Abstract
Objective
To evaluate the activity of interleukin-2 (IL-2) in combination with allogeneic large multivalent immunogen (LMI) vaccine, prepared by immobilizing SK23-CD80+ melanoma cell line plasma membrane on 5-μm-diameter silica beads, in patients with melanoma.
Methods
Twenty-one patients with metastatic melanoma were randomly assigned to an IL-2 alone control group or an IL-2 plus LMI vaccine treatment group. The primary objective was to evaluate the progression-free survival (PFS) of each group. Secondary clinical objectives included median overall survival (OS) and 1- and 2-year rates of OS.
Results
Treatment was very well tolerated. Median PFS was no different between the treatment arm (2.20 mo) and control arm (1.95 mo). Median OS was also similar for the treatment arm (11.89 mo) and control arm (9.97 mo).
Conclusions
This study failed to demonstrate that allogeneic LMI vaccine and low-dose IL-2 improved survival in patients with melanoma as compared with low-dose IL-2 alone.
Keywords: autologous vaccine, metastatic malignant melanoma, large multivalent immunogen vaccine
Melanoma has long been considered one of the most immunogenic cancers, with demonstrated responses to immune modulation by cytokines, such as high-dose inter-leukin-2 (IL-2) and interferon (IFN) α-2b, and more recently by ipilimumab, a monoclonal antibody to cytotoxic T-lymphocyte–associated 4 (CTLA-4) antigen, which abrogates the inhibitory immune checkpoint leading to T-cell activation.1–3 This innate immunogenicity and lack of responsiveness to chemotherapy suggests that vaccine therapy might be a promising strategy for the management of advanced melanoma. Although many vaccines have shown activity in early phase studies, a recent meta-analysis has shown that vaccines are associated with limited survival benefit compared with other forms of therapy; however, they do seem to be associated with a substantial increase in overall survival (OS) in the sub-population of patients with a melanoma-specific response.4
Various strategies have been used in the development of vaccines for melanoma ranging from use of tumor-derived peptides, irradiated autologous tumor cells, allogeneic cell lysates, dendritic cells, adoptive T-cell transfer, and gene-modified tumor or dendritic cells. These vaccines have often been combined with immune adjuvants, including growth factors such as granulocyte monocyte colony–stimulating factor, toll-like receptor agonists, CTLA-4 antibody, and cytokines such as IL-2. We have developed a novel approach to melanoma vaccine, termed large multivalent immunogen (LMI), in which cell-sized (5-mm diameter) latex or silica spheres serve as a support structure for presenting tumor antigens.5 Cytotoxic T lymphocytes (CTL) are unable to mount a significant response in vivo to soluble or subcellular forms of antigen and need cell-sized fragments for optimal activation.6 LMI provides a large contiguous surface area for immobilizing melanoma antigens. In a previous phase II study, patients with advanced melanoma were given LMI loaded with autologous tumor cell membranes and IL-2, with or without cyclophosphamide, resulting in a median OS of 20.4 months (95% CI, 8.0; NA) and progression-free survival (PFS) of 2.8 months (95% CI, 1.87-6.25).7 One of these patients is still in remission over 5 years since completing the treatment. Although autologous vaccine has the advantage of containing all MHC molecules and tumor-associated antigens specific to a patient, it is hampered by a limited quantity of tumor tissue.
In the current study, we addressed this limitation by using the SK23-CD80+ cell line to prepare an LMI vaccine. This cell line expresses all common melanoma antigens and was genetically modified to express human B7-1 (CD80). However, even if allogeneic LMI melanoma vaccine initiates an antimelanoma immune response, antigen presentation alone may be insufficient to sustain this response. Preclinical studies have shown that the CD8+ T-cell response declines after an initial robust response to tumor antigen production, but this anergy can be overcome by administration of IL-2.8 To test whether IL-2 improves CD8+ T-cell responses, we conducted a randomized phase II trial comparing allogeneic LMI vaccine plus IL-2 to IL-2 alone.
