Significance
d-xylose is the most abundant pentose in nature and is considered an attractive carbon source for bio-based fuel/chemical production through fermentation. The first step of d-xylose utilization, cross-membrane sensing, is mediated by the membrane complex LytS-XylFII in solventogenic clostridia, the mechanism of which remains unclear. We found that the perception of the environmental d-xylose by the LytS-XylFII complex is initiated through the d-xylose–specific binding to XylFII. Binding of d-xylose to XylFII induces the inactive LytS-XylFII heterodimer to form an active heterotetramer and transmits the signal to activate the downstream target genes responsible for d-xylose uptake and metabolism. The molecular mechanism revealed here will benefit the metabolic engineering to improve the d-xylose utilization efficiency.
Keywords: two-component system, molecular mechanism, d-xylose uptake, histidine kinase, cross-membrane signaling
Abstract
d-xylose, the main building block of plant biomass, is a pentose sugar that can be used by bacteria as a carbon source for bio-based fuel and chemical production through fermentation. In bacteria, the first step for d-xylose metabolism is signal perception at the membrane. We previously identified a three-component system in Firmicutes bacteria comprising a membrane-associated sensor protein (XylFII), a transmembrane histidine kinase (LytS) for periplasmic d-xylose sensing, and a cytoplasmic response regulator (YesN) that activates the transcription of the target ABC transporter xylFGH genes to promote the uptake of d-xylose. The molecular mechanism underlying signal perception and integration of these processes remains elusive, however. Here we purified the N-terminal periplasmic domain of LytS (LytSN) in a complex with XylFII and determined the conformational structures of the complex in its d-xylose–free and d-xylose–bound forms. LytSN contains a four-helix bundle, and XylFII contains two Rossmann fold-like globular domains with a xylose-binding cleft between them. In the absence of d-xylose, LytSN and XylFII formed a heterodimer. Specific binding of d-xylose to the cleft of XylFII induced a large conformational change that closed the cleft and brought the globular domains closer together. This conformational change led to the formation of an active XylFII-LytSN heterotetramer. Mutations at the d-xylose binding site and the heterotetramer interface diminished heterotetramer formation and impaired the d-xylose–sensing function of XylFII-LytS. Based on these data, we propose a working model of XylFII-LytS that provides a molecular basis for d-xylose utilization and metabolic modification in bacteria.
One of the major constituents of plant biomass, d-xylose, has been considered an attractive carbon source for bio-based fuel and chemical production through fermentation (1, 2). However, only a small number of bacteria can use d-xylose as an energy source, preventing fermentation efficiency. Usually, bacteria that metabolize d-xylose possess cross-membrane transporters, metabolic enzymes, and regulatory elements; therefore, metabolic engineering strategies have attempted to incorporate these functional modules into the genomes of target strains to improve the efficiency of fermentation (3).
In bacteria, the first step in the uptake of d-xylose is the ability to sense the pentose sugar. Two predominant systems are known to be responsible for the transmembrane transport of d-xylose in bacteria: the ABC-type transporter and proton symporter systems (4, 5). The ABC-type d-xylose transporter is an energy-consuming system consisting of the periplasmic d-xylose-binding protein XylF, the membrane permease XylH, and the ATP-binding protein XylG. Compared with the proton symporter, the ABC-type transporter has much higher affinity for d-xylose and thus is more efficient in d-xylose uptake. However, despite intensive studies, the mechanism by which bacteria sense an extracellular d-xylose signal and then activate its uptake, metabolism, and regulation remains understood.
Two-component systems are the major pathways for signal sensing and transduction in bacteria. These systems regulate a variety of physiological processes by sensing complicated extracellular stimuli, including nutrients, light, temperature, and redox potential (6–9). A prototypical two-component system consists of a membrane-integrated histidine kinase and a cytoplasmic response regulator. The histidine kinase usually forms a homodimer that can transmit external signals across the cell membrane. These kinases harbor an N-terminal periplasmic domain that can sense an extracellular signal, a transmembrane domain, and a C-terminal kinase domain that can activate the cytoplasmic response regulator (7, 10–12). In general, a conformational change induced by the binding of relevant ligands for the histidine kinase to the N-terminal sensing domain causes the signal to be transmitted across the membrane to activate a cytoplasmic phosphorelay. By comparing the ligand-free and ligand-bound structures of kinase sensors, the principle mechanisms of signal transduction by two-component systems have been studied in some cases. For example, the human gut symbiont Bacteroides hetaiotaomicron can sense the saccharides via the BT4663 sensor domain, which undergoes a scissors-like movement to transduce the signal across the membrane (13). Some sensor domains undergo a symmetry-to-asymmetry conformational switch in response to a signal (6, 14–16), whereas some other domains undergo a piston-like movement to transmit a signal (17, 18). Besides independently sensing the signals, auxiliary proteins that interact with histidine kinases that influence the activities of two-component systems have been identified (7, 8). In Bacillus subtilis, the periplasmic membrane-tethered proteins YycH and YycI were found to control the activity of YycG to regulate crucial cellular processes (19). In Escherichia coli, the fumarate responsive sensor histidine kinase DcuS required the C4-dicarboxylate transporter DctA to form a sensor complex in the cytoplasm to sense the level of fumarate under anaerobic conditions (20).
In a previous study, we reported a three-component system-based regulatory model for d-xylose sensing and transport in Clostridium beijerinckii, a typical species among solventogenic clostridia (21). This three-component system consists of a membrane-associated d-xylose sensor protein, XylFII, and a two-component system comprising a transmembrane histidine kinase (LytS) and a cytoplasmic response regulator (YesN). The periplasmic protein XylFII was found to act as a signal sensor to aid the response of the LytS/YesN system to extracellular d-xylose, enabling LytS/YesN to directly activate the transcription of the adjacent ABC transporter xylFGH genes and thereby promoting the uptake of d-xylose. The underlying mechanism remains elusive, however. In particular, how the d-xylose signal is perceived by XylFII and transmitted to LytS, and why XylFII specifically binds d-xylose, are still not clearly understood. To get a detailed understanding of this three-component-based d-xylose sensing and uptake pathway, we purified the N-terminal periplasmic domain of LytS (LytSN) in a complex with XylFII and determined the conformational structures of the complex in its d-xylose–free and d-xylose–bound forms. Structure-based biochemical analyses revealed the molecular basis for d-xylose sensing by the membrane tethering complex XylFII-LytS. Based on our findings, we proposed a plausible working model.
Results
Structure of XylFII-LytSN Bound with d-Xylose.
