Significance
Bottom trawling is the most widespread source of physical disturbance to the world’s seabed. Predictions of trawling impacts are needed to underpin risk assessment, and they are relevant for the fishing industry, conservation, management, and certification bodies. We estimate depletion and recovery of seabed biota after trawling by fitting models to data from a global data compilation. Trawl gears removed 6–41% of faunal biomass per pass, and recovery times posttrawling were 1.9–6.4 y depending on fisheries and environmental context. These results allow the estimation of trawling impacts on unprecedented spatial scales and for data poor fisheries and enable an objective analysis of tradeoffs between harvesting fish and the wider ecosystem effects of such activities.
Keywords: logistic recovery model, systematic review, metaanalysis, impacts, trawling
Abstract
Bottom trawling is the most widespread human activity affecting seabed habitats. Here, we collate all available data for experimental and comparative studies of trawling impacts on whole communities of seabed macroinvertebrates on sedimentary habitats and develop widely applicable methods to estimate depletion and recovery rates of biota after trawling. Depletion of biota and trawl penetration into the seabed are highly correlated. Otter trawls caused the least depletion, removing 6% of biota per pass and penetrating the seabed on average down to 2.4 cm, whereas hydraulic dredges caused the most depletion, removing 41% of biota and penetrating the seabed on average 16.1 cm. Median recovery times posttrawling (from 50 to 95% of unimpacted biomass) ranged between 1.9 and 6.4 y. By accounting for the effects of penetration depth, environmental variation, and uncertainty, the models explained much of the variability of depletion and recovery estimates from single studies. Coupled with large-scale, high-resolution maps of trawling frequency and habitat, our estimates of depletion and recovery rates enable the assessment of trawling impacts on unprecedented spatial scales.
Fisheries using bottom trawls are the most widespread source of anthropogenic physical disturbance to global seabed habitats (1, 2). Almost one-quarter of global seafood landings from 2011 to 2013 were caught by bottom trawls (3). Development of fisheries, conservation, and ecosystem-based management strategies requires assessments of the distribution and impact of bottom trawling and the relative status of benthic biota and habitats. There are many drivers for such assessments, including (i) policy commitments to an ecosystem approach to fisheries, (ii) requirements to take account of trawling impacts in fisheries and environmental management plans, (iii) demands from certification bodies to assess fisheries’ environmental impacts, and (iv) the need to evaluate the effects of alternate management measures to meet conservation and management objectives (4–6). These assessments are used to assess the sustainability of bottom trawl fisheries, formulate priorities for habitat protection, and ultimately, achieve a balance between fisheries production and environmental protection. The distribution of bottom trawling is increasingly well-characterized by vessel tracking and other monitoring systems (7), but impacts depend on the magnitude of trawling-induced mortality and recovery rates of biota, for which the current evidence base is incomplete, dispersed, and often contested (4, 8).
Bottom trawls [here defined as any towed bottom-fishing gear, including otter trawls (OTs), beam trawls (BTs), towed (scallop) dredges (TDs), and hydraulic dredges (HDs)] are used to catch fish, crustaceans, and bivalves living in, on, or above the seabed (9). Bottom trawling resuspends sediments (10, 11); reduces topographic complexity and biogenic structures (12–14); reduces faunal biomass, numbers, and diversity (15, 16); selects for communities dominated by fauna with faster life histories (17); and produces energy subsidies in the form of carrion (18). These effects lead to changes in community production, trophic structure, and function (19, 20). Given the patchy and dynamic distribution of bottom fishing (21), fished seabeds comprise a mosaic of undisturbed, recently impacted, and recovering benthic communities and habitats (22). The state of each patch within this mosaic depends on the history and frequency of past trawling impacts and the recovery rates of the biota present (23).
Recovery rates after trawling depend on recruitment of new individuals, growth of surviving biota, and active immigration from adjacent habitat. Most existing estimates of recovery rates come from experimental studies, with changes in abundance recorded before and after experimental trawling (15, 16). Although these experiments provide reliable estimates of immediate mortality, their small scale is likely to underestimate recovery time, in particular for mobile fauna. This underestimation is because immigration makes a greater contribution to recovery when biota are relatively more abundant around the impacted site and because most experiments have been conducted in infrequently and untrawled areas (16). On fishing grounds, impacts occur on larger scales, such that untrawled and infrequently trawled areas become scarce when there is more trawling activity. Furthermore, experiments typically focus on recovery after single trawling events rather than recovery from successive events typical of fishing grounds.
The development of satellite-based vessel monitoring systems has enabled scientists to map commercial fishing activity at high resolution (7). Such maps have been used to design studies of the comparative impacts of towed bottom-fishing gears across gradients of commercial fishing frequency (herein equals comparative studies). In contrast to experimental studies, these studies account for the spatial extent, frequency, and temporal variability in fishing activity and are expected to provide more representative estimates of recovery rates. When these estimates are coupled with estimates of the mortality of biota from experimental studies, they can be used to assess the status of impacted biota on fishing grounds. Presently, there are too few studies to adopt the alternative approach of analyzing large-scale studies directly recording recovery from trawling (24).
