Significance
When we touch one hand with the other, the touch feels less intense than identical touches generated by another person or robot. This is because our brain predicts the contact between our hands and attenuates the expected sensation. Here, we describe how the attenuation of self-touch depends on the experienced ownership of the touching hand. We found that illusory self-touch with a rubber hand that feels like one’s own is attenuated. We also found the reverse: The attenuation of real self-touch is reduced when the rubber hand that feels like one’s own is far from the receiving hand. These findings are important because they demonstrate that sensory predictions and sensory attenuation depend on the sense of ownership of the body.
Keywords: sensory attenuation, body ownership, forward models, state estimation, predictive motor control
Abstract
Self-perception depends on the brain’s abilities to differentiate our body from the environment and to distinguish between the sensations generated as a consequence of voluntary movement and those arising from events in the external world. The first process refers to the sense of ownership of our body and relies on the dynamic integration of multisensory (afferent) signals. The second process depends on internal forward models that use (efferent) information from our motor commands to predict and attenuate the sensory consequences of our movements. However, the relationship between body ownership and sensory attenuation driven by the forward models remains unknown. To address this issue, we combined the rubber hand illusion, which allows experimental manipulation of body ownership, and the force-matching paradigm, which allows psychophysical quantification of somatosensory attenuation. We found that a rubber right hand pressing on the left index finger produced somatosensory attenuation but only when the model hand felt like one’s own (illusory self-touch); reversely, the attenuation that was expected to occur during actual self-touch with the real hands was reduced when the participants simultaneously experienced ownership of a rubber right hand that was placed at a distance from their left hand. These results demonstrate that the sense of body ownership determines somatosensory attenuation. From a theoretical perspective, our results are important because they suggest that body ownership updates the internal representation of body state that provides the input to the forward model generating sensory predictions during voluntary action.
The distinction between self and nonself is fundamental for all biological organisms. External signals constitute potential threats and must be clearly distinguished from self-related signals. Imagine, for example, how dangerous it would be if you could not distinguish between a spider crawling up your neck and your own fingers scratching the same part of the skin. The central nervous system has therefore developed mechanisms to distinguish between self-related signals and non–self-related signals. Two neural mechanisms are considered particularly important for this differentiation. First, the integration of signals from different sensory modalities (e.g., vision, touch, proprioception; multisensory integration) leads to the formation of a central representation of one’s own body in space (1, 2). Sensory information attributed to this representation is experienced as originating from one’s own body (sense of body ownership). Second, when we actively move our body, the brain predicts the sensory consequences of the movements by using internal copies of the voluntary motor commands (efference copy) (3–5). This allows the central nervous system to disambiguate self-produced sensations from sensations arising from external causes. However, the relationship between these two basic self-mechanisms is unclear.
The importance of multisensory integration for the sense of body ownership is illustrated in the classical rubber hand illusion (RHI) (6). In this illusion, healthy participants experience a rubber hand as part of their own body when it is stroked synchronously with strokes administered to their real hand, which is hidden from view. The illusion obeys specific temporal and spatial rules that are reminiscent of the temporal and spatial principles of multisensory integration; the seen and felt brushstrokes must be synchronous and administered to homologous locations in the hands, and the rubber hand must be placed in a similar orientation to the hidden real hand for the illusion to be elicited. The illusion does not depend on specific sensory modalities; it is the spatial and temporal correlation between the signals from the different senses that matters. For example, the illusion is induced when the rubber hand moves synchronously with the real hidden hand, in the absence of touch (7, 8). Moreover, the strength of the illusion correlates with the activity in multisensory frontoparietal areas (9–12). Thus, the feeling of ownership of a limb is associated with the dynamic formation of a coherent representation of one’s own limb in space based on multisensory integration mechanisms (2, 13–15).
The importance of sensory predictions based on the efference copy is illustrated in the well-known phenomenon of “sensory attenuation.” Sensory attenuation refers to the decreased salience of self-generated sensations with respect to that of externally generated sensations. In the somatosensory domain, this is reflected in the fact that when one actively touches his/her own body (self-touch), the touch feels less intense (and less ticklish) compared with an identical touch applied by another person or a machine (16–19). Somatosensory attenuation can be effectively studied using the classical force-matching paradigm, which allows a psychophysical quantification of the degree of attenuation introduced by a simulated self-touch between the index fingers by means of a motor (20–23). The mechanism of sensory attenuation has been theorized to arise as a consequence of basic mechanisms of motor control. To accurately and speedily control our movements, the brain needs to have a central estimate of the spatial configuration of the body at any given point in time. Afferent sensory information alone is not sufficient to reliably compute this estimate because afferent signals are noisy and suffer from long delays due to axon conduction. To compensate for this, the brain uses computational units—called internal forward models—to predict the sensory consequences of our movements based on the efference copy (4, 24, 25). These sensory predictions are used both to attenuate self-produced sensations and to obtain a more accurate estimate of the state of the body (26–29). However, it is unknown whether body ownership plays a role in the sensory predictions of forward models and, thus, in somatosensory attenuation.
Here, we investigated the relationship between the sense of body ownership and somatosensory attenuation. We tested the hypothesis that the attenuation of somatosensation is determined by the ownership of the hand that is perceived to generate the touch on the body. To this end, we combined the RHI, which allows the experimental manipulation of ownership, and the force-matching paradigm, which allows the sensitive quantification of attenuation during simulated self-touch, in a single experimental paradigm. Our results demonstrate that somatosensory attenuation depends on the subjective feeling of body ownership.
Results
Experiment 1—Somatosensory Attenuation During Illusory Self-Touch with a Rubber Hand That Feels Like One’s Own.
In the first experiment, we tested the hypothesis that illusory ownership of a rubber hand leads to somatosensory attenuation when the artificial right hand touches the real left hand (illusory self-touch). By using the force-matching task, we could quantify the perceived intensity of forces applied to the left index finger and study how this changes as a function of whether the forces are experienced as occurring from a perceived self-touch or not by means of the RHI.
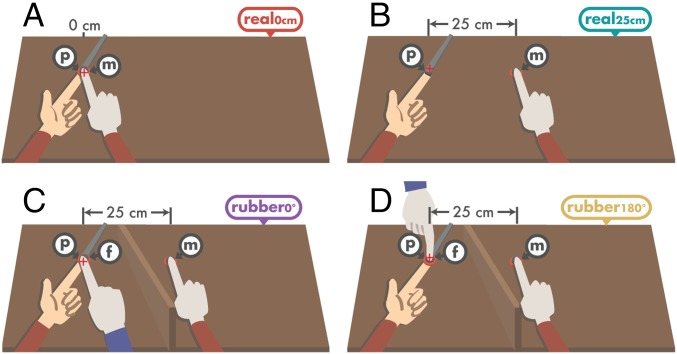
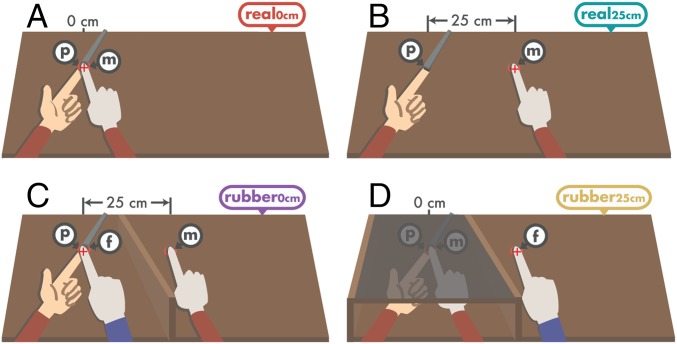
Experiment 1 had four conditions (Fig. 1). In all conditions, the participants rested their left hand palm up, with the index finger placed on a molded support. In each trial, they received a constant force (1 N, 1.5 N, 2 N, 2.5 N, 3 N, or 3.5 N) on the pulp of their relaxed left index finger that was generated by a motor (presented force). Immediately afterward, they were asked to generate a force that matched the intensity of the presented force (matched force) by pressing their right index finger against a force sensor that controlled the force output on their left index finger. In the two conditions without rubber hands, participants pressed their right index finger against a force sensor placed either directly above (but not in contact with) the left index finger (real0cm; Fig. 1A), thereby producing somatosensory attenuation by simulating real self-touch (20), or 25 cm to the right of the left index finger (real25cm; Fig. 1B)—a large distance that eliminates attenuation (30) because physical contact of the fingers is very unlikely. In the two rubber hand conditions, the force sensor and the participants’ real right hand were placed 25 cm to the right of their left index finger and were occluded from view. A rubber right hand holding its index finger on top of a fake force sensor was placed directly above (but not in contact with) the real left index finger (Fig. 1 C and D). To induce the RHI before the force-matching task, participants first repeatedly tapped their right index finger against the force sensor for 1 min while they observed the rubber hand making the corresponding finger movements on the fake force sensor synchronously. This is the RHI elicited by finger movements (7, 8). The critical experimental manipulation was presenting the rubber hand either in the same (anatomically congruent) orientation as the real right hand (rubber0°; Fig. 1C), which is a condition that elicits the illusion, or rotating the rubber hand by 180 degrees (rubber180°; Fig. 1D), which is a condition that prevents the induction of the illusion (7, 9). During the force trials of the two rubber hand conditions, every time participants pressed their right index finger on the force sensor to reproduce the presented force, the rubber index finger moved down to press the fake sensor synchronously. These very small but synchronous seen and felt movements provided additional multisensory cues that maintained the ownership illusion in the anatomically congruent condition.
