Significance
In mammals, long-term programming of offspring physiology occurring before birth has effects persisting into adulthood. Appropriate programming allows environmental preadaptation, while inappropriate programming has deleterious health and fitness consequences. To define the mechanistic pathways underlying maternal programming, this study focused on the programming of calendar time before birth, which sets subsequent trajectories for growth and reproduction. This phenomenon depends on day-length perception, encoded via the light-sensitive hormone melatonin from the mother. This study defines the pathway by which maternal melatonin engages with the fetal brain and shows how this prenatal signal leads to a long-term effect on brain thyroid hormone signaling, establishing a new paradigm for investigating epigenetic programming of brain function in utero.
Keywords: photoperiodism, developmental programming, hypothalamus, pars tuberalis, thyroid
Abstract
In wild mammals, offspring development must anticipate forthcoming metabolic demands and opportunities. Within species, different developmental strategies may be used, dependent on when in the year conception takes place. This phenotypic flexibility is initiated before birth and is linked to the pattern of day length (photoperiod) exposure experienced by the mother during pregnancy. This programming depends on transplacental communication via the pineal hormone melatonin. Here, we show that, in the Siberian hamster (Phodopus sungorus), the programming effect of melatonin is mediated by the pars tuberalis (PT) of the fetal pituitary gland, before the fetal circadian system and autonomous melatonin production is established. Maternal melatonin acts on the fetal PT to control expression of thyroid hormone deiodinases in ependymal cells (tanycytes) of the fetal hypothalamus, and hence neuroendocrine output. This mechanism sets the trajectory of reproductive and metabolic development in pups and has a persistent effect on their subsequent sensitivity to the photoperiod. This programming effect depends on tanycyte sensitivity to thyroid stimulating hormone (TSH), which is dramatically and persistently increased by short photoperiod exposure in utero. Our results define the role of the fetal PT in developmental programming of brain function by maternal melatonin and establish TSH signal transduction as a key substrate for the encoding of internal calendar time from birth to puberty.
In mammals, the maternal uterine environment exerts long-term influences on offspring phenotype, a phenomenon known as maternal programming. This subject has received extensive attention because exposure to poor nutrition or to abnormal levels of steroid hormones during pregnancy can lead to persistent metabolic and reproductive dysfunction extending into adult life (1, 2). Additionally, maternal programming effects may have adaptive value, enabling the physiology of the offspring to predict forthcoming environmental conditions based upon intrauterine experience (the so-called “predictive adaptive response”) (3, 4). This response has led to the concept that the observed deleterious effects of maternal programming may derive from a mismatch between environmental conditions as predicted in utero and the postnatal environment eventually encountered (3). For example, a “thrifty phenotype” established as a consequence of fetal malnutrition might preadapt the organism for a nutrient-poor postnatal environment, but lead to obesity and diabetes if subsequent food supply is plentiful. Hence the study of evolutionarily adaptive programming may give mechanistic insights into the fetal origins of life-long disease.
Maternal photoperiodic programming (MPP) (i.e., the establishment of a sense of calendar time before birth), is the archetypical adaptive programming mechanism. This phenomenon is best described in rodents, where it plays a major role in ensuring that offspring reproductive development proceeds rapidly in spring-born animals but is arrested to the following year in autumn-born animals (4). MPP has also been described in larger mammals (5) and is probably of broad adaptive significance for the setting of growth and maturation trajectories. This programming, which depends on the length of daily light exposure (photoperiod) experienced by the mother being relayed to the fetus, sets initial postnatal developmental trajectories as well as postnatal sensitivity to subsequent photoperiodic experience. This latter aspect is important because intermediate photoperiods are experienced in both the spring and in the autumn and need to be interpreted in the context of photoperiodic history.
The mother’s production of the hormone melatonin is an essential requirement for MPP (6, 7). Melatonin is produced by the pineal gland under circadian control generated by the suprachiasmatic nuclei (SCN), with a profile that represents the duration of the night. MPP does not occur if mothers are pinealectomized before pregnancy, and manipulation of the mother’s endogenous melatonin signal by melatonin injection during pregnancy alters neonatal growth trajectories and the perception of photoperiodic history (4, 6, 7). The pups themselves first become able to generate a nocturnal melatonin profile when efferent pathways from the SCN to the pineal gland are established shortly before weaning (8). Hence the interaction between gestational exposure to maternal melatonin and photoperiodic experience of the pup in the postweaning period determines MPP effects on growth and reproductive development in rodents (9, 10).
In juvenile and adult mammals, melatonin controls reproductive endocrine function through effects on the pars tuberalis (PT) of the anterior pituitary gland. This tissue contains type 1 melatonin receptor (MT1) expressing thyrotrophic endocrine cells (11), which produce thyroid stimulating hormone (TSH) (12, 13). TSH produced by the PT acts on ependymal cells in the neighboring basal hypothalamus, known as tanycytes, which express TSH receptors (TSHRs), and in turn regulate local thyroid hormone (TH) levels through seasonal changing expression of TH deiodinase enzymes (12, 13). Type 2 deiodinase (DIO2) generates active triiodothyronine (T3) from thyroxine (T4), whereas type 3 deiodinase (DIO3) generates inactive reverse T3 from T4, and degrades T3 to diiodothyronine (14). Through this conserved pathway, lengthening photoperiod increases the expression of dio2 relative to dio3, producing an euthyroid state in the basal hypothalamus and leading to expression of a spring/summer-like endocrine physiology (e.g., increased body mass and reproductive activation in rodents). Declining photoperiod is associated with the opposite effect (e.g., decrease in body mass, reproductive arrest, and hibernation in rodents) through a relative increase in dio3 expression (15, 16).
Here, we investigated the effect of MPP on the function of the TSH–DIO2/DIO3 axis in mediobasal hypothalamus (MBH) of the Siberian hamster (Phodopus sungorus), manipulating the interaction between prenatal and postweaning photoperiod exposure. Our data demonstrate first that this axis provides a route whereby the mother can influence offspring brain function via the fetal pituitary gland, and second that this leads to persistent changes in TSH sensitivity in the MBH, extending into adult life.
