Significance
New pain medications with novel mechanisms of action are needed. Here we show that sigma-1 antagonism decreases inflammatory pain hypersensitivity by enhancing the actions of endogenous opioid peptides produced by leukocytes in mice. Sigma-1 antagonism results in opioid analgesia only at the inflamed site, where immune cells naturally accumulate. This mechanism, which maximizes the analgesic potential of immune cells in painful inflamed sites, differs from that of conventional analgesics.
Keywords: sigma-1 receptors, inflammatory pain, endogenous opioid peptides, immune cells
Abstract
Sigma-1 antagonism potentiates the antinociceptive effects of opioid drugs, so sigma-1 receptors constitute a biological brake to opioid drug-induced analgesia. The pathophysiological role of this process is unknown. We aimed to investigate whether sigma-1 antagonism reduces inflammatory pain through the disinhibition of the endogenous opioidergic system in mice. The selective sigma-1 antagonists BD-1063 and S1RA abolished mechanical and thermal hyperalgesia in mice with carrageenan-induced acute (3 h) inflammation. Sigma-1–mediated antihyperalgesia was reversed by the opioid antagonists naloxone and naloxone methiodide (a peripherally restricted naloxone analog) and by local administration at the inflamed site of monoclonal antibody 3-E7, which recognizes the pan-opioid sequence Tyr–Gly–Gly–Phe at the N terminus of most endogenous opioid peptides (EOPs). Neutrophils expressed pro-opiomelanocortin, the precursor of β-endorphin (a known EOP), and constituted the majority of the acute immune infiltrate. β-endorphin levels increased in the inflamed paw, and this increase and the antihyperalgesic effects of sigma-1 antagonism were abolished by reducing the neutrophil load with in vivo administration of an anti-Ly6G antibody. The opioid-dependent sigma-1 antihyperalgesic effects were preserved 5 d after carrageenan administration, where macrophages/monocytes were found to express pro-opiomelanocortin and to now constitute the majority of the immune infiltrate. These results suggest that immune cells harboring EOPs are needed for the antihyperalgesic effects of sigma-1 antagonism during inflammation. In conclusion, sigma-1 receptors curtail immune-driven peripheral opioid analgesia, and sigma-1 antagonism produces local opioid analgesia by enhancing the action of EOPs of immune origin, maximizing the analgesic potential of immune cells that naturally accumulate in painful inflamed areas.
There is a need for new analgesics with innovative mechanisms of action (1). The sigma-1 receptor acts as a ligand-operated chaperone, which modifies the function of several receptors and channels important in neurotransmission (2), and has been the focus of intense preclinical research as a new pharmacological target for pain treatment (3, 4). The role of sigma-1 receptors in neuropathic pain has been extensively studied, and it has been widely reported that sigma-1 inhibition decreases central sensitization (3), which plays a key role in this type of pain (5). Among the selective sigma-1 antagonists, the best characterized are BD-1063 and S1RA (3). The latter compound is currently being evaluated in phase II clinical trials with a primary indication for neuropathic pain/neuropathy treatment (4), after successful positive phase I studies demonstrated its acceptable safety and tolerability in healthy people (6). A further potential indication for this sigma-1 antagonist is the enhancement of opioid analgesia (4). The potentiation of opioid antinociception by sigma-1 antagonism was described in the early 1990s (7). Later studies showed that the enhancement of opioid antinociception by sigma-1 antagonism is produced at central levels (8) and is particularly prominent at peripheral levels (9, 10). The marked potentiation of opioid antinociception by peripheral sigma-1 antagonism is consistent with its higher density in the dorsal root ganglion than in several central areas (10). Moreover, these receptors in the dorsal root ganglion are selectively located in sensory neurons and not in glial cells (11). It is now known that sigma-1 receptors can form a macromolecular complex with opioid receptors, tonically inhibiting receptor functioning, and that sigma-1 antagonism can protect opioid receptors from the tonic inhibitory effects of sigma-1 receptors, thus enhancing opioid analgesia (12, 13). However, although the ability of sigma-1 antagonism to potentiate the analgesic effects of opioid drugs is clear, the physiological and pathophysiological roles of sigma-1 receptors in opioid modulation remain unknown.
The role of sigma-1 receptors in pathological pain, apart from neuropathic pain, has been less well explored, but recent reports have shown that sigma-1 antagonism can ameliorate inflammatory hyperalgesia (14). Immune cells that infiltrate inflamed tissue produce and release algogenic chemicals that participate in the sensitization of nociceptors; thus, immune cells promote pain during inflammation (15). These immune cells can also produce endogenous opioid peptides (EOPs) (16), but despite the analgesic potential of these EOPs, the end result of inflammation is usually pain. It is unknown whether sigma-1 receptors curtail the antinociceptive effects of EOPs during inflammation and thereby facilitate inflammatory pain.
