum | 3.5 | 3 | 3 | 3 | 3.5 | 3 | 3.5 | 3 | 4.5 | 0 | 0 | 3.5 | ||
| Ipsilateral Entorhinal | Superficial | 3 | 2.5 | 2 | 3 | 2.5 | 3 | 3 | 3 | 4 | 3.5 | 0 | 3 | |
| Cortex | Deep | 3.5 | 4 | 2.5 | 4.5 | 2 | 2.5 | 3 | 1 | 3.5 | 3 | 1 | 3 | |
| Perirhinal | 3 | 3 | 3 | 2.5 | 2.5 | 1.5 | 1 | 3 | 3.5 | 3.5 | 1 | 2.5 | ||
| Ectorhinal | 3 | 3 | 3 | 2.5 | 2.5 | 2.5 | 1 | 4.5 | 4 | 3.5 | 1.5 | 3 | 0.017 | |
| Temp Ass | 3 | 3 | 3 | 2 | 0 | 2.5 | 1 | 4.5 | 3.5 | 3.5 | 1.5 | 3 | 0.017 | |
| Ipsilateral Cortex | Auditory | 2.5 | 2.5 | 0 | 0 | 0 | 0 | 0 | 3.5 | 3.5 | 3.5 | 1 | 3 | 0.017 |
| Sensorimotor | 2 | 2.5 | 0 | 0 | 0 | 0 | 0 | 3 | 3.5 | 3.5 | 0 | 3 | 0.017 | |
| Insular | 2 | 2.5 | 0 | 0 | 0 | 0 | 0 | 3 | 3.5 | 3.5 | 0 | 3 | 0.017 | |
| Piriform | 3 | 3 | 0 | 3 | 3 | 2 | 2 | 3 | 3 | 3 | 2 | 4.5 | ||
| Limbic | 2.5 | 3 | 0 | 2 | 1 | 1 | 2 | 4.5 | 4 | 4.5 | 0 | 2.5 | 0.017 | |
|
|
||||||||||||||
| Molecular | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Contralateral Dentate | Granular | 4 | 3.5 | 3 | 3.5 | 3 | 4.5 | 4.5 | 3.5 | 3.5 | 4.5 | 0 | 3.5 | |
| Polymorphic | 3.5 | 2.5 | 2.5 | 2 | 3 | 2.5 | 3 | 2.5 | 3.5 | 2.5 | 0 | 0 | ||
| Stratum Oriens | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Contralateral CA3 | Pyramidal Cell | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 0 | 3 | |
| SR/SL | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | ||
| Contralateral SLM | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Stratum Oriens | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Contralateral CA2 | Pyramidal Cell | 2.5 | 2.5 | 3 | 3 | 2.5 | 2.5 | 2.5 | 2.5 | 3 | 2.5 | 0 | 3 | |
| SR/SL | 1 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 2 | ||
| Stratum Oriens | 2 | 1 | 1 | 2 | 1 | 1 | 1 | 0 | 1 | 0 | 0 | 1 | ||
| Contralateral CA1 | Pyramidal Cell | 4 | 4 | 3.5 | 3.5 | 3 | 3.5 | 3.5 | 3.5 | 3.5 | 3.5 | 0 | 3.5 | |
| SR/SL | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 2 | 0.017 | |
| Contralateral | Superficial | 3 | 2.5 | 2.5 | 2.5 | 3 | 3 | 3 | 3 | 3 | 2.5 | 0 | 3 | |
| Subiculum | Deep | 3 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 0 | 3.5 | |
| Contralateral Presubiculum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ||
| Contralateral Parasubiculum | 3 | 2.5 | 3 | 1.5 | 1 | 2 | 2 | 3.5 | 3.5 | 3 | 0 | 0 | ||
| Contralateral | Superficial | 3.5 | 2 | 2 | 0 | 1 | 1 | 2 | 3 | 3 | 2.5 | 0 | 3.5 | |
| Entorhinal Cortex | Deep | 1.5 | 0 | 0 | 0 | 0 | 0 | 1 | 1.5 | 3 | 2.5 | 0 | 3.5 | 0.033 |
| Perirhinal | 1.5 | 1 | 0 | 0 | 0 | 1 | 1 | 3 | 3.5 | 2.5 | 1 | 2.5 | 0.017 | |
| Ectorhinal | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 3.5 | 3.5 | 3.5 | 1 | 3 | 0.017 | |
| Temporal Assoc. | 1 | 1 | 0 | 0 | 0 | 1 | 1 | 3.5 | 3.5 | 3.5 | 1 | 3 | 0.017 | |
| Contralateral Cortex | Auditory | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | 3.5 | 3.5 | 0 | 3 | 0.017 |
| Sensorimotor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3.5 | 3.5 | 0 | 3 | 0.017 | |
| Insular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.5 | 3.5 | 3.5 | 0 | 3 | 0.017 | |
| Piriform | 2.5 | 2 | 0 | 0 | 0 | 2.5 | 2 | 3 | 3.5 | 3.5 | 1 | 4.5 | 0.017 | |
| Limbic | 1 | 1.5 | 0 | 0 | 0 | 1 | 2 | 2.5 | 4 | 3.5 | 0 | 2.5 | 0.017 | |
| Brain Regions | (A) MSO Early Epileptogenesis | (B) MSO Late Epileptogenesis | (C) Controls | Statistics Early vs Late (P) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||
| Rat A(m) | Rat D (m) | Rat E (m) | RatJ (m) | Rat K (m) | Rat N (m) | RatR (m) | Rat H (m) | Rat O (m) | Rat S (m) | Rat C (pbs) | RatT (pilo) | |||
| Claustrum | 2* | 2.5** | 0 | 0 | 1 | 1.5 | 1.5 | 3 | 3 | 3.5 | 0 | 3 | 0.017 | |
| Endopiriform | Dorsal | 1.5* | 2.5** | 0 | 0 | 1 | 0 | 1 | 2.5 | 3 | 3 | 0 | 3 | 0.033 |
| Inter./Vent. | 1.5* | 2.5** | 0 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | 0 | 3 | ||
| Amygdalopiriform | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Amygdalohippoc. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Basal | 0 | 2** | 0 | 0 | 0 | 0 | 0 | 0 | 2.5* | 2.5* | 0 | 2.5 | ||
| Amygdala | Lateral | 3.5** | 3.5** | 4** | 2.5** | 3** | 1.5** | 0 | 3.5** | 3* | 3* | 1 | 1.5 | |
| Intercalated | 1** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | ||
| Central | 2.5** | 3** | 0 | 0 | 0 | 1.5 | 0 | 0 | 1.5* | 2* | 0 | 3 | ||
| Medial | 1** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5* | - | 0 | 2.5 | ||
| Ant. Olfactory Area | 4.5** | 3** | 3* | 4** | 3.5** | 0 | 2.5* | 3.5* | 4 | 4.5* | 0 | 4 | ||
| Olfactory Tubercle | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4.5 | ||
| Navicular Nuc | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | ||
| Tenia Tecta | 3.5 | 3.5** | 2.5** | 4** | 3.5 | 0 | 2 | 3 | 4 | 4.5* | 0 | 4.5 | ||
| Nucleus Accumbens | 1 | 1.5* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | 0 | 3.5 | ||
| Medial Septum | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Lateral Septum | 2 | 1 | 0 | 0 | 1 | 2 | 2 | 2* | 2.5* | 0 | 2 | 3 | ||
| Triangonal Septal Nucleus | 1.5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | ||
| Diagonal Band | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | ||
| Stria Terminalis | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 2** | 2.5* | 2.5* | 0 | 2.5 | 0.017 | |
| Median Preoptic | 0 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 1 | 0 | 0 | 2.5 | ||
| Medial Preoptic | 2 | 1.5 | 0 | 0 | 0 | 1 | 0 | 0 | 2.5* | 0 | 1.5 | 2.5 | ||
| Dorsomedial | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 2* | 3 | 2* | 0 | 3.5 | ||
| Hypothalamus | Posterior | 0 | 1 | 1 | 1 | 0 | 1 | 1 | 0 | - | 2* | 0 | 2.5 | |
| Paraventricular | 1 | 1.5 | 0 | 0 | 1 | 1 | 1 | 0 | 3* | 0 | 1.5 | 4 | ||
| Anterior | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 1.5 | 0 | 0 | 2.5 | ||
| Lateral | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | ||
| Globus Pallidus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Caudate/Putaman | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5 | 2* | 0 | 0 | 3.5 | ||
| Reticular | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | ||
| Reuniens | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | ||
| VM | 1.5* | 2** | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Rhomboid | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3.5 | ||
| Anterior | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| VPL/VPM | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Thalamus | VA/VL | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Po/Lat. Po. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Submedian | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.5* | 0 | 0 | ||
