Abstract
The study aimed to investigate whether social engagement predicted longitudinally objective and subjective physical health. Measures of social engagement, subjective and objective health were taken at three points in time, 4 years apart (T1, T2, T3). Three questions were examined: does social engagement at T1 predict objective/subjective health at T2, does social engagement at T2 predict objective/subjective health at T3, and does social engagement at T1 predict objective/subjective health at T3? Participants were 359 adults aged 65 and over. A fully cross-lagged structural equation model was examined. Social engagement at T1 was found to significantly predict subjective health at T2. However, social engagement at T1 did not significantly predict subjective health at T3, nor was subjective health at T3 predicted by social engagement at T2. Social engagement never significantly predicted objective health. Unexpectedly, objective health at T2 predicted social engagement at T3. Finally, post-hoc analyses suggest that age has a greater influence on social engagement at T2 than at T1. Social engagement is a useful predictor of subjective physical health. However, objective health was not predicted by social engagement—indeed, the converse was the case. It is suggested that the relationship between social engagement and subjective health is mediated by psychosocial factors which may not be present in the social engagement–objective health relationship. In conclusion, the results reflect the complex interplay of objective and subjective health and social engagement as people age.
Keywords: Social engagement, Subjective health, Objective health, Longitudinal
Introduction
Researchers have been interested for some time in examining factors which may predict health status amongst older people. One of the factors which has received attention is social activity and participation (Bowling and Browne 1991; Steinbach 1992; Mendes de Leon et al. 2003). There is evidence that social activity influences physical health. However, different studies have looked at different health measures, for example, objective and subjective health, disability and disease status, and at different measures of social activity, including social networks, social support, and social engagement (Ferrucci et al. 1996; Unger et al. 1999; Mendes de Leon et al. 2003; Zunzunegui et al. 2004).
A number of studies have examined the impact of social support and social networks on physical health and mortality within the context of stress, examining two hypotheses—the main effect hypothesis and the buffering hypothesis (for a review, see Uchino et al. 1996). The main effect hypothesis suggests that social support plays a health-promoting role, while the buffering hypothesis argues that support acts as a protective factor. However, the nature, quantity and quality of support are also important, according to Estes and Rundall (1992). Unger et al. (1999) found that those with a greater number of social ties experienced reduced levels of functional decline. Grundy and Sloggett (2003) reported that social support contributed significantly to self-perceived health. On the other hand, social support did not predict the number of prescribed medications in women, nor long-standing illnesses in men (both measures were regarded as an objective measure of health). Zunzunegui et al. (2004) found that social networks and social support (as well as social engagement discussed below) all contributed to subjective health, but each in a different way.
Recently, attention has also focussed on the role which social engagement plays in maintaining good health. Social engagement has been defined in a variety of ways. For example, Zunzunegui et al. (2004, p. 2070) define it as community involvement such as belonging to “neighbourhood groups, religious organizations, or non-governmental organizations”. On the other hand, Morgan et al. (1987) define it as how much a person participates in the social milieu. In the current study, it is this latter, broader definition which is used. Mendes de Leon et al. (2003) found that social engagement (in which they included productive as well as social activities) was associated cross-sectionally with disability. However, the effect was much weaker when examined longitudinally. These authors argued that the protective effect of social engagement diminishes slowly over time. Zunzunegui et al. (2004) reported that social engagement was associated with good subjective health. In a theoretical paper of the relationship between differing social influences and health, Berkman et al. (2000) proposed a cascading conceptual model which moves from macro-social processes to psychobiological ones. Their model suggests that social engagement, as defined as community involvement, lies between the macro-social factors and the psychosocial mechanisms. In this model, the well-recognised path showing that social networks contribute to physical health is not mediated solely by the behavioural mechanism of social support, but also by other psychosocial mechanisms, including social engagement.
