Significance
Molecular mechanisms that establish the body plans of multicellular animals are not fully understood. This study was focused on two key transcription factors, Runt (Run) and Suppressor of Hairless [Su(H)], that act on particular enhancers within cis-regulatory systems. We showed these factors impact anterior-posterior and dorsal-ventral patterning by supporting enhancers to coordinate their action. These repressors can simultaneously regulate pattering across orthogonal axes and act as a counterbalance to the action of ubiquitous activators such as Zelda. Shared use of broad transcription factors, like Su(H)/Run, across axes may help to integrate patterning throughout the embryo and support robust development. Roles for broadly expressed repressors in the regulation of enhancer timing is likely a conserved mechanism of action in higher animals.
Keywords: embryonic patterning, transcriptional repressor, enhancers, Su(H), Runt
Abstract
The role of spatially localized repressors in supporting embryonic patterning is well appreciated, but, alternatively, the role ubiquitously expressed repressors play in this process is not well understood. We investigated the function of two broadly expressed repressors, Runt (Run) and Suppressor of Hairless [Su(H)], in patterning the Drosophila embryo. Previous studies have shown that Run and Su(H) regulate gene expression along anterior-posterior (AP) or dorsal-ventral (DV) axes, respectively, by spatially limiting activator action, but here we characterize a different role. Our data show that broadly expressed repressors silence particular enhancers within cis-regulatory systems, blocking their expression throughout the embryo fully but transiently, and, in this manner, regulate spatiotemporal outputs along both axes. Our results suggest that Run and Su(H) regulate the temporal action of enhancers and are not dedicated regulators of one axis but, instead, act coordinately to pattern both axes, AP and DV.
Patterning of embryos is accomplished through the combinatorial action of transcription factors, many having spatially localized expression domains, but how broadly expressed, often ubiquitous, factors support gene expression is less well understood. In Drosophila embryos, the maternally deposited transcription factors Bicoid and Dorsal are present in gradients oriented along the anterior-posterior (AP) and dorsal-ventral (DV) axes, respectively (1, 2). These transcription factor gradients act as concentration-dependent inputs that pattern each axis, supporting their classification as morphogens. Patterning results from integration of positive and negative input from these and other spatially localized transcriptional activators and repressors to support gene expression within distinct domains along the two orthogonal body axes (1, 3). However, more recent studies have determined that broadly expressed, pioneering activators also play a role. The maternally deposited activator Zelda impacts patterning globally throughout the embryo, influencing gene expression along AP and DV axes (2). Zelda is able to augment the ability of Bicoid and Dorsal, and likely other transcription factors as well, to support activation of gene expression, in part, by increasing their access to DNA (4). Less is known regarding the mechanism of action of ubiquitous, or broadly expressed, repressors.
Broadly expressed repressors Runt (Run) and Suppressor of Hairless [Su(H)] have been linked to patterning the AP and DV axes, respectively (5–7). Run repressor activity influences Bicoid-mediated activation of gap genes by helping to establish posterior boundaries of genes expressed more anteriorly along the AP axis (6). Alternatively, Su(H) acts as a repressor to define boundaries of genes along the DV axis. Whereas Run sets positional boundaries in a particular domain of the early embryo (6), Su(H) acts broadly to counterbalance Dorsal-mediated activation along the DV axis (7). Su(H) and Dorsal binding sites exhibit overlap, and, moreover, increasing or decreasing the ratio of Su(H) to Dorsal binding sites when placed in tandem influences gene boundary positions across the DV axis, suggesting that these factors function antagonistically. These particular studies provided important insight into the roles for Run and Su(H) and also suggested that these transcription factors provide dedicated input to AP or DV axis patterning, respectively.
However, our data here show that Run and Su(H) have more widespread roles in patterning the embryo, as they act to transiently silence the activity of particular enhancers throughout the entire embryo. This leads to delayed action of select enhancers within cis-regulatory systems to regulate gene expression spatiotemporal dynamics across both axes, AP and DV.
Results and Discussion
Similarity of Su(H) DNA Binding Site Consensus to AP Enhancer-Associated Motif and Run Site.
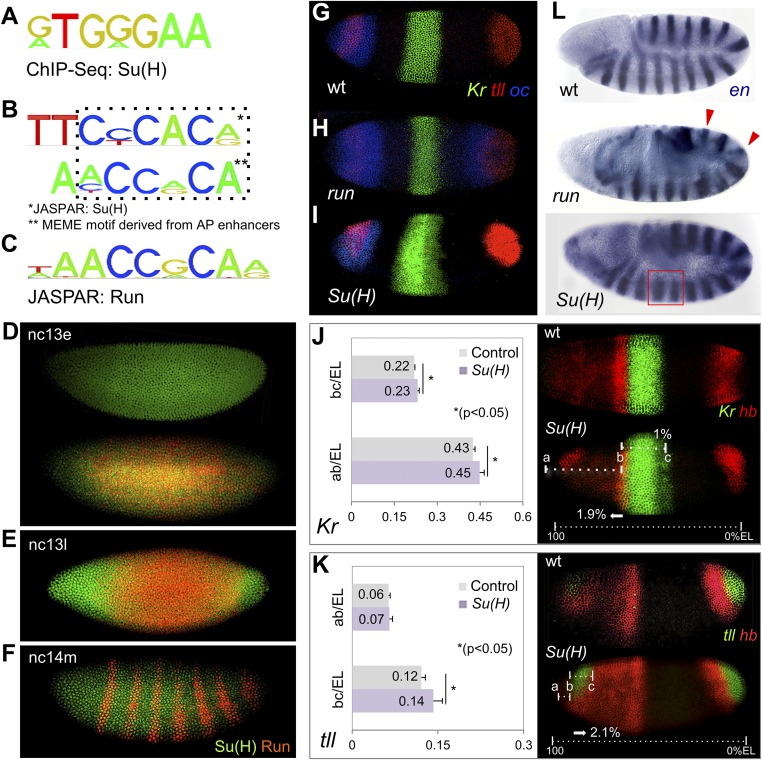
In a previous study, we conducted ChIP experiments coupled with high-throughput sequencing (ChIP-seq) to examine the in vivo binding occupancy of Su(H) transcription factor to DNA within Drosophila embryos (7). We noticed that the Su(H) binding site, derived in vivo from ChIP-seq–identified peaks (Fig. 1A) or in vitro studies (Fig. 1B, Top), overlaps with the MEME motif-derived site identified by Chen et al. (6) as an overrepresented sequence present in AP enhancers, AYCCRCA (Fig. 1B, Bottom). In this previous study, similarity between the Run DNA binding site sequence WAACCRCAR (JASPAR) and this AP enhancer motif led to identification of an earlier role for Run in antagonizing Bicoid-mediated activation (6) (Fig. 1B). We hypothesized that Su(H) might also support a role in regulating patterning along the AP axis, so we began by closely examining the expression pattern of Su(H) compared with that of Run in the early embryo.
Fig. 1.
Su(H) binding site and mutant phenotypes suggest a role for Su(H) in AP patterning. In this and all subsequent figures, Drosophila embryo images are depicted with anterior to the left and dorsal up unless otherwise noted. (A–C) Comparison of Su(H) DNA binding consensus site derived from ChIP-seq (A; 24% of called peaks compared with 6% background) (7), Drosophila Su(H) motif from JASPAR (B, Top; reverse complement relative to A), motif overrepresented within AP enhancers (B, Bottom) (6), and Drosophila Run consensus binding site (C) (6). (D–F) Anti-Su(H) (green) and anti-Run (red) protein staining of embryos at early stage nc 13 (nc13e; D), late nc 13 (nc13l; E), and mid-nc 14 (nc14m; F). (G–I) FISH using riboprobes to detect Kr (green), tll (red), and oc (blue) transcripts in WT embryos (G) as well as in run (H) and Su(H) (I) mutant embryos. (J and K) Ratio of Kr and tll transcript expression domains relative to total EL. Embryos processed by FISH using Kr, hb, and/or tll riboprobes to detect transcripts in WT and Su(H) mutants at early nc 14. The anterior boundaries of the central Kr (J) or anterior tll (K) domains are marked as “ab,” whereas “bc” demarcates the central, dorsal, Kr domain width (J), or anterior tll domain length (K). (L) en transcript expression in germband-elongated embryos of WT, run mutant, or Su(H) mutant genetic backgrounds. Red arrowheads mark odd en stripes in run− embryos, whereas a red box indicates the en interstripe distance in Su(H) mutants.
Even Though Su(H) Is Broadly Expressed, Mutant Embryos Exhibit AP Patterning Defects That Perdure.