Fig. 1.
The conditions of experiment 1. In each trial, a brief constant force was applied on the participants’ left index finger by a probe attached to a lever and measured by the sensor inside the probe p. Participants then pressed their right index finger on a mobile sensor m that controlled the force output of the probe on their left index finger. This sensor (m) was placed either on top of their left index finger (A) or 25 cm to the right of their left index finger (B–D). In two conditions, the sensor and the participants’ right arm were placed behind an occluder, and a rubber hand holding its index finger against a fake sensor f was placed just above their left index finger. The rubber hand was either oriented congruently with respect to the participants’ hand (C) or rotated by 180° (D). The rubber index finger pressed the sensor f at the same time the participants’ index finger pressed the sensor m. Red crosses denote the fixation point of participants. The arms with the red sleeves represent the participants’ arms, whereas the arm with the blue sleeve refers to the rubber arm. Both the participants’ right hand and the rubber hand wore identical plastic surgical gloves (light gray).
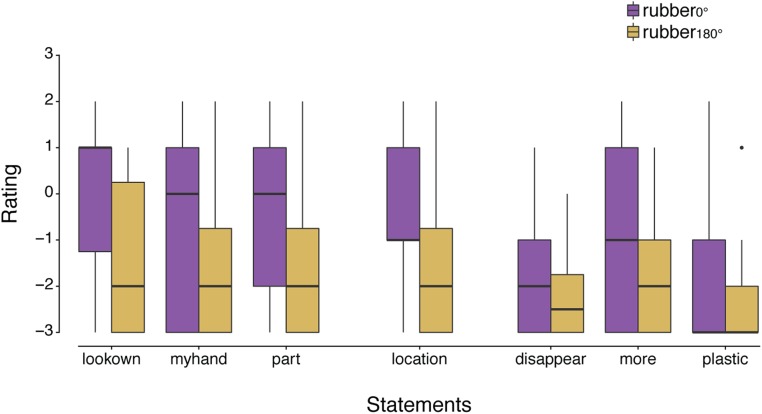
Upon completion of the force-matching task for all four conditions, we tested the RHI in a separate session: The participants performed the 1-min tapping task again for each of the two rubber hand conditions, and afterward, they completed a questionnaire to assess the strength of the illusion (adapted from refs. 6, 7) (Table S1).
Table S1.
Statements used in experiments 1 and 2 to measure the experience of participants toward the rubber hand and control statements
| Category | Statements | Abbreviation |
| Ownership | 1. I felt as if I was looking at my own right hand. | lookown |
| 2. I felt as if the fake hand was my right hand. | myhand | |
| 3. I felt as if the fake hand was part of my body. | part | |
| Location | 1. I felt as if my right hand was at the location where the fake hand was. | location |
| Control | 1. I felt as if my (real) right hand was turning plastic. | plastic |
| 2. It felt as if I had no longer a right hand, as if my right hand had disappeared. | disappear | |
| 3. I felt as if I had more than one right hand. | more | |
| 4. It felt as if my (real) right hand were drifting toward the fake hand. | drift |
Participants rated their level of agreement with each statement on a 7-point Likert-type scale, ranging from –3 (strongly disagree) to +3 (strongly agree), with 0 indicating “neither agree nor disagree.”
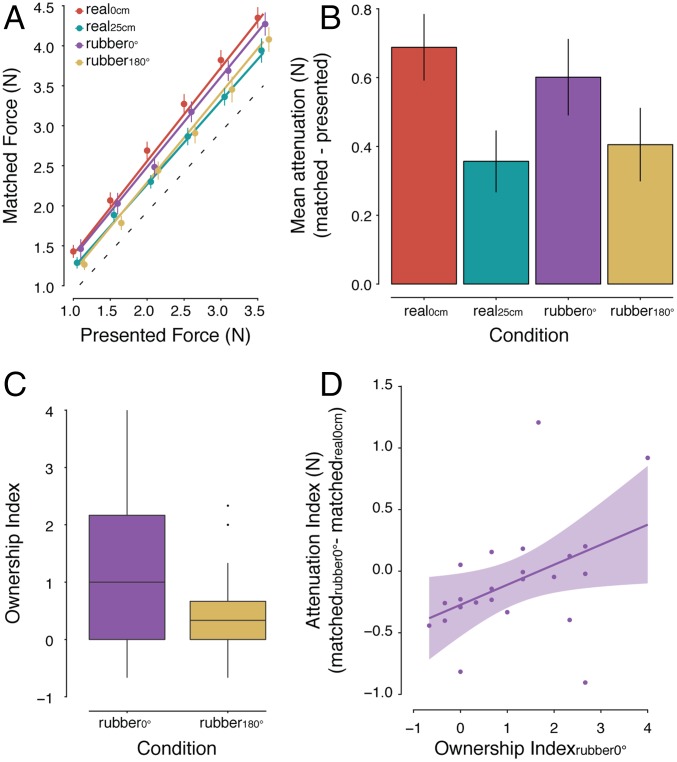
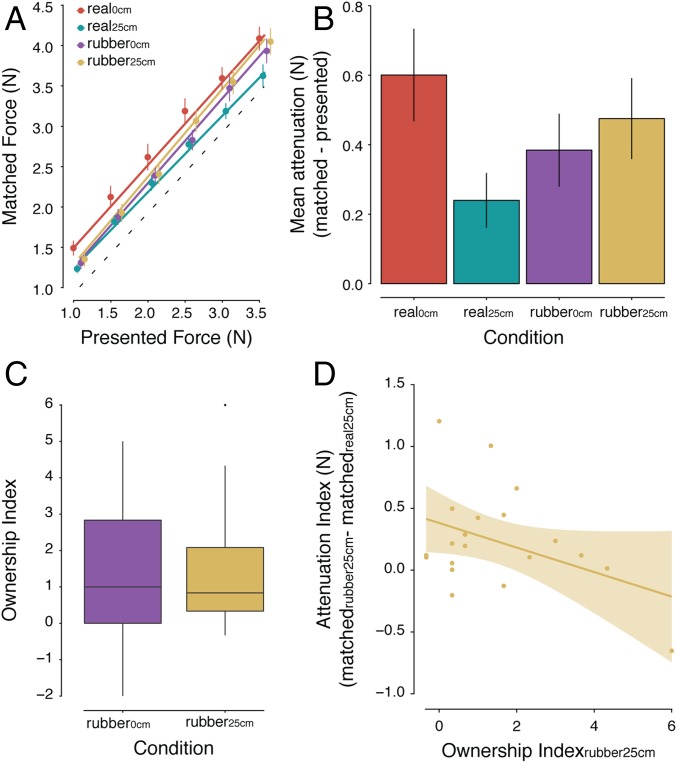
When we analyzed the force data (Fig. 2A), a main effect of condition was detected, F(3, 66) = 6.52, P < 0.001. Fig. 2B shows this main effect expressed as the difference between the mean forces participants applied in each condition and the presented forces. Unsurprisingly, in the conditions without the rubber hands, the forces the participants applied when their hands were spatially aligned (real self-touch; real0cm) were significantly stronger compared with those applied with their hands being separated (real25cm): P = 0.001 (Table S2). This result confirms previous findings showing that introducing a distance between the real hands greatly reduces the tactile attenuation (22, 30). Critically, we observed significantly greater forces in the congruent rubber hand condition (rubber0°) compared with the incongruent rubber hand condition (rubber180°), P = 0.039, and in the congruent rubber hand condition compared with the real25cm condition, P = 0.008. That is, despite the real hands being placed at the same large distance that would typically abolish somatosensory attenuation, the rubber hand placed in the congruent orientation eliciting the illusion produced somatosensory attenuation. Moreover, the congruent rubber hand condition (rubber0°) did not significantly differ from the condition of real self-touch (real0cm), P = 0.092, although there was a statistical trend for a difference. In contrast, forces in the incongruent rubber hand condition (rubber180°) were significantly weaker compared with the real self-touch condition (real0cm) (P = 0.004), but they were not significantly different from the real25cm condition (P = 0.505).
