Significance
Although the incidence of chronic pain is estimated at 20–25% worldwide, optimal drug treatment regimens with few side effects have yet to be developed. In this study, we demonstrated that clodronate is a potent and selective inhibitor of vesicular ATP release that attenuates neuropathic and inflammatory pain unrelated to bone abnormalities. Clodronate was more effective and associated with comparatively fewer side effects than existing drugs. Thus, the nonopioid agent clodronate may represent a unique treatment strategy for chronic pain via inhibition of vesicular ATP release, suggesting that clodronate may be effective in the treatment of several diseases involving purinergic chemical transmission, including inflammatory diseases, diabetes, and neurological disorders. Our study identifies a transporter-targeted analgesic drug.
Keywords: vesicular nucleotide transporter, purinergic chemical transmission, analgesic effect, antiinflammatory effect, clodronate
Abstract
Despite the high incidence of neuropathic and inflammatory pain worldwide, effective drugs with few side effects are currently unavailable for the treatment of chronic pain. Recently, researchers have proposed that inhibitors of purinergic chemical transmission, which plays a key role in the pathological pain response, may allow for targeted treatment of pathological neuropathic and inflammatory pain. However, such therapeutic analgesic agents have yet to be developed. In the present study, we demonstrated that clodronate, a first-generation bisphosphonate with comparatively fewer side effects than traditional treatments, significantly attenuates neuropathic and inflammatory pain unrelated to bone abnormalities via inhibition of vesicular nucleotide transporter (VNUT), a key molecule for the initiation of purinergic chemical transmission. In vitro analyses indicated that clodronate inhibits VNUT at a half-maximal inhibitory concentration of 15.6 nM without affecting other vesicular neurotransmitter transporters, acting as an allosteric modulator through competition with Cl−. A low concentration of clodronate impaired vesicular ATP release from neurons, microglia, and immune cells. In vivo analyses revealed that clodronate is more effective than other therapeutic agents in attenuating neuropathic and inflammatory pain, as well as the accompanying inflammation, in wild-type but not VNUT−/− mice, without affecting basal nociception. These findings indicate that clodronate may represent a unique treatment strategy for chronic neuropathic and inflammatory pain via inhibition of vesicular ATP release.
Although acute nociception is an important biological warning system, chronic neuropathic and inflammatory pain may result from nerve injury, inflammation, or other pathological processes (1–4). Although the incidence of chronic pain is estimated at 20–25% worldwide (1, 4), optimal drug treatment regimens with few side effects have yet to be developed. Common inflammatory pain medications, such as nonsteroidal antiinflammatory drugs (e.g., cyclooxygenase inhibitors), may cause side effects, such as gastrointestinal and renal dysfunction (5). Notably, opioids exert a strong analgesic effect against inflammatory pain but are associated with severe side effects, such as drug addiction, drowsiness, and vomiting (6, 7). Moreover, these drugs are ineffective for neuropathic pain. Drugs for neuropathic pain, which include voltage-dependent Ca2+ channel inhibitors, such as the anticonvulsants pregabalin and gabapentin, also have severe side effects, such as drowsiness and edema (8).
Pathological nociception is associated with purinergic chemical transmission (9). Vesicular nucleotide transporter (VNUT/SLC17A9) carries ATP into secretory vesicles in a membrane potential (Δψ)- and Cl−-dependent manner (10–12), is expressed in neurons at primary afferent nerve terminals and in the dorsal horn of the spinal cord, and is responsible for vesicular storage and release of ATP (13–15). Upon depolarization-evoked stimulation, released ATP binds to purinoceptors, which, in turn, cause pain responses (9). Experiments in VNUT knockout (KO) (VNUT−/−) mice have revealed that VNUT in spinal dorsal horn neurons is involved in neuropathic pain (13), and that VNUT inhibition leads to improvement of pathological conditions with no significant changes in phenotype (12). Such findings suggest that VNUT inhibitors may be effective analgesic agents with few side effects, although therapeutic inhibitors of purinergic chemical transmission have yet to be developed.
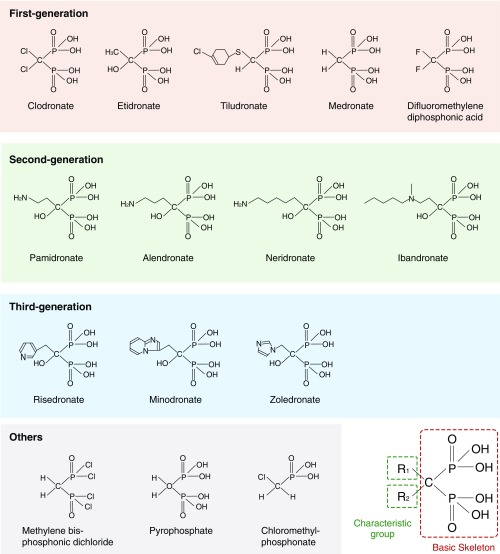
Bisphosphonates are widely used as therapeutic agents for osteoporosis; however, studies have demonstrated that bisphosphonates also have analgesic properties. Although they are used to treat bone diseases accompanied by chronic neuropathic or inflammatory pain (16–18), the mechanism underlying this analgesic effect is unknown. Notably, non–nitrogen-containing bisphosphonates (first generation) result in less inhibition of bone resorption but have fewer side effects than nitrogen-containing bisphosphonates (second and third generations) (19–21) (Fig. S1). Therefore, we hypothesized that non–nitrogen-containing bisphosphonates, which produce effects similar to the beneficial phenotypes observed in VNUT−/− mice, are candidate therapeutic drugs for chronic pain that may act via VNUT inhibition.
Fig. S1.
Basic structure of bisphosphonates. Bisphosphonates, which have the basic skeleton of methylene bisphosphonate (medronate), are classified based on the characteristic group attached to the central carbon atom. The structural formulae of bisphosphonates are shown.
In the present study, we demonstrate that clodronate, a non–nitrogen-containing bisphosphonate, is a potent and selective inhibitor of VNUT in vivo. In addition, our findings indicate that clodronate-evoked inhibition of vesicular ATP release is important for the treatment of chronic neuropathic and inflammatory pain, as well as the accompanying inflammation.
Results
Clodronate Is a Selective and Potent Inhibitor of VNUT.
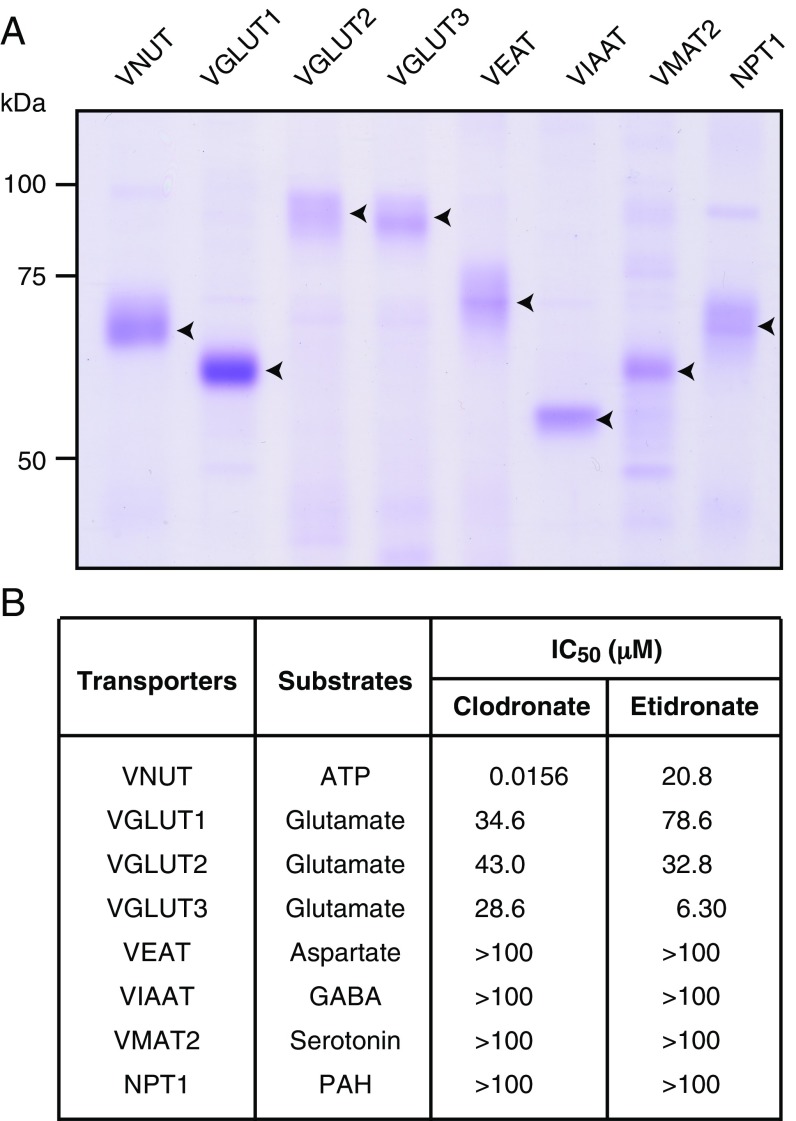
To identify the targets of the first-generation bisphosphonates clodronate and etidronate, we used a quantitative transport assay system involving proteoliposomes containing only purified protein (22, 23). The cDNAs of vesicular neurotransmitter transporters or SLC17 transporters were cloned into Escherichia coli or baculovirus expression vectors, and overexpressed in E. coli or insect cells. The membrane fractions were solubilized, and the transporters were purified via nickel-nitrilotriacetic acid (Ni-NTA) affinity column chromatography. The final fractions contained major protein bands of the expected apparent molecular masses, as determined via staining with Coomassie Brilliant Blue (Fig. 1A).
Fig. 1.
Clodronate is a potent and selective inhibitor of VNUT. (A) Purified fraction (5 μg) was analyzed via 10% SDS/PAGE and visualized via Coomassie Brilliant Blue staining. The positions of marker proteins are indicated on the left. The positions of recombinant proteins are indicated using arrowheads. (B) Clodronate and etidronate inhibition of various transporters was assayed in the presence of 10 mM Cl− (the physiological intracellular concentration of chloride) for 2 min, and is shown as the concentration required for 50% inhibition (IC50) (n = 3–12). PAH, p-aminohippurate; VEAT, vesicular excitatory amino acid transporter; VIAAT, vesicular inhibitory amino acid transporter.
These purified proteins were incorporated into proteoliposomes, following which the inhibitory effects of the first-generation bisphosphonates on these transporters were examined. We observed that at half-maximal inhibitory concentration of 15.6 nM, clodronate exerted a stronger inhibitory effect on VNUT-mediated ATP uptake than etidronate (Fig. 1B). Neither clodronate nor etidronate exhibited strong inhibitory effects on other vesicular neurotransmitter transporters or SLC17 family transporters (Fig. 1B). Our cis-inhibition analysis also indicated that clodronate was the strongest VNUT inhibitor among the bisphosphonates examined (Fig. 2).