| IAM | 2 | 2 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 3 | ||
| IMD/MD | 2 | 2.5 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 0 | 0 | ||
| CM/CL | 2.5** | 2.5* | 0 | 2 | 1 | 1.5 | 1 | 0 | 2 | 2 | 0 | 0 | ||
| Ethmoid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | - | - | 0 | 0 | ||
| PVA | 2.5 | 2 | 2 | 2 | 2 | 2 | 2.5 | 1.5 | 2.5 | 3 | 2 | 3.5 | ||
| Habenula | 0 | 2.5 | 0 | 0 | 2 | 0 | 0 | 2 | 0 | 0 | ||||
| Subthalamus | 1* | 1.5 | 0 | 0 | 0 | 0 | 1 | 0 | 3 | 2.5* | 0 | 3.5 | ||
| Interpeduncular Nuc | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3.5 | ||
| Supramammillary Nuc | 2 | 2.5 | 0 | 1 | 1 | 1 | 0 | 0 | 2.5 | 2 | 2 | 2.5 | ||
| Substantia Nigra | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Zona Incerta | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Geniculate Nuc | 2.5* | 3** | 1.5 | 2* | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2* | 1.5 | 3 | ||
| Inters. of Cajal | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 0 | 0 | 0 | 1 | 0 | ||
| Tectal/Pretectal | 2* | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Nuc Post Commissure | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Periaqueductal Gray Matter | 1 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2.5 | 0 | 1 | 2.5 | ||
| Dorsal Raphe | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 3 | ||
Results
MSO infusion location, seizures and neuronal loss
The MSO injection was targeted at the right entorhinal-hippocampal area (i.e. subiculum, deep entorhinal cortex and angular bundle) in the right, or hereafter, “ipsilateral” hemisphere (Table 1, asterisks in Fig. 3), and only rats with infusions into this area were included in the study. We have shown previously that targeting these areas result in a similar pattern of recurrent seizures that evolve in frequency and behavioral severity over a period of several weeks, consistent with a process of epileptogenesis (Dhaher et al., 2015). The MSO- and PBS-infused rats exhibited variable degrees of nonselective neuronal loss in a zone surrounding the infusion track. The loss was minimal in the PBS-infused rats, extending up to 100 μm from the center of the track. In the MSO-infused rats, the loss was minimal to moderate, consistent with prior studies (Eid et al., 2008), extending in some cases as far as 500 μm from the center of the track. The areas of neuronal loss were excluded from the c-Fos staining assessment.
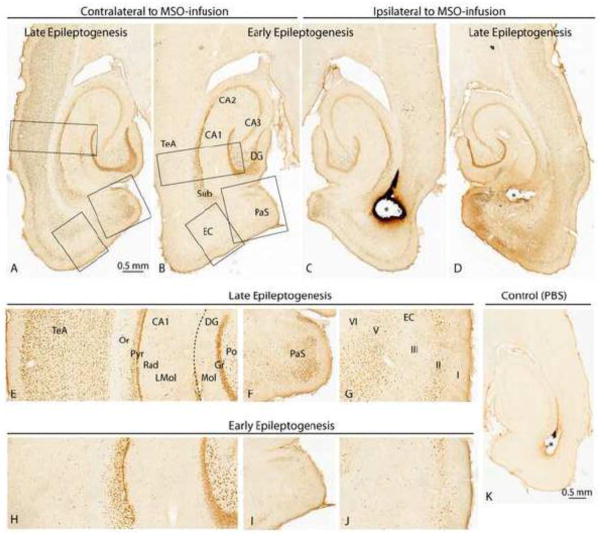
Figure 3.
C-Fos staining in the entorhinal-hippocampal area of representative MSO-infused rats during early [B, C; Rat K (m)] and late [A, D; Rat O (m)] epileptogenesis. There is strong c-fos staining of neurons in several regions ipsilateral to the MSO-infusion (asterisks) both early (C) and late (D) in epileptogenesis. Regions with particularly intense staining include granule cells (Gr) and polymorphic cells (Po) of the dentate gyrus (DG) as well as neurons in the CA1, subiculum (Sub), parasubiculum (PaS), and layers of the entorhinal cortex (EC) and temporal association cortex (TeA). In general, there is more extensive staining for c-Fos during late vs. early epileptogenesis ipsilateral to the MSO-infusion. Neurons in several areas contralateral to the MSO-infusion are stained as well with a marked increase in the number of stained cells late (A, E, F, G) vs. early (B, H, I, J) in epileptogenesis. In the contralateral hemisphere, numerous neurons are stained in the TeA, PaS, and layers V–VI of the EC during late epileptogenesis (E, F, G) whereas the corresponding areas show minimal if any staining during early epileptogenesis (H, I, J). A nonepileptic (PBS-infused) rat is shown in K [Rat C (pbs)] and is negative for c-Fos staining (K). Abbreviations: CA1-3, cornu ammonis subfields 1–3 of the hippocampus; LMol, stratum lacunosum-moleculare; Mol, molecular layer of the dentate gyrus; Or, stratum oriens; Pyr, pyramidal layer; Rad, stratum radiatum.
The entorhinal-hippocampal area and neocortex
Due to the location of the MSO infusion, it was expected that significant seizure-induced staining would be observed in this region, particularly in highly interconnected areas. While some variation in staining patterns between rats was observed in most brain regions, the staining in the ipsilateral hippocampus was remarkably reproducible with consistent and strong/dense staining of neurons in the dentate granular layer and the pyramidal cell layers of the CA fields (Table 2A, Fig 3C). While the polymorphic layer of the dentate hilus showed less dense packing of stained cells, each cell’s staining intensity was remarkably high (Table 2A, Fig. 3C). The subiculum showed some variation depending on the depth, with superficial areas (juxtaposed to the dentate) staining similarly to CA1 and deeper layers (adjacent to the angular bundle) exhibiting weaker and less dense staining. Far weaker staining was observed inconsistently in the strata radiatum, lucidum, and oriens of the CA fields (Fig. 3C), and the molecular layer of the dentate remained almost completely devoid of staining (Fig. 3C). Furthermore, this same staining pattern in the hippocampus remained consistent, not only in early (Fig. 3C) and late epileptogenesis (Fig. 3C) ipsilaterally, but also early (Fig. 3B, H) and late (Fig. 3A, E) in the contralateral hemisphere.
Beyond the hippocampus proper, increased variation in staining was observed, and a common pattern that will be repeated in the subsequent sections emerged. Despite the unilateral injection, c-Fos staining was most commonly bilateral, as in the hippocampus. However, a handful of regions were stained only ipsilaterally in early epileptogenesis, but staining extended bilaterally in MSO late epileptogenesis. The first examples of this pattern of ipsilateral concordance and contralateral discordance between early and late seizures was observed in the parasubiculum (Fig. 3F, I) and entorhinal cortex (Fig. 3G, J). Although the MSO infusion targeted the deep layers of the entorhinal cortex, it was the superficial layers that showed somewhat stronger staining on the ipsilateral side during early and late epileptogenesis (Fig. 3C, D). The parasubiculum also showed significant staining ipsilaterally during early and late epileptogenesis (Fig. 3C, D). However, contralateral superficial entorhinal and parasubiculum staining was less dense and deep entorhinal staining disappeared altogether during early epileptogenesis (Fig. 3G, J). While the contralateral parasubiculum was densely stained during late epileptogenesis (Fig. 3F), no staining was apparent at the early stage (Fig. 3I).
