Significance
Complement activation occurs when bacteria invade the circulating blood, leading not only to removal of the pathogen but also to inflammation, organ damage, and poor prognosis for septic patients. We used a baboon model of Escherichia coli bacteremia to determine the effects of a C5 inhibitor on bacteriolysis, bacteria clearance, and sepsis progression. We observed that complement-mediated bacteriolysis has a detrimental effect by inducing release of LPS and fulminant inflammation. Inhibition of C5 cleavage and subsequent formation of the lytic terminal complex C5b-9 diminished LPS release, blocked sepsis-induced inflammation, decreased the associated consumptive coagulopathy, and protected organ function. Overall, treatment with C5 inhibitor significantly improved the survival of septic baboons, suggesting a potentially important strategy to treat bacteremic sepsis.
Keywords: complement, coagulation, sepsis, Escherichia coli, organ failure
Abstract
Bacterial sepsis triggers robust activation of the complement system with subsequent generation of anaphylatoxins (C3a, C5a) and the terminal complement complex (TCC) that together contribute to organ failure and death. Here we tested the effect of RA101295, a 2-kDa macrocyclic peptide inhibitor of C5 cleavage, using in vitro whole-blood assays and an in vivo baboon model of Escherichia coli sepsis. RA101295 strongly inhibited E. coli-induced complement activation both in vitro and in vivo by blocking the generation of C5a and the soluble form of TCC, sC5b-9. RA101295 reduced the E. coli-induced “oxidative burst,” as well as leukocyte activation, without affecting host phagocytosis of E. coli. RA101295 treatment reduced plasma LPS content in E. coli-challenged baboons, implying reduced complement-mediated bacteriolysis, whereas treated animals showed slightly improved bacterial clearance during the bacteremic stage compared with controls. Treatment with RA101295 also improved consumptive coagulopathy and preserved endothelial anticoagulant and vascular barrier functions. RA101295 abolished sepsis-induced surges in proinflammatory cytokines and attenuated systemic circulatory and febrile responses, likely reflecting decreased systemic levels of LPS and C5a. Overall, RA101295 treatment was associated with significant organ protection and markedly reduced mortality compared with nontreated controls (four of five animals survived in a 100% lethal model). We therefore conclude that inhibition of C5 cleavage during the bacteremic stage of sepsis could be an important therapeutic approach to prevent sepsis-induced inflammation, consumptive coagulopathy, and subsequent organ failure and death.
Severe sepsis is a life-threatening systemic condition for which, besides intravenous antibiotics, there are no specific targeted therapies. Evidence from patients and animal models suggest that sepsis is a multistage, multifactorial disease in which early fulminant inflammatory responses to invading bacteria leads to hypoperfusion and ischemia-reperfusion injury that develops into multiple organ failure and death (1, 2). In its fulminant form, Escherichia coli infection is characterized by high bacterial load, especially in the very young and very old, and can produce circulatory collapse and death within hours. This variant of sepsis occurs in ∼15% of diagnosed patients. Sepsis is accompanied by robust activation of the coagulation (3, 4) and the complement (5) systems, triggered both by the pathogen itself and by damaged tissue. Whereas local and controlled activation of these pathways can promote host defense against pathogens, excessive or uncontrolled systemic activation is harmful (5, 6). The end products of complement activation are the anaphylatoxin C5a and the C5b-9 terminal complement complex, which inserts into membranes as the membrane attack complex and lyses bacteria or promotes inflammation at a sublytic level. The terminal complement complex formed in the fluid-phase, sC5b-9, is nonlytic and a marker of plasma complement activation. C5b-9 and C5a are major contributors to cell death, immune paralysis, cardiac dysfunction, and multiple organ failure (6, 7). C5a is a chemoattractant signal to neutrophils and other inflammatory cells, and exerts effects on various cell types, contributing to apoptosis, immune paralysis, activation of the coagulation and fibrinolytic systems, and multiorgan dysfunction (6). Besides lysis of bacteria, C5b-9 can damage the cells and tissues of the host. It can induce tissue factor activity on endothelial cells (8), and prothrombinase formation on platelets (9, 10) and endothelial cells (11), thus contributing to development of multiple organ failure (12). Activation of complement, inflammation, and coagulation together promote thrombocytopenia and disseminated intravascular coagulation (DIC), a potential lethal thrombo-hemorrhagic complication of sepsis (13).
Although inhibition of complement activation has been entertained for decades as a potential therapy for septic patients (reviewed in ref. 14), it is not yet clear which component might be the best target. There is also uncertainty regarding the optimal timing of such intervention to achieve maximum protection against complement-induced tissue damage, without compromising beneficial antimicrobial and tissue recovery effects. Previously, we successfully prevented multiple organ failure by blocking complement activation with a C3 inhibitor during the late stage of disease, and markedly improved blood pressure when we targeted early-stage events (15). However, blocking complement activation at the C3 level is considered unsafe because the activation fragments C3b and iC3b are required for bacteria opsonization and subsequent phagocytosis. We have shown that E. coli clearance in vitro in porcine whole blood was substantially reduced using a C3 inhibitor (16). Inhibition of C5 activation has been investigated primarily in rodent models (14) and a single statistically underpowered primate study (17). The studies on rodents assumed that inhibition of C5a, rather than C5, offers a better treatment strategy, as C5b and downstream C5b-9 are thought to have protective roles in lysing bacteria (6). However, other studies in rodents have suggested that both C5a and C5b-9 may not be absolutely required for host defense, because C5-deficient mice (7) or C5 inhibition in rats (18) were still protected against cecal ligation and puncture (CLP)-induced sepsis. Furthermore, C6-deficient rats that were able to form C5a, but not C5b-9, were also protected in the same CLP model (18), suggesting that C5b-9 is the primary contributor to sepsis-induced organ damage and death, and that it can be inhibited without major compromise of immune effector function. Well aware that mice are not men (19), translation from rodents to humans has its limitations. Pig models of complement inhibitory therapy in sepsis, in particular in combination with anti-CD14 (20–22), have yielded promising results. They are more relevant to human pathophysiology than rodent models, but the treatment was limited to only 8 h, as the animals were monitored under full anesthesia. Nonhuman primate studies are more clinically relevant and do not suffer from the same very small experimental treatment windows.
Here we have used a well-established baboon model of E. coli sepsis (2), coupled with in vitro whole-blood approaches, to test the protective effects of an inhibitor of C5 cleavage, RA101295, against sepsis-induced organ damage, and to determine whether C5 inhibition provides a survival benefit. Our data demonstrate that blockade of C5 in animals challenged with lethal doses of E. coli markedly improved survival by reducing the lysis of bacteria and the subsequent massive release of LPS, resulting in substantially reduced coagulopathy and organ damage. RA101295 treatment did not significantly impair host innate immune defense and clearance of the pathogen. C5 inhibition with RA101295 prevented death of four of five baboons exposed to a dose of bacteria that reproducibly leads to 100% lethality in untreated animals.
Results
Effect of RA101295 on Complement Activation in a Human Whole-Blood Assay.
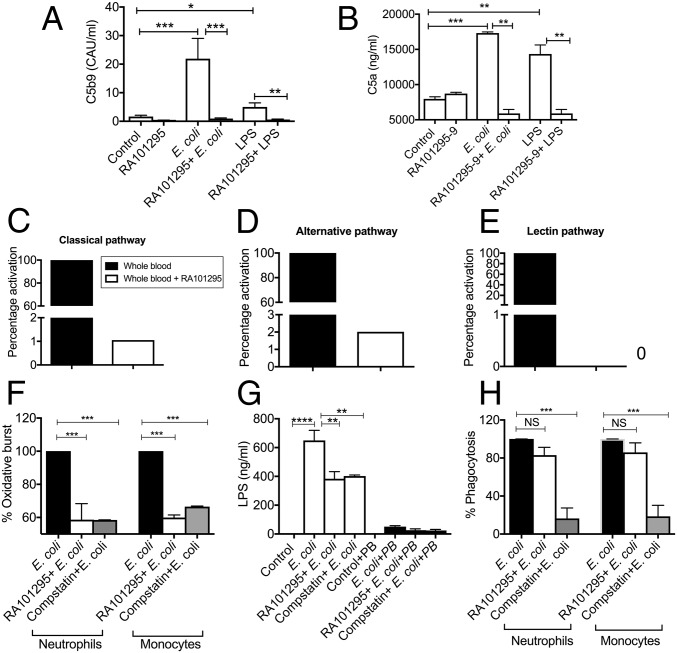
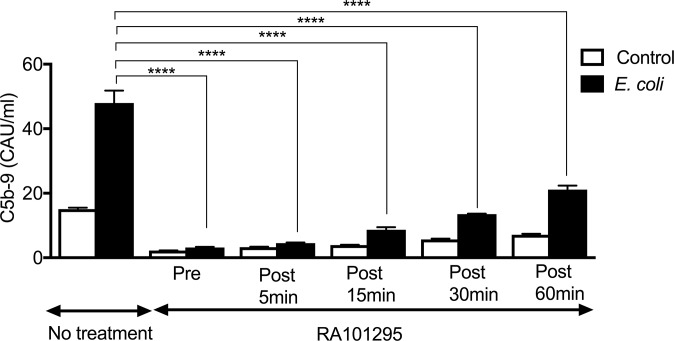
The effect of RA101295 on complement activation was studied in vitro by incubating human whole blood anticoagulated with the thrombin inhibitor lepirudin (23) for 30 min either with PBS (control), E. coli (5 × 107 cfu/mL), or LPS (500 ng/mL) in the presence or absence of 1 µM RA101295 (Fig. 1). RA101295 efficiently blocked sC5b-9 (Fig. 1A) and C5a (Fig. 1B) generation induced by E. coli or LPS in human whole blood. Baboon whole blood was also incubated with E. coli and RA101295, as above (pretreatment), or addition of RA101295 was delayed by 5, 15, 30, or 60 min. The results show that RA101295 inhibited E. coli-induced C5b-9 generation in a time-dependent manner (Fig. S1). Even when the treatment was delayed by 1 h, the inhibitory effect was still robust (approximately 50%), suggesting that posttreatment with RA101295 may be a viable therapeutic option. RA101295 fully inhibited deposition of C5b-9 induced via classic, alternative, or lectin pathways (Fig. 1 C–E) (24).
Fig. 1.