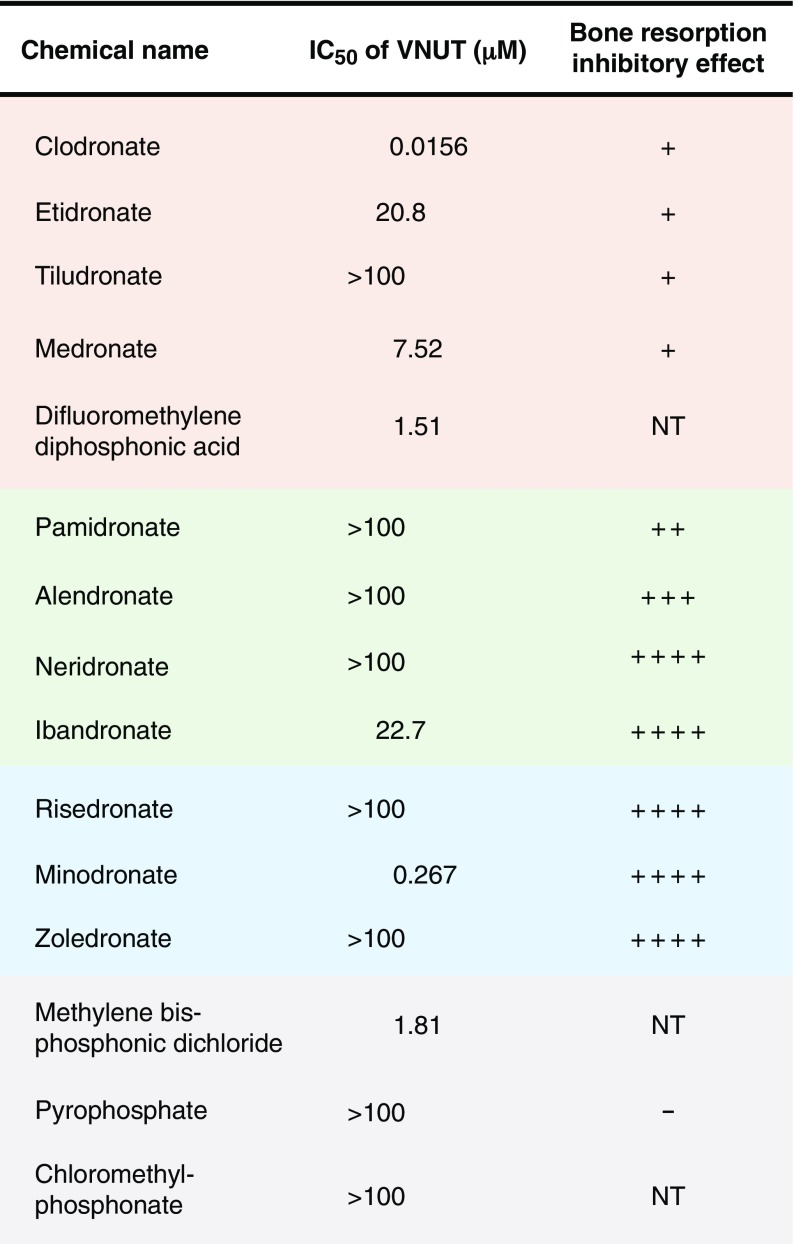
Fig. 2.
Clodronate is the strongest inhibitor of VNUT. The inhibitory potencies of bisphosphonates toward Δψ-dependent ATP uptake by proteoliposomes containing purified VNUT were assayed in the presence of 10 mM Cl− at 2 min and are shown as IC50 (n = 3–13). The effect of pyrophosphate on ATP uptake was examined in ref. 10. The degrees of bone resorption inhibitory effect are indicated as follows: −, none; +, weak; ++, moderate; +++, strong; ++++, very strong (18, 43). NT, not tested.
Clodronate Is an Allosteric Modulator of VNUT Cl− Dependence.
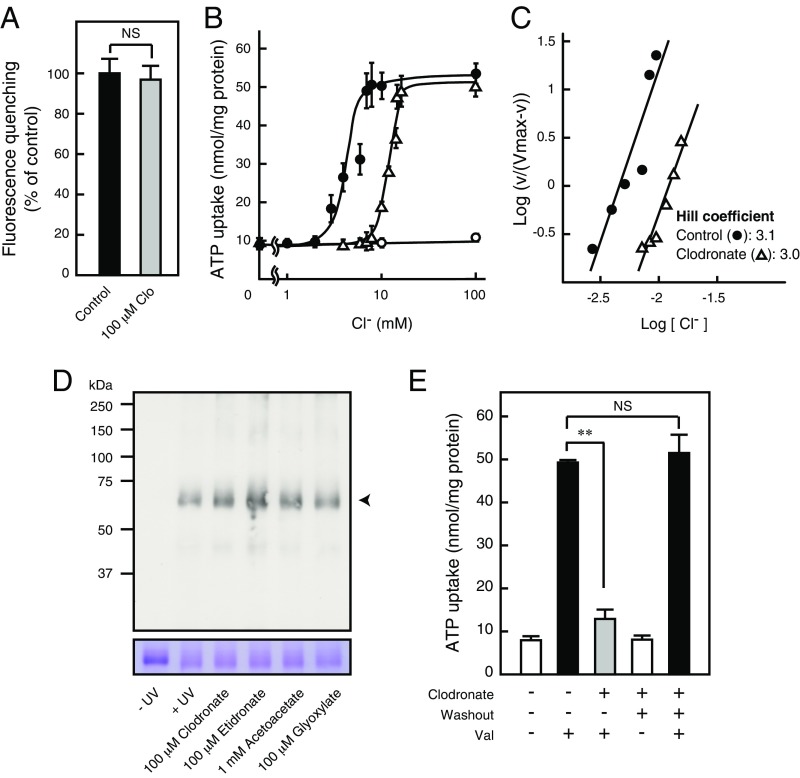
We further examined the mechanism underlying the inhibitory effect of clodronate on VNUT-mediated ATP uptake. Exposure to a high concentration of clodronate had no effect on the Δψ, based on oxonol-V fluorescence quenching (Fig. 3A). An analysis of Cl− dependency for ATP uptake revealed no change in ATP transport following exposure to up to 2 mM Cl−: ATP uptake markedly increased following treatment with 3–7 mM Cl− and plateaued beyond 8 mM Cl− (Fig. 3B). Notably, Cl−-dependent VNUT activation exhibited strong positive cooperativity for ATP transport, with a Hill coefficient of ∼3 for Cl− (Fig. 3C). Clodronate shifted Cl− concentration toward a higher activation level, suggesting a competitive interaction (Fig. 3 B and C). Photoaffinity labeling for ATP binding showed results almost identical to the results obtained for ATP transport-mediated substrate specificity (Fig. S2), and was not inhibited by either clodronate or etidronate (Fig. 3D). In addition, keto acids, such as acetoacetate or glyoxylate, which are known to inhibit SLC17 transporters in a Cl−-dependent manner (23, 24), did not inhibit ATP binding (Fig. 3D). These effects of clodronate were completely reversible (Fig. 3E).
Fig. 3.
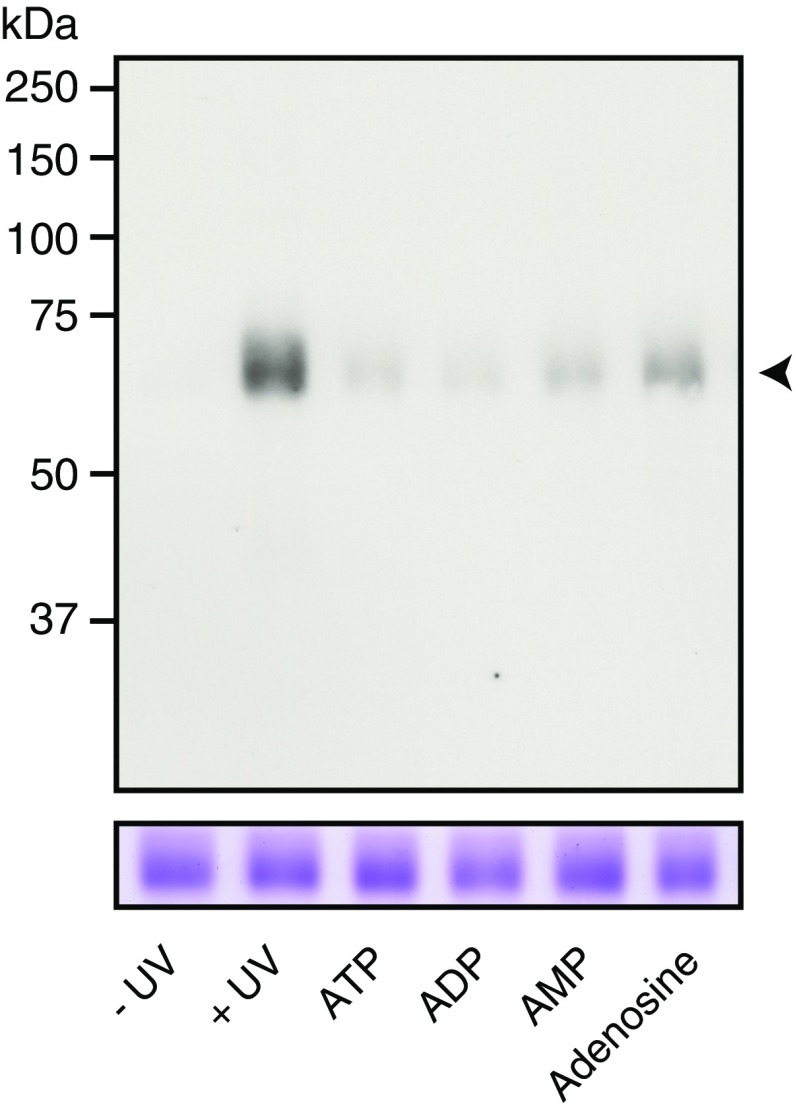
Clodronate (Clo) reversibly inhibits VNUT through Cl− competition. (A) Val-evoked Δψ formation was measured as a 2-min quench of oxonol V fluorescence in the absence or presence of 100 μM Clo. Control activity was subtracted from the quench in the presence of carbonyl cyanide m-chlorophenyl hydrazone (n = 7–8). (B) ATP uptake for 1 min at various [Cl−] values in the presence (△) or absence (●) of 100 nM Clo, or in the absence of valinomycin (○) (n = 3–12). (C) Hill plot of ATP uptake in the presence or absence of 100 nM clodronate. Data were taken from Fig. 3B. (D, Upper) Photoaffinity labeling of VNUT protein (4 μg) upon UV illumination with biotin-11–ATP in the presence or absence of the indicated concentrations of various compounds. (D, Lower) Each protein was analyzed using 10% SDS/PAGE and visualized using Coomassie Brilliant Blue staining. The arrowhead indicates the position of VNUT protein. (E) Inhibition due to 1 μM clodronate was fully reversed after washing the proteoliposomes (n = 4–8). In all cases, data are mean ± SEM (**P < 0.01; two-tailed paired Student’s t test). NS, not significant. NS, not significant.
Fig. S2.
Determination of substrate specificity by photoaffinity labeling of VNUT. (Upper) Photoaffinity labeling of VNUT protein (4 μg) upon UV illumination with 20 μM of biotin-11–ATP in the presence or absence of various compounds at a concentration of 1 mM. (Lower) Each purified protein sample (4 μg) was analyzed using 10% SDS/PAGE and visualized using Coomassie Brilliant Blue staining. The positions of marker proteins are indicated on the left. The position of VNUT protein is indicated using an arrowhead (n = 3).
Clodronate Modulates Vesicular ATP Release.