Fig. 2.
Results of experiment 1. (A) Forces generated by participants (matched forces) as a function of the forces generated externally by the motor (presented forces) (mean ± SE across participants). The dotted line indicates the theoretically perfect performance. The colored lines represent the fitted regression lines per condition. For illustration purposes, the position of the markers has been adjusted to the right to avoid overlapping points. (B) Mean force attenuation (matched forces – presented forces) across participants per condition. The forces participants applied in the rubber0° (purple) condition were significantly stronger than those in the rubber180° (yellow) or real25cm (cyan) condition, even though the distance between the participants’ hands was identical in all three conditions. In contrast, the forces applied in the rubber180° (yellow) condition did not significantly differ from those in the real25cm (cyan) condition. (C) The strength of the rated ownership of the rubber hand shown as boxplots for the two conditions. As can be seen, the illusion was significantly stronger when the rubber hand was congruently oriented. The horizontal black bars represent the medians, and the boxes denote the interquartile ranges and extend from the first (Q1) to the third (Q3) quartile. Whiskers represent Q1 – 1.5 × IQR and Q3 + 1.5 IQR, respectively. Data beyond the end of the whiskers are plotted as individual data points. (D) Regression plot (and 95% confidence bands) showing that the stronger the ownership participants experienced toward the congruent rubber hand (ownership index), the stronger the attenuation of the self-generated forces (attenuation index). An attenuation index of zero indicates that the attenuation in the rubber0° condition is of the same magnitude as the attenuation during the real0cm condition.
Table S2.
Pairwise comparisons for the main effect of condition in experiment 1 (n = 23)
| Condition | real0cm | real25cm | rubber0° |
| real25cm | P = 0.001 | ||
| rubber0° | P = 0.092 | P = 0.008 | |
| rubber180° | P = 0.004 | P = 0.505 | P = 0.039 |
As expected from earlier RHI studies, the participants reported experiencing a significantly stronger illusion in the condition wherein the rubber hand was placed in the anatomically congruent orientation compared with the incongruent orientation (7, 9) (Figs. S1 and S2). Specifically, the participants’ “ownership index” (Fig. 2C) was significantly stronger in the rubber0° than in the rubber180° condition (P = 0.027). The most interesting finding, however, was that the strength of the RHI was a significant positive predictor of the “attenuation index” in the rubber0° condition (b = 0.163, P = 0.039, R2 = 0.15) (Fig. 2D). That is, the more strongly the participants experienced the rubber hand as being their own, the stronger the attenuation of the touches produced by this model hand. In contrast, no relationship was found between the ownership and attenuation indices in the rubber180° condition (P = 0.693) (SI Materials and Methods and SI Results for further details). Collectively, the results of experiment 1 indicate that illusory self-touch with a rubber hand that feels like one’s own leads to significant somatosensory attenuation.
Fig. S1.
Questionnaire responses in experiment 1 (n = 24).
Fig. S2.
Individual subject data for differences in ownership index between the two rubber hand conditions of experiment 1 (n = 24).
Experiment 2—The RHI Can Reduce Somatosensory Attenuation During Real Self-Touch.
We reasoned that if body ownership truly determines somatosensory attenuation, then not only should illusory ownership produce attenuation during illusory self-touch with a rubber hand as demonstrated in experiment 1, but the opposite should also hold true: The classic somatosensory attenuation effect seen when the real right hand is touching the left one (real self-touch) should be reduced when the participants experience ownership of a rubber right hand placed at a distance from the left hand. Experiment 2 was designed to test this prediction using the same force-matching task as described above. There were four conditions, three of which were the same as in experiment 1 (Fig. 3 A–C). In the fourth condition, the participants’ real hands were spatially aligned (0 cm—real self-touch) and occluded by a screen placed above the hands, whereas the rubber hand and the fake sensor were placed 25 cm to the right of their left index finger (rubber25cm; Fig. 3D). The RHI was expected in both rubber hand conditions, as the rubber hand was always congruently oriented and moved in synchrony with the participants’ right hand in both conditions. The 1-min tapping task was performed to elicit the illusion before the force-matching task as in experiment 1.
Fig. 3.
The conditions of experiment 2. Three of the conditions in experiment 2 (A–C) were identical to conditions used in experiment 1 (rubber0cm is identical to rubber0°). In the fourth condition (rubber25cm), the rubber hand and the fake sensor f were placed 25 cm to the right of the participants’ left index finger (D). For illustration purposes, the occluder in D appears semitransparent, but it was actually fully opaque.
Analysis of the force data revealed a significant main effect of condition, F(3, 57) = 5.62, P = 0.002 (Fig. 4 A and B and Table S3). In accordance with experiment 1, we observed a trend for greater forces in the illusory self-touch condition (rubber0cm) compared with the condition without the rubber hand but with the same far distance between the real hands (real25cm), P = 0.065 (Fig. 3 B and C). The important comparison, however, was between the two conditions in which the real hands were simulating self-touch (0 cm) but either presented in full view without a rubber hand (real0cm) or occluded from view while a rubber hand was presented at 25 cm from the left hand (rubber25cm; Fig. 3 A and D). We found that the participants applied significantly weaker forces in the rubber25cm condition compared with the real0cm condition (P = 0.038), indicating significantly less somatosensory attenuation.
Fig. 4.
Results of experiment 2. (A) Forces generated by participants (matched forces) as a function of the forces generated externally by the motor (presented forces) (mean ± SE across participants). (B) Mean force attenuation (matched forces – presented forces) across participants per condition. Participants pressed significantly weaker forces in the rubber25cm (yellow) condition compared with the real0cm (red) condition, even though the distance between the participants’ hands was identical (0 cm) in both conditions. (C) There were no significant differences in the experienced ownership between the two conditions. (D) Regression plot (and 95% confidence bands) showing that the stronger the ownership participants experienced toward the rubber hand in the rubber25cm condition, the less they attenuated their self-generated forces. An attenuation index of zero indicates that the attenuation in the rubber25cm condition is of the same magnitude as the attenuation during the real25cm condition.
Table S3.