Effect of RA101295 on E. coli-induced complement activation, oxidative burst, LPS release, and phagocytosis in whole blood in vitro assays. Lepirudin-anticoagulated whole blood was incubated with or without 1 µM RA101295 for 20 min, then 5 × 107 E. coli/mL or 500 ng/mL LPS was added for 30 min. RA101295 inhibits E. coli or LPS-induced complement activation as shown by soluble C5b-9 (A) and C5a (B). Data are presented as mean ± SEM (n = 3); multiple comparisons were made by one-way ANOVA with Bonferroni post hoc test. *P < 0.05, **P < 0.01, ***P < 0.001. (C–E) The effect of RA101295 on the classic (C), alternative (D), and lectin (E) pathways of complement activation were examined in Lepirudin-anticoagulated plasma. RA101295 completely attenuates complement activation on all three pathways. Data are presented as mean ± SEM (n = 3); two-tailed Student t test. ***P < 0.001. (F) Whole blood was incubated with 1 µM RA101295 or 0.2 mg/mL compstatin for 20 min, then with E. coli for 30 min. Oxidative burst was determined using DHR123 dye. (G) Whole blood was incubated with RA101295 or compstatin before E. coli incubation. Plasma was collected and, to differentiate the LPS-mediated effect with other TLR4 ligands, in one set polymyxin B (10 μg/mL) was added. LPS content in plasma was quantified using HEK-blue TLR4 reporting cells. (H) Phagocytosis in the presence of compstatin or RA101295 was determined using FITC-labeled E. coli. Data shown in F and G are presented as mean ± SEM (n = 3); multiple comparisons were made by one-way ANOVA followed by Bonferroni post hoc test: *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S1.
Effect of delayed treatment with RA101295 on E. coli induced complement activation in whole-blood in vitro model. Lepirudin-anticoagulated whole baboon blood was incubated with 5 × 107 E. coli/mL. Treatment with 1 µM RA101295 was done 15 min before (pretreatment) or 5, 15, 30, 60 min after E. coli challenge. Samples where E. coli was replaced by saline served as control. The total incubation time was 2 h. Data are presented as mean ± SEM (n = 3). Data are compared between E. coli no treatment and E-coli+RA101295 using two-tailed Student t test: ****P < 0.0001.
To determine the differential effect of complement inhibition at the C3 vs. C5 levels, we incubated human whole blood, preincubated with the C3 inhibitor compstatin or the C5 blocking peptide RA101295, with E. coli. The C3 and C5 inhibitors were equally effective in blocking the E. coli-induced oxidative burst both in neutrophils and monocytes (Fig. 1F). The C3 and C5 inhibitors also decreased LPS release to a similar extent, consistent with reduced complement-mediated bacteriolysis (Fig. 1G). However, inhibition of C3, but not C5, substantially impaired phagocytosis of E. coli by neutrophils and monocytes (Fig. 1H), suggesting that formation of C3b/C3bi opsonins are required for E. coli clearance by blood leukocytes.
Drug Exposure and Efficacy of RA101295 After Subcutaneous Injection in Baboons.
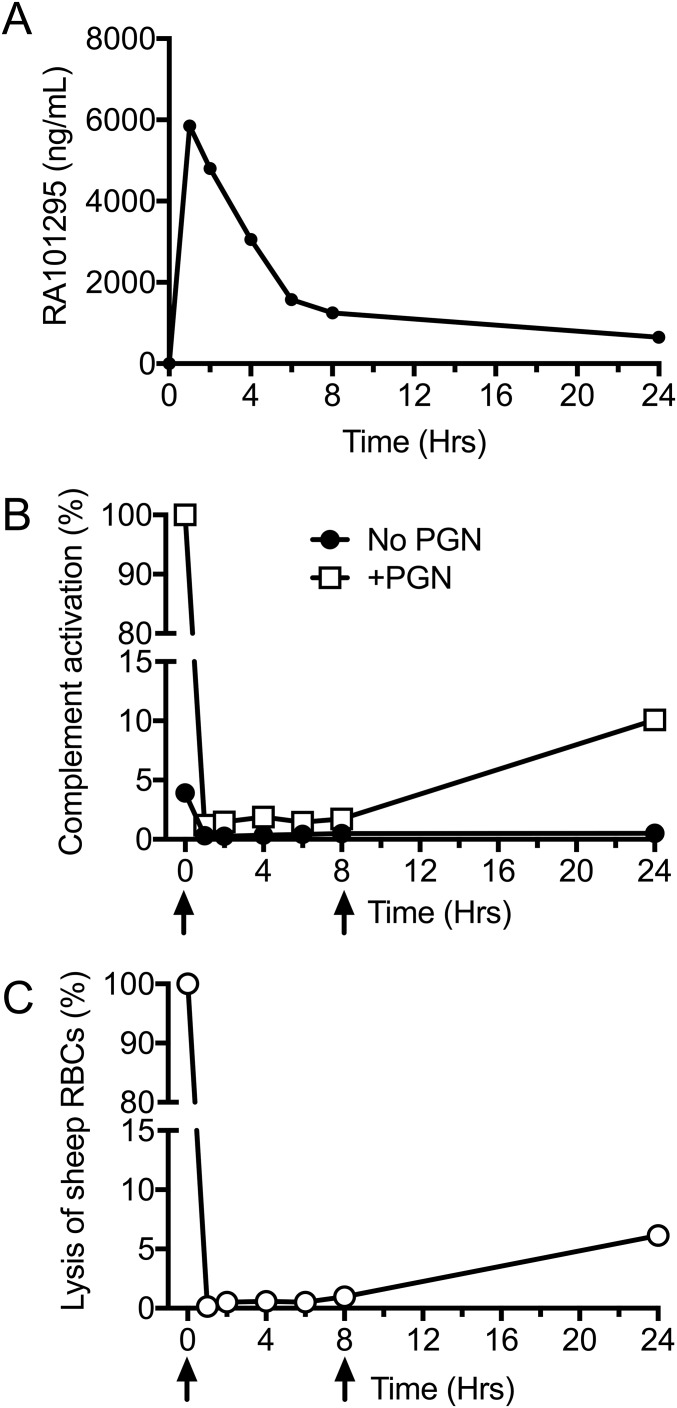
To determine the in vivo efficacy of RA101295, the drug was administered via subcutaneous injection, at 10 mg/kg body weight, and blood samples were collected in lepidurin anticoagulant at 1, 2, 4, 6, 8, and 24 h postinjection. The amount of RA101295 available in plasma indicated that the half-life of the compound is ∼4 h (Fig. S2A). To determine the duration of complement blockade, we determined plasma complement levels by measuring C5b-9 levels after in vitro incubation with bacterial peptidoglycan (PGN), a robust activator of complement (9) (Fig. S2B), or using a hemolytic assay (Fig. S2C). Both assays showed that RA101295 reached maximum inhibitory levels after 1 h postinjection, provided full C5 inhibition during the first 8 h, and ∼90% inhibition at 24 h (Fig. S2 B and C).
Fig. S2.
Time-course of RA101295 exposure and efficacy. (A) A noninfected baboon was given a subcutaneous dose of 10 mg/kg RA101295 at t = 0 h and plasma levels were determined using LC-MS. (B) A 10 mg/kg dose of RA101295 was subcutaneously injected at T0 and at T+8 h (arrows). Blood samples were collected at indicated time-points and PGN-induced complement activation was measured as sC5b-9 generation. (C) Complement levels in the blood samples collected in B were tested using a classic pathway-induced hemolysis assay.
Effects of RA101295 on Complement Activation Products in Vivo.
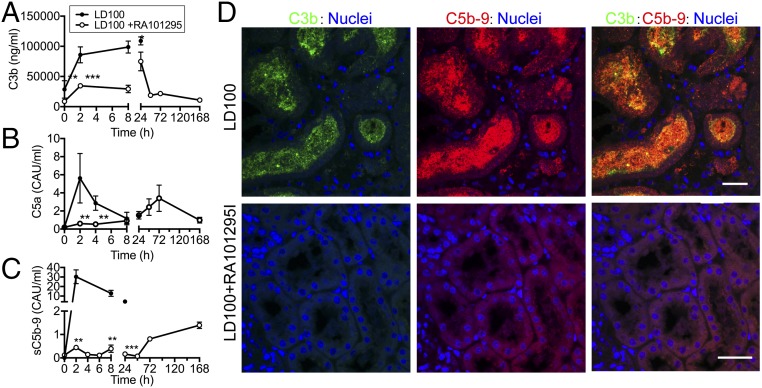
E. coli infusion induced rapid activation of the complement system in vivo: C3b (Fig. 2A), C5a (Fig. 2B), and sC5b-9 (Fig. 2C) reached maximal levels as early as 2 h postchallenge. Immunostaining of kidney sections showed substantial deposition of C3b and C5b-9 in the kidney tubules (Fig. 2D), a typical feature of kidney damage (25). RA101295 administered as four subcutaneous injections at 8-h intervals provided potent inhibition of sepsis-induced complement activation for ∼48 h, as shown by plasma levels of C5a and sC5b-9 (Fig. 2 B and C). The treatment also reduced C3 activation, as shown by C3b levels (Fig. 2A), especially during the early, highly bacteremic stage. Moreover, C3b and C5b-9 immunostaining in kidney was abrogated in the presence of RA101295 (Fig. 2D), suggesting decreased complement activation in the treated group.
Fig. 2.
Complement activation in plasma and kidney biopsies of baboons challenged with E. coli with/without RA101295. Complement activation products were quantified in plasma samples: (A) soluble C3b, (B) C5a, (C) C5b-9. Data are presented as mean ± SEM (n = 5 per group). Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test. *P < 0.05, **P < 0.01, ***P < 0.001. (D) Immunostaining for C3b (green) and C5b-9 (red) in the kidney of septic baboons (LD100; Upper) and septic baboons treated with C5 inhibitor (LD100+RA101295; Lower). To facilitate recognition of microscopical structures, nuclear staining (blue) was included. Right column shows merged green and red channels; colocalization of C3b and C5b9 staining is shown as yellow. (Scale bars, 50 µm.)
In contrast to the RA101295-treated group, nontreated animals became terminally ill and were humanely killed no later than 36 h; therefore, there are no control plasma samples for late time points (48, 72, and 168 h postchallenge) in all biomarkers assays assessed in this study.
Effect of RA101295 on Sepsis-Induced Cytokine Inflammation.