Methods
Study Design
LMI vaccine preparation was done as previously described,7 with the exception that the SK23-CD80+ melanoma cell line was used as the source of cell membranes in this study. This phase II randomized clinical trial compared LMI vaccination plus IL-2 to a control arm of IL-2 alone in a 1:1 ratio. Cross-over to the LMI vaccination plus IL-2 arm was allowed for patients progressing on IL-2 alone. The primary objective was to evaluate the PFS of each treatment group. Secondary clinical objectives included overall clinical response and survival at 1 and 2 years. The correlative objective was to determine whether an immune response was generated after the treatment. Immune responses were assessed by IFN-γ production by CD8+ T cells using the ELISpot assay, CD8+ T-cell binding to HLA-A2 multimers complexes with melanoma-derived peptides, and detection of antibodies that bind to the SK23-CD80+ cell line in patient serum by flow cytometry. The study was approved by the Institutional Review Board at the University of Minnesota and was followed by a data safety monitoring board. The study was halted after enrolling 21 patients after a preplanned analysis established that it was unlikely that the study would meet its primary objective of improved PFS with additional accrual.
Patients
Eligible criteria included a diagnosis of metastatic melanoma with measurable disease as defined by RECIST criteria, an ECOG performance status of 0 or 1, and normal organ function. Prior systemic therapies were allowed if 4 weeks had passed since prior treatment and the patient had recovered from the effects of previous therapy. Patients had to agree to vaccination with tetanus toxoid and keyhole limpet hemocyanin (KLH) to assess antigen-specific immune responses. Patients on immune suppressants (including prednisone) or patients with autoimmune disease or brain metastases were excluded from the study.
Treatment
All patients were vaccinated with KLH and tetanus toxoid on the first day of therapy initiation to assess each patient's primary and secondary immune response to control antigens. Patients in the control arm self-administered IL-2 subcutaneously at a dose of 106 IU once a day for 2 days and repeated this regimen every 4 weeks until disease progression or a maximum of 12 treatment cycles. Patients were premedicated with acetaminophen (650 mg) and ibuprofen (400 mg) before each dose of IL-2 followed 4 hours later by another dose of ibuprofen (400 mg). Patients in the treatment arm received 0.2 mL of allogeneic tumor cell membrane–coated LMI (1 × 107, 5-μm silica spheres) as an intradermal injection at 4-week intervals until disease progression or a maximum of 12 treatment cycles. On the seventh day after LMI vaccination, IL-2 and premedication were self-administered in the treatment arm according to the same regimen as the control arm (ie, 2 doses repeated each cycle). Patients in the control arm were allowed to cross over to the treatment arm if they met the eligibility criteria.
Assessments
Response to treatment was assessed with imaging CT or MRI scans every 8 weeks for the first year and every 12 weeks thereafter until disease progression. In the treatment group, delayed-type hypersensitivity (DTH) to LMI (1 × 107) and human serum albumin-coated silica beads (1 × 107) administered intradermally was assessed to evaluate immune response after the third vaccination. Patients in both groups received KLH and tetanus toxoid as control antigens at the start of therapy. Toxicity and adverse events were classified according to NCI's Common Terminology Criteria for Adverse Events V 3.0.
ELISpot assays were performed to detect T cells that produce IFN-γ in response to tumor-specific antigens. Briefly, Millipore MSHAS Multiscreen HTS nitrocellulose plates (Millipore Corp., Billerica, MA) were coated overnight with capture antibody to IFN-γ (BD Pharmingen, San Diego, CA). Patient's peripheral blood mononuclear cells (PBMC) were added to the antibody-coated plates along with peptide pools consisting of complete protein-spanning mixtures of over-lapping peptides derived from 1 or more of the melanoma-associated proteins: Mart-1, MAGEA1, MAGEA3, MAGEA4, and Tyrosinase (JPT Peptide Technologies GmbH, Berlin, Germany); cells were cultured with medium alone as a negative control and with Pokeweed mitogen (Sigma-Aldrich, St Louis, MO) as a positive control. After overnight culture, cells were removed from the wells, and spots where IFN-γ–producing cells were present were detected by sequential addition of biotinylated detection antibody to IFN-γ (BD Pharmingen), alkaline phosphatase–conjugated streptavidin (Jackson ImmunoResearch Laboratories, West Grove, PA), and BCIP/NBT substrate (Sigma-Aldrich). Spots were enumerated using a CTL-Immunospot analyzer (Shaker Heights, OH).