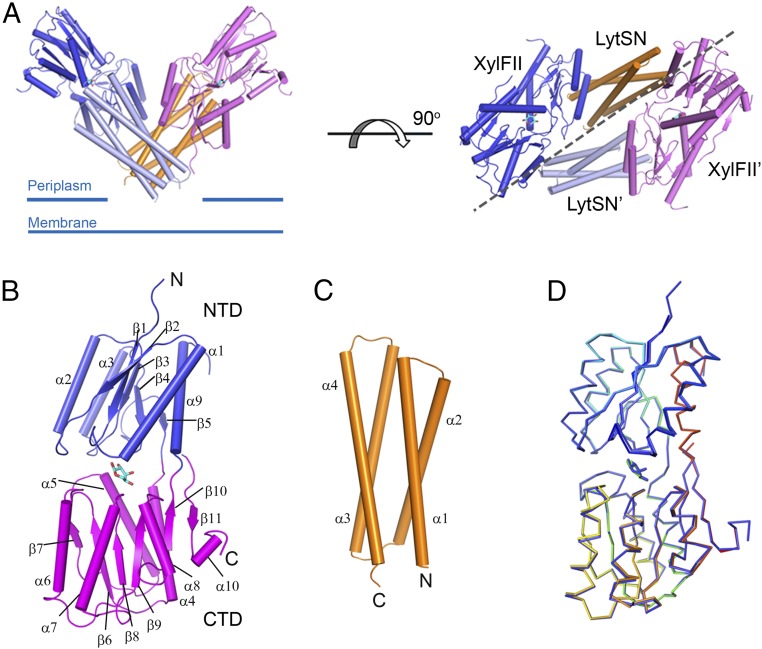
The soluble region of XylFII (residues 25–326) and the periplasmic domain of LytS (residues 1–121 of LytS, namely LytSN) were coexpressed and purified as a XylFII-LytSN complex (with a molar ratio of 1:1) by a two-step purification protocol (Fig. S1, peak 1). The XylFII-LytSN complex with bound d-xylose (XylFII-LytSN-xyl) was crystallized, and its structure was determined to 2.2-Å resolution (Table S1). The XylFII structure consists of two Rossmann fold-like globular domains at the N and C termini, connected by a three-stranded hinge region (Fig. 1 A and B). The N-terminal domain (NTD) contains five β-strands and four α-helices (residues 36–137 and 277–303, respectively), and the C-terminal domain (CTD) contains six β-strands and six α-helices (residues 138–276 and 304–315, respectively). Between the two domains is a deep cleft in which the d-xylose molecule is bound (Fig. 1B and Fig. S2). The LytSN structure contains a four-helix bundle, with the two ends of the bundle bound to two XylFII molecules (XylFII and XylFII′, respectively) (Fig. 1 A and C). One LytSN molecule and one XylFII molecule form a heterodimer, and two symmetrical XylFII-LytSN heterodimers form a tetramer, which has a “V” shape when viewed from the front side (Fig. 1A). The N and C termini of LytSN, which form the bottom of the V-shaped tetramer, may be associated with the membrane, because LytSN is flanked by a transmembrane region followed by a cytoplasmic kinase domain. We also solved the structure of XylFII bound with d-xylose and found that it has a very similar conformation to that in the XylFII-LytSN-xyl complex (rmsd of 0.44 Å) (Fig. 1D), suggesting that assembly of the XylFII-LytS-xyl complex has only a minor effect on the conformation of XylFII.
Fig. S1.
Coexpression and purification of XylFII-LytSN. Gel filtration profiles of XylFII-LytSN-xyl are shown. The fractions of peak1 and peak2 were run separately on SDS/PAGE gel.
Table S1.
Data collection and structure refinement statistics
| Statistic | XylFII-LytSN-xyl | XXylFII-LytSN-D103A | XylFII | Se-Met* |
| Wavelength, Å | 0.9798 | 0.9798 | 0.9798 | 0.9798 |
| Resolution, Å | 2.2–50.0 | 2.5–50.0 | 2.1–50.0 | 2.6–50.0 |
| Space group | C2221 | P41 21 2 | P1 | C2221 |
| Until cell | ||||
| a, b, c, Å | 105.6, 143.6, 87.1 | 144.9, 144.9, 103.1 | 67.5, 68.1, 76.7 | 105.6, 143.6, 87.1 |
| α, β, γ, ° | 90, 90, 90 | 90, 90, 90 | 89.9, 65.1, 87.3 | 90, 90, 90 |
| Unique reflections | 33,221 (2,748)† | 38,385 (3,768) | 70,577 (6,701) | 20,879 (1,726) |
| Multiplicity | 6.4 | 5.7 | 3.8 | 12.1 |
| I/σ(I) | 14.61 (3.0) | 20.46 (5.1) | 15.8 (7.8) | 32.9 (8.3) |
| Completeness, % | 97.87 (81.9) | 99.73 (100.0) | 96.77 (91.6) | 100 (100) |
| Rwork/Rfree‡ | 0.187/0.215 | 0.229/0.253 | 0.180/0.214 | |
| Number of atoms | 3,320 | 5,144 | 9,153 | |
| Ligand | 10 | 40 | ||
| Water | 231 | 140 | 816 | |
| Protein residues | 397 | 647 | 1,087 | |
| RMS, bonds | 0.008 | 0.011 | 0.011 | |
| Angles | 1.06 | 2.03 | 1.32 | |
| Ramachandran favored, % | 95 | 100 | 97 | |
| Outliers, % | 0 | 0 | 0 | |
| Average B factor | 39.1 | 57.0 | 23.1 | |
| Protein | 38.7 | 57.1 | 22.5 | |
| Ligand | 55.1 | 14.6 | ||
| Water | 43.9 | 55.2 | 30.6 |
The Se-Met data were collected from the XylFII-LytSN-xyl form.
Numbers in parentheses represent the highest-resolution shell.
R = Σhkl||Fo| − |Fc||/Σhkl|Fo|.
Fig. 1.
Structure of the XylFII-LytSN-xyl complex. (A) Structure of the WT XylFII-LytSN complex with bound d-xylose (XylFII-LytSN-xyl). (Left) Side view of the XylFII-LytSN heterotetramer. (Right) Top view of the XylFII-LytSN heterotetramer. XylFII, XylFII′, LytSN, and LytSN′ are shown in blue, magenta, orange, and gray, respectively. d-xylose molecules are shown as cyan sticks. (B) Structure of WT XylFII with bound d-xylose. The NTD and CTD are shown in blue and magenta, respectively. (C) Structure of WT LytSN. (D) Superimposed structures of XylFII-xyl and XylFII in the XylFII-LytSN-xyl complex. XylFII-xyl and XylFII in the XylFII-LytSN-xyl complex are shown in rainbow and blue, respectively.
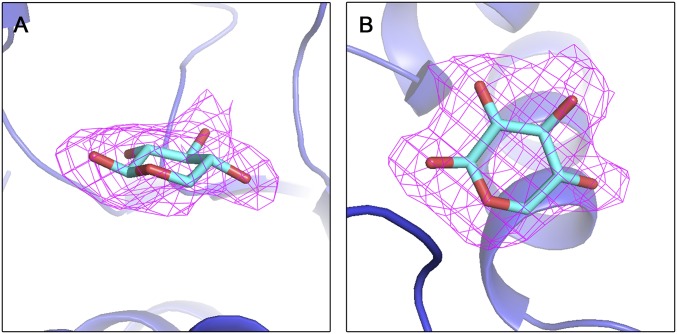
Fig. S2.
Electron density of d-xylose at the ligand-binding cleft of XylFII (Fo-Fc density contoured at the 2.0 σ level). A shows a view of the side and B shows a view of the top.
The d-Xylose Binding Site and Specificity.