Pairwise comparisons for the main effect of condition in experiment 2 (n = 20)
| Condition | real0cm | real25cm | rubber0cm |
| real25cm | P = 0.004 | ||
| rubber0cm | P = 0.036 | P = 0.065 | |
| rubber25cm | P = 0.038 | P = 0.018 | P = 0.284 |
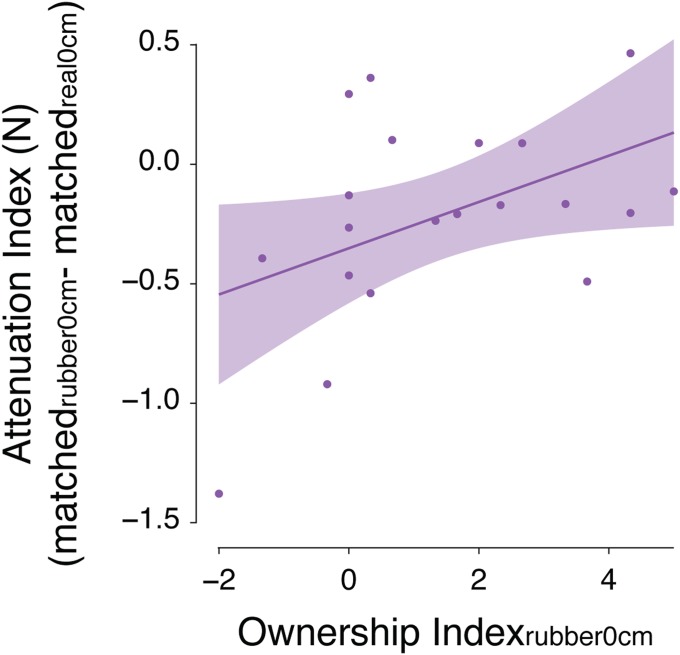
As expected, both rubber hand conditions elicited reliable illusions (Fig. S3), and there were no significant differences in the strength of ownership between them (Fig. 4C; P = 0.727). As in experiment 1, the strength of the ownership illusion significantly and positively predicted the strength of attenuation in the rubber0cm condition (b = 0.097, P = 0.024, R2 = 0.16; Fig. S4). Critically, we found the opposite relationship for the rubber25cm condition: The strength of the ownership illusion significantly but negatively predicted the strength of somatosensory attenuation (b = –0.099, P = 0.037, R2 = 0.12; Fig. 4D). That is, the more the participants experienced the rubber hand placed at a distance from their real left hand as their own, the less they attenuated the self-generated forces (SI Results for further details). Collectively, the results of experiment 2 indicate that illusory ownership of the rubber hand placed at a distance significantly reduces somatosensory attenuation, despite the two real hands being aligned simulating self-touch.
Fig. S3.
Questionnaire responses in experiment 2 (n = 24).
Fig. S4.
Regression plot (and 95% confidence bands) of ownership and attenuation indices for the condition rubber0cm of experiment 2 (n = 20). The plot shows that the stronger the ownership the participants experienced toward the rubber hand placed on top of their left index fingers (ownership index), the stronger the attenuation of the self-generated forces (attenuation index). An attenuation index of zero indicates that the attenuation in the rubber hand condition (rubber0cm) was of the same magnitude as the attenuation during real self-touch (real0cm).
SI Materials and Methods
General Procedure.
In all conditions, participants placed their left index finger on a molded support, with the palm of their hand up. Each condition consisted of 42 force trials (seven repetitions of each presented force level). In each trial, a force was applied to the pulp of the finger by a cylindrical probe (25 mm height) with a flat aluminum surface (20 mm diameter). The probe was attached to a lever that was controlled by a DC electric motor (Maxon EC Motor EC 90 flat; manufactured in Switzerland). A small force sensor (FSG15N1A, Honeywell Inc.; diameter, 5 mm; minimum resolution, 0.01 N; response time, 1 ms; measurement range, 0–15 N) was placed inside the probe and measured the force produced by the lever (sensor p in Figs. 1 and 3). After receiving the external force generated by the motor, the participants were instructed to press their right index finger against an identical force sensor (m) to produce a force of the same intensity. Participants were instructed to match the presented force as accurately as possible, and no feedback was ever provided to participants concerning their performance. The mobile sensor m was fixed on top of a small wooden support and could be placed at different locations independently of the position of the motor. The mobile sensor m controlled the force output of the lever with an intrinsic delay of ∼25 ms.
In both experiments, the participants wore a plastic glove on their right hand. White noise was presented through headphones to prevent the participants from hearing any noise produced by the motor that could otherwise have served as a cue for task performance. Auditory “go” and “stop” signals indicated the onset and the offset of the periods of the presented and matched forces (3 s each). Eye movements and visual input were controlled in all experimental conditions by asking the participants to fixate their gaze on a fixation point (Figs. 1 and 3).
Procedures in the Rubber Hand Conditions.
The artificial hand used in both experiments was a 3D-printed right hand, designed by Gyrobot (www.gyrobot.co.uk/) and printed and assembled by Fixers (https://fixers.gr/). A servomotor (Hitec HS-81) was used to move the plastic index finger in synchrony with the participants’ unseen right index finger movements: When the participants’ index finger pressed the sensor m, the plastic index finger moved down to press the fake sensor f, which was visually identical to the sensor m (Figs. 1 and 3). Analogously, when the participants stopped pressing the sensor, the fake index finger also stopped pressing the fake sensor. Technically speaking, as soon as participants pressed the mobile sensor with a force greater than 0.3 N, the small movement of the fake index finger was triggered immediately by the servomotor, and as soon as the force was less than or equal to 0.3 N, the fake index finger stopped pressing. The small movement of the plastic index finger pressing the fake force sensor was always the same regardless of the force the participant applied. The delay between the participant’s movements and the fake hand’s movement was <70 ms, a value substantially below the threshold for detecting visuomotor delays (56), thus ensuring synchronous conditions for illusion induction (7). This delay was calculated in a separate experiment by placing one light emitting diode (LED) on the controller, which was set to turn on as soon as the force applied exceeded 0.3 N, and a second LED on top of the plastic finger. We then recorded a video at 1,000 frames per second (Casio Exilim ZR100 digital camera) and calculated the delay between the frame in which the force exceeded the threshold and the frame in which the rubber hand started to move. On the basis of 10 recordings, the average delay was 61.80 ms. Before each experiment, participants were explicitly told that their task would be the same for all four conditions and that the only difference in the rubber hand conditions would be their fixation point. The order of conditions was counterbalanced in each experiment.
Fixation Points.
In experiment 1, participants were instructed to fixate on their right index finger when their hands were spatially aligned (Fig. 1A) or on the rubber index finger when the rubber hand was present (Fig. 1 C and D). In the condition when there was a 25-cm distance between their hands and no rubber hand present, participants were asked to fixate on a marked spot that corresponded to the same eye angle as in the other conditions (Fig. 1B). Because in experiment 2 the rubber hand was placed in different positions, we asked participants to fixate always on the visible pressing finger—that is, on their right index finger when the rubber hand was absent (Fig. 3 A and B) or on the rubber index finger when it was present (Fig. 3 C and D). In all conditions of both experiments, the pulp of the left index finger and the endpoint of the lever were occluded by a small wooden support.
Tapping Task.
Before the force-matching task in the rubber hand conditions of each experiment, participants were asked to perform a 1-min tapping task. They were instructed to intermittently press their right index finger on the sensor (m in Figs. 1 and 3) with a frequency of ∼1 Hz, while looking at the rubber index finger moving synchronously. This tapping task served to induce the body ownership illusion as shown by previous studies (7, 8). Once the tapping task was finished, the force trials started immediately.
Once all four conditions were finished in each of the two force-matching experiments, participants repeated this tapping task once for each of the two rubber hand conditions (rubber0° and rubber180° in experiment 1, rubber0cm and rubber25cm in experiment 2), and they then completed a questionnaire about their experience, adapted from refs. 6, 7. The two questionnaires included the same statements but in two different orders (Table S1). The order of the rubber hand conditions and the order of the versions of the questionnaires were counterbalanced across participants.
Analysis of the Forces.
For the analysis of the force data, we calculated the mean of the force data recorded by the sensor of the probe p during the period 2,000–2,500 ms after the go signal—that is, when the responses had stabilized and before participants released the sensor. Participants were explicitly told that only the last second of their response would be analyzed and that they were allowed to adjust their force in the beginning of each trial to find the force level that best matched the presented force. We then averaged the matched forces across the seven repetitions of each presented force level in accordance with earlier studies (22, 30). The data were processed in Matlab R2015a and analyzed using R (R version 3.3.2, RStudio Version 1.0.136).
Calculation of Ownership Index.
One control statement (statement “drift”, Table S1) was excluded from the analysis of both experiments because it was accidently given in two different formulations between the two conditions of experiment 1. To be consistent, we excluded the item also from the analysis of experiment 2.
The ownership index was calculated by subtracting the average score of the (remaining) three control questions from the average score of the three ownership items. Taking into account the participants’ responses to these control items reduces any effects of suggestibility or compliance (SI Results) and thus gives a more reliable estimate of the illusion strength. The minimum possible value of this ownership index was –6 and the maximum 6.
Calculation of Attenuation Index.