PBMCs from patients who were HLA-A2+ were stained with pentamers (Proimmune Ltd, Oxford, UK) to detect melanoma-specific CD8+ T cells. Cells were stained following the protocol provided by the vendor. The pentamers tested were complexed with peptides derived from melanoma proteins Mart-1 (ELAGIGILTV), gp100 (IMDQVPFSV), or peptides derived from viral proteins from CMV (NLVPMVATV) or EBV (GLCTLVAML). A negative control pentamer was also included.
Patient serum samples were tested for antibodies that recognized the SK23-CD80+ cell line used to prepare the LMI vaccine. Briefly, serum was added to live SK23-CD80+ cells at a final dilution of 1:200 and 1:800, incubated for 1 hour at 4°C, followed by incubation with fluorescent antibody to human immunoglobulin (Jackson ImmunoResearch). Cells were analyzed on a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA).
Statistical Analyses
Sample size was estimated by using the Lachin's method9 (S-PLUS 7.0; Insightful Corporation, Seattle, WA) on the basis of PFS. Assuming time to progression followed an exponential distribution and median PFS was 16 weeks for the LMI plus IL-2 arm and 8 weeks for IL-2 alone arm, the 1-year PFS rate would be 10.5% and 1.1% for the 2 arms, respectively. It was estimated that a sample size of 102 patients (51/arm) was needed to detect a difference in the 1-year PFS rate between treatment and control arms with 90% power at a significance level of 0.05. Block randomization was used to generate randomization schedules for the trial. Predictive probability interim analysis for PFS was conducted using “Predictive Probabilities” version 1.4.10 A frequentist test was used to calculate the predictive probability of declaring superiority for each treatment arm. The probability of type I error was 0.05, and 5000 simulations were conducted for the calculation. Data analysis followed the intention-to-treat principle. Baseline patient characteristics were summarized by descriptive statistics and compared between the 2 arms with the Wilcoxon rank-sum test or the Fisher exact test. Probabilities of OS and PFS were estimated with the Kaplan-Meier method and compared between arms by the log-rank test. The impact of treatment on both OS and PFS were evaluated through Cox proportional hazard models. A P-value of <0.05 was significant. SAS 9.2 (SAS Institute Inc., Cary, NC) was used for all data analysis.
Results
Twenty-one patients were enrolled in this clinical trial from July 2008 through August 2010, with 11 randomly assigned to the LMI plus IL-2 arm and 10 to the IL-2 alone arm (Table 1). One patient was found to have a brain metastasis after being randomized to the LMI plus IL-2 arm and did not receive any therapy on trial; this patient was included in the intention-to-treat analysis for efficacy but not in the safety analysis. Treatments were well tolerated, with similar side effects in both groups. The only grade IV nontreatment-related toxicity was deep vein thrombosis in 1 patient in the LMI vaccine plus IL-2 arm. Other side effects included grade III syncope, generalized weakness, back pain, abdominal pain, and dyspnea seen in 1 patient on the LMI plus IL-2 arm. A single patient experienced grade III nausea in the IL-2 alone arm.