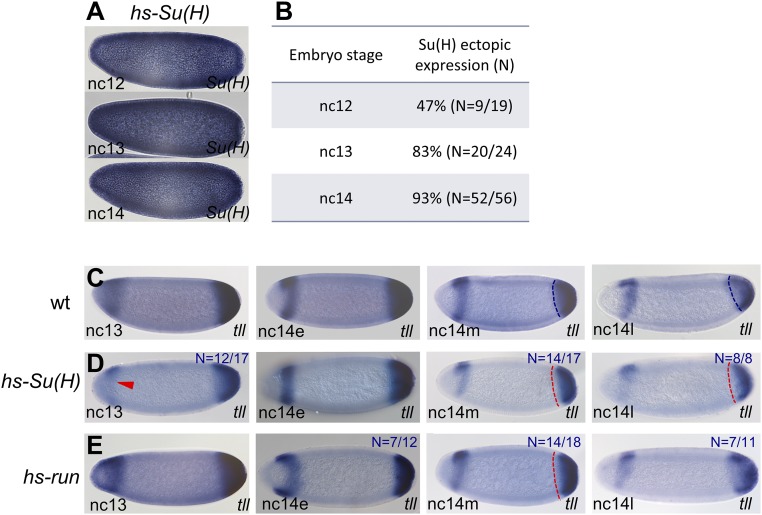
Run and Su(H) transcription factors exhibit dynamic expression patterns, which, at times, include patterns that are localized broadly throughout the embryo (8, 9). Previous studies had shown that Run is expressed in the trunk of Drosophila embryos but excluded from the terminal ends, early during nuclear cycle (nc) 13 (Fig. 1 D and E) (9), whereas, later, at nc 14, the pattern refines into a pair-rule expression pattern composed of seven stripes oriented along the AP axis (Fig. 1F). Most Run studies had focused on its role as regulator of pair-rule expression (10), but it was shown more recently that broadly expressed Run, at early stages, functions as a repressor of gap genes to position the boundary of genes expressed more anteriorly along the AP axis (6). In contrast, the Su(H) protein is broadly expressed in the early embryo (Fig. 1 D–F), even though lower levels of Su(H) are present at the anterior, specifically at nc 13 (Fig. 1D, Top). Su(H) repression activity regulates genes along the DV axis, except in ventrolateral and, possibly, also in ventral regions where input from Notch signaling pathway activation switches Su(H) from repressor to activator (5). It was not clear at first how these broadly expressed repressors could impact patterning spatially across both axes, which are orthogonal. Nevertheless, we investigated mutants for patterning phenotypes.
Gap gene expression along the AP axis was examined in embryos derived from Su(H) mutant germline clone females and compared with phenotypes described previously for zygotic runt (run) mutants. Run had been shown to regulate the expression of a subset of genes expressed along the AP axis (1). For example, in run mutants, the expression domain of the ocelliless (oc) gene is expanded at the posterior end, whereas the domain of Krüppel (Kr) expression is decreased in width (Fig. 1H vs. Fig. 1G). However, not all patterns are changed, as anterior tailless (tll) expression remains essentially unchanged in run mutants. We analyzed Su(H) mutant embryos lacking maternal and zygotic gene function obtained from female germline clones and found that they also exhibit AP patterning defects in addition to the DV patterning phenotypes described previously (5, 7). In Su(H) mutant embryos, oc expression is unchanged; however, the central Kr and anterior tll expression domains are expanded (Fig. 1I vs. Fig. 1G). When these expression domains were measured and normalized to embryo length, we found evidence that the boundary positions of Kr and tll were expanded posteriorly in the Su(H) mutants (Fig. 1J,K). Although run and Su(H) mutant embryo phenotypes are different, these results suggest that both genes play a role in patterning the AP axis.
Furthermore, these mutants exhibit phenotypes affecting expression of engrailed (en) at later stages (Fig. 1L and Fig. S1). In embryos undergoing germband elongation, En transcription factor, a segment polarity factor, is expressed in 14 stripes along the length of embryos and controls segmentation (11). We found that en phenotypes are exhibited by Su(H) mutants, as shown previously for run mutants (12), but the phenotypes differ. En stripes are broadened in Su(H) mutants (Fig. 1L), and the interstripe distance is increased upon Su(H) ectopic expression (Fig. S1C vs. Fig. S1 A and B). These results show that Run and Su(H) mutations exhibit lasting effects on AP patterning.
Fig. S1.
Ectopic expression of Runt and Su(H) impacts En expression in germband-elongated embryos. Germband-elongated embryos are oriented with anterior aspect to the left. In situ hybridization using a riboprobe to en shows expression within 14 stripes in WT embryos undergoing germband elongation (A). Upon ectopic expression of Runt, en is down-regulated in odd-numbered stripes (B; even stripes marked by arrowhead), whereas ectopic expression of Su(H) has a distinct phenotype resulting in an increase in interstripe distance (C, red box). hs-Su(H) and Su(H) mutant embryos exhibit opposite phenotypes: compare red boxes in C and in Fig. 1L.
Su(H) Regulates All Three Types of Bicoid-Bound AP Enhancers Compared with a More Targeted Role for Run.
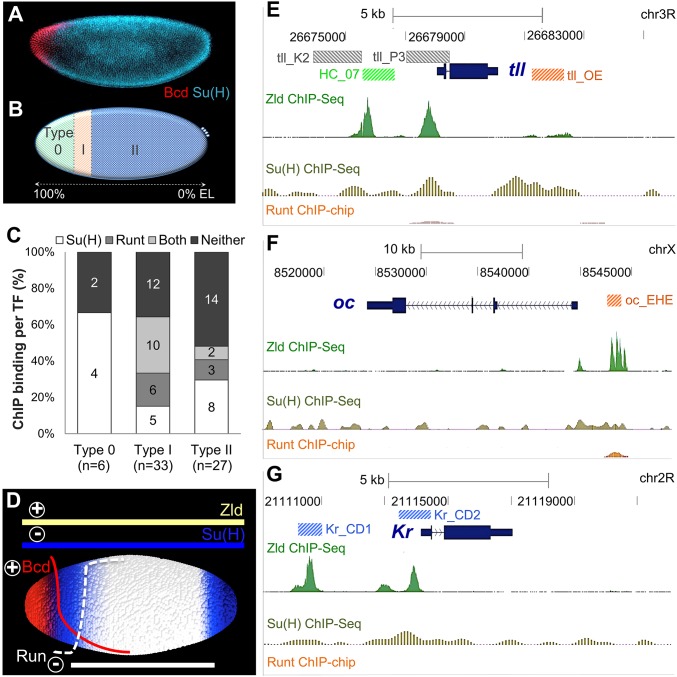
As embryos derived from Su(H) germline clone mutant females exhibit alterations in AP patterning (Fig. 1), we examined whether Su(H) regulates AP enhancers. The Bicoid gradient supports gene expression along the AP axis (Fig. 2A), but posterior boundaries of targets, which fall into a broad domain within the embryo, are likely specified by other factors, possibly Su(H) (Fig. 2B). In a study by Chen et al., 66 enhancers were characterized that support expression along the AP axis, and many were identified based on ChIP-defined occupancy of Bicoid transcription factor to these DNA sequences in vivo (6). This collection of enhancers was classified into three groups based on the position of posterior boundaries of expression supported by these reporter constructs: six type 0 patterns [boundaries in 100–85% egg length (EL) domain]; 33 type I patterns (boundaries in 85–75% EL); and 27 type II patterns (boundaries in 75–0% EL domain; Fig. 2B) (6). Zelda is a ubiquitous activator that binds most cis-regulatory sequences in the early Drosophila embryo (13). Cooccupancy of Zelda with other transcription factors, including Bicoid and Run (14), is often associated with many of these AP enhancer sequences. In a previous study, we identified occupancy of Su(H) to many enhancers, supporting expression along the DV axis (7), but, in the course of this work, we also noticed binding to AP enhancers as well. Comparing binding of Zelda, Su(H), and Run shows that these factors often, but not always, exhibit cooccupancy on the DNA in regions shown to act as enhancers (Fig. 2 E–G).
Fig. 2.