Results
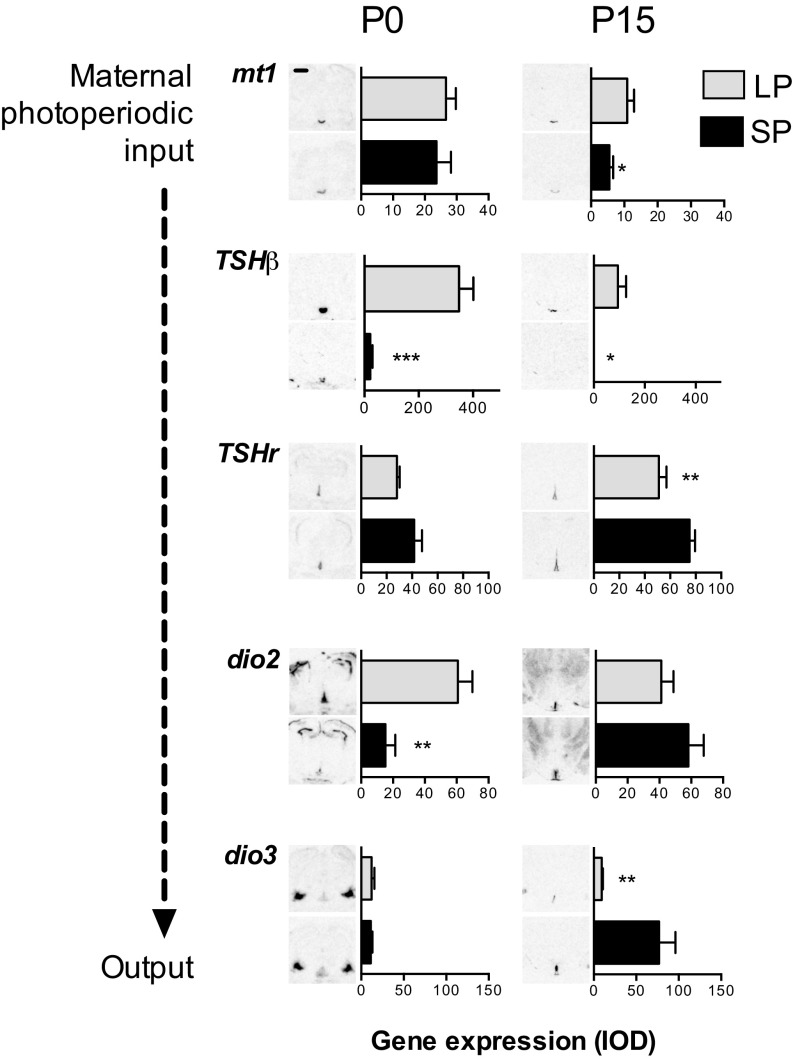
We first examined pituitary and hypothalamic gene expression in male newborn [postnatal day 0 (P0)] pups gestated under either long photoperiod (LP, 16 h light/24 h) or short photoperiod (SP, 8 h light/24 h). Pups from the two groups were superficially similar with no significant difference in birth weight. The high levels of mt1 mRNA present in the PT of these animals, were also unaffected by the gestational photoperiod (Fig. 1). Contrastingly, we observed a strong effect on the expression of RNA encoding TSH β-subunit (TSHβ) mRNA expression in the neonatal PT, with elevated expression under LP and nearly undetectable expression in SP (P < 0.001, Fig. 1). This observation indicates that the fetal PT is responsive to maternal photoperiod mediated by the maternal melatonin secretion pattern. In P0 pups, TSHr mRNA expression was present in the MBH surrounding the base of the third ventricle with no photoperiodic difference (Fig. 1), but in this region, dio2 mRNA expression was four times higher in LP- than in SP-gestated pups (P < 0.01). Contrastingly, in surrounding brain areas lacking TSHr expression, we saw no effect of gestational photoperiod on deiodinase expression [dio2, subventricular zone of the lateral ventricle: integrated optic density (IOD): LP = 157.6 ± 14.6, SP = 119.8 ± 13.64; dio3, amygdala: LP = 130.50 ± 17.45, SP = 127.5 ± 14.85]. Because dio3 mRNA expression is barely detectable in the MBH of P0 animals, despite being clearly present in other brain areas (Fig. 1), it is likely that maternal photoperiod effects on hypothalamic TH status are initially dio2 mediated. Hence these data indicate that maternal photoperiod acts through the maternal melatonin signal and the fetal PT to determine dio2 status in the newborn hypothalamus.
Fig. 1.
Effects of maternal photoperiod exposure on brain and pituitary gene expression in the neonatal period. Gene expression is shown at postnatal day 0 (P0, Left) and at P15 (Right). For each gene, representative images from in situ hybridization are shown next to integrated optical density (IOD) measurements. (Upper) Type 1 melatonin receptor (mt1) and thyroid stimulating hormone subunit-β (TSHβ) in the pars tuberalis; (Lower) TSH receptor (TSHr), type 2 deiodinase (dio2) and type 3 deiodinase (dio3) in the tanycytes. Data are mean ± SEM of n = 6 individuals. Expression differs significantly between groups. *P < 0.05, **P < 0.01, ***P < 0.001. (Scale bar, 0.5 mm.)
We next examined pituitary and hypothalamic gene expression after the first 2 wk of lactation, during which period we held litters and nursing mothers on the same photoperiods as during gestation. This neonatal window constitutes a “dead zone” for photoperiodic melatonin signaling because pups lose transplacental access to the maternal melatonin signal before their own pineal gland becomes sensitive to the photoperiodic information around the time of weaning (8). By P15, the initial difference in dio2 expression between LP- and SP-gestated pups observed at P0 had disappeared (Fig. 1), despite TSHβ expression remaining higher in LP- compared with SP-gestated pups (P < 0.05). Conversely a pronounced difference in dio3 mRNA appeared in this window, with expression increasing strongly between P0 and P15 in the MBH of SP-gestated pups, but remaining low in their LP-gestated counterparts (P < 0.01, Fig. 1). Associated with these photoperiodic history-dependent differences in deiodinase gene expression, testes at P15 were more than a third heavier in relation to body mass in LP- than in SP-gestated pups [LP gonadosomatic index (GSI) = 0.22 ± 0.01, SP GSI = 0.16 ± 0.02; P < 0.05].
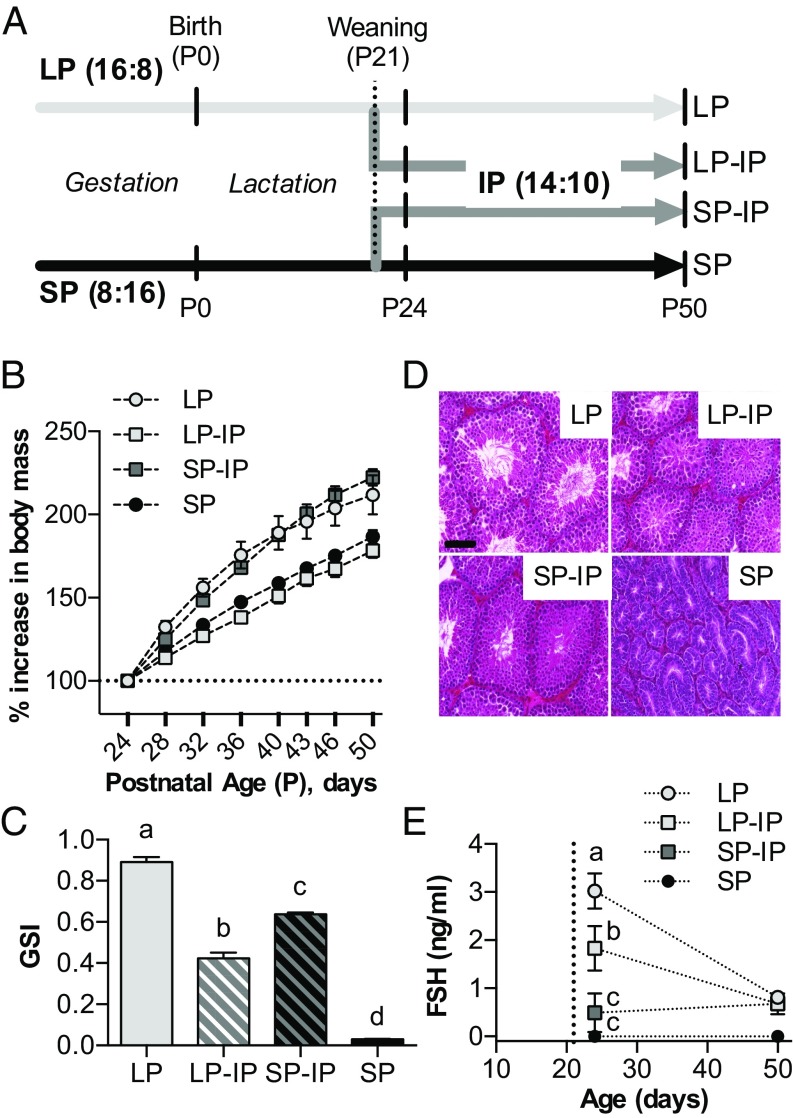
We next asked how maternal photoperiodic history affected subsequent pup photoperiodic sensitivity from weaning until puberty. Following exposure to either LP or SP from conception to weaning 21 d after birth (P21), half of the pups from each group were transferred to intermediate photoperiod (IP, 14 h light/24 h, giving rise to SP-IP and LP-IP groups as shown in Fig. 2A), a well-validated approach for studying photoperiodic history-dependent effects (9, 17). The remainder continued on their gestational photoperiods. The experiment then continued until pups reached P50, with repeated body weight measurement and sampling for testes weight at selected intervals.
Fig. 2.