As has been discussed, a variety of health outcome measures have been used in investigating the role of social engagement. In the current study, two health outcome measures were available for examination, one a measure of objective health, the other a measure of subjective health. The objective health measure was a health index taking into account symptoms, disability and medication use. Other studies have used outcome measures which examine aspects contained within this measure (Ferrucci et al. 1996; Mendes de Leon et al. 2003). Subjective health is widely recognised to be an important global measure of health status. For example, it has been shown to be an effective predictor of mortality (Deeg and Bath 2003), and is correlated with other health outcome measures (Pinquart 2001). Social engagement has been found to predict subjective as well as objective health (Zunzunegui et al. 2004). However, thus far studies have not examined the effects of social engagement on objective and subjective health within one and the same dataset or with the same analyses. Thus, it cannot be assumed that objective and subjective physical health respond to social participation in the same way or through the same mechanisms. However, in the present study it was possible to examine the effects of social engagement on both subjective and objective health.
The Nottingham Longitudinal Study of Activity and Ageing (NLSAA) has provided the dataset for this study. The current work aims to provide further evidence of the relationship between subjective and objective health and social engagement in survivors from the NLSAA. It assesses the impact of social engagement levels of physical health after 4 and 8 years, controlling for baseline measures of health. Consequently, the study examines three hypotheses:
Social engagement at T1 predicts objective/subjective health at T2;
Social engagement at T2 predicts objective/subjective health at T3;
Social engagement at T1 predicts objective/subjective health at T3.
Method
Data were derived from the NLSAA, full details of which are presented elsewhere (Morgan 1998). Briefly, the NLSAA is an 8-year survey of activity, health and well-being within a representative sample of community-dwelling people originally aged 65 and over. The baseline survey for the NLSAA was conducted between May and September 1985 (T1), during which time 1,042 people, randomly sampled from general practitioners’ lists, were interviewed in their own homes (a response rate of 80%). The sample was demographically representative of the British elderly population.
Follow-up surveys were conducted at 4-year intervals in 1989 (T2) and 1993 (T3), with re-interview rates among survivors of 88% (n=690) and 78% (n=410) respectively. At T1 there were 406 men and 636 women. At T2 there were 259 men and 431 women. Finally, at T3 there were 139 men and 267 women.
Questionnaire assessment
At each survey wave, general health was assessed using a 14-item health index scored from zero (no health problems) to 14 (multiple health problems), covering the presence or absence of heart, stomach, eyesight, sleep or foot problems; giddiness, headaches, urinary incontinence, arthritis and falls; long-term disabilities and drug and walking aid use, and contact with (primary and secondary care) medical services (Morgan 1998).
Subjective health was assessed by the question “How would you rate your present health?”. There were five response categories: poor (scored 1), fair, average, good, and excellent (scored 5).
Levels of social activity were assessed using the brief assessment of social engagement (BASE) scale developed specifically for this study (Morgan et al. 1987). This additive scale contained 20 dichotomously rated items (yes=1; no=0) covering both actual (e.g. voting, attending religious services, taking holidays, library attendance) and virtual (e.g. writing letters, reading newspapers/magazines, TV and radio access) engagement.
Participants
To conduct the analyses outlined below, only those respondents with complete data on the variables of interest were included. There were 119 men and 240 women. The mean age was 72.4 (range 65–92). It is important to note that those who were included in these analyses were younger, more socially engaged, and had better objective and subjective health at baseline than those who were excluded. Reasons for exclusion included mortality at T2 or T3, substantial missing data (for example, where proxy interviews had been conducted), and people refusing interviews or not having been traced. As a consequence, the analyses presented here concern survivors, or what can be termed an elite sample. The implications of this are considered in the discussion.
Analyses
Structural equation modelling techniques were employed using EQS (Multivariate Software, Inc.). The analyses presented are path analyses. In order to examine the impact of social engagement on health, a stability model and a cross-lagged model were compared. Stability models examine the relationship between a variable, for example, health, at T1, T2 and T3, without examining the influence of the variable of interest, for example, social engagement (see Fig. 1a for the objective health example). For both objective and subjective health, stable models are expected. The cross-lagged models examine the influence, over time (for example, between T1 and T2), of the predictor variable (social engagement) on the predicted variable (health). For completeness, the reverse relationship was also examined, that is, the influence of health on social engagement (see Fig. 1b for the objective health example; see de Jonge et al. 2001 for a clear description of cross-lagged models). Note that because the cross-lagged models include all possible paths, they test all three of these hypotheses.