Transcription factors binding to three different enhancer types that drive expression along the AP axis. (A) Su(H) (cyan) and Bicoid (Bcd; red) protein expression in early nc 14 embryos. (B) Schematics of three Bcd-defined enhancer types, type 0 (green), type I (orange), and type II (light blue), classified by the positions of their Bcd-dependent posterior boundaries based on Chen et al. (6). Left end of schematic represents anterior tip of the embryo (100% EL). (C) A total of 66 Bcd-dependent enhancers derived from Chen et al. (6) were classified based on whether endogenous sequences exhibit Su(H) and/or Run ChIP binding or a lack thereof (“Neither”). (D) Representation of Zld, Su(H), Bcd, and Run expression patterns within a schematic of an early embryo. Colored lines represent regions of expression for each of these factors. “+” defines input by activators Zld and Bcd, and “−“ defines input by repressors Su(H) and Run. (E–G) ChIP binding data for Zelda (13), Su(H) (7), and Run (14) showing occupancy of these factors at three loci: tll (E), oc (F), and Kr (G). Location for previous characterized enhancers active in the early embryo are shown and colored if associated with type 0 (green), type I (orange), or type II (blue) AP enhancers (B) or none (gray).
We investigated whether ChIP binding could be used to infer roles for Su(H) and Run in regulating enhancer activity. Run has been shown to modulate type I patterns. The sequence of the Run DNA binding site is enriched within type I enhancers, and run mutants exhibit alterations of these patterns (6). As would be expected, Run ChIP-defined binding is enriched in enhancers supporting type I patterns, but we also found that it is associated with enhancers of type II patterns (Fig. 2C). In contrast, the ChIP-defined binding of Su(H) is broadly associated with enhancer sequences representing all three classes (i.e., types 0, I, and II; Fig. 2C). The broad occupancy of Su(H) to AP enhancers of types 0, I, and II classes and their wide expression range on the AP axis (Fig. 2 A and B) suggested that this factor may play an expanded role in patterning the embryo.
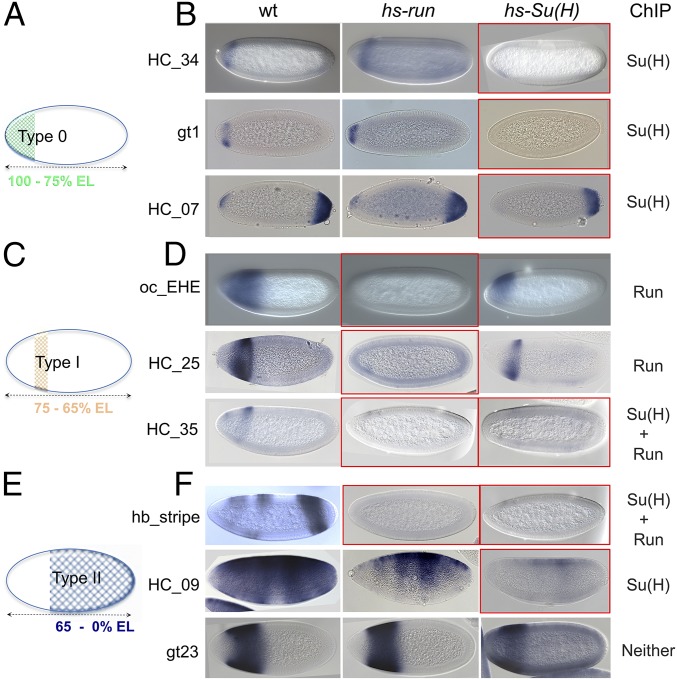
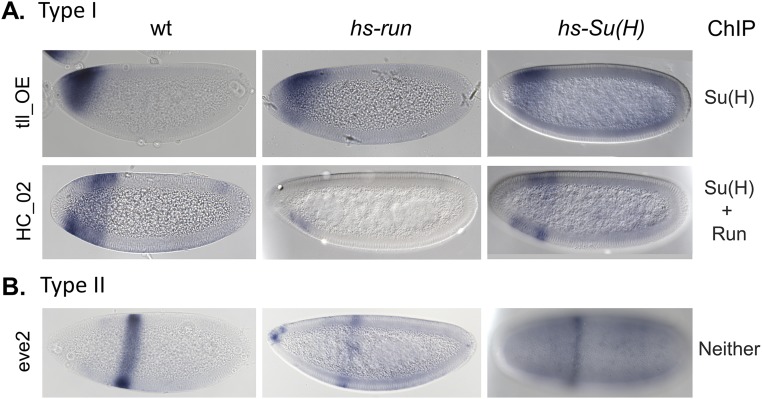
We hypothesized that repressors Su(H) and Run both regulate patterning along the AP axis by binding to the AP enhancers to counterbalance activation by Bicoid and Zelda (Fig. 2D). To test this idea, the effect of ectopic Su(H) or Run on expression of lacZ reporters supporting type 0, I, or II patterns was examined (Fig. 3). Ectopic expression was accomplished by using heat-shock expression constructs (12, 15). Three type 0 enhancers were assayed, and all exhibited repression of anterior patterns by ectopic Su(H); in contrast, ectopic Run had no effect on their expression (Fig. 3 A and B). Five enhancers of type I were assayed (Fig. 3C). Four were repressed by Run, including one that was additionally repressed by Su(H) (i.e., HC_35; Fig. 3D and Fig. S2A). Six enhancers of type II were assayed (Fig. 3E): two were repressed by Su(H) but not by Run (Fig. 3F, HC_09, and Fig. 4F, hb_shadow), two were repressed by Su(H) and Run (Fig. 4G, hb_stripe), and three were not repressed by either (Fig. 3F, gt23, Fig. 4E, hb_P2, and Fig. S2B, eve2). These results suggest that (i) Su(H) plays a major role in regulating type 0 patterns, whereas Run has marginal, if any, effect; (ii) Run plays a major role in regulating type I patterns, as described previously by Chen et al. (6), but Su(H) also can support this role; and (iii) Su(H) and Run both can regulate type II patterns and their roles in regulation of this particular class are variable, as one, both, or neither were found to support repression. Furthermore, the ability of Su(H) or Run to completely silence expression from these reporters correlated well with binding of these factors to enhancer sequences at the endogenous loci in vivo as determined by ChIP (Fig. 3 B, D, and F, Right). The exceptions were a few cases in which Su(H) or Run was found to decrease levels of expression but were not able to abolish expression completely [Fig. S2, HC_02 partial repression by Su(H), and Fig. 4F, hb_shadow partial repression by Run]. In summary, these experiments showed that Su(H) can repress type 0, I, and II patterns, whereas effects by Run are limited to type I as well as some type II patterns.
Fig. 3.
Ectopic expression of Su(H) or Runt has variable effects on AP enhancers. (A, C, and E) Schematic of domains of expression for gap genes expressed along the AP axis of Drosophila embryos that fall into the type 0 (A), type I (C), or type II (E) enhancer categories. (B, D, and F) Embryos containing the indicated enhancer constructs of type 0 (B), type I (D), or type II (F) classification within three different genetic backgrounds, WT embryos, hs-run, and hs-Su(H), were equivalently heat-shocked, and lacZ reporter expression examined by in situ hybridization. Embryos outlined in red from experiments showing loss of expression associated with heat shock. ChIP-defined binding to these enhancers sequences (endogenous locations) as assayed for occupancy by Su(H), Run, Su(H) + Run, or neither factor is shown (Right).
Fig. S2.
Runt and Su(H) ChIP occupancy of genomic enhancer sequences does not always correlate with the ability of these factors to repress reporter constructs. Expression patterns of some reporter genes from type I (A) and type II (B) enhancers (Left) in embryos collected from three different genetic backgrounds: WT, hs-run, or hs-Su(H). All embryos were equivalently processed, including heat shock of WT, which serves as control. Runt and Su(H) ectopic expression does not completely silence the expression of tll_OE, HC_02 (slp2 enhancer), and eve2 reporters, despite detection of ChIP binding to these sequences at their endogenous locations. No binding was detected to the eve2 locus.
Fig. 4.
