Abstract
We examined the patterns, variability, and predictors of urinary bisphenol A (BPA) concentrations in 337 children from the Cincinnati, Ohio HOME Study. From 2003 to 2014, we collected two urine samples from women at 16 and 26 weeks of pregnancy and six urine samples from children at 1–5 and 8 years of age. We used linear mixed models to calculate intraclass correlation coefficients (ICCs) as a measure of within-person BPA variability and to identify sociodemographic and environmental predictors. For the 8-year visit, we used multivariable linear regression to explore associations between urinary BPA concentrations and exposure- related factors. We calculated daily intakes using equations estimating creatinine excretion rates and creatinine-standardized BPA concentrations. Urinary BPA concentrations, which decreased over childhood, had a low degree of reproducibility (ICC<0.2). Estimated daily intakes decreased with age and were below the reference dose of 50 μg/kg body weight/day. BPA concentrations were positively associated with consuming food stored or heated in plastic, consuming canned food and beverages, and handling cash register receipts. Our results suggest that there are multiple sources of BPA exposure in young children. Etiological studies should collect serial urine samples to accurately classify BPA exposure and consider sociodemographic and environmental factors as possible confounders.
Graphical Abstract
Introduction
Bisphenol A (BPA) is a monomeric compound used to produce plastics and resins that are found in a variety of consumer products, including food can linings, plastic food and beverage storage containers, medical equipment, dental sealants, thermal paper receipts, children’s toys, and cigarette filters.1, 2 Its widespread use has made BPA exposure ubiquitous in both developed and developing countries.3–5 The primary source of non-occupational BPA exposure is diet, while individuals employed in industries using BPA or routinely handling BPA- containing products may also be exposed via inhalation and dermal absorption.6–11 The United States Environmental Protection Agency (EPA) has established an oral reference dose (RfD) for BPA of 50 g/kg body weight/day.12
Epidemiological studies suggest that BPA exposure may lead to adverse health outcomes.1 Early life exposures to BPA are of particular interest since major windows of developmental vulnerability exist during gestation and through early childhood.13, 14 BPA, which is structurally similar to several endogenous hormones, may disrupt normal hormone signaling and metabolism and affect children’s growth and neurodevelopment.15
A growing number of studies have reported urinary concentrations of BPA in children worldwide, ranging in ages from infancy to early adolescence (Table S1, Supplement).3–4, 16–30 Many of these studies have considered the effects of sociodemographic factors, such as parental education and household income, on childhood urinary BPA concentrations, while only a few have explored associations with dietary sources of BPA exposure.20, 21, 25 Additional studies are needed to identify other potentially modifiable sources of BPA exposure. Further, BPA has a short biological half-life (<6 hours),31 and urinary BPA concentrations can vary substantially within a day, resulting in significant within-person variability. This variation may lead to misclassification bias in studies relying on one BPA measurement.25, 32–33 Characterizing this variability will help design better studies and aid in interpreting studies that only had a single BPA measurement.
The objectives of the present study were to characterize the patterns and variability of serial urinary BPA concentrations in a cohort of children from Cincinnati, Ohio, between 1 and 8 years of age, and to explore associations with sociodemographic, environmental, dietary, and dental factors. Further, we used creatinine-adjusted BPA concentrations in conjunction with equations for age- and sex-specific creatinine excretion rates to estimate daily BPA intakes.
Materials and Methods
Study Participants
We used data collected from mothers and their children who were participating in the Health Outcomes and Measures of the Environment (HOME) Study, a prospective pregnancy and birth cohort in the Cincinnati, Ohio, metropolitan area designed to study general population environmental exposures. Eligibility criteria and participant recruitment have been described previously.34 Briefly, of 1,263 eligible women, 468 enrolled in our study (37%) between March 2003 and January 2006. The current analysis was restricted to 337 children who had at least one urinary BPA measurement from one of six study visits conducted between 1 and 8 years of age. The institutional review boards at Cincinnati Children’s Hospital Medical Center and the Centers for Disease Control and Prevention (CDC) approved this study. All mothers provided written informed consent for themselves and their children.
Urinary BPA Concentrations
We collected spot urine samples from children during study visits between 2004 and 2014, which included annual clinic visits at 1–5 and 8 years of age and annual home visits at 1–3 years of age. A subset of 60 children provided urine samples at both the clinic and home visits at 1 (n=16), 2 (n=24), and 3 (n=27) years of age (7 children provided both clinic and home visit samples at more than one year). For the present analysis, we used the sample collected at the clinic visit if a child provided a urine sample at both visits. We collected urine directly into polypropylene specimen cups for children who were toilet trained. For non-toilet trained children, we collected urine into Kendall abdominal pads placed inside the diaper and excluded inserts contaminated with stool. BPA was not detected in the inserts prior to sample collection.35 Urine samples were stored at −20°C until shipment to the CDC Environmental Health Laboratory, where they were stored at or below −20°C until analysis. For sample storage and analysis, we followed all provisions described in Ye et al. (2013) to minimize external contamination.36
The concentrations of total (free plus conjugated) BPA were measured at the CDC using analytical chemistry methods described previously.37 The limit of detection (LOD) was 0.4 ng/mL for samples collected at the 1–5 year visits and 0.1 ng/mL for the 8-year visit; concentrations below the LOD were assigned a value of LOD/√2.38 Each analytic batch included low and high concentration quality control (QC) samples, and the coefficients of variation of repeated QC samples were less than 10%. In the first batch, we measured free BPA to evaluate potential external contamination, but free BPA concentrations were either undetectable or very close to the LOD. We repeated total extractions only to confirm higher than expected results in all analytic batches,39 but results never suggested contamination. Urinary creatinine concentrations were measured using a previously described assay.40 We compared different methods of accounting for urine dilution, including: 1) creatinine-standardized values (individual BPA concentrations divided by creatinine) and 2) urinary BPA adjusted for creatinine or age- specific creatinine z-score as a covariate.
Predictors of Child BPA Concentrations
We examined various predictors, including sociodemographic, environmental, dietary, and dental variables collected from a combination of questionnaires and biological samples. Sociodemographic information was obtained using hospital medical charts (child’s sex) and a computer-assisted questionnaire administered by trained research staff to participating mothers at each study visit (maternal and child race, maternal education, and household income).
We examined other environmental exposures, including prenatal BPA exposure and childhood tobacco smoke and phthalate exposures. We measured maternal urinary BPA concentrations at 16- and 26-weeks of pregnancy. 32 For the present study, these two BPA measurements were averaged together to obtain an estimate of prenatal BPA exposure. Serum cotinine concentrations were measured at the 1, 2, and 3 year visits and averaged together to obtain a summary measure of second-hand tobacco smoke exposure for each child. Eleven phthalate monoester metabolites were measured in child urine samples collected at the study visits.41 These included: mono-n-butyl phthalate (MBP), monoisobutyl phthalate (MiBP), monobenzyl phthalate (MBzP), mono-3-carboxypropyl phthalate (MCPP), monoethyl phthalate (MEP), monocarboxynonyl phthalate (MCNP), and monocarboxyoctyl phthalate (MCOP). We also examined a summary measure of di-2-ethylhexyl phthalate (ΣDEHP), which was the molar sum of mono-2-ethylhexyl phthalate (MEHP), mono-2-ethyl-5-hydroxyhexyl phthalate (MEHHP), mono-2-ethyl-5-oxohexyl phthalate (MEOHP), and mono-2-ethyl-5-carboxypentyl phthalate (MECPP). Because we detected MEHP, MBP, and MiBP in the inserts used for sample collection at the 1, 2, and 3 year visits, we did not measure these analytes in urine at those ages.