To obtain a representative index of sensory attenuation for each rubber hand condition, we calculated the difference between the mean force participants exerted in the rubber hand condition and the force that they exerted in a reference condition without the rubber hand for each presented force level (1 N, 1.5 N, 2 N, 2.5 N, 3 N, and 3.5 N). We then averaged across the six force levels. For the two identical conditions in which the rubber hand was placed at 0 cm from the left hand (rubber0° in experiment 1 and rubber0cm in experiment 2), we used, as a reference, the condition when the participants’ real hands were aligned (real0cm)—the condition of “maximum” attenuation. The rationale was that under illusory self-touch, we would expect the participants’ perception to resemble that of real self-touch. Negative attenuation indices indicate weaker attenuation in the rubber hand conditions than that during real self-touch, whereas an attenuation index equal to 0 indicates the same degree of attenuation. In contrast, for the condition rubber25cm of experiment 2, we used, as reference, the condition when the participant’s real hands were placed at a 25 cm distance from each other (real25cm) —the condition of “minimum” attenuation. The rationale was that under ownership of the distant rubber hand, we expected that the participants’ behavior would resemble that when their real right hands were at the same distance from the left hands. Positive attenuation indices indicate stronger attenuation in the rubber hand condition (rubber25cm) than when the two real hands were placed far apart (real25cm), whereas an attenuation index equal to zero indicates the same degree of attenuation between these two conditions.
Statistical Analysis for Experiment 1 and Experiment 2.
The forces were analyzed using repeated-measures ANOVA. Normality was assessed using the Shapiro–Wilk test. Post hoc comparisons were performed using paired t tests or Wilcoxon signed-rank tests depending on the normality of the distribution of the conditions being compared. These were two-tailed unless stated otherwise. Questionnaire data were analyzed with nonparametric statistics—that is, Wilcoxon signed-rank tests. Linear regression analyses were performed between the participants’ ownership index and their attenuation index. Regression coefficients were tested against zero with two-tailed t tests for experiment 1, whereas in experiment 2, we used one-tailed t tests because we had strong prior directed hypotheses from the findings of experiment 1.
SI Results
Analysis of Forces in Experiment 1.
In all four conditions of experiment 1, participants reproduced the presented force by pressing the mobile sensor m with their right index finger (Fig. 1). In the first two conditions, the sensor m was placed either on top of the probe p just above their left index finger (real0cm) or 25 cm to the right (real25cm). In the other two conditions, the distance between the participants’ hands remained 25 cm, but their right arm was placed behind an occluder, and a rubber hand was placed in front of them, on top of a fake sensor that was placed above their left index finger. The rubber hand was either oriented congruently with the participants’ arm (rubber0°) or rotated by 180° (rubber180°).
A repeated-measures ANOVA with condition (real0cm, real25cm, rubber0°, rubber180°) and presented force (1 N, 1.5 N, 2 N, 2.5 N, 3 N, 3.5 N) as within-subjects factors was conducted to analyze the force data. The initial ANOVA on the data of experiment 1 (n = 24) did not leave normally distributed residual errors (Shapiro–Wilk normality test, P = 0.021). Visual inspection of the residual errors plot revealed the existence of one outlier value corresponding to one participant. After this participant was removed, the ANOVA was rerun (n = 23), and the errors confirmed normality (P = 0.894). It should be mentioned, though, that the ANOVA results are the same whether the outlier is included or not.
A main effect of condition was detected, F(3, 66) = 6.52, P < 0.001. Table S2 shows the P values of the post hoc comparisons between the four conditions. Analytically, the forces in the real0cm condition were significantly higher than those in the real25cm condition (paired t test, P = 0.001, CI = [0.142, 0.520]) or in the rubber180° condition (paired t test, P = 0.004, CI = [0.101, 0.465]) but not compared with those in the rubber0° condition, although a trend was detected toward less somatosensory attenuation in the latter comparison (Wilcoxon signed-rank test, P = 0.092, CI = [–0.038, 0.275]). Despite both involving the same distance of 25 cm between the participants’ hands and the rubber hand, the rubber0° condition led to significantly stronger forces than the rubber180° condition (paired t test, P = 0.039, CI = [0.010, 0.382]). The rubber0° condition produced stronger forces also compared with the real25cm condition, which involved the same distance but no rubber hand present (paired t test, P = 0.008, CI = [0.068, 0.420]). Finally, the forces applied in the rubber180° condition did not significantly differ from those in the real25cm condition (paired t test, P = 0.505, CI = [–0.197, 0.099]), suggesting that just the view of the rubber hand is not sufficient to modulate force perception.
There was also a main effect of presented force, F(5, 110) = 403.9, P < 0.001, that indicated significant differences between each pair of force levels (all P values < 0.001 for all 15 comparisons) and further confirmed that participants could discriminate each force level. Finally, a significant interaction between the level of presented force and condition was found, F(15, 330) = 1.95, P = 0.018.
Analysis of Questionnaires in Experiment 1.
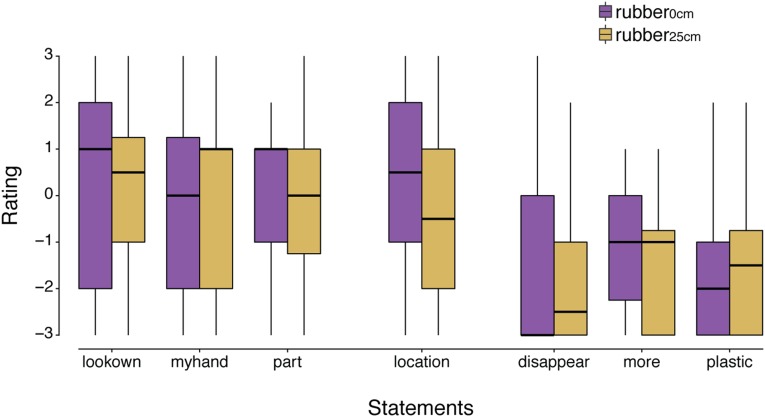
The questionnaire responses for each condition of experiment 1 are shown in Fig. S1 for all 24 participants. All three ownership questions were rated significantly higher in the congruent condition (rubber0°) than in the rotated condition (rubber180°): Wilcoxon signed-rank test, lookown, P = 0.004; myhand, P = 0.017; part, P = 0.003. The same was true for the location item, P = 0.039. The three control statements were rated low (negatively) and did not significantly differ between conditions (disappear, P = 0.376; plastic, P = 0.080) except for the item more, which was rated significantly higher in the congruent than in the rotated condition (P = 0.020).
The ownership index was significantly higher in the congruent rubber hand condition than in the incongruent rubber hand condition (Fig. S2): Wilcoxon signed-rank test, P = 0.021. The same was also found when the index was calculated without the more item (which significantly differed between conditions) but with the other two control items only—that is, disappear and plastic: P = 0.003. Finally, the same results were found when we removed the participant with the outlier value in the force matching task: P = 0.027, n = 23.
Analysis of Forces in Experiment 2.
As in experiment 1, participants reproduced the presented force by pressing their right index finger against the mobile sensor m. In the first two conditions, the sensor m was placed either on top of the probe p above their left index finger (real0cm) or 25 cm to the right (real25cm)—identically to experiment 1. The third condition (rubber0cm) was also identical to the condition rubber0° of experiment 1. In the fourth condition, the participants’ right hand and the mobile sensor m were placed on top of their left index finger and were occluded, whereas the rubber hand and fake sensor f were placed at 25 cm to the right of their hand (rubber25cm).
The initial ANOVA conducted for the full sample (n = 24) did not leave normally distributed residual errors (Shapiro–Wilk normality test, P < 0.001). Visual inspection of the residual errors plot revealed the existence of outlier values corresponding to four different participants. After removing the outliers, we reran the ANOVA (n = 20), and the errors confirmed normality (P = 0.123). It should be emphasized, though, that the same main effects were obtained in the ANOVA with and without these outliers.