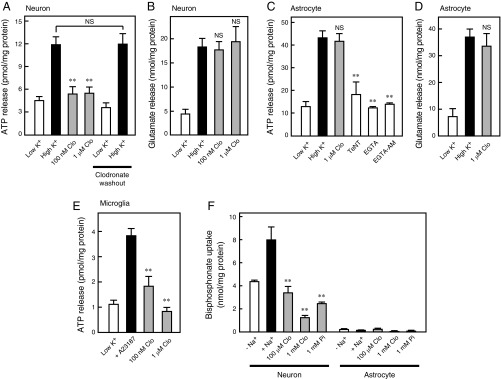
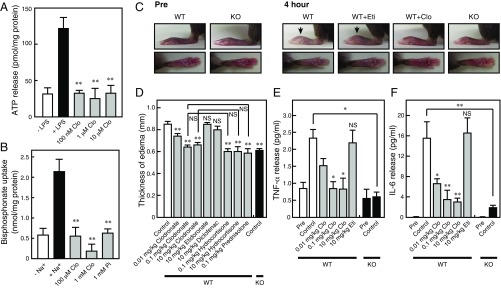
ATP is primarily released from neurons, astrocytes, and microglia via VNUT-mediated exocytosis (12, 25–28). Previous studies have demonstrated that depolarization-dependent ATP release from neurons is inhibited by tetanus neurotoxin or the cell-permeable Ca2+ chelator EGTA-tetraacetoxymethyl ester (AM), both of which are known inhibitors of exocytosis (25). In the present study, we observed complete inhibition of such ATP release following treatment with a low concentration (100 nM) of clodronate, compared with the concentration described for bone resorption inhibition (29) (Fig. 4A). In a parallel experiment, 1 μM clodronate did not inhibit glutamate release, suggesting that clodronate selectively inhibits vesicular ATP release (Fig. 4B). The effects of clodronate on ATP release were also completely reversible (Fig. 4A). Previous researchers have proposed various mechanisms of astrocytic ATP release, such as those mechanisms involving vesicular transporter or plasma membrane channel-mediated pathways (9, 26). In the present study, depolarization-dependent ATP release from astrocytes was inhibited by tetanus neurotoxin, the extracellular Ca2+ chelator EGTA, and EGTA-AM, supporting the notion that astrocytic ATP release occurs via a vesicular mechanism (26) (Fig. 4C). However, our findings indicate that neither ATP nor glutamate release from astrocytes was inhibited by clodronate, even at 1 μM (Fig. 4 C and D). In microglia, ATP release is known to be inhibited by botulinum neurotoxin A or the cell-permeable Ca2+ chelator O,O′-bis(2-aminophenyl)ethyleneglycol-N,N,N′,N′-tetraacetic acid-AM (27). In the present study, we also observed that a low concentration of clodronate resulted in complete inhibition of vesicular ATP release from microglia, similar to the effect observed in neurons (Fig. 4E).
Fig. 4.
Clodronate (Clo) inhibits vesicular ATP release from neurons. KCl-dependent release of ATP (A) and glutamate (B) from cultured neurons after 20 min was assayed in the presence (gray bars) or absence (filled bars) of the indicated Clo concentrations. Inhibition due to 100 nM Clo was fully reversed after washing the cultured neurons. For the washout experiments, Clo-treated cells were washed with medium and incubated for an additional 24 h (n = 4–8). KCl-dependent release of ATP (C) and glutamate (D) from cultured astrocytes after 20 min was assayed in the presence (gray bars) or absence (filled bars) of the indicated concentration of Clo. ATP release from cultured astrocytes was assayed in the presence of 15 μg/mL tetanus neurotoxin (TeNT), 1 mM EGTA, or 50 μM EGTA-AM (open bars) (n = 3–8). (E) Ca2+-dependent ATP release from cultured microglia after 5 min was assayed in the presence (gray bars) or absence (filled bar) of the indicated concentration of Clo (n = 4–5). (F) Alendronate uptake into cultured neurons or astrocytes for 10 min in the presence (gray bars) or absence (filled bar) of 100 μM or 1 mM Clo or 1 mM Pi (n = 4–6). Data are mean ± SEM (**P < 0.01, one-way ANOVA followed by Dunnett’s test). NS, not significant.
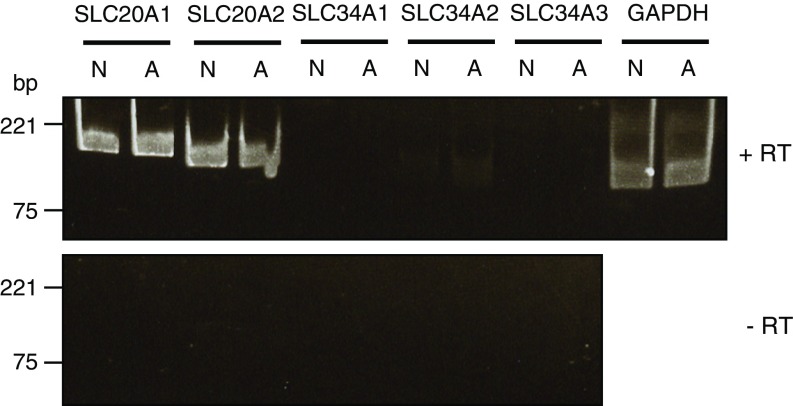
To examine the accessibility of bisphosphonate to neurons and astrocytes, we measured the uptake of a commercially available radiolabeled bisphosphonate. Na+-dependent bisphosphonate transport activity was detected in neurons but not astrocytes, and this activity was completely inhibited by clodronate and inorganic phosphate (Pi), suggesting that Pi transport involves bisphosphonate uptake into neurons (Fig. 4F). We further examined the gene expression pattern of Na+-dependent phosphate transporters, which physiologically consist of the SLC20 and SLC34 families, between neurons and astrocytes (30). However, no neuron-specific signals were detected, suggesting that cellular uptake of bisphosphonate occurs via a novel phosphonate transporter or other transport mechanisms (Fig. S3).
Fig. S3.
Gene expression of SLC20 and SLC34 phosphate transporter families in neurons and astrocytes. Gene expression was examined via RT-PCR analysis of total RNA from primary cultured neurons (N) and astrocytes (A) using probes specific to SLC20 and SLC34 transporters after RT (+RT, Upper) and without RT (−RT, Lower). The resultant PCR products of SLC20A1 (186 bp), SLC20A2 (168 bp), SLC34A1 (180 bp), SLC34A2 (132 bp), SLC34A3 (131 bp), and (GAPDH, 138 bp) were analyzed via electrophoresis.
Clodronate Controls Chronic Inflammatory Pain Through VNUT.
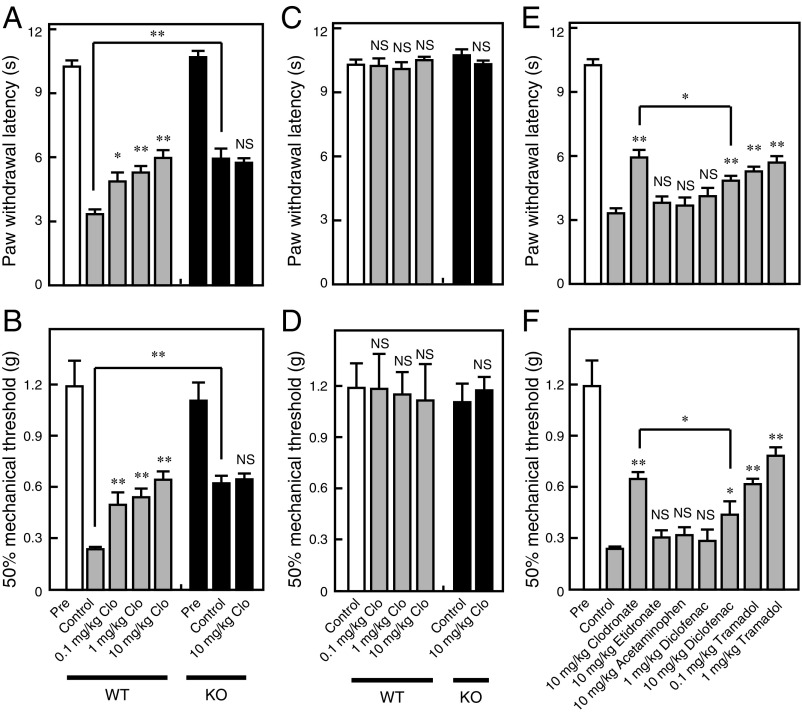
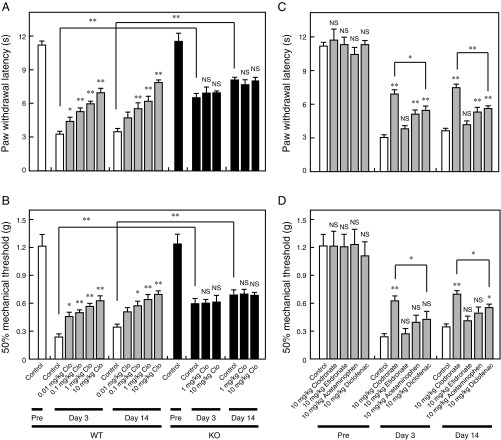
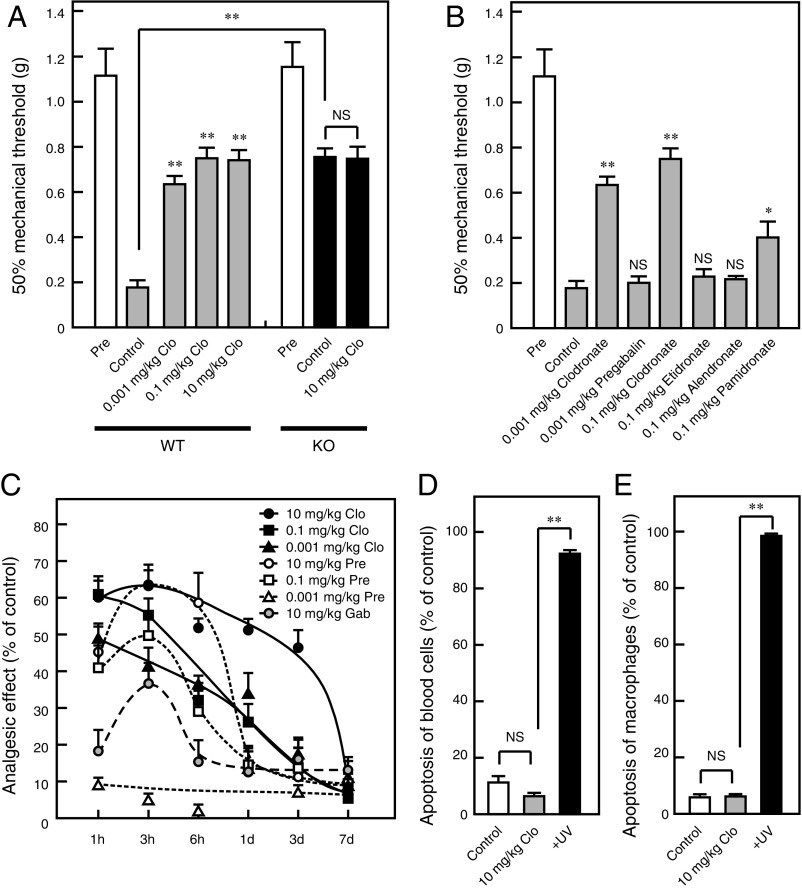
We analyzed the effects of clodronate on inflammatory and neuropathic pain unrelated to bone abnormalities. Approximately 40% attenuation of carrageenan- or complete Freund’s adjuvant (CFA)-evoked inflammatory pain was observed following injection of 10 mg/kg clodronate (Fig. 5 A and B and Fig. S4 A and B). In addition, VNUT−/− mice exhibited reduced hyperalgesia relative to wild-type controls, and the analgesic effect of clodronate was lost in VNUT−/− mice (Fig. 5 A and B and Fig. S4 A and B). Notably, clodronate did not alter baseline sensory thresholds (Fig. 5 C and D). Clodronate-mediated analgesia was stronger than the analgesic effect induced by acetaminophen and diclofenac (first-choice drugs), and comparable to analgesia of the nonnarcotic opioid tramadol in the therapeutic range (Fig. 5 E and F and Fig. S4 C and D). Etidronate, which exerted a lower inhibitory effect of VNUT, had no analgesic effect (Fig. 5 E and F and Fig. S4 C and D).
Fig. 5.
Clodronate (Clo) attenuates inflammatory pain via VNUT inhibition. The plantar test and von Frey test were performed 60 min after an i.v. injection of saline or Clo at the indicated concentrations in wild-type (WT; open or gray bars) and VNUT−/− mice (filled bars) at 4 h after (A and B) or before (C and D) carrageenan injection (n = 5–6 mice). (E and F) Various compounds at the indicated concentrations were assayed (gray bars), and this dataset is the same as the dataset in A and B (n = 5–7 mice). In all cases, data are mean ± SEM (*P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test). NS, not significant.
Fig. S4.