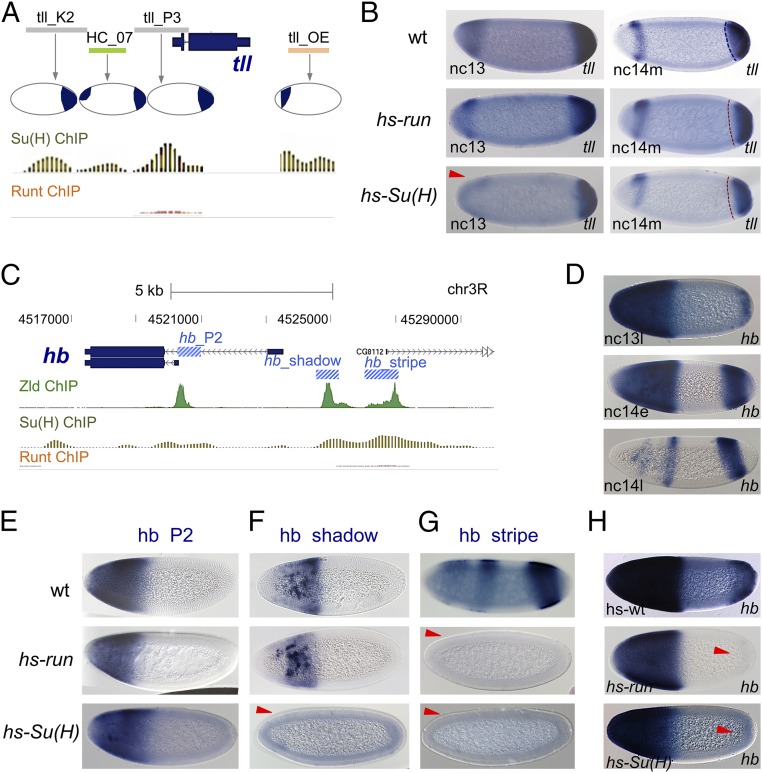
Broadly expressed repressors affect gap gene patterns via impacting the timing of action for a subset of their enhancers. (A) Relative location of four tll enhancer sequences (tll_K2, HC_07, tll_P3, tll_OE) to tll gene as well as the pattern of expression supported by each enhancer diagramed within the embryo schematics (26) compared with Su(H) and Run ChIP-defined in vivo occupancy to these sequences. (B) tll gene expression in heat-shocked WT and hs-run or hs-Su(H) embryos at nc 13 and mid-nc 14. (C) ChIP data for Zelda, Su(H), and Run binding to the hb locus relative to position of three enhancers (blue boxes) (19), supporting early embryonic hb expression. (D) Endogenous hb expression at three stages in the early embryo detected by in situ hybridization shows that the pattern is very dynamic. (E–H) Expression associated with hb enhancer reporter constructs using lacZ riboprobe (E–G) or endogenous hb (H) in heat-shocked WT, hs-run, and hs-Su(H) embryos at mid-nc 14 (E–G) or at the end of nc 13 (H). Delayed hb phenotype exhibited in 12 of 15 hs-run and 11 of 15 hs-Su(H) embryos (H). Red arrowheads mark domains where patterns exhibit alterations.
Run and Su(H) Target Particular Enhancers Within the tll and hb cis-Regulatory Systems.
To provide insight into the mechanism of action used by Run and Su(H) repressors, we investigated how these factors regulate enhancer function in their native genomic context within cis-regulatory systems in which multiple enhancers function coordinately (e.g., refs. 16, 17).
For example, multiple enhancers act to support embryonic expression of the gene tll (Fig. 4A). Su(H) mutant embryos exhibit expanded anterior tll expression (Fig. 1K), whereas it has been shown that run mutants exhibit expansion of anterior and posterior tll (18). To provide insight into the mechanism by which these repressors impact tll patterning, we first examined sensitivity of particular enhancers (Fig. 4A) to ectopic Su(H) or Run. The HC_07 tll-associated enhancer was repressed in anterior regions by ectopic expression of Su(H) but not Run (Fig. 3B, HC_07), whereas the tll_OE enhancer was not repressed by either factor (Fig. S2A). Upon heat shock-mediated ectopic expression of Run or Su(H), changes in endogenous tll expression were also observed (Fig. 4B and Fig. S3). Run and Su(H) decrease posterior tll, whereas only Su(H) represses anterior tll (Fig. 4B). This effect is consistent with the ChIP binding data, which showed that enhancers that support anterior tll expression are bound by Su(H) but not by Run (e.g., HC_07), whereas at least one enhancer that supports posterior tll expression is bound by both factors (e.g., tll_P3; Fig. 4A). These data suggest that Run and Su(H) target particular enhancers.
Fig. S3.
tll expression at the anterior and posterior poles is dynamic and differentially impacted by hs-Su(H) and hs-run. (A) Hs-Su(H) embryos overexpressing Su(H) gene at different stages of early development. (B) Quantification of ectopic expression assay efficiency at different ncs (nc 12–14). (C–E) Developmental time-course of tll expression within early embryos of indicated stages detected by in situ hybridization using tll riboprobes. All embryos were heat-shocked, including WT for comparative purposes. tll expression is shown for embryos of WT (C), hs-Su(H) (D), or hs-run (E) background at nc 13 until late nc 14. tll expression at the anterior and posterior poles is dynamic. Quantification of embryos that exhibit similar to the depicted phenotypes are present on the right corner of each image. Fifteen embryos were examined and the ratio was 14/15 embryos or greater if it is not presented. Red arrowhead and dashed lines indicate domains of reduced expression. SI Materials and Methods includes details about how tll posterior expression measurements were made.
Furthermore, tll anterior expression appears delayed, rather than completely abolished, by ectopic Su(H) (Fig. S3D vs. Fig. S3C), and tll repression by Run at the posterior is also only transient, as the tll pattern appears similar to WT at late nc 14 (Fig. S3E vs. Fig. S3C). Although these results may relate to changes in the timing of enhancer action by regulating exchange from one enhancer to the next, little is known about the temporal order of action of tll enhancers. Therefore, we turned our focus to another gap gene, hunchback (hb), which exhibits dynamic expression (Fig. 4D) that is supported by the coordinated activity of three enhancers.
hb is regulated by three distinct noncoding regions, enhancers hb_P2, hb_shadow, and hb_stripe (Fig. 4E, Top) (19). Early expression is supported by the hb_P2 within a cap at the anterior 40% of embryos. The hb_shadow pattern overlaps in expression with hb_P2 but also exhibits a sharper posterior boundary than hb_P2. Finally, the hb_stripe enhancer supports expression in a stripe localized at ∼40% EL (from the anterior pole) as well as in a domain at the posterior of the embryo. Several previous studies support the view that these three enhancers function together to support the dynamic hb gene expression pattern (Fig. 4D) (16, 19). We investigated how Run and Su(H) affect expression of hb and these three embryonic enhancers. hs-run expression acts to silence expression from hb_stripe, but has only a marginal effect on expression of hb_P2 and hb_shadow (Fig. 4E). In turn, hs-Su(H) expression acts to silence expression of hb_shadow in addition to hb_stripe, but has only minimal effect on expression of hb_P2 (Fig. 4E). Although binding of Su(H) was detected at the hb_P2 sequence (Fig. 4C), silencing of this sequence was not observed. Our assay of repression through heat shock-mediated ectopic expression can be interpreted with confidence only at nc 13 and nc 14, but not earlier (Fig. S3 A and B), and suggests an inability to assay repression of hb_P2, which emerges early. Collectively, these results show that Run and Su(H) differentially repress hb-associated enhancers, and sensitivity to repression for the most part correlates with ChIP-detected occupancy of these factors to enhancer sequences at the endogenous hb locus (Fig. 4C).
Next, we investigated how these sensitivities to Run and Su(H) at the enhancer level relate to changes in endogenous hb expression. Ectopic expression of these factors also affected expression of endogenous hb, most clearly at the early stage. At late nc 13, anterior hb expression appears shifted anteriorly upon ectopic expression of Run and Su(H), whereas posterior expression of hb associated with the hb_stripe enhancer specifically was completely absent, supporting the view that the hb_stripe enhancer is not active yet (i.e., delayed; Fig. 4H). However, the effect of ectopic expression of Su(H) on anterior expression of hb is stronger than that observed with Run and likely relates to the delay of hb_stripe enhancer as well as hb_shadow in the case of Su(H). Nevertheless, these effects on hb expression appeared transient because, by mid-nc 14, hb expression is similar to WT (Fig. S4D). Heat shock-mediated ectopic expression is only effective starting at nc 13 (Fig. S3B). It is possible that ectopic expression at earlier stages would be necessary to support lasting effects on hb (and tll) expression, but we favor the view that Su(H) and Run regulate the timing of enhancer switching because of the results of mutant analysis.
Fig. S4.
Su(H) and Runt affect hb spatiotemporal patterning. (A and B) hb expression shown in WT, run, and Su(H) embryos detected by using in situ hybridization with an intronic hb riboprobe to embryos of nc 12, nc 13, and early nc 14 as indicated (A). Two more stages (mid-late nc 14) identified by using hb exon riboprobe (B). (C) FISH using riboprobes to ftz (gray), hb (red), and sog (green) to examine transcript expression domain in WT vs. run mutant embryos (late nc 13). (D) Endogenous hb expression in heat-shocked WT and hs-run or hs-Su(H) embryos at mid- and late nc 14. Red arrowhead indicates reduced expression upon ectopic expression of hs-SuH. Quantification of the depicted phenotypes are present in the right corner of each image unless the ratio is 14/15 embryos or greater.