There was a main effect of condition, F(3, 57) = 5.62, P = 0.002. Table S3 shows the P values of the post hoc comparisons between the four conditions. Specifically, the forces applied in the real0cm condition were significantly higher than those in the real25cm (Wilcoxon signed-rank test, P = 0.004, CI = [0.140, 0.522]) or the rubber0cm condition (paired t test, P = 0.036, CI = [0.016, 0.416]). Interestingly, the forces in the real0cm conditions were also stronger than those in the rubber25cm condition (paired t test, P = 0.038, CI = [0.007, 0.243]), which included the same distance between the real hands (0 cm) but differed in the presence of the rubber hand. Confirming the main finding of experiment 1, the forces participants exerted in the real25cm condition were weaker than those in the rubber0cm condition, although the comparison did not reach statistical significance (one-tailed paired t test, P = 0.065). Forces in the real25cm condition were significantly weaker than the forces in the rubber25cm condition (paired t test, P = 0.018, CI = [–0.426, –0.044]). Finally, the forces in the rubber0cm condition were not significantly different from those in the rubber25cm condition (paired t test, P = 0.284, CI = [–0.263, 0.081]).
As in experiment 1, a main effect of the presented force was detected, F(5, 95) = 462.6, P < 0.001, and all force levels were significantly different from each other (all P values < 0.001 for all 15 comparisons). In addition, the interaction between condition and presented force was significant, F(15, 285) = 1.88, P = 0.025.
Analysis of Questionnaires in Experiment 2.
The questionnaire responses for experiment 2 are shown in Fig. S3 for all 24 participants. The ownership items were rated significantly higher than the control items in both the rubber0cm (P = 0.002) and rubber25cm (P < 0.001) conditions, thus showing that the ownership illusion was effectively induced in both conditions. In line with this, the ownership index did not significantly differ between the two conditions (P = 0.721), and the same was true also when we excluded the outlier participants detected in the force-matching task, P = 0.727, n = 20. Finally and as expected, there were no significant differences in any of the ownership items (lookown, P = 0.884; myhand, P = 0.566; part, P = 0.765) or control items (disappear, P = 0.753; more, P = 0.336; plastic, P = 0.260), nor in the location item (P = 0.281), between the two conditions.
Discussion
The present study investigated the relationship between two fundamental mechanisms of self-perception: the multisensory sense of body ownership and the sensory attenuation arising from efference-based sensory predictions by the forward models. Our results show that the sense of ownership can facilitate or impede somatosensory attenuation depending on the perceived contact or separation between one’s own hands, over and above any effects related to the actual positions of the real upper limbs. The first experiment demonstrated that when the participants experienced the RHI for a rubber right hand that touched the real left index finger (illusory self-touch), the perceived forces were attenuated, even when the real right hand was placed at a far distance that made physical contact impossible (25 cm). Thus, during the illusion, the forward models generated somatosensory predictions for contact between the subjects’ own hands based on the rubber hand rather than the real right hand. The second experiment demonstrated that when the participants touched their left index finger with the real right one (real self-touch), the attenuation was reduced when they simultaneously experienced ownership of a rubber right hand placed at a far distance from the left hand (25 cm). Thus, here the forward models used the sensory information about the rubber hand that was felt as the subject’s own hand rather than the real right hand and consequently did not reliably predict self-touch. Taken together, these results provide compelling evidence for a basic link between body ownership and somatosensory attenuation. From a theoretical perspective, these findings are important because they suggest that the dynamic multisensory representation of one’s own body that is associated with a subjective feeling of ownership also acts as a source of central state information for the forward models that generate sensory predictions during voluntary action.
How do the present results advance our understanding about body ownership? We can identify three important advances. First, our results suggest that the RHI affects the motor system. Previous research on body ownership has not reached a consensus on whether the subjective illusion “deceives” the motor system. For example, some studies did not find effects of the RHI on reaching (31) or pointing (32) behavior, whereas others found such influence (33, 34). Interestingly, two recent studies found that ownership of an artificial hand influences the endpoint errors (35) and the initial direction (36) of reaching movements in such a way as if the movement were planned from a starting position that had been shifted toward the seen fake hand. Our study not only confirms the idea that body ownership affects the motor system but also explains how it does so from a computational perspective: The central multisensory representation of the owned limb in space provides critical input information about the body state to the forward model that generates the predictions about the sensory consequences of the limb’s voluntary action.
Second, our results showed that somatosensory attenuation is a marker of body ownership, at least when it is combined with active movements and the sense of “agency”—that is, the sense of being the author of voluntary action (37, 38). Common wisdom in the field assumes an intimate relationship between agency and sensory attenuation, because the former is believed to depend (at least in part) on comparisons between the predicted sensory consequences of action and actual sensory feedback (“comparator mechanisms”) (39). In the present experiments, there was a significant correlation between the strength of ownership and the degree of somatosensory attenuation, but agency was experienced in all conditions, because the matched forces were always instantaneously controlled by the action of the right index finger. Thus, at least in the somatosensory domain, attenuation seems to occur for only sensory predictions that are related to one’s own body and not to sensory events that are caused by movements through remote and arbitrary mechanisms such as pressing a button to produce touch over a distance with a motor. This is not to say that agency could not be a necessary factor for somatosensory attenuation, but when agency is present, body ownership determines whether touch will be attenuated. These findings suggest that the forward models [in the cerebellum (26, 40, 41)] responsible for somatosensory predictions during active self-touch have been developed and finessed to operate for the human body rather than to learn arbitrary mappings between motor commands and sensory feedback.
Third and not least, the results from our second experiment provide a rare demonstration of “disownership” of the real hand during the RHI. Earlier studies suggest that the skin temperature of the stimulated hand drops during the RHI (42) (but see ref. 43 for a critical analysis) and that physical threats applied to the real hand elicit weaker emotional responses during subjective disownership (11). Our observation that somatosensory attenuation during actual self-touch is reduced when participants simultaneously experience ownership of the rubber hand that is placed at a distance from their left hand supports the hypothesis that the real hand is “disowned” and the rubber hand replaces it in the central body representation. From this, it also follows the interesting prediction that one should be able to tickle oneself with the disowned real hand—a hypothesis that we aim to examine in future studies.
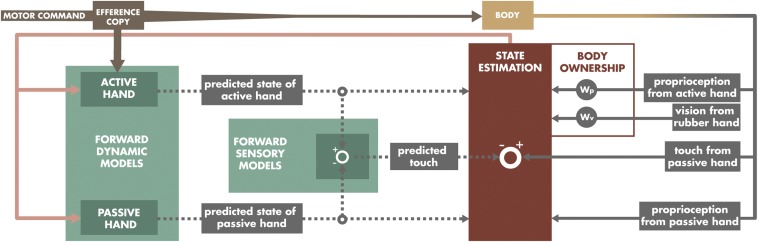
How can the interplay between body ownership and sensory predictions be implemented from a computational perspective? When the participant moves his or her right index finger to match the presented force, a forward dynamic model predicts the future position of the right index finger based on its currently estimated state (e.g., where it is now) and the efference copy specifying the movement. A forward sensory model then predicts the sensory consequences associated with this predicted state (5). For instance, if the right index finger is predicted to be sufficiently close to the left index after the movement as to (likely) touch it, then the forward sensory model predicts tactile feedback from the contact between the fingers (22, 30). However, the present study showed that the forward sensory model predicted touch under illusory self-touch with the rubber hand, whereas in fact the real right hand was placed at such a far distance (25 cm) that touch would not have been predicted in the absence of a rubber hand. Moreover, we found that the forward sensory model did not reliably predict touch when the rubber hand was placed far from the left index finger during the illusion (25 cm), even when in fact the two real hands simulated self-touch (0 cm). Because the prediction of the forward sensory model depends on the future position of the right index finger that is predicted by the forward dynamic model, which in turn depends on the currently estimated state of the right hand and index finger, it follows that body ownership exerts its influence on the forward models by affecting their input—that is, the currently estimated state of the upper limb and its digits (Fig. 5).
Fig. 5.
A model for predicting the tactile consequences of movement during illusory self-touch with the rubber hand. The active (right) hand is hidden from view and actively causes the touch on the passive (left) hand through the sensor connected to the motor. The rubber hand placed in full view just above the left hand is perceived as the subject’s own during the illusion. The forward dynamic model predicts the future position of the active hand (dotted gray arrow) on the basis of the efference copy (solid brown arrow) and the currently estimated position (solid pink arrow). The future state of the passive left hand is similarly predicted, although this state should remain the same, because that hand does not move. If these two predicted positions are close, as is the case during the illusion, the forward sensory model predicts tactile feedback (left comparator). The motor command makes the body move, which in turn generates new sensory feedback (solid gray arrows) including tactile signals. After the actual touch is received, it is attenuated because it was predicted (right comparator). The resulting afferent visual and proprioceptive information is used to estimate the new state of the right hand. Body ownership influences the degree to which visual and proprioceptive cues are relevant for the state estimation by modifying the relative weights so that the stronger the illusion, the more relevant the visual information from the model hand. The estimated state is then fed back to the forward model (solid pink arrow), and new states are predicted after new motor commands are generated.