Clodronate (Clo) attenuates CFA-evoked inflammatory pain via VNUT inhibition. The plantar test (A) and von Frey test (B) were performed 60 min after i.v. injection of saline (open bars) or Clo at the indicated concentrations (gray bars) in wild-type (WT) mice (Left) and saline (filled bars) or Clo at the indicated concentrations (filled bars) in VNUT−/− mice at days 3 and 14 after CFA injection (Right) (n = 9–11 mice). The plantar test (C) and von Frey test (D) were performed 60 min after i.v. injection of saline (open bars) or the indicated concentrations of various compounds (gray bars) at the time of maximal effect in WT mice at days 3 and 14 after CFA injection (n = 8–10 mice). This dataset is the same as the dataset in A and B. In all cases, data are mean ± SEM (*P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test). NS, not significant.
VNUT-Mediated Immune Control by Clodronate.
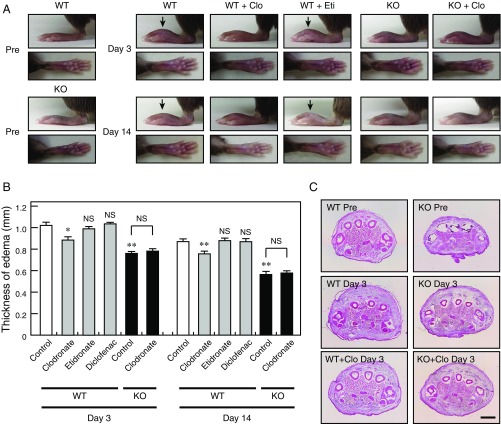
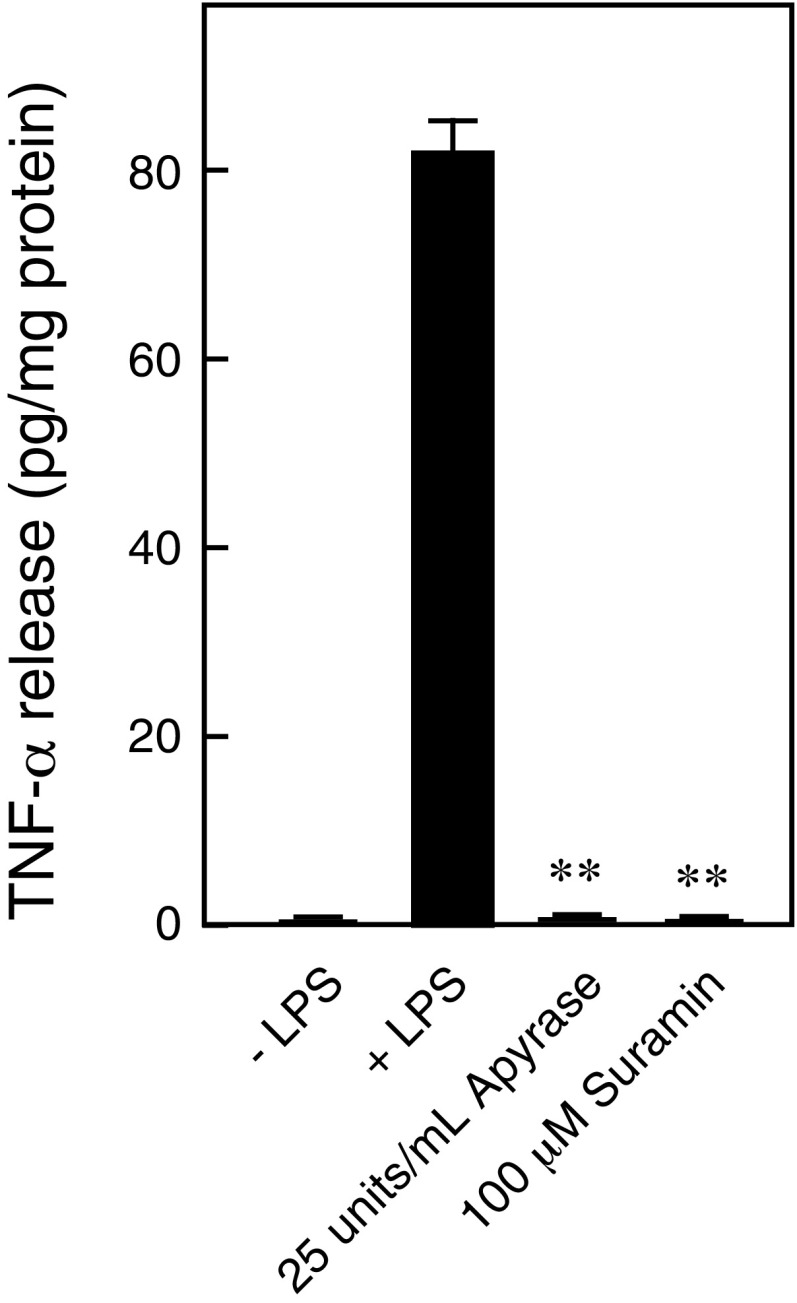
Immune cells contribute to chronic inflammatory pain through the release of inflammatory mediators and infiltration of inflammatory cells (31). In the human monocyte cell line THP-1 (32), clodronate inhibited vesicular ATP release in a manner similar to the vesicular ATP release observed in neurons, suggesting that clodronate exerts an antiinflammatory effect (Fig. 6 A and B). In vivo, carrageenan-evoked or CFA-evoked hind-paw edema was attenuated following exposure to clodronate, but not to etidronate (Fig. 6 C and D and Fig. S5 A and B). The antiinflammatory effect of clodronate was stronger than the effect of diclofenac in the therapeutic range, comparable to the maximal effect of the moderate steroid hydrocortisone and to the effect of the strong steroid prednisolone in the therapeutic range (Fig. 6D and Fig. S5B). Glucocorticoids exert a strong antiinflammatory effect but can cause a wide range of severe side effects, such as metabolic dysfunction (diabetes, hyperlipidemia, and osteoporosis) and increased susceptibility to infections (33). In VNUT−/− mice, carrageenan- or CFA-evoked hind-paw edema was also attenuated to ∼70% of the edema observed in wild-type controls (Fig. 6 C and D and Fig. S5 A and B). Moreover, the inflammatory response was decreased in VNUT−/− mice compared with the inflammation observed in wild-type controls (Fig. S5C). Notably, TNF-α and IL-6 were detected in the blood after carrageenan injection, and the release of these cytokines was strongly inhibited by clodronate (Fig. 6 E and F). Similarly, VNUT−/− mice exhibited markedly decreased blood cytokine levels compared with wild-type controls (Fig. 6 E and F). Release of inflammatory mediators from THP-1 cells was inhibited by apyrase (ATP diphosphohydrolase) or suramin (nonselective inhibitor of purinoceptors), suggesting that cytokine release requires autocrine ATP release (Fig. S6).
Fig. 6.
Clodronate (Clo) attenuates inflammation via VNUT inhibition. (A) LPS-dependent release of ATP from THP-1 cells after 10 min was assayed in the presence (gray bars) or absence (filled bar) of the indicated concentrations of Clo (n = 4–6). (B) Alendronate uptake into THP-1 cells at 10 min in the presence (gray bars) or absence (filled bar) of 100 μM or 1 mM Clo or 1 mM Pi (n = 3–5). (C) Hind paws of WT and VNUT−/− mice were injected i.v. with saline or the indicated various compound concentrations, and were photographed at 4 h after carrageenan injection. Edema is indicated using arrows. Eti, etidronate. (D) Summary of edema thickness in C. The values were determined by subtracting the thickness before carrageenan injection from the thickness after injection (n = 5–11 mice). (E and F) Serum cytokines levels in WT and VNUT−/− mice were measured 2 h after carrageenan injection in the presence of saline. The indicated concentrations of Clo or Eti were also measured 2 h after carrageenan injection (n = 3–5 mice). In all cases, data are mean ± SEM (*P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test). NS, not significant.
Fig. S5.
Clodronate (Clo) attenuates CFA-evoked inflammation via VNUT inhibition. (A) Hind paws of WT mice were injected s.c. with saline (open bars in B) or various compounds at 10 mg/kg (gray bars in B) per day, and hind paws of VNUT−/− mice were injected s.c. with saline (filled bars in B) or 10 mg/kg Clo (filled bars in B) per day. The hind paws were photographed at days 3 and 14 after injection of CFA. Edema is indicated using arrows. (B) Summary of the edema thickness shown in A. The values were determined by subtracting the thickness before CFA injection from the thickness after injection (n = 9–10 mice). (C) Hematoxylin/eosin staining of hind-paw sections from WT and VNUT−/− mice injected s.c. with saline or 10 mg/kg Clo per day at day 3 after CFA injection. (Scale bar: 1 mm.) In all cases, data are mean ± SEM (*P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test). NS, not significant.
Fig. S6.
Cytokine release requires autocrine ATP release. LPS-dependent release of TNF-α from THP-1 cells after 6 h was assayed in the presence (gray bars) or absence (filled bar) of the indicated concentration of apyrase or suramin (n = 5–8). Data are mean ± SEM (**P < 0.01, one-way ANOVA followed by Dunnett’s test).
VNUT-Mediated Chronic Neuropathic Pain Control by Clodronate.
Approximately 60% attenuation of neuropathic pain was observed after a prior clodronate injection at a dose lower than the dose used for inflammatory pain (Fig. 7A). VNUT−/− mice also exhibited reduced hyperalgesia compared with wild-type controls, and this analgesic effect of clodronate was lost in VNUT−/− mice (Fig. 7A). The analgesic effect of clodronate was stronger than analgesia induced by pregabalin and gabapentin (Fig. 7 B and C), both of which are in widespread clinical use. Some bisphosphonates attenuate the neuropathic pain associated with complex regional pain syndrome 1 in conditions involving bone abnormalities (16). In the present study, bisphosphonate compounds other than clodronate exerted weak or no analgesic effects, similar to their inhibitory effects on VNUT, suggesting that the analgesic effect of other bisphosphonates depends on the inhibition of bone resorption (Fig. 7B). The analgesic effect of clodronate has both fast- and long-acting properties compared with the effect of pregabalin and gabapentin and was completely reversible, suggesting that clodronate at this dose is not toxic and is without side effects (Fig. 7C). Notably, pregabalin at the effective dose (0.1–10 mg/kg) seemingly induced drowsiness and reduced exploratory behavior, although these effects were not observed for clodronate, even at a dose of 10 mg/kg (Movie S1). Although pregabalin did not induce drowsiness at a dose of 0.001 mg/kg, administration at this dose did not attenuate neuropathic pain (Fig. 7C).
Fig. 7.
Clodronate (Clo) attenuates neuropathic pain via VNUT inhibition. (A) The von Frey test was performed 60 min after an i.v. injection of saline (open bar) or Clo at the indicated concentrations (gray bars) in WT mice (Left) and VNUT−/− mice (Right) at 10 d after nerve injury (filled bars) (n = 7 mice). (B) Various compounds at the indicated concentration were assayed, and this dataset is the same as the dataset in A. The injection of compounds was performed at the time of maximal effect (n = 7 mice). (C) The von Frey test was performed the indicated time after i.v. injection of saline, Clo, pregabalin (Pre), or gabapentin (Gab) at the indicated concentrations in WT mice (n = 6–10 mice). Blood cells (D) or macrophages (E) were prepared 60 min after an i.v. injection of saline (open bars) or 10 mg/kg Clo (gray bars) in WT mice, and the apoptosis assay was performed (n = 3–5 mice). In all cases, data are mean ± SEM (*P < 0.05, **P < 0.01; one-way ANOVA followed by Dunnett’s test or two-tailed paired Student’s t test). NS, not significant.