Transient effects on hb expression were also identified in mutants. We found that hb expression is turned on earlier in Su(H) and run mutants, suggesting regulation of this enhancer by these factors (Fig. S4A). hb is expressed in an expanded domain at the anterior region of Su(H) mutants compared with WT, but this expansion appears transient, as, in fully cellularized embryos, the expression in mutants is similar to WT (Fig. S4B, Su(H) vs. WT). It is likely that this phenotype relates to prolonged action of the hb_shadow or hb_P2 enhancers, which support expression at the anterior cap. On the contrary, in run mutants, a stripe of hb expression is observed at the anterior of embryos in early nc 14, stronger in expression than in WT embryos (Fig. S4 A–C, run vs. WT). This result suggests that the hb_stripe enhancer comes on earlier and is possibly derepressed in run mutants.
Run and Su(H) Regulate Patterning Throughout the Embryo, Along the AP Axis as Well as the DV Axis.
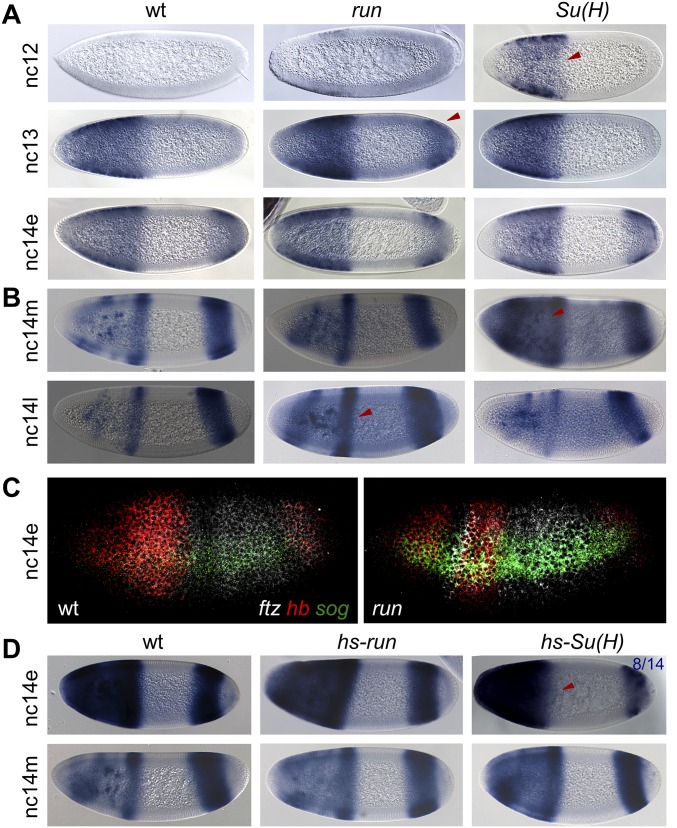
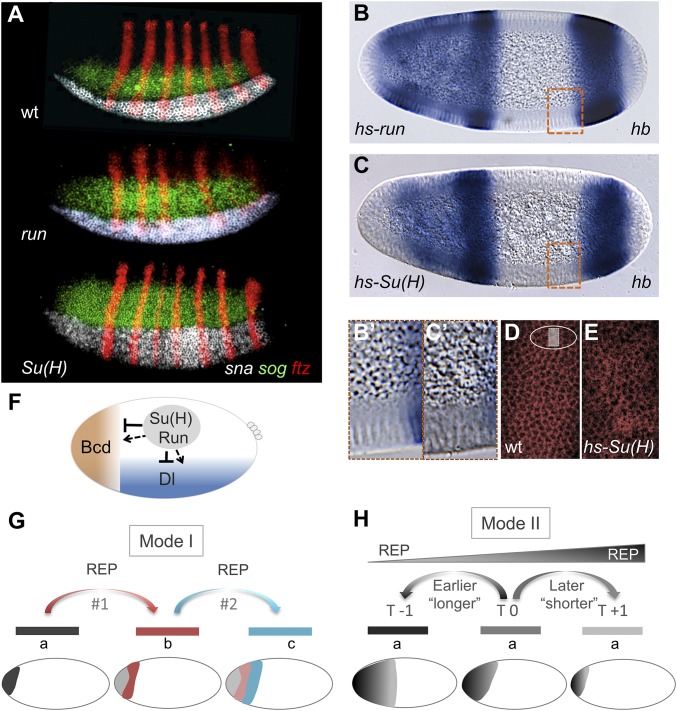
As these results support the idea that Su(H), in addition to Run, regulates gene expression along the AP axis, we investigated whether, inversely, Run in addition to Su(H) might support DV patterning. snail (sna) and short gastrulation (sog) are genes expressed in ventral and lateral regions of Drosophila early embryos (3). Previously, we showed that expression of these genes is altered in mutant embryos derived from Su(H) germline clone females: the sna boundary is unsharp and levels of expression are lower, whereas the sog expression domain appears expanded dorsally (7) (Fig. 5A). In run mutant embryos, sna expression domain appears relatively unaffected, but, in contrast, sog is expanded relative to WT, but not to the extent observed in Su(H) mutants (Fig. 5A).
Fig. 5.
Ubiquitous repressors regulate enhancer action across embryonic axes. (A) FISH using riboprobes to sna (white), sog (green), and ftz (red) show transcript expression domains within WT as well as run− and Su(H)− mutant embryos (mid-nc 14). (B and C) Ectopic expression of Su(H) through heat shock of hs-SuH embryos results in cellularization defects at late cycle 14 (C and C’). In contrast, no such cellularization phenotypes are observed upon heat shock of hs-run (B and B′) or WT embryos (Fig. S7C). (B′ and C′) Magnified views of B and C, respectively. (D and E) Fluorescent staining of embryos shows anomalous distribution of cell membranes within hs-Su(H) embryos (E) compared with WT (D) at mid-nc 14. Embryos in B–E processed by in situ hybridization using hb (B and C) or Kr (D and E) probes. Although expression of these genes is not necessarily relevant to cellularization defects, this confirms that the embryos are fertilized and development had progressed. (F) Broadly acting transcription factors Su(H) and Run encompass multiple roles in patterning by acting as repressors to regulate gene expression along AP and DV axes together with Bicoid (Bcd) and/or Dorsal (Dl) morphogens, respectively. As these factors are known to exhibit dual function, their roles as activators may also be more widespread. (G and H) Two different mechanisms by which broadly expressed repressors may impact spatiotemporal patterning are depicted. Repressors may regulate the timing of action for different enhancers acting in series (G) or, alternatively, repressors may influence the length of time a single enhancer is active (H) to impact spatiotemporal outputs.
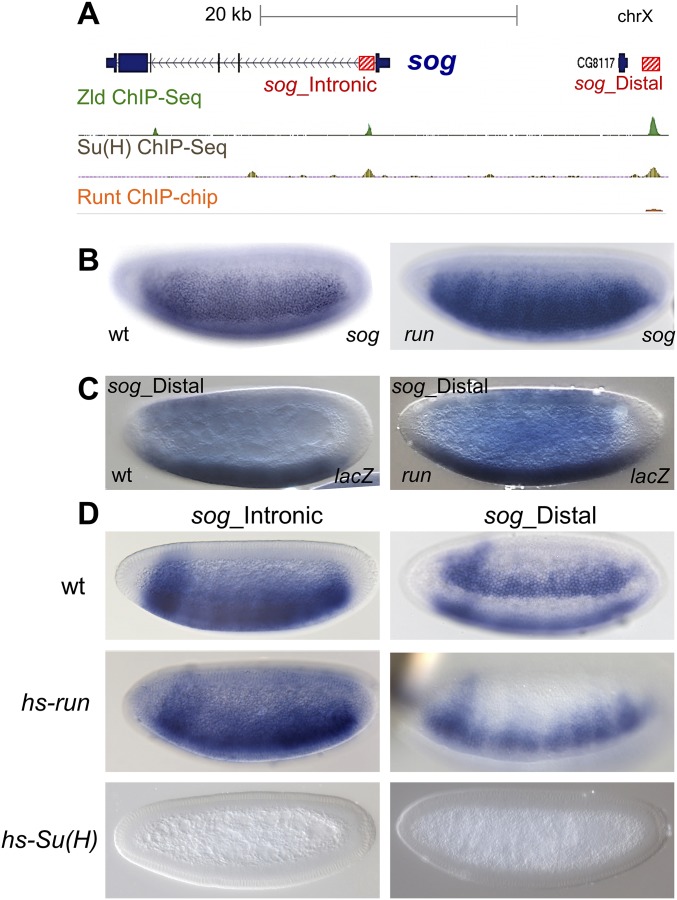
We next assayed whether Run and Su(H) function coordinately to regulate sog expression by acting on particular enhancers with the sog cis-regulatory system, as observed for tll and hb. Two enhancers, sog_Intronic and sog_Distal, control sog gene expression in the early embryo (20, 21). Ectopic expression of hs-Su(H) throughout the embryo leads to complete down-regulation of expression from both enhancers, sog_Intronic and sog_Distal; on the contrary, expression of hs-run fails to down-regulate either (Fig. S5C). Repression of both enhancers by Su(H) likely explains why expansion of sog is observed in Su(H) mutant embryos. In contrast, the failure of ectopic Run to repress either enhancer made it unclear why sog is expanded in run mutants (Fig. 5A). However, we found evidence that the sog_Distal enhancer exhibited expanded expression in run mutants, and this enhancer sequence also showed binding of Run by ChIP (Fig. S5 A and C). It is possible that Run’s ability to repress DV genes is context-dependent, depending on the binding of other factors to enhancer sequences in tandem on DNA to support Run’s activity as a repressor (or activator).