In light of these antecedents, the aim of this study was to explore whether the mechanisms underlying the antihyperalgesic effects induced by sigma-1 antagonism during inflammation involve the disinhibition of these endogenous opioidergic mechanisms in the periphery. If this were the case, it would constitute an innovative mechanism of analgesia that might expand the therapeutic potential of sigma-1 antagonists.
Results and Discussion
Effects of Sigma-1 Antagonists on Acute Inflammatory Hyperalgesia Are Sensitive to Opioid Antagonism.
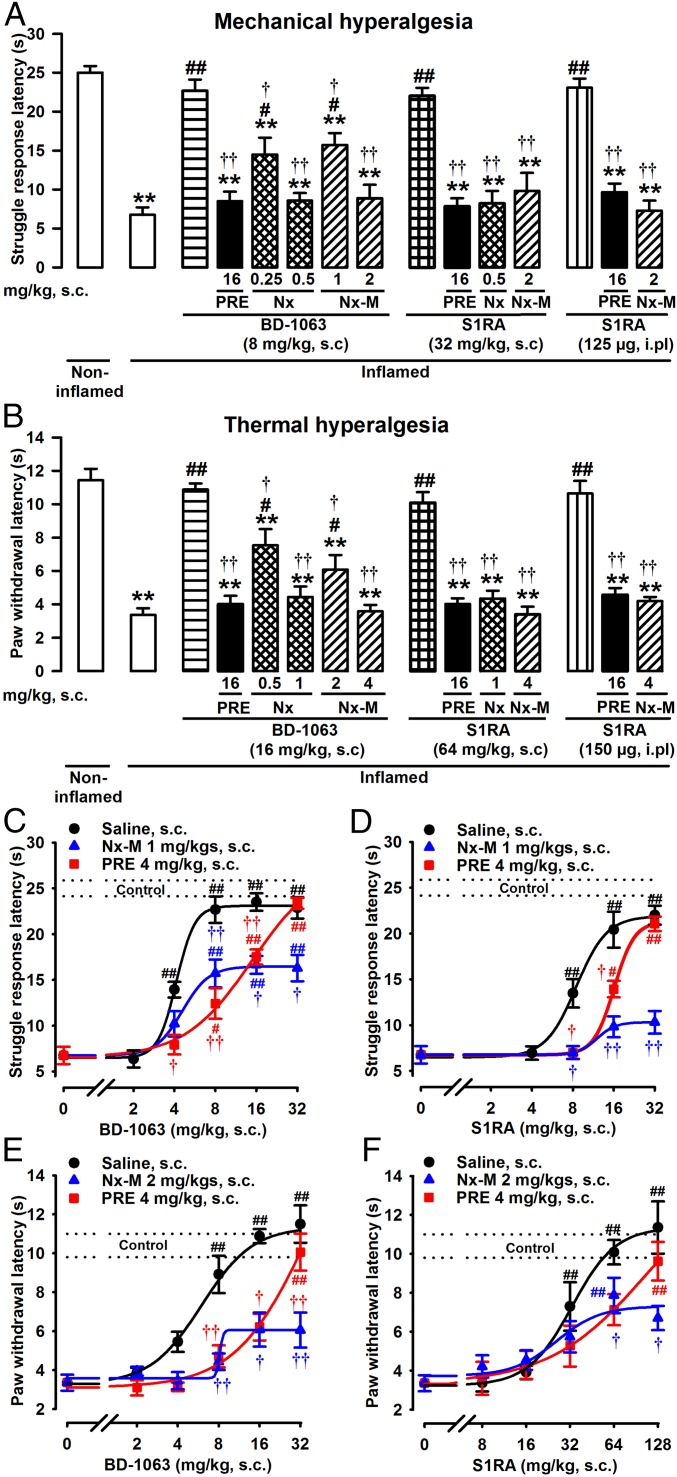
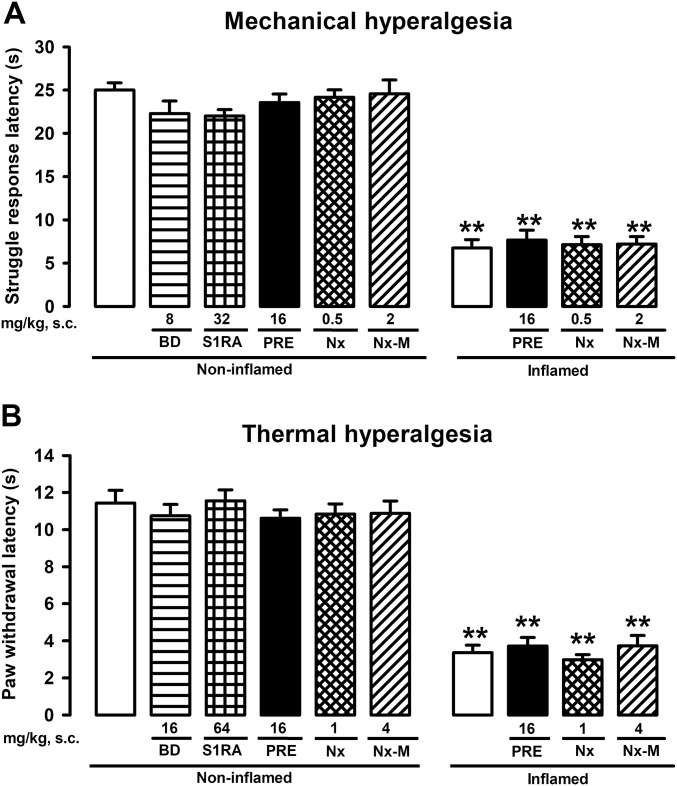
Mice showed a significant decrease in the struggle response latency to mechanical pressure 3 h after carrageenan-induced acute inflammation (Fig. 1A) as well as a decrease in paw withdrawal latency to radiant heat (Fig. 1B). These results indicate the development of inflammatory mechanical and thermal hyperalgesia, respectively. The systemic administration of sigma-1 antagonists BD-1063 and S1RA fully attenuated this inflammatory hyperalgesia to mechanical and thermal stimuli (Fig. 1 A and B), at doses that did not modify the latency to mechanical or thermal stimulation in mice without inflammation (Fig. S1 A and B). These results agree with both the known absence of effects of sigma-1 antagonism on nociceptive pain induced by mechanical or thermal stimuli (9, 17) and the recently reported amelioration of inflammatory hyperalgesia by sigma-1 antagonism (14). Although the results for thermal and mechanical hyperalgesia were qualitatively equivalent, the doses of sigma-1 antagonists required to fully reverse thermal hypersensitivity were higher than those needed to reverse mechanical hyperalgesia (Fig. 1 A and B, respectively) and are consistent with our previous study (14). In contrast to the sigma-1 antagonists, the selective sigma-1 agonist PRE-084 had no effect on the response latency in mice without inflammation or in mice with inflammation stimulated with either pressure or heat (Fig. S1 A and B, respectively) but abolished the inhibitory effects of BD-1063 and S1RA on mechanical and thermal hyperalgesia (Fig. 1 A and B). These results favor the involvement of sigma-1 receptors in the antihyperalgesic effects of both drugs.
Fig. 1.
Reversion of the antihyperalgesic effects of sigma-1 antagonists by both opioid antagonism and sigma-1 agonism during acute inflammation. Mice were evaluated 3 h after i.pl. injection with carrageenan (inflamed) or saline (noninflamed). Sigma-1 antagonists were administered either s.c. or i.pl.; PRE-084 (PRE), naloxone (Nx), and naloxone methiodide (Nx-M) were administered s.c. Effects induced by a single dose of sigma-1 antagonists BD-1063 (BD) or S1RA on hyperalgesia to mechanical (A) or thermal (B) stimuli and reversion by sigma-1 agonist PRE and opioid antagonists Nx and Nx-M. Dose–response curves of the effects induced by BD-1063 (C) and S1RA (D) on carrageenan-induced mechanical hyperalgesia and by BD-1063 (E) and S1RA (F) on inflammatory thermal hyperalgesia. Sigma-1 antagonists were administered alone or with fixed doses of PRE or Nx-M, or solvent controls. The dashed lines (control) represent the mean ± SEM in mice without inflammation. Bars or points show means ± SEM from 8 to 10 animals. **P < 0.01, mice without vs. mice with inflammation (for clarity these comparisons are omitted in C–F); #P < 0.05, ##P < 0.01, mice with inflammation treated with sigma-1 antagonists vs. mice with inflammation treated with solvent controls; †P < 0.05, ††P < 0.01, mice with inflammation treated with sigma-1 antagonist alone vs. mice with inflammation treated with PRE, Nx, or Nx-M; one-way ANOVA followed by Bonferroni test.
Fig. S1.
Absence of effects of sigma-1 agonism and opioid antagonism in mice with or without inflammation. Mice were injected i.pl. with carrageenan (inflamed) or saline (noninflamed) and s.c. with sigma-1 agonist PRE-084, opioid antagonists naloxone and naloxone methiodide, or solvent controls and subjected to mechanical (A) or thermal (B) stimulation. Bars show means ± SEM from 8 to 10 animals. **P < 0.01, mice without vs. mice with inflammation; no significant differences between values from mice without inflammation treated with the drugs or their solvent or between the values from mice with inflammation treated with the drugs or their solvent; one-way ANOVA followed by Bonferroni test.
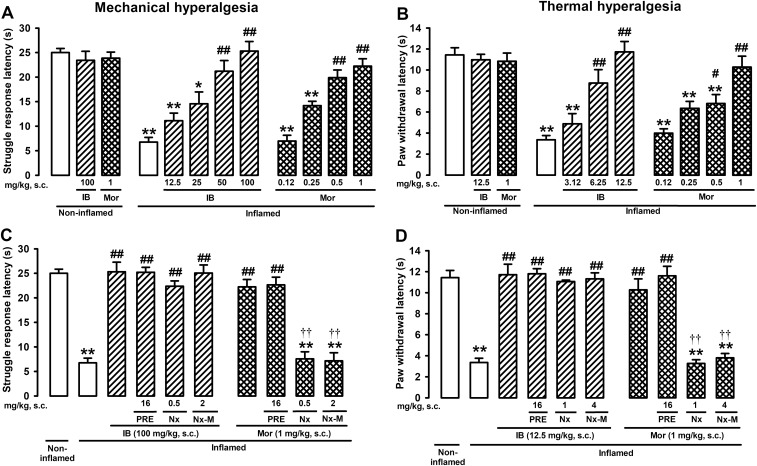
Interestingly, the ameliorative effects induced by BD-1063 or S1RA on inflammatory hyperalgesia to mechanical or thermal stimuli were also reversed by the opioid antagonists naloxone and naloxone methiodide (Fig. 1 A and B). To control for the specificity of the effects induced by these opioid antagonists, experiments in which antihyperalgesic effects were induced with the nonsteroidal anti-inflammatory drug ibuprofen and the opioid agonist morphine were conducted. Both ibuprofen and morphine induced dose-dependent antihyperalgesic effects to mechanical and thermal stimuli (Fig. S2 A and B). Naloxone and naloxone methiodide reversed the antihyperalgesic effects induced by morphine (at the same doses that reversed the effects of the sigma-1 antagonists) but had no effect on ibuprofen-induced antihyperalgesia (Fig. S2 C and D). These results indicate that the opioid antagonists used in this study are unable to reverse the analgesic effects induced by drugs without opioid action. This is a report of a reversion by opioid antagonism of the ameliorative effects of sigma-1 antagonists on pathological pain.
Fig. S2.