We used the logistic growth equation (25) to describe recovery of benthic fauna, because it provides an effective abstraction of the complex recovery dynamics of populations and communities and can be fitted to available data (22, 23, 26). This model is identical to the Schaefer models commonly used in fisheries management when the data to implement full age or size-structured models are not available (27). If we assume that the recovery of biomass or numbers (hereafter abundance) of biota B after trawling is described by the logistic growth equation, then the equilibrium solution can be used to estimate B as a fraction of carrying capacity K in an environment subject to chronic fishing disturbance (28):
| [1] |
where F is trawling frequency, d is the depletion of biota caused by each trawl pass (expressed as a proportion), and r is rate of increase interpreted here as the recovery rate. Eq. 1 only requires estimates of F, d , and r to estimate relative abundance B/K (28). Eq. 1 suggests that r is constant, but in communities composed of species with a range of r values, trawling selects for species with faster life histories that are more resilient, and therefore, r can be expected to increase with F. We found that the relationship between community B/K and F for communities is well-approximated by a log-linear relationship (SI Appendix). We, therefore, estimated r at F = 0 and assuming a log-linear relationship between B/K and F (Eq. 2). More sophisticated models of recovery can account for differential responses of groups with contrasting life histories and other aspects of community dynamics and thus, provide a better description of underlying processes (19, 29), but higher parameter demands limit their application to systems with a substantial amount of available data. Conversely, if d and r can be estimated and if associated uncertainties can be quantified, the logistic model would facilitate assessment of trawling impacts in most marine systems. Different gears and substrata will have different levels of seabed contact or penetration, and these factors will influence d. Penetration depth is, however, largely independent of the towing speed (6). If a strong relationship exists between the penetration depth and d, this relationship can be used to obtain estimates of depletion for trawl gears for which no empirical depletion estimates are available. Trawling frequency F is defined as the swept area ratio, which is the area trawled annually divided by the studied area (kilometers2 kilometer–2 year–1; simplified to year−1); it should ideally be calculated for small cells (∼1 km2), because trawling tends to be spatially clustered at larger scales.
Here, we conduct a metaanalysis of experimental studies of trawling impacts to estimate depletion of biota after trawling. We report the effect on the abundance of whole benthic macroinvertebrates communities, including infauna and epifauna. We combine this with a metaanalysis of results from large-scale comparative studies of trawling effects on fishing grounds to estimate recovery rates of seabed biota and describe how they vary with gear characteristics and environment. All data were collated from studies that were quality assured after systematic review methodology, thereby avoiding selection bias (30).
Results
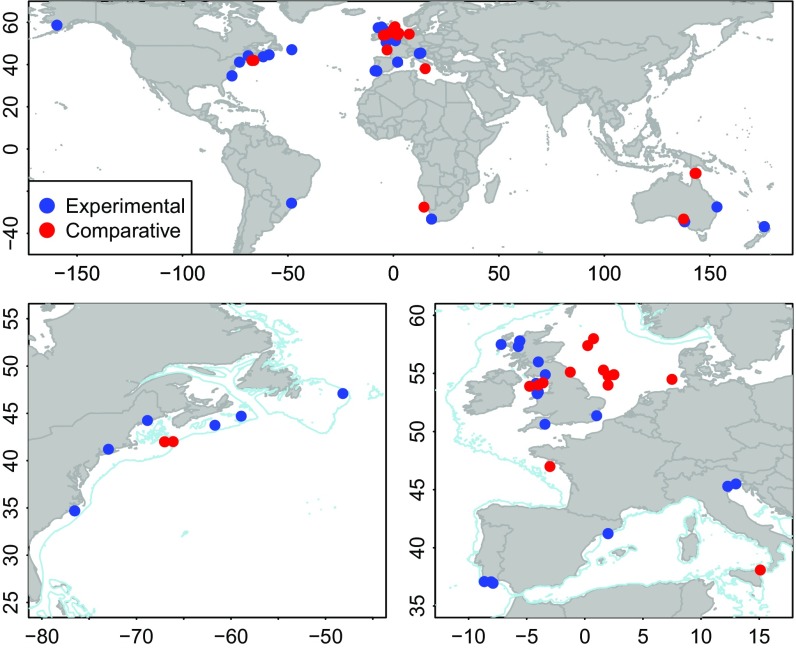
Twenty-four comparative and 46 experimental studies met the criteria for inclusion in our analyses (SI Appendix, Tables S1–S3). Studies were mostly temperate and concentrated in northwestern Europe and the northeastern United States (Fig. 1). None of the studies that met the criteria examined the effect of trawling on biogenic habitats, but there were sufficient studies in other habitats. Many gear–habitat combinations were not represented in the studies reviewed, because many fishing gears are only suitable for fishing on particular seabed types or species associated with those habitats (SI Appendix, Table S1) and because some habitats are less widespread than others (7).
Fig. 1.
Maps of the locations of the studies. The higher-resolution maps of the northwest and northeast Atlantic give more detail for two areas with high concentration of studies. The 200-m depth contour is shown in blue.