Table 1. Patients' Demographics on Both Arms of Study.
| Characteristics | IL-2 (n = 10) | LMI+IL-2 (n=11) | Test | P |
|---|---|---|---|---|
| LDH level | 674.8 ± 290.4 Median = 603.5 |
516.1 ± 90.5 Median = 513.5 |
Rank Sum | 0.0917 |
| No. prior therapies | 1.2 ± 1.2 Median =1.0 |
2.1 ± 2.0 Median = 1.5 |
Rank Sum | 0.3088 |
| Time from last prior therapy (mo) | 8.3 ± 15.1 Median = 2.4 |
8.4 ± 15.5 Median = 2.0 |
Rank Sum | 0.9702 |
| Time from diagnosis to study therapy (mo) | 31.0 ± 21.2 Median = 30.4 |
70.3 ± 121.3 Median= 35.0 |
Rank Sum | 0.4814 |
| Primary site | ||||
| Eye | 1 (50%) | 1 (50%) | Fisher exact | 1.0000 |
| Mucosal | 1 (50%) | 1 (50%) | ||
| Skin | 8 (50%) | 8 (50%) | ||
| ECOG performance | ||||
| 0 | 9 (90%) | 7 (70%) | Fisher exact | 0.5820 |
| 1 | 1 (10%) | 3 (30%) | ||
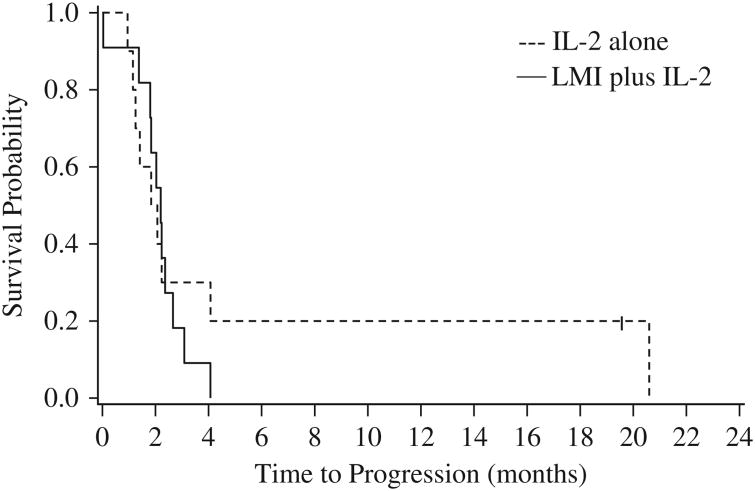
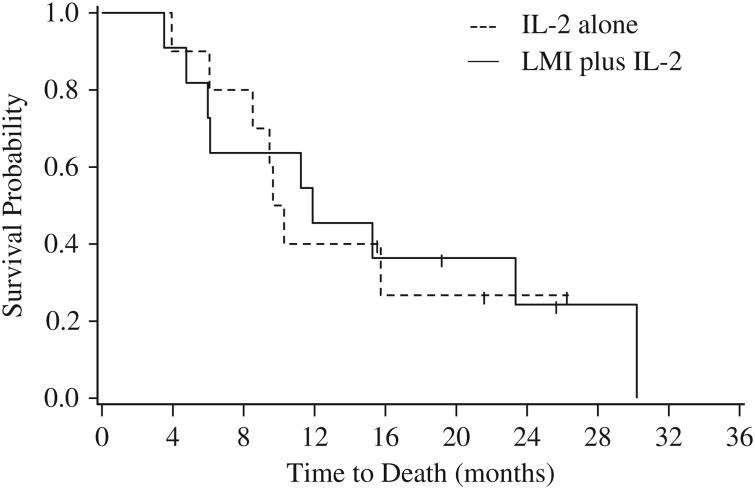
Within the first 2 months of treatment, disease progression was evident in 4 of the 11 patients receiving LMI vaccine plus IL-2 and 5 of the 10 patients receiving only IL-2. Further, 12 patients in total (6 from each arm) died during the first year of treatment. When the predictive probability interim analysis was conducted, all 11 patients on the LMI plus IL-2 arm had progressed, as did 7 out of 10 patients from IL-2 alone arm. The median PFS was 2.2 months for the LMI plus IL-2 arm and 2.0 months for the IL-2 alone arm (Table 2; Fig. 1), and there was no significant difference in PFS between the 2 arms (log-rank test, P = 0.5673). The median OS was 11.9 and 10.0 months for LMI plus IL-2 and IL-2 alone arms, respectively, (Table 2; Fig. 2), and there was no significant difference in OS between the 2 arms (log-rank test, P = 0.9071). Six patients who progressed on the IL-2 arm crossed over to receive LMI vaccine. Survival outcomes were comparable for patients who crossed over and for those remaining in the IL-2 only arm, with 2-month PFS rates of 50% [95% confidence interval (CI), 11%-80%] and 50% (95%CI, 6%-84%), respectively, and 1-year OS rates of 33% (95% CI, 5%-68%) and 50% (95% CI, 6%-84%). In addition, the accrual rate was slow at only 0.67 patients per month, making attainment of the recruitment goal of 102 patients in 4 years unlikely. On the basis of these data, it was calculated that at the end of the trial the probability of the study concluding in favor of IL-2 alone would be 85.6%, whereas the probability of concluding in favor of LMI plus IL-2 would be only 1.5%. The study was therefore terminated early.