Opioid antagonism reverses the antihyperalgesic effect induced by morphine but not the effect induced by ibuprofen. Inhibitory effects of ibuprofen (IB) and morphine (Mor) on mechanical (A) and thermal (B) inflammatory hyperalgesia. Effects of sigma-1 agonist PRE-084 (PRE) or opioid antagonists naloxone (Nx) or naloxone methiodide (Nx-M) with IB or Mor on mechanical (C) or thermal (D) hyperalgesia. Mice were injected i.pl. with carrageenan (inflamed) or saline (noninflamed) and s.c. with the drugs or solvent controls. Bars show means ± SEM from 8 to 10 animals. *P < 0.05, **P < 0.01, mice without vs. mice with inflammation; #P < 0.05, ##P < 0.01, mice with inflammation treated with IB or Mor vs. mice with inflammation treated with solvent controls; ††P < 0.01, mice treated with Nx or Nx-M vs. mice treated with solvent controls, among those mice with inflammation and treated with Mor; one-way ANOVA followed by Bonferroni test.
In contrast to the known central penetrability of naloxone, naloxone methiodide (a quaternary derivative of naloxone) is unable to cross the blood–brain barrier and is therefore a peripherally restricted opioid antagonist (18). Naloxone methiodide fully reversed the antihyperalgesic effects induced by systemic sigma-1 antagonists, highlighting the importance of peripheral actions on the analgesic effects of these drugs. In fact, the intraplantar (i.pl.) administration of S1RA to the inflamed site fully reversed the observed hyperalgesia to mechanical and thermal stimuli, and these effects were also reversed by PRE-084 and by the peripherally restricted drug naloxone methiodide (Fig. 1 A and B).
Effects of Sigma-1 Antagonists on Acute Inflammatory Hyperalgesia Are Noncompetitively Inhibited by Peripheral Opioid Antagonism.
To determine whether the inhibition by PRE-084 or naloxone methiodide of the antihyperalgesic effects induced by BD-1063 or S1RA on carrageenan-induced acute inflammation was of a competitive or noncompetitive nature, we tested whether the effects of fixed doses of PRE-084 or naloxone methiodide were overcome by increasing doses of sigma-1 antagonists (19). Inhibition by PRE-084 of the antihyperalgesic effects induced by BD-1063 or S1RA, to either mechanical (Fig. 1 C and D) or thermal (Fig. 1 E and F) stimuli, was fully overcome by increasing the dose of sigma-1 antagonists. This indicates a competitive interaction between PRE-084 and both sigma-1 antagonists. These results agree with the pharmacological profile of these drugs, as it is known that PRE-084, BD-1063, and S1RA bind to sigma-1 receptors (17, 20).
In contrast, increasing the dose of BD-1063 or S1RA was unable to overcome the inhibition by naloxone methiodide of the antihyperalgesic effects of these sigma-1 antagonists to mechanical (Fig. 1 C and D) or thermal (Fig. 1 E and F) stimuli, indicating that naloxone methiodide inhibits the effects of the sigma-1 antagonists in a noncompetitive manner. Naloxone methiodide does not bind to sigma-1 receptors (10), and conversely, the sigma-1 antagonists BD-1063 and S1RA do not bind to opioid receptors (9, 17). However, sigma-1 receptors can form macromolecular complexes with opioid receptors to produce tonic inhibition of receptor functioning (12, 13). Sigma-1 antagonism is well known to increase opioid agonist-induced signaling, resulting in the potentiation of opioid analgesia by sigma-1 antagonists (12, 13). Therefore, a possible explanation for the sensitivity of sigma-1–mediated antihyperalgesic effects to opioid antagonism is that naloxone methiodide antagonizes peripheral opioid receptors, thereby impeding the action of endogenous opioid agonists produced at the site of inflammation (whose action is maximized by sigma-1 antagonism), resulting in noncompetitive inhibition of the antihyperalgesic effects induced by sigma-1 antagonism. This hypothesis necessarily implies that during inflammation, the production of EOPs that can be modulated by sigma-1 receptors is increased at the site of inflammation.
Sigma-1 Antagonism and EOPs During Acute Inflammation.
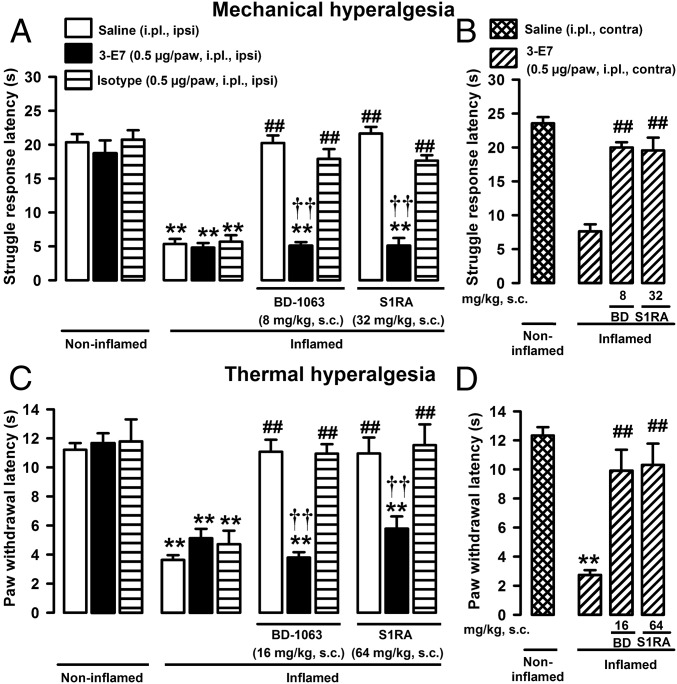
The effects of inhibiting the action of EOPs on the antihyperalgesic effects induced by sigma-1 antagonists were investigated. The actions of EOPs at the inflamed site were neutralized by local administration of the monoclonal antibody 3-E7, which recognizes the panopioid sequence Tyr–Gly–Gly–Phe at the N terminus of most EOPs (21). I.pl. administration of 3-E7 (0.5 µg per paw) did not alter the behavioral responses to mechanical stimuli in mice with or without inflammation (Fig. 2A), indicating that even if EOPs were present in the inflamed paw, the levels were not sufficient to alleviate hyperalgesia under inflammatory conditions. However, administration of 3-E7 into the inflamed paw abolished the antihyperalgesic effects in response to mechanical stimuli induced by systemic administration of BD-1063 or S1RA (Fig. 2A). The same dose of an isotype control antibody did not alter the behavioral responses to mechanical stimuli in animals with or without inflammation regardless of whether they were treated or not with sigma-1 antagonists (Fig. 2A). The effect of 3-E7 antibody on the antihyperalgesic effect induced by sigma-1 antagonism was seen exclusively in the injected paw: Both BD-1063 and S1RA still induced maximal mechanical antihyperalgesia in the inflamed paw when the antibody was injected in the noninflamed paw (Fig. 2B). Identical results were seen for the effects of sigma-1 antagonists on thermal hyperalgesia: 3-E7 administration in the inflamed paw abolished the anthyperalgesic effects of BD-1063 and S1RA, without altering the responses to heat stimuli in control mice with or without inflammation, and the isotype control was without effect under all experimental conditions (Fig. 2C). Injection of 3-E7 in the paw contralateral to the site of inflammation also had no effect on the antihyperalgesia induced by sigma-1 antagonists, indicating again a local effect of this antibody (Fig. 2D). These results show that the antihyperalgesic effects of sigma-1 antagonists are due to the action of EOPs at the inflamed site.
Fig. 2.
EOPs at the inflamed site participate in the antihyperalgesic effects of sigma-1 antagonists. Mice were evaluated 3 h after i.pl. injection with carrageenan (inflamed) or saline (noninflamed). Animals were treated s.c. with sigma-1 antagonists (BD-1063 and S1RA) or solvent controls and subjected to mechanical (A and B) or thermal stimulation (C and D). Mice were also treated i.pl. with 3-E7 anti-EOP monoclonal antibody, its isotype control, or solvent control in the same paw (ipsi) as carrageenan or saline (A and C) or in the paw contralateral (contra) to carrageenan (B and D). All mice received sensory stimulation in the paw into which carrageenan or its solvent was injected. Bars show means ± SEM from 8 to 10 animals. **P < 0.01, mice without inflammation treated with the solvent of the drugs or antibodies vs. mice with inflammation; ##P < 0.01, mice with inflammation treated with sigma-1 antagonists vs. mice with inflammation treated with solvent control; ††P < 0.01, mice with inflammation treated with a sigma-1 antagonist alone vs. mice with inflammation treated with 3-E7 antibody in the inflamed paw; one-way ANOVA followed by Bonferroni test.