We administered an extensive chemical exposure questionnaire to mothers at the time of the 8-year sample collection. Mothers were asked about their child’s frequency of eating food stored or heated in plastic, use of plastic serving ware, and consumption of canned food and beverages in the past year and past 24 hours. We also asked mothers if their child handled any cash register receipts in the last 24 hours and about the child’s dental history, including the number of plastic/composite white or silver/amalgam fillings, crowns, and sealants and how old the child was when he or she received them.
Statistical Analysis
Log10-transformed urinary BPA concentrations were the outcome in all statistical analyses. We used box-and-whisker plots and linear mixed models to assess the trend in log10-transformed urinary BPA concentrations over 1 to 8 years of age with and without creatinine standardization. Further, we explored correlations between log10-transformed, maternal urinary BPA concentrations during pregnancy and children’s urinary BPA concentrations at each age, both unstandardized and creatinine standardized, using Pearson correlation coefficients.
We calculated intraclass correlation coefficients (ICCs) to examine the reproducibility of urinary BPA concentrations across visits and to compare methods of standardizing or adjusting for urinary creatinine. The ICC is a measure of the reproducibility of a measurement within an individual, where a value of zero indicates no reproducibility and a value of one indicates perfect reproducibility. We used random intercept linear mixed models with an unstructured covariance matrix to estimate between- and within-subject variability of log10-transformed urinary BPA concentrations. ICCs were calculated for urinary BPA concentrations over the entire study period (long-term ICCs) using participants with a BPA measurement from at least two visits (n=297). Short-term ICCs were calculated for the subset of 60 children that provided urine samples at both the clinic and home visits at 1, 2, or 3 years of age. Models were adjusted for child’s age.
To account for repeated measures of BPA, we used linear mixed models to examine associations between urinary BPA concentrations and sociodemographic and environmental factors. Log10-transformed urinary BPA concentrations were the outcome in each model. Sociodemographic factors included child’s sex, child’s race, household income, and maternal education. We entered child’s sex and race as fixed effects, while maternal education and household income were allowed to vary over time. Each sociodemographic variable was examined individually in crude models adjusted only for child’s age and creatinine z-score. Adjusted models further accounted for all other sociodemographic covariates. We examined log10-transformed concentrations of mean serum cotinine and urinary phthalate metabolites as continuous variables and adjusted for the sociodemographic factors above.
We used multivariable linear regression to explore associations between log10- transformed urinary BPA concentrations and possible sources of BPA exposure (e.g. food storage practices) at the 8-year visit. To examine the combined effect of multiple sources of BPA exposure, we created a summary variable for consuming canned food, canned beverages, beverages in carton or pouch, and receipt handling. We compared those with 1, 2, and 3–4 of these exposures to a reference group that did none of the above activities in the last 24 hours. Models were adjusted for creatinine z-score, child age, sex, race, maternal education, and household income. Since our outcome was log10-transformed, we exponentiated beta coefficients to obtain the percent difference in BPA concentrations compared to the reference group for categorical variables or for a given increase in continuous variables.
We also explored possible temporal trends in urinary BPA concentrations. Over the time period of the study, awareness of the potential adverse health effects of BPA likely increased, which may have impacted manufacturer practices and personal behaviors to reduce BPA exposure. To investigate these trends, we created a dichotomous year of birth variable to compare children born in 2003–2004 to those born in 2005–2006 and modeled urinary BPA concentrations as a function of year of birth, age, an interaction term (age x year of birth), and sociodemographic covariates.41 In addition, we ran separate models for each visit (year 1, year 2, etc.) with year of sample collection as a continuous predictor of urinary BPA concentrations.
The final analysis consisted of calculating daily BPA intakes (ng BPA/kg body weight/day) using creatinine-standardized BPA concentrations (μg BPA/g creatinine) and estimates of daily creatinine excretion (CE) rates. We calculated CE rates (mg/day) for each participant using equations for healthy, non-obese children based on age, sex, and height (Supplement, Table S2).42 After the CE rate was calculated, we estimated the daily intake by multiplying the creatinine-standardized BPA concentration by the CE rate and then dividing by the child’s body weight in kilograms. Daily intakes were compared to the EPA’s RfD of 50 μg/kg bw/day and EFSA’s Allowable Daily Intake (ADI) of 4 ug/kg bw/day.12, 43 All analyses were performed using R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria). Type one error was set to 0.05.
Results
There were 337 children with at least one, 297 with at least two, and 93 with all six urine samples from 1–8 years of age. Of the children who provided at least one urine sample, 317 (1,266 repeated measures) had complete covariate information. At age 8, 213 children provided a urine sample and had complete covariate data.
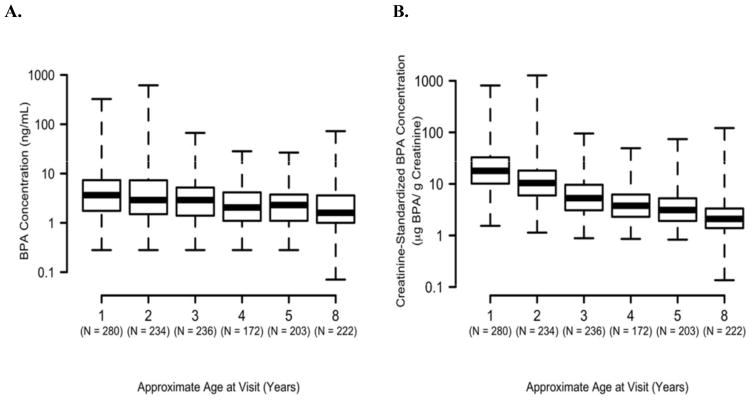
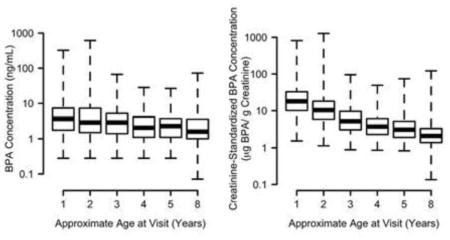
Percentages of non-detectable urinary BPA concentrations were <6% for all ages (Table S3, supplement). We observed a statistically significant decrease in unstandardized and creatinine-standardized BPA concentrations as child’s age increased (Figure 1). Because urinary creatinine increased with age (Figure S1, supplement), the decreasing trend between 1 and 8 years of age was more pronounced for the creatinine-standardized than the unstandardized urinary BPA concentrations.
Figure 1.
Distribution of log-transformed (A) urinary BPA (ng/mL) and (B) creatinine- standardized BPA (μg BPA/g creatinine) concentrations in HOME Study children ages 1–8 years.
* p-values for child age trend<0.0001. Increases in urinary creatinine with age resulted in the more pronounced decrease in creatinine-standardized BPA concentrations (see Figure S1 in the supplement).