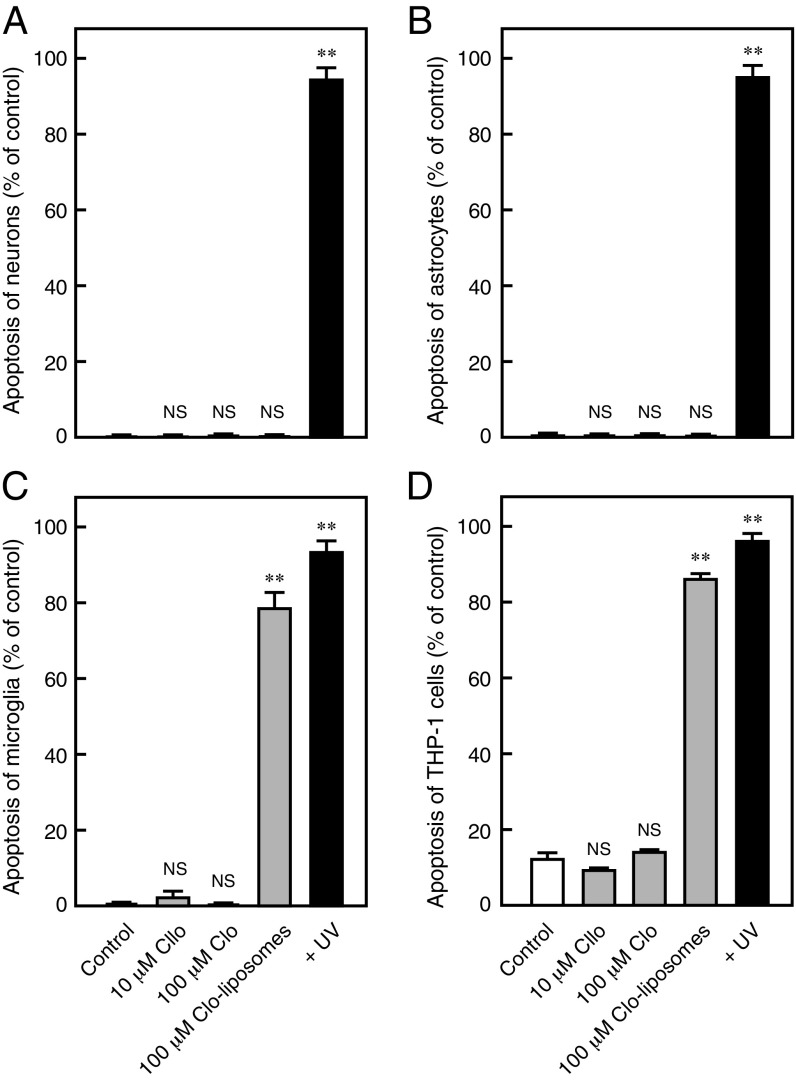
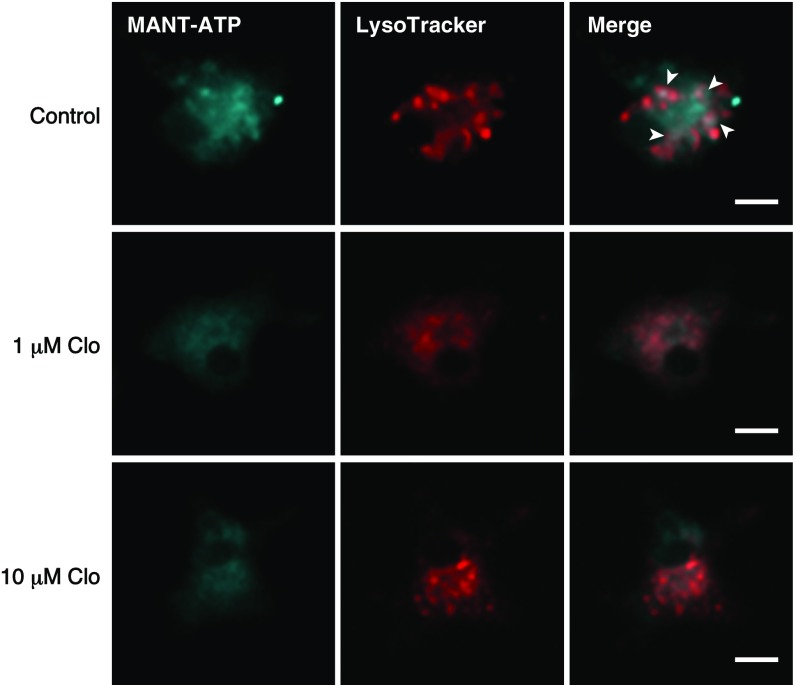
Moreover, previous studies have indicated that clodronate-containing liposomes induce macrophage apoptosis by selective delivery of a high concentration of clodronate into macrophages (34), which may be involved in the clodronate analgesic effect. However, as expected, the low concentration of clodronate (10 mg/kg) did not induce apoptosis of blood cells, including macrophages (Fig. 7 D and E). Similarly, low concentrations of clodronate, which resulted in complete inhibition of vesicular ATP release, did not induce apoptosis in neurons, astrocytes, microglia, or THP-1 cells. As expected, clodronate-containing liposomes induced complete apoptosis in phagocytic microglia and THP-1 cells, but not in neurons or astrocytes (34, 35) (Fig. S7). Recent reports have suggested that ATP is also stored in microglial lysosomes in a VNUT-mediated manner, and that inhibition of this process leads to cell death (36). Although lysosomal storage of ATP in microglia was inhibited by clodronate, clodronate did not induce microglial apoptosis, suggesting the existence of other functions of lysosomal ATP release (Fig. S8).
Fig. S7.
The Clodronate (Clo)-evoked effect is independent of apoptosis. Apoptosis assays were performed in neurons (A), astrocytes (B), microglia (C), and THP-1 cells (D) following incubation with the indicated concentrations of Clo or Clo-containing liposomes for 24 h (n = 5–7). Data are mean ± SEM (**P < 0.01, one-way ANOVA followed by Dunnett’s test). NS, not significant.
Fig. S8.
Clodronate (Clo) inhibits MANT-ATP signals in LysoTracker-positive organelles. MANT-ATP signals in cultured microglia were inhibited following treatment with 1 μM or 10 μM Clo. MANT-ATP signals (blue) partially colocalized with LysoTracker staining (red). The colocalized signals are indicated using arrowheads. (Scale bars: 10 μm.)
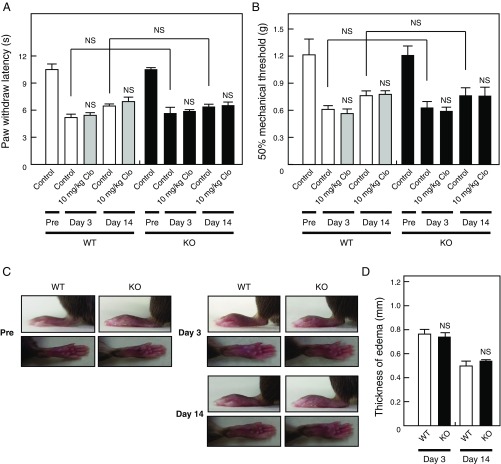
Finally, the pathogenesis of neuropathic pain, but not inflammatory pain, is thought to involve VNUT (13). Our analysis revealed that VNUT is involved in pathological inflammatory pain in two well-characterized models. Consistent with the findings of a previous study (13), we observed that basal nociception and weak inflammation with less chronicity were not affected by clodronate in an inflammatory pain model (one-fourth volume of CFA compared with Figs. S4 and S5) (Fig. S9). These observations strongly suggest that VNUT inhibition improves chronic pathogenesis more extensively than pain under physiological conditions.
Fig. S9.
Severity of CFA-evoked inflammation is associated with the significance of VNUT in inflammatory pain. The plantar test (A) and von Frey test (B) were performed 60 min after i.v. injection of saline (open bar) or clodronate (Clo) at a concentration of 10 mg/kg (gray bars) in WT mice (Left) and saline (filled bar) or Clo at a concentration of 10 mg/kg (filled bars) in VNUT−/− mice (Right) at days 3 and 14 after injection of 5 μL of 1 mg/mL CFA (one-fourth volume of Figs. S4 and S5) (n = 6–7 mice). (C) Hind paws of WT and VNUT−/− mice were photographed at days 3 and 14 after injection of 5 μL of 1 mg/mL CFA. (D) Summary of the edema thickness shown in C. The values were determined by subtracting the thickness before CFA injection from the thickness after injection (n = 6 mice). In all cases, data are mean ± SEM (two-tailed paired Student’s t test). NS, not significant.
Discussion
Previous attempts to develop new therapeutic drugs for the treatment of chronic neuropathic and inflammatory pain with reduced side effects have been unsuccessful. In the present study, we observed that clodronate is a potent and selective inhibitor of vesicular storage and release of ATP, which is mediated by allosteric modulation at the VNUT Cl− binding site. In vivo, clodronate was more effective than other agents in attenuating chronic neuropathic and inflammatory pain and the accompanying inflammation without affecting basal nociception in wild-type mice. Consistent with these observations, VNUT−/− mice exhibited reductions in chronic pain and inflammation, for which clodronate was ineffective. These observations indicated that clodronate-evoked inhibition of purinergic chemical transmission is important for the treatment of neuropathic and inflammatory pain with reduced side effects. Furthermore, the present study identifies a transporter-targeted analgesic and antiinflammatory drug.
Cl− dependency is a unique feature of SLC17 transporters (23). VNUT-mediated ATP transport is activated by Cl−, and this activation is inhibited competitively and reversibly by keto acids, such as acetoacetate and glyoxylate (23, 24). Regulation of the metabolic anion switch between Cl− and keto acids safely controls purinergic chemical transmission in response to changes in metabolic state. However, keto acids are metabolized in the body, and they do not show high specificity among SLC17 transporters (∼10-fold) (23, 24). Our findings suggest that clodronate is the strongest allosteric modulator of VNUT Cl− dependence, because the stoichiometric ratio of VNUT protein in proteoliposomes and clodronate was 1:1, with no change in the Hill coefficient for Cl− even in the presence of clodronate (Figs. 1 and 3). Although regulation of Cl− is highly conserved in both mammals and plants (37), clodronate has been shown to interact selectively with VNUT Cl− binding sites. Because the VNUT inhibitory effects of halogen atoms from the characteristic bisphosphonate groups are correlated with VNUT activation in a halogen-dependent manner, the chlorines of clodronate may bind to the VNUT Cl− binding site, and the bisphosphonate skeleton may enhance affinity to this binding site (10) (Fig. 2 and Fig. S1). Further structural studies of VNUT are required to clarify this structure–activity relationship.
We elucidated two significant phenotypes associated with the loss of vesicular ATP release using clodronate and VNUT−/− mice. First, VNUT was involved in pathological neuropathic and inflammatory pain in vivo (Figs. 5 and 7). Although VNUT gene defects were not associated with basal nociception and weak inflammation with low chronicity, VNUT contributed to chronic hyperalgesia (∼40–60% of the total). Consistent with these observations, a recent study has reported that VNUT gene expression in the spinal cord is significantly up-regulated in pathological conditions (13). The study further reported that VNUT in spinal dorsal horn neurons, but not in astrocytes and microglia, is responsible for the pathogenesis of neuropathic pain, suggesting that VNUT may be an important target for the treatment of neuropathic pain (13). In the present study, clodronate was consistently taken up by neurons, inhibiting neuronal vesicular ATP release and thereby attenuating neuropathic pain (Figs. 4 and 7). Our results strongly support the notion that VNUT is involved in the pathogenesis of not only neuropathic pain but also inflammatory pain, and that a specific inhibitor may serve as an extensive and effective analgesic drug in this patient population. Further studies are required to clarify in vivo VNUT function in primary afferent nerve terminals of the spinal dorsal horn, which may be involved in inflammatory pain.