Since the early 1990s, sigma-1 antagonists have been known to potentiate the analgesic effects of opioid drugs, and it was therefore suggested that an antiopioid sigma-1 system tonically inhibits opioid drug-induced analgesia in the central nervous system (7). Our data show that peripheral tonic inhibition of opioid analgesia is produced physiologically during inflammation by sigma-1 receptors, limiting the ability of EOPs to induce endogenous opioid analgesia, thereby facilitating inflammatory pain.
Neutrophils and EOPs.
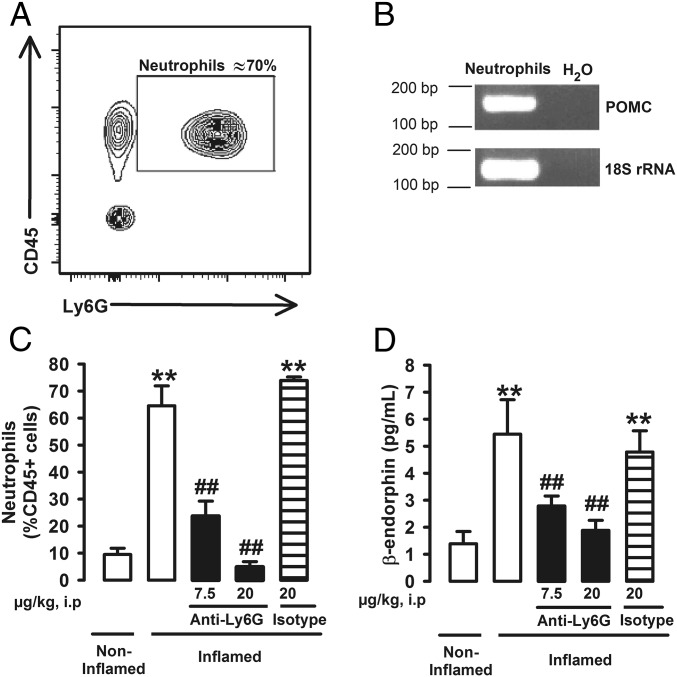
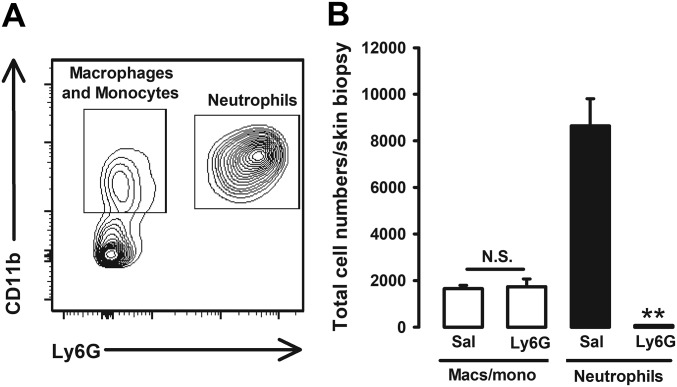
We wanted to identify the source of the endogenous opioid agonists responsible for the antihyperalgesic effects induced by sigma-1 antagonism. Immune cells are known to produce and secrete EOPs (16, 22), and they naturally accumulate at inflamed sites. Fluorescence-activated cell sorting (FACS) with cell-specific markers in tissue from the inflamed paw was used to determine the predominant types of hematopoietic cells (CD45+ cells) in the paw during carrageenan-induced acute inflammation. Neutrophils (CD45+Ly6G+ cells) constituted the majority (about 70%) of hematopoietic cells in the inflamed paws 3 h after carrageenan administration (Fig. 3A), as expected in acute inflammation. Macrophages/monocytes (CD45+CD11b+Ly6G– cells) were also present but to a lesser extent (about 10% CD45+ cells) (Fig. S3A). As neutrophils were the predominant type of myeloid cell in the inflamed paw and β-endorphin is known to be produced by these immune cells (23), we determined whether neutrophils were able to produce this EOP under our experimental conditions. We found that neutrophils express pro-opiomelanocortin (POMC) mRNA (Fig. 3B), the precursor of β-endorphin (16). Ly6G is selectively present in neutrophils and is needed for migration and recruitment of these immune cells (24). The actions of Ly6G can be inhibited in vivo by the systemic administration of an anti-Ly6G antibody (24). The in vivo administration of anti-Ly6G antibody (7.5–20 µg) resulted in complete, dose-dependent inhibition of neutrophil infiltration in the inflamed paw, whereas the administration of its isotype control (20 µg) had no effect on neutrophil levels (Fig. 3C). However, treatment with anti-Ly6G had no impact on macrophage/monocyte infiltration in the inflamed tissue (Fig. S3B), indicating the specificity of this approach to reduce neutrophil levels. Mice were found to have increased β-endorphin in the inflamed paw, and in vivo administration of anti-Ly6G dose-dependently reduced the levels of this EOP, whereas the isotype control antibody had no effect (Fig. 3D). These results mirrored the effects of neutrophil depletion in response to anti-Ly6G administration and suggest that neutrophils contribute to the production of this EOP in carrageenan-induced acute inflammation. β-endorphin is thought to be the predominant EOP produced by immune cells (16), and consequently, here we tested its levels to exemplify that under our experimental conditions immune cells can produce EOPs. However, leukocytes have also been shown to produce enkephalins and dynorphins (22), and they might also play a role in our results. We therefore hypothesize that EOP production by neutrophils may participate in the naloxone-sensitive antihyperalgesic effects of sigma-1 antagonists during acute inflammation.
Fig. 3.
Neutrophils contribute to the production of β-endorphin in the carrageenan-injected paw during acute inflammation. (A) Representative FACS diagram showing CD45+Ly6G+ cells from the inflamed paw, corresponding to neutrophils. (B) Real-time PCR products for POMC mRNA and 18S ribosomal RNA (18S rRNA) as an internal standard (predicted band sizes 165 bp and 133 bp, respectively) from FACS-purified neutrophils. (C) Effects of in vivo treatment with anti-Ly6G on the population of neutrophils in the inflamed paw, determined by FACS. (D) Effects of in vivo treatment with anti-Ly6G on β-endorphin levels in the inflamed paw, measured by fluorescent enzyme immmunoassay. Mice were treated i.p. with anti-Ly6G antibody, solvent control, or isotype control antibody and injected i.pl. with carrageenan (inflamed) or saline (noninflamed) 3 h before obtaining the samples. Graphs show means ± SEM from n = 6–10 determinations. **P < 0.01, mice without vs. mice with inflammation; ##P < 0.01, mice with inflammation treated with anti-Ly6G vs. mice with inflammation treated with solvent control.
Fig. S3.
The in vivo treatment with anti-Ly6G decreases neutrophil load in the paw during carrageenan-induced acute inflammation without altering the number of macrophages/monocytes. (A) Representative FACS diagram of CD45+ cell populations showing that neutrophils CD11b+Ly6G+ are the most abundant leukocytes in the inflamed paw 3 h after carrageenan injection, whereas macrophages/monocytes (CD11b+Ly6G–) constitute a minor population. (B) Effects of in vivo treatment with anti-Ly6G on neutrophils and macrophages/monocytes (macs/mono) in the inflamed paw. Mice were treated intraperitoneally with anti-Ly6G antibody or its solvent control and injected i.pl. with carrageenan 3 h before obtaining the samples. Graph shows means ± SEM from n = 4 determinations. **P < 0.01, number of neutrophils in mice treated with anti-Ly6G vs. solvent control. There were no statistical differences (N.S.) between the number of macrophages from mice treated with anti-Ly6G vs. solvent control; one-way ANOVA followed by Bonferroni test.
Neutrophils, Edema, and Hyperalgesia.