Variability
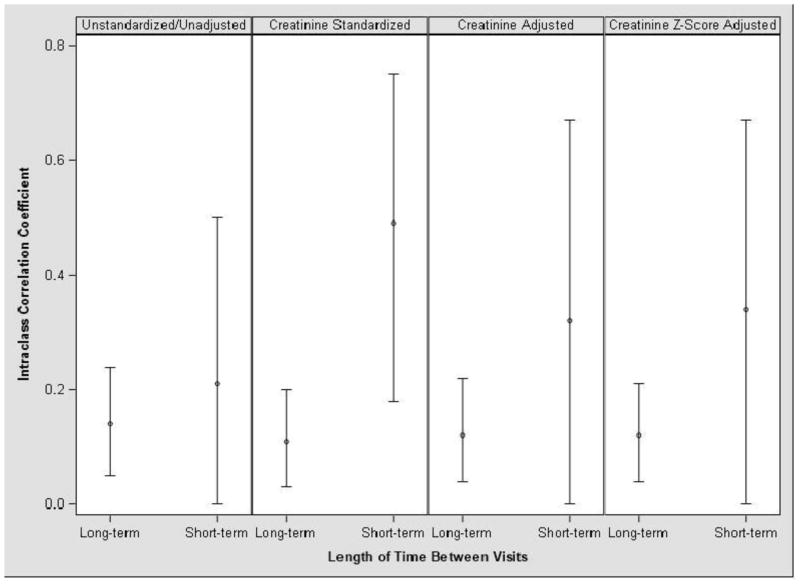
The reproducibility of urinary BPA concentrations within individuals was low, with long-term ICCs ranging from 0.11 (creatinine-standardized) to 0.14 (unstandardized/unadjusted) (Figure 2). The short-term ICCs ranged from 0.21 (unstandardized/ unadjusted) to 0.49 (creatinine standardized), indicating that BPA concentrations in these samples, collected approximately 2 weeks apart, were less variable than concentrations in samples collected over long time periods. In general, the three methods used to account for urine dilution did not substantially change the ICCs, although the short-term ICCs were more sensitive to creatinine standardization compared to the long-term ICCs.
Figure 2.
Age-adjusted intraclass correlation coefficients (ICCs, with 95% confidence intervals) of HOME Study children’s urinary BPA concentrations collected from 1–8 years of age (long- term, N=297, repeated samples=1,307) and ~2 weeks apart from 1–3 years of age (short-term, N=60, repeated samples=134).1
1Long-term ICCs derived from BPA concentrations in urine collected at 1, 2, 3, 4, 5, and 8 years of age. Short-term ICCs derived from BPA concentrations taken 2-weeks apart at 1, 2, and 3 years of age.
Demographic Predictors
Urinary BPA concentrations were associated with child race, household income, and maternal education, but not male or female sex (Table 1). Compared to white children, black children had 20% (95% CI: 6, 34) higher urinary BPA concentrations after adjustment for confounders. Compared to households with annual incomes ≤$20,000, BPA concentrations were 16% lower (95% CI: −27, −3) for children living in households making between $40,000–80,000 and 9% lower (95% CI: −22, 6) for children in the highest income bracket (>$80,000). Children of mothers with higher education also had lower urinary BPA concentrations than those of mothers with less than a grade 12 education.
Table 1.
Geometric mean (GM) and percent difference (% diff.) in HOME Study children’s urinary BPA concentrations at 1–8 years of age, according to sociodemographic and household factors (N = 317 with a total of 1,266 repeated measures).
| Variable | N children (%) | Number of measurements | Unadjusted GM (ng/mL)1 | Unadjusted % diff. (95% CI)1 | Adjusted GM (ng/mL)2 | Adjusted % diff. (95% CI)2 |
|---|---|---|---|---|---|---|
| Child Race | ||||||
|
| ||||||
| Non-Hispanic White | 200(63.1) | 830 | 2.4 | Ref. | 2.7 | Ref. |
| Non-Hispanic Black | 96 (30.3) | 353 | 3.4 | 43 (28, 59) | 3.2 | 20 (6, 34) |
| Other | 21(6.6) | 83 | 2.9 | 22 (−1, 52) | 3.2 | 19 (−7, 50) |
|
| ||||||
| Child Sex | ||||||
|
| ||||||
| Female | 173(54.6) | 689 | 2.8 | Ref. | 3.1 | Ref. |
| Male | 144(45.4) | 577 | 2.5 | −11 (−18, −3) | 2.9 | −9 (−20, 5) |
|
| ||||||
| Household Income | ||||||
|
| ||||||
| $20,000 | 59 (18.6) | 207 | 3.7 | Ref. | 3.4 | Ref. |
| >$20,000–40,000 | 42 (13.2) | 152 | 3.0 | −20 (−31, −7) | 3.0 | −12 (−26, 3) |
| >$40,000–80,000 | 110(34.7) | 403 | 2.4 | −36 (−41, −29) | 2.9 | −16 (−27, −3) |
| >$80,000 | 106(33.4) | 504 | 2.4 | −35 (−41, −30) | 3.1 | −9 (−22, 6) |
|
| ||||||
| Maternal Education | ||||||
|
| ||||||
| Less than grade 12 | 27(8.5) | 99 | 4.0 | Ref. | 3.7 | Ref. |
| High school graduate | 37 (11.7) | 129 | 3.2 | −20 (−32, −5) | 3.1 | −18 (−33, −1) |
| Some college | 85 (26.8) | 355 | 3.0 | −24 (−32, −16) | 3.1 | −18 (−29, −5) |
| College graduate | 168 (53.0) | 683 | 2.2 | −43 (−47, −39) | 2.4 | −36 (−47, −24) |
Adjusted for creatinine z-score and age
Adjusted for creatinine z-score, age, child race, sex, household income, and maternal education. Income and education are included as time-varying factors in the model.
Environmental Predictors
Unstandardized maternal BPA concentrations during pregnancy were positively correlated with unstandardized children’s BPA concentrations at age 1 (Pearson correlation coefficient: 0.1, 95% CI: 0.0–0.2) and reached statistical significance at ages 2 through 4, with year 4 exhibiting the strongest correlation (Pearson correlation coefficient: 0.3, 95% CI: 0.2, 0.4). However, creatinine-standardized maternal and child urinary BPA concentrations were not correlated (data not shown). For example, the correlation with year 4 was almost null (Pearson correlation coefficient: 0.01, 95% CI: −0.1, 0.2).
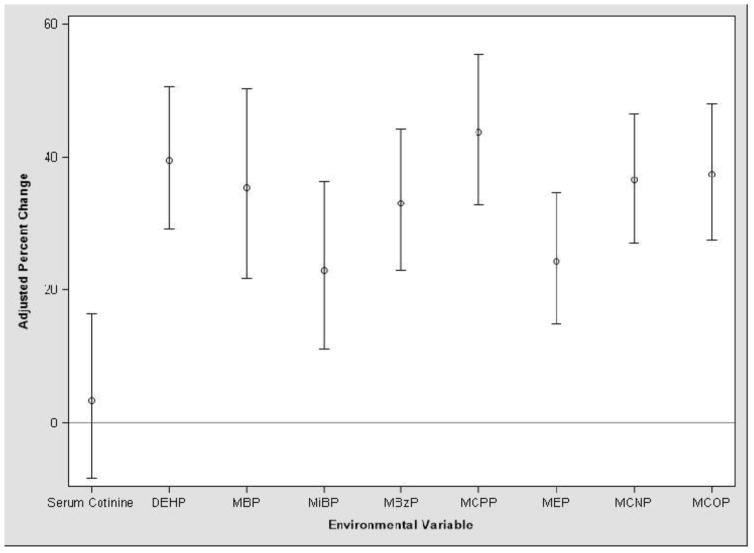
Urinary BPA concentrations were 25% (95% CI: 15, 35) higher per interquartile range (IQR) increase in children’s serum cotinine levels in unadjusted models, but the association was attenuated after adjusting for covariates (% change: 3, 95% CI: −8, 16) (Figure 3). An IQR increase in the concentration of all the urinary phthalate metabolites was associated with greater urinary BPA concentrations after adjustment for covariates. MCNP, MCPP, MCOP, and ΣDEHP had the strongest correlations with BPA, while the associations with MBP, MBzP, MEP, and MiBP were relatively weaker.