Table 2. Univariate Analysis of PFS and OS.
| PFS | Censored | Dead | Total | Median PFS (95% CI)* | 2-mo PFS Rate (95% CI) | 4-mo PFS Rate (95% CI) | Hazard Ratio (95% CI) |
|---|---|---|---|---|---|---|---|
| All | 1 | 20 | 21 | 2.1 (1.4, 2.4) | 57% (34%-75%) | 19% (6%-38%) | — |
| Treatment | |||||||
| LMI+IL-2 | 0 | 11 | 11 | 2.2 (1.4, 2.7) | 64% (30%-85%) | 9% (1%-33%) | 1.3 (0.5, 3.4)† |
| IL-2 | 1 | 9 | 10 | 2.0 (1.0, 4.1) | 50% (18%-75%) | 30% (7%-58%) | 1 |
| KLH 10-fold | |||||||
| Response | 0 | 9 | 9 | 1.8 (1.0, 2.4) | 33% (8%-62%) | 11% (1%-39%) | 2.9 (1.1, 7.6)§ |
| No response | 1 | 9 | 10 | 2.4 (1.8, 4.1) | 90% (47%-99%) | 30% (7%-58%) | 1 |
|
| |||||||
| OS | Censored | Dead | Total | Median OS (95% CI)* | 1-y OS Rate (95% CI) | 2-y OS Rate (95% CI) | Hazard Ratio (95% CI) |
|
| |||||||
| All | 5 | 16 | 21 | 11.2 (6.1, 23.4) | 43% (22%-62%) | 24% (8%-46%) | — |
| Treatment | |||||||
| LMI+IL-2 | 2 | 9 | 11 | 11.9 (4.8, 30.2) | 45% (17%-71%) | 24% (4%-53%) | 0.9 (0.3, 2.6)‡ |
| IL-2 | 3 | 7 | 10 | 10.0 (3.9, NA) | 40% (12%-67%) | 27% (5%-56%) | 1 |
| KLH 10-fold | |||||||
| Response | 1 | 8 | 9 | 9.5 (3.5, 15.7) | 33% (8%-62%) | 0% (1%-49%) | 2.8 (0.9, 8.8)‖ |
| No response | 4 | 6 | 10 | 22.7 (6.0, 30.2) | 60% (25%-83%) | 50% (18%-75%) | 1 |
Time is in months.
P = 0.5535.
P = 0.9069.
P = 0.0348.
P = 0.0701.
CI indicates confidence interval; IL-2, interleukin-2; KLH, keyhole limpet hemocyanin; LMI, large multivalent immunogen; OS, overall survival; PFS progression-free survival.
Figure 1.
Kaplan-Meier curves of progression-free survival for the 2 treatment arms.
Figure 2.
Kaplan-Meier curves of overall survival for the 2 treatment arms.