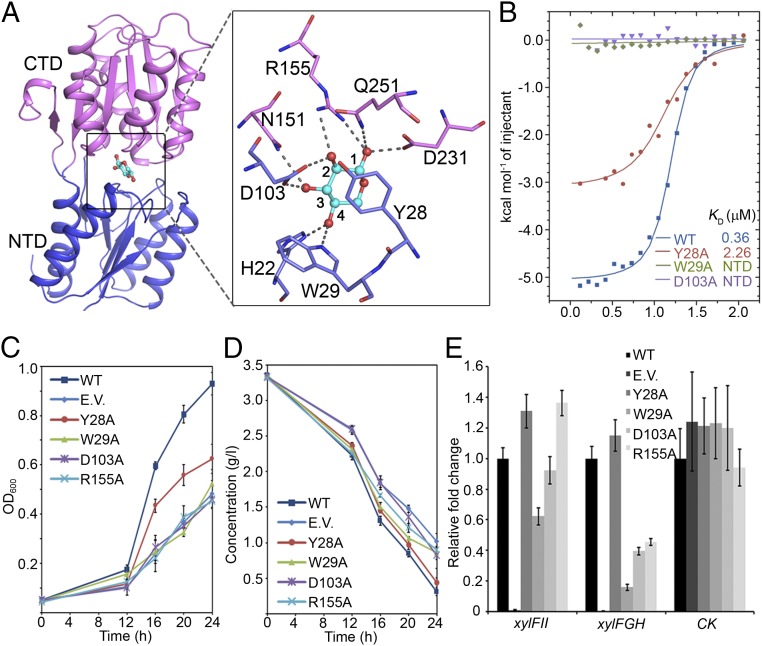
In the deep cleft formed by the NTD and CTD of XylFII, the pyranose ring of the d-xylose molecule adopts a chair conformation, and residues from both terminal domains of XylFII form hydrophobic and hydrogen-bonding interactions with the ring. Among these interactions, residue Tyr28 forms a π–π interaction with the pyranose ring; residues Trp29 and His22 form two hydrogen bonds with the C4 hydroxyl; residues Asp103, Asn151, and Arg155 form four hydrogen bonds with the C2 and C3 hydroxyls; and residues Arg155, Asp231, and Gln251 form three hydrogen bonds with the C1 hydroxyl (Fig. 2A and Fig. S2). These interactions may explain the tight binding of d-xylose to XylFII-LytSN; the dissociation constant is KD = 368 nM (Fig. 2B).
Fig. 2.
The d-xylose binding site of XylFII. (A) Interaction of amino acid residues with d-xylose in the binding site. The bound d-xylose (cyan) is depicted as a ball-and-stick model. The side chains of the residues involved in the specific binding of d-xylose are shown in a zoom-in view. (B) Binding affinities of d-xylose to XylFII-LytSN and mutants measured using ITC. Binding curves of XylFII-LytSN (WT) and mutants XylFIIY28A-LytSN (Y28A), XylFIIW29A-LytSN (W29A), and XylFIID103A-LytSN (D103A) measured by ITC are shown in blue, red, green, and purple, respectively. (C) Growth of C. beijerinckii affected by the d-xylose binding site mutations. The growth rates were monitored by transforming XylFII (WT), empty pXY1 vector (EV), XylFIIY28A (Y28A), XylFIIW29A (W29A), XylFIID103A (D103A), or XylFIIR155A (R155A) genes to the xylFII mutant strain and growing them in YP2 medium with d-xylose as the sole carbon source. The bars indicate the SDs of three independent experiments. (D) d-xylose consumption of curve of C. beijerinckii affected by the d-xylose binding site mutations. The residual d-xylose in the medium were monitored by transforming XylFII (WT), empty pXY1 vector (EV), XylFIIY28A (Y28A), XylFIIW29A (W29A), XylFIID103A (D103A), or XylFIIR155A (R155A) genes to the xylFII mutant strain and growing them in YP2 medium with d-xylose as the sole carbon source. The bars indicate the SDs of three independent experiments. (E) Expression levels of genes affected by the d-xylose binding site mutations. The expression of xylFII, the downstream target d-xylose ABC transporter xylFGH genes, and a gene encoding pullulanase (control; CK) were measured by qRT-PCR. Gene expression levels are represented as fold differences normalized to WT. The error bars indicate the SDs of three independent experiments.
To characterize the effect of these residues on d-xylose binding, we mutated them and determined the d-xylose binding affinity of the XylFII-LytSN complex in vitro using isothermal titration calorimetry (ITC). The results show that the mutations H22A, Y28A, and Q251A significantly decreased d-xylose binding, whereas the mutations W29A, D103A, N151A, R155A, and D231A abolished d-xylose binding (Fig. 2B and Fig. S3). To test the functional roles of these residues in vivo, the XylFII wild-type (WT) and mutant genes were transformed to the C. beijerinckii xylFII mutant strain, then the cell growth and d-xylose consumption were measured (Fig. 2 C and D). The results show that the growth rates of the W29A, D103A, and R155A mutants were comparable to those of the xylFII knockout strain, all were similarly impaired, and the growth rate of the Y28A mutant was between the growth rates of WT and the xylFII knockout strain. The consumption of d-xylose was considered consistent with the growth rate. We found that the utilization of d-xylose by the above mutants, except for Y28A, was significantly reduced.
Fig. S3.
d-xylose binding affinity of XylFII-LytSN and its binding site mutants assayed by ITC.
We also detected the expression levels of the xylFGH genes, which encode a d-xylose ABC transporter and are the downstream targets of XylFII-LytS, using qRT-PCR (Fig. 2E). The results demonstrate that the W29A, D103A, and R155A mutations significantly reduce the transcription levels of xylFGH, whereas the transcription level of xylFII itself was mildly to moderately affected by these mutations. As a negative control, the transcription of the gene encoding pullulanase was not affected by the xylFII mutations. These data suggest that d-xylose binding to XylFII is essential for activating the three-component system to regulate d-xylose uptake and metabolism.