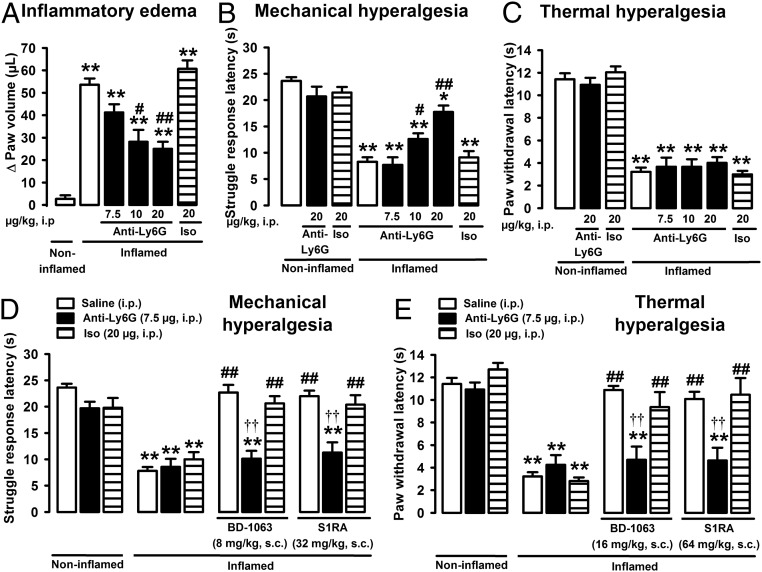
Although neutrophils (and other immune cells) can produce EOPs and therefore may participate in decreasing pain during inflammation (16), it is conventionally accepted that they promote pain by synthesizing and releasing algogenic chemicals (15) and also participate in the development of edema (24). In turn, edema can increase pressure on nociceptive nerve endings also involved in pain during inflammation (25). We therefore tested the effects of anti-Ly6G treatment on inflammatory edema and hyperalgesia. Carrageenan induced prominent edema 3 h after its administration, which was monitored as the increase in volume of the injected paw (Fig. 4A). This edema was decreased in a dose-dependent manner by anti-Ly6G treatment, although only partly and at high doses (10–20 µg), whereas the isotype control antibody (20 µg) lacked effect (Fig. 4A). These results are in agreement with previous reports, where high doses of anti-Ly6G antibody were needed to ameliorate inflammatory edema (24). Neither treatment with anti-Ly6G nor the isotype control modified the response latencies in mice without inflammation subjected to mechanical or thermal stimuli (Fig. 4 B and C, respectively), indicating that neutrophils do not play a role in acute nociception to either type of stimulus. Treatment with anti-Ly6G, but not with the isotype control, increased the response latency to mechanical stimuli in mice with inflammation (Fig. 4B) at doses that decreased edema, whereas neither anti-Ly6G nor the isotype control antibody had an effect on thermal hyperalgesia at any dose tested (Fig. 4C). Peripheral sensory neurons are specialized in detecting specific sensory stimuli, and therefore, the mechanisms for thermal and mechanical nociception are not fully overlapping (25). Our data suggest that neutrophils may participate in the development of mechanical hyperalgesia by promoting edema, with a consequent increase in the stimulation of pressure-sensitive nociceptors, but that other sources of algogenic chemicals apart from these immune cells account for carrageenan-induced thermal hypersensitivity.
Fig. 4.
Neutrophils contribute to both inflammatory hyperalgesia and the antihyperalgesic effects of sigma-1 antagonists during acute inflammation. Effect of the administration of anti-Ly6G antibody on inflammatory edema (A), mechanical hyperalgesia (B), thermal hyperalgesia (C), and on the effects of sigma-1 antagonists on mechanical (D) and thermal (E) hyperalgesia. Mice were treated i.p. with anti-Ly6G, solvent control or isotype (iso) control, and injected i.pl. with carrageenan (inflamed) or saline (noninflamed) 3 h before the evaluation (A–E). Mice were treated s.c. with sigma-1 antagonists (BD-1063 and S1RA) or solvent controls (D and E). Bars show means ± SEM from 8 to 10 animals. *P < 0.05, **P < 0.01, mice without inflammation treated with solvent controls or antibodies vs. mice with inflammation; #P < 0.05, ##P < 0.01, mice with inflammation treated with anti-Ly6G or sigma-1 antagonists alone vs. mice with inflammation treated with solvent control; ††P < 0.01, mice with inflammation treated with a sigma-1 antagonist alone vs. with inflammation treated with anti-Ly6G antibody; one-way ANOVA followed by Bonferroni test.
Influence of Neutrophils on the Effects Induced by Sigma-1 Antagonists.
The influence of neutrophils on the antihyperalgesic effects induced by sigma-1 antagonists during carrageenan-induced acute inflammation was then explored. For these experiments, a submaximal dose of anti-Ly6G (7.5 µg), which was enough to markedly decrease neutrophil infiltration at the inflamed site without significantly altering inflammatory edema or the behavioral responses of mice with or without inflammation to mechanical or thermal stimuli (Fig. 4 D and E, respectively), was used. This dose of anti-Ly6G abolished the ameliorative effects of BD-1063 and S1RA on inflammatory mechanical and thermal hypersensitivity (Fig. 4 D and E, respectively), whereas a high dose of isotype control (20 µg) had no effect (Fig. 4 D and E). These results suggested that the observed effects were specific. Together, our findings show that the naloxone-sensitive antihyperalgesic effects induced by sigma-1 antagonism on carrageenan-induced acute inflammation require the presence of EOPs produced by neutrophils (which constitute the majority of the immune infiltrate) at the inflamed site.
Naloxone-Sensitive Effects of Sigma-1 Antagonists in Sustained Inflammation.
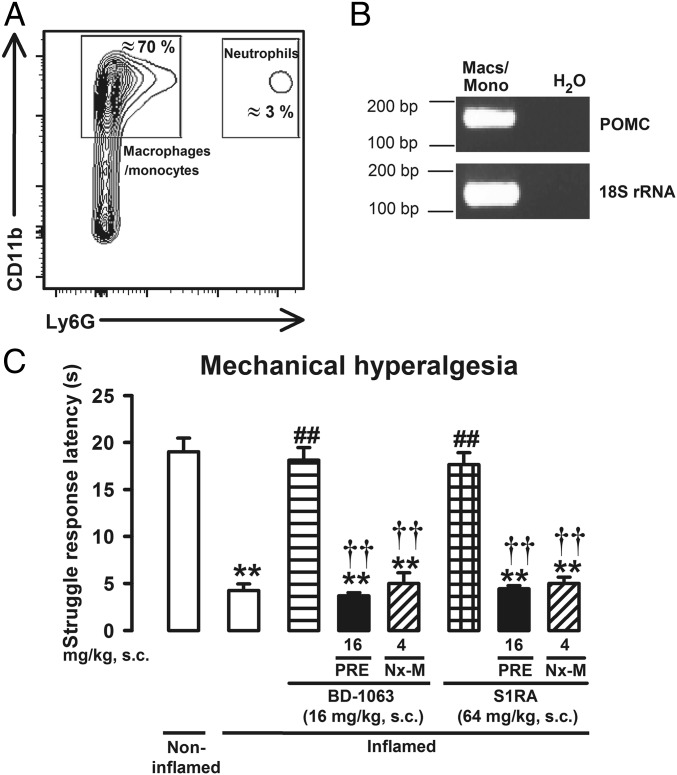
As the predominant immune cell types vary with the time course of the inflammation (21), we sought to determine whether the naloxone-sensitive antihyperalgesic effects of sigma-1 antagonists were preserved when the predominant myeloid cells during inflammation differ from neutrophils. The presence of neutrophils 5 d after carrageenan administration was almost negligible, whereas the presence of macrophages/monocytes (CD11b+Ly6G– cells) was largely increased, constituting the majority (about 70%) of CD45+ cells (Fig. 5A). Similar to neutrophils, macrophages/monocytes were found to express POMC mRNA (Fig. 5B). These data are consistent with previous reports showing that all immune cell subpopulations produce EOPs (22) and that distinct leukocyte lineages are the main source of these peptides at different stages of inflammation (21). Under this sustained inflammatory condition, animals showed a prominent mechanical hyperalgesia that was reversed by the sigma-1 antagonists S1RA and BD-1063 (Fig. 5C). The effects of these sigma-1 antagonists were abolished by both the sigma-1 agonist PRE-084 and the peripheral opioid antagonist naloxone methiodide (Fig. 5C), indicating that both sigma-1 receptors and peripheral opioid receptors are involved in the effects induced by these drugs during sustained inflammation and support that immune-driven peripheral opioid analgesia induced by sigma-1 antagonism is not limited to neutrophilic inflammation.