Effects of photoperiodic history on growth and testicular development. (A) Schematic representation of the timeline and photoperiodic conditions of the experiment. Small vertical bars indicate sampling points for tissue collection. LP = 16 h light/24 h for whole study, SP = 8 h light/24 h for whole study. LP-IP, SP-IP = transfer to 14 h light/24 h at weaning, from LP and SP, respectively. P, postnatal day. (B) Graph depicting offspring increase in body mass from P24 to P50. (C) Offspring gonadosomatic index (GSI) at P50. Data are mean ± SEM of n = 12–14 individuals. (D) Hemotoxylin and eosin-stained testis sections from P50 animals. (Scale bar, 100 μm.) (E) Serum FSH levels following weaning. The vertical dotted line indicates the weaning date (P21). Data are mean ± SEM of n = 6 individuals. Different letters indicate significant differences between groups (P < 0.05).
This experiment revealed a strong history-dependent effect of IP on somatic growth rate, which was markedly increased in SP-IP pups compared with LP-IP pups (P < 0.001, Fig. 2B). In addition, testicular growth was accelerated in SP-IP pups, but arrested in LP-IP pups, and by P50, results showed markedly larger testes in the former group, even after differences in somatic growth were taken into account (P < 0.001 Fig. 2C). Moreover, histological examination of testes at P50 revealed clear differences (Fig. 2D), with LP and SP-IP seminiferous tubules showing large lumen diameter and many mature spermatids, indicative of gonadal activation, whereas SP and LP-IP tubules had small lumens and lacked mature spermatids, indicative of reproductive quiescence. Testicular growth arrest in LP-IP pups commenced within 3 d of IP exposure and was associated with markedly reduced follicle-stimulating hormone (FSH) levels relative to LP control (P < 0.05, Fig. 2E); in contrast, the accelerating effect in SP-IP pups developed more slowly, becoming evident between P24 and P50. Overall these data demonstrate that gestational photoperiodic history has a large impact on photoperiod-dependent somatic and reproductive development in the postweaning period.
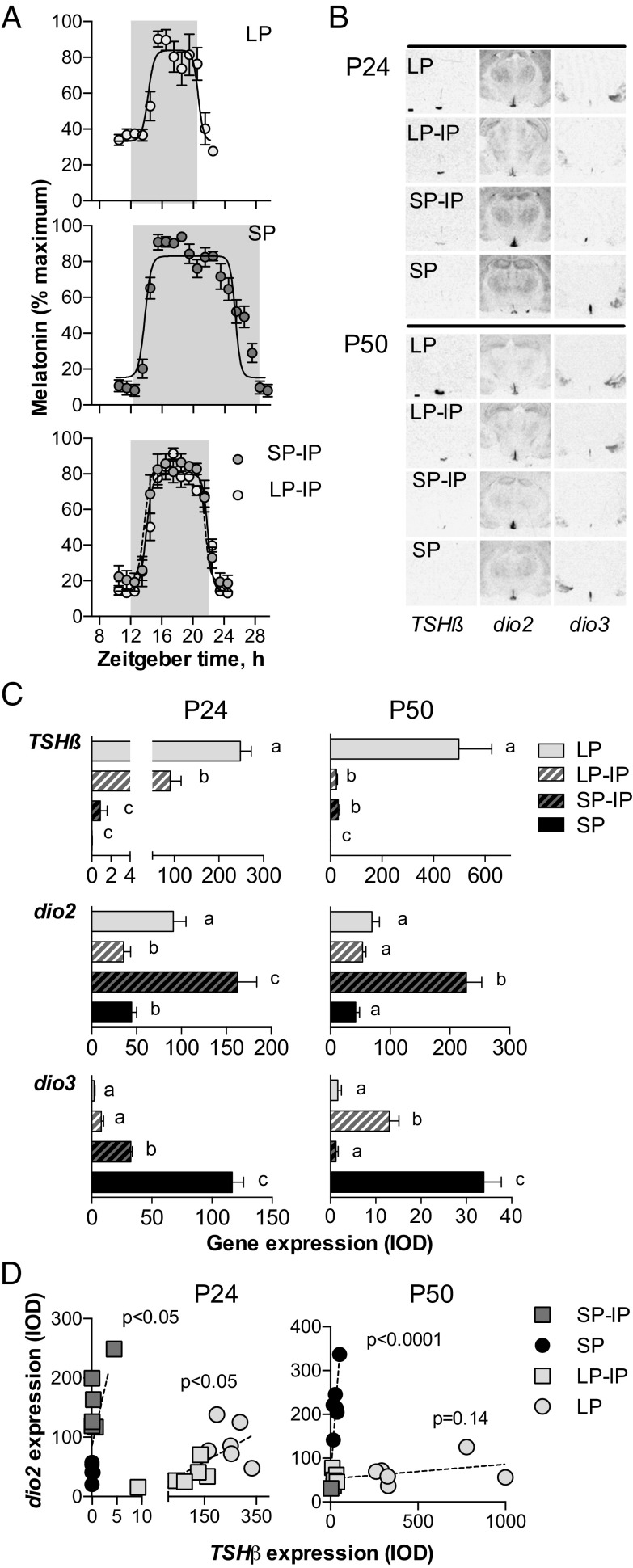
Because photoperiodic effects in mammals are mediated by the pineal production of melatonin, we next asked whether gestational photoperiod affects the profile of melatonin secretion by the pups in response to IP exposure. We implanted microdialysis probes into the pineal gland of weaned pups, allowing individual melatonin profiles to be observed in the window from P26 to P31 (Fig. 3A). As predicted, the longest melatonin profiles were seen in SP-exposed pups (11.65 ± 0.52 h), and the shortest were seen in the LP-exposed pups (6.46 ± 0.16 h), whereas those in IP pups were intermediate, and not significantly affected by gestational history (LP-IP: 7.71 ± 0.39 h; SP-IP: 7.71 ± 0.18 h). This result indicates that gestational photoperiodic history modulates photoperiodic sensitivity at a level beyond pineal melatonin synthesis, either in the melatonin-responsive PT or further downstream.
Fig. 3.
Effects of photoperiodic history at different levels of the photoperiodic response pathway. (A) Profiles of pineal melatonin concentrations obtained by microdialysis. Data are mean ± SEM of n = 5–7 individuals. The shaded area represents the dark period. The time axis is specified as “zeitgeber time”(ZT) where ZT12 is lights off. (B) Autoradiographs of TSHβ, dio2, and dio3 gene expression determined by radioactive in situ hybridization at postnatal day 24 (P24, Left) and P50 (Right). (Scale bar, 500 μm.) (C) Integrated optical density (IOD) measurements of TSHβ in the pars tuberalis, and dio2/dio3 expression in the hypothalamus at P24 and P50. Data are mean ± SEM of n = 6 animals. (D) Scatterplots showing the correlations between TSHβ and dio2 expression at P24 and P50, in offspring gestated on short photoperiod (SP and SP-IP groups) or long photoperiod (LP and LP-IP groups). P values indicate probability that variables are independent.
To assess whether gestational photoperiodic history dependence arises within the PT, we again looked at TSHβ gene expression. At P24, within 3 d of transfer of LP-gestated pups to IP, TSHβ levels fell by more than 50% compared with LP controls (P < 0.001, Fig. 3 B and C). Nevertheless, probably because of the dynamics of TSHβ RNA turnover and the highly divergent initial TSHβ values between SP and LP, LP-IP TSHβ levels remained almost two orders of magnitude higher than in SP-IP (P < 0.01). Consistent with this interpretation, by P50 TSHβ levels in the two IP groups had converged (P = 1, Fig. 3 B and C). This result implies that gestational photoperiod history has a negligible impact on the postweaning response of the PT to melatonin.