The neocortex was consistently stained during early and late epileptogenesis with notable differences in staining patterns. In general, staining was restricted to the ipsilateral perirhinal, ectorhinal and temporal association cortex in early epileptogenesis (Fig. 3B, C, H), whereas large portions of the neocortex were stained bilaterally in late epileptogenesis (Fig. 3A, D, E). The neocortical staining during late epileptogenesis was particularly pronounced in sensorimotor areas (Table 2). This finding corroborates with the more severe, stage 5 motor seizures that were preferentially observed in the chronic stage rats. The nonepileptic PBS-infused control rats were negative or only very faintly stained for c-fos staining in all of the above regions (Fig. 3K).
Other cortical regions
Strong staining was observed in the anterior olfactory nucleus (Fig. 4A–E), the olfactory cortex, the piriform cortex (Fig. 4A–E) and the tenia tecta (Fig. 4A–E) in both early and late epileptogenesis. Similar to other regions, the staining was strong ipsilaterally (Fig. 4C, D) and weak or absent contralaterally early in epileptogenesis (Fig. 4B), and strong bilaterally late in epileptogenesis (Fig. 4A). Interestingly, despite their related function, the olfactory tubercle and navicular nucleus showed complete absence of staining in all MSO-infused rats (Table 2).
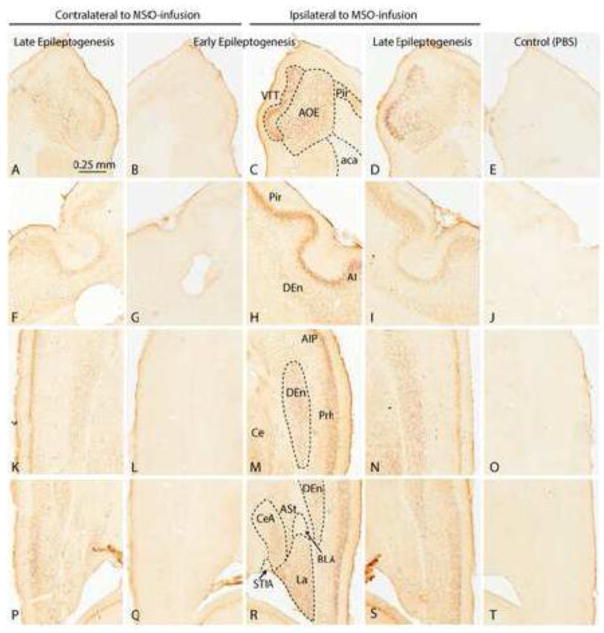
Figure 4.
C-Fos staining in other cortical regions of representative MSO-infused rats during early [Rat D (m)] and late [Rat O (m)]) epileptogenesis (left four columns). C-Fos staining ipsilateral to the infusion of a nonepileptic (PBS-infused) control is shown in the rightmost column [Rat C (pbs)]. A–D: Abundant neuronal staining is evident in the ventral tenia tecta (VTT) and anterior olfactory area, external part (AOE) ipsilateral to the infusion during early (C) and late (D) epileptogenesis. Contralateral to the infusion, staining is weaker during late epileptogenesis and absent during early epileptogenesis. F–I: Neurons in the piriform cortex (Pir), agranular insular cortex (AI) and dorsal endopiriform nucleus (Den) are strongly stained ipsilateral to the infusion during early epileptogenesis (H) and weakly stained during late epileptogenesis (I). Contralateral to the infusion, staining is weak during late epileptogenesis and absent during early epileptogenesis. K–O: There is abundant staining of neurons in the dorsal endopiriform nucleus (Den), central nucleus of the amygdala (Ce), and posterior part of the agranular insular cortex (AIP) ipsilateral to the infusion during early (M) and late (N) epileptogenesis. Contralateral to the infusion, staining is weak during late epileptogenesis (K) and absent during early epileptogenesis (L). P–S: Discrete nuclei in the amygdala are strongly stained ipsilateral to the infusion during early epileptogenesis (R), particularly the lateral (LA) and parts of the central (CeA) nuclei. The dorsal endopiriform nucleus (Den) and amygdalostriatal transition area (ASt) were stained as well. Staining in these areas was less pronounced ipsilateral (S) and contralateral (P) to the infusion during late epileptogenesis. Staining was absent ipsilateral to the infusion during early epileptogenesis (Q). There is complete absence of c-Fos staining in all areas shown in the PBS infused control rat (E–T). Abbreviations: aca, anterior commissure, anterior part; BLA, basolateral amygdaloid nucleus, anterior part; STIA, bed nucleus of the stria terminalis, intraamygdaloid division.
The ipsilateral dorsal endopiriform nucleus, claustrum and agranular insular cortex were stained in a subset of the early stage rats (Fig. 4H, M) and in all of the late stage rats (Fig. 4I, N). Except from a small patch of strongly stained cells in 2 of 3 rats with late/stage 5 seizures, the majority of neurons in the caudate putamen and globus pallidus were not consistently stained.
There was some variation in the staining of the various sub-regions of the amygdala, but staining was most consistently seen in the lateral nucleus, ipsilateral to MSO-infusion, in the majority of early (Fig. 4R) and late stage (Fig. 4S) rats. While staining in the lateral nucleus was usually present in the contralateral amygdala in the late stage rats (Fig. 4P), contralateral staining was usually absent in the early stage animals (Fig. 4Q). Staining was also observed, but with less frequency, in the unilateral central nucleus and the amygdalostriatal transition area in the early and late stage rats. A similar staining pattern was also observed in the contralateral hemisphere in the late stage rats (Fig. 4P) whereas staining was absent in the early stage rats (Fig. 4Q). The nonepileptic PBS-infused control rats were negative or only very faintly stained for c-fos staining in all of the above regions (Fig. 4E, J, O, T).
The thalamus and hypothalamus
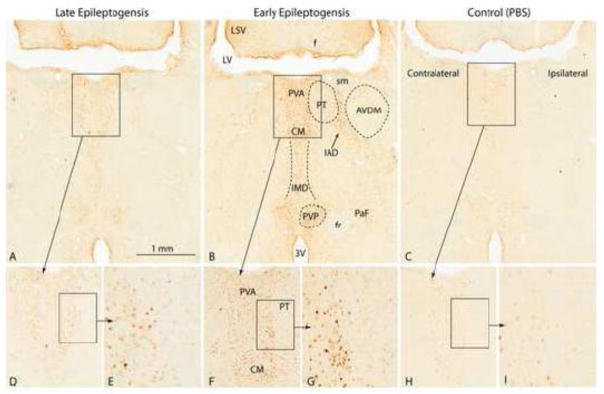
Perhaps one of the most notable and generalizable aspects was a lack of strong staining in most regions of the thalamus or hypothalamus in any of the MSO-infused rats. While some staining was present in these regions, it was weaker and less consistent than in most of the aforementioned areas. Weak to moderate staining was observed, though inconsistently, in the midline thalamic nuclei such as the interomediodorsal (Fig. 5A, B), rhomboid, reuniens, and central medial (Fig. 5A. B, D, E, F, G). The only thalamic nuclei with staining observed in more than 4 of the 10 treatment rats was the central medial. While the paraventricular nuclei of the thalamus (Fig. 5A, B, D, E, F, G) and hypothalamus were consistently stained in the MSO-infused rats, both nuclei were stained in the PBS control rats (Fig. 5C, H, I). Thus, the staining is probably not specific for seizures, but likely to be related to stress, such as change in environment in the hour between the seizure occurrence and fixation. Involvement of these nuclei in stress is consistent with previous studies (Benarroch, 2005).
Figure 5.
C-Fos staining in midline thalamic nuclei of representative MSO-infused rats during early [B, F, G; Rat D (m)] and late epileptogenesis [A, D, E; Rat O (m)]. A stained nonepileptic, PBS-infused control [Rat C (pbs)] is shown in C, H and I. There is intense staining of neurons in the anterior part of the periventricular thalamic nucleus (PVA), the central medial thalamic nucleus (CM), the intermediodorsal thalamic nucleus (IMD), the interanteromdial thalamic nucleus and the posterior part of the paraventricular thalamic nucleus (PVP) during early epileptogenesis and slightly less intense staining in the same areas during late epileptogenesis. Weak staining of very few cells were occasionally encountered in the PBS control (C, H, I). Abbreviations: 3V, third ventricle; AVDM, anteroventral thalamic nucleus, dorsomedial part; f, fornix; fr, fasciculus retroflexus; LSV, lateral septal nucleus, ventral part; LV, lateral ventricle; PT, parathenial thalamic nucleus; sm, stria medullaris.