Fig. 5.
The peripheral opioid-dependent effects of sigma-1 antagonists are preserved during sustained inflammation. (A) Representative FACS blot of CD45+ cell populations in the inflamed paw: CD11b+Ly6G– (macrophages/monocytes) and CD11b+Ly6G+ (neutrophils). (B) Real-time PCR products for POMC mRNA and 18S ribosomal RNA (18S rRNA) as an internal standard (predicted band sizes 165 bp and 133 bp, respectively) from FACS-purified macrophages/monocytes (macs/mono). (C) Effects induced by the sigma-1 antagonists BD-1063 (BD) or S1RA on mechanical hyperalgesia. Mice were injected i.pl. with carrageenan (inflamed) or saline (noninflamed) 5 d before the evaluation and administered s.c. with the sigma-1 antagonists PRE-084 (PRE) and naloxone methiodide (Nx-M). Bars show means ± SEM from 8 to 10 animals. **P < 0.01, mice without vs. mice with inflammation; ##P < 0.01, mice with inflammation treated with sigma-1 antagonists vs. mice with inflammation treated with solvent controls; ††P < 0.01, mice with inflammation treated with sigma-1 antagonist alone vs. mice with inflammation treated with PRE or Nx-M; one-way ANOVA followed by Bonferroni test.
Effects of Sigma-1 Antagonism on Formalin-Induced Pain.
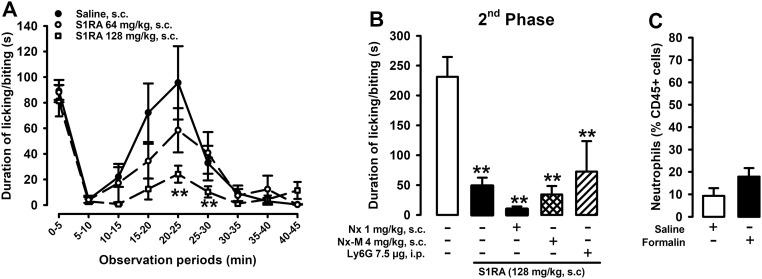
We also tested whether the ameliorative effects of sigma-1 antagonism in other pain models also involve the actions of EOPs of immune origin. S1RA dose-dependently decreased the second phase of formalin-induced pain (Fig. S4A), as previously described for this and other sigma-1 antagonists (3). However, the administration of opioid antagonists or anti-Ly6G did not modify the antinociceptive effects of S1RA (Fig. S4B). Interestingly, at the peak of the nociceptive behaviors (20–25 min), formalin was unable to recruit neutrophils to the injected paw (Fig. S4C). These results suggest that sigma-1 antagonism requires the presence of immune cells harboring EOPs to induce their opioid-dependent effects but that this is not the only mechanism used by sigma-1 antagonists to ameliorate pain. Our results are consistent with previous findings that the ameliorative effects of nonselective sigma-1 antagonists (i.e., haloperidol and its metabolites) in behavioral models involving central sensitization (such as the second phase of formalin-induced pain or capsaicin-induced secondary mechanical hypersensitivity) are not reversed by naloxone (26, 27). The sigma-1 receptor is a ligand-regulated chaperone that participates in pain neurotransmission through multiple pathways (3). Although we show here that the predominant mechanism of action of sigma-1 antagonists in ameliorating inflammatory hyperalgesia involves the modulation of EOPs from immune cells at the site of inflammation, this is not necessarily the case in all pain conditions. Further research is needed to fully characterize the mechanisms involved in the actions of sigma-1 antagonists in different types of pain.
Fig. S4.
EOPs of immune origin do not play a role in the antinociceptive effects induced by S1RA in the formalin test. (A) Time course of pain-like responses (duration of licking or biting the affected paw) induced by the i.pl. injection of formalin (2.5%) in mice treated s.c. with S1RA or solvent control. **P < 0.01, mice treated with S1RA vs. mice treated with solvent control; two-way repeated measures ANOVA followed by Bonferroni test. (B) Effects of administration of S1RA (s.c.) with the administration of naloxone (Nx; s.c.) or naloxone methiodide (Nx-M; s.c.), and administration of anti-Ly6G antibody (i.p.) on the second phase of formalin-induced pain (15–35 min after injection). **P < 0.01, mice treated with S1RA vs. mice treated with solvent control; no significant differences between mice treated with S1RA alone vs. mice treated with S1RA and other agents were observed; one-way ANOVA followed by Bonferroni test. (C) Effects of formalin on neutrophil levels (CD45+Ly6G+ cells) in the injected paw, determined by FACS. No significant differences between mice treated with formalin vs. mice treated with saline were observed; unpaired Student’s t test. Bars or points show means ± SEM from 8 to 10 animals.
Conclusions
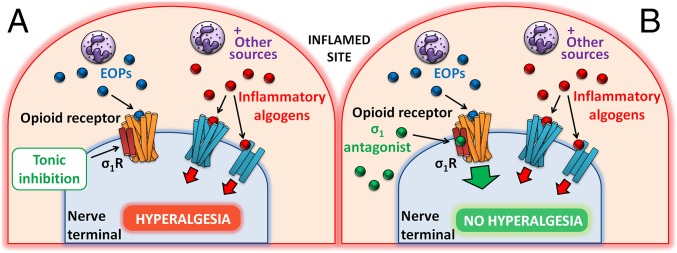
Peripheral sigma-1 receptors constitute a biological brake to immune-driven opioid analgesia during inflammatory conditions in which immune cells and other sources of algogenic chemicals promote inflammatory pain (Fig. 6A). This biological brake to opioid antinociception can be released pharmacologically by sigma-1 antagonists, which promote opioid analgesia at the site of inflammation by the disinhibition of the effects of EOPs of immune origin (Fig. 6B). This mechanism, which maximizes the analgesic potential of immune cells that naturally accumulate in painful inflamed sites, differs from that of conventional analgesics. Our findings suggest that sigma-1 antagonists merit further research as potential agents for the treatment of inflammatory pain.
Fig. 6.
Proposed mechanism of action for the effects of sigma-1 antagonism on inflammatory hyperalgesia. (A) Immune cells (for instance, neutrophils) infiltrating the inflamed paw (and other sources) release algogenic chemicals that sensitize nociceptors but also EOPs. These EOPs of immune origin do not relieve inflammatory pain, because of tonic inhibition of opioid functioning by sigma-1 receptors (σ1R). The balance between the effects of algogenic chemicals and EOPs favors increased sensitivity to pain characteristic of inflammation. (B) Sigma-1 antagonists protect opioid receptors from the tonic inhibition induced by sigma-1 receptors, potentiating the effects of EOPs of immune origin and producing opioid-mediated antihyperalgesic effects during inflammation.
Materials and Methods
Details of our methods and providers are shown in SI Materials and Methods and are outlined here. Animal protocols were approved by regional (Junta de Andalucía) and institutional (Research Ethics Committee of the University of Granada, Spain) authorities.
Carrageenan-Induced Inflammation.
Paw inflammation was induced with an i.pl. injection of carrageenan (14) to female CD-1 mice. Paw edema was measured with a plethysmometer (14). Experiments were performed during acute (3 h) or sustained (5 d) inflammation.
Drugs and Antibodies.
We tested the effects of the sigma-1 antagonists BD-1063 and S1RA (17, 20) and the sigma-1 agonist PRE-084 (20). The opioid antagonists used were the centrally penetrant naloxone hydrochloride and its peripherally restricted analog naloxone methiodide (18). The 3-E7 monoclonal antibody, which recognizes the panopioid sequence Tyr–Gly–Gly–Phe at the N terminus of most EOPs (21), was administered i.pl. to block the effects of the EOPs. An anti-Ly6G antibody was administered intraperitoneally to inhibit neutrophil infiltration (24).