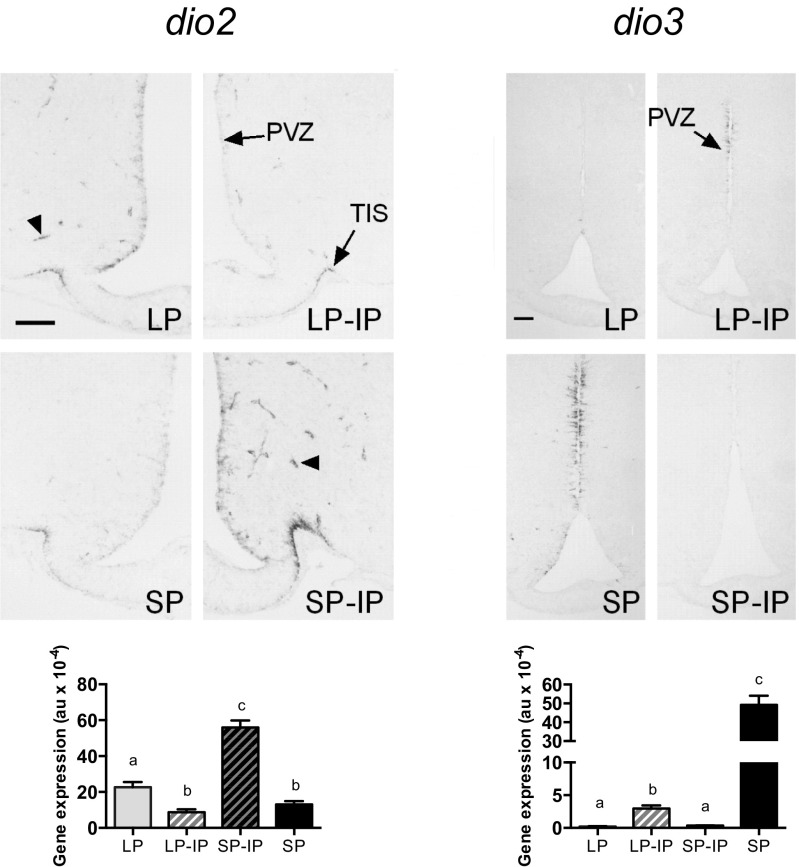
Contrastingly, we observed persistent marked effects of gestational photoperiodic history on the postweaning photoperiodic response at the level of hypothalamic deiodinase gene expression. For dio2, this difference was apparent by P24 with a dramatic stimulation of dio2 seen in SP-IP animals compared with SP controls (P < 0.001)—such that levels of dio2 mRNA in this group were also higher than in the LP control group (P < 0.01, Fig. 3 B and C). Contrastingly, LP-IP animals at P24 had the lowest dio2 expression levels, not significantly different from SP control levels. This pattern was maintained until P50 (Fig. 3 B and C). For dio3, the gestational history-dependent effect was approximately the inverse of that seen for dio2, but the dynamic was slower in some aspects. By P24, transfer of SP-gestated pups to IP caused a dramatic reduction in dio3 levels compared with SP controls (P < 0.001), but levels in LP and LP-IP animals at P24 remained much lower at this time point (Fig. 3 B and C). Contrastingly by P50, SP-IP animals showed the lowest dio3 expression levels, and these were an order of magnitude lower than in LP-IP animals (P < 0.01, Fig. 3 B and C). In line with this reciprocal control of dio2 and dio3 expression, we observed modest changes in total T3 content in hypothalamic blocks (LP: 1.63 ± 0.09 pmol/g; LP-IP: 1.41 ± 0.13 pmol/g; SP-IP: 2.04 ± 0.27 pmol/g; and SP: 1.78 ± 0.01 pmol/g), but not in the cortex (LP: 1.72 ± 0.07; LP-IP: 1.51 ± 0.08; SP-IP: 1.73 ± 0.07; and SP: 1.67 ± 0.36). These data, and the restricted distribution of dio2/dio3-expressing cells (Fig. S1), imply highly localized control of T3 levels within the developing hypothalamus.
Fig. S1.
Dio2 and dio3 gene expression determined by digoxigenin in situ hybridization in offspring at postnatal day 50 (P50). Detailed representative images of dio2 and dio3 expression in the mediobasal hypothalamic region in offspring at P50 in long photoperiod (LP), short photoperiod (SP), or transferred to intermediate photoperiod (IP) on the day of weaning (LP-IP and SP-IP, respectively). (Bottom) Quantification of dio2 and dio3 expression shown above (au, arbitrary units). Different letters indicate differences between groups. Data are plotted as mean ± SEM of n = 6–7 individuals. PVZ, paraventricular zone; TIS, tuberoinfundibular sulcus. Arrowheads point to dio2 expression at tanycyte endfeet surrounding capillary vessels. (Scale bars, 100 μm.)
The marked history dependence of the hypothalamic dio2/dio3 response to IP exposure, despite identical responsiveness at the level of melatonin synthesis and the PT TSHβ response, suggested to us that sensitivity to TSH might lie at the crux of MPP. We therefore compared the correlation between TSHβ and dio2 expression in animals of SP- and LP-gestational history, at both P24 and P50 (Fig. 3D). At P24, we observed a significant positive relationship between these two variables for both SP- (P < 0.05, R2 = 0.43) and LP-gestated (P < 0.05, R2 = 0.37) animals; however, linear regression analysis yielded a slope coefficient (b) about two orders of magnitude larger in SP-gestated animals (P < 0.01, for comparison of b). This disparity became even more pronounced at P50, with LP-gestated animals showing no significant relationship between TSHβ and dio2 expression (P < 0.001, R2 = 0.87 for SP-gestated animals; P = 0.14, R2 = 0.20 for LP-gestated animals).
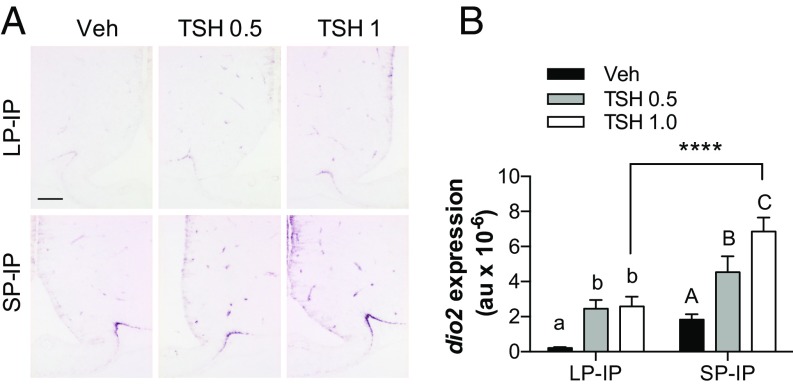
This shifting relationship between TSHβ and dio2 expression as a function of gestational photoperiod is consistent with the hypothesis that photoperiodic history dependence arises as a consequence of shifts in hypothalamic sensitivity to TSH. To test this hypothesis, we injected TSH into the cerebral ventricles of LP-IP and SP-IP pups at P50 and assessed the effect on dio2 gene expression in hypothalamic tanycytes. A TSH dose of 0.5 mIU stimulated dio2 expression in both LP-IP and SP-IP groups compared with the vehicle-infused controls in each photoperiod (P < 0.05 in LP-IP; P < 0.05 in SP-IP; Fig. 4 A and B). However, a higher dose of 1 mIU TSH further increased dio2 expression in the SP-IP (P < 0.05), but not in the LP-IP animals (P = 1), and the resulting level of dio2 expression was more than twofold higher in SP-IP compared with the LP-IP animals (Fig. 4 A and B). Hence the dio2 response to TSH stimulation saturates at a lower level following LP exposure during gestation. This effect is not accounted for by changing TSHr expression, because at P50 this effect was lower in SP-gestated pups (IOD LP = 72.54 ± 5.64; SP = 48.53 ± 5.32, P < 0.05). Moreover, it cannot be accounted for by history-dependent changes in systemic T3 levels feeding back on dio2 mRNA expression (18), because circulating T3 levels at P50 are similar in LP, LP-IP, and SP-IP offspring (ng/mL: LP = 1.09 ± 0.03; LP-IP = 0.89 ± 0.11; SP-IP = 0.83 ± 0.09; and SP = 1.24 ± 0.09; P > 0.05). These data suggest that reduced exposure to TSH during gestation under SP increases the intrinsic capacity of the pup hypothalamus to respond to TSH, through mechanisms lying downstream of TSHr mRNA expression.