Fig. S5.
Assays to test the role for Runt, in addition to Su(H), in the regulation of the sog gene. (A) ChIP data for Zld, Su(H), and Runt showing occupancy at the sog gene locus. Position of two enhancers controlling sog early embryonic expression, sog_Intronic and sog_Distal, are indicated by red boxes. All three factors exhibit binding to sog_Distal, but only two of the three factors [Zld and Su(H)] exhibit binding to sog_Intronic. (B–D) Expression of transcripts in embryos was obtained using in situ hybridization and specific riboprobes. (B) Expression of sog in WT or run mutant embryos at late nc 14. sog is expanded in run mutants. (C) Expression of lacZ in embryos, either WT or run mutants, containing the sog_Distal reporter at late nc 13. The reporter is more broadly expressed in the run mutant compared with WT embryos. (D) Expression of sog_Intronic or sog_Distal in WT, hs-run, and hs-Su(H) embryos that have been heat-shocked. Ectopic Su(H) represses expression of both reporters, suggesting a role for Su(H) in regulation of both sog enhancers. Alternatively, ectopic Runt was not able to repress expression of either reporter.
Run and Su(H) are dual-function transcription factors that can function as repressors or activators. The binding of other transcription factors locally to enhancers may cause Run and/or Su(H) factors to locally flip in activity from repressor to activator and vice versa (Fig. 5F) (5, 10, 22). It is possible that the spatially localized repression of some enhancers observed in this study (e.g., Fig. 3 B and F, HC_07, HC_09, and Fig. S2A, HC_02) may relate to such context-dependent action of these factors.
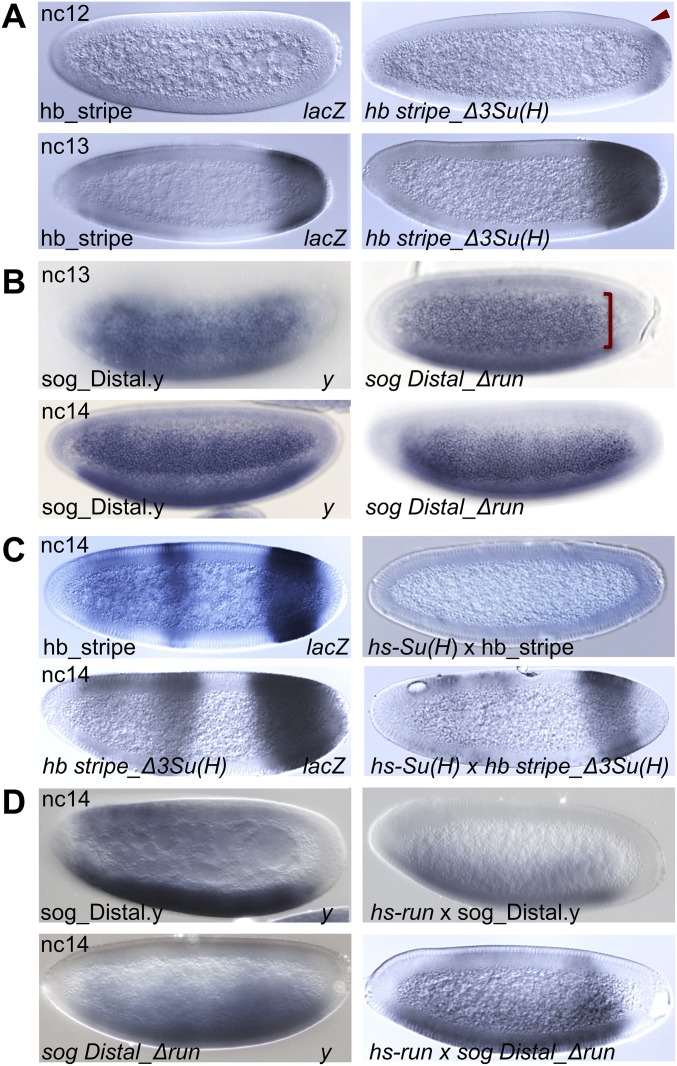
To test whether Run and Su(H) apply their repressive effects via direct binding to specific enhancers, we mutated their binding sites to other nucleotides (Fig. S6). Three Su(H)-binding sites within the hb stripe enhancer (Fig. S6A) and one Run-binding site within the sog_Distal enhancer were mutated (Fig. S6B). Finally, we crossed all hb_stripe and sog_Distal reporter constructs into hs-run and hs-Su(H) backgrounds (Fig. S6 C and D), showing that the ability of these factors to repress expression of reporters was dependent on presence of binding sites. Together, these experiments show that the aforementioned transcription factors have direct repressive activity along the two axes.
Fig. S6.
Mutagenesis of Su(H) and Run binding sites within enhancers demonstrates a direct role for these factors in the regulation of hb and sog gene expression. (A and B) Reporter gene expression in embryos of indicated stages driven by hb_stripe (A) and sog_Distal.y (B) WT or mutated enhancers assayed using riboprobes to reporters lacZ (A) or yellow (B). Three Su(H) sites (matches to the binding site consensus) were mutated in hb_stripe enhancer sequence [hb stripe_Δ3Su(H)], and one Run site was mutated in sog_Distal enhancer sequence [sogDistal_Δrun]. Mutation of Su(H) sites results in the h_stripe enhancer reporter supporting earlier expression relative to WT, whereas mutation of the Run site in the sog_Distal enhancer reporter results in expansion of expression at nc 13 but has little effect at nc 14. Red arrowhead and bracket indicate domains where patterns exhibit alterations. (C and D) Heat shock-mediated ectopic expression of Su(H) or Run on WT or mutated hb_stripe and sog_Distal enhancer sequences. Whereas ectopic expression of Su(H) is able to silence expression of the hb_stripe reporter, the hb stripe_Δ3Su(H) reporter was refractory to repression (C). Similarly, whereas ectopic expression of Run down-regulates expression of the sog_Distal.y reporter, the sogDistal_Δrun reporter was refractory to repression (D). The control enhancers [hb_stripe, hb stripe_Δ3Su(H), sog_Distal.y, sogDistal_Δrun] were similarly treated as the hs-Su(H) samples (i.e., heat-shocked).
Ectopic Expression of Su(H) Leads to Defective Cellularization as Exhibited by Zelda Mutants.
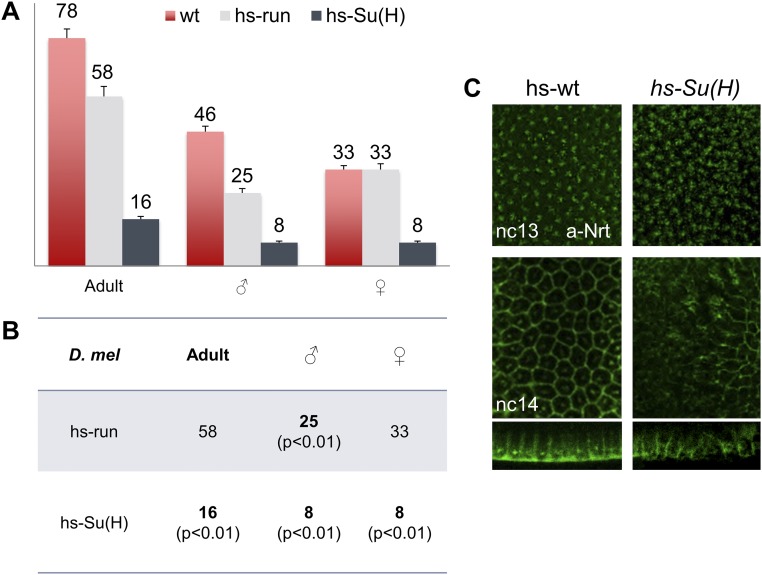
Surprisingly, we identified shared phenotypes between zelda mutants and overexpression of Su(H), as both exhibit cellularization defects (Fig. 5 C, C′, and E and Fig. S7C) (23). This phenotype was not associated with ectopic Run expression (Fig. 5 B, B′, and D), suggesting that Su(H) likely supports a distinct and likely wider role in embryonic patterning than Run. In addition, lethality was higher upon ectopic expression of Su(H) compared with Run (Fig. S7), but ectopic Run causes a reduction in male viability, likely because of its previously characterized role in the regulation of the sex-determinant gene Sex lethal (24) (Fig. S7). It is possible that Su(H) and Zelda share targets and that Su(H)-mediated repression acts to counterbalance Zelda-mediated activation.