To optimally estimate the position of the limb, the brain combines sensory information—that is, visual and proprioceptive signals—with central information about the expected position of the hand (5, 28, 44). Thus, the most likely explanation is that the RHI heavily weights the contribution of visual signals to the update of the central estimate of the hand position, favoring a position that is displaced toward the artificial hand. Forward models use this estimate to generate sensory predictions when new motor commands are generated (Fig. 5). This proposal is strongly consistent with earlier observations that the RHI causes a shift in the perceived hand position toward the model hand (6, 45, 46) and that the stronger the subjective illusion of ownership, the stronger this “proprioceptive recalibration” toward the model hand (7, 47).
Based on theoretical considerations and behavioral observations in a patient with lesions in the posterior parietal cortex (48, 49), it has been argued that the internal state of the body in terms of spatial configuration is maintained in the posterior parietal cortex. This is consistent with earlier electrophysiological evidence from monkeys and neuroimaging evidence from humans that have implicated active neuronal populations in the posterior parietal cortex as well as the ventral premotor cortex, which is anatomically connected to this parietal region, in the formation of a central multisensory representation of the limb in space (9–12, 50, 51). Interestingly, these areas are active during the RHI (9, 10, 52), and there is a correlation between the shift in perceived hand position toward the rubber hand and neural responses in the posterior parietal cortex indexing a shift in (peri-)hand space toward the rubber hand (52). Information from the posterior parietal representation of one’s own hand in space could reach the cerebellum—where forward models are likely situated (26, 40, 41)—through the anatomical connections between these structures (53, 54). Thus, the multisensory representation of the body in space maintained by active areas in the premotor and posterior parietal cortices could both be associated with the subjective experience of ownership and act as a source of input data to the forward models that generate self-specific sensory predictions during voluntary movement.
Materials and Methods
Participants.
After providing written informed consent, 24 naïve participants (15 women and 9 men, 22 right-handed and 2 ambidextrous) aged 22–37 y participated in experiment 1, and 24 naïve participants (13 women and 11 men, 22 right-handed and 2 ambidextrous) aged 20–36 y participated in experiment 2. Handedness was assessed using the Edinburgh Handedness Inventory (55). The Regional Ethical Review Board of Stockholm approved both experiments. One participant was excluded from experiment 1 and four participants from experiment 2 as indicated by the nonnormality of the residual errors from the two ANOVAs. It should be noted, however, that we obtained the same ANOVA effects with and without the inclusion of the outliers (SI Results).
Procedures in the Rubber Hand Conditions.
The artificial hand used in both experiments was a 3D-printed right hand. The model hand could move in synchrony with the participants’ unseen right index finger movements by means of a servomotor: When the participants’ index finger pressed the sensor m, the plastic index finger moved down to press the fake sensor f, which was visually identical to the sensor m (Figs. 1 and 3). The movement of the plastic index finger was always the same regardless of the force the participants were applying. Analogously, when the participants stopped pressing the sensor, the fake index finger also stopped pressing the fake sensor. Before each experiment, the participants were explicitly told that their task would be the same for all four conditions and that the only difference in the rubber hand conditions was that they were to look at the rubber index finger. The order of conditions was counterbalanced in each experiment. Further information is provided in SI Materials and Methods.
Acknowledgments
We thank Elias Giannopoulos for help with the illustrations and Paul Rousse for technical support. K.K. was supported by Marie Skłodowska-Curie Intra-European Individual Fellowship 704438 and the Swedish Wenner-Gren Foundations scholarship for foreign postdoctoral researchers. The project was funded by the Swedish Research Council, the James McDonnell Foundation, Torsten Söderbergs Stiftelse, and Hjärnfonden.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1703347114/-/DCSupplemental.
References
- 1.Graziano MSA, Botvinick M. 2002. How the brain represents the body: Insights from neurophysiology and psychology. Common Mech Percept Action Atten Perform XIX:136–157.
- 2.Ehrsson HH. The concept of body ownership and its relation to multisensory integration. In: Stein BE, editor. The New Handbook of Multisensory Process. MIT Press; Cambridge, MA: 2012. pp. 775–792. [Google Scholar]
- 3.Wolpert DM, Flanagan JR. Motor prediction. Curr Biol. 2001;11:R729–R732. doi: 10.1016/s0960-9822(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 4.Shadmehr R, Smith MA, Krakauer JW. Error correction, sensory prediction, and adaptation in motor control. Annu Rev Neurosci. 2010;33:89–108. doi: 10.1146/annurev-neuro-060909-153135. [DOI] [PubMed] [Google Scholar]
- 5.Wolpert DM, Ghahramani Z. Computational principles of movement neuroscience. Nat Neurosci. 2000;3:1212–1217. doi: 10.1038/81497. [DOI] [PubMed] [Google Scholar]
- 6.Botvinick M, Cohen J. Rubber hands ‘feel’ touch that eyes see. Nature. 1998;391:756. doi: 10.1038/35784. [DOI] [PubMed] [Google Scholar]
- 7.Kalckert A, Ehrsson HH. Moving a rubber hand that feels like your own: A dissociation of ownership and agency. Front Hum Neurosci. 2012;6:40. doi: 10.3389/fnhum.2012.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalckert A, Ehrsson HH. The moving rubber hand illusion revisited: Comparing movements and visuotactile stimulation to induce illusory ownership. Conscious Cogn. 2014;26:117–132. doi: 10.1016/j.concog.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 9.Ehrsson HH, Spence C, Passingham RE. That’s my hand! Activity in premotor cortex reflects feeling of ownership of a limb. Science. 2004;305:875–877. doi: 10.1126/science.1097011. [DOI] [PubMed] [Google Scholar]
- 10.Ehrsson HH, Holmes NP, Passingham RE. Touching a rubber hand: Feeling of body ownership is associated with activity in multisensory brain areas. J Neurosci. 2005;25:10564–10573. doi: 10.1523/JNEUROSCI.0800-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gentile G, Guterstam A, Brozzoli C, Ehrsson HH. Disintegration of multisensory signals from the real hand reduces default limb self-attribution: An fMRI study. J Neurosci. 2013;33:13350–13366. doi: 10.1523/JNEUROSCI.1363-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Limanowski J, Blankenburg F. Integration of visual and proprioceptive limb position information in human posterior parietal, premotor, and extrastriate cortex. J Neurosci. 2016;36:2582–2589. doi: 10.1523/JNEUROSCI.3987-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Makin TR, Holmes NP, Ehrsson HH. On the other hand: Dummy hands and peripersonal space. Behav Brain Res. 2008;191:1–10. doi: 10.1016/j.bbr.2008.02.041. [DOI] [PubMed] [Google Scholar]
- 14.Kilteni K, Maselli A, Kording KP, Slater M. Over my fake body: Body ownership illusions for studying the multisensory basis of own-body perception. Front Hum Neurosci. 2015;9:141. doi: 10.3389/fnhum.2015.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Samad M, Chung AJ, Shams L. Perception of body ownership is driven by Bayesian sensory inference. PLoS One. 2015;10:e0117178. doi: 10.1371/journal.pone.0117178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wolpert DM, Ghahramani Z, Flanagan JR. Perspectives and problems in motor learning. Trends Cogn Sci. 2001;5:487–494. doi: 10.1016/s1364-6613(00)01773-3. [DOI] [PubMed] [Google Scholar]