Fig. 1a–c.
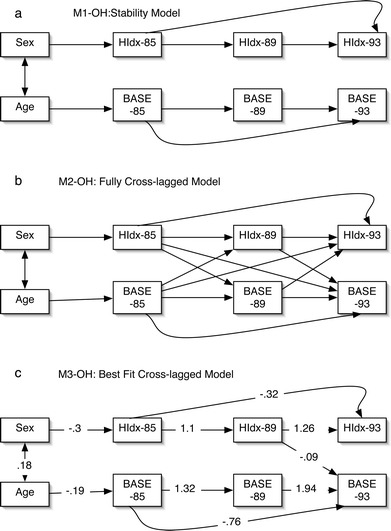
Objective health (Hldx) predicted by social engagement (BASE): a M1-OH: stability model. b M2-OH: fully cross-lagged model. c M3-OH: best fit cross-lagged model
In these analyses both social engagement and health are endogenous variables. Age and gender were included as exogenous variables. Following an analysis of correlations, and consideration of residuals from preliminary analyses, it was considered that age would predict social engagement and gender predict health. In addition, it was considered that gender and age would covary.
In turn, objective health and subjective health were assessed using the following steps:
the stability model was tested for objective (subjective) health;
the cross-lagged model was tested for objective (subjective) health;
the stability and the cross-lagged model models were compared for best fit and parsimony, including post-hoc modifications where necessary. This third step is critical, as it combines the information from the first two to establish the critical factors.
Since considerable debate exists as to which indices are preferable, three fit indices are reported—the comparative fit index, the Bentler-Bonnet normed fit index, and chi-square (Tabachnick and Fidell 2001). Both the comparative fit index and the Bentler-Bonnet normed fit index should be over 0.9 and ideally over 0.95, and the chi-square should be non-significant. However, the chi-square is not always reliable, especially in smaller samples where the assumptions of the test may be violated (Bentler 1995). Thus, if the other indices are satisfactory, a significant chi-square is not considered too problematic. Comparisons between models were undertaken using the chi-square difference test (Δχ2=χ21−χ22, df=df1–df2).
In Figs. 1, 2 and 3, rectangles represent measured variables. Single arrows represent a unidirectional effect, and double arrows represent covarying variables. Absence of line connecting two variables suggests the lack of a hypothesised direct effect. In order to account for shared measurement error (Maruyama 1998), in all analyses errors were correlated between T1 and T2 and between T2 and T3 (data not shown).
Fig. 2a–c.
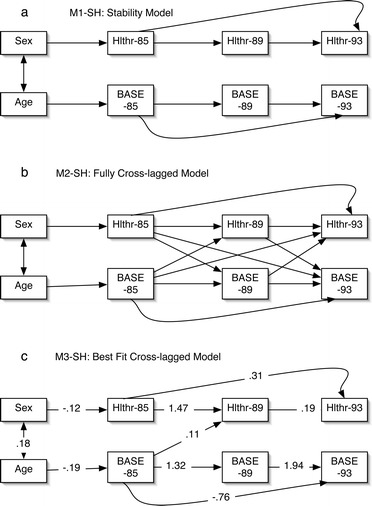
Subjective health (Hlthr) predicted by social engagement (BASE): a M1-SH: stability model. b M2-SH: fully cross-lagged model. c M3-SH: best fit cross-lagged model
Fig. 3.