Figure 3.
Percent change (with 95% confidence interval) in HOME Study children’s urinary BPA concentrations per interquartile range increase in serum cotinine1 and urinary phthalate metabolite concentrations.2,3
1Mean of available serum cotinine concentrations at 1–3 years were used to estimate secondhand tobacco smoke exposure.
2Urinary phthalate metabolites included MBP, MiBP, MBzP, MCPP, MEP, MCNP, MCOP, and the sum of DEHP metabolites (MEHP + MEHHP + MEOHP + MECPP).
3Adjusted for creatinine z-score, age, race, sex, income, and education
Food Packaging and Dental Predictors
Of the dietary predictors we examined, consuming food stored or heated in plastic, canned foods, and canned beverages tended to be associated with increased BPA (Table 2). Children who ate food stored in plastic at least once per day within the past year had higher urinary BPA than those who ate such food less than 3 times per month (% difference: 29, 95% CI: −9, 83), although the difference was not statistically significant. However, eating food stored in plastic in the past 24 hours was not associated with urinary BPA. We observed a similar finding between consuming food heated in plastic in the past year versus the last 24 hours. Eating more than half the contents of a can of canned food in the past 24 hours was associated with increased BPA (% difference: 79%, 95% CI: 7, 200). For specific types of canned food, canned pasta exhibited the strongest relationship with BPA concentrations (% difference: 96, 95% CI: −6, 308).
Table 2.
Geometric mean (GM) and adjusted percent difference (% diff.) in HOME Study children’s urinary BPA concentrations at 8 years of age according to parent-reported child food packaging use, diet, and receipt handling (N = 210–213).1
| Variable | N (%) | Adjusted GM | Adjusted % diff. (95% CI) | Variable | N (%) | Adjusted GM | Adjusted % diff. (95% CI) |
|---|---|---|---|---|---|---|---|
| Food stored in plastic past year | Type of canned food past 24 h | ||||||
|
| |||||||
| <3 times a month | 16 (7) | 1.6 | Ref. | None | 161 (77) | 1.8 | Ref. |
| 1–6 times a week | 102 (48) | 1.6 | 3 (−28, 45) | Vegetables | 23 (11) | 2.0 | 10 (−32, 77) |
| At least once a day | 95 (45) | 2.0 | 29 (−9, 83) | Fruit | 7 (3) | 1.9 | 9 (−45, 118) |
|
|
|||||||
| Food stored in plastic past 24 h | Pasta | 7(3) | 3.5 | 96 (−6, 308) | |||
|
|
|||||||
| No | 83 (39) | 1.8 | Ref. | Other | 12 (6) | 2.0 | 12 (−38, 99) |
| Yes | 129 (61) | 1.8 | 1 (−28, 43) | ||||
|
| |||||||
| Food heated in plastic past year | Bottled beverages past 24 h | ||||||
|
| |||||||
| <3 times a month | 127 (60) | 1.6 | Ref. | No | 46 (22) | 1.9 | Ref. |
| Once a week or more | 85 (40) | 2.2 | 34 (−6, 90) | Yes | 167 (78) | 1.8 | −7 (−33, 29) |
|
| |||||||
| Food heated in plastic past 24 h | Canned beverages past year | ||||||
|
| |||||||
| No | 193 (91) | 1.8 | Ref. | <3 times a month | 148 (70) | 1.7 | Ref. |
| Yes | 19 (9) | 1.5 | −21 (−51, 29) | At least once a week | 64 (30) | 1.9 | 11 (−23, 59) |
|
| |||||||
| Plastic serving ware past year | Canned beverages past 24 h | ||||||
|
| |||||||
| <3 times a month | 65 (31) | 1.7 | Ref. | No | 181 (85) | 1.7 | Ref. |
| 1–6 times a week | 65 (30) | 1.7 | 2 (−30, 47) | Yes | 31 (15) | 2.3 | 38 (−8, 108) |
|
|
|||||||
| At least once a day | 82 (39) | 2.0 | 19 (−17, 71) | Beverages carton or pouch past year | |||
|
|
|
||||||
| Plastic serving ware past 24 h | Less than 3 times a month | 40 (19) | 1.7 | Ref. | |||
|
|
|||||||
| No | 82 (39) | 1.7 | Ref. | 1–6 times a week | 94 (44) | 1.8 | 6 (−25, 51) |
| Yes | 131 (61) | 1.9 | 11 (−21, 57) | At least once a day | 79 (37) | 1.8 | 3 (−27, 46) |
|
| |||||||
| Canned food past 24 h | Beverages carton or pouch past 24 h | ||||||
|
| |||||||
| No | 161 (76) | 1.7 | Ref. | No | 100 (48) | 1.7 | Ref. |
| Yes | 51 (24) | 2.1 | 19 (−19, 76) | Yes | 110 (52) | 1.9 | 13 (−21, 60) |
|
| |||||||
| Cans of canned food past 24 h | Receipt handling past 24 h | ||||||
|
| |||||||
| 0 | 161 (77) | 1.7 | Ref. | No | 183 (86) | 1.6 | Ref. |
| >0 to ≤ 0.25 | 17 (8) | 1.8 | 5 (−37, 75) | Yes | 30 (14) | 2.3 | 45 (−1, 113) |
| >0.25 to ≤0.50 | 15 (7) | 1.8 | 5 (−38, 75) | ||||
| >0.50 | 17 (8) | 3.0 | 79 (7, 200) | ||||
Adjusted for creatinine z-score, age, child race, sex, household income, and maternal education.
Before covariate adjustment, children who handled cash register receipts in the past 24 hours had 39% (95% CI: 3, 88) higher urinary BPA concentrations compared to those who did not, and this association was similar, but less precise, after adjustment for child’s sex, race, maternal education, and income (% difference: 45, 95% CI: −1, 113) (see Table 2).
Considering the combined effect of multiple exposures in the past 24 hours (consumption of canned food, canned beverages, beverages in carton or pouch, and receipt handling), participants with 1 exposure (N=99, % difference: 32, 95% CI: −8, 90) and 2 exposures (N=42, % difference: 37, 95% CI: −9, 105) had higher BPA concentrations than those with none (N=56); these differences did not reach conventional levels of statistical significance. Participants with 3–4 of these exposures (N=12) had 111% (95% CI: 25, 258) higher BPA concentrations compared to those with none.
There were no associations between urinary BPA concentrations and ever having a dental filling, crown, or sealant (Table 3). Urinary BPA concentrations increased 22% (95% CI: −2, 53) for each additional resin crown; however, only 8 children had crowns. We did not observe any associations between urinary BPA and the current number of plastic fillings or sealants, or time since most recent filling or sealant.
Table 3.
Adjusted percent difference or change in HOME Study children’s urinary BPA concentrations at 8 years of age according to parent-reported dental history of child (N = 213).1
| Variable | N (%) or Median (10th, 90th percentile) | Adjusted GM | % diff. or change per unit increase (95% CI) |
|---|---|---|---|
| Ever had a dental filling | |||
| No | 131 (62) | 1.8 | Ref. |
| Yes | 82 (38) | 1.8 | 3 (−26, 45) |
|
| |||
| Current number of composite fillings | 0 (0, 1) | 3 (−10, 18) | |
|
| |||
| Time since most recent filling (years) | 2 (1, 4) | 4 (−8, 18) | |
|
| |||
| Ever had a resin crown | |||
| No | 205 (96) | 1.8 | Ref. |
| Yes | 8 (4) | 2.1 | 20 (−38, 134) |
|
| |||
| Current number of resin crowns | 0 (0, 0) | 22 (−2, 53) | |
|
| |||
| Ever had a sealant | |||
| No | 119 (56) | 1.7 | Ref. |
| Yes | 91 (43) | 2.0 | 17 (−18, 67) |
|
| |||
| Number of teeth treated with sealant | 0 (0, 8) | 0 (−2, 3) | |
|
| |||
| Time since most recent sealant (years) | 1 (0, 3) | 2 (−11, 17) | |
Adjusted for creatinine z-score, age, child race, sex, household income, and maternal education.