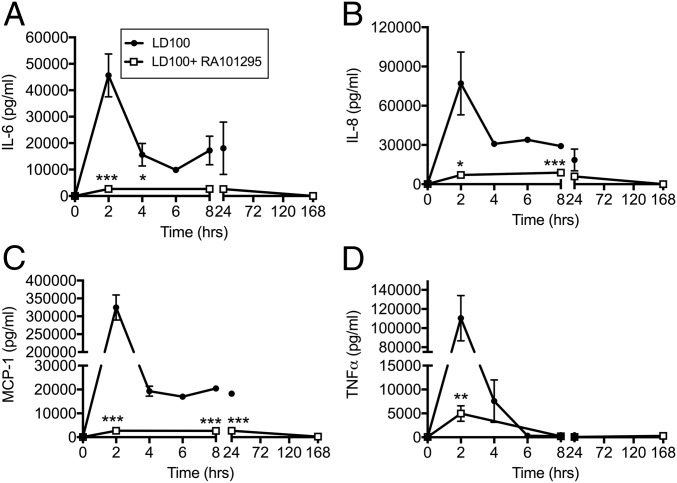
Although E. coli sepsis strongly induced the expression of the proinflammatory cytokines IL-6, IL-8, MCP-1, and TNF, treatment with RA101295 lead to a substantial inhibition of these cytokines (Fig. 3).
Fig. 3.
Effect of RA101295 treatment on the time-course production of cytokines during E. coli sepsis. (A) IL-6, (B) IL-8, (C) MCP-1, and (D) TNF in plasma of septic baboons treated with or without RA101295. Data are presented as mean ± SEM (n = 5 per group). Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Effects of RA101295 on Sepsis-Induced Coagulopathy/DIC.
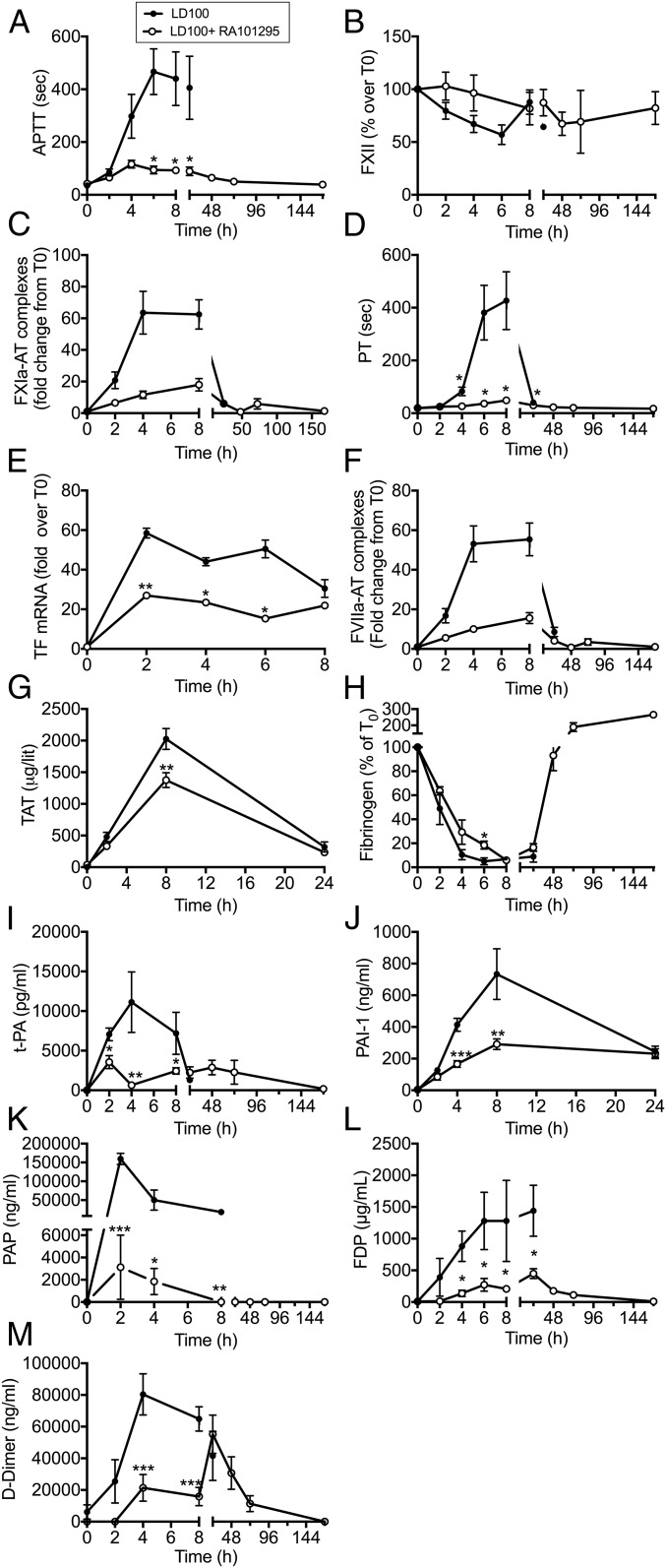
E. coli challenge in baboons is known to lead to pronounced activation of the coagulation and fibrinolytic systems (26). Treatment with RA101295 lead to decreased activated partial thromboplastin time (APTT) (Fig. 4A), reduced consumption of plasma Factor XII (Fig. 4B), and lower levels of Factor XIa-antithrombin complexes (Fig. 4C), together supporting decreased activation of coagulation via the intrinsic pathway. Moreover, RA101295-treated animals showed decreased partial thromboplastin time (PT) (Fig. 4D), lower expression of tissue factor mRNA (Fig. 4E), and lower levels of Factor VIIa–antithrombin complexes (Fig. 4F), all supporting a reduced activation of the tissue factor-dependent extrinsic pathway of coagulation in RA101295-treated vs. untreated animals. Accordingly, thrombin–antithrombin (TAT) complexes were significantly decreased at 8 h in the treated animals compared with untreated controls (Fig. 4G).
Fig. 4.
Effect of RA101295 treatment on hemostatic biomarkers. Time course of: (A) APTT, (B) total Factor XII protein, (C) Factor XIa-antithrombin (FXIa-AT) complexes, (D) PT. (E) tissue factor mRNA, (F) Factor VIIa-antithrombin complexes (FVIIa-AT), (G) TAT complexes, (H) fibrinogen, (I) tissue plasminogen activator (t-PA); (J) PAI-1, (K) PAP, (L) FDP, and (M) d-dimer in septic baboons treated with or without RA101295. Data are presented as mean ± SEM (n = 5 per group); Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01, ***P < 0.001.
Fibrinogen consumption was slightly delayed in the treated group and recovered after 48 h to reach baseline levels at 7 d postchallenge (Fig. 4H). Furthermore, plasma levels of the fibrinolysis biomarkers, tissue plasminogen activator (Fig. 4I), plasminogen activator inhibitor 1 (PAI-1) (Fig. 4J), and plasmin–antiplasmin complex (PAP) (Fig. 4K) were significantly reduced in the treated vs. untreated group. Taken together, these findings support decreased coagulopathic and fibrinolytic responses to E. coli challenge in the RA101295-treated group, suggesting a substantial protection against DIC. Concordantly, fibrinogen/fibrin degradation products (FDP) (Fig. 4L) and d-dimer (Fig. 4M) levels were lower in the treated vs. untreated group, suggesting decreased fibrin formation and degradation.
Effects of RA101295 on Hematologic Parameters.
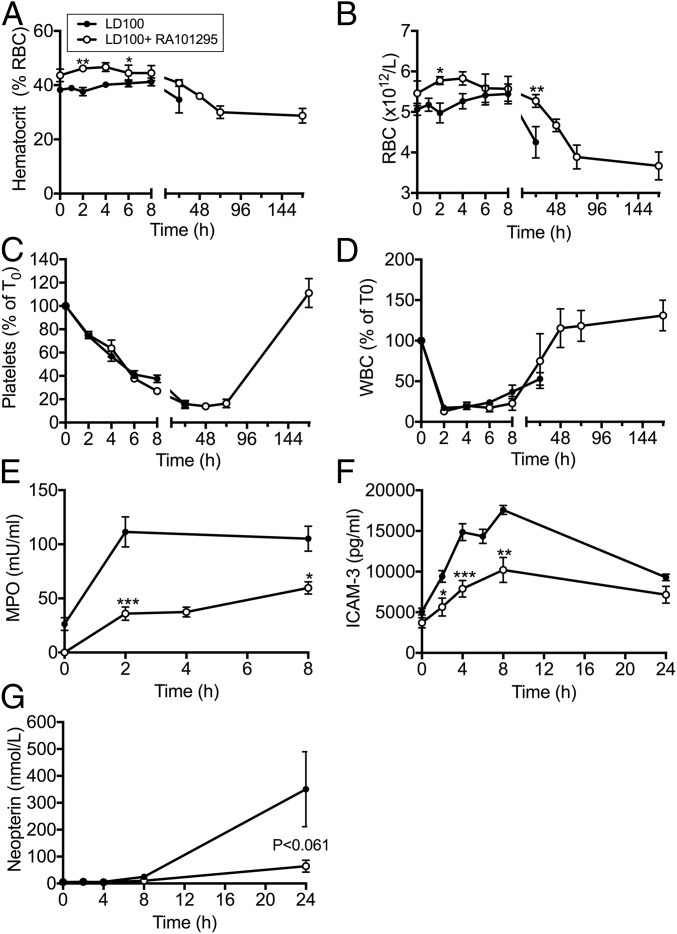
E. coli sepsis lead to a gradual decrease of hematocrit (Fig. 5A) and in red blood cell (RBC) count (Fig. 5B). RA101295 treatment slightly reduced RBC consumption during the first 24 h. Hematocrit and RBC count continued to decrease after cessation of the treatment (T+36 h) and in the absence of visible intravascular hemolysis, and had not recovered by the end of the experiment, at 7 d.
Fig. 5.
Effect of RA101295 treatment on blood cell counts and markers of leukocyte activation. Time course changes of (A) hematocrit, (B) RBC, (C) platelets, (D) leukocytes (WBC), (E) myeloperoxidase (MPO), (F) ICAM-3, and (G) neopterin in the blood of septic baboons without (LD100) or with treatment (LD100+RA101295). Data are presented as mean ± SEM (n = 5 per group). Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01, ***P < 0.001.