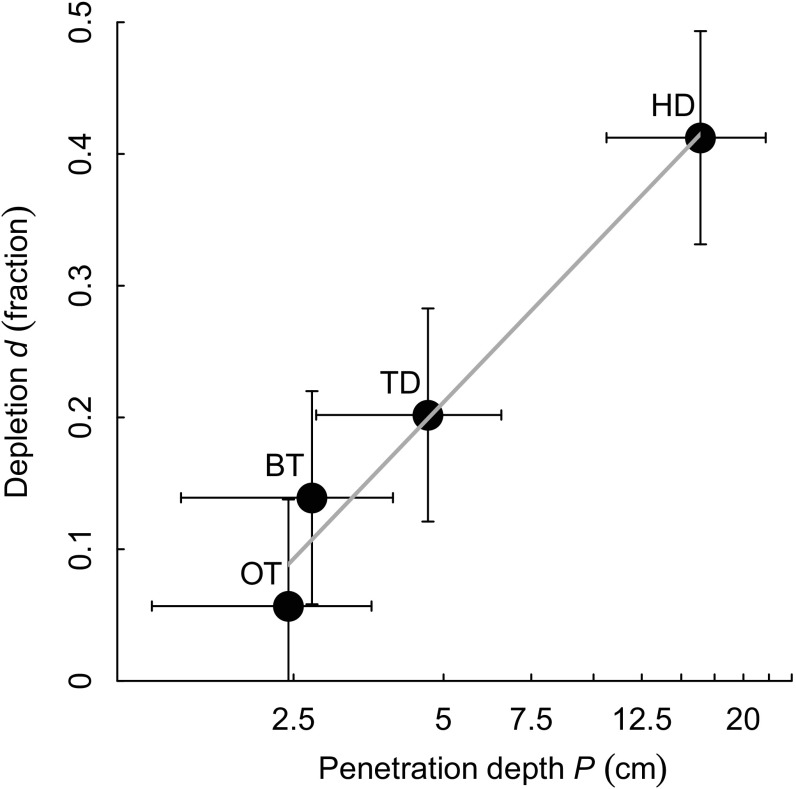
Depletion rates estimated from the experimental studies for biomass and numbers were not significantly different. Thus, the pooled estimates of d (SI Appendix, Table S4) apply to both biomass and numbers. Estimates of depletion d and penetration depth P by gear type were very closely correlated (Fig. 2) (Pearson’s r = 0.980, P = 0.020). OTs had the smallest impact, removing on average 6% of organisms per trawl pass and penetrating on average 2.4 cm into the sediment. Median penetration depths were 2.7 and 5.5 cm for BTs and TDs, respectively, and the corresponding median depletions per trawl pass were 14 and 20%, respectively. HDs had the largest impact, removing on average 41% of organisms per pass and penetrating 16.1 cm.
Fig. 2.
The relationship between the penetration depth P and depletion d of macrofaunal community biomass and numbers caused by a single trawl pass for different trawl gears (means ± SD).
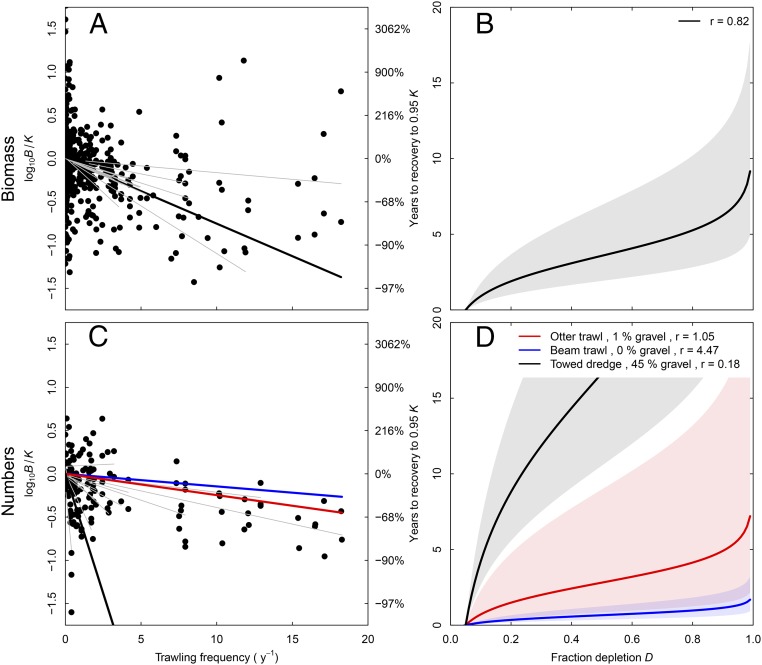
The effect of trawling frequency on relative biomass estimated from the comparative studies showed a log-linear relationship, with each unit increase in swept area ratio linked to a mean fall in biomass of 15.5% (Fig. 3A). None of the other environmental variables significantly affected this response (Table 1). The effect of sediment composition on community biomass depletion was not significant (Table 1, community biomass and SI Appendix, Table S5), but the model estimates for gravel are nevertheless shown in Table 1 for community biomass to allow comparison with the significant effects of gravel found for community numbers (Table 1, community numbers and SI Appendix, Table S5). Mean community r (estimated using SI Appendix, Eqs. S4.1 and S4.2 from d and b) increased with trawling frequency from 0.82 y−1 when there was no trawling (5–95% uncertainty intervals = 0.42–1.53) to 1.73 (0.89–3.23) y−1 when the trawling frequency was 10 y−1 (using the mean estimated d across gears OT, BT, and TD; d = 0.13) (SI Appendix, Fig. S1 and Table S6). The increase in r, which results from changes in community composition to favor biota with faster life histories, is, therefore, relatively slight across ranges of trawling frequencies that dominate those on real fishing grounds (e.g., 0–1 y−1) (7, 31, 32). The r estimate of 0.82 y−1 enables estimates of median time to recovery (T) to 0.95K for a range of levels of depletion (Fig. 3B). For example, if the fraction depleted D = 0.5K, then recovery time is 3.6 y (5–95% uncertainty intervals = 1.9–6.4 y).
Fig. 3.
The relationship between trawling frequency and total community (A) biomass and (C) numbers. The thicker lines are the fixed effects, and grey lines are the random effects of the individual studies (not all visible, because many studies had small ranges and low trawling frequencies). Recovery time to 0.95K for depleted total community (B) biomass and (D) numerical abundance as a function of estimated r and initial depletion D. In A and B, lines are the median estimate based on the mean d across all gears. In C and D, lines are the median estimates for three different gear types based on the mean gravel content in the areas where studies using these gear types were carried out. The shaded areas indicate the 5–95% uncertainty intervals for estimates.