Second, our study demonstrates that VNUT is involved in the immune response in vivo (Fig. 6). We propose that the following mechanism underlies the antiinflammatory effect of VNUT inhibition: VNUT is also localized in secretory vesicles in immune cells (e.g., monocytes, macrophages, T cells) and is responsible for vesicular storage and release of ATP (32, 38). Released ATP or degraded ADP and adenosine bind to various purinoceptors in an autocrine or paracrine manner, stimulating the release of inflammatory mediators and thus leading to inflammation (39). We observed that clodronate completely inhibited the release of ATP from immune cells and reduced blood levels of inflammatory mediators, such as TNF-α and IL-6, which are released mainly from macrophages and T cells (Fig. 6). These observations indicated that VNUT is a key molecule for the induction of pathological pain and inflammation, and that VNUT inhibition is therefore essential for improving pathological symptoms.
Because purinergic chemical transmission is involved in disease pathogenesis (9), clodronate-evoked purinergic chemical transmission blockade may be effective in the treatment of several chronic diseases, including chronic auto-inflammatory diseases, diabetes, and neurological disorders, among others. It should be stressed that the therapeutic effects of clodronate were stronger than the effects of widespread drugs for neuropathic pain, such as pregabalin or gabapentin (Fig. 7C). In addition, these effects were comparable to the effects of widespread drugs for inflammatory pain or inflammation, such as tramadol or prednisolone, in the therapeutic range (Figs. 5 E and F and 6D). However, tramadol and prednisolone are associated with severe side effects, strongly suggesting that clodronate is promising for the treatment of intractable diseases associated with abnormalities in purinergic transmission, with few side effects. Notably, VNUT−/− mice exhibit an improvement in blood glucose homeostasis, insulin sensitivity, and other pathological conditions, with no significant changes in phenotype (12). These observations suggest that clodronate may improve a wide range of diabetic symptoms, such as neuropathic pain, inflammation, hyperglycemia, and insulin sensitivity. Because no effective drugs or therapies for these diabetic symptoms have yet been developed (40), further studies regarding the wide range of applications of clodronate are currently in progress in our laboratories.
In summary, our findings indicate that clodronate selectively and robustly inhibits VNUT and can safely regulate purinergic chemical transmission in vivo, thereby attenuating pathological neuropathic and inflammatory pain and the accompanying inflammation in conditions without bone abnormalities. Notably, clodronate is approved for clinical use in the treatment of osteoporosis in many countries, and its clinical safety in humans is well established (21). Therefore, it is important to evaluate analgesic effects of clodronate for painful diseases independent of bone abnormalities in humans. Given its potency and the side effects of existing analgesics, clodronate, a nonopioid and nonsteroidal drug, might serve well as a novel analgesic or antiinflammatory drug.
Materials and Methods
Animal experiments were performed in accordance with the guidelines set by the Animal Care and Use Committees of Okayama University and Ajinomoto Co., Inc. All experiments were carried out in accordance with the approved institutional guidelines. Additional information on experimental methods is included in SI Materials and Methods.
Expression and Purification of Transporters in E. coli.
Vesicular neurotransmitter transporters were expressed and purified as previously described (22). Briefly, E. coli C43 (DE3) cells were transformed with the expression vectors and grown in Terrific Broth medium containing 30 μg/mL kanamycin sulfate at 37 °C. E. coli cells were grown until A600 reached 0.6–0.8, following which isopropyl-β-d-thiogalactopyranoside was added to a final concentration of 1 mM, followed by incubation for a further 16 h at 18 °C. The cells were then harvested by centrifugation and suspended in buffer consisting of 70 mM Tris⋅HCl (pH 8.0), 100 mM NaCl, 10 mM KCl, 15% glycerol, and 2 mM PMSF. The cell suspension was then disrupted by sonication with a TOMY UD200 tip sonifier (OUTPUT4) and centrifuged at 5,856 × g at 4 °C for 10 min to remove large inclusion bodies and cell debris. The resultant supernatant was carefully collected and centrifuged again at 150,000 × g for 1 h at 4 °C. The pellet was suspended in the same buffer, and the protein concentration was adjusted to 10 mg/mL. The membranes were then treated with 2% Fos-choline 14 (Affymetrix) and centrifuged at 150,000 × g at 4 °C for 1 h. The supernatant containing recombinant protein was obtained and diluted twofold with buffer consisting of 70 mM Tris⋅HCl (pH 8.0), 100 mM NaCl, 10 mM KCl, 15% glycerol, and 2 mM PMSF, following which it was applied to a column containing 1 mL of Ni-NTA Superflow resin (Qiagen) equilibrated with buffer consisting of 70 mM Tris⋅HCl (pH 8.0), 100 mM NaCl, 10 mM KCl, and 15% glycerol. After incubation for 3 h at 4 °C, the column was washed with 20 mL of washing buffer consisting of 70 mM Tris⋅HCl (pH 8.0), 20 mM imidazole, 100 mM NaCl, 10 mM KCl, 20% glycerol, and 0.1% n-decyl-β-d-thiomaltopyranoside (DTM; Affymetrix). The protein was eluted with 3 mL of buffer consisting of 20 mM Tris⋅HCl (pH 8.0), 250 mM imidazole, 100 mM NaCl, 10 mM KCl, 20% glycerol, and 0.1% DTM, following which it was stored at −80 °C, at which temperature it was stable without loss of activity for at least a few months.
Reconstitution.
Aliquots of 20 μg of purified protein were mixed with 550 μg of liposomes and frozen at −80 °C for at least 15 min. The mixture was thawed quickly by holding the sample tube in the hand and diluted 60-fold with reconstitution buffer containing 20 mM 3-(N-morpholino)propanesulfonic acid (MOPS)–Tris (pH 7.0), 150 mM sodium acetate, and 5 mM magnesium acetate. The buffer composition was altered as necessary. Reconstituted proteoliposomes were pelleted via centrifugation at 200,000 × g for 1 h at 4 °C and then suspended in 0.2 mL of reconstitution buffer. Asolectin liposomes were prepared as previously described (37). Soybean lecithin (10 mg/mL, Type IIS; Sigma) was suspended in buffer containing 20 mM MOPS-NaOH (pH 7.0) and 1 mM DTT. The mixture was sonicated until clear in a bath-type sonicator and stored at −80 °C until use.
In another experiment, 20 μg of VMAT2 was mixed with liposomes (550 μg of lipid), frozen at −80 °C, and left at this temperature for at least 15 min. The mixture was diluted 60-fold with reconstitution buffer containing 40 mM MES-Tris (pH 5.7), 150 mM potassium acetate, and 5 mM magnesium acetate. Reconstituted proteoliposomes were pelleted via centrifugation at 200,000 × g for 1 h at 4 °C and then suspended in 0.2 mL of reconstitution buffer.
Transport Assay.
The reaction mixture (130 μL) consisting of 0.3 μg of protein incorporated into proteoliposomes, 20 mM MOPS-Tris (pH 7.0), 140 mM potassium acetate, 5 mM magnesium acetate, 10 mM KCl, and 2 μM valinomycin, as well as 100 μM [3H] ATP (0.5 MBq/μmol; PerkinElmer), 100 μM [2,3-3H] l-glutamate (0.5 MBq/μmol; PerkinElmer), 100 μM [2,3-3H] l-aspartate (0.5 MBq/μmol; PerkinElmer), 100 μM [2,3-3H] GABA (0.5 MBq/μmol; PerkinElmer), or 100 μM p-[glycyl-2-3H] p-aminohippuric acid (0.5 MBq/μmol; PerkinElmer), was incubated at 27 °C. At the indicated time points, the proteoliposomes were separated from the external medium using centrifuge columns containing Sephadex G-50 (fine) to terminate transport. The radioactivity in the eluate was measured via liquid scintillation counting (PerkinElmer).
For serotonin transport by VMAT2, proteoliposomes containing VMAT2 (0.3 μg of protein) were incubated in 20 mM MOPS-Tris (pH 7.5), 140 mM potassium acetate, 5 mM magnesium acetate, 10 mM KCl, and 10 μM [2-3H] serotonin (0.5 MBq/μmol; PerkinElmer) at 27 °C.
ATP and Glutamate Release from Neurons, Astrocytes, and Microglia.
Primary cultured neurons or astrocytes (2.0 × 105 cells per 3.5-cm dish) were washed three times with Krebs–Ringer bicarbonate buffer composed of 128 mM NaCl, 1.9 mM KCl, 1.2 mM KH2PO4, 1.3 mM MgSO4, 26 mM NaHCO3, 10 mM d-glucose, 10 mM Hepes-NaOH (pH 7.4), 2.4 mM CaCl2, and 0.2% (wt/vol) BSA. After the cells had been incubated in Krebs–Ringer bicarbonate buffer at 37 °C for 3 h, 55 mM KCl was added to stimulate ATP and glutamate release. After incubation at 37 °C for 20 min, aliquots were collected and the amount of ATP was measured using an ATP bioluminescent assay kit (Sigma–Aldrich), whereas the amount of glutamate was measured via HPLC on a COSMOSIL5 C18-ARII column (4.6 × 150 mm; Nacalai Tesque) and fluorescence detection, as previously described (12). In primary cultured microglia (1.0 × 104 cells per 96-well plate), 5 μM Ca2+ ionophore A23187 was added to stimulate ATP release. Aliquots were collected after 5 min, and the amount of ATP was measured. The addition of clodronate at our experimental concentration exerted no impact on the ATP bioluminescence assay. The slopes of the standard curves in the absence and presence of clodronate at 10 μM were as follows: 96.4 ± 9.0 and 92.8 ± 5.7 relative luminescence unit/fmol of ATP, respectively (n = 3, not significant in two-tailed paired Student’s t test).
Plantar Test.
The plantar test was performed as previously described (41). C57BL/6 mice (male, weighing 22–30 g at the time of the test) were acclimatized to an elevated acrylic observation chamber (14.0 × 17.0 × 11.0 cm) for 60 min before the plantar test. Heat hyperalgesia was assessed using a Hargreaves radiant heat apparatus (Ugo Basile). The heat source, a mobile infrared photobeam, was positioned under the plantar surface of the left hind paw. The cutoff was set to 20 s to prevent tissue damage in untreated control mice. Clodronate was injected i.v. via the tail vein 60 min before the plantar test in a volume of 100 μL per 10 g of body weight. We calculated the percent maximum possible effect = [(PL − BL2)/(BL1 − BL2)] × 100, where BL1 represents baseline latency before inflammation, BL2 represents baseline latency after inflammation but before drug injection, and PL represents latency after drug injection.
The von Frey Test.
The von Frey test was performed as previously described (41). C57BL/6 mice (male, weighing 22–30 g at the time of the test) were acclimatized to an elevated metal mesh floor chamber (10.0 × 16.0 × 9.0 cm) for 60 min before the von Frey test. Mechanical hyperalgesia was assessed by measuring the left hind-paw withdrawal response to stimulation with a series of von Frey filaments (0.04–2.0 g; Aesthesio) presented perpendicular to the plantar surface. We determined the 50% paw withdrawal threshold using Dixon’s up-down method (42). Clodronate was injected i.v. via the tail vein 60 min before the von Frey test in a volume of 100 μL per 10 g of body weight.
SI Materials and Methods
Chemicals.
Clodronate, etidronate, tildronate, medronate, pamidronate, neridronate, ibandronate, zoledronate, methylene bisphosphonic dichloride, glyoxylate, pregabalin, gabapentin, apyrase, suramin, and tetanus neurotoxin were purchased from Sigma–Aldrich. Difluoromethylene diphosphonic acid was purchased from Toronto Research Chemicals, Inc. Alendronate and risedronate were purchased from LKT Laboratories, Inc. Minodronate and acetoacetate were purchased from the Tokyo Chemical Industry Co., Ltd. Chloromethyl phosphonate was purchased from Santa Cruz Biotechnology, Inc. The compounds were dissolved in distilled water. Acetaminophen and prednisolone were purchased from Sigma–Aldrich and dissolved in ethanol. Diclofenac was purchased from Wako Pure Chemical Industries, Ltd. Hydrocortisone was purchased from Sigma–Aldrich. Tramadol was purchased from Toronto Research Chemicals, Inc. The compounds were dissolved in methanol. A23187 was purchased from Sigma–Aldrich and dissolved in dimethyl sulfoxide. Clophosome-N (clodronate-containing liposomes) was purchased from FormuMax Scientific, Inc.
cDNA.