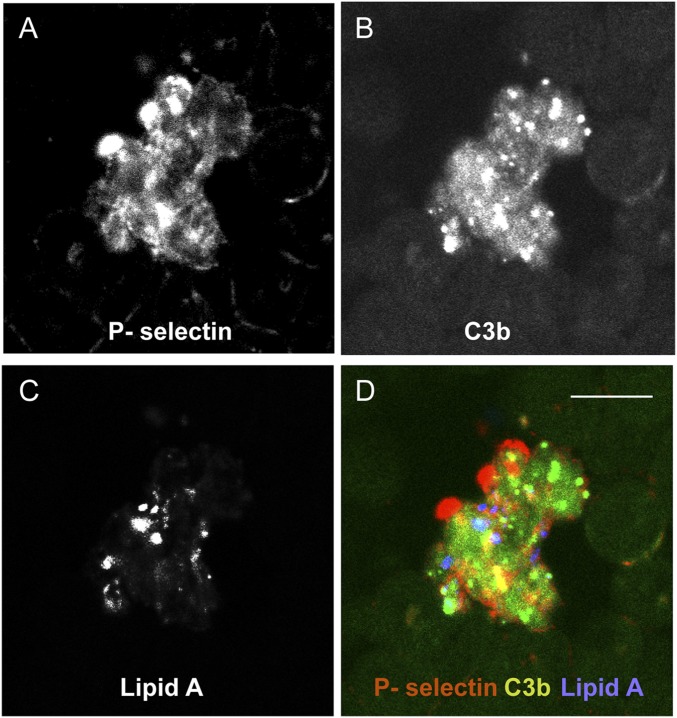
E. coli infusion induced a steady decline of platelets (Fig. 5C). Platelet consumption was similar between the two groups during the first 24 h. Platelet consumption continued in the treated group up to 72 h, but had fully recovered at 7 d. Immunostaining of blood smears collected during the bacteremic state revealed the presence of C3b on circulating RBC and platelet aggregates, some of which were associated with bacteria (Fig. S3), suggesting that platelets and bacteria may be opsonized and both cleared by blood and tissue phagocytes.
Fig. S3.
C3b binding on circulating aggregated platelets of E. coli challenged baboons. Blood collected at T+2 h was smeared on slides and triple-labeled with antibodies against P-selectin as platelet marker (A), lipid A as LPS/bacteria marker (B), and C3b (C). (D) Merged image show colocalization of C3b with aggregated platelets and bacteria. (Scale bar, 10 µm.)
E. coli infusion induced a rapid fall in white blood cell (WBC) count, reaching the lowest values at 2 h, then slowly increasing (Fig. 5D). RA101295 treatment did not protect against sepsis-induced leukopenia during the first 8 h but WBC count fully recovered after 48–72 h. Plasma levels of myeloperoxidase (Fig. 5E) and soluble intercellular adhesion molecule 3 (ICAM3) (Fig. 5F), two proteins released upon neutrophil activation, were decreased in the treated group. Similarly, the increase in neopterin (Fig. 5G), released by activated macrophages, was much reduced in the treated group. Taken together, these data demonstrate that C5 blockade leads to an overall inhibition of leukocyte activation.
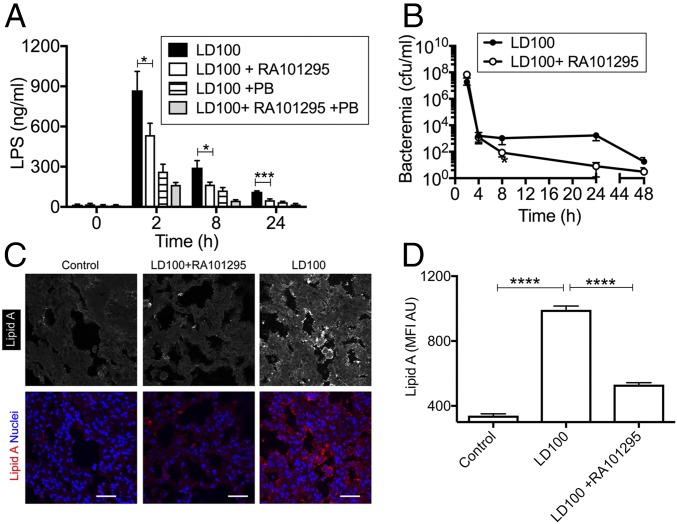
Effect of RA101295 on Bacteriolysis, LPS Release, and Bacteria Clearance.
LPS in plasma was quantified using a reporter cell assay that measure LPS-mediated signaling via the NF-κB pathway. Quenching of LPS signaling with polymyxin B was used to differentiate between the specific effects of LPS vs. other NF-κB agonists. Baboons treated with RA101295 had a significantly lower plasma LPS than nontreated septic controls (Fig. 6A), consistent with decreased bacteriolysis. RA101295 did not impair, but slightly increased the clearance of bacteria from blood (Fig. 6B), particularly after 8 h postchallenge. Staining for lipid A showed lower amounts of LPS associated with lung tissue (Fig. 6 C and D) in animals treated with RA101295 vs. untreated septic animals.
Fig. 6.
Treatment with RA101295 inhibits LPS release from lysed bacteria without impairing bacteria clearance. (A) Time course of LPS in plasma, quantified by using the HEK-Blue TLR4 reporting cells. Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, ***P < 0.001. (B) Time course of bacteremia as determined by counting the colony forming units on agar plate. (C) Immunostaining of Lipid A component of LPS in the lung from healthy (Control), septic treated (LD100+RA101295), and septic not-treated (LD100) baboons. To better see the difference in fluorescence intensity between the three specimens, staining for lipid A is shown in grayscale (Upper). (Lower) Same microscopic fields (lipid A in red) are shown with nuclear counterstaining (blue). (Scale bars, 50 µm.) (D) Quantitation of whole-field MFI of 20 images for each experimental condition illustrated in C. ****P < 0.0001.
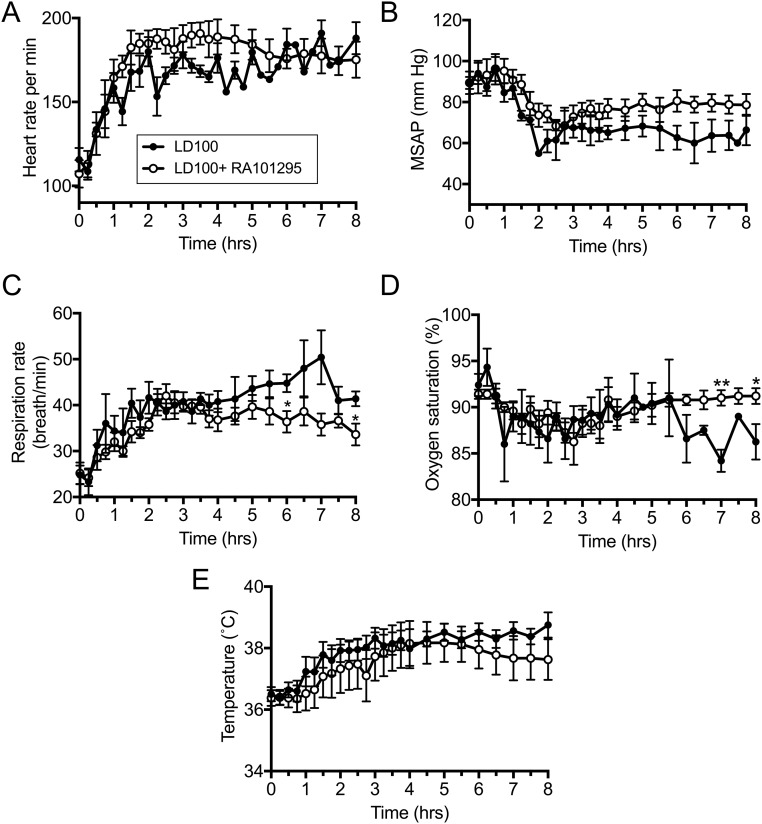
Effects of RA101295 on Vital Signs and Organ Function in E. coli-Challenged Baboons.
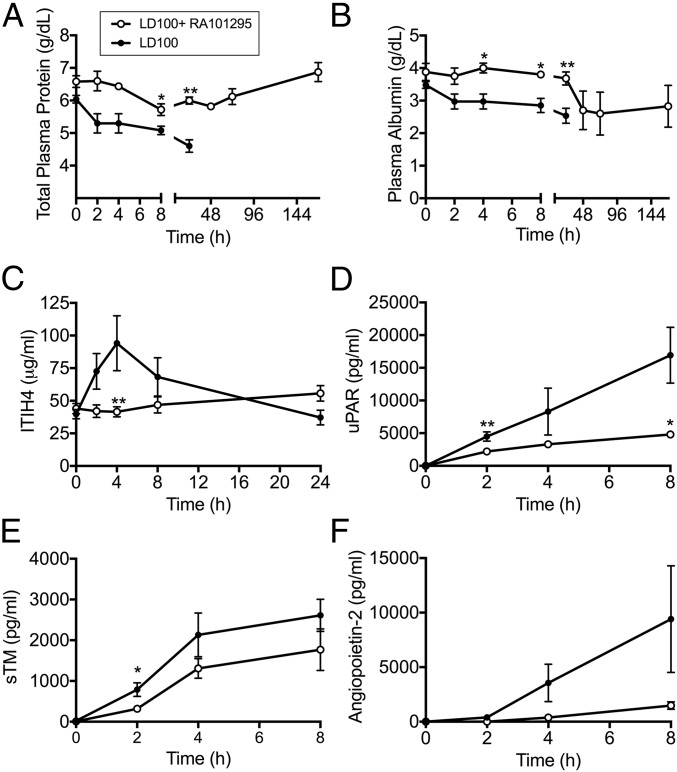
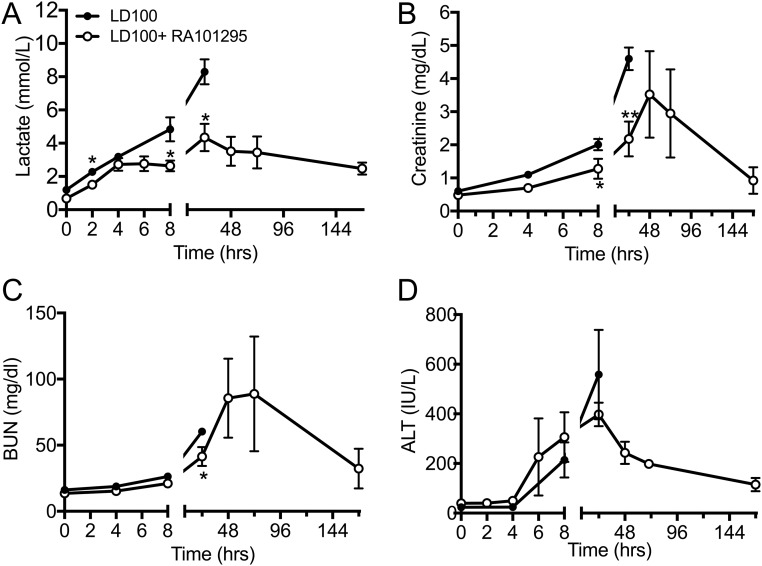
Compared with nontreated septic baboons, RA101295-treated animals had slightly improved vital signs while under anesthesia (T0 to T+8), as reflected by heart and respiration function, including heart and respiration rates, mean systemic arterial pressure, oxymetry, and body temperature (Fig. S4). Treated animals showed maintenance of plasma total proteins and albumin levels, indicating reduced capillary leak, and intact endothelial barrier function (Fig. 7 A and B). Plasma lactate was lower in the RA101295-treated animals, suggesting enhanced vascular perfusion and clearance of anaerobic metabolites (Fig. S5A). Creatinine and blood urea nitrogen (BUN) biomarkers showed improved kidney function during the treatment period (0–48 h), with additional improvement at 72 h and full recovery at 7 d (Fig. S5 B and C). Liver transaminases were increased during the first 24–48 h but partially recovered at 7 d (Fig. S5D). Marker of hepatocyte synthetic activities ITIH4, a positive active-phase protein produced by the liver (27), was substantially increased in the nontreated animals but was undetectable in RA101295-treated animals, reflecting both decreased liver inflammation and systemic inflammation (Fig. 7C).