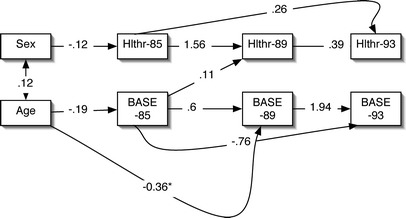
M4-SH subjective health (Hlthr) predicted by social engagement (BASE) and the effects of age
Results
Table 1 shows descriptive statistics for variables at T1, T2 and T3 and the correlations. Gender is shown to significantly correlate with both measures of health and with social engagement, whilst age was found to correlate with social engagement and objective health, but not with subjective health. The correlation between objective and subjective health at T1, while significant, demonstrates that these two variables do not measure exactly the same factor (r=0.46, p<0.001).
Table 1.
Means, standard deviations and Pearson correlation coefficients of the variables (* denotes p<0.05)
| Variable | Mean | SD | Sex | Age | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (1) | 1.7 | 0.5 | |||||||||||
| Age (2) | 72.4 | 5.0 | 0.18* | ||||||||||
| T1 | Social engagement (3) | 13.4 | 2.6 | −0.12* | −0.19* | ||||||||
| Objective health (4) | 4.2 | 2.7 | 0.30* | 0.20* | −0.22* | ||||||||
| Subjective health (5) | 3.7 | 1.0 | −0.12* | −0.02 | 0.30* | −0.46* | |||||||
| T2 | Social engagement (6) | 12.3 | 3.1 | −0.11* | −0.25* | 0.7* | −0.17* | 0.2* | |||||
| Objective health (7) | 5.0 | 2.5 | 0.33* | 0.22* | −0.16* | 0.7* | −0.39* | −0.18* | |||||
| Subjective health (8) | 3.6 | 1.1 | −0.19* | 0.02 | 0.27* | −0.46* | 0.55* | 0.27* | −0.54* | ||||
| T3 | Social engagement (9) | 12.3 | 3.0 | −0.18* | −0.34* | 0.6* | −0.28* | 0.2* | 0.65* | −0.24* | 0.24* | ||
| Objective health (10) | 6.2 | 2.3 | 0.32* | 0.16* | −0.16* | 0.6* | −0.39* | −0.16* | 0.67* | −0.45* | −0.24* | ||
| Subjective health | 3.3 | 1.1 | −0.08 | −0.02 | 0.12* | −0.32* | 0.42* | 0.16* | −0.36* | 0.48* | 0.21* | −0.44* |
Objective health
The stability model (M1-OH, without cross-lagged structural paths) is presented in Fig. 1a. It was found to fit the data well (χ2 (15, n=359)=39.146, p<0.001, comparative fit index=0.977, Bentler-Bonnet normed fit=0.963). The cross-lagged model (M2-OH) is presented in Fig. 1b. It also fitted the data well (χ2 (10, n=359)=51.33, p<0.001, comparative fit index=0.96, Bentler-Bonnet normed fit=0.950). However, only one of the cross-lagged paths was found to be significant. Interestingly, the significant path was not one of the proposed relationships. Rather, it indicated that objective health at T2 predicted social engagement at T3. In order to achieve parsimony, post-hoc modifications were undertaken, whereby non-significant paths were removed (M3-OH). This provided the most parsimonious fit (χ2 (14, n=359)=33.959, p=0.002, comparative fit index=0.981, Bentler-Bonnet normed fit=0.968; see Fig. 1c). This final model differed significantly from the stability model (Δχ2(1)=5.187, p<0.05).
Subjective health
Figure 2a represents the stability model (M1-SH). The fit of the data was only just adequate (χ2 (17, n=359)=76.731, p<0.001, comparative fit index=0.926, Bentler-Bonnet normed fit=0.908). The cross-lagged model (M2-SH) is presented in Fig. 2b. This model fitted the model more effectively (χ2 (11, n=359)=67.04, p<0.001, comparative fit index=0.931, Bentler-Bonnet normed fit=0.920). Only one cross-lagged path was significant—that between social engagement at T1 and subjective health at T2. Once more, to achieve parsimony, post-hoc modifications were undertaken by removing non-significant paths (M3-SH). This fitted the data both well and parsimoniously (χ2 (16, n=359)=71.069, p<0.001, comparative fit index=0.931, Bentler-Bonnet normed fit=0.915). This final model differed significantly from the stability model (Δχ2(1)=5.662, p<0.02).