- 17.Blakemore SJ, Frith CD, Wolpert DM. Spatio-temporal prediction modulates the perception of self-produced stimuli. J Cogn Neurosci. 1999;11:551–559. doi: 10.1162/089892999563607. [DOI] [PubMed] [Google Scholar]
- 18.Weiskrantz L, Elliott J, Darlington C. Preliminary observations on tickling oneself. Nature. 1971;230:598–599. doi: 10.1038/230598a0. [DOI] [PubMed] [Google Scholar]
- 19.Blakemore SJ, Wolpert D, Frith C. Why can’t you tickle yourself? Neuroreport. 2000;11:R11–R16. doi: 10.1097/00001756-200008030-00002. [DOI] [PubMed] [Google Scholar]
- 20.Shergill SS, Bays PM, Frith CD, Wolpert DM. Two eyes for an eye: The neuroscience of force escalation. Science. 2003;301:187. doi: 10.1126/science.1085327. [DOI] [PubMed] [Google Scholar]
- 21.Shergill SS, Samson G, Bays PM, Frith CD, Wolpert DM. Evidence for sensory prediction deficits in schizophrenia. Am J Psychiatry. 2005;162:2384–2386. doi: 10.1176/appi.ajp.162.12.2384. [DOI] [PubMed] [Google Scholar]
- 22.Bays PM, Wolpert DM. Predictive attenuation in the perception of touch. In: Haggard EP, Rosetti Y, Kawato M, editors. Sensorimotor Foundations of Higher Cognition. Oxford Univ Press; Oxford: 2008. pp. 339–358. [Google Scholar]
- 23.Bays PM, Wolpert DM, Flanagan JR. Perception of the consequences of self-action is temporally tuned and event driven. Curr Biol. 2005;15:1125–1128. doi: 10.1016/j.cub.2005.05.023. [DOI] [PubMed] [Google Scholar]
- 24.Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279. doi: 10.1016/s0893-6080(96)00035-4. [DOI] [PubMed] [Google Scholar]
- 25.Davidson PR, Wolpert DM. Widespread access to predictive models in the motor system: A short review. J Neural Eng. 2005;2:S313–S319. doi: 10.1088/1741-2560/2/3/S11. [DOI] [PubMed] [Google Scholar]
- 26.Wolpert DM, Miall RC, Kawato M. Internal models in the cerebellum. Trends Cogn Sci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 27.Wolpert DM, Kawato M. Multiple paired forward and inverse models for motor control. Neural Netw. 1998;11:1317–1329. doi: 10.1016/s0893-6080(98)00066-5. [DOI] [PubMed] [Google Scholar]
- 28.Wolpert DM, Diedrichsen J, Flanagan JR. Principles of sensorimotor learning. Nat Rev Neurosci. 2011;12:739–751. doi: 10.1038/nrn3112. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan JR, Bowman MC, Johansson RS. Control strategies in object manipulation tasks. Curr Opin Neurobiol. 2006;16:650–659. doi: 10.1016/j.conb.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 30.Kilteni K, Ehrsson HH. Sensorimotor predictions and tool use: Hand-held tools attenuate self-touch. Cognition. 2017;165:1–9. doi: 10.1016/j.cognition.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 31.Kammers MPM, de Vignemont F, Verhagen L, Dijkerman HC. The rubber hand illusion in action. Neuropsychologia. 2009;47:204–211. doi: 10.1016/j.neuropsychologia.2008.07.028. [DOI] [PubMed] [Google Scholar]
- 32.Kammers MPM, Longo MR, Tsakiris M, Dijkerman HC, Haggard P. Specificity and coherence of body representations. Perception. 2009;38:1804–1820. doi: 10.1068/p6389. [DOI] [PubMed] [Google Scholar]
- 33.Kammers MPM, Kootker JA, Hogendoorn H, Dijkerman HC. How many motoric body representations can we grasp? Exp Brain Res. 2010;202:203–212. doi: 10.1007/s00221-009-2124-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Heed T, et al. Visual information and rubber hand embodiment differentially affect reach-to-grasp actions. Acta Psychol (Amst) 2011;138:263–271. doi: 10.1016/j.actpsy.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Newport R, Pearce R, Preston C. Fake hands in action: Embodiment and control of supernumerary limbs. Exp Brain Res. 2010;204:385–395. doi: 10.1007/s00221-009-2104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zopf R, Truong S, Finkbeiner M, Friedman J, Williams MA. Viewing and feeling touch modulates hand position for reaching. Neuropsychologia. 2011;49:1287–1293. doi: 10.1016/j.neuropsychologia.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haggard P. Conscious intention and motor cognition. Trends Cogn Sci. 2005;9:290–295. doi: 10.1016/j.tics.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 38.Gallagher S. The natural philosophy of agency. Philos Compass. 2007;2:347–357. [Google Scholar]
- 39.Frith CD, Blakemore SJ, Wolpert DM. Abnormalities in the awareness and control of action. Philos Trans R Soc Lond B Biol Sci. 2000;355:1771–1788. doi: 10.1098/rstb.2000.0734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blakemore SJ, Frith CD, Wolpert DM. The cerebellum is involved in predicting the sensory consequences of action. Neuroreport. 2001;12:1879–1884. doi: 10.1097/00001756-200107030-00023. [DOI] [PubMed] [Google Scholar]
- 41.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9:304–313. doi: 10.1038/nrn2332. [DOI] [PubMed] [Google Scholar]
- 42.Moseley GL, et al. Psychologically induced cooling of a specific body part caused by the illusory ownership of an artificial counterpart. Proc Natl Acad Sci USA. 2008;105:13169–13173. doi: 10.1073/pnas.0803768105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rohde M, Wold A, Karnath H-O, Ernst MO. The human touch: Skin temperature during the rubber hand illusion in manual and automated stroking procedures. PLoS One. 2013;8:e80688. doi: 10.1371/journal.pone.0080688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Körding KP, Wolpert DM. Bayesian decision theory in sensorimotor control. Trends Cogn Sci. 2006;10:319–326. doi: 10.1016/j.tics.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 45.Tsakiris M, Haggard P. The rubber hand illusion revisited: Visuotactile integration and self-attribution. J Exp Psychol Hum Percept Perform. 2005;31:80–91. doi: 10.1037/0096-1523.31.1.80. [DOI] [PubMed] [Google Scholar]
- 46.Abdulkarim Z, Ehrsson HH. No causal link between changes in hand position sense and feeling of limb ownership in the rubber hand illusion. Atten Percept Psychophys. 2016;78:707–720. doi: 10.3758/s13414-015-1016-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Longo MR, Schüür F, Kammers MPM, Tsakiris M, Haggard P. What is embodiment? A psychometric approach. Cognition. 2008;107:978–998. doi: 10.1016/j.cognition.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 48.Shadmehr R, Krakauer JW. A computational neuroanatomy for motor control. Exp Brain Res. 2008;185:359–381. doi: 10.1007/s00221-008-1280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wolpert DM, Goodbody SJ, Husain M. Maintaining internal representations: The role of the human superior parietal lobe. Nat Neurosci. 1998;1:529–533. doi: 10.1038/2245. [DOI] [PubMed] [Google Scholar]
- 50.Graziano MS. Where is my arm? The relative role of vision and proprioception in the neuronal representation of limb position. Proc Natl Acad Sci USA. 1999;96:10418–10421. doi: 10.1073/pnas.96.18.10418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graziano MS, Cooke DF, Taylor CS. Coding the location of the arm by sight. Science. 2000;290:1782–1786. doi: 10.1126/science.290.5497.1782. [DOI] [PubMed] [Google Scholar]
- 52.Brozzoli C, Gentile G, Ehrsson HH. That’s near my hand! Parietal and premotor coding of hand-centered space contributes to localization and self-attribution of the hand. J Neurosci. 2012;32:14573–14582. doi: 10.1523/JNEUROSCI.2660-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bostan AC, Dum RP, Strick PL. Cerebellar networks with the cerebral cortex and basal ganglia. Trends Cogn Sci. 2013;17:241–254. doi: 10.1016/j.tics.2013.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prevosto V, Graf W, Ugolini G. Cerebellar inputs to intraparietal cortex areas LIP and MIP: Functional frameworks for adaptive control of eye movements, reaching, and arm/eye/head movement coordination. Cereb Cortex. 2010;20:214–228. doi: 10.1093/cercor/bhp091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 56.Franck N, et al. Defective recognition of one’s own actions in patients with schizophrenia. Am J Psychiatry. 2001;158:454–459. doi: 10.1176/appi.ajp.158.3.454. [DOI] [PubMed] [Google Scholar]