Fig. S7.
Ectopic expression of Run or Su(H) within embryos results in decreased viability of adult flies, including sex-specific differences, as well as cellularization defects in embryos. (A) Flies survived after heat-shocking WT embryos, as well as embryos carrying hs-run or hs-Su(H) reporter constructs. The overall survival rate decreased to 20.5% in hs-Su(H) embryos. These data support a role for Runt in sex determination, as there was a 46% decrease in male progeny in the hs-run experiment. (B) P value analysis presented in B. (C) Anti-Nrt staining of early hs-WT and hs-Su(H) Drosophila embryos (nc 13 and nc 14) as indicated. Lateral views and cross-sections of nc 14 hs-Su(H) embryos showed cellularization defects compared with hs-WT of the same stage.
Shared use of key transcription factors, such as Su(H) and Run, across orthogonal axes may help to integrate patterning throughout the embryo and support robust development. Although a standard mechanism of repression involving spatial regulation of activator function is used to establish the posterior boundaries of particular patterns, for instance in the repression of type I patterns by Run (6), our results provide evidence of an additional mechanism of action in which the broadly expressed repressors Run and Su(H) function to fully repress expression of enhancers throughout the embryo to regulate enhancer timing of action and thereby impact spatiotemporal outputs of gene expression. This could be accomplished in at least two ways: regulation of the timing of enhancer initiation for multiple elements acting in series within cis-regulatory systems (Fig. 5G) or by controlling the length of time that one particular enhancer is active (Fig. 5H). A role for broadly expressed repressors in the regulation of enhancer timing is likely a conserved mechanism of action and may extend beyond patterning to temporal regulation of gene expression in general (25).
Materials and Methods
Fly Stocks and Crosses.
yw was used as WT if not otherwise noted. Su(H)Δ47 FRT40A P[l(2)35Bg +]/CyO (5), run3/FM7 (Bloomington stock no. 56499), hs-run (12), and hs-Su(H) (15) fly stocks were used. Details regarding generation of germline clones and heat-shock protocol for ectopic expression are in SI Materials and Methods.
Reporter Constructs Analyzed.
A total of 14 reporter constructs containing AP enhancers from all three types (0, I, II) of enhancer sequences occupied by Bicoid in vivo were randomly selected from the 2012 study of Chen et al. (6) and assayed. Su(H)- and Run-mutated binding sites of hb_stripe and sog_Distal enhancers were chemically synthesized (GenScript). Mutated site sequences and their WT equivalent fragments are listed in SI Materials and Methods (Table S1).
Table S1.
Mutagenesis of enhancer sequence variants (Fig. S6)
 |
Mutated Su(H) and Run binding sites are in red, WT sites in blue.
In Situ Hybridizations, Immunohistochemistry, and Image Processing.
Embryos were collected, fixed, and stained by using standard conditions (20). Additional information is provided in SI Materials and Methods.
SI Materials and Methods
Identification of an Su(H) in Vivo DNA Binding Site Consensus Sequence.
ChIP-seq to identify Su(H) protein in vivo within Drosophila early embryos was previously published (7). ChIP-Seq defined peaks were identified, and overrepresented motifs were identified by using Hypergeometric Optimization of Motif EnRichment (HOMER) (27). The second most common motif (second to a GAGA repeat sequence that likely represents an open chromatin signature) (21) was a sequence similar to Su(H) from JASPAR and was present in 24% of called peaks, which suggests this motif is the explanatory site for ChIP and represents an in vivo DNA binding site consensus sequence for Drosophila Su(H) in the early embryo.
Fly Crosses and Generation of Su(H) Germline Clones.
Flies were reared under standard conditions at 23 °C. run− flies were rebalanced with CyO ftz-lacZ marked balancer. For ectopic expression experiments, AP or DV enhancer reporter-line males were crossed to yw, hs-run, or hs-Su(H) females.
Su(H)Δ47 is a null allele and used previously for generation of females containing germline clones using the Flp-FRT system as described previously (5,7). SuHΔ47 FRT40A P[l(2)35Bg +]/CyO hb-lacZ males were crossed to females with germline clones of Su(H)Δ47 FRT40A P[l(2)35Bg +].
Heat Shock-Mediated Ectopic Expression.
For heat-shock experiments, 1–3-h embryos carrying one copy of hs-Su(H) (15) or hs-run (12) and one copy of a given reporter gene were collected and transferred into a 37 °C incubator for 20–25 min, allowed to recover at 25 °C for 35–40 min, and fixed immediately. Controls include comparisons of reporter expression associated with (i) embryos containing the hs-Su(H) or hs-run construct without the heat-shock treatment as well as (ii) heat-shocked yw embryos construct lacking either construct.
Candidate Enhancers and Mutagenesis.
hb_stripe, sog_Distal, and sog_Intronic enhancer sequences were cloned into BglII/KpnI, BglII/NotI, or NaeI/NotI sites, respectively, associated with the eve.promoter-lacZ-attB vector (20), using standard techniques. Primers used to perform PCR of genomic fragments representing hb_stripe, sog_Distal, and sog_Intronic enhancers are listed as follows:
hb_stripeF5′-ATTAAGATCTTTTCATTGTCCGCCTTAATGG-3′;
hb_stripeR5′-ATTAGGTACCCTTTCGGACGCTCAACAATG-3′;
sog_DistalF5′-ATTAGCGGCCGCGACAGATTCCCGGG-3′;
sog_DistalR5′-ATTAAGATCTAACTGACAGGGGCAAGTGCG-3′;
sog_IntronicF5′-ATTAGCCGGCGTTGCCAATGCCA-3′; and
sog_IntronicR5′-ATTAGCGGCCGCGCTTTATGGTCC-3′.
Site-directed transgenesis was carried out using a Drosophila melanogaster stock containing attP insertion site at position ZH-86Fb (Bloomington stock no. 23648).
The 14 AP reporter enhancer assayed include 13 provided by S. Small (i.e., HC_11, HC_34, gt1, HC_07, oc_EHE, HC_25, HC_35, HC_09, gt_23, hb_P2, tll_OE, HC_02, and eve2; Chen et al. (6)] as well as the hb_stripe, which was remade for the purpose of this study.
The sog_Distal and sog_DistalΔrun yellow reporters (sog_Distal.y and sog_DistalΔrun.y) were created by inserting gene-synthesized WT sog_Distal and Run-binding site-mutated sog_Distal sequences into the eve2promoter-MS2.yellow-attB vector (28). A yellow gene intronic probe was used to detect expression in fixed tissues. sog_Distal.y and sog_DistalΔrun reporter sequences are included in Table S1. The mutated Su(H) and Run binding sites are marked with red, compared with blue for the WT sites (Table S1).
In Situ Hybridizations, Immunohistochemistry, and Image Processing.
Enzymatic in situ hybridizations were performed with antisense RNA probes labeled with digoxigenin, biotin, or FITC-UTP to detect reporter or in vivo gene expression (7, 20). Kr, tll, oc, hb, en, sna, sog, and eve riboprobes were used for multiplex FISH, as well as hb and yellow intronic riboprobes. Images were taken under the same settings, 15 z-sections at 0.5-μm intervals, on a Zeiss Pascal confocal. P values across the study were calculated by using a one-tailed t test.
Antibody staining was performed by incubating fixed embryos with mouse anti-Nrt (BP 106 Developmental Studies Hybridoma Bank) at 1:50 dilutions; rat anti-Bcd (no. 807) (29), goat anti-Su(H) (sc-15813; Santa Cruz Biotechnology), and guinea pig anti-Runt antibodies (no. 638) (29) at 1:200 dilutions followed by incubation with Alexa Fluor 488 donkey anti-rat IgG secondary antibodies (1:500 dilutions; Invitrogen). Heat/methanol-fixed embryos were used for the anti-Nrt staining.
Measurement of Gene Expression Boundary Positions.