The question arises why there was a significant path between social engagement at T1 and subjective health at T2, but not between social engagement at T2 and subjective health at T3. Theoretically, it was believed that age might have an increased influence as the whole sample became older (for example, at T2 compared with T1). To test this hypothesis, the stability model was re-examined, but an arrow was added between age and social engagement at T2. This amended model (M4-SH) is presented in Fig. 3. This model also fitted the data well (χ2 (17, n=359)=76.78, p<0.001, comparative fit index=0.925, Bentler-Bonnet normed fit=0.908), but of most interest is the comparison of the standardised coefficients between age and social engagement at T1 (−0.19) and at T2 (−0.36). This indicates that as people grow older, age plays an increasingly larger influence on social engagement.
Discussion
The relationship between social engagement and objective and subjective health over an 8-year period was examined. Three hypotheses were tested: (1) that social engagement at T1 would predict objective/subjective health at T2; (2) that social engagement at T2 would predict objective/subjective health at T3; and (3) that social engagement at T1 would predict objective/subjective health at T3. Analysis of the structural equation models provided support for only the first hypothesis and, only in relationship to subjective health, the hypothesis that social engagement at T1 predicts subjective health at T2. Higher levels of social engagement predicted better subjective health, 4 years later. There was no significant support for the other hypotheses. There was also one unexpected finding, which was that objective health at T2 significantly predicted social engagement at T3. In this case, better objective health at 4 years predicted higher levels of social engagement at 8 years after the study began.
The most important finding of this study was that social engagement significantly predicted future subjective health, confirming previous research (Zunzunegui et al. 2004). Indeed, even when prior subjective health is taken account of, those who are more socially engaged will report higher levels of subjective health than those with lower levels of social engagement. This provides evidence of a causal link. It is likely that, as Mendes de Leon et al. (2003) argue, social engagement exerts a protective influence on health. In this study, however, it has a protective effect on subjective, rather than objective health. At a practical level, it is possible that by encouraging older adults to participate socially and to maintain their social engagement, they will be more likely to consider themselves healthy at a later stage.
It was not, however, found that baseline (T1) social engagement significantly predicted subjective health at T3, nor was a significant relationship found between subjective health and social engagement at T3. From a statistical point of view, Maruyama (1998) would argue that for a finding to be stable, one would expect the T1–T2 relationship to be the same as a T2–T3 relationship. However, in an ageing population this may not necessarily be the case. Age might influence social engagement differently in a sample whose mean age was 72.4 (at T1), compared with a sample whose mean age was 76.4 (T2) and 80.4 (at T3), indicating a non-linear relationship. This hypothesis was examined in post-hoc analyses, and it was found that the age–social engagement coefficient increased between T1 and T2. This suggests, in turn, that age has an indirect effect on subjective health, via social engagement, which changes as the sample ages. These findings also provide support for the observations of Mendes de Leon et al. (2003) that the protective effects of social engagement diminish with time. A more detailed analysis of the impact of age on social engagement and subjective health would be valuable, especially in a sample not confined to survivors. Three questions in particular remain outstanding. First, since the data were collected at 4-year intervals, it is not possible to examine the effects of change over shorter periods, for example, annual or biennial. As Maruyama (1998) has argued, choosing the correct time lag is an important consideration. Second, one cannot be certain that the age–social engagement relationship is linear (as mentioned above). Indeed, the study by Mendes de Leon et al. (2003) provides evidence that this is not the case. Thirdly, the sample is an elite sample, one of survivors. There was no measure of mortality in these analyses, and so one cannot be certain that the pattern of results would remain the same if all participants had been included. Structural equation modelling has difficulties dealing with missing data, an issue discussed briefly below.