Nineteen patients underwent the KLH test, of which 9 presented a 10-fold KLH response. Results from univariate analysis (Table 2) show that presence of KLH response paradoxically correlated with worse PFS (log-rank test, P = 0.0286) and OS (log-rank test, P = 0.0589). The correlation of KLH response with PFS and OS was not altered by type of treatment received (interaction of treatment × KLH response, P = 0.1224 for PFS and 0.5205 for OS); however, when a 2-fold difference was used as the cutoff response, KLH responders on the IL-2 arm had significantly worse survival than those treated with LMI and IL-2 (log-rank test, P = 0.0388; Cox regression, P = 0.0684).
ELISpot assays were performed for all patients who received the LMI vaccine (either initially or after crossover from the IL-2 alone arm) and from whom we received at least 1 postvaccine blood sample. Baseline and 1 to 3 postvaccine samples were tested in the same assay. All PBMCs responded to the positive control (Pokeweed mitogen). One patient had a high response to the MAGEA4 peptide pool at baseline, but the number of spots did not increase in postvaccination samples; none of the other patients had a positive response (at least 10 spots) to any of the peptide pools. Pentamer staining was performed for all HLA-A2+ patients, in either arm of the study, from whom we received at least 1 posttreatment blood sample. These tests did not reveal any evidence of CD8+ T cells that bound the pentamers complexed with Mart-1 or gp100 peptides in the blood of patients, either at baseline or after treatment. Sera from all patients who received the LMI vaccine and from whom we received at least 1 postvaccine blood sample were tested for the presence of antibodies that would bind to the SK23-CD80+ cells line used to produce the LMI vaccine. This screening failed to detect evidence of an antibody response to the vaccine in any patients.
Discussion
The present study was designed to build upon the success with autologous LMI vaccine in a previous phase II trial where the subgroup of patients receiving LMI vaccine alone had not reached median survival after 45.9 months.7 In contrast to this previous trial, the current randomized phase II trial used LMI beads coated with immunogen derived from allogeneic melanoma SK23-CD80+ cells and tested whether IL-2 combined with LMI vaccine would be beneficial. Unfortunately, allogeneic LMI vaccine in combination with IL-2 failed to prolong PFS in patients with advanced refractory melanoma when compared with IL-2 alone. Although late responses to vaccine therapies are known, it is possible that the design of this study powered for assessment of PFS did not allow for the evaluation of late benefits of our vaccine. In addition, the lack of treatment benefit could be due to the use of allogeneic melanoma cells instead of autologous tissue tumor tissue or the use of IL-2. Indeed, in a previous phase II trial, patients receiving IL-2, cyclophosphamide, and autologous LMI vaccine exhibited a median OS of 20.4 months compared with a median survival of >45.9 months in patients receiving only autologous LMI vaccine. There could be several reasons why autologous vaccine could be superior over allogeneic vaccine. Preclinical studies using murine tumor models demonstrated that the immuno-therapeutic effects of LMI depend, at least in part, on recognition of antigen and class I MHC complex on the surface of the LMI.6,11 For this reason, we required for inclusion in our trial that a patient share at least 1 class I MHC allele with the SK23-CD80+ cell line (HLA-A1, A2, B7, B8, C7) used to prepare the LMI. In contrast, the full spectrum of class I alleles and tumor epitopes that can potentially be recognized by the patient's CD8 T cells is displayed when plasma membranes from an autologous tumor are used to prepare the LMI. In addition, there is also evidence that T-cell responses are mostly dictated by tumor rejection neoantigens created by mutations in the patient's tumor that are unlikely to be represented in the allogeneic LMI used in our study.7,12
With an improved understanding of immunotherapy, new agents are rapidly being developed that have the potential to enhance vaccines.4 Despite this rapid progress, however, we continue to adhere to response evaluations that are best suited for cytotoxic chemotherapies rather than immunotherapies or targeted therapies. To better measure responses, immune-related response criteria have been proposed in patients with melanoma.13 Similarly, the Prostate Cancer Working Group has recommended that therapy continue for 12 weeks despite biochemical PSA progression, which has been the traditional marker of response.14 Assessment of late responses seen in immune therapies and decisions regarding further development of such therapy based on OS should be incorporated in future trials. We used KLH as control antigen to assess the intactness of the immune system. This approach showed that in KLH 2-fold responders, vaccine treatment was associated with better survival. This has to be further investigated. The lack of any other correlates of immune response in this study was disappointing. As new immunotherapy agents are being developed, we will have to utilize novel trial designs, identify intermediary markers for response, utilize immune-related response criteria, and better select patients most likely to respond.