Temporal Predictors
Although geometric mean urinary BPA concentrations were higher among children born in 2005–2006, their concentrations declined more rapidly compared to children born in 2003–2004 (age x year of birth interaction p=0.016) (Figure 4). When separate models were run for each visit with year of collection as the predictor of interest, urinary BPA tended to increase with collection year for the 1-year visit (% change: 14, 95% CI: − 1, 32) but decrease for the 8-year visit (% change: −15, 95% CI: −26, 1). There were no clear associations between BPA concentrations and collection year for the other visits (data not shown).
Figure 4.
Unadjusted geometric mean and adjusted smoothed regression of urinary BPA concentrations according to child age and year of birth (2003–2004 or 2005–2006).1
1Unadjusted models include age, year of birth, and the interaction between age and year of birth. Adjusted models additionally adjust for child’s sex and race, mother’s education, household income, and creatinine z-score.
Daily Intake
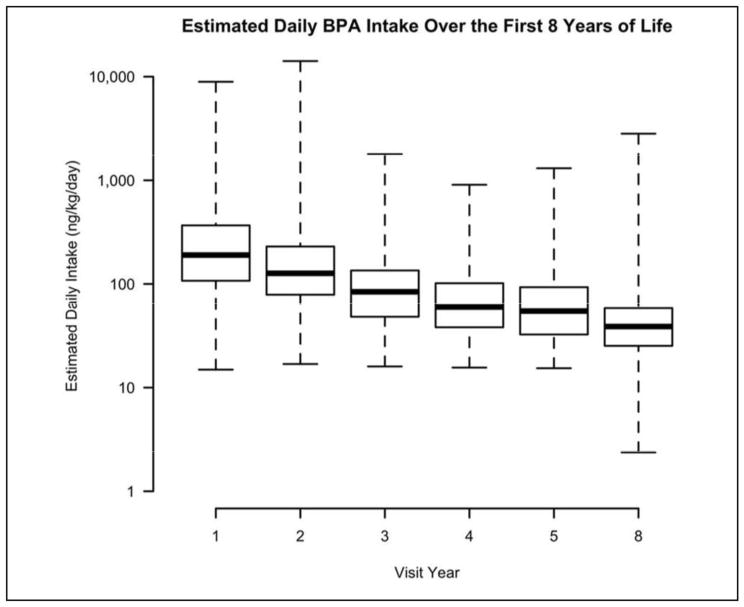
Daily intakes ranged from 2 ng/kg bw/day to a maximum of 14,135 ng/kg bw/day (Table S4, supplement). Estimated daily intakes decreased as child age increased, with median intakes of 190 and 39 ng/kg bw/day for 1- and 8-year-olds, respectively (Figure 5). Most of the intakes were below a 10-fold margin of the RfD (i.e., 5 μg/kg bw/day), except for the maximum values at 1 and 2 years of age (see Table S4). All but these two values were below the European Food Safety Authority’s recently revised ADI for BPA of 4 μg/kg bw/day.43
Figure 5.
Distribution of estimated daily BPA intakes (ng/kg body weight/day) in HOME Study children at 1–8 years of age.1
1The current EPA RfD for BPA is 50 μg/kg body weight/day (50,000 ng/kg body weight/day).
The EFSA ADI is 4 μg/kg body weight/day (4,000 ng/kg body weight/day).
Discussion
In the present study, we investigated longitudinal patterns and predictors of urinary BPA concentrations and calculated daily BPA intakes in a cohort of children from Cincinnati, Ohio. We found that urinary BPA concentrations decreased as children got older and had a low degree of reproducibility. Factors associated with urinary BPA concentrations included child race, maternal education, household income, urinary phthalate concentrations, food storage and heating practices, consumption of canned beverages and certain types of canned foods, and the handling of cash register receipts. The median and maximum daily intakes for our cohort were all below the current EPA RfD of 50 μg/kg bw/day.12
Other studies of childhood or adolescent exposure to BPA have also observed decreasing urinary BPA concentrations with increasing age,16, 18–20 while a positive association with age was reported for school-aged children in China.27–28 Our BPA concentrations generally agree with those reported for similar age groups in previous studies (see Table S1), although they are slightly higher than those reported for children in Sweden,20 Mexico,21 Egypt,25 and China.27–28 Differences in study design and trends in BPA usage and personal behaviors influencing exposure may complicate the comparison of childhood BPA concentrations across studies.
Serial urinary BPA measurements taken approximately 2 weeks apart had a higher degree of reproducibility than those taken annually. Prior research suggests that repeated urinary BPA measurements exhibit low to moderate reproducibility in children26 and adults.32–33, 44 This high degree of within-person variation may lead to misclassification bias in studies relying on a single BPA measurement.33 Resources permitting, future studies should collect multiple samples on multiple days to characterize BPA exposure more accurately and reduce misclassification bias.
Our results suggest that sociodemographic factors such as race, education, and household income may influence childhood exposure to BPA. Children who were non-Hispanic black, living in lower income households, or whose mothers were less educated had higher urinary BPA concentrations than other children. Prior studies have reported that non-Hispanic black children have higher urinary BPA concentrations compared to other racial groups,3, 19 but the literature is inconsistent regarding associations with income and education, with some studies reporting inverse,32 positive,45 or no associations.19 Discrepancies across studies could be due to differences in diet and other behaviors related to BPA exposure specific to the populations under investigation (e.g., canned food consumption).
Serum cotinine concentrations were not associated with urinary BPA in our study after adjustment for sociodemographic covariates. However, urinary phthalate metabolite and BPA concentrations were positively associated with each other. Associations with BPA were strongest for MCPP, MCNP, MCOP, and DEHP metabolites, likely due to shared sources used in food packaging and processing. We previously found positive correlations between maternal urinary concentrations of BPA and certain phthalates in pregnant women in the HOME study.32 We found positive but relatively weaker associations for the metabolites of phthalate diesters found in some personal care products (MBP, MiBP, and MEP).
Urinary BPA concentrations in our cohort tended to be higher for children who consumed food stored or heated in plastic more frequently in the past year than for other children. However, consuming food stored or heated in plastic in the past 24 hours was not positively associated with BPA concentrations. Nahar et al. reported that eating food stored in plastic increased urinary BPA concentrations in a group of 10 to 13-year old Egyptian girls.25 We found higher urinary BPA concentrations in children who drank canned beverages and consumed more than half of a can of canned food in the past 24 hours. BPA is used in the manufacture of some coatings found in the lining of food and beverage cans.1 Eating canned pasta had the strongest correlation with BPA concentrations compared to other types of canned foods. We previously reported that a mother enrolled in the HOME Study, who had urinary BPA concentrations two orders of magnitude higher than those documented in previous biomonitoring studies, consumed canned ravioli frequently during her pregnancy.39 Although BPA can be used to make some plastic beverage containers,1 we did not find an association between drinking bottled water and urinary BPA concentrations, which is consistent with another study of 8 to 13-year-old Mexican children.21 However, Carwile et al. observed a subtle increase in urinary BPA concentrations among college students after one week of drinking most cold beverages from a polycarbonate bottle.46 We also found that exposure to 3–4 BPA sources in the past 24 hours increased urinary BPA concentrations for our participants, although the sample size in this group was small (N=12).