Table 1.
Linear mixed model (SI Appendix, Eq. S3.1) fits for the analysis of data from comparative studies of changes in biomass and numbers
| Model | Slope (b) | SE | df | t Value | P value | AIC |
| Community biomass | ||||||
| TF | −0.07522 | 0.0158 | 503 | −4.732 | <0.0001 | 566.9 |
| TF | −0.07142 | 0.0172 | 502 | −4.148 | <0.0001 | 568.4 |
| TF: gravel | −0.00067 | 0.0010 | 502 | −0.648 | 0.5168 | |
| TF | −0.08623 | 0.0325 | 502 | −2.653 | 0.0082 | 568.8 |
| TF: d/PP | 125.6879 | 373.7966 | 502 | 0.336 | 0.7368 | |
| Community numbers | ||||||
| TF | −0.21185 | 0.1342 | 141 | −1.577 | 0.1169 | 89.5 |
| TF | −0.01451 | 0.0942 | 140 | −0.153 | 0.8778 | 81.1 |
| TF: gravel content | −0.01206 | 0.0035 | 140 | −3.377 | 0.0009 | |
| TF | 0.25300 | 0.2145 | 140 | 1.048 | 0.2964 | 86.1 |
| TF: d/PP | −6,892.96900 | 2,676.5453 | 140 | −2.575 | 0.0111 |
For community biomass, the model with the lowest AIC included no explanatory variables other than trawling frequency, but for community abundance, both gravel content and d/PP improved the AIC in relation to a model without other explanatory variables. Results for these variables are given under community biomass for comparative purposes. d, Depletion estimate from experimental studies (fraction per trawl pass) (SI Appendix, Table S4); gravel, sediment composition in percentage by weight; PP, primary production (milligrams C meter–2 day–1); TF, trawling frequency.
The effect of trawling on community numbers, estimated from the comparative studies, increased significantly with the gravel content of the sediment (Fig. 3C, Table 1, community numbers, and SI Appendix, Table S5), and this effect persisted when examined among gears. The reductions in benthic community numbers for each unit increase in trawling frequency were 3.1% at 0% gravel content (typical for BT studies), 5.5% at 1% gravel content (typical for OT studies), and 72% at 45% gravel content (typical for TD studies). The estimates of r for community abundance range from 0.18 y−1 for TD on 45% gravel to 4.47 y−1 for BT on 0% gravel, with high uncertainty. These r estimates result in a median recovery time T from 0.5K to 0.95K of 0.7–16.6 y (Fig. 3D). Other than gravel content, the inclusion of the ratio of d over primary production also resulted in reduced Akaike information criterion (AIC) compared with the model with no additional explanatory variables, with the effect of trawling on numbers increasing with d and decreasing at higher levels of primary production (Table 1, community numbers and SI Appendix, Table S5).
Discussion
This study is an attempt to quantify the impacts of bottom trawling and recovery of seabed biota by synthesizing data from trawling studies after a systematic review of the available evidence base. We developed a method to derive the recovery rates of benthic macrofaunal invertebrate communities from trawling by combining results from experimental and comparative studies and provide estimates of depletion and recovery, including a quantification of uncertainty based on all available data. The method for estimating the recovery rate from comparative studies is unique. Given that realistic and robust r estimates have been largely unavailable previously, this work is critically important. Recovery rates were estimated from changes in the biomass and numbers of biota across fishing grounds, and therefore, estimates are likely applicable to trawled shelf seas in general (at least in temperate waters where most of the studies were carried out). Our estimates of depletion and recovery enable the parameterization of models to predict the state of the benthic biota as a function of trawling frequency and levels of primary production and percentage gravel (28). Coupled with the emergence of large-scale estimates of trawling frequency (7), these models will support assessment of trawling impacts on unprecedented spatial scales, because our approach provides a quantitative estimate of status with minimal data requirements (28). The method is widely applicable, because it requires relatively few data inputs and could be applied worldwide, including for fisheries where trawl impacts remain unassessed. The r and d values that we estimate here with a broad geographic basis are based on the full body of available evidence and therefore, the most robust estimates available. The generality of our approach means that the outputs of assessments are accurate when averaging over larger scales but that biases may exist when used for local assessments. These results have global policy relevance for conservation and food security policy development, because they enable an objective analysis of the efficacy of different methods of harvesting food from the ocean to be considered in the light of the wider ecosystem effects of such activities on the marine environment. The results enable managers to understand the variable resilience of benthic systems to trawl fisheries and set limits of fishing accordingly.
Most continental shelves consist of relatively small intensively trawled areas, where the trawling frequency is in the range of 1–10 y−1, and extensive infrequently trawled areas, where the trawling frequency is <1 y−1 and predominantly <0.25 y−1 (7). Our results show that trawling frequencies of 1 y−1 cause average declines of 15.5% in the biomass of benthic biota. Communities on gravel may be more sensitive to trawling, because they, on average, have a larger proportion of larger, long-lived, and sessile epifauna (33) that are particularly sensitive to trawling (34). Effects were greater for gears that kill a larger fraction of the biota (larger d), because they penetrate the sediment more deeply and weaker in areas of higher primary production, where higher food supply to the benthos may result in a higher recovery rate.