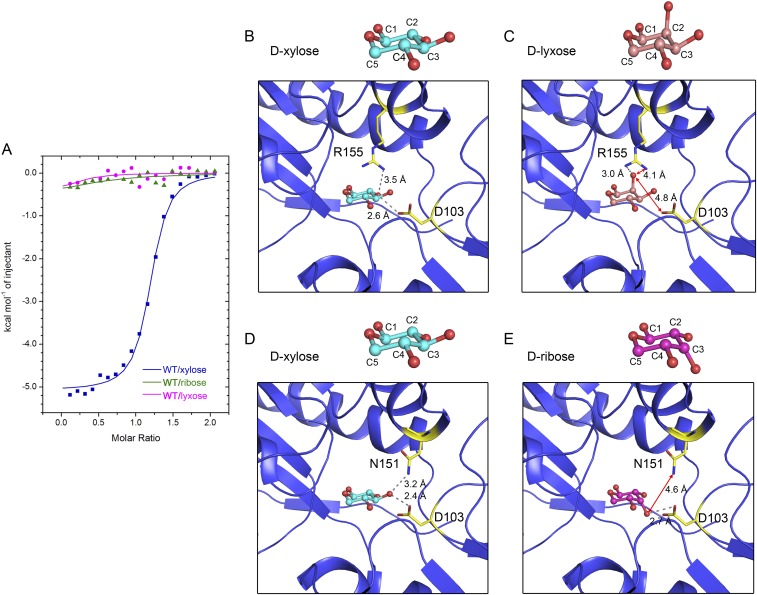
l-arabinose cannot be sensed by XylFII-LytS in vivo (21), probably because its C4 hydroxyl has a different orientation than the C4 hydroxyl of d-xylose. Consistent with this observation, we found that l-arabinose did not bind to the XylFII-LytSN complex in vitro (Fig. 3A). To understand the molecular basis of the binding specificity of XylFII, we fitted the l-arabinose molecule to the d-xylose binding site in the XylFII-LytSN structure, and then compared the model with the XylFII-LytSN-xyl structure. The results show that most of the interactions between the binding site residues and d-xylose or l-arabinose were similar, except that the C4 hydroxyl of d-xylose could form a hydrogen bond with Trp29, whereas the C4 hydroxyl of l-arabinose was 4.7 Å away from the -NH of the Trp29 indole ring in the modeled structure (Fig. 3 B and C). The W29A mutation of Trp29 abolished the binding of d-xylose to the XylFII-LytSN complex (Fig. 2B), indicating the importance of this hydrogen bond in substrate binding. This result may explain why XylFII-LytS can sense d-xylose but not l-arabinose. To further test the specificity, we chose d-lyxose and d-ribose, other two isomers of d-xylose for examination. Compared with d-xylose, d-lyxose and d-ribose have different orientations at C2 and C3 hydroxyls, respectively. The binding abilities of d-lyxose and d-ribose with XylFII-LytSN were tested in vitro by ITC, which showed no obvious binding (Fig. S4A). To understand the underlying mechanism, d-lyxose and d-ribose were modeled to the XylFII-LytSN structure. From the modeling results and comparison with the XylFII-LytSN-xyl structure, the hydrogen bond between residue Asp103 and C2 hydroxyl was lost in d-lyxose (Fig. S4 B and C), whereas the hydrogen bond between residue Asn151 and C3 hydroxyl was lost in d-ribose (Fig. S4 D and E). The foregoing data suggest that the C2, C3, and C4 hydroxyls of d-xylose are critical for its specific binding with XylFII-LytSN.
Fig. 3.
Specificity of the d-xylose binding site of XylFII. (A) Binding affinities of d-xylose and l-arabinose to XylFII-LytSN measured by ITC. The binding curves for d-xylose and l-arabinose are in blue and red, respectively. (B) Interactions dictating the specificity of d-xylose binding. The conformation of d-xylose and the interaction of the C4 hydroxyl with residues His22 and Trp29 in the binding cleft are shown. d-xylose is represented as a ball-and-stick model (cyan), and the side chains of His22 and Trp29 are shown. (C) Modeled structure of l-arabinose in the d-xylose binding site of XylFII. l-arabinose is represented as a ball-and-stick model (green).
Fig. S4.
Specificity of the d-xylose binding site of XylFII. (A) Binding curves of d-xylose, d-lyxose, and d-ribose to XylFII-LytSN. (B and D) d-xylose binding site in XylFII. (C) Modeled structure showing the detail of d-lyxose binding with XylFII. (E) Modeled structure showing the detail of d-ribose binding with XylFII.
Conformational Change Induced by d-Xylose Binding.
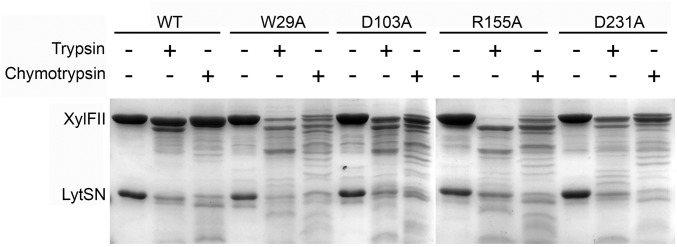
In a limited proteolysis experiment, we found that WT XylFII-LytSN complex was somewhat resistant to trypsin or chymotrypsin but not with W29A, D103A, R155A, and D231A mutants, which showed a similar digestion pattern (Fig. S5). This implies that d-xylose binding might induce a conformational change within the XylFII-LytSN complex. To track the conformational change induced by d-xylose binding, we aimed to obtain the d-xylose–free XylFII-LytSN structure. First, we used the minimum medium to obtain the protein, but when we solved the structure, we found that it still contained bound d-xylose, possibly due to trace amounts of d-xylose in the carbon source medium. Next, we tried to crystallize protein complexes containing binding site mutations. Finally, we solved a d-xylose–free XylFII-LytSN structure by molecular replacement using the D103A mutant (XylFII-LytSN-D103A) (Fig. 4A and Table S1). There are two XylFII molecules and one LytSN molecule in one asymmetrical unit, and XylFII molecules adopt an open conformation. One XylFII molecule (XylFIIA) binds LytSN via interface ITF1 (NTD), whereas the other XylFII molecule (XylFIIB) contacts back-to-back with XylFIIA (possibly owing to the weakened interactions between XylFII and LytSN) (Fig. S6). Thus, the XylFIIA-LytSN complex in the asymmetrical unit could represent the d-xylose–free complex (XylFII-LytSN-apo). Superposition of XylFII-LytSN-apo and XylFII-LytSN-xyl structures revealed that the CTD of XylFII, as a rigid body, underwent a 40-degree rotation through the hinge region in the d-xylose–bound and d-xylose–free structures (Fig. 4 A and B). In the XylFII-LytSN-apo structure, the NTD and CTD were separated and formed an open conformation, whereas in the XylFII-LytSN-xyl structure, the d-xylose molecule held the NTD and CTD together to form a closed conformation. Although XylFII underwent a significant conformational change on the binding and release of d-xylose, this movement did not seem to be transmitted to LytSN, the periplasmic domain of the histidine kinase (Fig. 4A). In addition, the conformation of the NTD of XylFII and its interaction with LytSN were relatively unchanged within one XylFII-LytS heterodimer.
Fig. S5.
SDS/PAGE results of limited proteolysis experiment. Samples of XylFII-LytSN WT and W29A, D103A, R155A, and D231A mutants were run on SDS/PAGE after digestion with trypsin and chymotrypsin.
Fig. 4.
Conformational change induced by d-xylose binding and release. (A) Superimposition of the structures of d-xylose–bound XylFII-LytSN-xyl and d-xylose-free XylFII-LytSN-apo (containing the D103A mutation). XylFII-LytSN-xyl is shown in gray, and XylFII and LytSN in the XylFII-LytSN-apo complex are shown in blue and orange, respectively. (B) Conformational change of XylFII induced by d-xylose binding and release. The CTD of XylFII underwent a 40-degree rotation during d-xylose binding and release.
Fig. S6.
Structure of XylFII-LytSN-D103A in one asymmetrical unit.
d-Xylose Binding Induced the Formation of an Active Heterotetramer.