Other brain regions
Other areas of the brain and brainstem showed weak and inconsistent staining, such as the habenula, tectal/pretectal nucleus, periaqueductal gray and dorsal raphe nuclei (Table 2). None of the above regions stained in PBS-infused rats; however, the following areas were labeled in both PBS- and MSO-infused rats, making their involvement in seizures less likely: the geniculate, lateral septal, and supramammilary nuclei (Table 2).
Discussion
We have used the intrahippocampal MSO-infusion model of MTLE to demonstrate how increasing populations of neurons are activated by seizures as the disorder progresses over a period of several weeks. Our main finding is that seizure activation is preferential to selected limbic structures during early stages of the disorder, whereas involvement of more extensive brain areas occurs later in epileptogenesis. This finding supports our working hypothesis that epileptogenesis caused by unilateral hippocampal GS inhibition evolves over time, from a highly focal disorder to a pathological process of widespread neuronal network activation.
C-Fos and neuronal activation
Immediate early gene expression has been instrumental in identifying activated neurons in different brain regions. The prototypical and most characterized immediate early gene-encoded transcription factor is c-Fos which is expressed with a peak of 30 to 90 minutes after stimulation (Herrera and Robertson, 1996). There is ample literature describing neuronal c-Fos induction following kindling and other seizure models (Barone et al., 1993; Bozzi et al., 2011; Chiasson et al., 1995; Dragunow and Faull, 1989; Dragunow and Robertson, 1988; Morgan and Curran, 1991a, b; Motte et al., 1997). Furthermore, several studies have demonstrated a correlation between the expansion of c-Fos induction from limbic areas to the cerebral cortex and the progression of epileptic seizures (Andre et al., 1998; Clark et al., 1991; Ebert and Loscher, 1995; Hiscock et al., 2001; Simler et al., 1999; Szyndler et al., 2009; Willoughby et al., 1997). These studies and others have capitalized on the following advantages of c-Fos as a marker of neuronal activity: ease of detection, good sensitivity following neuronal stimulation, cellular mapping that enables the examination of all neurons in a particular network spanning multiple brain regions, and the ability to combine c-Fos labeling with other immunohistochemical markers and/or retrograde axonal tracing for connectivity studies.
Nonetheless, c-Fos labeling has a number of limitations that can restrict its scope of use in some contexts (Hoffman and Lyo, 2002). First, not all neurons that are known to be active by other techniques express c-Fos. Second, the temporal pattern of c-Fos expression makes it ineffective for parsing out short-lasting from long-lasting activity bursts. Third, c-Fos can only provide information on neurons which are activated, and not those which are inhibited. Fourth, this information can only be collected at a single time point in each animal, necessitating the use of multiple cohorts of animals for a time course study. Some groups, however, have developed transgenic mice that allow for the persistent tagging of activated neurons during a given time window by placing reporter genes under the control of the c-Fos promoter (Collaco and Geusz, 2003; Sanders et al., 2013). Despite these limitations, c-Fos expression remains the most commonly used method for assessing neuronal activation, given its outlined advantages and the time limits and technical difficulties of other techniques such as electrophysiological recordings and imaging.
Several discrete areas were consistently activated by seizures regardless of the stage of epileptogenesis
Regardless of the stage of epileptogenesis and severity of seizures, neuronal c-Fos staining in the MSO model was consistently observed in several strongly interconnected areas, hereby defined as primary activation circuits, within the limbic system. Perhaps one of the best characterized such circuit is the entorhinal-hippocampal-entorhinal network, which facilitates the flow of signals via the following neuronal connections: layer II of the entorhinal cortex → granule cell layer of the dentate gyrus → CA3 → CA2 → CA1 → subiculum → layers V-VI of the entorhinal cortex → layer II of the entorhinal cortex (Witter et al., 1989). Activation of this circuit by seizures has been reported in other models of MTLE, such as the systemic kainic acid (Popovici et al., 1990) and pilocarpine models (Harvey and Sloviter, 2005) and in models of amygdala kinding (Dragunow et al., 1988). Depth electrode EEG recordings from patients with MTLE also suggest that this circuit is preferentially involved during seizures (Spencer and Spencer, 1994). Finally, a large number of cellular, molecular and chemical alterations have been demonstrated within this network in humans with MTLE and animal models of the disorder (reviewed in (O’Dell et al., 2012)).
C-Fos staining was also consistently present, regardless of seizure severity, in other regions of the limbic system, particularly presubiculum, parasubiculum, lateral amygdaloid nucleus, perirhinal cortex, tenia tecta, anterior olfactory area, dorsal endopiriform nucleus, claustrum, and midline thalamic nuclei. Most of these regions communicate with the entorhinal-hippocampal circuit, or with each other, via unidirectional or reciprocal connections (Witter et al., 1989). Some of the regions, particularly the amygdala, endopiriform nucleus, claustrum and midline thalamic nuclei, have been implicated in the pathophysiology of MTLE in humans and animal models. Many of these areas also express c-Fos after seizures in the systemic pilocarpine (Barone et al., 1993) and kainic acid models (Silveira et al., 2002).
Amygdala is exquisitely sensitive to kindling in several species (Goddard et al., 1969; McIntyre et al., 1982) and is characterized by sclerosis, i.e. neuron loss and proliferation of astrocytes, in animal models and patients with MTLE (Hudson et al., 1993; Pitkanen et al., 1998). Notably, spontaneous discharges occur in the lateral amygdala in human epilepsy specimens in vitro (Graebenitz et al., 2011) and also in epileptic, but not control animal specimens (Benini and Avoli, 2006). Similar to the MSO-model, both the systemic kainic acid and pilocarpine models exhibit seizure-induced c-Fos staining in different regions of amygdala, such as the lateral and central nuclei (Barone et al., 1993; Silveira et al., 2002). Interestingly, while the basolateral nucleus is often reported to be stained in the kainic acid and pilocarpine models, this nucleus appears to be less consistently stained in the MSO-model.
The endopiriform nucleus also appears to have a role in seizure initiation. Injection of small quantities of proconvulsants into or near the rostral endopiriform nucleus (a.k.a. “area tempestas”) triggers generalized tonic-clonic seizures (Piredda and Gale, 1985) whereas infusion of γ-vinyl GABA into the same area suppresses amygdala kindled seizures (Stevens et al., 1988). The endopiriform nucleus is also consistently stained by c-fos in the kainic acid model (Willoughby et al., 1997).
The claustrum is located deep to the insular cortex in the human brain, and electrical stimulation of the claustrum leads to secondarily generalized seizures in the kindling model of MTLE (Mohapel et al., 2001). Lesions of the claustrum delay the development of amygdala kindling and reduces the severity of kindled seizures from generalized to partial seizures (Wada and Kudo, 1997). Moreover, magnetic resonance spectroscopy imaging has shown decreased N-acetylaspartate/creatine (NAA/Cr) ratios bilaterally in the claustrum in humans with MTLE vs. control subjects, suggestive of neuronal mitochondrial dysfunction in this area in MTLE.
Finally, studies have shown that the midline thalamic nuclei are important for seizure activity in the limbic system. Depth electrode EEG recordings of rats with kindled seizures and chronic limbic epilepsy show early seizure activity in the midline thalamus and degeneration of neurons in the medial dorsal, reuniens, and rhomboid nuclei (Bertram et al., 2001). Thalamic neurons from these rats have changes in synaptically mediated and voltage-gated responses; moreover, lidocaine infusion into the midline thalamus shortens the after-discharge duration of the cells (Bertram et al., 2001) and infusion of the sodium channel blocker tetrodotoxin suppresses the limbic seizures (Sloan et al., 2011). C-fos staining of thalamic nuclei, including midline thalamus, also occurs in the pilocarpine (Barone et al., 1993) and kainic acid models (Beer et al., 1998) of MTLE.