Acknowledgments
The authors thank Michael J. Franklin (University of Minnesota) for editorial support.
Supported by University of Minnesota Comprehensive Cancer Center.
Footnotes
The authors declare no conflicts of interest.
References
- 1.Robert C, Thomas L, Bondarenko I, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364:2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 2.Sparano JA, Fisher RI, Sunderland M, et al. Randomized phase III trial of treatment with high-dose interleukin-2 either alone or in combination with interferon alfa-2a in patients with advanced melanoma. J Clin Oncol. 1993;11:1969–1977. doi: 10.1200/JCO.1993.11.10.1969. [DOI] [PubMed] [Google Scholar]
- 3.Creagan ET, Ahmann DL, Frytak S, et al. Three consecutive phase II studies of recombinant interferon alfa-2a in advanced malignant melanoma. Updated analyses. Cancer. 1987;59(suppl):638–646. doi: 10.1002/1097-0142(19870201)59:3+<638::aid-cncr2820591312>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 4.Chi M, Dudek AZ. Vaccine therapy for metastatic melanoma: systematic review and meta-analysis of clinical trials. Melanoma Res. 2011;21:165–174. doi: 10.1097/CMR.0b013e328346554d. [DOI] [PubMed] [Google Scholar]
- 5.Mescher MF. Surface contact requirements for activation of cytotoxic T lymphocytes. J Immunol. 1992;149:2402–2405. [PubMed] [Google Scholar]
- 6.Rogers J, Mescher MF. Augmentation of in vivo cytotoxic T lymphocyte activity and reduction of tumor growth by large multivalent immunogen. J Immunol. 1992;149:269–276. [PubMed] [Google Scholar]
- 7.Dudek AZ, Mescher MF, Okazaki I, et al. Autologous large multivalent immunogen vaccine in patients with metastatic melanoma and renal cell carcinoma. Am J Clin Oncol. 2008;31:173–181. doi: 10.1097/COC.0b013e3181573e6b. [DOI] [PubMed] [Google Scholar]
- 8.Mescher MF, Popescu FE, Gerner M, et al. Activation-induced non-responsiveness (anergy) limits CD8 T cell responses to tumors. Semin Cancer Biol. 2007;17:299–308. doi: 10.1016/j.semcancer.2007.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lachin JM, Foulkes MA. Evaluation of sample size and power for analyses of survival with allowance for nonuniform patient entry, losses to follow-up, noncompliance, and stratification. Biometrics. 1986;42:507–519. [PubMed] [Google Scholar]
- 10.M.D. Anderson Cancer Center, Department of Biostatistics. Predictive Probabilities. [Accessed October 15, 2010]; Available at: https://biostatistics.mdanderson.org/SoftwareDownload/
- 11.Goldberg J, Shrikant P, Mescher MF. In vivo augmentation of tumor-specific CTL responses by class I/peptide antigen complexes on microspheres (large multivalent immunogen) J Immunol. 2003;170:228–235. doi: 10.4049/jimmunol.170.1.228. [DOI] [PubMed] [Google Scholar]
- 12.Lennerz V, Fatho M, Gentilini C, et al. The response of autologous T cells to a human melanoma is dominated by mutated neoantigens. Proc Natl Acad Sci U S A. 2005;102:16013–16018. doi: 10.1073/pnas.0500090102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wolchok JD, Hoos A, O'Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res. 2009;15:7412–7420. doi: 10.1158/1078-0432.CCR-09-1624. [DOI] [PubMed] [Google Scholar]
- 14.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]