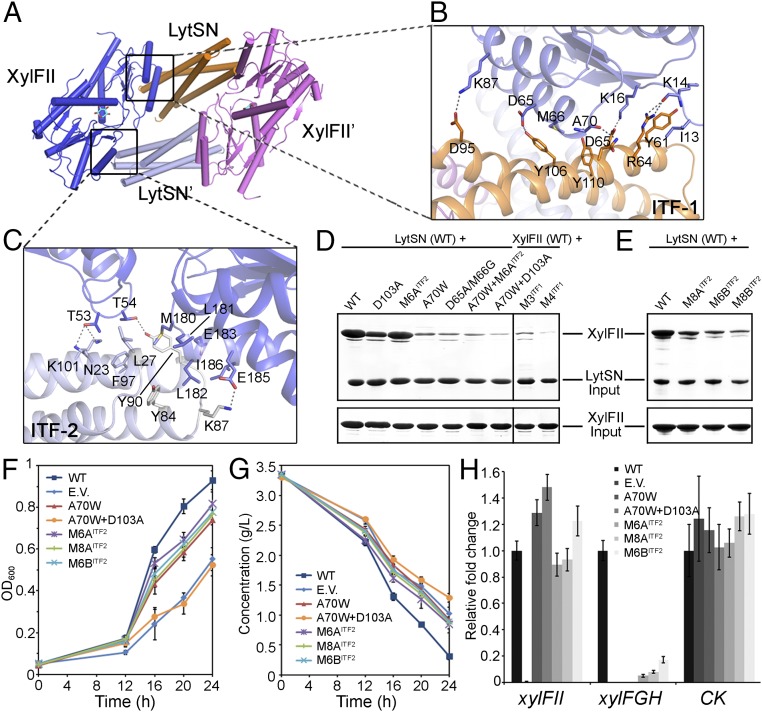
Although d-xylose binding to XylFII did not affect the interactions between the NTD domain of XylFII and LytSN within one XylFII-LytSN heterodimer (ITF1), it did generate another interaction surface between the CTD domain of XylFII and a neighboring LytSN′ (ITF2). This interaction induced the formation of a heterotetramer comprising two XylFII-LytSN heterodimers (XylFII-LytSN/XylFII′-LytSN′) (Fig. 5 A–C). This observation is reminiscent of typical two-component system histidine kinases, which typically form homodimers that transmit external signals across membranes (10–12). Therefore, we suggest that the XylFII-LytS/XylFII′-LytS′ heterotetramer may be the active form.
Fig. 5.
d-xylose binding induced the formation of an active tetramer. (A) Interaction interfaces within the active tetramer. The tetramer is a homodimer of XylFII-LystN/XylFII′-LystN′ heterodimers. The interfaces 1 and 2 (ITF-1 and ITF-2) are indicated. (B) Zoom-in view showing the interactions at ITF-1. Residues from XylFII (slate blue) and LytsN (orange) at ITF-1 are shown with their side chains. The dashed lines indicate hydrogen bonding interactions. (C) Zoom-in view showing the interactions at ITF-2. Residues from XylFII (slate blue) and LytsN′ (gray) at ITF-2 are shown with their side chains. The dashed lines indicate hydrogen-bonding interactions. (D and E) SDS/PAGE showing the effects of the ITF-1 and ITF-2 mutations on complex formation determined by the in vitro pull-down experiments. (F) Growth of C. beijerinckii affected by the ITF-1 and ITF-2 mutations. The growth rates were monitored by transforming XylFII (WT), empty pXY1 vector (EV), or XylFII genes containing mutations at ITF-1 and ITF-2 [i.e., XylFIIA70W (A70W), XylFIIA70W+D103A (A70W+D103A), XylFIIM6A (M6AITF2), XylFIIM6B (M6BITF2), or XylFIIM8A (M8AITF2)] to the xylFII mutant strain and growing them in YP2 medium with d-xylose as the sole carbon source. The bars indicate the SDs of three independent experiments. (G) d-xylose consumption of C. beijerinckii affected by the ITF-1 and ITF-2 mutations. The residual d-xylose in the medium were monitored by transforming XylFII (WT), empty pXY1 vector (EV), or XylFII genes containing mutations at ITF-1 and ITF-2 [i.e., XylFIIA70W (A70W), XylFIIA70W+D103A (A70W+D103A), xylFIIM6A (M6AITF2), XylFIIM6B (M6BITF2), or XylFIIM8A (M8AITF2)] to the xylFII mutant strain and growing them in YP2 medium with d-xylose as the sole carbon source. The bars indicate the SDs of three independent experiments. (H) Expression levels of genes affected by the ITF-1 and ITF-2 mutations. The expression levels of XylFII, the downstream target d-xylose ABC transporter xylFGH genes, and a gene encoding pullulanase (CK) were measured using qRT-PCR. Gene expression levels are represented as fold differences normalized to WT. The error bars indicate the SDs of three independent experiments.
The ITF1 contains residues Ile13, Lys14, Lys16, Asp65, Met66, Ala70, and Lys87 from XylFII (or XylFII′), which mainly form hydrogen-bonding interactions with residues Tyr61, Arg64, Asp65, Asn68, Asp95, Tyr106, and Tyr110 from LytS (or LytS′) (Fig. 5B). The ITF2 contains residues Met180, Leu181, Leu182, and Ile186 from XylFII (or XylFII′), which form hydrophobic interactions with residues Leu27, Tyr84, Tyr90, and Phe97 from LytS′ (or LytS), and residues Thr53, Thr54, Glu183 and Glu185 from XylFII (or XylFII′), which form hydrogen-bonding interactions with residues Asn23, Lys87, Tyr90, and Lys101 from LytS′ (or LytS) (Fig. 5C). To verify the importance of the interaction surfaces for the biological function of XylFII-LytS, we sought to generate mutations at the interfaces. Four mutants were generated with ITF1: A70W, which induces steric conflict at the interface; D65A/M66G, which breaks the hydrogen bond between Asp65 and Tyr106 and disrupts the hydrophobic interactions among Met66 and surrounding residues; M3, in which the surface residues Asn68, Thr69, and Asp71 of LytS are replaced with Ala; and M4, in which residues Gln60, Tyr61, Arg64, and Asp65 of LytS are replaced with Ala. Five mutants were generated with ITF2: M6AITF2, which contains M180A, L181A, L182A, E183A, E185A, and I186A mutations; M6BITF2, which contains M180E, L181A, L182E, E183A, E185A, and I186A mutations; M8AITF2, which contains T53A, T54A, M180A, L181A, L182A, E183A, E185A, and I186A mutations; M8BITF2, which contains T53A, T54A, M180E, L181A, L182E, E183A, E185A, and I186A mutations; and D103A, which abolishes d-xylose binding and impairs ITF2.
In the in vitro pull-down experiments, we found that the interactions between XylFII and LytSN were largely decreased by the M6BITF2, M8AITF2, M8BITF2, and D103A mutations; severely impaired by the A70W, D65A/M66G, A70W/M6A, and M3 mutations; and almost destroyed by the A70W/D103A and M4 mutations (Fig. 5 D and E). Consistently, the A70W/D103A mutation had the most severe effect on cell growth and d-xylose consumption in vivo, which is comparable to the results obtained for the xylFII knockout strain. The other mutations tested, including A70W, M6AITF2, M6BITF2, and M8AITF2, also had significant effects on cell growth and d-xylose consumption (Fig. 5 F and G). As expected, these mutations failed to activate expression of the downstream target xylFGH genes (Fig. 5H). These data suggest that both ITF1 and ITF2 are essential for the biological functions of XylFII-LytS, and validate the idea that the XylFII-LytS/XylFII′-LytS′ heterotetramer induced by d-xylose binding is the active form.