Behavioral Experiments.
Mechanical and thermal hyperalgesia were assessed using the paw pressure test and the Hargreaves test (14). Formalin-induced pain was assessed by injecting i.pl. a formalin solution (26). Paw samples were collected for FACS analysis.
β-endorphin Levels in the Paw.
β-endorphin levels were determined in the soft tissue from the paws by a fluorescent enzyme immunoassay.
FACS Analysis.
We used antibodies recognizing CD45 (hematopoietic cells), Ly6G (neutrophils), and CD11b (myeloid cells). The population of macrophages/monocytes was determined by using the combination of these two last markers (CD11b+Ly6G– cells).
PCR Analysis.
Transcripts encoding POMC and 18S ribosomal RNA (as an internal standard) were amplified using real-time PCR in samples from FACS-purified neutrophils and macrophages/monocytes.
SI Materials and Methods
Experimental Animals.
All experiments were performed in female CD-1 mice (Charles River) weighing 25–30 g. Mice were housed in a temperature-controlled room (22 ± 2 °C) with an automatic 12-h light/dark cycle (08:00–20:00 h). Mice were allowed to acclimatize to their environment for at least 4 d before testing. The animals were fed a standard laboratory diet (Harlan Teklad Research Diet) and tap water ad libitum. Experiments were performed during the light phase (from 9:00 AM to 3:00 PM) and at random times throughout the estrous cycle. For the in vitro experiments, animals were killed by cervical dislocation before collecting tissue samples. Animal care was in accordance with institutional (Research Ethics Committee of the University of Granada, Spain), regional (Junta de Andalucía), and international standards (European Communities Council Directive 2010/63).
Carrageenan-Induced Inflammation.
Paw inflammation was induced with an i.pl. injection of a carrageenan solution (50 µL, 1% wt/vol in saline; Sigma-Aldrich). Carrageenan solution was freshly prepared on the day and injected into s.c. tissue of the plantar surface of the right hindpaw using a Hamilton microsyringe with a 301/2-gauge needle. The control groups were similarly injected with saline (24). Experiments were performed during acute (3 h) or sustained (5 d) inflammation induced by carrageenan.
Drugs and Antibodies.
The sigma-1 receptor antagonists used were BD-1063 (1-[2-(3,4-dichlorophenyl)ethyl]-4-methylpiperazine dihydrochloride; Tocris Cookson Ltd.) and S1RA (4-[2-[[5-methyl-1-(2-naphthalenyl)1H-pyrazol-3-yl] oxy] ethyl] morpholine hydrochloride; kindly supplied by Laboratorios Esteve). Both sigma-1 antagonists are considered to be selective for sigma-1 receptors (9, 27). PRE-084 (2-[4-morpholinethyl]1-phenylcyclohexanecarboxylate hydrochloride; Tocris Cookson) was used as a selective sigma-1 receptor agonist (27). The doses used of these drugs were chosen based on our previous study (24). The opioid antagonists used were centrally penetrant naloxone hydrochloride and its peripherally restricted analog naloxone methiodide (25) (both from Sigma-Aldrich). To study the effects of systemic treatments, drug solutions (5 mL/kg) were administered s.c. into the interscapular zone. To test the effect of local drug treatments, drug solutions (20 μL) were administered i.pl.
To block the effects of the EOPs produced during the inflammatory process, 20 µL of a solution containing 3-E7 monoclonal antibody, which recognizes the pan-opioid sequence Tyr–Gly–Gly–Phe at the N terminus of most EOPs (28) (MAB5276; Merck Millipore), was administered i.pl. An anti-Ly6G antibody (BE0075-1; Bio X Cell) was administered intraperitoneally (10 mL/kg) to inhibit neutrophil infiltration (30). Saline injections and the administration of nonreactive isotype antibodies (obtained from the same providers as the active antibodies) were used as a control for the effects of 3-E7 (PP102; Merck Millipore) or anti-Ly6G (BE0089; Bio X Cell).
Sigma-1 antagonists or 3-E7 antibody (or their solvents/isotype control) were administered 30 min before the evaluation. For the sigma-1 antagonists with 3-E7 antibody studies, the sigma-1 antagonist was administered first and the 3-E7 (or its isotype control) immediately after. To test for the effects of sigma-1 antagonists with PRE-084, naloxone or naloxone methiodide (or their solvent), the sigma-1 agonist, or the opioid antagonists were injected s.c. 5 min before the sigma-1 antagonist solution (or solvent). Anti-Ly6G or its isotype control was injected 24 h before inflammation was induced. Saline injections by the same routes as the drugs or antibodies tested were used as controls.
Mechanical Hyperalgesia.
Mice were gently restrained, and a blunt cone-shaped paw-presser (Model 37215; Ugo-Basile) was applied at a constant intensity of 100 g to the dorsal surface of the hindpaw until the animal showed a struggle response. Immediately thereafter, the stimulus was stopped, and the response latency was recorded. The test was performed twice with a 1-min interval between stimulations, and the average of the two trials was recorded (24).
Thermal Hyperalgesia.
Mice were habituated for 2 h to individual chambers placed on a glass floor at 30 °C. A beam of radiant heat (90 mW/cm2) was applied to the plantar surface of the right hindpaw with a plantar test apparatus (IITC), and paw withdrawal latencies were recorded. Withdrawal latencies were measured three times with at least a 30-s interval between consecutive measurements, and the latencies were averaged for each animal (24).
Formalin-Induced Pain.
The formalin test was performed as previously described (33). Formalin solution (20 μL, 2.5%) was injected i.pl. in the right hindpaw, using a Hamilton microsyringe with a 301/2-gauge needle. Immediately thereafter, the mouse was put into a glass cylinder and observed. The time spent licking or biting the injected paw during the 45-min period after the injection (divided into periods of 5 min each) was measured. The first phase of formalin-induced pain was recorded for 0–5 min after the injection. The second phase of pain was recorded for 15–35 min after injection. Pain-like responses in the second phase peaked at 20–25 min (see Results and Discussion for details), and paw samples were collected at this time for FACS analysis to determine the immune cell content of the paw tissue.
Paw Edema.
Hindpaw volume was measured with a plethysmometer (Ugo-Basile). The volume of edema was determined as the difference between the values obtained before and after i.pl. injection of carrageenan or its solvent (24).
β-Endorphin Levels in the Paw.
The paw was excised at the ankle, and the bones were removed. Soft tissue was homogenized in 1 mL RIPA buffer containing 0.05% protease inhibitors (P8340; Sigma-Aldrich) and 0.1% phosphatase inhibitors (P0044; Sigma-Aldrich). β-endorphin levels were determined with a fluorescent enzyme immunoassay kit (Phoenix Pharmaceuticals) according to the manufacturer’s instructions.
FACS Analysis.
Plantar tissue was dissected and digested with collagenase/DNase for 1 h at 37 °C (1 mg/mL collagenase IV and 0.1% DNase I; Worthington). Samples were filtered (pore size, 70 μm) and incubated with antibodies recognizing the hematopoietic cell marker CD45 (14-0451-85, clone 30-F11; eBioscience), the neutrophil-specific marker Ly6G (127602, clone 1A8; Biolegend), and the myeloid marker CD11b (101243, clone M1/70; Biolegend) for 1 h on ice. The population of macrophages/monocytes was determined by using the following markers: CD45+CD11b+Ly6G– cells. All antibodies were used at a concentration of 2 µg/mL. Cell viability was determined with DAPI (Sigma-Aldrich). Before and after incubation with the antibodies, the cells were washed three times in 1% FCS/PBS (FACS buffer). Samples were acquired with a BD LSRII flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (Treestar). Cells were pregated in a DAPI-negative population to analyze only living cells.
PCR Analysis.