The results regarding objective health were contrary to the predictions of this study. Objective health was not significantly predicted by social engagement for any of the cross-lags. This is contrary to the findings of, for example, Mendes de Leon et al. (2003). These data suggest no causal link between social engagement and objective physical health. If one had confirmed Mendes de Leon’s findings, one would have expected a significant path between baseline (T1) and T2, if not between baseline and T3. In their study, however, data were collected annually over 9 years. It may be the case that with respect to objective health, the time lag of 4 years used in the current study was too long. It would be valuable to look at this relationship again but with more closely spaced time periods.
One significant causal path was identified in the objective health model—objective health was found to predict social engagement between T2 and T3. This suggests that the poorer one’s health is, in particular at older ages, the less likely one is to engage in social activities. These results confirm those of Harwood et al. (2000) who examined the effects of physical health on social engagement in older men in a cross-sectional study. They found that better subjective health, fewer diagnoses and the absence of disability were associated with higher levels of social engagement. The present findings add longitudinal support to their cross-sectional study. There is further evidence to support this view from Sidell (1995). She reviewed work examining the effects of chronic illness in later life, and suggested that poor physical health presents a number of barriers to social participation.
The different relationship between social engagement and subjective and objective health suggests that the two types of health outcomes reflect two different aspects of health. A number of studies have found, for example, that whilst older people’s objective health might be poor, their subjective health remains good (see Pinquart 2001 for a meta-analysis; see also Leonard and Burns 2000). How might this divergence be explained? Many people have argued that subjective health is not only a perception of one’s physical health but also an aspect of one’s psychological well-being (see Benyamini et al. 2003 for a discussion). If subjective health is a broader measure of health which includes a psychological component, then explanations for these divergent findings may lie in an exploration of the psychological or behavioural component of subjective health. In Berkman et al.’s (2000) model, the authors suggested that social engagement is one of the psychosocial mechanisms mediating the social networks–health relationship. Social engagement is also associated with psychological well-being, and indeed is often viewed as a component of psychological well-being (Morgan et al. 1987; Maier and Smith 1999; Zunzunegui et al. 2003). Theoretically, therefore, it is possible that the difference in strength of predictions of subjective and objective health by social engagement (the former significant, the latter non-significant) can be accounted for by the shared psychological well-being component of social engagement and subjective health.
While the present findings regarding subjective health resemble those of Zunzunegui et al. (2003), the analyses shown here and, therefore, the conclusions drawn from these results differ in a number of ways. First, the data presented here are longitudinal. Longitudinal data allow firmer conclusions about causal mechanisms than do cross-sectional data. Second, because of the longitudinal nature of the dataset, it was possible to examine the influence of baseline levels of subjective health. Third, a cross-lagged design was used, which examined the influence of subjective health on social engagement at different time points, and the reverse relationship between subjective health and social engagement. These, it is believed, are strengths of the current analyses results, and provide evidence for the causal hypothesis that social engagement exerts a protective influence on later subjective health.
The study raises a number of interesting methodological issues. The use of a cross-lagged design was found to be particularly useful, since it allows one to exam not only the effects of social engagement on health, but also those of health on social engagement. As a consequence, it was possible to identify the significant impact of objective health on social engagement discussed above. This raises the important issue of the impact of time lag in the measurement of longitudinal variables, also discussed briefly above. For example, if only 8-year differences had been considered, it would not have been possible to identify that social engagement predicted subjective health. It was also believed that it was important to account for baseline measures of health, in that previous levels of health are likely to have a stronger impact on later health than other variables of interest, such as social engagement. If baseline health had not been included, the relationship between social engagement and health might have been inflated. Finally, it was also important to correlate the errors between T1 and T2 and between T2 and T3 in order to account for shared measurement error.
The nature of structural equation modelling presents also some challenges, and inherent limitations, to the analysis of longitudinal data, particularly in an older sample where attrition is marked, and where missing data are not uncommon. This type of analysis has difficulties assessing incomplete datasets. As a consequence, only 359 participants were available for study, representing an elite sample, i.e. survivors. A larger sample would have been available had one been interested only in T1 to T2 data, but then some interesting findings would have been overlooked. Nevertheless, it would be interesting in future to reconsider the data in two ways. First, by examining T1 to T2 data only and second, by introducing an outcome variable such as survival into the analyses.