Dental-related factors were not associated with urinary BPA concentrations in our study, except for a suggestive association between the current number of resin crowns and BPA level. Dental materials made from BPA derivatives, such as bisphenol-A-dimethacrylate and bisphenol-A-diglycidyl-dimethacrylate, have been used in dentistry as alternatives to mercury amalgams.47 BPA impurities left over from resin synthesis or resulting from resin degradation can leach from composite materials.48 However, recent work shows that newly placed composite restorations were not associated with elevated urinary BPA concentrations in children’s urine samples 2 weeks to 6 months after restoration placement, suggesting these composites might not be a source of chronic BPA exposure.49
Handling cash register receipts in the last 24 hours was associated with higher BPA concentrations in our study. Handling printed receipts may result in BPA exposure from dermal absorption in the skin as well as ingestion via hand-to-mouth transfer.7 Urinary BPA concentrations were significantly higher in occupational groups (e.g. restaurant and retail workers) who frequently handle receipts compared to non-occupational groups.6, 9–10 To our knowledge, this is the first study to consider receipt handling as a possible source of BPA exposure in children.
Our analyses suggest that exposure to BPA decreased over the period of the study. Urinary BPA concentrations among children born in 2005–2006 decreased significantly faster than those born in 2003–2004. A similar trend was observed for urinary concentrations of some phthalate metabolites in HOME study children,41 perhaps related to the implementation of regulations such as the 2008 Consumer Product Safety Improvement Act, which may have reduced children’s phthalate exposure.50 Although similar legislation does not exist for BPA, increased awareness of BPA’s potential adverse health effects and/or changes in manufacturing practices, such as the use of BPA alternatives (e.g. bisphenol S), could have reduced BPA exposure during the more recent years of our study.9, 51 However, Hoepner et al. did not observe temporal trends in geometric mean BPA concentrations over collection years from 2001 to 2010 in children from an urban minority birth cohort.19
Estimating daily intake from human biomonitoring data takes into account contributions from all sources and allows us to interpret urinary concentrations in the context of thresholds for toxicity established by risk assessors. All but 2 children in our study had estimated daily intakes that were below 1 order of magnitude of the RfD and no children had intakes exceeding the RfD. In several recent studies, the estimated daily BPA intakes for children from the United States, Germany, and China were typically lower than the RfD values established by US or European Union regulatory agencies.11, 23, 27–28 Our results were comparable to von Goetz et al., who estimated total BPA intake rates of 72 ng/kg bw/day for 1 to 5-year-old children and 36 ng/kg bw/day for 6 to 12-year-olds, using an exposure model with consumer- and source-related inputs.11 In another study of preschool-aged children in Ohio, daily BPA intake was found to be mostly due to dietary ingestion (median: 109 ng/kg bw/day), while daily intakes via other exposure routes were <1 ng/kg bw/day.23
This study has several strengths. First, we obtained serial urinary BPA concentrations during several sensitive periods of development from the time our participants were infants to elementary school-aged. These serial measurements allowed us to examine the patterns and variability in BPA concentrations from early to middle childhood. We also developed and administered an extensive exposure questionnaire to investigate a variety of dietary and dental factors as possible predictors of childhood urinary BPA concentrations. We were able to collect detailed information on the frequency of certain behaviors, such as consuming food stored or heated in plastic, as well as numbers and types of packaged foods and beverages consumed. Previous studies of the predictors of childhood urinary BPA concentrations have not considered dental history or receipt handling as possible exposure sources.
Our study also has several limitations. As we and others have demonstrated, single spot urine measurements have the potential to result in misclassification of BPA exposure.26,32–33 Further, we used creatinine to correct for urine dilution, which could over- or underestimate exposure at different ages since physiology changes considerably throughout childhood.42 To help address this, we adjusted for age-specific creatinine z-scores in our regression models as an alternative to using the creatinine-standardized BPA concentrations.41 Our estimates of BPA daily intake assume that 100% of BPA intake would be excreted in urine. Previous studies in adults have found that almost all BPA is excreted in urine after 24 hours of oral administration.30, 52–53 Although the pharmacokinetics of BPA in children are not fully known and inter-species comparisons must be made carefully, studies in rats have found that neonates are less efficient at metabolizing BPA compared to female adult rats.54 If BPA metabolism in children is also less efficient than in adults, then our results may underestimate actual BPA daily intakes.
In addition, the relatively small number of children who engaged in some behaviors may have reduced our statistical power to detect associations between urinary BPA concentrations and some sources of exposure. Urinary BPA concentrations tended to be higher for several of these predictors, such as eating canned pasta, but did not reach conventional levels of statistical significance. An inherent limitation in the study design is the nature of the exposure recall interview, which relied on mothers to report this information on behalf of their child. Thus, their answers may not reflect the child’s actual exposure. This is particularly true for 8-year-old children, who start spending more time outside of the home and may be exposed to BPA- containing products without the parents’ knowledge.
In conclusion, we found that urinary BPA concentrations decrease over childhood, possibly due to age-related changes in physiology and exposure. As BPA concentrations have high within-person variability during childhood, future studies should obtain serial urinary BPA measurements to more accurately characterize exposure and reduce misclassification bias. Future work should consider race, education, income, and phthalate exposure as possible confounding factors because they may be associated with both childhood urinary BPA concentrations and health outcomes. Consistent with previous work in children and adults, we found that consuming food stored or heated in plastic as well as canned foods and beverages may be important sources of BPA exposure among school-aged children. We also found that receipt handling is an additional significant source of BPA exposure for children. Additional research is needed to quantify the relative contribution of these sources to childhood BPA exposure.
Supplementary Material
Acknowledgments
Financial Support: This work was supported by grants R00 ES020346, R01 ES024381, P42 ES007381, P30 ES023515, R01 ES009718, R01 ES020349, and R01 ES022955 from the National Institute of Environmental Health Sciences.
Footnotes
Disclaimer: Dr. Lanphear has served as an expert witness and a consultant to the California Attorney General’s Office for the plaintiffs in a public nuisance case related to childhood lead poisoning, but he has not personally received any compensation for these services. Dr. Lanphear has also served as a paid consultant on a US Environmental Protection Agency research study related to childhood lead poisoning. Dr. Braun was financially compensated for conducting a re-analysis of a study of child lead exposure for the plaintiffs in a public nuisance case related to childhood lead poisoning. None of these activities are directly related to the present study.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Conflicts of Interest: The authors have no actual or potential competing financial interests.