Discussion
In this study, we determined the structure of XylFII in complex with the periplasmic domain of histidine kinase LytS in d-xylose–free and d-xylose–binding forms. Biochemical analyses revealed that XylFII formed a heterodimer with LytS and only XylFII specifically bounds d-xylose. d-xylose binding induced a heterodimer-to-heterotetramer transition, which we consider necessary for cytoplasmic signal transmission and d-xylose use.
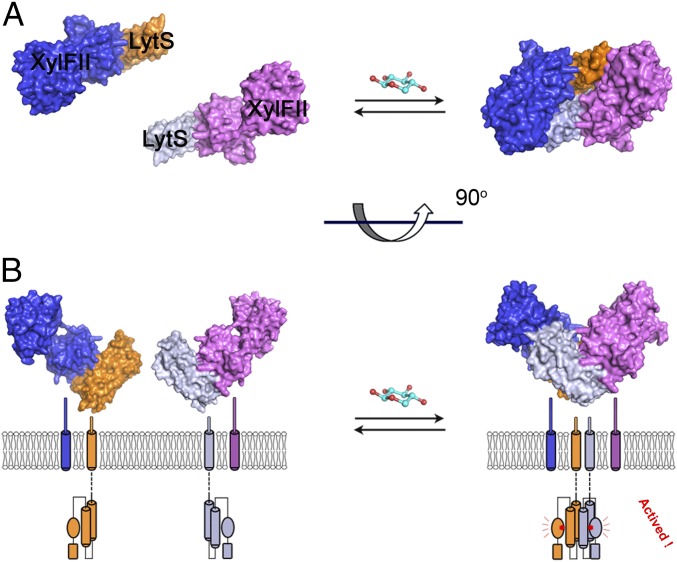
Based on these findings, we propose the following working model of how XylFII-LytS senses environmental d-xylose. When d-xylose is deficient in the environment, XylFII forms a heterodimer with the periplasmic domain of LytS; however, the kinase activity of LytS and the transcription activity of the response regulator YesN are not activated. The presence of d-xylose in the environment is perceived immediately by XylFII through tight specific binding. The binding of d-xylose induces a conformational change in XylFII, which brings two LytS together to form an active heterotetramer on the membrane. The kinase activity of LytS is activated, which further induces the phosphorylation of the response regulator YesN and activation of the downstream target XylFGH genes for d-xylose uptake and consumption (Fig. 6 A and B).
Fig. 6.
A possible working model of the XylFII-LytS complex. Top view (A) and side view (B) of the model. In the absence of d-xylose, XylFII forms an inactive heterodimer with the periplasmic domain of LytS, and kinase activity is inactivated. d-xylose binding induces two XylFII-LytS dimers to form a symmetrical heterotetramer. This conformational change is transmitted across the membrane to favor autophosphorylation of LytS, and then the signal is transmitted to the downstream response regulator YesN and activation of the downstream target XylFGH genes for d-xylose uptake and consumption.
d-xylose is the most abundant fermentable pentose in nature, and engineering the signal sensing or using system into bacteria that are incapable of using d-xylose as a fermentable carbon source is a promising strategy. Although uptake or transport of d-xylose across membranes is a prerequisite for its metabolism, our data provide a molecular basis for the d-xylose cross-membrane sensing process, which may benefit the engineering of bacteria that can then use d-xylose for the production of bio-based fuel or other chemicals in the future.
Materials and Methods
All experiments are described in detail in SI Materials and Methods. In brief, the XylFII-LytSN protein complex was expressed in E. coli and purified to homogeneity for crystallization. Diffraction data were collected and processed with HKL3000 (22). The structures were determined using PHENIX (23), and structural models were built with Coot (24) (Table S1). The binding of d-xylose, l-arabinose, d-lyxose, and d-ribose with the XylFII-LytSN complex was determined using ITC. Cell growth and d-xylose consumption assays were carried in C. beijerinckii strains. Mutations were generated using one-step PCR (Table S2).
Table S2.
Primers used in this study
| Primer name | Sequence (5′ to 3′) |
| For protein expression constructs (pET-DUET vector) | |
| XylFII-F-BglII | CCCAGATCTAATTTATTAAGAGACAAGAAAG |
| XylFII-R-XhoI | AAACTCGAGTTAGTTTTCAACTTTATCATC |
| LytS-F-BamHI | AAAGGATCCATGCTTAACAATATGCTCATTAC |
| LytS-R-SalI | AAAGTCGACTTAGCTGCTATTTCTATTTAGTTC |
| For protein expression constructs (pXY1 vector) | |
| XylFII-Full-F-BamHI | AGGAGGTTAGTTAGAGGATCCATGAAATTCTTAAAGAGCGTTT |
| XylFII-Full-R-BamHI | CCAGTGAATTCCCGGGGATCCTTAGTTTTCAACTTTATCATCTCC |
| XylFII-F1 | AATTTATTAAGAGACAAGAAAGTAG |
| XylFII-R1 | CTACTTTCTTGTCTCTTAATAAATT |
| For protein expression constructs (pET-28a vector) | |
| LytS-F-BamHI | AAAGGATCCATGCTTAACAATATGCTCATTAC |
| LytS-R-SalI | AAAGTCGACTTAGCTGCTATTTCTATTTAGTTC |
| For protein expression constructs (pGEX4T-1 vector) | |
| XylFII-F-BglII | CCCAGATCTAATTTATTAAGAGACAAGAAAG |
| XylFII-R-XhoI | AAACTCGAGTTAGTTTTCAACTTTATCATC |
| For site-directed mutagenesis experiments | |
| tXylFII-H22A-F | GTGCTGATTTCTGCTATAAAGACTAATC |
| XylFII-H22A-R | GATTAGTCTTTATAGCAGAAATCAGCAC |
| XylFII-Y28A-F | AAAGACTAATCCAGCTTGGCTTGATATAAA |
| XylFII-Y28A-R | TTTATATCAAGCCAAGCTGGATTAGTCTTT |
| XylFII-W29A-F | CTAATCCATATGCGCTTGATATAAAAG |
| XylFII-W29A-R | CTTTTATATCAAGCGCATATGGATTAG |
| XylFII-D103A-F | GTAGTTACAATAGCTTCAGATGAAGAAG |
| XylFII-D103A-R | CTTCTTCATCTGAAGCTATTGTAACTAC |
| XylFII-N151A-F | GAAAAACGTAAAGGCTCAGAAAGAAAGG |
| XylFII-N151A-R | CCTTTCTTTCTGAGCCTTTACGTTTTTC |
| XylFII-R155A-F | GAATCAGAAAGAAGCGGTTGAAGGCTTTAC |
| XylFII-R155A-R | GTAAAGCCTTCAACCGCTTCTTTCTGATTC |
| XylFII-D231A-F | AAATTATATGCTTTGCTGATTTGGATGATAC |
| XylFII-D231A-R | GTATCATCCAAATCAGCAAAGCATATAATTT |
| XylFII-Q251A-F | GCAACAATAGTGGCGAAAAGTAATGAA |
| XylFII-Q251A-R | TTCATTACTTTTCGCCACTATTGTTGC |
| XylFII-A70W-F | GATATGGCTACATCATGGAAGGTGAGTGGC |
| XylFII-A70W-R | GCCACTCACCTTCCATGATGTAGCCATATC |
| XylFII-D65A/M66G-F | GGGCTAAAACTTTTTGCTGGGGCTACATCAGCAAAG |
| XylFII-D65A/M66G-R | CTTTGCTGATGTAGCCCCAGCAAAAAGTTTTAGCCC |
| XylFII-M6A-F | GATTCATCGGATGCGGCGGCTGCTGCGGCAGCAGCCATAACAAGAAAAATATTAAATAG |
| XylFII-M6A-R | CTATTTAATATTTTTCTTGTTATGGCTGCTGCCGCAGCAGCCGCCGCATCCGATGAATC |
| LytS-M4-F | CTGATGATGTAAATGCAGCTATTTTAGCAGCTCTAGATAATACTTTAG |
| LytS-M4-R | CTAAAGTATTATCTAGAGCTGCTAAAATAGCTGCATTTACATCATCAG |
| LytS-M3-F | TTAAGAGATCTAGATGCTGCTTTAGCTTCTTATATAGAAAG |
| LytS-M3-R | CTTTCTATATAAGAAGCTAAAGCAGCATCTAGATCTCTTAA |
| XylFII-T53A/T54A-F | GAATTTTTAGGACCTGCAGCTGCAAGCACTGAAG |
| XylFII-T53A/T54A-R | CTTCAGTGCTTGCAGCTGCAGGTCCTAAAAATTC |
| XylFII-M6B-F | GATTCATCGGATGCGGAGGCTGAGGCGGCAGCAGCC |
| XylFII-M6B-R | GGCTGCTGCCGCCTCAGCCTCCGCATCCGATGAATC |
SI Materials and Methods
Cloning, Expression, and Purification of the XylFII:LytS Complex.