Human VNUT (accession no. NM001302643.1), rat VGLUT1 (accession no. NM053859.2), rat VGLUT2 (accession no. NM053427.1), human VGLUT3 (accession no. NM001145288.1), mouse VEAT (accession no. NM172773), mouse VIAAT (accession no. BC052020), rat VMAT2 (accession no. NM013031.1), and mouse NPT1 (accession no. NM001170638.1) PCR was used to clone cDNAs (22, 23).
Expression and Purification of Transporters in Insect Cells.
Vesicular neurotransmitter transporters were expressed in insect cells and purified as previously described (23). Recombinant baculovirus containing the genes of interest were constructed using the Bac-to-Bac baculovirus expression system (Invitrogen) in accordance with the manufacturer’s protocol. The cells (1–2 × 108) were suspended in a buffer containing 20 mM Tris⋅HCl (pH 8.0), 100 mM sodium acetate, 10% glycerol, 0.5 mM DTT, 10 μg/mL pepstatin A, and 10 μg/mL leupeptin, and were disrupted by sonication with a TOMY UD200 tip sonifier. Cell lysates were centrifuged at 700 × g for 10 min to remove debris, and the resultant supernatant was centrifuged at 160,000 × g for 1 h at 4 °C. The pellet (membrane fraction) was suspended in a buffer containing 20 mM Tris⋅HCl (pH 8.0), 10% glycerol, 10 μg/mL pepstatin A, and 10 μg/mL leupeptin at ∼1.5 mg of protein per milliliter and solubilized with 2% octyl glucoside (Dojindo). After centrifugation at 260,000 × g for 30 min, the supernatant was collected and applied to 1 mL of Ni-NTA Superflow resin (Qiagen). After incubation for 4 h at 4 °C, the resin was washed with 20 mL of 20 mM MOPS-Tris (pH 7.0), 4 mM imidazole, 20% glycerol, and 1% octyl glucoside. The protein was eluted from the resin with 3 mL of the same buffer containing 60 mM imidazole and could be stored at −80 °C without loss of activity for at least a few months.
Measurement of Δψ via Fluorescence Quenching.
We assayed Δψ (inside-positive) by measuring the fluorescence quenching of oxonol V (Sigma–Aldrich) as previously described (44). The reaction mixture (450 μL) consisting of 1 μg of protein incorporated into proteoliposomes, 20 mM MOPS-Tris (pH 7.0), 140 mM potassium acetate, 5 mM magnesium acetate, 10 mM KCl, and 1 μM oxonol V was incubated for 50 s at 27 °C. The reaction was initiated by addition of 2 μM valinomycin in the absence or presence of the listed inhibitors and was terminated by addition of 2 μM carbonyl cyanide m-chlorophenyl hydrazone.
ATP Binding Assay.
Photoaffinity labeling of VNUT proteins was performed essentially as previously described (45). Biotin-11–ATP (PerkinElmer) at a concentration of 20 μM was mixed with 4 μg of VNUT protein in 50 μL of a buffer containing 20 mM MOPS-Tris (pH 7.4), 50 mM potassium acetate, 2 mM magnesium acetate, 10 mM KCl, and 0.1% DTM on ice in the dark. After incubation for 3 min on ice, the solutions were irradiated with a handheld UV lamp at 254 nm for 10 min. After irradiation, the cross-linking reaction was stopped by adding sample buffer containing 10% SDS, 50% glycerol, 0.3% EDTA, 6% Tris, and bromophenol blue. The samples were then subjected to SDS/PAGE, followed by immunoblotting with antistreptavidin antibody (Sigma–Aldrich).
Animal Experiments.
C57BL/6 mice and Wistar rats were purchased from Japan SLC, Inc. VNUT−/− mice were generated as previously described (12). These animals were housed individually or in groups of no more than four animals per cage at a temperature of 23 ± 1 °C under a 12-h light/dark cycle, and given ad libitum access to food and water. For the chronic inflammatory pain model, C57BL/6 mice (male, weighing 22–30 g at the time of the test) received an injection of 20 μL of 1 mg/mL CFA (Sigma–Aldrich) or 1% carrageenan solution (Sigma–Aldrich) into the plantar surface of the left hind paw using a 100-μL Hamilton microsyringe with a 27-gauge needle (41). For the chronic neuropathic pain model, C57BL/6 mice (male, weighing 22–30 g at the time of the test) underwent unilateral ligation of approximately one-half of the sciatic nerve high in the thigh (46). In all experiments, animals were randomly divided into each experimental group.
Cell Cultures and Isolation of Neurons, Astrocytes, and Microglia.
Rat fetal hippocampal neurons were isolated and cultured as previously described (47). After isolation, the hippocampus was incubated in Hanks’ solution containing 0.25% trypsin and 0.01% DNaseI for 15 min at 37 °C. The cells were washed twice with DMEM and cultured at 2.0 × 105 cells per 3.5-cm dish in Neurobasal medium (GIBCO) supplemented with 0.5 mM glutamine, 100 units/mL penicillin, 100 μg/mL streptomycin, 0.25 μg/mL Fungizone, and B27 supplement (GIBCO) in 5% CO2/95% air at 37 °C. Hippocampal astrocytes were isolated using a procedure similar to the method used for neurons and cultured in DMEM containing 10% FBS in 5% CO2/95% air at 37 °C.
Rat microglia from cerebral cortices were prepared on embryonic day 17 using a procedure similar to the method used for neurons. Cells were cultured in DMEM containing 10% FBS for 10–17 d. The culture flasks of mixed glial cells were shaken gently, following which the floating microglia were collected (27).
Uptake of Alendronate into Neurons and Astrocytes.
Primary culture cells (2.0 × 105 cells per 3.5-cm dish) were washed twice with uptake solution composed of 128 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 10 mM Hepes-Tris (pH 7.4). After incubation in uptake solution at 37 °C for 20 min, the cells were washed again twice with uptake solution composed of 128 mM NaCl or 256 mM sucrose, 2 mM KCl, 1 mM MgCl2, and 10 mM Hepes-Tris (pH 7.4). Uptake of alendronate was allowed for 10 min at 37 °C in uptake solution containing 10 μM [1-14C] alendronate (0.5 MBq/μmol; Moravek). After incubation, the cells were washed three times in uptake solution and lysed with 1% SDS. The radioactivity in the lysate was measured via liquid scintillation counting (PerkinElmer).
ATP Imaging.
Cultured microglia (1.0 × 104 cells per 96-well plate) were washed twice with DMEM. The cells were incubated in DMEM in the presence or absence of clodronate at 37 °C for 3 h, further incubated following the addition of 300 μM MANT-ATP (Anaspec) at 37 °C for 8 h, and finally incubated at 37 °C for 1 h following the addition of 50 nM LysoTracker (Molecular Probes) after washing with Hanks’ solution. After the cells were washed with Hanks’ solution, they were observed using a BZ-X700 microscope (Keyence).
RT-PCR.
Total RNA was prepared from cultured neurons and astrocytes using an ISOGEN Kit (Nipon Gene Co., Ltd). A PrimeScript RT Reagent Kit (Takara Bio, Inc.) was used to generate cDNA from total RNA, using 1 μg of total RNA as the template. RT-PCR was performed with specific forward and reverse primers at 0.4 μM and 5 units⋅μL−1 of SYBR Premix Ex Taq II (Takara Bio, Inc.). Denaturation was performed for 15 s at 95 °C (35 cycles), whereas annealing/extension was performed for 30 s at 60 °C. The primer sets used for the detection of SLC20A1, SLC20A2, SLC34A1, SLC34A2, and SLC34A3 were as follows: 5′-GTGTAGTGACCCTGAAGCAAGC-3′ and 5′-GCCACACAGCAGAACCAAACA-3′, 5′-AATGGTCGGCTCAGCGGTC-3′ and 5′-GATATGAACCAGGAGGCAACGATC-3′, 5′-GTCAAGGACTCATTGTGGGTGC-3′ and 5′-ACTGGAGATGGCATAGGTGGTT-3′, 5′-TGGTTGCCTCCTCCTTGCTG-3′ and 5′-ATGCCCTTCTGAACTCATTTCTGTC-3′, or 5′-GCTGTGAAGACCGTTATCAATGC-3′ and 5′-ACAATGGCTGCCGTGAAGAC-3′, respectively. The primer set used for detection of glyceraldehyde 3-phosphate dehydrogenase (GAPDH, housekeeping gene) was as follows: 5′-ACTTTGTGAAGCTCATTTCCTGGT-3′ and 5′-TCTCTTGCTCTCAGTATCCTTGCTG-3′.
Release of ATP and Cytokines from THP-1 Cells.
THP-1 cells (JCRB Cell Bank) were grown in RPMI 1640 medium containing 10% FBS, 100 units/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL Fungizone in 5% CO2/95% air at 37 °C (30). Cultured cells (1.0 × 106 cells) were washed twice with Krebs–Ringer bicarbonate buffer consisting of 128 mM NaCl, 1.9 mM KCl, 1.2 mM KH2PO4 1.3 mM MgSO4, 26 mM NaHCO3, 10 mM d-glucose, 10 mM Hepes-NaOH (pH 7.4), 2.4 mM CaCl2, and 0.2% (wt/vol) BSA. After incubation in Krebs–Ringer bicarbonate buffer at 37 °C for 3 h, 10 μg/mL LPS from Escherichia coli 055:B5 (Sigma–Aldrich) was added to the cells to stimulate ATP release. After incubation at 37 °C for 10 min, aliquots were collected and the amount of ATP was measured using an ATP bioluminescent assay kit (Sigma–Aldrich). After incubation at 37 °C for 6 h, aliquots were collected and the amount of cytokine was measured using a cytometric bead array enhanced sensitivity flex set system (Becton Dickinson), in accordance with the manufacturer’s protocol. The beads were analyzed using a MACS Quant analyzer (Miltenyi Biotec).
Uptake of Alendronate into THP-1 Cells.
THP-1 cells (1.5 × 105 cells) were washed twice with uptake solution composed of 128 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 10 mM Hepes-Tris (pH 7.4). After incubation in uptake solution at 37 °C for 20 min, the cells were washed again with uptake solution composed of 128 mM NaCl or 256 mM sucrose, 2 mM KCl, 1 mM MgCl2, and 10 mM Hepes-Tris (pH 7.4). Uptake of alendronate was allowed for 10 min at 37 °C in uptake solution containing 10 μM [1-14C] alendronate (0.5 MBq/μmol; Moravek). After incubation, aliquots of the mixture (180 μL) were filtered through 0.45-μm HA membrane filters (Millipore). Each filter was washed twice with ice-cold uptake solution, and the radioactivity on the filter was measured via liquid scintillation counting (PerkinElmer).
Assessment of Edema and Inflammation.
CFA-induced or carrageenan-induced edema was assessed using electronic calipers to measure the thickness of the hind paw. For histological examination (hematoxylin/eosin staining), the hind paws were decalcified with 19% EDTA, fixed in 4% paraformaldehyde, cut into 10-μm-thick sections, and stained first with Mayer’s hematoxylin and then with 1% eosin Y solution (Wako). Samples were mounted with Mount-Quick (Daido Sangyo) and observed using a Biozero microscope (Keyence). Histological analyses were performed using BZ-X Analyzer software (Keyence).
Measurement of Cytokines in Serum.
Serum was incubated for 1 h at ∼23 °C and centrifuged at 900 × g for 15 min. Levels of cytokines in the serum were measured using a cytometric bead array-enhanced sensitivity flex set system. The beads were analyzed using a MACS Quant analyzer (Miltenyi Biotec).
Apoptosis Assay.