FACS-purified neutrophils and macrophages/monocytes were sorted directly into TRIzol, and total RNA was extracted using phenol-chloroform method. Equal amounts of total RNA were added to the reverse transcriptase enzyme mix (iScript Reverse Transcription Supermix, BioRad). Templates and primers were added to SYBR Green enzyme mix (iQ SYBR Green Supermix, BioRad), and transcripts encoding POMC and 18S ribosomal RNA (18srRNA) (as an internal standard) were amplified using Real Time System (CFX Connect, BioRad). Reactions were run for 50 cycles, and the specificity was determined by subsequent melting curve analysis. PCR reactions were run on 2% agarose gel for determination of product size. The following forward and reverse primers (NCBI Primer Blast) were used for amplification: 5′-GGCCGTTCTTAGTTGGTGGAGCG-3′ and 5′-CTGAACGCCACTTGTCCCTC-3′ for 18srRNA (predicted band size, 133 bp), 5′-TAGCGGGAGAGAAAGCCGAG-3′ and 5′-TAGCGGGAGAGAAAGCCGAG-3′ for POMC (predicted band size, 165 bp).
Data Analysis.
All statistical analyses were carried out using one-way analysis of variance (ANOVA) followed by a Bonferroni post hoc test, except in Fig. S4A, where a two-way repeated measures ANOVA followed by Bonferroni test was used, and in Fig. S4C, where an unpaired Student’s t test was used. The differences between values were considered significant when the P value was <0.05. Data were analyzed with SigmaPlot 12.0 software (Systat Software Inc.).
Acknowledgments
The authors thank the reviewers at Mundipharma Research for their useful comments on and critical discussion of the manuscript as well as K. Shashok for editing the English in the manuscript. This work was supported by the Spanish Ministry of Economy and Competitiveness Grants SAF2013-47481P (to E.J.C.) and SAF2016-80540-R (to E.J.C. and J.M.B.), European Regional Development Funds (to E.J.C.), and the Research Program of the University of Granada (to M.A.T. and E.J.C.). This research was done in partial fulfillment of the requirements for the doctoral thesis of M.A.T.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620068114/-/DCSupplemental.
References
- 1.Kissin I. The development of new analgesics over the past 50 years: A lack of real breakthrough drugs. Anesth Analg. 2010;110:780–789. doi: 10.1213/ANE.0b013e3181cde882. [DOI] [PubMed] [Google Scholar]
- 2.Su TP, Su TC, Nakamura Y, Tsai SY. The sigma-1 receptor as a pluripotent modulator in living systems. Trends Pharmacol Sci. 2016;37:262–278. doi: 10.1016/j.tips.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamanillo D, Romero L, Merlos M, Vela JM. Sigma 1 receptor: A new therapeutic target for pain. Eur J Pharmacol. 2013;716:78–93. doi: 10.1016/j.ejphar.2013.01.068. [DOI] [PubMed] [Google Scholar]
- 4.Vela JM, Merlos M, Almansa C. Investigational sigma-1 receptor antagonists for the treatment of pain. Expert Opin Investig Drugs. 2015;24:883–896. doi: 10.1517/13543784.2015.1048334. [DOI] [PubMed] [Google Scholar]
- 5.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10:895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abadias M, Escriche M, Vaqué A, Sust M, Encina G. Safety, tolerability and pharmacokinetics of single and multiple doses of a novel sigma-1 receptor antagonist in three randomized phase I studies. Br J Clin Pharmacol. 2013;75:103–117. doi: 10.1111/j.1365-2125.2012.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chien CC, Pasternak GW. Functional antagonism of morphine analgesia by (+)-pentazocine: Evidence for an anti-opioid sigma 1 system. Eur J Pharmacol. 1993;250:R7–R8. doi: 10.1016/0014-2999(93)90650-7. [DOI] [PubMed] [Google Scholar]
- 8.Mei J, Pasternak GW. Sigma1 receptor modulation of opioid analgesia in the mouse. J Pharmacol Exp Ther. 2002;300:1070–1074. doi: 10.1124/jpet.300.3.1070. [DOI] [PubMed] [Google Scholar]
- 9.Sánchez-Fernández C, et al. Potentiation of morphine-induced mechanical antinociception by σ1 receptor inhibition: Role of peripheral σ1 receptors. Neuropharmacology. 2013;70:348–358. doi: 10.1016/j.neuropharm.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 10.Sánchez-Fernández C, et al. Modulation of peripheral μ-opioid analgesia by σ1 receptors. J Pharmacol Exp Ther. 2014;348:32–45. doi: 10.1124/jpet.113.208272. [DOI] [PubMed] [Google Scholar]
- 11.Mavlyutov TA, et al. Sigma-1 receptor expression in the dorsal root ganglion: Reexamination using a highly specific antibody. Neuroscience. 2016;331:148–157. doi: 10.1016/j.neuroscience.2016.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim FJ, et al. Sigma 1 receptor modulation of G-protein-coupled receptor signaling: Potentiation of opioid transduction independent from receptor binding. Mol Pharmacol. 2010;77:695–703. doi: 10.1124/mol.109.057083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez-Muñoz M, et al. The σ1 receptor engages the redox-regulated HINT1 protein to bring opioid analgesia under NMDA receptor negative control. Antioxid Redox Signal. 2015;22:799–818. doi: 10.1089/ars.2014.5993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tejada MA, et al. Sigma-1 receptor inhibition reverses acute inflammatory hyperalgesia in mice: Role of peripheral sigma-1 receptors. Psychopharmacology (Berl) 2014;231:3855–3869. doi: 10.1007/s00213-014-3524-3. [DOI] [PubMed] [Google Scholar]
- 15.Ji RR, Xu ZZ, Gao YJ. Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. 2014;13:533–548. doi: 10.1038/nrd4334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hua S, Cabot PJ. Mechanisms of peripheral immune-cell-mediated analgesia in inflammation: Clinical and therapeutic implications. Trends Pharmacol Sci. 2010;31:427–433. doi: 10.1016/j.tips.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Romero L, et al. Pharmacological properties of S1RA, a new sigma-1 receptor antagonist that inhibits neuropathic pain and activity-induced spinal sensitization. Br J Pharmacol. 2012;166:2289–2306. doi: 10.1111/j.1476-5381.2012.01942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menéndez L, Lastra A, Meana A, Hidalgo A, Baamonde A. Analgesic effects of loperamide in bone cancer pain in mice. Pharmacol Biochem Behav. 2005;81:114–121. doi: 10.1016/j.pbb.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Rang HP, Dale MM, Ritter JM, Flower R. Rang and Dale's Pharmacology. 6th Ed. Churchill Livingstone; Edinburgh: 2007. How drugs act: General principles; pp. 8–24. [Google Scholar]
- 20.Cobos EJ, Entrena JM, Nieto FR, Cendán CM, Del Pozo E. Pharmacology and therapeutic potential of sigma(1) receptor ligands. Curr Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rittner HL, et al. Opioid peptide-expressing leukocytes: Identification, recruitment, and simultaneously increasing inhibition of inflammatory pain. Anesthesiology. 2001;95:500–508. doi: 10.1097/00000542-200108000-00036. [DOI] [PubMed] [Google Scholar]
- 22.Rittner HL, Stein C. Involvement of cytokines, chemokines and adhesion molecules in opioid analgesia. Eur J Pain. 2005;9:109–112. doi: 10.1016/j.ejpain.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Sahbaie P, Li X, Clark JD. Roles of Gr-1+ leukocytes in postincisional nociceptive sensitization and inflammation. Anesthesiology. 2012;117:602–612. doi: 10.1097/ALN.0b013e3182655f9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JX, et al. Ly6G ligation blocks recruitment of neutrophils via a β2-integrin-dependent mechanism. Blood. 2012;120:1489–1498. doi: 10.1182/blood-2012-01-404046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Julius D, Basbaum AI. Molecular mechanisms of nociception. Nature. 2001;413:203–210. doi: 10.1038/35093019. [DOI] [PubMed] [Google Scholar]
- 26.Cendán CM, Pujalte JM, Portillo-Salido E, Baeyens JM. Antinociceptive effects of haloperidol and its metabolites in the formalin test in mice. Psychopharmacology (Berl) 2005;182:485–493. doi: 10.1007/s00213-005-0127-z. [DOI] [PubMed] [Google Scholar]
- 27.Entrena JM, et al. Antagonism by haloperidol and its metabolites of mechanical hypersensitivity induced by intraplantar capsaicin in mice: Role of sigma-1 receptors. Psychopharmacology (Berl) 2009;205:21–33. doi: 10.1007/s00213-009-1513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]