The ranking of different fishing gears with respect to their magnitude of impact reported here is similar to the ranking in previous metaanalyses of small-scale experimental studies (15, 16), although our estimates of d are smaller, probably because we adjusted for the number of trawl passes, whereas previous analyses did not. The use of depletion to primary production ratio as a proxy for community resilience to trawling has the advantages of being easily understandable and easy to estimate for new areas and fisheries. The ratio of depletion over primary production might support rapid preliminary large-scale risk assessments of potential trawling impacts on community abundance to guide more region-specific studies. The close relationship between penetration depth and depletion can be used to estimate depletion resulting from the pass of a given trawl gear when no direct depletion estimate is available. Accurate estimates of penetration depth are much easier and cheaper to obtain than estimates of depletion, would support preliminary impact assessments by gear type, and can even be generated using numerical models (11).
Our analyses did not identify any variables other than trawling frequency that affected community biomass. This finding is surprising given the contrasting results for numbers and that some comparative studies and past metaanalyses of experimental studies have shown interaction effects between gear type and habitat type (16, 29). The relatively small number of studies included in the biomass analysis and the high variability associated with benthic sampling, which cannot be fully controlled in a metaanalysis, may have contributed to this discrepancy. Our results for biomass imply that a single estimate of recovery rate r is appropriate when assessing impacts on the different habitat types studied here. They also suggest that differences in time to recovery and expected biomass (B/K) will be driven primarily by gear type (and hence, d) and trawling frequency (F).
Our estimates of biomass recovery times are similar to empirical measurements of recovery taken in three areas where commercial trawling was stopped (4–5 y) (24) but longer than estimates from small-scale experimental studies, which are on the order of 25−500 d (15, 16). The scale dependency of recovery times has important implications for management, because recovery will be faster when trawled areas are closer to less impacted areas from which individuals can recruit or migrate (as also shown in ref. 22). We found that biomass recovery rates were slower and that recovery times were longer than those for numbers. This result is expected based on the population dynamics of seabed biota. Recovery in numbers is driven more strongly by recruitment than recovery of biomass, which is driven by increases in the size and age structure of the population through growth of individuals. We recommend the use of recovery rates for community biomass when modeling trawl impacts and their consequences. This approach will give due weight to recovery of body size and age structure as well as numbers and take account of energy flow through food webs and other ecosystem processes that are linked closely to biomass. Recovery times as estimated from the logistic model nevertheless do not imply that the communities will recover over these times to the species, size, and age composition that existed before trawling, but they do imply the recovery of total biomass or numbers and related cross-species ecosystem processes, such as aggregate secondary production.
Uncertainties around mean/median estimates of penetration depth, recovery, and depletion were high, despite the careful screening of included data (which also decreased the sample size and potentially, power to detect effects) (30). However, our approach allows us to address directly some aspects of uncertainty, and the broad distribution of resulting depletion and recovery estimates show that large site-specific differences in the response of seabed communities to trawling are expected. The advantage of characterizing uncertainty is that it can be propagated in future risk and impact analyses. Given the unexplained variance in r, percentiles from the distribution of plausible values might be selected to reflect the degree of risk aversion in the management system. The extent of risk aversion is a nonscientific decision (although it would be informed by science) that would likely be made by managers and other stakeholders. Risk aversion would likely depend on the perceived value of a habitat type. A risk-averse approach might adopt a value of r from a lower percentile of the distribution (e.g., the 10 or 25%) rather than the median (SI Appendix, Table S6 shows a selection of values).
Our use of comparative studies provides improved estimates of recovery compared with those from previous small-scale experiments studies, because they are based on larger-scale measurements from fishing grounds. Comparative studies may, however, be affected by “shifting baselines” (35), where historical trawling has removed the most sensitive organisms and only resilient organisms remain. Because trawling selects for species with faster life histories that are more resilient, recovery time will increase with trawling frequency. Our finding that mean community r increases with F conforms with previous observations of shifts toward species with faster life histories in disturbed communities (36). This effect is apparent across a range of plausible trawling frequencies from >0 to 10 y−1 but would be small for the great proportion of most fishing grounds, where swept area ratio is less than 1 y−1 (7). Although this shift means that previously trawled communities may be more resilient to additional trawling, it does not mean that they will recover any faster to the original pretrawling state. For this reason, we used the r estimate of untrawled communities for estimating recovery times. Selective effects linked to trawling history are likely to be strongest for long-lived sessile epifauna that build biogenic reefs, such as sponges and corals. The estimates of r and T presented here are applicable to invertebrate communities living in sedimentary habitats but not biogenic habitats, because no studies of trawling impacts on biogenic habitats met the rigorous selection criteria imposed by the systematic review.
In summary, we apply widely applicable methods to estimate depletion and recovery rates of benthic invertebrate communities after trawling. By accounting for the effects of gear type and penetration, environmental variation, and uncertainty, our analysis explained much of the variability of depletion and recovery estimates from single studies. Coupled with large-scale, high-resolution maps of trawling frequency and habitat, our estimates of depletion and recovery rates will enable analysis of trawling impacts on unprecedented spatial scales to inform best practices to achieve sustainable fishing and will be of use to policymakers, conservation planners, and fisheries managers for risk assessment and the evaluation of management strategies.
Methods
We present analyses for whole-community biomass and numbers of benthic invertebrates. Changes in the abundance of seabed biota after trawling depend on the mortality caused by each pass of a trawl and the rate of recovery of the biota between trawl passes. We estimated the immediate depletion of biota (d) caused by a trawl pass from a metaanalysis of experimental studies of trawling impacts. We estimated the recovery rates (r) of biota from a metaanalysis of comparative studies of trawling impacts. The analyses were structured to assess the effects of gear type, penetration depth, and environmental variables (e.g., depth and sediment composition) on depletion and recovery.
Depletion.