Apoptosis was measured using a propidium iodide (PI) staining solution kit (Becton Dickinson) in accordance with the manufacturer’s protocol. Briefly, blood from the mouse was hemolyzed with lysis buffer and centrifuged at 200 × g for 5 min at ∼23 °C. The blood cells were incubated with 1.25 μg/mL rat anti-mouse CD16/CD32 antibody at 4 °C for 15 min, and then incubated with 5 μg/mL Brilliant Violet 421 (BV421) rat anti-mouse F4/80 antibody or 5 μg/mL BV421 rat IgG2a at 4 °C for 40 min. Blood cells were washed with stain buffer and incubated with 0.5 μg/mL PI staining solution at room temperature for 10 min. The apoptotic cells were analyzed using a MACSQuant analyzer (Miltenyi Biotec). Positive control of apoptosis induction was performed via irradiation of blood cells with a handheld UV lamp at 254 nm for 30 min. In the case of adherent cultured cells, PI-positive cells were counted using a BZ-X700 microscope (Keyence).
Data Analysis.
The sample size was chosen to allow a statistical analysis of the results based on the results of previous animal experiments (41). All numerical values are shown as the mean ± SEM. Statistical significance was determined using a two-tailed paired Student’s t test or one-way ANOVA, followed by Dunnett’s test for multiple comparisons conducted using GraphPad Prism 6 software (GraphPad Software). Significance was defined as *P < 0.05 and **P < 0.01.
Supplementary Material
Acknowledgments
We thank Prof. M. Tominaga, Dr. H. Furue, and Dr. Y. Takayama (National Institute for Physiological Sciences, Okazaki, Japan); Prof. N. Nelson (Tel Aviv University, Tel Aviv, Israel); Dr. S. Sakamoto (Kyushu University, Japan); Dr. N. Juge, Dr. K. Sawada, Mr. S. Itano, and Mr. T. Sekiya (Okayama University, Japan); and the Central Research Laboratory at the Medical School and Advanced Science Research Center, Okayama University, Japan, for their help in this study. This work was supported, in part, by a Grant-in-Aid for Research Activity Start-up (Grant 26893154 to Y.K.); a Grant-in-Aid for Scientific Research (A) (Grant 25253008 to Y.M.); and the Advanced Research and Development Programs for Medical Innovation of the Japan Agency for Medical Research and Development (T.M.), a Grant-in-Aid for Scientific Research (C) (Grant 26460067 to T.M.), the Astellas Foundation for Research on Metabolic Disorders (T.M.), the Salt Science Foundation (Grant 1554 to T.M.), the Smoking Research Foundation (T.M.), and the Takeda Science Foundation (T.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704847114/-/DCSupplemental.
References
- 1.Gold MS, Gebhart GF. Nociceptor sensitization in pain pathogenesis. Nat Med. 2010;16:1248–1257. doi: 10.1038/nm.2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ren K, Dubner R. Interactions between the immune and nervous systems in pain. Nat Med. 2010;16:1267–1276. doi: 10.1038/nm.2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dworkin RH, et al. Advances in neuropathic pain: Diagnosis, mechanisms, and treatment recommendations. Arch Neurol. 2003;60:1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- 4.Tsuda M, Inoue K, Salter MW. Neuropathic pain and spinal microglia: A big problem from molecules in “small” glia. Trends Neurosci. 2005;28:101–107. doi: 10.1016/j.tins.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 5.Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: An update of gastrointestinal, cardiovascular and renal complications. J Pharm Pharm Sci. 2013;16:821–847. doi: 10.18433/j3vw2f. [DOI] [PubMed] [Google Scholar]
- 6.Woolf CJ. μ and δ opioid receptors diverge. Cell. 2009;137:987–988. doi: 10.1016/j.cell.2009.05.033. [DOI] [PubMed] [Google Scholar]
- 7.Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications, and management options. Pain Med. 2009;10:654–662. doi: 10.1111/j.1526-4637.2009.00583.x. [DOI] [PubMed] [Google Scholar]
- 8.Jensen TS, Madsen CS, Finnerup NB. Pharmacology and treatment of neuropathic pains. Curr Opin Neurol. 2009;22:467–474. doi: 10.1097/WCO.0b013e3283311e13. [DOI] [PubMed] [Google Scholar]
- 9.Burnstock G. Purinergic signalling and disorders of the central nervous system. Nat Rev Drug Discov. 2008;7:575–590. doi: 10.1038/nrd2605. [DOI] [PubMed] [Google Scholar]
- 10.Sawada K, et al. Identification of a vesicular nucleotide transporter. Proc Natl Acad Sci USA. 2008;105:5683–5686. doi: 10.1073/pnas.0800141105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyaji T, Sawada K, Omote H, Moriyama Y. Divalent cation transport by vesicular nucleotide transporter. J Biol Chem. 2011;286:42881–42887. doi: 10.1074/jbc.M111.277269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sakamoto S, et al. Impairment of vesicular ATP release affects glucose metabolism and increases insulin sensitivity. Sci Rep. 2014;4:6689. doi: 10.1038/srep06689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masuda T, et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat Commun. 2016;7:12529. doi: 10.1038/ncomms12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishida K, Nomura Y, Kawamori K, Moriyama Y, Nagasawa K. Expression profile of vesicular nucleotide transporter (VNUT, SLC17A9) in subpopulations of rat dorsal root ganglion neurons. Neurosci Lett. 2014;579:75–79. doi: 10.1016/j.neulet.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 15.Jung J, Shin YH, Konishi H, Lee SJ, Kiyama H. Possible ATP release through lysosomal exocytosis from primary sensory neurons. Biochem Biophys Res Commun. 2013;430:488–493. doi: 10.1016/j.bbrc.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 16.Brunner F, Schmid A, Kissling R, Held U, Bachmann LM. Biphosphonates for the therapy of complex regional pain syndrome I–Systematic review. Eur J Pain. 2009;13:17–21. doi: 10.1016/j.ejpain.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 17.Costa L, Major PP. Effect of bisphosphonates on pain and quality of life in patients with bone metastases. Nat Clin Pract Oncol. 2009;6:163–174. doi: 10.1038/ncponc1323. [DOI] [PubMed] [Google Scholar]
- 18.Kim S, et al. Analgesic effects of the non-nitrogen-containing bisphosphonates etidronate and clodronate, independent of anti-resorptive effects on bone. Eur J Pharmacol. 2013;699:14–22. doi: 10.1016/j.ejphar.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 19.Rogers MJ, Crockett JC, Coxon FP, Mönkkönen J. Biochemical and molecular mechanisms of action of bisphosphonates. Bone. 2011;49:34–41. doi: 10.1016/j.bone.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 20.Pazianas M, Abrahamsen B. Safety of bisphosphonates. Bone. 2011;49:103–110. doi: 10.1016/j.bone.2011.01.003. [DOI] [PubMed] [Google Scholar]
- 21.Muratore M, Quarta E, Grimaldi A, Calcagnile F, Quarta L. Clinical utility of clodronate in the prevention and management of osteoporosis in patients intolerant of oral bisphosphonates. Drug Des Devel Ther. 2011;5:445–454. doi: 10.2147/DDDT.S12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leviatan S, Sawada K, Moriyama Y, Nelson N. Combinatorial method for overexpression of membrane proteins in Escherichia coli. J Biol Chem. 2010;285:23548–23556. doi: 10.1074/jbc.M110.125492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juge N, et al. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiasa M, et al. Essential role of vesicular nucleotide transporter in vesicular storage and release of nucleotides in platelets. Physiol Rep. 2014;2:e12034. doi: 10.14814/phy2.12034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsson M, et al. Functional and anatomical identification of a vesicular transporter mediating neuronal ATP release. Cereb Cortex. 2012;22:1203–1214. doi: 10.1093/cercor/bhr203. [DOI] [PubMed] [Google Scholar]
- 26.Oya M, et al. Vesicular nucleotide transporter is involved in ATP storage of secretory lysosomes in astrocytes. Biochem Biophys Res Commun. 2013;438:145–151. doi: 10.1016/j.bbrc.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 27.Imura Y, et al. Microglia release ATP by exocytosis. Glia. 2013;61:1320–1330. doi: 10.1002/glia.22517. [DOI] [PubMed] [Google Scholar]
- 28.Shinozaki Y, et al. Microglia trigger astrocyte-mediated neuroprotection via purinergic gliotransmission. Sci Rep. 2014;4:4329. doi: 10.1038/srep04329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahni M, Guenther HL, Fleisch H, Collin P, Martin TJ. Bisphosphonates act on rat bone resorption through the mediation of osteoblasts. J Clin Invest. 1993;91:2004–2011. doi: 10.1172/JCI116422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forster IC, Hernando N, Biber J, Murer H. Phosphate transporters of the SLC20 and SLC34 families. Mol Aspects Med. 2013;34:386–395. doi: 10.1016/j.mam.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 31.Marchand F, Perretti M, McMahon SB. Role of the immune system in chronic pain. Nat Rev Neurosci. 2005;6:521–532. doi: 10.1038/nrn1700. [DOI] [PubMed] [Google Scholar]
- 32.Sakaki H, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Autocrine regulation of macrophage activation via exocytosis of ATP and activation of P2Y11 receptor. PLoS One. 2013;8:e59778. doi: 10.1371/journal.pone.0059778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 34.Rogers MJ, et al. Bisphosphonates induce apoptosis in mouse macrophage-like cells in vitro by a nitric oxide-independent mechanism. J Bone Miner Res. 1996;11:1482–1491. doi: 10.1002/jbmr.5650111015. [DOI] [PubMed] [Google Scholar]
- 35.Kumamaru H, et al. Liposomal clodronate selectively eliminates microglia from primary astrocyte cultures. J Neuroinflammation. 2012;9:116. doi: 10.1186/1742-2094-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cao Q, et al. SLC17A9 protein functions as a lysosomal ATP transporter and regulates cell viability. J Biol Chem. 2014;289:23189–23199. doi: 10.1074/jbc.M114.567107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miyaji T, et al. AtPHT4;4 is a chloroplast-localized ascorbate transporter in Arabidopsis. Nat Commun. 2015;6:5928. doi: 10.1038/ncomms6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tokunaga A, Tsukimoto M, Harada H, Moriyama Y, Kojima S. Involvement of SLC17A9-dependent vesicular exocytosis in the mechanism of ATP release during T cell activation. J Biol Chem. 2010;285:17406–17416. doi: 10.1074/jbc.M110.112417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 40.Gerstein HC, et al. Action to Control Cardiovascular Risk in Diabetes Study Group Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008;358:2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu ZZ, et al. Resolvins RvE1 and RvD1 attenuate inflammatory pain via central and peripheral actions. Nat Med. 2010;16:592–597. doi: 10.1038/nm.2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dixon WJ. Efficient analysis of experimental observations. Annu Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- 43.Dominguez LJ, Di Bella G, Belvedere M, Barbagallo M. Physiology of the aging bone and mechanisms of action of bisphosphonates. Biogerontology. 2011;12:397–408. doi: 10.1007/s10522-011-9344-5. [DOI] [PubMed] [Google Scholar]
- 44.Kato Y, Omote H, Miyaji T. Inhibitors of ATP release inhibit vesicular nucleotide transporter. Biol Pharm Bull. 2013;36:1688–1691. doi: 10.1248/bpb.b13-00544. [DOI] [PubMed] [Google Scholar]
- 45.Shimada-Shimizu N, Hisamitsu T, Nakamura TY, Wakabayashi S. Evidence that Na+/H+ exchanger 1 is an ATP-binding protein. FEBS J. 2013;280:1430–1442. doi: 10.1111/febs.12138. [DOI] [PubMed] [Google Scholar]
- 46.Seltzer Z, Dubner R, Shir Y. A novel behavioral model of neuropathic pain disorders produced in rats by partial sciatic nerve injury. Pain. 1990;43:205–218. doi: 10.1016/0304-3959(90)91074-S. [DOI] [PubMed] [Google Scholar]
- 47.Banker GA, Cowan WM. Rat hippocampal neurons in dispersed cell culture. Brain Res. 1977;126:397–442. doi: 10.1016/0006-8993(77)90594-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.