Fig. 4.
Photoperiodic history influences the response to central TSH injections. (A) Representative images of dio2 expression in the mediobasal hypothalamic region, detected by digoxigenin (DIG)-based in situ hybridization. Images are from P50 offspring held on IP after gestation on LP or SP, 4 h after infusion with vehicle (Veh) or the indicated doses of TSH in milliinternational units. (Scale bar, 100 µm.) (B) Quantitation of dio2 expression from DIG-based in situ hybridization. Data are mean ± SEM of n = 4–8 individuals. ****, significantly increased expression compared with corresponding treatment in LP-IP animals. Within each photoperiodic history group different superscripts are statistically different, P < 0.05.
Discussion
Our data demonstrate that maternal melatonin acts via the fetal PT to regulate levels of locally acting TSH, and this leads in turn to modulation of TH metabolism in the hypothalamus. Because TH is a key hormonal regulator of brain development, these data provide a mechanistic pathway for the fetal programming effects of maternal melatonin. Within this pathway, we show that deiodinase-expressing tanycytes are the anatomical substrate through which photoperiodic history dependence emerges, mediated via persistent shifts in TSH sensitivity (Fig. 5).
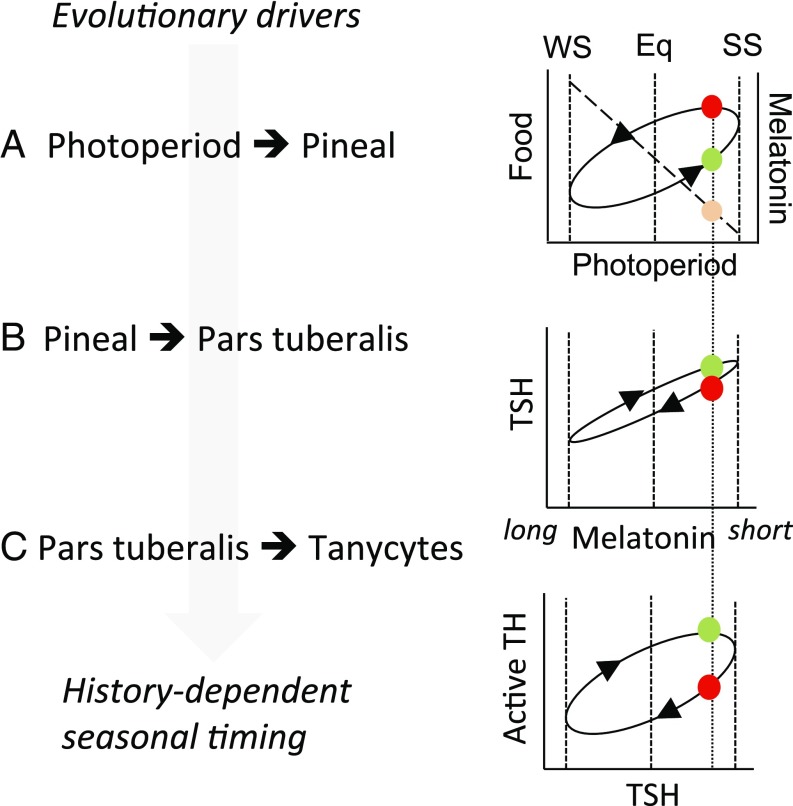
Fig. 5.
Encoding of photoperiodic history dependence. (A) Environmental conditions influencing bioenergetic status (e.g., food) follow an elliptical history-dependent relationship to photoperiod. At a given photoperiod in the spring (green circle), there is less food available, but favorable conditions in prospect, compared with the corresponding photoperiod in the autumn, when more food is available but unfavorable conditions are in prospect. This difference between spring and autumn is the evolutionary driver for history-dependent interpretation of photoperiod. The nocturnal melatonin signal duration is proportional to night length regardless of history, so melatonin signals on equivalent spring and autumn photoperiods are indistinguishable (orange symbol). (B) The pars tuberalis transduces melatonin signal duration into production of TSH. No history dependence was observed at this level in the present study, but effects have been reported in other seasonal paradigms (27, 28) and may contribute to ellipsis in the encoded response to photoperiod. (C) Strong history dependence is seen in the hypothalamic response to TSH, giving a history-dependent internal representation of calendar time. Eq, equinox; SS, summer solstice; WS, winter solstice.
Melatonin receptor expression in the fetal pituitary is under tight spatiotemporal control (19), with distinctive regulation of expression in pars distalis gonadotrophs and PT-specific thyrotrophs (20). The former are transiently expressed in the perinatal period and appear to directly regulate LH secretion, before establishment of descending hypothalamic control through GnRH secretion (20). In contrast to this transitory influence of melatonin, the present study defines a pathway through which melatonin exerts persistent developmental effects. This pathway starts before birth, because large photoperiodic differences in both PT TSHβ and hypothalamic dio2 expression were seen in newborn animals, and these were associated with carry-over effects through the neonatal period before pups become autonomously sensitive to the photoperiod.
Although in the present study we focused primarily on the reproductive axis, seasonal changes in hypothalamic T3 availability are also linked to body mass change and the capacity to express torpor (15, 21). Independent of seasonal studies, the ependymal zone in which dio2/dio3-expressing tanycytes reside is a recognized hypothalamic stem cell niche, implicated in maintenance of normal metabolic regulation (22). These observations lead us to propose that the effects of MPP on T3 levels exert persistent influences on the development of hypothalamic neuronal networks controlling multiple aspects of metabolic physiology. Consistent with this hypothesis, knockout of dio3 in mice affects T3 titers in the developing arcuate and paraventricular nuclei and leads to the appearance of a lean metabolic phenotype in later life (23). Finally it is interesting to note that, neonatal leptin-dependent effects on the development of metabolic regulation appear to be mediated via leptin receptors transiently expressed in the same ependymal region in which dio2/dio3-expressing tanycytes are found (24). These observations suggest that the tanycyte region is a convergence point for different influences on the programming of hypothalamic function in early life.
MPP does not alter circadian control of pineal melatonin production in the offspring, because their nocturnal melatonin peak was not affected by the gestational photoperiod. This result mirrors the lack of history dependence in melatonin secretion and in the acute response of the PT to melatonin in adult mammals (25). Rather, our results support the idea that photoperiodic programming arises from the downstream neuroendocrine interpretation of the offspring’s melatonin pattern. Subsequent to the neonatal period, we observed persistent maternal photoperiodic history-dependent effects on the sensitivity of tanycytes to TSH. We and others have also studied the effects of photoperiodic history on reproduction and the TSH–DIO2/DIO3 axis in adult sheep (26, 27). Here, the levels at which history dependence arise differ for the spontaneous summer–autumn reactivation of reproduction (studied by holding animals on LP for extended periods) compared with winter–spring reproductive inhibition (studied by holding animals on SP for extended periods). For the former, history-dependent effects at the level of PT TSH production appear to be important (26, 27), whereas, for the latter, echoing the present study, large increases in dio2 expression are associated with a slight rise in PT TSH expression (26). Similarly, European hamsters maintained in constant LP show sustained cycles in PT TSH and hypothalamic dio2 expression, correlated with changes in physiological status (28). Collectively, these observations suggest that the internal representation of calendar time (i.e., circannual timekeeping) may emerge through photoperiodic history dependence both in the PT response to photoperiod and in the tanycyte response to the PT. The relative importance of these effects may vary between species and seasons, and effects at more than one level may enhance contrast in the internal representation of spring and autumn (Fig. 5).