Fig. S4.
Changes in vital signs during E. coli sepsis in baboons in the presence/absence of RA101295. (A) Heart rate, (B) mean systemic arterial pressure (MSAP), (C) respiration rate, (D) oxygen saturation, (E) body temperature. Data are presented as mean ± SEM. Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01.
Fig. 7.
Effect of RA101295 treatment on plasma markers of capillary leak (A and B), liver (C), and endothelial cell (D and E) dysfunction. (A) Total proteins; (B) albumin; (C) inter–α-trypsin inhibitor 4 (ITH4); (D) soluble uPAR; (E) soluble thrombomodulin (s-TM); (F) Angiopoietin-2. Data are presented as mean ± SEM (n = 5 per group). Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01.
Fig. S5.
Effect of RA101295 treatment on plasma markers of organ function. (A) Lactate, (B) creatinine, (C) BUN, (D) alanine aminotransferase (ALT). Data are presented as mean ± SEM. Same time-point data are compared between LD100 and LD100+RA101295 using two-tailed Student t test: *P < 0.05, **P < 0.01.
Effect of RA101295 on Markers of Endothelial Cell Dysfunction.
Detection in plasma of proteins exposed on the endothelial cell surface or secreted in response to inflammatory stimuli are established markers of endothelial activation and dysfunction. We observed a significant decrease in plasma levels of soluble urokinase receptor (uPAR) (Fig. 7D), thrombomodulin (Fig. 7E), and angiopoietin-2 (Fig. 7F) in plasma of RA101295-treated vs. nontreated baboons, suggesting that C5 inhibition protects the endothelium against sepsis-induced injury.
Effect of RA101295 Against Sepsis-Induced Multiple Organ Failure.
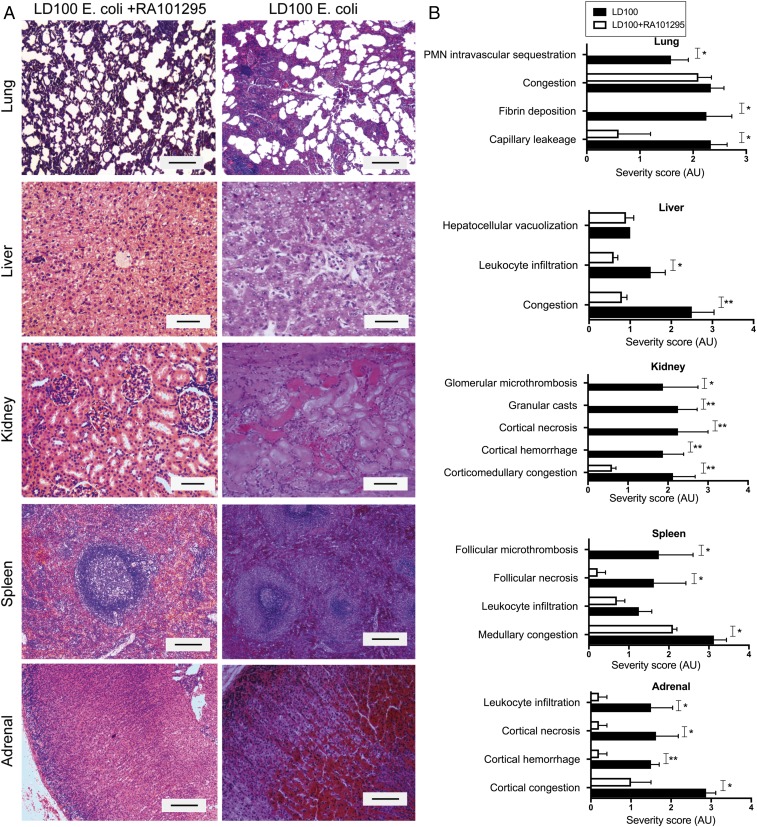
Unbiased semiquantitative histological analysis of organs collected at killing confirmed that RA101295 provided organ protection. Unlike the nontreated group, RA101295-treated animals showed near normal microscopic anatomy of the lung, liver, kidney, spleen, and adrenals (Fig. 8A). The scoring of histological lesions showed no detectible microvascular thrombosis, decreased capillary leakage, no detectible leukocyte infiltration, and no detectible tissue necrosis in the above organs (Fig. 8B).
Fig. 8.
Effect of RA101295 on the histological changes of vital organs. (A) Histological images of organs collected at the time of necropsy stained with H&E. (Scale bars, 100 μm.) (B) Semiquantitative evaluations of the pathological features performed in a blinded fashion and graded on a scale from 0 to 4, with 0 being normal and 4 being severe. Data are presented as mean ± SEM (n = 5). Data are compared between LD100 and LD100+RA101295 using two-tailed Student t test. *P < 0.05, **P < 0.01.
There is a significant time difference between the samples taken from the nontreated animals, killed after 24–36 h (for ethical reasons), and those taken from treated animals, killed at 7 d. Direct comparison of the histopathological features at time-points after 36 h was thus not possible; as well as raising additional ethical questions, to do so would have required substantially increasing the number of animals used.
Effect of RA101295 on Sepsis-Induced Mortality.
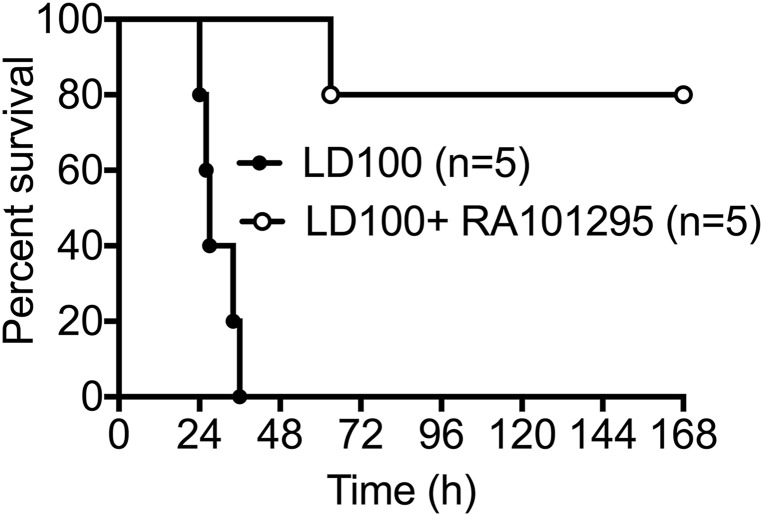
Treatment with RA101295 provided a marked survival benefit, with four of five treated animals reaching the 7 d end-point, compared with 100% mortality in the five control animals challenged with the same dose of bacteria (1010 cfu/kg) (Mantel–Cox test P = 0.0018; the result is significant at P < 0.005) (Fig. 9). Based on our previous experience with this LD100 model, animals that survive 7 d are considered permanent survivors (2).
Fig. 9.
Effect of RA101295 on sepsis-induced mortality. Survival plot of animals challenged with LD100 E. coli and treated with RA101295 (n = 5) vs. control animals challenged with LD100 E. coli without treatment (n = 5). Survival distribution of the two groups was determined using a log-rank Mantel–Cox test; the result is significant at P = 0.0018.
Discussion
We have used a well-established model of Gram-negative bacteria sepsis to test the therapeutic potential of a macrocyclic peptide RA101295 that blocks C5 activation and thus the generation of C5a and C5b-9. Treatment with this C5 inhibitor: (i) blocked sepsis-induced inflammation, (ii) attenuated sepsis-induced coagulopathy, (iii) preserved endothelial function, (iv) decreased leukocyte activation, (v) reduced the lysis of bacteria in the systemic circulation and the subsequent massive release of LPS, and (vi) did not affect the clearance of bacteria by phagocytosis. C5 inhibition was associated with organ protection and significantly reduced mortality in animals challenged with a reproducibly lethal dose of E. coli.
Sepsis in the clinical setting is a complex multifactorial syndrome that presently cannot be fully recapitulated by a single animal model. Our group has developed nonhuman primate models of sepsis, where the intravenous challenge with defined doses of pathogen can produce the various pathophysiologic syndromes observed in clinical practice (2). Here we have used a model of severe sepsis that mimics the progression of shock with DIC in humans to test a therapy targeting the terminal pathway of complement activation. Using this approach, we observed a blunting of the early inflammatory response and DIC-induced by Gram-negative LPS-containing bacteria, as well as reduced ischemia-reperfusion–associated tissue injury occurring during late sepsis that leads to multiple organ failure and death (2).
Baboons were challenged by intravenous infusion of 1010cfu/kg live E. coli, a dose that in our hands reliably leads to 100% mortality. The model mimics the clinical variant of fulminant sepsis with Gram-negative bacteria, which is the most deadly form of the disease (2). This LD100 model results in rapid and severe inflammation, complement activation, and coagulopathy, leading to irreversible shock and death within 24–36 h postchallenge (2). During the bacteremic stage, which lasts ∼6–8 h postbacterial infusion, complement is activated directly by the pathogen through the classic, lectin, and alternative pathways. Because bacteria and their pathogen-associated molecular patterns (e.g., LPS) are major complement activators, this model is well suited for studying C5 inhibition during the rapid progression of the bacteremic stage of sepsis. This model allowed us to determine that early administration of a C5 inhibitor-attenuated disease progression, and prevented multiple organ failure and death without affecting host bacteria clearance.