Includes additional information on equations for calculating creatinine excretion rates, distributions of urinary BPA and creatinine concentrations in the HOME study, and estimated BPA daily intakes
References
- 1.Braun JM, Hauser R. Bisphenol A and children's health. Current Opinion in Pediatrics. 2011;23(2):233–239. doi: 10.1097/MOP.0b013e3283445675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chapin RE, Adams J, Boekelheide K, Gray LE, Hayward SW, Lees PS, McIntyre BS, Portier KM, Schnorr TM, Selevan SG. NTP-CERHR expert panel report on the reproductive and developmental toxicity of bisphenol A. Birth Defects Research Part B: Developmental and Reproductive Toxicology. 2008;83(3):157–395. doi: 10.1002/bdrb.20147. [DOI] [PubMed] [Google Scholar]
- 3.Calafat AM, Ye X, Wong L-Y, Reidy JA, Needham LL. Exposure of the US population to Bisphenol A and 4-tertiary-Octylphenol: 2003–2004. Environmental Health Perspectives. 2008;116(1):39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casas L, Fernández MF, Llop S, Guxens M, Ballester F, Olea N, Irurzun MB, Rodríguez LSM, Riaño I, Tardón A. Urinary concentrations of phthalates and phenols in a population of Spanish pregnant women and children. Environment International. 2011;37(5):858–866. doi: 10.1016/j.envint.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 5.Vandenberg LN, Chahoud I, Heindel JJ, Padmanabhan V, Paumgartten FJ, Schoenfelder G. Urinary, circulating, and tissue biomonitoring studies indicate widespread exposure to bisphenol A. Environmental Health Perspectives. 2010;118(8):1055–1070. doi: 10.1289/ehp.0901716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehrlich S, Calafat AM, Humblet O, Smith T, Hauser R. Handling of thermal receipts as a source of exposure to bisphenol A. JAMA. 2014;311(8):859–860. doi: 10.1001/jama.2013.283735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zalko D, Jacques C, Duplan H, Bruel S, Perdu E. Viable skin efficiently absorbs and metabolizes bisphenol A. Chemosphere. 2011;82:424–430. doi: 10.1016/j.chemosphere.2010.09.058. [DOI] [PubMed] [Google Scholar]
- 8.Li D, Zhou Z, Qing D, He Y, Wu T, Miao M, Wang J, Weng X, Ferber JR, Herrinton LJ, Zhu Q, Gao E, Checkoway H, Yuan W. Occupational exposure to bisphenol-A (BPA) and the risk of self-reported male sexual dysfunction. Human Reproduction. 2010;25(2):519–27. doi: 10.1093/humrep/dep381. [DOI] [PubMed] [Google Scholar]
- 9.Thayer KA, Taylor KW, Garantziotis S, Schurman S, Kissling GE, Hunt D, Herbert B, Church R, Jankowich R, Churchwell MI. Bisphenol A, Bisphenol S, and 4- Hydroxyphenyl 4-Isoprooxyphenylsulfone (BPSIP) in Urine and Blood of Cashiers. Environmental Health Perspectives. 2016;124(4):437–444. doi: 10.1289/ehp.1409427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hehn RS. NHANES Data Support Link between Handling of Thermal Paper Receipts and Increased Urinary Bisphenol A Excretion. Environmental Science & Technology. 2016;50:397–404. doi: 10.1021/acs.est.5b04059. [DOI] [PubMed] [Google Scholar]
- 11.Von Goetz N, Wormuth M, Scheringer M, Hungerbühler K. Bisphenol A: how the most relevant exposure sources contribute to total consumer exposure. Risk Analysis. 2010;30(3):473–487. doi: 10.1111/j.1539-6924.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- 12.United States Environmental Protection Agency Integrated Risk Information System (IRIS) [accessed November 17, 2015];Bisphenol A. http://cfpub.epa.gov/ncea/iris/index.cfm?fuseaction=iris.showQuickview&substance_nmbr=0356.
- 13.Barker DJ. The origins of the developmental origins theory. Journal of Internal Medicine. 2007;261(5):412–417. doi: 10.1111/j.1365-2796.2007.01809.x. [DOI] [PubMed] [Google Scholar]
- 14.Landrigan PJ, Miodovnik A. Children's health and the environment: an overview. Mount Sinai Journal of Medicine: A Journal of Translational and Personalized Medicine. 2011;78(1):1–10. doi: 10.1002/msj.20236. [DOI] [PubMed] [Google Scholar]
- 15.Rochester JR. Bisphenol A and human health: a review of the literature. Reproductive Toxicology. 2013;42:132–155. doi: 10.1016/j.reprotox.2013.08.008. [DOI] [PubMed] [Google Scholar]
- 16.Braun JM, Kalkbrenner AE, Calafat AM, Yolton K, Ye X, Dietrich KN, Lanphear BP. Impact of early-life bisphenol A exposure on behavior and executive function in children. Pediatrics. 2011;128(5):873–882. doi: 10.1542/peds.2011-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Casas M, Valvi D, Luque N, Ballesteros-Gomez A, Carsin AE, Fernandez MF, Koch HM, Mendez MA, Sunyer J, Rubio S. Dietary and sociodemographic determinants of bisphenol A urine concentrations in pregnant women and children. Environment International. 2013;56:10–18. doi: 10.1016/j.envint.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 18.Frederiksen H, Aksglaede L, Sorensen K, Nielsen O, Main KM, Skakkebaek NE, Juul A, Andersson A-M. Bisphenol A and other phenols in urine from Danish children and adolescents analyzed by isotope diluted TurboFlow-LC MS/MS. International Journal of Hygiene and Environmental Health. 2013;216(6):710–720. doi: 10.1016/j.ijheh.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Hoepner LA, Whyatt RM, Just AC, Calafat AM, Perera FP, Rundle AG. Urinary concentrations of bisphenol A in an urban minority birth cohort in New York City, prenatal through age 7 years. Environmental Research. 2013;122:38–44. doi: 10.1016/j.envres.2012.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Larsson K, Björklund KL, Palm B, Wennberg M, Kaj L, Lindh CH, Jönsson BA, Berglund M. Exposure determinants of phthalates, parabens, bisphenol A and triclosan in Swedish mothers and their children. Environment International. 2014;73:323–333. doi: 10.1016/j.envint.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, Téllez-Rojo MM. Predictors of urinary bisphenol A and phthalate metabolite concentrations in Mexican children. Chemosphere. 2013;93(10):2390–2398. doi: 10.1016/j.chemosphere.2013.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendonca K, Hauser R, Calafat A, Arbuckle T, Duty S. Bisphenol A concentrations in maternal breast milk and infant urine. International Archives of Occupational and Environmental health. 2014;87(1):13–20. doi: 10.1007/s00420-012-0834-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morgan MK, Jones PA, Calafat AM, Ye X, Croghan CW, Chuang JC, Wilson NK, Clifton MS, Figueroa Z, Sheldon LS. Assessing the quantitative relationships between preschool children’s exposures to bisphenol A by route and urinary biomonitoring. Environmental Science & Technology. 2011;45(12):5309–5316. doi: 10.1021/es200537u. [DOI] [PubMed] [Google Scholar]