In addition to the practical difficulties associated with structural modelling and missing data, the use of an elite sample also raises some important theoretical issues. The conclusions which can be drawn from this study can be generalised only to a population of community-dwelling (at the outset of the study), and not cognitively impaired individuals who remained alive for the duration of the study. As a consequence, this is a study of the importance of social engagement in a population of relatively healthy older people. On the other hand, there are a number of papers which have examined social engagement as a predictor of mortality and longevity, and which have drawn similar conclusions (Steinbach 1992; Sugisawa et al. 1994; Benyamini and Idler 1999; Bennett 2002). Steinbach (1992) reported that low levels of social participation have also been found to be predictive of both institutionalisation and mortality. In previous work by Bennett (2002), using the NLSAA, it was demonstrated that social engagement also predicted mortality. Taken together, the evidence thus suggests that social engagement is an important predictor of health status amongst those who are longer lived and those who are not, such that those who have lower levels of social engagement are more likely to die and, if they do survive, are likely to be in poorer health.
Finally, a brief mention should be made about the age of the dataset. Although data for the NLSAA were collected between 1985 and 1993 and are, therefore, more than a decade old, the dataset is valuable for a number of reasons. First, it contains a specific measure of social engagement, unlike many other studies which have studied social engagement in a post-hoc fashion (e.g. Mendes de Leon et al. 2003). Second, the study also contains measures of objective and subjective health, allowing an exploration of the two in relationship to social engagement. Third, the relationship between social engagement and health in the NLSAA has only been studied thus far in relationship to mortality (Bennett 2002), and with respect to service use (Bath and Gardiner 2005, this issue). Most importantly, structural equation modelling, and cross-lagged models in particular, have only recently become widely available, and structural equation modelling has to date not been used with NLSAA data. The use of structural equation modelling and cross-lagged models has made it possible to identify simultaneously the relationships between social engagement and health, taking into account gender and age. In these analyses, more than one dependent variable (or, in structural equation modelling terms, endogenous variable) was considered, which is not possible with other techniques. It is thus proposed, with more certainty, that social engagement predicts subjective health and, conversely, that objective health predicts social engagement. In addition, analyses of longitudinal data in general are often carried out some time after the data have been collected (see, for example, Mendes de Leon et al. 2003). There have not been substantial changes either in physical health of older people or in the activities measured in social engagement since 1993. Some changes in the activities measured in social engagement may change with the advent of new technology, for example, with the use of the internet. However, it is considered that these advances have not made an impact on social engagement yet. It is also suggested that while the activities might change, the role of social engagement in health would probably remain the same.
Conclusion
Baseline (T1) social engagement was found to predict subjective health at T2 (4 years later). However, baseline social engagement did not predict subjective health at T3 (8 years later), nor did T2 social engagement predict T3 subjective health. Objective health was not predicted by social engagement at any point. Thus, social engagement appears to operate differentially on subjective and objective health. It is suggested that social engagement may act on subjective health through a psychosocial pathway which may be absent in its relationship with objective health. Unexpectedly, objective health at T2 predicted social engagement at T3, perhaps through mechanisms such as functional capacity and physical symptoms. Certainly, these findings reflect the complex interplay between objective and subjective health and social engagement. It is clear that these are worthy of further, more detailed examination in a larger population, with the addition of mortality as an outcome variable.
Acknowledgements
The Nottingham Longitudinal Study of Activity and Ageing is supported by grants from The Grand Charity, Help the Aged, the PPP Medical Trust and from the Trent Regional Health Authority. I would like to thank the anonymous reviewer for comments on an earlier draft of this paper.
Footnotes
This article was previously published with DOI s10433-005-0016-7, which was mistakenly created twice and has therefore been replaced by the current DOI.