Although our data exclude a change in TSHr mRNA expression as the cause of the changing capacity of tanycytes to respond to TSH, we observed persistent attenuation of TSHR signaling in LP-gestated pups. The large extracellular domain of the TSHR is subject to extensive posttranslational modification, including glycosylation and proteolytic cleavage, and these processes are linked to shifts in TSH sensitivity (29). Photoperiodic history-dependent regulation of TSH sensitivity may operate through similar mechanisms or through downstream effects on G protein-dependent activation of the cAMP–CREB pathway linking TSHR to transcriptional control of dio2 gene expression. In thyroid tissue, refractoriness of the cAMP-dependent pathway to TSH-dependent stimulation, following chronic exposure to TSH has been described previously (30). Additionally, early life hormonal programming has been associated with epigenetic mechanisms, involving changes in chromatin state or DNA methylation (31). Both deiodinases are known targets of epigenetic regulation. During muscle cell differentiation, dio2 gene expression is up-regulated by histone demethylation and acetylation leading to myoblast differentiation (32), whereas dio3 sits in an imprinted locus, tightly regulated by epigenetic mechanisms essential for normal growth and viability (33). In addition, it has been reported that photoperiodic information regulates dio3 gene expression by acting upon promoter methylation in adult Siberian hamsters (34). In view of these results, we favor a hypothesis where TSHR-mediated changes in the epigenetic regulation of dio2 gene expression underlie the shift in TSH sensitivity caused by the MPP effect on fetal TSH signaling. Other PT-secreted factors acting upon tanyctes, for example neuromedin U (35) may also contribute to this epigenetic process.
TSH-mediated modulation of tanycyte function has emerged as the lynchpin for seasonal neuroendocrine regulation (12, 15, 36), reflecting the manifold actions of tanycytes as metabolic regulators (37). These include actions as leptin and glucose sensors relaying metabolic feedback signals to the arcuate nucleus (38, 39) and as physical barriers controlling access of blood/CSF signals to the brain and release of hypothalamic neuropeptides to the pituitary portal system (40, 41). Hence photoperiodic history-dependent adjustment of tanycyte sensitivity to TSH constitutes an effective proximate mechanism to meet the ultimate evolutionary drive to exploit day length as a calendar synchronizer for annual life-history programs (Fig. 5). Defining the mechanisms through which TSH sensitivity shifts arise and persist, and the breadth of other maternal programming influences mediated by tanycytes, will be important avenues for understanding how early life environments shape metabolic physiology.
Methods
MPP Experiment.
Animal experiments were approved by the Université de Strasbourg institutional review board (Comité d'Éthique en Matière d'Expérimentation Animale de Strasbourg). Siberian hamsters were mated on LP, before transfer of half of the pregnant females to SP (Fig. 2A). Dams and pups remained on the same photoperiod until weaning. From weaning, half of the animals in each litter remained on the same photoperiod and half were transferred to IP. All sample groups contained offspring from at least three different litters. Animals were killed in the midlight phase and weighed before blood sampling and harvesting of tissues.
Pineal Microdialysis.
Pineal microdialysis was performed between P26 and P31 (42). Animals were allowed to recover for 3 d after implantation of dialysis probes before dialysis perfusion. Dialysates were sampled hourly from 2 h before lights off until 2 h after lights on. Details of cannula placement and dialysis procedures can be found in SI Materials and Methods.
Intracerebroventricular TSH Injection.
Male offspring were implanted with guide cannulae into the lateral ventricles at P43, and received intracerebroventricular (ICV) injections (5 µL) of Ringer solution containing specified doses of TSH at P50. Four hours later, animals were killed and perfused with 4% paraformaldehyde, and brains were dissected.
In Situ Hybridization.
Coronal sections (16 µm) of snap-frozen brain tissue, covering the MBH at 160-µm resolution (128 µm in P0 animals) were processed for in situ hybridization using 35S- or digoxigenin-labeled probes (26, 28). Details of probe labelling, hybridization and image analysis can be found in SI Materials and Methods.
Hormone Analysis.
Plasma FSH, T3 levels, and T3 content in brain tissue blocks were determined by radioimmunoassay (43, 44).
Testis Histology.
Fixed and embedded testis samples were sectioned (5 µm) on a microtome and stained with hematoxylin-eosin.
Statistics.
Data were assessed for normality by Kolmogorov–Smirnov test, and treatment effects were assessed by ANOVA. Post hoc comparisons were made by Bonferroni tests. The relationship between the expression of TSHβ and dio2 was assessed by F test to determine whether slope coefficients (b) differed between LP- and SP-gestated animals. The threshold for statistical significance was set at P < 0.05.
SI Materials and Methods
Animals.
Animals were kept in type 2 Macrolon cages in rooms with constant temperature of 22 ± 2 °C and humidity of 55 ± 5%, with food (105 Rodents diet, Safe-diets) and water access ad libitum. Sexually mature Siberian hamsters (Phodopus sungorus) maintained in controlled LP were selected for coupling in several cohorts along the experiment.
All animal experiments were conducted at the Chronobiotron (CNRS-UMS 3415) at the University of Strasbourg in accordance with the French regulations (décret 2013–118) in application of European directive UE/63/2010 on the protection of animals used for scientific purposes. All protocols were submitted to the local ethical committee (Comité d’Éthique en Matière d’Expérimentation Animale de Strasbourg) (CREMEAS) and authorized by the French National Law for animal experimentation (ref. 02015011315455522).
Experimental Design.
Sexually mature females received a 100-µL i.p. injection of 10 units of human chorionic gonadotropin (hCG), Chorulon 1500 (Intervet) in saline at the beginning of the afternoon to stimulate ovulation (45) and immediately coupled to sexually mature males. Pregnant females and offspring after birth and after weaning were maintained in the experimental conditions described in the main text. A series of cohorts was used for the different studies as detailed below.
A first cohort of animals was used for gene expression studies, as indicated in the main text. Frozen hypothalamic and brain cortex blocks from females in this cohort were used for tissue T3 determination as explained below.
A second cohort of offspring was used for determination of pineal melatonin content by pineal microdialysis.
A third cohort of animals was sampled at P50 only for a more detailed anatomical analysis of hypothalamic gene expression by nonradioactive in situ hybridization. After death, these animals were fixed by transcardiac perfusion with paraformaldehyde (PFA) 4% in 0.1 M phosphate buffer (pH 7.4). Brains were immediately dissected from the skull, postfixed in the same fixative for 24 h, and dehydrated in serial alcohols.
A fourth cohort of offspring of the LP-IP and SP-IP groups only was used for intracerebroventricular (ICV) TSH injections.
Radioactive in Situ Hybridization and Image Analysis.
In situ hybridizations were performed as described previously (26). Coronal sections of 16 µm were cut on a cryostat and mounted on polylysine-coated slides and stored at −80 °C. Briefly, frozen sections were fixed in ice-cold PFA 4% followed by dehydration in ethanols. Riboprobes were prepared by plasmid linearization and transcribed in vitro using 35S-UTP, followed by column purification to remove unincorporated nucleotides. Sections were hybridized overnight at 60 °C using ∼5 × 105 cpm labeled probe per slide. The next day, slides were subjected to digestion with RNase-A and stringency washes with decreasing concentrations of sodium citrate buffer to remove nonspecific probe binding. Slides were dehydrated and exposed to film autoradiography (Kodak), with exposure between 3 and 10 d according to intensity of hybridization signal. Films were scanned with an Epson 1640XL transmittance scanner at 720 dpi along with a calibrated series of optical density (OD) standards. Calibrated integrated OD (IOD) measurements of labeling above a threshold defined by slide background OD were made for each defined region of interest using ImageJ software (NIH).
Probes used were: antisense Siberian hamster Dio2 (36), Dio3, and TSH-r (46); antisense rat TSHβ (GenBank accession M10902); and antisense mouse MT1 (11).
Pineal Microdialysis.
The active dialysis area measured 1 mm and the probe was implanted 0.5 mm ventral to the skull surface and 0.4 mm posterior to lambda. After surgery, animals were allowed to recover for 3 d in individual cages before dialysis perfusion. Pineal dialysates were sampled every hour from 2 h before dark onset until 2 h after dark offset during each photoperiod schedule.