- 24.Nachman RM, Fox SD, Golden WC, Sibinga E, Groopman JD, Lees PSJ. Serial free bisphenol A and bisphenol A glucuronide concentrations in neonates. Journal of Pediatrics. 2015;167(1):64–69. doi: 10.1016/j.jpeds.2015.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nahar MS, Soliman AS, Colacino JA, Calafat AM, Battige K, Hablas A, Seifeldin IA, Dolinoy DC, Rozek LS. Urinary bisphenol A concentrations in girls from rural and urban Egypt: a pilot study. Environ Health. 2012;11:20. doi: 10.1186/1476-069X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teitelbaum S, Britton J, Calafat A, Ye X, Silva M, Reidy J, Galvez M, Brenner B, Wolff M. Temporal variability in urinary concentrations of phthalate metabolites, phytoestrogens and phenols among minority children in the United States. Environmental Research. 2008;106(2):257–269. doi: 10.1016/j.envres.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang B, Wang H, Zhou W, He Y, Zhou Y, Chen Y, Jiang Q. Exposure to bisphenol A among school children in eastern China: A multicenter cross-sectional study. Journal of Exposure Science and Environmental Epidemiology. 2014;24:657–664. doi: 10.1038/jes.2014.36. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Zhou Y, Tang C, Wu J, Chen Y, Jiang Q. Association between bisphenol A exposure and body mass index in Chinese school children: a cross-sectional study. Environ Health. 2012;11(1):79. doi: 10.1186/1476-069X-11-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolff MS, Britton JA, Boguski L, Hochman S, Maloney N, Serra N, Liu Z, Berkowitz G, Larson S, Forman J. Environmental exposures and puberty in inner-city girls. Environmental Research. 2008;107(3):393–400. doi: 10.1016/j.envres.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wolff MS, Teitelbaum SL, Pinney SM, Windham G, Liao L, Biro F, Kushi LH, Erdmann C, Hiatt RA, Rybak ME. Investigation of relationships between urinary biomarkers of phytoestrogens, phthalates, and phenols and pubertal stages in girls. Environmental Health Perspectives. 2010;118(7):1039–1046. doi: 10.1289/ehp.0901690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Völkel W, Colnot T, Csanády GA, Filser JG, Dekant W. Metabolism and kinetics of bisphenol A in humans at low doses following oral administration. Chemical Research in Toxicology. 2002;15(10):1281–1287. doi: 10.1021/tx025548t. [DOI] [PubMed] [Google Scholar]
- 32.Braun JM, Kalkbrenner AE, Calafat AM, Bernert JT, Ye X, Silva MJ, Barr DB, Sathyanarayana S, Lanphear BP. Variability and predictors of urinary bisphenol A concentrations during pregnancy. Environ Health Perspect. 2011;119(1):131–7. doi: 10.1289/ehp.1002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cox KJ, Porucznik CA, Anderson DJ, Szczotka KM, Bailey NM, Wilkins DG, Stanford JB. Exposure classification and temporal variability in urinary bisphenol-A concentrations among couples in Utah The HOPE study. Environ Health Perspect. 2015 doi: 10.1289/ehp.1509752. (advanced publication) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, Xu Y, Yolton K, Lanphear BP. Cohort Profile: The Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol. 2016:1–10. doi: 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Braun JM, Lanphear BP, Calafat AM, Deria S, Khoury J, Howe CJ, Venners SA. Early-Life Bisphenol A Exposure and Child Body Mass Index: A Prospective Cohort Study. Environmental Health Perspectives. 2014;122(11):1239–1245. doi: 10.1289/ehp.1408258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ye X, Zhou X, Hennings R, Kramer J, Calafat AM. Potential external contamination with bisphenol A and other ubiquitous organic environmental chemicals during biomonitoring: an elusive laboratory challenge. Environmental Health Perspectives. 2013;121(3):283–286. doi: 10.1289/ehp.1206093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ye X, Kuklenyik Z, Needham LL, Calafat AM. Automated on-line column-switching HPLC-MS/MS method with peak focusing for the determination of nine environmental phenols in urine. Analytical Chemistry. 2005;77(16):5407–5413. doi: 10.1021/ac050390d. [DOI] [PubMed] [Google Scholar]
- 38.Hornung RW, Reed LD. Estimation of average concentration in the presence of nondetectable values. Applied Occupational and Environmental Hygiene. 1990;5(1):46–51. [Google Scholar]
- 39.Sathyanarayana S, Braun JM, Yolton K, Liddy S, Lanphear BP. Case report: high prenatal bisphenol a exposure and infant neonatal neurobehavior. Environ Health Perspect. 2011;119(8):1170–5. doi: 10.1289/ehp.1003064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Larsen K. Creatinine assay in the presence of protein with LKB 8600 Reaction Rate Analyser. Clin Chim Acta. 1972;38(2):475–476. doi: 10.1016/0009-8981(72)90146-5. [DOI] [PubMed] [Google Scholar]
- 41.Watkins DJ, Eliot M, Sathyanarayana S, Calafat AM, Yolton K, Lanphear BP, Braun JM. Variability and Predictors of Urinary Concentrations of Phthalate Metabolites during Early Childhood. Environmental Science & Technology. 2014;48(15):8881–8890. doi: 10.1021/es501744v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mage DT, Allen RH, Kodali A. Creatinine corrections for estimating children's and adult's pesticide intake doses in equilibrium with urinary pesticide and creatinine concentrations. Journal of Exposure Science and Environmental Epidemiology. 2008;18(4):360–368. doi: 10.1038/sj.jes.7500614. [DOI] [PubMed] [Google Scholar]
- 43.European Food Safety Authority (EFSA) [accessed April 24, 2016];Bisphenol A. http://www.efsa.europa.eu/en/topics/topic/bisphenol.
- 44.Nepomnaschy PA, Baird DD, Weinberg CR, Hoppin JA, Longnecker MP, Wilcox AJ. Within-person variability in urinary bisphenol A concentrations: measurements from specimens after long-term frozen storage. Environmental Research. 2009;109(6):734–737. doi: 10.1016/j.envres.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ye X, Pierik FH, Hauser R, Duty S, Angerer J, Park MM, Burdorf A, Hofman A, Jaddoe VW, Mackenbach JP. Urinary metabolite concentrations of organophosphorous pesticides, bisphenol A, and phthalates among pregnant women in Rotterdam, the Netherlands: the Generation R study. Environmental Research. 2008;108(2):260–267. doi: 10.1016/j.envres.2008.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environmental Health Perspectives. 2009;117(9):1368–1372. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fleisch AF, Sheffield PE, Chinn C, Edelstein BL, Landrigan PJ. Bisphenol A and related compounds in dental materials. Pediatrics. 2010;126(4):760–768. doi: 10.1542/peds.2009-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Van Landuyt K, Nawrot T, Geebelen B, De Munck J, Snauwaert J, Yoshihara K, Scheers H, Godderis L, Hoet P, Van Meerbeek B. How much do resin-based dental materials release? A meta-analytical approach. Dental Materials. 2011;27(8):723–747. doi: 10.1016/j.dental.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 49.Maserejian NN, Trachtenberg FL, Wheaton OB, Calafat AM, Ranganathan G, Kim HY, Hauser R. Changes in urinary bisphenol A concentrations associated with placement of dental composite restorations in children and adolescents. J Am Dent Assoc. 2016 doi: 10.1016/j.adaj.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zota AR, Calafat AM, Woodruff TJ. Temporal trends in phthalate exposure: findings from the National Health and Nutrition Examination Survey, 2001–2010. Environmental Health Perspectives. 2014;122(3):235–241. doi: 10.1289/ehp.1306681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ye X, Wong LY, Kramer J, Zhou X, Jia T, Calafat AM. Urinary concentrations of bisphenol A and three other bisphenols in convenience samples of U.S. adults during 2000–2014. Environmental Science and Technology. 2015;49(19):11834–11839. doi: 10.1021/acs.est.5b02135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dekant W, Völkel W. Human exposure to bisphenol A by biomonitoring: Methods, results and assessment of environmental exposures. Toxicology and Applied Pharmacology. 2008;228:114–134. doi: 10.1016/j.taap.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Thayer KA, Doerge DR, Hunt D, Schuman SH, Twaddle NC, Churchwell MI, Garantziotis S, Kissling GE, Easterling MR, Bucher JR, Birnbaum LS. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environmental International. 2015;83:107–115. doi: 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doerge DR, Twaddle NC, Vanlandingham M, Brown RP, Fisher JW. Distribution of bisphenol A into tissues of adult, neonatal, and fetal Sprague-Dawley rats. Toxicology and Applied Pharmacology. 2011;255:261–270. doi: 10.1016/j.taap.2011.07.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.