Our study is not an exact mimic of a clinical model for sepsis treatment in that we pretreated the animals with RA101295 1 h before infusion of bacteria. This pretreatment was carried out to allow absorption of the drug into the systemic circulation following subcutaneous administration. Subcutaneous delivery allowed us to maintain a therapeutic level of drug for 2 d without using intravenous infusion, which is not feasible in nonsedated animals. As the pharmacokinetics properties of the drug become better established, future studies should combine continuous infusion of the drug during anesthesia with subcutaneous delivery during postanesthesia.
Although our results clearly demonstrate that C5 inhibition efficiently prevented organ damage and death, future studies are needed to test if the therapy works in a postinfection model. The in vitro data suggest that RA101295 can provide robust complement inhibition even when the treatment is carried out postinfection. However, delayed treatment cannot be tested in high-load bacteremic challenge studies because the LD100 challenge leads to early, rapid, and irreversible organ damage as a result of shock, DIC, and capillary leak. We also know from other therapies tested in the LD100 model (28, 29) that the chance of success decreases rapidly when the treatment is delayed longer than 1 h postinfection. Experiments testing delayed treatment with C5 inhibitor will likely require the use of a less-severe challenge, such as an LD50 model, and will also require a larger number of animals to achieve statistical significance in determining survival benefit. It has been established that the first 6 (“golden”) hours after the diagnosis of sepsis represent a small but critical therapeutic window in which prevention of inflammation, coagulopathy, and complement-induced damage are possible and during which progression to organ failure can be averted. Thus, we regard C5 inhibitory treatment relevant only to early stages of bacteremic sepsis, or even as a prophylactic in patients at high-risk of development of sepsis: for example, anastomosis leakage after surgery or major burn and trauma patients.
We determined that RA101295 is an effective inhibitor of complement activation. Treatment with RA101295 reduced the level of sC5b-9 by over 99% and C5a by 90% in plasma at the peak of bacteremia (T+2 h). Moreover, C3b levels were significantly decreased in the treated group, suggesting that C5 blockade prevented the C3 amplification loop that lies at the core of all complement pathways (30). LPS is a known activator of the alternative pathway; thus, the reduced C3b levels may also reflect the lower amount of LPS released in the treatment group.
Complement blockade both at the C3 or C5 levels inhibited the oxidative burst, a C5a-dependent process that leads to rapid generation of peroxide, which is highly toxic for both pathogen and host. Similarly, both the C3 and C5 inhibitors decreased the explosive bacteriolysis associated with release of LPS, a process induced by C5b-9. However, only C3 blockade diminished bacterial clearance by phagocytosis.
The in vitro data correlated well with in vivo results, where treatment with RA101295 in septic baboons decreased bacteriolysis and LPS release but did not affect bacterial clearance. Baboons challenged with E. coli and treated with RA101295 showed very low levels of live bacteria in their circulation after 6–8 h, whereas nontreated animals showed significant residual cfu at 24 h postchallenge. The role of C5 in removal of bacteria has not been clearly established. It has been suggested that C5 deficiency increases the susceptibility to meningococcal infections in humans (31), and C5 deficiency in mice is associated with increased bacteremia in a CLP peritonitis model (32). In vitro assays using whole blood from patients with C5 deficiency suggested that C5 is essential for bacterial killing and that C5a is required in bacterial phagocytosis and oxidative burst production by up-regulating CR3 (CD11b/CD18) on leukocytes (33). Other studies report that C5 inhibition with antibodies reduced the bacterial load in the blood and organs and provided protection against CLP-induced sepsis in rats with a genetic deficiency of C6, which can generate C5a and C5b but not C5b-9 (18). Our data showed that C5 inhibition decreased bacterial lysis and reduced the activation of neutrophils and monocytes, but did not interfere with removal of bacteria by phagocytosis. The leukocyte count in the blood of treated and nontreated animals was decreased to a similar extent during the first 24–48 h despite a significant decrease in C5a in the treated group, suggesting similar levels of leukocyte margination and extravasation. Leukocyte counts gradually returned to normal in the animals that survived.
Discrepancies between rodent peritonitis and our bacteremia model could be because of the location of the pathogen. In our model, bacteria are infused directly into the blood where they are rapidly lysed, causing a massive release of proinflammatory LPS into the systemic circulation. C5 inhibition blocks the massive release of LPS and subsequent induction of proinflammatory cytokines and prothrombotic mediators, without affecting bacterial clearance by phagocytosis. In the CLP model of peritonitis, where the pathogen invades and proliferates in a body cavity, lysis of the bacteria can lead to local rather than systemic release of LPS, and the lack of complement-mediated lysis can promote the infection. It is plausible that the small amounts of C5a produced in our treated animals was enough to support phagocytosis-promoting signaling, whereas robust inhibition of the C5b-9 lytic complex decreased the amount of LPS released by over 50% with potent antiinflammatory and organ protective effects in septic baboons.
In a previous study, using the C3 inhibitor compstatin, we successfully prevented multiple organ failure and markedly improved blood pressure through blocking complement activation in a less severely bacteremic (109 cfu/kg E. coli; LD50) model (15). This study was limited to the first 24 h postchallenge and did not monitor survival benefit. We also demonstrated that complement activation products contribute to the fibro-proliferative response, and we used pharmacological inhibition of complement to attenuate fibrosis, by decreasing the expression of several profibrotic pathways, including TGF-β (34). The only E. coli sepsis study in primates targeting C5a, using rabbit polyclonal antibodies, was published three decades ago (17). Although showing attenuation of lung dysfunction, and some systemic responses, this investigation collected data for only 4 h postchallenge (17), therefore missing information on long-term organ function and survival benefit.
RA101295 substantially decreased sepsis-induced coagulopathy. The functions of complement and coagulation pathways in sepsis are closely interlinked (13). C5a and C5b-9 increase the thrombogenicity of the blood by simultaneous induction of procoagulant (35) and antifibrinolytic proteins, and inhibition of natural anticoagulants (5). C5b-9 induces (5) or decrypts tissue factor (36), and induces exposure of phosphatidylserine on platelets to provide a catalytic surface for prothrombinase (9, 10). C5a up-regulates tissue factor and PAI-1 (37) on monocytes, thereby enhancing blood thrombogenicity and decreasing dissolution of fibrin clots (38). We previously demonstrated that C3 inhibition reduced thrombocytopenia and microvascular thrombosis, and preserved endothelial anticoagulant properties (15). Here we show that C5 inhibition also substantially decreases the activation of both intrinsic and extrinsic pathways of coagulation, as well as the fibrinolytic system in septic baboons. These data highlight the close link between the coagulation and complement systems in sepsis (13). Moreover, besides the pronounced antithrombotic effect, our data demonstrate that C5 inhibition offers endothelial cell protection, as revealed by the decrease in markers of endothelial dysfunction (s-TM, s-uPAR, and angiopoietin-2).
RA101295 did not prevent the decrease in platelets and RBCs induced by sepsis, suggesting that complement activation upstream of C5 contributes to platelet and RBCs clearance. Platelets support complement activation, via CP, by binding C1q through a specific receptor (39, 40), and they also bind C3b, a major opsonin involved in the clearance of bacteria and dead cells. Coating of platelets and RBCs with C3b and iC3b can render them susceptible to phagocytosis by macrophages in the spleen or liver. Indeed, we detected C3b on circulating aggregates of platelets and bacteria in baboons challenged with E. coli, suggesting that platelets and bacteria may be opsonized and cleared together, by blood and tissue phagocytes. We and others showed that C5b-9 deposition on platelets leads to platelet activation and aggregation (9, 10). Future work should establish the relative contribution of activated complement to platelet and RBC clearance in sepsis.
Similar to C3 inhibition (15), blocking C5 ameliorated sepsis-induced cardiomyopathy, one of the main predictors of poor outcome in septic patients. C5a is a major cardio-depressant (41). CLP sepsis in rodents induces expression of C5aR on cardiomyocytes and increased generation of C5a causes dysfunction of cardiomyocytes (42). Complement inhibition with compstatin prevented sepsis-induced hypotension when administered at T0 in the baboon LD50 model (15).
We observed that early C5 blockade significantly decreased sepsis-induced inflammation. C5a is a strong inducer and enhancer of inflammation during sepsis (reviewed in ref. 38). RA101295 significantly decreased the release of the proinflammatory LPS, substantially inhibited the production of proinflammatory cytokines, dampened plasma-activation markers of neutrophils, and reduced leukocyte infiltration in vital organs.
Overall, the treatment provided significantly reduced mortality, as four of five baboons reached the 7-d end-point after challenge with a bacterial dose that reproducibly leads to 100% lethality within 24–36 h in untreated animals.
These results demonstrate that the complement system plays a pivotal role in sepsis progression and offers proof-of-concept that C5 inhibition can protect against organ damage and mortality.
Materials and Methods
Study Design.
The objective of this study was to test whether inhibiting complement function by preventing C5 cleavage protects against sepsis-induced inflammation, consumptive coagulopathy, and organ failure, and reduces mortality. We used well-established in vitro and in vivo models of sepsis. The in vitro whole-blood sepsis model was used to investigate the effect of the RA101295 C5 inhibitor on complement activation (C5a and sC5b-9 formation), bacteriolysis, and leukocyte functions, such as oxidative burst and phagocytosis.
The in vivo effects of the C5 inhibitor on sepsis-induced complement activation, inflammation, consumptive coagulopathy, organ functions, and injury were studied in a LD100 baboon model of E. coli sepsis.
The Baboon Model of E. coli Sepsis.
Our study was carried out in compliance with the Animal Welfare Act, the Guide for the Care and Use of Laboratory Animals (43), and the NIH Office of Laboratory Animal Welfare. The study protocol received prior approvals by the Institutional Animal Care and Use Committees of both the Oklahoma Medical Research Foundation and the University of Oklahoma Health Science Center. The animals had free access to water and primate diet; fruits and dietary enrichments were given during the course of the study. Tuberculosis-free, healthy Papio anubis baboons, males and females, 3–4 y old, 6–10 kg body weight, with hemoglobin greater than 10 gm/dL and WBC counts less than 12,000 were included in the study. Two experimental groups were studied: (i) E. coli challenge only (n = 5) and (ii) E. coli plus RA101295 treatment (n = 5). The group size was used based on our previous experience (15) to account for biological variability among animals and the need for reliable statistical analysis and balanced ethical use of animals. Animals were sedated with ketamine (14–20 mg/kg, intramuscularly), then pentobarbital (2 mg/kg, i.v.) was administered periodically to maintain a light level of anesthesia during the experimental procedure. Sepsis was induced by intravenous infusion of 1–2 × 1010 E. coli (LD100 dose, serotype B7-086a:K61; ATCC) for 2 h. Blood samples and physiological parameters were collected before the E. coli infusion (T0), then after 2, 4, 6, and 8 h postbacterial challenge. The time after n hours of E. coli infusion was referred as T+n h. Stock solutions of RA101295, (20 mg/mL) were prepared in PBS, pH = 7. RA101295 (10 mg/kg body weight) was filter-sterilized and subcutaneously injected at four time points: 1 h before the challenge, then at 8, 24, and 36 h after the E. coli challenge, based on the bioavailability data obtained in pilot experiments (Fig. S1). Physiological parameters, including mean systemic arterial pressure, heart rate, respiratory rate, tissue oxygenation, and temperature, and blood samples were collected during first 8 h as above, and then at 24, 48, and 72 h. Animals were continuously monitored during the study period and humanely killed with Euthasol (50 mg/kg, i.v.) when the condition deteriorated or when the animals reached the primary end-point (7-d survival).