The mean body weight of the animals at the time of surgery was 16.33 ± 0.40 g. For surgery, animals received a s.c. injection of buprenorphine (0.05 mg/kg, Buprecare, Axience). They were anesthetized with i.p. injections of xylazine (2.5 mg/kg, Rompun 2%, Bayer) and tiletamine/zolazepam (50 mg/kg, Zoletil 50, Virbac).
Melatonin concentrations in dialysates were determined in duplicate 25-μL samples by radioimmunoassay using a specific rabbit antiserum (R 19540) provided by Institut National de la Recherche Agronomique at a final dilution of 1/100,000 and [125I]-2-iodomelatonin labeling. The sensitivity limit of the assay was 0.50 pg per tube. The direct melatonin assay has been previously validated for pineal dialysates (42).
For each animal, melatonin concentrations were expressed as a function to external zeitgeber time (ZT, where ZT12 = lights off). To characterize the pattern of melatonin secretion, the obtained individual profiles were fitted with a nonlinear regression analysis performed with SigmaPlot version 12 (Systat Software) with the following equation:
where y was the nth data point, x represents the time point of the nth point, Y0 the basal level during the daytime, and Yampl the amplitude of the nocturnal peak over the basal level. The multiplicative factors in the exponentials (2.5 and 3) represent the slopes and were chosen to fit the observed profiles in this study. The duration of the melatonin peak was determined as the difference between half decrease (DT50) and half increase (IT50) of the peak.
The mean peak length obtained from the individual regressions for the four photoperiodic groups (LP, SP, LP-IP, and SP-IP) was compared by a one-way ANOVA followed by a Tukey’s multiple comparison test using SigmaPlot version 12 (Systat Software). The threshold for statistical significance was set at P < 0.05.
Nonradioactive in Situ Hybridization and Semiquantitative Image Analysis.
For a better anatomical detail, dio2 and dio3 expression was analyzed at P50 in the four photoperiodic conditions of the experiments using the antisense Siberian hamster probes described above. In situ hybridization was performed as in ref. 28. Briefly, the probes were digoxigenin (DIG) labeled using the DIG RNA labeling kit (Roche), according to the manufacturer’s instructions. Fixed brains were cut into serial 12-µm coronal sections using a microtome (Leica Microsystems). One in ten sections through the hypothalamus were mounted on SuperFrost ultraplus slides (Menzel-Glaser) and stored at −80 °C. On the day of hybridization, sections were postfixed in PFA 4% in 0.1 M phosphate buffer for 10 min at room temperature, rinsed with PBS, treated with 0.5 µg/mL proteinase K (Roche) for 30 min at 37 °C, rinsed with ice-cold PFA 2% in phosphate buffer, rinsed again in PBS, acetylated twice for 10 min with 0.25% acetic anhydride in 100 mM triethanolamine, and finally equilibrated in 5× saline sodium citrate (SSC) 0.05% Tween-20 twice for 5 min at room temperature. Sections were hybridized with 4 µg/mL of antisense probe in a medium containing 50% formamide, 5× SSC, 5× Denhardt’s solution, 0.1% Tween 20 (Tw20), 0.04% DEPC, and 1 mg/mL salmon sperm DNA for 40 h at 60 °C. High-stringency washes were performed with 0.1× SSC 0.05% Tw20 at 72 °C to reduce nonspecific labeling. DIG-labeled riboprobes were detected using an alkaline phosphatase (AP)-labeled antidigoxigenin antibody at 1:5,000 (Roche). The AP activity was detected with nitroblue tetrazolium and bromo-chloro-indolyl phosphate. After detection, slides were premounted using Crystalmount aqueous mounting medium (Sigma-Aldrich) and mounted with Eukitt (Sigma-Aldrich).
Acknowledgments
The authors thank Dominique Ciocca and Aurore Senser (Chronobiotron, UMS 3415, Strasbourg) for excellent animal care; Manuel Tena Sempere for plasma FSH assay; Veerle Darras for T3 assays; and Kevin Mackenzie, Gillian Milne, and Mike Birnie for histology. This work was supported by the University of Strasbourg Institute of Advanced Studies (Project “Epigenetic Light” to D.H.). C.S.d.M. was supported by a doctoral fellowship funded by the Région d’Alsace and the University of Aberdeen.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1702943114/-/DCSupplemental.
References
- 1.Barker DJ. Fetal growth and adult disease. Br J Obstet Gynaecol. 1992;99:275–276. doi: 10.1111/j.1471-0528.1992.tb13719.x. [DOI] [PubMed] [Google Scholar]
- 2.Wilson PR, Tarttelin MF. Studies on sexual differentiation of sheep. I. Foetal and maternal modifications and post-natal plasma LH and testosterone content following androgenisation early in gestation. Acta Endocrinol (Copenh) 1978;89:182–189. [PubMed] [Google Scholar]
- 3.Gluckman PD, Hanson MA, Spencer HG, Bateson P. Environmental influences during development and their later consequences for health and disease: Implications for the interpretation of empirical studies. Proc R Soc Biol Sci Ser B. 2005;272:671–7. doi: 10.1098/rspb.2004.3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horton TH. Fetal origins of developmental plasticity: Animal models of induced life history variation. Am J Hum Biol. 2005;17:34–43. doi: 10.1002/ajhb.20092. [DOI] [PubMed] [Google Scholar]
- 5.Bassett JM, Curtis N, Hanson C, Weeding CM. Effects of altered photoperiod or maternal melatonin administration on plasma prolactin concentrations in fetal lambs. J Endocrinol. 1989;122:633–643. doi: 10.1677/joe.0.1220633. [DOI] [PubMed] [Google Scholar]
- 6.Weaver DR, Reppert SM. Maternal melatonin communicates daylength to the fetus in Djungarian hamsters. Endocrinology. 1986;119:2861–2863. doi: 10.1210/endo-119-6-2861. [DOI] [PubMed] [Google Scholar]
- 7.Horton TH, Stachecki SA, Stetson MH. Maternal transfer of photoperiodic information in Siberian hamsters. IV. Peripubertal reproductive development in the absence of maternal photoperiodic signals during gestation. Biol Reprod. 1990;42:441–449. doi: 10.1095/biolreprod42.3.441. [DOI] [PubMed] [Google Scholar]
- 8.Yellon SM, Tamarkin L, Goldman BD. Maturation of the pineal melatonin rhythm in long- and short-day reared Djungarian hamsters. Experientia. 1985;41:651–652. doi: 10.1007/BF02007704. [DOI] [PubMed] [Google Scholar]
- 9.Stetson MH, Elliott JA, Goldman BD. Maternal transfer of photoperiodic information influences the photoperiodic response of prepubertal Djungarian hamsters (Phodopus sungorus sungorus) Biol Reprod. 1986;34:664–669. doi: 10.1095/biolreprod34.4.664. [DOI] [PubMed] [Google Scholar]
- 10.Horton TH. Growth and reproductive development of male Microtus montanus is affected by the prenatal photoperiod. Biol Reprod. 1984;31:499–504. doi: 10.1095/biolreprod31.3.499. [DOI] [PubMed] [Google Scholar]
- 11.Dardente H, Klosen P, Pévet P, Masson-Pévet M. MT1 melatonin receptor mRNA expressing cells in the pars tuberalis of the European hamster: Effect of photoperiod. J Neuroendocrinol. 2003;15:778–786. doi: 10.1046/j.1365-2826.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 12.Hanon EA, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 13.Ono H, et al. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA. 2008;105:18238–18242. doi: 10.1073/pnas.0808952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gereben B, et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr Rev. 2008;29:898–938. doi: 10.1210/er.2008-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett P, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 16.Hazlerigg DG, Simonneaux V. Seasonal regulation of reproduction in mammals. In: Plant T, Zeleznik A, editors. Knobil and Neill’s Physiology of Reproduction. 4th Ed. Elsevier; Boston: 2015. pp. 1575–1604. [Google Scholar]