Depletion was estimated using data collated from experimental studies of trawling impacts identified using systematic review methodology. A comprehensive literature search of journal papers, book chapters, and grey literature reports was carried out. Details of literature search terms, databases, and study inclusion criteria are provided in the systematic review protocol by Hughes et al. (30). All included studies quantified the immediate mortality of biota after one or multiple trawling events. Each identified study had to pass quality assurance criteria before data from the study were included in the collated dataset.
We classified gear types as OTs, BTs, TDs, or HDs (SI Appendix). The reduction in abundance of biota resulting from one pass of a trawling gear depends on the characteristics and operation mode of the gear. Different gears are designed to have different levels of seabed contact or penetration depending on the target species and seabed type, and these factors will influence mortality (37). Consequently, we assessed the relationship between mortality and penetration depth of the gear. Some of these studies were conducted in previously trawled areas with a lowered abundance of biota, but because we are estimating the fraction of organisms removed rather than the absolute amount, we expect that this will have had little effect on our estimates of d. Depletion d was estimated using a generalized linear mixed model implemented in the package nlme in R (38, 39), with the log of the ratio of the biomass or abundance in trawled over untrawled areas (lnRR) as the response variable, log2 (time t in days since trawling) and gear type as fixed factors, and the study as a random effect assuming a Gaussian error distribution. We weighted lnRR values by the inverse of their variance, which is normal practice in metaanalyses. We estimated d as the intercept for the different gears at t = 0.
Predicted penetration depth of each gear type into the seabed was estimated from values in the literature by averaging the reported penetration depths of the individual components of the gear (e.g., doors, sweeps, and bridles of an OT) weighted by the width of these components (details are in SI Appendix).
Recovery.
Recovery rates were estimated using data collated from comparative studies of trawling impacts. All included studies sampled the biomass or numbers of whole communities of benthic invertebrates at two or more sites subject to different trawling intensities on commercial fishing grounds. Contributing studies were identified following the same procedure as for experimental studies (SI Appendix). In the analyses of the comparative studies, we assume that both K and observed gradients of trawling effort were unrelated to other environmental drivers and that the observed state of the biota is in equilibrium with the reported trawling effort. Gradients in trawling effort may be driven by regulation and seabed obstructions but are also observed in areas of homogenous habitat (29). Spatial patterns of trawling effort are also shown to be relatively stable over time in the few fisheries where high-resolution time series have been analyzed (40). K could vary across the trawl grounds because of environmental variations, and this source of variation will increase the uncertainty around relationships between B and F.
In the comparative studies, conversions between units of abundance were not always possible (e.g., biomass per unit sediment volume could not be converted to biomass per unit sediment area given sampling gears with different but unknown efficiencies), and therefore, absolute B or K could not be estimated. We normalized the data by expressing relative biomass or numbers as the B/K ratio and used a log-linear approximation for the relationship between community B/K and F:
| [2] |
where b is the slope of the relationship (derivation taking account of the log-linear relationship between B/K and F and the distribution of trawling is in SI Appendix). After fitting a linear relationship to log10B vs. F for each comparative study, K was estimated as the 10intercept of this relationship.
The data collated from comparative studies were initially used to estimate relative changes in abundance (B/K) as a function of trawling frequency F. This approach differs from the aforementioned analyses of depletion, because the change in abundance with trawling is a response to both depletion (per trawl pass) and recovery. Because b = d/r (Eq. 1), after d is estimated from experimental data, recovery rate r can be estimated from the slope b of Eq. 2 after taking account of the log-linear nature of this relationship, which implies that r increases with F. To propagate uncertainty in the estimates of b and d into the estimate of r, we sampled the distributions of b and d estimates to derive the distribution of r (SI Appendix). Time to recovery from a given level of depletion D to a defined proportion ϕ of K at which recovery is deemed to have occurred (e.g., 0.95) was derived from the approach of Lambert et al. (22) (SI Appendix). When reporting recovery times, we report recovery from 0.5K to 0.95K.
Variables That Determine the Effect of Trawling in Comparative Studies.
The effect of trawling on seabed biota in comparative studies could be influenced by different variables. Thus, we evaluated the explanatory power of several potential factors by including them as covariates in a linear mixed model (39) based on Eq. 2 and selecting the most parsimonious model using AIC. According to Eq. 2 the community response to trawling in log10 scale is approximately proportional to F, with slope a function of the ratio of d/r. The fixed part of the mixed models was, therefore,
where the response variable is community biomass or numbers and the “other variables” can be covariates for d, r, or their ratio (4). The intercept was removed, because log10(B/K) with no impact = 0. We modeled “study” as a random effect, allowing the slope to vary per study. This approach accounted for the nonindependence of observations within a study. We checked the assumptions of the linear mixed model by visual inspection of the normalized residuals (38).
We expected that factors that lead to a higher d would strengthen the effect of trawling (e.g., higher penetration depth), whereas factors that lead to a higher r by affecting growth rates of individuals and populations (higher flow of energy to the seabed because of a higher production, shallower depth, or higher temperature) would weaken the effect. The closely related penetration depth P (continuous) and gear type (categorical) were examined as covariates for d. The following covariates for r were examined: primary production estimated from the vertically generalized productivity model (milligrams C meter−2 day−1) (41) and particulate organic carbon flux to depth (grams Corg meter−2 year−1) (42) as proxies for energy availability, mean sea bottom temperature calculated from monthly mean bottom temperature for 2009–2011 provided in MyOcean Product (GLOBAL-REANALYSIS-PHYS-001–009), depth (from GEBCO if not reported in the original study), habitat type, and sediment composition (gravel, sand, and mud content). Habitat types were classified as biogenic habitats, gravel, sand, muddy sand/sandy mud, and mud. Sediment gravel, sand, and mud content were extracted from the source studies by converting the sediment description to the Folk classification (43) and then converting the Folk classification to percentages based on the means in each category. In addition to analyses using covariates of d or r, we also conducted analyses using covariates of the d/r ratio; here, the d/r ratio was approximated as the ratio of d or P to the continuous r covariates. The effect of trawling is expected to increase with water depth owing to the lower levels of natural disturbance in deeper water and the corresponding increase in the relative abundance of individuals with slower life histories (low r), and therefore, d × depth was examined as a covariate for d/r, with depth expressed as a negative number.