The primary end-point was prospectively selected based on extensive previous experience with this model (2). RA101295-treated animals that survived for 7 d after E. coli challenge reached the primary end-point of the study. To reduce the number of animals used, the study was stopped when statistical significance of the primary end-point (P < 0.005 Mantel–Cox test when compared survival between treated vs. nontreated controls) was achieved.
At the end of the experiment, animals were killed and tissue samples were snap-frozen in liquid nitrogen and stored at −80 °C or fixed in 4% paraformaldehyde for microscopy (15).
Biochemical Assays.
Lactate level was measured by Lactate Scout (EKF Diagnostics). Serum metabolites (BUN, creatinine, albumin, total proteins, and alanine aminotransferase) were measured by using a comprehensive diagnostic profile rotor and VetScan VS2 (Abaxis Veterinary Diagnostics) chemistry analyzer.
ELISA.
Plasma cytokine levels were measured using the MILLIPLEX MAP Non-Human Primate Cytokine Magnetic Bead Panel (EMD Millipore) as per the manufacturer’s instructions. The complement activation product sC5b-9 was measured as described previously (44). Briefly the monoclonal antibody aE11 (Enzo Life Sciences), which binds to a neoepitope exposed in C9 after incorporation in the C5b-9 complex, was used as capture antibody. Anti-human C6 biotinylated antibody (Quidel) was used as detection antibody. Pooled human serum activated with heat-aggregated IgG (1 mg/mL) and zymosan (10 mg/mL) containing 1,000 CAU/mL of C5b-9, was used as standard (44). C5a was measured by using mouse anti-human C5a/C5a(desArg), clone 17/5 capture antibody (BioLegend) and biotinylated anti-human C5a/C5a(desArg)/C5, clone G25/2 detection antibody (BioLegend). Zymosan-activated (10 mg/mL) baboon serum containing 1,000 CAU/mL C5a was used as standard (44). C3b/c was measured using mouse monoclonal anti-human C3b/c IgG, clone C3-28, which recognize a neoepitope not exposed in native C3 (Cell Sciences), for capture, and goat anti human C3 (Complement Technologies) as detection antibody. Purified C3 protein (Complement Technologies) was used as standard. TAT complex was measured using the Enzygnost-TAT ELISA kit (Siemens). Inhibitory complexes of Factor XIa and Factor VIIa with antithrombin were detected by sandwich ELISA, in which affinity-purified goat polyclonal antibodies against human Factor XI or Factor VII were used for antigen capture and biotinylated goat polyclonal antibodies against human antithrombin (all from Affinity Biologicals) and Streptavidin- peroxidase for detection. For these two assays, optical density values were reported as a percentage of T0. Kits for soluble thrombomodulin, ICAM-3, PAI-1, and uPAR were from R&D Systems; neopterin from Immune-Biological Laboratories; d-dimer from Diagnostica Stago; and PAP from Technoclone. All assays were performed according to manufacturers’ instructions.
Coagulation Tests.
APTT and PT were measured as described previously (15). FDP were measured by latex agglutination assay (45). Fibrinogen concentration was determined using a clotting based functional assay (45).
Myeloperoxidase Activity Assay.
Myeloperoxidase in plasma samples was determined by using Fluoro MPO myeloperoxidase detection kit (Cell Technology) as per the manufacturer’s instructions.
Cell-Based Assay for LPS.
HEK-Blue hTLR4 cells (InvivoGen) were cultured in DMEM, 4.5 gm/L glucose, 10% (vol/vol) FBS, 50 U/mL penicillin, 50 µg/mL streptomycin, 100 µg/mL normocin, 2 mM l-glutamine and 1× HEK blue selection medium (InvivoGen). Baboon plasma was diluted in PBS and in one sample polymyxin B was added (10 µg/mL). LPS from E. coli, serotype O55:B5 S-form was used as standard. HEK-Blue hTLR4 cells were suspended in HEK-Blue detection medium to measure alkaline phosphatase secreted in the medium. Twenty microliters of LPS or diluted plasma was added to the respective well of a 96-well plate, and then 180-µL cell suspensions were added. The plate was incubated at 37 °C in 5% CO2 incubator for 12–14 h. Absorbance was recorded at 620–650 nm.
Morphologic Analysis.
For immunofluorescence, tissues were fixed in 4% paraformaldehyde, washed in 15% sucrose/PBS, embedded in OCT, snap-frozen in liquid nitrogen, and stored at −80 °C.
Cryosections (∼10 µm) were incubated with primary antibodies: goat anti-LPS (Meridian Life Sciences); mouse monoclonal anti C3b/c, clone C3-28 (Cell Sciences); monoclonal anti C5b-9 neoepitope, clone aE11 (Enzo Life Sciences), overnight at 4 °C, followed by appropriate detection antibodies coupled to FITC or Cy3 and mounted with VectaShield hardset mounting medium (Vector Laboratories) supplemented with ToPro3 (Invitrogen) as nuclear counterstaining (15, 46).
Histopathologic analysis was done on paraffin sections stained with phosphotungstic acid, Prussian blue, or H&E by an experienced veterinary pathologist (S. Kosanke, Oklahoma University Health Sciences Center, Oklahoma City, OK), who was blinded to the experimental conditions (45).
In Vitro Whole-Blood Model Incubated with E. coli.
Blood was collected from consenting healthy volunteers in polypropylene tubes containing lepirudin (50 µg/mL blood), a thrombin inhibitor that does not interfere with the complement activation (23). Whole blood was preincubated with vehicle, RA101295 (1 µM; Ra Pharmaceuticals) or Compstatin (0.2 mg/mL; Tocris Bioscience) for 20 min and then incubated with E. coli (5 × 107 cfu/mL) for 30 min. In a different set of experiments, RA101295 (1 µM) was added at 0, 5, 15, 30, or 60 min after E. coli (5 × 107 cfu/mL) inoculation to lepirudin-anticoagulated baboon blood, to determine the effect of posttreatment on complement activation. For the complement assay, 10 mM EDTA was added to stop further activation and samples were centrifuged at 3,500 × g for 15 min at 4 °C. Plasma was stored at −80 °C until further analysis (23).
Phagocytosis Assay.
Lepirudin-anticoagulated whole blood was preincubated with RA101295 (1 µM), then treated with FITC labeled E. coli for 30 min. RBCs were lysed using 1× RBC lysis buffer (150 mM ammonium chloride, 10 mM sodium bicarbonate, 0.1 mM EDTA). Leukocytes were resuspended in ice-cold 1% PFA-PBS and analyzed on FACS Calibur flow cytometer (BD Biosciences). Monocytes and neutrophils were gated on the basis of side scatter and forward scatter. To differentiate between phagocytized and adherent bacteria, Trypan blue (250 µg/mL) was added and samples were read within 2 min. Mean fluorescence intensity of E. coli challenged sample was considered as 100%.
Oxidative Burst Assay.
Lepirudin anticoagulated whole blood was preincubated with RA101295 (1 µM) and then treated with E. coli (5 × 107 cfu/mL) for 15 min. Free-radical binding dye DHR 123 (10 µM) was added to each tube and incubated for 15 min. RBCs were lysed and samples were measured using a flow cytometer as described above. Mean fluorescence intensity (MFI) of E. coli challenged sample was considered as 100%.
Statistical Analysis.
For statistical analyses, we used Prism (GraphPad Software 6.0b). Values are given as mean ± SEM. Comparisons between two groups were performed using a two-tailed Student t test and multiple comparisons were made by one-way ANOVA followed by Bonferroni post hoc test. Results were considered significant at P < 0.05 (*P < 0.05, **P < 0.01, ***P < 0.001). Comparison of survival data were done using log-rank Mantel–Cox test. P value tests the null hypothesis that the survival curves are identical in the two groups (the treatment did not change survival). Results obtained were significantly different at P = 0.0018.
Data reported in this article were presented in abstract form at two annual meetings of the American Society of Hematology (47, 48). RA101295 can be obtained from Ra Pharmaceuticals through a material transfer agreement.
SI Materials and Methods
To determine the half-life of RA101295 in circulation, one dose (10 mg/kg) was injected subcutaneously. Blood samples were collected on lepirudin anticoagulant before (T0), then at 1, 2, 4, 6, 8, and 24 h postinjection and the amount of RA101295 in plasma was determined by LC-MS.
To determine the duration of RA101295 inhibitory activity, two doses of RA101295 (10 mg/kg) were injected subcutaneously at T+5 min and T+8 h. Blood samples were collected on lepirudin anticoagulant before (T0), then at 1, 2, 4, 6, 8, and 24 h postinjection. The plasma was incubated in vitro with 25 μg/mL PGN, a potent complement activator (9), for 30 min at 37 °C. The formation of sC5b-9 was quantified using ELISA as described in the main text. sC5b-9 formation in sample collected at T0 and treated with PGN was considered as 100%. On the basis of the sC5b-9 formation, the treatment time of RA101295 was decided in LD100 Escherichia coli sepsis model. The time course of bioavailability of C5 inhibitor was also examined by using the classic pathway-mediated hemolysis assay. Plasma was diluted (1:10) with GVB++ buffer (Complement Technology). Next, 50 μL of diluted plasma was mixed with 50 μL of antibody-sensitized sheep erythrocytes (Complement Technology) and incubated for 30 min at 37 °C. Reactions were stopped by adding 150 μL ice-cold GVB++/5 mM EDTA. The plate was centrifuged and supernatants were transferred to a new plate. The plate was read at 412 nm. The value of the T0 plasma sample was considered 100% and percent hemolysis was calculated (49).