Most areas were activated bilaterally; however, some areas were preferentially activated in the hemisphere that received MSO
It is not surprising that most areas were activated bilaterally by the seizures even though MSO was infused unilaterally. Both hippocampi are extensively interconnected in the rat via several commissural pathways, thus ensuring efficient spread of seizures among limbic structures in both hemispheres (Blackstad, 1956). However, a small number of areas were preferentially activated ipsilateral to the MSO infusion, specifically the parasubiculum, layers II and V-VI of the entorhinal cortex, the lateral amygdala, the anterior olfactory area, and the tenia tecta. A hypothetical explanation for the unilateral preference is that these areas may not receive seizure input; rather they project to and activate the other, bilaterally involved areas. The unilaterally involved areas are therefore potentially regions of seizure initiation, rather than regions of seizure spread.
While future studies are necessary to establish whether these and other areas are critical for seizure initiation, published data provide some clues. The parasubiculum receives major inputs from glutamatergic cells of the CA1 and cholinergic cells of the medial septum as well as minor inputs from the subiculum, anterior thalamus and basolateral amygdala. The main efferents project to layer II of the entorhinal cortex, which in turn projects to the dentate granule cells and CA3 pyramidal neurons as the perforant pathway (Caballero-Bleda and Witter, 1993, 1994; Funahashi and Stewart, 1997; Swanson and Cowan, 1979; Wouterlood et al., 1990). Furthermore, presubicular neurons can generate theta frequency EEG activity, probably via membrane potential oscillations that are synchronized by local inhibitory inputs (Glasgow and Chapman, 2007). Theta activity is important for modulation of hippocampal cognitive function, emotional behavior, stress and anxiety–modalities that are often affected in MTLE (Pan and McNaughton, 2002; Richmond et al., 1999). Hippocampal theta activity is also altered in human MTLE (Bettus et al., 2008) and in relevant animal models of the disease (Chauviere et al., 2009). Finally, neurons in layers II and III of the parasubiculum, including parvalbumin-positive cells, are lost in animal models of MTLE, suggesting that local network changes involving the parasubiculum occur in epilepsy (Pitkanen et al., 1995; van Vliet et al., 2004). The significance of the entorhinal cortex and amygdala for MTLE has already been discussed.
More extensive neuronal activation occurred late vs. early in epileptogenesis
The last observation is that seizure-induced activation extended beyond the limbic system in the later stages of the MSO model. The extended areas of activation include large portions of the neocortex, the nucleus of the stria terminalis, the mediodorsal thalamus, and the central nucleus of the amygdala. The widespread involvement of other brain areas, particularly the neocortex, is consistent with the more severe seizure phenotype seen late in epileptogenesis in the MSO model. A progressive worsening of the severity of seizures has been reported in other animal models (Norwood et al., 2010; Sloviter and Bumanglag, 2013) and in humans with MTLE (Pitkanen and Sutula, 2002), suggesting that the development of MTLE (epileptogenesis) is a process of brain plasticity that may start focally, but eventually extends to involve multiple brain areas.
The current study provides novel and important information about regions of seizure involvement in the MSO model in a fairly unbiased fashion throughout the brain. However, the c-fos approach cannot determine the exact onset and propagation of the seizures. Multiple depth EEG or single cell in vivo recordings carried out over days to weeks in the same animals, are required to address these issues. Nevertheless, the c-fos approach is important because it can be used to guide the electrode placement in future electrophysiological recordings.
The role of GS in epilepsy
Our findings demonstrate that inhibition of astroglial GS focally in the entorhinal-hippocampal area of rats triggers a process of epileptogenesis characterized by gradual worsening of seizure severity and involvement of progressively larger neuronal populations over time. As mentioned above there is increasing evidence to suggest that GS is implicated in the causation of epilepsy due to its loss in patients with MTLE (Eid et al., 2004; van der Hel et al., 2005) and neocortical epilepsies (Steffens et al., 2005), and the fact that inhibiting GS using MSO in rats results in recurrent seizures (Eid et al., 2008; Wang et al., 2009), comorbid depressive behavior (Gruenbaum et al., 2015), and neuropathological features similar to human MTLE (Eid et al., 2008; Lauritzen et al., 2012). Several other findings from humans and animals support the notion that a deficiency in GS is implicated in the causation of epilepsy. Humans with mutations in the GS gene develop epilepsy (Haberle et al., 2005) and heterozygous mutations of the astroglial GS gene in mice reduces the threshold to induced seizures (van Gassen et al., 2009). Ingestion of domoic acid by California sea lions reduces astroglial GS and leads to epilepsy (Madl et al., 2014) and loss of astroglial GS occurs early in epileptogenesis in the hilus of the fascia dentate in the juvenile pilocarpine model of epilepsy (van der Hel et al., 2014). However, the role of GS in epileptogenesis is complex. Studies have shown that hippocampal GS is temporarily upregulated in epileptogenesis in the kainic acid and kindling models of MTLE (Hammer et al., 2008; Sun et al., 2013), and extremely low doses of MSO given during epileptogenesis may, in some cases, protect against development of epilepsy (Sun et al., 2013). These seemingly discrepant features of GS in the causation of may be due to the model used and the anatomical distribution and timing of the GS perturbation. While further studies are needed to resolve these issues, the current findings clearly support the notion that inhibiting GS focally in the hippocampal formation induces epileptogenesis with worsening of seizures and involvement of progressively larger neuronal networks over time.
With respect to the current findings, future studies are needed to (a) to determine the parts of the network that are responsible for initiation, maintenance and spread of seizures respectively, (b) to characterize the causative mechanisms at play in the developing and developed network for seizure initiation, maintenance and spread, (c) to understand the vulnerability of the different parts of this network, and the network as a whole, to specific interventions at different points in the epileptogenic process, and (d) to determine whether there are any changes in the frequency, patterns and brain involvement of the seizures after the MSO-infusion pump has been discharged.
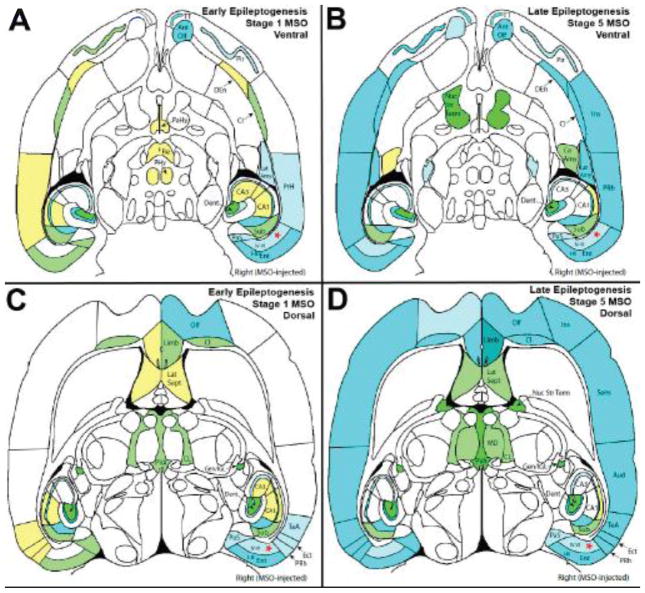
Figure 2.
Visual summary representation of median levels of c-Fos staining in early and late epileptogenesis in horizontal sections at two different points on the dorso-ventral axis. Each brain region was graded for density and intensity of staining in each section in which it appeared. The median level of staining was determined within each rat, then within each rat subclass. These median levels of staining are visualized based on the images from “The Rat Brain in Stereotactic Coordinates” (Paxinos and Watson, 2007). (A) and (B) represent the staining at a ventral section (Bregma −6.82 mm, Figure 192, page 235) from MSO stage 1 seizure rats and stage 5 seizure rats, respectively. (C) and (D) represent the staining at a dorsal section (Bregma −5.32 mm, Figure 198, p 241) from MSO stage 1 seizure rats and stage 5 seizure rats, respectively. Color coding follows the legend presented in Figure 1.
Highlights.