References
- Bath PA, Gardiner A (2005) Social engagement and health and social care use and medication use among older people. Eur J Ageing 2 DOI 10.1007/s10433-005-0022-9 [DOI] [PMC free article] [PubMed]
- Bennett Age Ageing. 2002;31:165. doi: 10.1093/ageing/31.3.165. [DOI] [PubMed] [Google Scholar]
- Bentler PM (1995) EQS structural equations program manual. Multivariate Software, Inc, Encino, CA
- Benyamini Res Aging. 1999;21:392. doi: 10.1177/0164027599213002. [DOI] [Google Scholar]
- Benyamini Soc Sci Med. 2003;56:1661. doi: 10.1016/S0277-9536(02)00175-2. [DOI] [PubMed] [Google Scholar]
- Berkman Soc Sci Med. 2000;51:843. doi: 10.1016/S0277-9536(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Bowling J Gerontol. 1991;46:S20. doi: 10.1093/geronj/46.1.s20. [DOI] [PubMed] [Google Scholar]
- Deeg Gerontologist. 2003;43:369. doi: 10.1093/geront/43.3.369. [DOI] [PubMed] [Google Scholar]
- de J Occup Organ Psychol. 2001;74:29. doi: 10.1348/096317901167217. [DOI] [Google Scholar]
- Estes CL, Rundall TG (1992) Social characteristics, social structure, and health in the aging population. In: Ory M, Ableles RP, Lipman PD (eds) Aging, health and behaviour. Sage, Newbury Park, pp 299–326
- Ferrucci J Gerontol. 1996;51A:M123. doi: 10.1093/gerona/51a.3.m123. [DOI] [PubMed] [Google Scholar]
- Grundy Soc Sci Med. 2003;56:935. doi: 10.1016/S0277-9536(02)00093-X. [DOI] [PubMed] [Google Scholar]
- Harwood Psychol Health Med. 2000;5:75. doi: 10.1080/135485000106025. [DOI] [Google Scholar]
- Leonard R, Burns A (2000) The paradox of older women’s health. In: Ussher J (ed) Women’s health: contemporary international perspectives. British Psychological Society, Leicester, pp 485–489
- Maier J Gerontol Psychol Sci. 1999;54B:P44. doi: 10.1093/geronb/54b.1.p44. [DOI] [PubMed] [Google Scholar]
- Maruyama GM (1998) Basics of structural equation modeling. Sage, Thousand Oakes
- Mendes Am J Epidemiol. 2003;157:633. doi: 10.1093/aje/kwg028. [DOI] [PubMed] [Google Scholar]
- Morgan Age Ageing Suppl. 1998;27:5. doi: 10.1093/ageing/27.1.5. [DOI] [Google Scholar]
- Morgan Br J Psychiatr. 1987;150:801. doi: 10.1192/bjp.150.6.801. [DOI] [PubMed] [Google Scholar]
- Pinquart Psychol Aging. 2001;16:414. doi: 10.1037/0882-7974.16.3.414. [DOI] [PubMed] [Google Scholar]
- Sidell Health in old. 1995;age:myth. [Google Scholar]
- Steinbach J Gerontol. 1992;47:S183. doi: 10.1093/geronj/47.4.s183. [DOI] [PubMed] [Google Scholar]
- Sugisawa J Gerontol. 1994;49:S3. doi: 10.1093/geronj/49.1.s3. [DOI] [PubMed] [Google Scholar]
- Tabachnick BG, Fidell LS (2001) Using multivariate statistics, 4th edn. Allyn and Bacon, Boston
- Uchino Psychol Bull. 1996;119:488. doi: 10.1037/0033-2909.119.3.488. [DOI] [PubMed] [Google Scholar]
- Unger J Gerontol. 1999;54B:S245. doi: 10.1093/geronb/54b.5.s245. [DOI] [PubMed] [Google Scholar]
- Zunzunegui J Gerontol Ser B Psychol Sci Soc Sci. 2003;58:S93. doi: 10.1093/geronb/58.2.s93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zunzunegui Soc Sci Med. 2004;58:2069. doi: 10.1016/j.socscimed.2003.08.005. [DOI] [PubMed] [Google Scholar]