Acknowledgments
We thank Fletcher Taylor and Gary Kinasewitz for expert advice; Gary White for veterinary oversight; and Guy Riddihough (Life Science Editors) for editorial assistance. This work was supported by NIH Grants GM097747, GM116184, GM121601, U19AI062629, and P30GM114731; The Research Council of Norway (BIOTEK2021-244390/O30); and Ra Pharmaceuticals. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Ra Pharmaceuticals.
Footnotes
Conflict of interest statement: F.L. has received research support from Ra Pharmaceuticals. S.J.D. is employed by Ra Pharmaceuticals. T.E.M. is a consultant for Ra Pharmaceuticals.
This work was presented in part at the 56th Annual Meeting of the American Society of Hematology, San Francisco, December 6–9, 2014, and at the 57th Annual Meeting of the American Society of Hematology, Orlando, FL, December 5–8, 2015.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1706818114/-/DCSupplemental.
References
- 1.Angus DC, van der Poll T. Severe sepsis and septic shock. N Engl J Med. 2013;369:840–851. doi: 10.1056/NEJMra1208623. [DOI] [PubMed] [Google Scholar]
- 2.Taylor FB, Jr, Kinasewitz GT, Lupu F. Pathophysiology, staging and therapy of severe sepsis in baboon models. J Cell Mol Med. 2012;16:672–682. doi: 10.1111/j.1582-4934.2011.01454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levi M, Schultz M, van der Poll T. Sepsis and thrombosis. Semin Thromb Hemost. 2013;39:559–566. doi: 10.1055/s-0033-1343894. [DOI] [PubMed] [Google Scholar]
- 4.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8:776–787. doi: 10.1038/nri2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Markiewski MM, DeAngelis RA, Lambris JD. Complexity of complement activation in sepsis. J Cell Mol Med. 2008;12:2245–2254. doi: 10.1111/j.1582-4934.2008.00504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ward PA. The dark side of C5a in sepsis. Nat Rev Immunol. 2004;4:133–142. doi: 10.1038/nri1269. [DOI] [PubMed] [Google Scholar]
- 7.Olson LM, Moss GS, Baukus O, Das Gupta TK. The role of C5 in septic lung injury. Ann Surg. 1985;202:771–776. doi: 10.1097/00000658-198512000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tedesco F, et al. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–1627. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun D, et al. Bacillus anthracis peptidoglycan activates human platelets through FcγRII and complement. Blood. 2013;122:571–579. doi: 10.1182/blood-2013-02-486613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 1988;263:18205–18212. [PubMed] [Google Scholar]
- 11.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement—Their role in inflammation. Semin Immunopathol. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hesselvik JF, Blombäck M, Brodin B, Maller R. Coagulation, fibrinolysis, and kallikrein systems in sepsis: Relation to outcome. Crit Care Med. 1989;17:724–733. doi: 10.1097/00003246-198908000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Lupu F, Keshari RS, Lambris JD, Coggeshall KM. Crosstalk between the coagulation and complement systems in sepsis. Thromb Res. 2014;133:S28–S31. doi: 10.1016/j.thromres.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ward PA, Guo RF, Riedemann NC. Manipulation of the complement system for benefit in sepsis. Crit Care Res Pract. 2012;2012:427607. doi: 10.1155/2012/427607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silasi-Mansat R, et al. Complement inhibition decreases the procoagulant response and confers organ protection in a baboon model of Escherichia coli sepsis. Blood. 2010;116:1002–1010. doi: 10.1182/blood-2010-02-269746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorgersen EB, et al. Inhibition of complement and CD14 attenuates the Escherichia coli-induced inflammatory response in porcine whole blood. Infect Immun. 2009;77:725–732. doi: 10.1128/IAI.01305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stevens JH, et al. Effects of anti-C5a antibodies on the adult respiratory distress syndrome in septic primates. J Clin Invest. 1986;77:1812–1816. doi: 10.1172/JCI112506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buras JA, et al. Inhibition of C5 or absence of C6 protects from sepsis mortality. Immunobiology. 2004;209:629–635. doi: 10.1016/j.imbio.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 19.Warren HS, et al. Mice are not men. Proc Natl Acad Sci USA. 2015;112:E345. doi: 10.1073/pnas.1414857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barratt-Due A, et al. Combined inhibition of complement C5 and CD14 markedly attenuates inflammation, thrombogenicity, and hemodynamic changes in porcine sepsis. J Immunol. 2013;191:819–827. doi: 10.4049/jimmunol.1201909. [DOI] [PubMed] [Google Scholar]
- 21.Egge KH, et al. Organ inflammation in porcine Escherichia coli sepsis is markedly attenuated by combined inhibition of C5 and CD14. Immunobiology. 2015;220:999–1005. doi: 10.1016/j.imbio.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 22.Skjeflo EW, et al. Combined inhibition of complement and CD14 improved outcome in porcine polymicrobial sepsis. Crit Care. 2015;19:415. doi: 10.1186/s13054-015-1129-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollnes TE, et al. Essential role of the C5a receptor in E coli-induced oxidative burst and phagocytosis revealed by a novel lepirudin-based human whole blood model of inflammation. Blood. 2002;100:1869–1877. [PubMed] [Google Scholar]
- 24.Seelen MA, et al. Functional analysis of the classical, alternative, and MBL pathways of the complement system: Standardization and validation of a simple ELISA. J Immunol Methods. 2005;296:187–198. doi: 10.1016/j.jim.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 25.Khan TN, Sinniah R. Role of complement in renal tubular damage. Histopathology. 1995;26:351–356. doi: 10.1111/j.1365-2559.1995.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 26.de Boer JP, et al. Activation patterns of coagulation and fibrinolysis in baboons following infusion with lethal or sublethal dose of Escherichia coli. Circ Shock. 1993;39:59–67. [PubMed] [Google Scholar]
- 27.Piñeiro M, et al. ITIH4 serum concentration increases during acute-phase processes in human patients and is up-regulated by interleukin-6 in hepatocarcinoma HepG2 cells. Biochem Biophys Res Commun. 1999;263:224–229. doi: 10.1006/bbrc.1999.1349. [DOI] [PubMed] [Google Scholar]
- 28.Creasey AA, et al. Tissue factor pathway inhibitor reduces mortality from Escherichia coli septic shock. J Clin Invest. 1993;91:2850–2860. doi: 10.1172/JCI116529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Taylor FB, Jr, et al. Protein C prevents the coagulopathic and lethal effects of Escherichia coli infusion in the baboon. J Clin Invest. 1987;79:918–925. doi: 10.1172/JCI112902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lachmann PJ. The amplification loop of the complement pathways. Adv Immunol. 2009;104:115–149. doi: 10.1016/S0065-2776(08)04004-2. [DOI] [PubMed] [Google Scholar]
- 31.Chaudhuri AK, Banatvala N, Caugant DA, Fallon RJ, Whaley K. Phenotypically similar clones of serogroup B Neisseria meningitidis causing recurrent meningitis in a patient with total C5 deficiency. J Infect. 1994;29:236–238. doi: 10.1016/s0163-4453(94)91010-3. [DOI] [PubMed] [Google Scholar]
- 32.Flierl MA, et al. Functions of the complement components C3 and C5 during sepsis. FASEB J. 2008;22:3483–3490. doi: 10.1096/fj.08-110595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lappegård KT, et al. Human genetic deficiencies reveal the roles of complement in the inflammatory network: Lessons from nature. Proc Natl Acad Sci USA. 2009;106:15861–15866. doi: 10.1073/pnas.0903613106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silasi-Mansat R, et al. Complement inhibition decreases early fibrogenic events in the lung of septic baboons. J Cell Mol Med. 2015;19:2549–2563. doi: 10.1111/jcmm.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ritis K, et al. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 36.Langer F, et al. Rapid activation of monocyte tissue factor by antithymocyte globulin is dependent on complement and protein disulfide isomerase. Blood. 2013;121:2324–2335. doi: 10.1182/blood-2012-10-460493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kastl SP, et al. The complement component C5a induces the expression of plasminogen activator inhibitor-1 in human macrophages via NF-kappaB activation. J Thromb Haemost. 2006;4:1790–1797. doi: 10.1111/j.1538-7836.2006.02046.x. [DOI] [PubMed] [Google Scholar]
- 38.Guo RF, Ward PA. Role of C5a in inflammatory responses. Annu Rev Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 39.Peerschke EI, Yin W, Ghebrehiwet B. Complement activation on platelets: Implications for vascular inflammation and thrombosis. Mol Immunol. 2010;47:2170–2175. doi: 10.1016/j.molimm.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peerschke EI, Ghebrehiwet B. Human blood platelet gC1qR/p33. Immunol Rev. 2001;180:56–64. doi: 10.1034/j.1600-065x.2001.1800105.x. [DOI] [PubMed] [Google Scholar]
- 41.Atefi G, et al. Complement dependency of cardiomyocyte release of mediators during sepsis. FASEB J. 2011;25:2500–2508. doi: 10.1096/fj.11-183236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Niederbichler AD, et al. An essential role for complement C5a in the pathogenesis of septic cardiac dysfunction. J Exp Med. 2006;203:53–61. doi: 10.1084/jem.20051207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 44.Bergseth G, et al. An international serum standard for application in assays to detect human complement activation products. Mol Immunol. 2013;56:232–239. doi: 10.1016/j.molimm.2013.05.221. [DOI] [PubMed] [Google Scholar]
- 45.Taylor FB, Jr, et al. The endothelial cell protein C receptor aids in host defense against Escherichia coli sepsis. Blood. 2000;95:1680–1686. [PubMed] [Google Scholar]
- 46.Lupu C, et al. Tissue factor-dependent coagulation is preferentially up-regulated within arterial branching areas in a baboon model of Escherichia coli sepsis. Am J Pathol. 2005;167:1161–1172. doi: 10.1016/S0002-9440(10)61204-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Keshari R, et al. A novel C5 complement inhibitor protects against sepsis-induced activation of complement, coagulation and inflammation and provides survival benefit in E. coli sepsis. Blood. 2014;124:112. [Google Scholar]
- 48.Keshari RS, et al. Complement C5 inhibition blocks the cytokine storm and consumptive coagulopathy by decreasing lipopolysaccharide (LPS) release in E. coli sepsis. Blood. 2015;126:765. [Google Scholar]
- 49.Harder MJ, et al. Incomplete inhibition by eculizumab: Mechanistic evidence for residual C5 activity during strong complement activation. Blood. 2017;129:970–980. doi: 10.1182/blood-2016-08-732800. [DOI] [PMC free article] [PubMed] [Google Scholar]