- 17.Ebling FJP, Wood RI, Suttie JM, Adel TE, Foster DL. Prenatal photoperiod influences neonatal prolactin secretion in the sheep. Endocrinology. 1989;125:384–391. doi: 10.1210/endo-125-1-384. [DOI] [PubMed] [Google Scholar]
- 18.Tu HM, et al. Regional distribution of type 2 thyroxine deiodinase messenger ribonucleic acid in rat hypothalamus and pituitary and its regulation by thyroid hormone. Endocrinology. 1997;138:3359–3368. doi: 10.1210/endo.138.8.5318. [DOI] [PubMed] [Google Scholar]
- 19.Carlson LL, Weaver DR, Reppert SM. Melatonin receptors and signal transduction during development in Siberian hamsters (Phodopus sungorus) Brain Res Dev Brain Res. 1991;59:83–88. doi: 10.1016/0165-3806(91)90032-e. [DOI] [PubMed] [Google Scholar]
- 20.Johnston JD, et al. Gonadotrophin-releasing hormone drives melatonin receptor down-regulation in the developing pituitary gland. Proc Natl Acad Sci USA. 2003;100:2831–2835. doi: 10.1073/pnas.0436184100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy M, et al. Effects of manipulating hypothalamic triiodothyronine concentrations on seasonal body weight and torpor cycles in Siberian hamsters. Endocrinology. 2012;153:101–112. doi: 10.1210/en.2011-1249. [DOI] [PubMed] [Google Scholar]
- 22.Rizzoti K, Lovell-Badge R. Pivotal role of median eminence tanycytes for hypothalamic function and neurogenesis. Mol Cell Endocrinol. 2017;445:7–13. doi: 10.1016/j.mce.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 23.Wu Z, Martinez ME, St Germain DL, Hernandez A. Type 3 deiodinase role on central thyroid hormone action affects the leptin-melanocortin system and circadian activity. Endocrinology. 2017;158:419–430. doi: 10.1210/en.2016-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cottrell EC, Mercer JG, Ozanne SE. Postnatal development of hypothalamic leptin receptors. In: Litwack G, editor. Vitamins and Hormones. Elsevier; Burlington, MA: 2010. pp. 201–217. [DOI] [PubMed] [Google Scholar]
- 25.Lincoln GA, Johnston JD, Andersson H, Wagner G, Hazlerigg DG. Photorefractoriness in mammals: Dissociating a seasonal timer from the circadian-based photoperiod response. Endocrinology. 2005;146:3782–3790. doi: 10.1210/en.2005-0132. [DOI] [PubMed] [Google Scholar]
- 26.Sáenz de Miera C, et al. Circannual variation in thyroid hormone deiodinases in a short-day breeder. J Neuroendocrinol. 2013;25:412–421. doi: 10.1111/jne.12013. [DOI] [PubMed] [Google Scholar]
- 27.Wood SH, et al. Binary switching of calendar cells in the pituitary defines the phase of the circannual cycle in mammals. Curr Biol. 2015;25:2651–2662. doi: 10.1016/j.cub.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sáenz de Miera C, et al. A circannual clock drives expression of genes central for seasonal reproduction. Curr Biol. 2014;24:1500–1506. doi: 10.1016/j.cub.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 29.Kleinau G, Neumann S, Grüters A, Krude H, Biebermann H. Novel insights on thyroid-stimulating hormone receptor signal transduction. Endocr Rev. 2013;34:691–724. doi: 10.1210/er.2012-1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field JB, Chou MC, Titus G, Worden W. Recovery from thyroid-stimulating hormone-induced refractoriness in thyroid slices: Effect of removal of hormone and new protein synthesis. Endocrinology. 1982;110:820–824. doi: 10.1210/endo-110-3-820. [DOI] [PubMed] [Google Scholar]
- 31.Godfrey KM, et al. Epigenetic gene promoter methylation at birth is associated with child’s later adiposity. Diabetes. 2011;60:1528–1534. doi: 10.2337/db10-0979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ambrosio R, et al. Epigenetic control of type 2 and 3 deiodinases in myogenesis: Role of lysine-specific demethylase enzyme and FoxO3. Nucleic Acids Res. 2013;41:3551–3562. doi: 10.1093/nar/gkt065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Charalambous M, Hernandez A. Genomic imprinting of the type 3 thyroid hormone deiodinase gene: Regulation and developmental implications. Biochim Biophys Acta. 2013;1830:3946–3955. doi: 10.1016/j.bbagen.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad Sci USA. 2013;110:16651–16656. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Helfer G, Ross AW, Morgan PJ. Neuromedin U partly mimics thyroid-stimulating hormone and triggers Wnt/β-catenin signalling in the photoperiodic response of F344 rats. J Neuroendocrinol. 2013;25:1264–1272. doi: 10.1111/jne.12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Klosen P, Sébert ME, Rasri K, Laran-Chich M-P, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB J. 2013;27:2677–2686. doi: 10.1096/fj.13-229559. [DOI] [PubMed] [Google Scholar]
- 37.Rodríguez EM, et al. Hypothalamic tanycytes: A key component of brain-endocrine interaction. Int Rev Cytol. 2005;247:89–164. doi: 10.1016/S0074-7696(05)47003-5. [DOI] [PubMed] [Google Scholar]
- 38.Balland E, et al. Hypothalamic tanycytes are an ERK-gated conduit for leptin into the brain. Cell Metab. 2014;19:293–301. doi: 10.1016/j.cmet.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frayling C, Britton R, Dale N. ATP-mediated glucosensing by hypothalamic tanycytes. J Physiol. 2011;589:2275–2286. doi: 10.1113/jphysiol.2010.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yamamura T, Hirunagi K, Ebihara S, Yoshimura T. Seasonal morphological changes in the neuro-glial interaction between gonadotropin-releasing hormone nerve terminals and glial endfeet in Japanese quail. Endocrinology. 2004;145:4264–4267. doi: 10.1210/en.2004-0366. [DOI] [PubMed] [Google Scholar]
- 41.Prevot V, et al. Definitive evidence for the existence of morphological plasticity in the external zone of the median eminence during the rat estrous cycle: Implication of neuro-glio-endothelial interactions in gonadotropin-releasing hormone release. Neuroscience. 1999;94:809–819. doi: 10.1016/s0306-4522(99)00383-8. [DOI] [PubMed] [Google Scholar]
- 42.Herwig A, Pévet P, Bothorel B, Steinlechner S, Saboureau M. Trans-pineal microdialysis in the Djungarian hamster (Phodopus sungorus): a tool to study seasonal changes of circadian clock activities. J Pineal Res. 2006;40:177–183. doi: 10.1111/j.1600-079X.2005.00296.x. [DOI] [PubMed] [Google Scholar]
- 43.García-Galiano D, Pinilla L, Tena-Sempere M. Sex steroids and the control of the Kiss1 system: Developmental roles and major regulatory actions. J Neuroendocrinol. 2012;24:22–33. doi: 10.1111/j.1365-2826.2011.02230.x. [DOI] [PubMed] [Google Scholar]
- 44.Reyns GE, Venken K, Morreale de Escobar G, Kühn ER, Darras VM. Dynamics and regulation of intracellular thyroid hormone concentrations in embryonic chicken liver, kidney, brain, and blood. Gen Comp Endocrinol. 2003;134:80–87. doi: 10.1016/s0016-6480(03)00220-x. [DOI] [PubMed] [Google Scholar]
- 45.Theuring F, Hansmann I. Follicular development in immature Djungarian hamsters (Phodopus sungorus) and the influence of exogenous gonadotropins. Biol Reprod. 1986;35:407–412. doi: 10.1095/biolreprod35.2.407. [DOI] [PubMed] [Google Scholar]
- 46.Herwig A, et al. Hypothalamic ventricular ependymal thyroid hormone deiodinases are an important element of circannual timing in the Siberian hamster (Phodopus sungorus) PLoS One. 2013;8:e62003. doi: 10.1371/journal.pone.0062003. [DOI] [PMC free article] [PubMed] [Google Scholar]