Genes encoding the peripalsmic regions of XylFII (25–326 aa) and LytS (1–134 aa) were directly PCR-amplified from C. beijerinckii NCIMB 8052 genomic DNA and then inserted into the pETDuet-1 vector. The constructs containing point mutations were generated by PCR overlap extension (Table S2) and verified by DNA sequencing. The plasmid was transformed into the E. coli Rosette (DE3) strain for protein expression. The bacterial cells were grown at 37 °C to an OD600 of 0.8, after which protein expression was induced by adding 0.5 mM isopropyl β-d-thiogalactoside for 16 h at 16 °C. The cells were collected, resuspended in buffer A (20 mM Tris⋅HCl, 100 mM NaCl, pH 8.0), and then lysed by French press. The lysate was centrifuged at 20,000 × g for 1 h, and the supernatant was loaded through a Ni-NTA column. After washing with buffer A plus 25 mM imidazole, the protein was eluted from the column using buffer A plus 250 mM imidazole and was concentrated for further purification by gel filtration (Superdex-200; GE Healthcare) in buffer A. The peak fractions were collected and concentrated to 10 mg/mL for crystallization. For SeMet-derived protein expression, the constructs were transformed into E. coli B834 (DE3) strain, and the cells were cultured in M9 medium containing 50 mg/L selenomethionine.
To test the effect of mutations located in the interaction surface of XylFII and LytSN, the DNA fragments of the peripalsmic region of XylFII and its mutations were cloned into the pGEX4T-1 vector, with a GST tag at the N terminus, and the DNA fragments of the peripalsmic region of LytS and its mutations were cloned into pET-28a vector, with a 6×His tag at the N terminus. XylFII and LytSN (or their mutants) were coexpressed and purified by GST resin for input and purified by Ni-NTA His resin for His pull-down to test the interactions. Genes encoding the full length of XylFII and its mutations were obtained by PCR overlap extension from the aforementioned pETDuet constructs. These genes were then cloned into the pXY1 vector and then used for the construction of pXY1-XylFII WT and pXY1-XylFII mutants to perform cell growth and xylose consumption analysis. The primers used are listed in Table S2.
Crystallization, Diffraction Data Collection, and Structure Determination.
Crystals were grown at 20 °C using the sitting-drop vapor diffusion method. Equal volumes (0.8 μL) of protein sample (10 mg/mL) and the reservoir solution were mixed and equilibrated against 100 μL of the reservoir solution. To obtain crystals of XylFII-LytSN-xyl and XylFII-xyl complexes, the purified protein was incubated with a 10-fold excess amount of d-xylose for crystallization. The XylFII-LytSN-xyl and SeMet-derived XylFII-LytSN-xyl crystals were grown under 0.2 M lithium sulfate, 2.0 M ammonium sulfate, and 0.1 M Mes, pH 6.6. The XylFII-xyl crystals were grown under 20% (wt/vol) polyethylene glycol 8000, 0.1 M phosphate-citrate pH 4.2, and 0.2 M NaCl. The XylFII-LytSN-D103A crystals were grown under 0.1 M lithium sulfate, 0.1 M sodium citrate tribasic dihydrate pH 5.5, and 20% (wt/vol) polyethylene glycol 1000. Crystals used for data collection were directly flash-frozen in a cold nitrogen stream at 100 K. All data were collected at BL17U beamline of the Shanghai Synchrotron Radiation Facility and processed with HKL3000 (22).
The XylFII-LytSN-xyl complex structure was solved by the single wavelength anomalous dispersion method. The selenium sites were determined, and initial phases were calculated using the HKL3000 package (22). Structures of the XylFII-xyl and XylFII-LytSN-D103A complex were solved by molecular replacement using Phenix (23), using the XylFII-LytSN-xyl structure as an initial model. All of the models were refined with Phenix and built manually with Coot (24). The data collection and refinement statistics are summarized in Table S1.
ITC Analysis.
ITC experiments were performed with the MicroCal ITC200 system (Malvern Instruments) at 20 °C in buffer A. Proteins were purified as described above; d-xylose, l-arabinose, d-lyxose and d-ribose were dissolved in buffer A. For detection of d-xylose binding to XylFII-LytSN complex and its mutants, the syringe was filled with 0.3 mM d-xylose, and the cell was filled with 30 μM protein. The sample in the syringe was added to the sample cell by sequential injections of 2-μL aliquots followed by 120 s of equilibration after each injection. There were a total of 20 injections. For analysis, the heat released by each injection was integrated, and the background was subtracted. The data were fit to the Wiseman isotherm with the Origin ITC analysis package. The experiments were repeated at least three times for each sample. The binding detection of l-arabinose, d-lyxose, and d-ribose with XylFII-LytSN complex follows a same protocol.
Analysis of Cell Growth and Xylose Concentration.
C. beijerinckii strains were cultivated anaerobically at 37 °C in Clostridium growth medium (CGM) (Thermo Fisher Scientific). YP2 medium, modified from P2 medium, containing 3 g/L d-xylose as the carbon source and 0.05 g/L yeast extract, was used for fermentation. C. beijerinckii strains grown in CGM medium were transferred into YP2 medium for inoculum preparation and then incubated into YP2 medium for fermentation. Cell growth was indicated by optical density (OD600), and the d-xylose concentration was determined by HPLC (model 1200; Agilent) with a Sugar-PakTM I column (Waters) and a refractive index detector.
Gene Expression Analysis by qRT-PCR.