Supplementary Material
Acknowledgments
The study was funded by the David and Lucile Packard Foundation; the Walton Family Foundation; The Alaska Seafood Cooperative; American Seafoods Group; Blumar Seafoods Denmark; Clearwater Seafoods; Espersen Group; Glacier Fish Company LLC; Gortons Inc.; Independent Fisheries Limited N.Z.; Nippon Suisan (USA), Inc.; Pacific Andes International Holdings, Ltd.; Pesca Chile, S.A.; San Arawa, S.A.; Sanford Ltd. N.Z.; Sealord Group Ltd. N.Z.; South African Trawling Association; and Trident Seafoods. Additional funding was provided by United Kingdom Department of Environment, Food and Rural Affairs Project MF1225 (to S.J.), European Union Project BENTHIS EU-FP7 312088, The International Council for the Exploration of the Sea Science Fund, and the Food and Agriculture Organisation of the United Nations.
Footnotes
Conflict of interest statement: The study was funded by a variety of organizations that include nongovernmental organizations that promote conservation and sustainable use and fish producers.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1618858114/-/DCSupplemental.
References
- 1.Halpern BS, et al. A global map of human impact on marine ecosystems. Science. 2008;319:948–952. doi: 10.1126/science.1149345. [DOI] [PubMed] [Google Scholar]
- 2.Foden J, Rogers SI, Jones AP. Human pressures on UK seabed habitats: A cumulative impact assessment. Mar Ecol Prog Ser. 2011;428:33–47. [Google Scholar]
- 3.FAO . The State of World Fisheries and Aquaculture 2016. FAO; Rome: 2016. [Google Scholar]
- 4.Rice J. Challenges, objectives, and sustainability: Benthic community, habitats and management decision-making. Am Fish Soc Symp. 2005;41:41–58. [Google Scholar]
- 5.Rice J, et al. Indicators for Sea-floor Integrity under the European Marine Strategy Framework Directive. Ecol Indic. 2012;12:174–184. [Google Scholar]
- 6.Rijnsdorp AD, et al. Towards a framework for the quantitative assessment of trawling impact on the seabed and benthic ecosystem. ICES J Mar Sci. 2016;73:i127–i138. [Google Scholar]
- 7.Eigaard OR, et al. The footprint of bottom trawling in European waters: Distribution, intensity and seabed integrity. ICES J Mar Sci. 2016;74:847–865. [Google Scholar]
- 8.Gray JS, Dayton P, Thrush S, Kaiser MJ. On effects of trawling, benthos and sampling design. Mar Pollut Bull. 2006;52:840–843. doi: 10.1016/j.marpolbul.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 9.Sainsbury JC. Commercial Fishing Methods. Fishing News Books; Oxford: 1986. [Google Scholar]
- 10.Churchill JH. The effect of commercial trawling on sediment resuspension and transport over the Middle Atlantic Bight continental shelf. Cont Shelf Res. 1989;9:841–864. [Google Scholar]
- 11.O'Neill F, Ivanović A. The physical impact of towed demersal fishing gears on soft sediments. ICES J Mar Sci. 2016;73:i5–i14. [Google Scholar]
- 12.Peterson CH, Summerson HC, Fegley SR. Ecological consequences of mechanical harvesting of clams. Fish Bull. 1987;85:281–298. [Google Scholar]
- 13.Collie JS, Escanero GA, Valentine PC. Effects of bottom fishing on the benthic megafauna of Georges bank. Mar Ecol Prog Ser. 1997;155:159–172. [Google Scholar]
- 14.Collie JS, Hermsen JM, Valentine PC, Almeida FP. Effects of fishing on gravel habitats: Assessment and recovery of benthic megafauna on Georges Bank. In: Barnes P, Thomas J, editors. Benthic Habitats and the Effects of Fishing. Vol 41. American Fisheries Society; Bethesda, MD: 2005. pp. 325–343. [Google Scholar]
- 15.Collie JS, Hall SJ, Kaiser MJ, Poiner IR. A quantitative analysis of fishing impacts on shelf-sea benthos. J Anim Ecol. 2000;69:785–798. doi: 10.1046/j.1365-2656.2000.00434.x. [DOI] [PubMed] [Google Scholar]
- 16.Kaiser MJ, et al. Global analysis and prediction of the response of benthic biota to fishing. Mar Ecol Prog Ser. 2006;311:1–14. [Google Scholar]
- 17.van Denderen PD, et al. Similar effects of bottom trawling and natural disturbance on composition and function of benthic communities across habitats. Mar Ecol Prog Ser. 2015;541:31–43. [Google Scholar]
- 18.Ramsay K, Kaiser MJ, Moore PG, Hughes RN. Consumption of fisheries discards by benthic scavengers: Utilization of energy subsidies in different marine habitats. J Anim Ecol. 1997;66:884–896. [Google Scholar]
- 19.Duplisea DE, Jennings S, Warr KJ, Dinmore TA. A size-based model of the impacts of bottom trawling on benthic community structure. Can J Fish Aquat Sci. 2002;59:1785–1795. [Google Scholar]
- 20.Hiddink JG, et al. Bottom trawling affects fish condition through changes in the ratio of prey availability to density of competitors. J Appl Ecol. 2016;53:1500–1510. [Google Scholar]