Inhibition of glutamine synthetase in the hippocampal formation leads to epilepsy
The epileptic seizures involve a small number of brain areas initially
The seizures are mostly low grade (not severe) during the early phase
The seizures progressively worsen over the next several weeks
The worsening is accompanied by seizure involvement of multiple brain areas
Acknowledgments
The authors would like to thank Ms. Ilona Kovacs for excellent technical assistance. TE, RD and HZ are supported by grants from the National Institutes of Health (NIH): NINDS K08 NS058674 and R01 NS070824. This work was also made possible by grants from the National Center for Advancing Translational Sciences (NCATS; UL1 RR024139), a component of the NIH and NIH roadmap for Medical Research, and from the National Center for Research Resources (NCRR) and NCATS (TL1 RR024137), components of the NIH. The authors have no conflicts of interest to declare.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Amaral DG, Insausti R. Hippocampal formation. In: Paxinos G, editor. The human nervous system. Academic Press; San Diego: 1990. pp. 711–756. [Google Scholar]
- Andre V, Pineau N, Motte JE, Marescaux C, Nehlig A. Mapping of neuronal networks underlying generalized seizures induced by increasing doses of pentylenetetrazol in the immature and adult rat: a c-Fos immunohistochemical study. Eur J Neurosci. 1998;10:2094–2106. doi: 10.1046/j.1460-9568.1998.00223.x. [DOI] [PubMed] [Google Scholar]
- Avoli M, Gloor P. Pathophysiology of focal and generalized convulsive seizures versus that of generalized non-convulsive seizures. In: Wolf P, editor. Epileptic Seizures and Syndromes. John Libby Eurotext Ltd; Montrouge, France: 1994. pp. 547–561. [Google Scholar]
- Barone P, Morelli M, Cicarelli G, Cozzolino A, DeJoanna G, Campanella G, DiChiara G. Expression of c-fos protein in the experimental epilepsy induced by pilocarpine. Synapse. 1993;14:1–9. doi: 10.1002/syn.890140102. [DOI] [PubMed] [Google Scholar]
- Beer J, Mielke K, Zipp M, Zimmermann M, Herdegen T. Expression of c-jun, junB, c-fos, fra-1 and fra-2 mRNA in the rat brain following seizure activity and axotomy. Brain Res. 1998;794:255–266. doi: 10.1016/s0006-8993(98)00233-9. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Paraventricular nucleus, stress response, and cardiovascular disease. Clinical autonomic research : official journal of the Clinical Autonomic Research Society. 2005;15:254–263. doi: 10.1007/s10286-005-0290-7. [DOI] [PubMed] [Google Scholar]
- Benini R, Avoli M. Altered inhibition in lateral amygdala networks in a rat model of temporal lobe epilepsy. J Neurophysiol. 2006;95:2143–2154. doi: 10.1152/jn.01217.2005. [DOI] [PubMed] [Google Scholar]
- Bertram EH. Monitoring for Seizures in Rodents. In: Pitkanen A, Schwartzkroin PA, Moshe SL, editors. Models of Seizures and Epilepsy. Elsevier; Amsterdam: 2006. [Google Scholar]
- Bertram EH, Mangan PS, Zhang DX, Scott CA, Williamson JM. The midline thalamus: alterations and a potential role in limbic epilepsy. Epilepsia. 2001;42:967–978. doi: 10.1046/j.1528-1157.2001.042008967.x. [DOI] [PubMed] [Google Scholar]
- Bettus G, Wendling F, Guye M, Valton L, Regis J, Chauvel P, Bartolomei F. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008;81:58–68. doi: 10.1016/j.eplepsyres.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Bezzi P, Carmignoto G, Pasti L, Vesce S, Rossi D, Rizzini BL, Pozzan T, Volterra A. Prostaglandins stimulate calcium-dependent glutamate release in astrocytes. Nature. 1998;391:281–285. doi: 10.1038/34651. [DOI] [PubMed] [Google Scholar]
- Blackstad TW. Commissural connections of the hippocampal region in the rat, with special reference to their mode of termination. J Comp Neurol. 1956;105:417–537. doi: 10.1002/cne.901050305. [DOI] [PubMed] [Google Scholar]
- Bozzi Y, Dunleavy M, Henshall DC. Cell signaling underlying epileptic behavior. Frontiers in behavioral neuroscience. 2011;5:45. doi: 10.3389/fnbeh.2011.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero-Bleda M, Witter MP. Regional and laminar organization of projections from the presubiculum and parasubiculum to the entorhinal cortex: an anterograde tracing study in the rat. J Comp Neurol. 1993;328:115–129. doi: 10.1002/cne.903280109. [DOI] [PubMed] [Google Scholar]
- Caballero-Bleda M, Witter MP. Projections from the presubiculum and the parasubiculum to morphologically characterized entorhinal-hippocampal projection neurons in the rat. Exp Brain Res. 1994;101:93–108. doi: 10.1007/BF00243220. [DOI] [PubMed] [Google Scholar]
- Chauviere L, Rafrafi N, Thinus-Blanc C, Bartolomei F, Esclapez M, Bernard C. Early deficits in spatial memory and theta rhythm in experimental temporal lobe epilepsy. J Neurosci. 2009;29:5402–5410. doi: 10.1523/JNEUROSCI.4699-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiasson BJ, Dennison Z, Robertson HA. Amygdala kindling and immediate-early genes. Brain Res Mol Brain Res. 1995;29:191–199. doi: 10.1016/0169-328x(94)00250-i. [DOI] [PubMed] [Google Scholar]
- Clark M, Post RM, Weiss SR, Cain CJ, Nakajima T. Regional expression of c-fos mRNA in rat brain during the evolution of amygdala kindled seizures. Brain Res Mol Brain Res. 1991;11:55–64. doi: 10.1016/0169-328x(91)90021-o. [DOI] [PubMed] [Google Scholar]
- Collaco AM, Geusz ME. Monitoring immediate-early gene expression through firefly luciferase imaging of HRS/J hairless mice. BMC physiology. 2003;3:8. doi: 10.1186/1472-6793-3-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Dhaher R, Wang H, Gruenbaum SE, Tu N, Lee TS, Zaveri HP, Eid T. Effects of site-specific infusions of methionine sulfoximine on the temporal progression of seizures in a rat model of mesial temporal lobe epilepsy. Epilepsy Res. 2015;115:45–54. doi: 10.1016/j.eplepsyres.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. Journal of neuroscience methods. 1989;29:261–265. doi: 10.1016/0165-0270(89)90150-7. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA. Seizure-inducible c-fos protein(s) in mammalian neurons. Trends Pharmacol Sci. 1988;9:5–6. doi: 10.1016/0165-6147(88)90229-5. [DOI] [PubMed] [Google Scholar]
- Dragunow M, Robertson HA, Robertson GS. Amygdala kindling and c-fos protein(s) Exp Neurol. 1988;102:261–263. doi: 10.1016/0014-4886(88)90103-3. [DOI] [PubMed] [Google Scholar]
- Ebert U, Loscher W. Strong induction of c-fos in the piriform cortex during focal seizures evoked from different limbic brain sites. Brain Res. 1995;671:338–344. doi: 10.1016/0006-8993(94)01401-3. [DOI] [PubMed] [Google Scholar]
- Eid T, Ghosh A, Wang Y, Beckstrom H, Zaveri HP, Lee TS, Lai JC, Malthankar-Phatak GH, de Lanerolle NC. Recurrent seizures and brain pathology after inhibition of glutamine synthetase in the hippocampus in rats. Brain. 2008;131:2061–2070. doi: 10.1093/brain/awn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Falconer MA, Taylor DC. Surgical treatment of drug-resistant epilepsy due to mesial temporal sclerosis. Etiology and significance. Arch Neurol. 1968;19:353–361. doi: 10.1001/archneur.1968.00480040019001. [DOI] [PubMed] [Google Scholar]
- Foldvary N, Nashold B, Mascha E, Thompson EA, Lee N, McNamara JO, Lewis DV, Luther JS, Friedman AH, Radtke RA. Seizure outcome after temporal lobectomy for temporal lobe epilepsy: a Kaplan-Meier survival analysis. Neurology. 2000;54:630–634. doi: 10.1212/wnl.54.3.630. [DOI] [PubMed] [Google Scholar]