Cells for qRT-PCR were grown in YP2 medium containing 3 g/L d-xylose at 37 °C for 16 h. Samples were harvested, immediately frozen in liquid nitrogen, and then ground into powder. RNA was isolated by TrizolTM (Invitrogen) extraction according to the manufacturer’s instructions. Contaminant DNA was removed by treatment with DNase I (TaKaRa), and cDNA was synthesized by reverse transcription for qRT-PCR. qRT-PCR was performed in a MyiQ2 two-color real-time PCR detection system (Bio-Rad) using iQTM SYBR Green Supermix (Bio-Rad). The 16s rRNA gene served as the internal control.
Limited Proteolysis Assay.
XylFII-LytSN complex protein WT and W29A, D103A, R155A, and D231A mutants (1 mg/mL) were digested with trypsin (1 μg/mL) or chymotrypsin (1 μg/mL) in buffer containing 20 mM Tris⋅HCl and 100 mM NaCl at 20 °C for 1 h. The digestion was stopped by the addition of 4×SDS loading buffer and heated at 95 °C for 10 min. The samples were run on SDS/PAGE.
Acknowledgments
We thank the staff members at BL17U of Shanghai Synchrotron Radiation Facility and BL19U1/U2 of National Center for Protein Science Shanghai for technical assistance in data collection, and the staff at the core facility center of Institute of Plant Physiology and Ecology for X-ray diffraction experiment analysis. This work was supported by grants from the National Natural Science Foundation of China (31670755 and 31322016, to P.Z.; 31630003 and 31421061, to W.J.) and the Chinese Academy of Sciences (QYZDB-SSW-SMC006 and XDPB0402).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. N.Y. is a guest editor invited by the Editorial Board.
Data deposition: The atomic coordinates and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID codes 5XSD, 5XSJ, and 5XSS).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620183114/-/DCSupplemental.
References
- 1.Kim JH, Block DE, Mills DA. Simultaneous consumption of pentose and hexose sugars: An optimal microbial phenotype for efficient fermentation of lignocellulosic biomass. Appl Microbiol Biotechnol. 2010;88:1077–1085. doi: 10.1007/s00253-010-2839-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang GC, Liu JJ, Kong II, Kwak S, Jin YS. Combining C6 and C5 sugar metabolism for enhancing microbial bioconversion. Curr Opin Chem Biol. 2015;29:49–57. doi: 10.1016/j.cbpa.2015.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Kim SR, Ha SJ, Wei N, Oh EJ, Jin YS. Simultaneous co-fermentation of mixed sugars: A promising strategy for producing cellulosic ethanol. Trends Biotechnol. 2012;30:274–282. doi: 10.1016/j.tibtech.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Davis EO, Henderson PJ. The cloning and DNA sequence of the gene xylE for xylose-proton symport in Escherichia coli K12. J Biol Chem. 1987;262:13928–13932. [PubMed] [Google Scholar]
- 5.Song S, Park C. Organization and regulation of the d-xylose operons in Escherichia coli K-12: XylR acts as a transcriptional activator. J Bacteriol. 1997;179:7025–7032. doi: 10.1128/jb.179.22.7025-7032.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, et al. Structural identification of a bacterial quorum-sensing signal containing boron. Nature. 2002;415:545–549. doi: 10.1038/415545a. [DOI] [PubMed] [Google Scholar]
- 7.Buelow DR, Raivio TL. Three (and more) component regulatory systems: Auxiliary regulators of bacterial histidine kinases. Mol Microbiol. 2010;75:547–566. doi: 10.1111/j.1365-2958.2009.06982.x. [DOI] [PubMed] [Google Scholar]
- 8.Tetsch L, Jung K. The regulatory interplay between membrane-integrated sensors and transport proteins in bacteria. Mol Microbiol. 2009;73:982–991. doi: 10.1111/j.1365-2958.2009.06847.x. [DOI] [PubMed] [Google Scholar]
- 9.Mascher T, Helmann JD, Unden G. Stimulus perception in bacterial signal-transducing histidine kinases. Microbiol Mol Biol Rev. 2006;70:910–938. doi: 10.1128/MMBR.00020-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock AM, Robinson VL, Goudreau PN. Two-component signal transduction. Annu Rev Biochem. 2000;69:183–215. doi: 10.1146/annurev.biochem.69.1.183. [DOI] [PubMed] [Google Scholar]
- 11.Gao R, Stock AM. Biological insights from structures of two-component proteins. Annu Rev Microbiol. 2009;63:133–154. doi: 10.1146/annurev.micro.091208.073214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheung J, Hendrickson WA. Sensor domains of two-component regulatory systems. Curr Opin Microbiol. 2010;13:116–123. doi: 10.1016/j.mib.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowe EC, Baslé A, Czjzek M, Firbank SJ, Bolam DN. A scissor blade-like closing mechanism implicated in transmembrane signaling in a Bacteroides hybrid two-component system. Proc Natl Acad Sci USA. 2012;109:7298–7303. doi: 10.1073/pnas.1200479109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moore JO, Hendrickson WA. An asymmetry-to-symmetry switch in signal transmission by the histidine kinase receptor for TMAO. Structure. 2012;20:729–741. doi: 10.1016/j.str.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neiditch MB, et al. Ligand-induced asymmetry in histidine sensor kinase complex regulates quorum sensing. Cell. 2006;126:1095–1108. doi: 10.1016/j.cell.2006.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Neiditch MB, Federle MJ, Miller ST, Bassler BL, Hughson FM. Regulation of LuxPQ receptor activity by the quorum-sensing signal autoinducer-2. Mol Cell. 2005;18:507–518. doi: 10.1016/j.molcel.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 17.Sevvana M, et al. A ligand-induced switch in the periplasmic domain of sensor histidine kinase CitA. J Mol Biol. 2008;377:512–523. doi: 10.1016/j.jmb.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 18.Cheung J, Hendrickson WA. Structural analysis of ligand stimulation of the histidine kinase NarX. Structure. 2009;17:190–201. doi: 10.1016/j.str.2008.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szurmant H, Mohan MA, Imus PM, Hoch JA. YycH and YycI interact to regulate the essential YycFG two-component system in Bacillus subtilis. J Bacteriol. 2007;189:3280–3289. doi: 10.1128/JB.01936-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witan J, et al. Interaction of the Escherichia coli transporter DctA with the sensor kinase DcuS: Presence of functional DctA/DcuS sensor units. Mol Microbiol. 2012;85:846–861. doi: 10.1111/j.1365-2958.2012.08143.x. [DOI] [PubMed] [Google Scholar]
- 21.Sun Z, et al. A novel three-component system-based regulatory model for d-xylose sensing and transport in Clostridium beijerinckii. Mol Microbiol. 2015;95:576–589. doi: 10.1111/mmi.12894. [DOI] [PubMed] [Google Scholar]
- 22.Minor W, Cymborowski M, Otwinowski Z, Chruszcz M. HKL-3000: The integration of data reduction and structure solution—from diffraction images to an initial model in minutes. Acta Crystallogr D Biol Crystallogr. 2006;62:859–866. doi: 10.1107/S0907444906019949. [DOI] [PubMed] [Google Scholar]
- 23.Adams PD, et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr D Biol Crystallogr. 2010;66:213–221. doi: 10.1107/S0907444909052925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Emsley P, Cowtan K. Coot: Model-building tools for molecular graphics. Acta Crystallogr D Biol Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]