- 21.Rijnsdorp AD, Buys AM, Storbeck F, Visser EG. Micro-scale distribution of beam trawl effort in the southern North Sea between 1993 and 1996 in relation to the trawling frequency of the sea bed and the impact on benthic organisms. ICES J Mar Sci. 1998;55:403–419. [Google Scholar]
- 22.Lambert GI, et al. Quantifying recovery rates and resilience of seabed habitats impacted by bottom fishing. J Appl Ecol. 2014;51:1326–1336. [Google Scholar]
- 23.Ellis N, Pantus F, Pitcher CR. Scaling up experimental trawl impact results to fishery management scales—a modelling approach for a “hot time.”. Can J Fish Aquat Sci. 2014;71:733–746. [Google Scholar]
- 24.Hiddink JG, Jennings S, Kaiser MJ. Indicators of the ecological impact of bottom-trawl disturbance on seabed communities. Ecosystems. 2006;9:1190–1199. [Google Scholar]
- 25.Schaefer MB. Some aspects of the dynamics of populations important to the management of the commercial marine fisheries. Inter-American Tropical Tuna Commission Bull. 1954;1:23–56. [Google Scholar]
- 26.McClanahan TR, Graham NA, Calnan JM, MacNeil MA. Toward pristine biomass: Reef fish recovery in coral reef marine protected areas in Kenya. Ecol Appl. 2007;17:1055–1067. doi: 10.1890/06-1450. [DOI] [PubMed] [Google Scholar]
- 27.Costello C, et al. Global fishery prospects under contrasting management regimes. Proc Natl Acad Sci USA. 2016;113:5125–5129. doi: 10.1073/pnas.1520420113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pitcher CR, et al. Estimating the sustainability of towed fishing-gear impacts on seabed habitats: A simple quantitative risk assessment method applicable to data-poor fisheries. Methods Ecol Evol. 2017;8:472–480. [Google Scholar]
- 29.Hiddink JG, et al. Cumulative impacts of seabed trawl disturbance on benthic biomass, production and species richness in different habitats. Can J Fish Aquat Sci. 2006;63:721–736. [Google Scholar]
- 30.Hughes KM, et al. Investigating the effects of mobile bottom fishing on benthic biota: A systematic review protocol. Environ Evidence. 2014;3:23. doi: 10.1186/s13750-024-00348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baird S, Wood BA, Bagley NW. Nature and Extent of Commercial Fishing Effort on or Near the Seafloor Within the New Zealand 200 N. Mile Exclusive Economic Zone, 1989–90 to 2004–05. Ministry of Fisheries; Wellington, New Zealand: 2011. [Google Scholar]
- 32.Pitcher R, et al. 2016. Implications of Current Spatial Management Measures for AFMA ERAs for Habitats. (CSIRO Oceans & Atmosphere, Brisbane, Australia), FRDC Project 2014/204.
- 33.Bolam SG, et al. Differences in biological traits composition of benthic assemblages between unimpacted habitats. Mar Environ Res. 2017;126:1–13. doi: 10.1016/j.marenvres.2017.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Tillin HM, Hiddink JG, Kaiser MJ, Jennings S. Chronic bottom trawling alters the functional composition of benthic invertebrate communities on a sea basin scale. Mar Ecol Prog Ser. 2006;318:31–45. [Google Scholar]
- 35.Brown CJ, Trebilco R. Unintended cultivation, shifting baselines, and conflict between objectives for fisheries and conservation. Conserv Biol. 2014;28:677–688. doi: 10.1111/cobi.12267. [DOI] [PubMed] [Google Scholar]
- 36.Jennings S, Greenstreet SPR, Reynolds JD. Structural change in an exploited fish community: A consequence of differential fishing effects on species with contrasting life histories. J Anim Ecol. 1999;68:617–627. [Google Scholar]
- 37.Eigaard OR, et al. Estimating seabed pressure from demersal trawls, seines, and dredges based on gear design and dimensions. ICES J Mar Sci. 2015;73:i27–i43. [Google Scholar]
- 38.Zuur AF, et al. Mixed Effects Models and Extensions in Ecology with R. Springer; New York: 2009. [Google Scholar]
- 39.Pinheiro J, Bates D, DebRoy S, Sarkar D. 2013. nlme: Linear and Nonlinear Mixed Effects Models. (R Package), Version 3.1-113.
- 40.Jennings S, Lee J, Hiddink JG. Assessing fishery footprints and the tradeoffs between landings value, habitat sensitivity and fishing impacts to inform marine spatial planning and an ecosystem approach. ICES J Mar Sci. 2012;69:1053–1063. [Google Scholar]
- 41.Behrenfeld MJ, Falkowski PG. Photosynthetic rates derived from satellite-based chlorophyll concentration. Limnol Oceanogr. 1997;42:1–20. [Google Scholar]
- 42.Lutz MJ, Caldeira K, Dunbar RB, Behrenfeld MJ. Seasonal rhythms of net primary production and particulate organic carbon flux to depth describe the efficiency of biological pump in the global ocean. J Geophys Res Oceans. 2007;112:C10011. [Google Scholar]
- 43.Folk RL. The distinction between grain size and mineral composition in sedimentary-rock nomenclature. J Geol. 1954;62:344–359. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.