- Funahashi M, Stewart M. Presubicular and parasubicular cortical neurons of the rat: functional separation of deep and superficial neurons in vitro. J Physiol. 1997;501( Pt 2):387–403. doi: 10.1111/j.1469-7793.1997.387bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasgow SD, Chapman CA. Local generation of theta-frequency EEG activity in the parasubiculum. J Neurophysiol. 2007;97:3868–3879. doi: 10.1152/jn.01306.2006. [DOI] [PubMed] [Google Scholar]
- Goddard GV, McIntyre DC, Leech CK. A permanent change in brain function resulting from daily electrical stimulation. Exp Neurol. 1969;25:295–330. doi: 10.1016/0014-4886(69)90128-9. [DOI] [PubMed] [Google Scholar]
- Graebenitz S, Kedo O, Speckmann EJ, Gorji A, Panneck H, Hans V, Palomero-Gallagher N, Schleicher A, Zilles K, Pape HC. Interictal-like network activity and receptor expression in the epileptic human lateral amygdala. Brain. 2011;134:2929–2947. doi: 10.1093/brain/awr202. [DOI] [PubMed] [Google Scholar]
- Gruenbaum SE, Wang H, Zaveri HP, Tang AB, Lee TS, Eid T, Dhaher R. Inhibition of glutamine synthetase in the central nucleus of the amygdala induces anhedonic behavior and recurrent seizures in a rat model of mesial temporal lobe epilepsy. Epilepsy Behav. 2015;51:96–103. doi: 10.1016/j.yebeh.2015.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberle J, Gorg B, Rutsch F, Schmidt E, Toutain A, Benoist JF, Gelot A, Suc AL, Hohne W, Schliess F, Haussinger D, Koch HG. Congenital glutamine deficiency with glutamine synthetase mutations. N Engl J Med. 2005;353:1926–1933. doi: 10.1056/NEJMoa050456. [DOI] [PubMed] [Google Scholar]
- Hader WJ, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Wiebe S, Kwon CS, Jette N. Complications of epilepsy surgery: a systematic review of focal surgical resections and invasive EEG monitoring. Epilepsia. 2013;54:840–847. doi: 10.1111/epi.12161. [DOI] [PubMed] [Google Scholar]
- Hammer J, Alvestad S, Osen KK, Skare O, Sonnewald U, Ottersen OP. Expression of glutamine synthetase and glutamate dehydrogenase in the latent phase and chronic phase in the kainate model of temporal lobe epilepsy. Glia. 2008;56:856–868. doi: 10.1002/glia.20659. [DOI] [PubMed] [Google Scholar]
- Harvey BD, Sloviter RS. Hippocampal granule cell activity and c-Fos expression during spontaneous seizures in awake, chronically epileptic, pilocarpine-treated rats: implications for hippocampal epileptogenesis. J Comp Neurol. 2005;488:442–463. doi: 10.1002/cne.20594. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA. Activation of c-fos in the brain. Prog Neurobiol. 1996;50:83–107. doi: 10.1016/s0301-0082(96)00021-4. [DOI] [PubMed] [Google Scholar]
- Hiscock JJ, Mackenzie L, Medvedev A, Willoughby JO. Kainic acid and seizure-induced Fos in subtypes of cerebrocortical neurons. J Neurosci Res. 2001;66:1094–1100. doi: 10.1002/jnr.1252. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lyo D. Anatomical markers of activity in neuroendocrine systems: are we all ‘fos-ed out’? Journal of neuroendocrinology. 2002;14:259–268. doi: 10.1046/j.1365-2826.2002.00775.x. [DOI] [PubMed] [Google Scholar]
- Hudson LP, Munoz DG, Miller L, McLachlan RS, Girvin JP, Blume WT. Amygdaloid sclerosis in temporal lobe epilepsy. Ann Neurol. 1993;33:622–631. doi: 10.1002/ana.410330611. [DOI] [PubMed] [Google Scholar]
- Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess-Walsh P, Morris HH, Dinner DS, Nair D, Foldvary-Schaeffer N, Prayson RA, Comair Y, O’Brien R, Bulacio J, Gupta A, Luders HO. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66:1938–1940. doi: 10.1212/01.wnl.0000219810.71010.9b. [DOI] [PubMed] [Google Scholar]
- Lauritzen F, Perez EL, Melillo ER, Roh JM, Zaveri HP, Lee TS, Wang Y, Bergersen LH, Eid T. Altered expression of brain monocarboxylate transporter 1 in models of temporal lobe epilepsy. Neurobiology of disease. 2012;45:165–176. doi: 10.1016/j.nbd.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorente de Nó R. Studies on the structure of the cerebral cortex. II. Continuation of the study of the ammonic system. J Psychol Neurol (Leipzig) 1934;46:113–177. [Google Scholar]
- Madl JE, Duncan CG, Stanhill JE, Tai PY, Spraker TR, Gulland FM. Oxidative stress and redistribution of glutamine synthetase in California sea lions (Zalophus californianus) with domoic acid toxicosis. Journal of comparative pathology. 2014;150:306–315. doi: 10.1016/j.jcpa.2013.07.012. [DOI] [PubMed] [Google Scholar]
- McIntyre DC, Nathanson D, Edson N. A new model of partial status epilepticus based on kindling. Brain Res. 1982;250:53–63. doi: 10.1016/0006-8993(82)90952-0. [DOI] [PubMed] [Google Scholar]
- Mohapel P, Zhang X, Gillespie GW, Chlan-Fourney J, Hannesson DK, Corley SM, Li XM, Corcoran ME. Kindling of claustrum and insular cortex: comparison to perirhinal cortex in the rat. Eur J Neurosci. 2001;13:1501–1519. doi: 10.1046/j.0953-816x.2001.01532.x. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Proto-oncogene transcription factors and epilepsy. Trends Pharmacol Sci. 1991a;12:343–349. doi: 10.1016/0165-6147(91)90594-i. [DOI] [PubMed] [Google Scholar]
- Morgan JI, Curran T. Stimulus-transcription coupling in the nervous system: involvement of the inducible proto-oncogenes fos and jun. Annu Rev Neurosci. 1991b;14:421–451. doi: 10.1146/annurev.ne.14.030191.002225. [DOI] [PubMed] [Google Scholar]
- Motte JE, da Silva Fernandes MJ, Marescaux C, Nehlig A. Effects of pentylenetetrazol-induced status epilepticus on c-Fos and HSP72 immunoreactivity in the immature rat brain. Brain Res Mol Brain Res. 1997;50:79–84. doi: 10.1016/s0169-328x(97)00174-5. [DOI] [PubMed] [Google Scholar]
- Norwood BA, Bumanglag AV, Osculati F, Sbarbati A, Marzola P, Nicolato E, Fabene PF, Sloviter RS. Classic hippocampal sclerosis and hippocampal-onset epilepsy produced by a single “cryptic” episode of focal hippocampal excitation in awake rats. J Comp Neurol. 2010;518:3381–3407. doi: 10.1002/cne.22406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell CM, Das A, Wallace Gt, Ray SK, Banik NL. Understanding the basic mechanisms underlying seizures in mesial temporal lobe epilepsy and possible therapeutic targets: a review. J Neurosci Res. 2012;90:913–924. doi: 10.1002/jnr.22829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olney JW, Sharpe LG, Feigin RD. Glutamate-induced brain damage in infant primates. J Neuropathol Exp Neurol. 1972;31:464–488. doi: 10.1097/00005072-197207000-00006. [DOI] [PubMed] [Google Scholar]
- Pan WX, McNaughton N. The role of the medial supramammillary nucleus in the control of hippocampal theta activity and behaviour in rats. Eur J Neurosci. 2002;16:1797–1809. doi: 10.1046/j.1460-9568.2002.02267.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6. Academic Press; Massachusettes: 2006. [Google Scholar]
- Piredda S, Gale K. A crucial epileptogenic site in the deep prepiriform cortex. Nature. 1985;317:623–625. doi: 10.1038/317623a0. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Sutula TP. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1:173–181. doi: 10.1016/s1474-4422(02)00073-x. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Tuunanen J, Halonen T. Subiculum, Presubiculum and Parasubiculum Have Different Sensitivities to Seizure-Induced Neuronal Damage in the Rat. Neuroscience Letters. 1995;192:65–68. [Google Scholar]