To measure boundary positions of Kr central and tll anterior expression domain, lateral images of alkaline phosphatase-stained embryos were taken by using a 20× objective on an Axioplan microscope. Eight to 15 embryos of each genotype [WT, Su(H)− mutant, hs-Su(H), hs-run] of appropriate ages and orientation were then analyzed for expression patterns. A line was drawn at the center of each embryo from the anterior edge to the posterior edge to measure EL. AP positions were displayed as a percentage of EL with the anterior pole as 100%. Following the same logic, a line was drawn from the anterior pole to the anterior boundary of the central Kr domain (marked as “ab”), whereas the “bc” line demarcates the central Kr domain width. Similar to Kr, “ab” is the length of the anterior boundary of the tll anterior domain and “bc” is the anterior tll domain length. Each of the “ab” and “bc” measurements was then divided by EL, resulting in the distance from the anterior pole or the width of the expression domain as a percentage. Significance was tested by Student’s two-tailed t test and was designated by a P value of <0.05. For the tll posterior domain, all expressed cells of the blastoderm periphery were counted in WT, hs-Su(H), and hs-run embryos. All comparisons were made by number of cells counted, and the analysis revealed an average of seven fewer cells expressed per embryo upon ectopic expression of either Su(H) or run.
Viability Experiments.
For each of the genotypes WT, hs-run, and hs-Su(H), 10 male and 10 virgin female D. melanogaster flies were crossed, and the number of progeny counted to establish a measure of viability resulting after heat shock and ectopic expression of run or Su(H). After a 24-h egg-laying period, the adult flies were transferred away, and the eggs were then allowed to develop at 23 °C. This procedure was performed in triplicates. The number of adult flies that emerged from each genotype was counted, and an average of three experiments is presented in Fig. S6.
Acknowledgments
We thank Peter Gergen, Mike Levine, James Posakony, and Stephen Small for providing plasmids and fly stocks and the A.S. laboratory, Lijia Ma, Andres Collazo, and Henry Amrhein for experimental support and helpful discussions. This study was supported by NIH Grants R35GM118146 and GM077668 (to A.S.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703001114/-/DCSupplemental.
References
- 1.Briscoe J, Small S. Morphogen rules: Design principles of gradient-mediated embryo patterning. Development. 2015;142:3996–4009. doi: 10.1242/dev.129452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rushlow CA, Shvartsman SY. Temporal dynamics, spatial range, and transcriptional interpretation of the Dorsal morphogen gradient. Curr Opin Genet Dev. 2012;22:542–546. doi: 10.1016/j.gde.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 3.Reeves GT, Stathopoulos A. Graded dorsal and differential gene regulation in the Drosophila embryo. Cold Spring Harbor Perspect Biol. 2009;1(4):a000836. doi: 10.1101/cshperspect.a000836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, et al. Zelda overcomes the high intrinsic nucleosome barrier at enhancers during Drosophila zygotic genome activation. Genome Res. 2015;25:1703–1714. doi: 10.1101/gr.192542.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morel V, Schweisguth F. Repression by suppressor of hairless and activation by Notch are required to define a single row of single-minded expressing cells in the Drosophila embryo. Genes Dev. 2000;14:377–388. [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Xu Z, Mei C, Yu D, Small S. A system of repressor gradients spatially organizes the boundaries of Bicoid-dependent target genes. Cell. 2012;149:618–629. doi: 10.1016/j.cell.2012.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozdemir A, Ma L, White KP, Stathopoulos A. Su(H)-mediated repression positions gene boundaries along the dorsal-ventral axis of Drosophila embryos. Dev Cell. 2014;31:100–113. doi: 10.1016/j.devcel.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schweisguth F, Posakony JW. Suppressor of Hairless, the Drosophila homolog of the mouse recombination signal-binding protein gene, controls sensory organ cell fates. Cell. 1992;69:1199–1212. doi: 10.1016/0092-8674(92)90641-o. [DOI] [PubMed] [Google Scholar]
- 9.Gergen JP, Butler BA. Isolation of the Drosophila segmentation gene runt and analysis of its expression during embryogenesis. Genes Dev. 1988;2:1179–1193. doi: 10.1101/gad.2.9.1179. [DOI] [PubMed] [Google Scholar]
- 10.Hang S, Gergen JP. Different modes of enhancer-specific regulation by Runt and Even-skipped during Drosophila segmentation. Mol Biol Cell. 2017;28:681–691. doi: 10.1091/mbc.E16-09-0630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fujioka M, Jaynes JB, Goto T. Early even-skipped stripes act as morphogenetic gradients at the single cell level to establish engrailed expression. Development. 1995;121:4371–4382. doi: 10.1242/dev.121.12.4371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tsai C, Gergen JP. Gap gene properties of the pair-rule gene runt during Drosophila segmentation. Development. 1994;120:1671–1683. doi: 10.1242/dev.120.6.1671. [DOI] [PubMed] [Google Scholar]
- 13.Harrison MM, Li XY, Kaplan T, Botchan MR, Eisen MB. Zelda binding in the early Drosophila melanogaster embryo marks regions subsequently activated at the maternal-to-zygotic transition. PLoS Genet. 2011;7:e1002266. doi: 10.1371/journal.pgen.1002266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.MacArthur S, et al. Developmental roles of 21 Drosophila transcription factors are determined by quantitative differences in binding to an overlapping set of thousands of genomic regions. Genome Biol. 2009;10:R80. doi: 10.1186/gb-2009-10-7-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schweisguth F, Posakony JW. Antagonistic activities of Suppressor of Hairless and Hairless control alternative cell fates in the Drosophila adult epidermis. Development. 1994;120:1433–1441. doi: 10.1242/dev.120.6.1433. [DOI] [PubMed] [Google Scholar]
- 16.Perry MW, Boettiger AN, Levine M. Multiple enhancers ensure precision of gap gene-expression patterns in the Drosophila embryo. Proc Natl Acad Sci USA. 2011;108:13570–13575. doi: 10.1073/pnas.1109873108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dunipace L, Saunders A, Ashe HL, Stathopoulos A. Autoregulatory feedback controls sequential action of cis-regulatory modules at the brinker locus. Dev Cell. 2013;26:536–543. doi: 10.1016/j.devcel.2013.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsai CC, Kramer SG, Gergen JP. Pair-rule gene runt restricts orthodenticle expression to the presumptive head of the Drosophila embryo. Dev Genet. 1998;23:35–44. doi: 10.1002/(SICI)1520-6408(1998)23:1<35::AID-DVG4>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 19.Perry MW, Bothma JP, Luu RD, Levine M. Precision of hunchback expression in the Drosophila embryo. CurrBiol. 2012;22:2247–2252. doi: 10.1016/j.cub.2012.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liberman LM, Stathopoulos A. Design flexibility in cis-regulatory control of gene expression: Synthetic and comparative evidence. Dev Biol. 2009;327:578–589. doi: 10.1016/j.ydbio.2008.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ozdemir A, et al. High resolution mapping of Twist to DNA in Drosophila embryos: Efficient functional analysis and evolutionary conservation. Genome Res. 2011;21:566–577. doi: 10.1101/gr.104018.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barolo S, Posakony JW. Three habits of highly effective signaling pathways: Principles of transcriptional control by developmental cell signaling. Genes Dev. 2002;16:1167–1181. doi: 10.1101/gad.976502. [DOI] [PubMed] [Google Scholar]
- 23.Liang HL, et al. The zinc-finger protein Zelda is a key activator of the early zygotic genome in Drosophila. Nature. 2008;456:400–403. doi: 10.1038/nature07388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Torres M, Sánchez L. The segmentation gene runt is needed to activate Sex-lethal, a gene that controls sex determination and dosage compensation in Drosophila. Genet Res. 1992;59:189–198. doi: 10.1017/s0016672300030470. [DOI] [PubMed] [Google Scholar]
- 25.Hansen IA, Attardo GM, Rodriguez SD, Drake LL. Four-way regulation of mosquito yolk protein precursor genes by juvenile hormone-, ecdysone-, nutrient-, and insulin-like peptide signaling pathways. Front Physiol. 2014;5:103. doi: 10.3389/fphys.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim Y, et al. Context-dependent transcriptional interpretation of mitogen activated protein kinase signaling in the Drosophila embryo. Chaos. 2013;23:025105. doi: 10.1063/1.4808157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heinz S, et al. Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell. 2010;38:576–589. doi: 10.1016/j.molcel.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garcia HG, Tikhonov M, Lin A, Gregor T. Quantitative imaging of transcription in living Drosophila embryos links polymerase activity to patterning. CurrBiol. 2013;23:2140–2145. doi: 10.1016/j.cub.2013.08.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kosman D, Small S, Reinitz J. Rapid preparation of a panel of polyclonal antibodies to Drosophila segmentation proteins. Dev Genes Evol. 1998;208:290–294. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]