- Pitkanen A, Tuunanen J, Kalviainen R, Partanen K, Salmenpera T. Amygdala damage in experimental and human temporal lobe epilepsy. Epilepsy Res. 1998;32:233–253. doi: 10.1016/s0920-1211(98)00055-2. [DOI] [PubMed] [Google Scholar]
- Popovici T, Represa A, Crepel V, Barbin G, Beaudoin M, Ben-Ari Y. Effects of kainic acid-induced seizures and ischemia on c-fos-like proteins in rat brain. Brain Res. 1990;536:183–194. doi: 10.1016/0006-8993(90)90024-6. [DOI] [PubMed] [Google Scholar]
- Racine RJ, Burnham WM, Gartner JG, Levitan D. Rates of motor seizure development in rats subjected to electrical brain stimulation: strain and inter-stimulation interval effects. Electroencephalogr Clin Neurophysiol. 1973;35:553–556. doi: 10.1016/0013-4694(73)90033-3. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Yee BK, Pouzet B, Veenman L, Rawlins JN, Feldon J, Bannerman DM. Dissociating context and space within the hippocampus: effects of complete, dorsal, and ventral excitotoxic hippocampal lesions on conditioned freezing and spatial learning. Behavioral neuroscience. 1999;113:1189–1203. doi: 10.1037/0735-7044.113.6.1189. [DOI] [PubMed] [Google Scholar]
- Robert SM, Buckingham SC, Campbell SL, Robel S, Holt KT, Ogunrinu-Babarinde T, Warren PP, White DM, Reid MA, Eschbacher JM, Berens ME, Lahti AC, Nabors LB, Sontheimer H. SLC7A11 expression is associated with seizures and predicts poor survival in patients with malignant glioma. Science translational medicine. 2015;7:289ra286. doi: 10.1126/scitranslmed.aaa8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders J, Mayford M, Jeste D. Empathic fear responses in mice are triggered by recognition of a shared experience. PLoS One. 2013;8:e74609. doi: 10.1371/journal.pone.0074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira DC, Sogawa Y, Holmes GL. The expression of Fos following kainic acid-induced seizures is age-dependent. Eur J Neurosci. 2002;15:329–344. doi: 10.1046/j.0953-816x.2001.01849.x. [DOI] [PubMed] [Google Scholar]
- Simler S, Vergnes M, Marescaux C. Spatial and temporal relationships between C-Fos expression and kindling of audiogenic seizures in Wistar rats. Exp Neurol. 1999;157:106–119. doi: 10.1006/exnr.1999.7036. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Zhang D, Bertram EH., 3rd Excitatory amplification through divergent-convergent circuits: the role of the midline thalamus in limbic seizures. Neurobiology of disease. 2011;43:435–445. doi: 10.1016/j.nbd.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS, Bumanglag AV. Defining “epileptogenesis” and identifying “antiepileptogenic targets” in animal models of acquired temporal lobe epilepsy is not as simple as it might seem. Neuropharmacology. 2013;69:3–15. doi: 10.1016/j.neuropharm.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer DD, Spencer SS, Mattson RH, Williamson PD, Novelly R. Access to posterior medial temporal lobe structures in the surgical treatment of temporal lobe epilepsy. Neurosurg. 1984;15:667–671. doi: 10.1227/00006123-198411000-00005. [DOI] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Steffens M, Huppertz HJ, Zentner J, Chauzit E, Feuerstein TJ. Unchanged glutamine synthetase activity and increased NMDA receptor density in epileptic human neocortex: implications for the pathophysiology of epilepsy. Neurochem Int. 2005;47:379–384. doi: 10.1016/j.neuint.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Stevens JR, Phillips I, de Beaurepaire R. gamma-Vinyl GABA in endopiriform area suppresses kindled amygdala seizures. Epilepsia. 1988;29:404–411. doi: 10.1111/j.1528-1157.1988.tb03739.x. [DOI] [PubMed] [Google Scholar]
- Sun HL, Zhang SH, Zhong K, Xu ZH, Feng B, Yu J, Fang Q, Wang S, Wu DC, Zhang JM, Chen Z. A Transient Upregulation of Glutamine Synthetase in the Dentate Gyrus Is Involved in Epileptogenesis Induced by Amygdala Kindling in the Rat. PLoS One. 2013;8:e66885. doi: 10.1371/journal.pone.0066885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson LW, Cowan WM. The connections of the septal region in the rat. J Comp Neurol. 1979;186:621–655. doi: 10.1002/cne.901860408. [DOI] [PubMed] [Google Scholar]
- Szyndler J, Maciejak P, Turzynska D, Sobolewska A, Taracha E, Skorzewska A, Lehner M, Bidzinski A, Hamed A, Wislowska-Stanek A, Krzascik P, Plaznik A. Mapping of c-Fos expression in the rat brain during the evolution of pentylenetetrazol-kindled seizures. Epilepsy Behav. 2009;16:216–224. doi: 10.1016/j.yebeh.2009.07.030. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Hel WS, Hessel EV, Bos IW, Mulder S, Verlinde SA, van Eijsden P, de Graan PN. Persistent reduction of hippocampal glutamine synthetase expression after status epilepticus in immature rats. Eur J Neurosci. 2014;40:3711–3719. doi: 10.1111/ejn.12756. [DOI] [PubMed] [Google Scholar]
- van der Hel WS, Notenboom RG, Bos IW, van Rijen PC, van Veelen CW, de Graan PN. Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology. 2005;64:326–333. doi: 10.1212/01.WNL.0000149636.44660.99. [DOI] [PubMed] [Google Scholar]
- van Gassen KL, van der Hel WS, Hakvoort TB, Lamers WH, de Graan PN. Haploinsufficiency of glutamine synthetase increases susceptibility to experimental febrile seizures. Genes Brain Behav. 2009;8:290–295. doi: 10.1111/j.1601-183X.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- van Vliet EA, Aronica E, Tolner EA, Lopes da Silva FH, Gorter JA. Progression of temporal lobe epilepsy in the rat is associated with immunocytochemical changes in inhibitory interneurons in specific regions of the hippocampal formation. Exp Neurol. 2004;187:367–379. doi: 10.1016/j.expneurol.2004.01.016. [DOI] [PubMed] [Google Scholar]
- Wada JA, Kudo T. Involvement of the claustrum in the convulsive evolution of temporal limbic seizure in feline amygdaloid kindling. Electroencephalogr Clin Neurophysiol. 1997;103:249–256. doi: 10.1016/s0013-4694(97)96160-5. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zaveri HP, Lee TS, Eid T. The development of recurrent seizures after continuous intrahippocampal infusion of methionine sulfoximine in rats: a video-intracranial electroencephalographic study. Exp Neurol. 2009;220:293–302. doi: 10.1016/j.expneurol.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willoughby JO, Mackenzie L, Medvedev A, Hiscock JJ. Fos induction following systemic kainic acid: early expression in hippocampus and later widespread expression correlated with seizure. Neuroscience. 1997;77:379–392. doi: 10.1016/s0306-4522(96)00462-9. [DOI] [PubMed] [Google Scholar]
- Witter MP, Groenewegen HJ, Lopes da Silva FH, Lohman AHM. Functional organization of the extrinsic and intrinsic circuitry of the parahippocampal region. Prog Neurobiol. 1989;33:161–253. doi: 10.1016/0301-0082(89)90009-9. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG, Saldana E, Witter MP. Projection from the nucleus reuniens thalami to the hippocampal region: light and electron microscopic tracing study in the rat with the anterograde tracer Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1990;296:179–203. doi: 10.1002/cne.902960202. [DOI] [PubMed] [Google Scholar]
- Yoon HH, Kwon HL, Mattson RH, Spencer DD, Spencer SS. Long-term seizure outcome in patients initially seizure-free after resective epilepsy surgery. Neurology. 2003;61:445–450. doi: 10.1212/01.wnl.0000081226.51886.5b. [DOI] [PubMed] [Google Scholar]