Abstract
Background
Sorafenib and sunitinib are widely used as first-line targeted therapy for metastatic renal cell carcinoma (mRCC) in China. This study aimed to compare the efficacy, safety, and quality of life (QoL) in Chinese mRCC patients treated with sorafenib and sunitinib as first-line therapy.
Methods
Clinical data of patients with mRCC who received sorafenib (400 mg twice daily; 4 weeks) or sunitinib (50 mg twice daily; on a schedule of 4 weeks on treatment followed by 2 weeks off) were retrieved. Primary outcomes were overall survival (OS), progression-free survival (PFS), adverse events (AEs), and QoL (SF-36 scores), and secondary outcomes were associations of clinical characteristics with QoL.
Results
Medical records of 184 patients (110 in the sorafenib group and 74 in the sunitinib group) were reviewed. PFS and OS were comparable between the sorafenib and sunitinib groups (both P > 0.05). The occurrence rates of leukocytopenia, thrombocytopenia, and hypothyroidism were higher in the sunitinib group (36.5% vs. 10.9%, P < 0.001; 40.5% vs. 10.9%, P < 0.001; 17.6% vs. 3.6%, P = 0.001), and that of diarrhea was higher in the sorafenib group (62.7% vs. 35.2%, P < 0.001). There was no significant difference in SF-36 scores between the two groups. Multivariate analysis indicated that role-physical and bodily pain scores were associated with the occurrence rate of grade 3 or 4 AEs (P = 0.017 and 0.005).
Conclusions
Sorafenib has comparable efficacy and lower toxicity profile than sunitinib as first-line therapy for mRCC. Both agents showed no significant impact on QoL of patients.
Keywords: Metastatic renal cell carcinoma, Sorafenib, Sunitinib, Quality of life
Background
Molecular targeted therapy has shown promising results in different clinical trials and clinical practice, and it has been the preferred therapeutic option for patients with metastatic renal cell carcinoma (mRCC) [1–5]. Among new therapeutic agents, tyrosine kinase inhibitors (TKIs) such as sorafenib and sunitinib are used as first-line treatment agents for mRCC in China [6]. Although the efficacy of targeted therapies in terms of tumor growth control at metastatic sites is definite, their adverse events (AEs) are often major limitations. Furthermore, it is estimated that the severity of hand-foot syndrome, hypertension, diarrhea, and alopecia was higher in Japanese patients [7] than in Western patients [8, 9]. Thus, comparison of safety in clinical practice would provide important information for patient counseling and treatment decision-making.
According to the data in the Japanese study [7], it is plausible that the quality of life (QoL) of Japanese patients might have been significantly impaired with the use of TKIs. Moreover, because the Chinese ethnicity closely resembles the Japanese ethnicity, the TKI therapy-associated QoL patterns might share similarities. QoL has been a curative effect index and an important parameter to evaluate treatment efficacy in recent clinical practices. Many QoL assessment tools are available. Among them, the Chinese version of the 36-Item Short Form Health Survey Questionnaire (SF-36) has been validated and is the most commonly used tool in Chinese patients [10]. A Japanese study on QoL of patients receiving different TKIs using SF-36 reported that neither sorafenib nor sunitinib was associated with significant impairments in QoL [11].
Efficacy evaluation is important for targeted therapy. Although phase III trials compared the efficacy between pazopanib and sunitinib [12] and between axitinib and sorafenib [13], no such attempts to compare efficacy, safety, and QoL impairments simultaneously between sorafenib and sunitinib as first-line treatment for patients with mRCC have been made. Only three retrospective studies directly compared the efficacy but not QoL impairments between sorafenib and sunitinib. Choueiri et al. [5] reported that the overall response rate was higher in the sunitinib group (37%) than in the sorafenib group (9%) in two oncology centers in the US. In contrast, no significant differences in overall survival (OS) and progression-free survival (PFS) between sorafenib- and sunitinib-treated patients were observed in an Asian population (both P > 0.05), although sorafenib was concluded to be more favorable than sunitinib because of minimal grade 3 or 4 toxicities [14]. Recently, Sheng et al. [15] reported similar results in terms of OS and PFS of Chinese patients with mRCC (both P > 0.05) [15]. However, these studies were conducted with a small sample size and aimed to compare the efficacy and safety of sorafenib and sunitinib, but not the effect of these drugs on QoL.
In the present study, we retrospectively compared the efficacy, safety, and QoL impairments between sorafenib and sunitinib in Chinese patients with mRCC to further guide clinical treatment.
Methods
Study design and population
Data were retrieved from the electronic medical records of patients with mRCC who visited the Department of Urology in Renji Hospital affiliated to Shanghai Jiao Tong University School of Medicine (Shanghai, China) between March 2006 and July 2015. The study protocol was in accordance with the Chinese guidelines on the management of renal cell carcinoma (2015 edition) [16] and conformed to the principles of the Declaration of Helsinki.
mRCC patients who received sorafenib or sunitinib as first-line therapy with a Karnofsky performance status (KPS) score of 70–100 and completed the SF-36 questionnaire (Chinese version) at baseline and 3 months after treatment were included in the study. Informed consent was obtained from all patients. Patients who did not comply with the above criteria or had unstable or severe cardiac disease, uncontrolled brain metastases, concurrent malignancies, or incomplete data files were excluded. Personal information from medical records was anonymized and de-identified before analysis. Ethical approval was obtained from the Institutional Ethics Committee of Renji Hospital.
QoL assessment
The filled SF-36 questionnaires were assessed independently by two authors (Wen Cai and Wen Kong), and a third person (Jiwei Huang) was consulted to resolve any disagreements.
Clinicopathologic evaluation
Demographic information was retrieved from the medical record database. All patients underwent a pretreatment baseline evaluation including complete medical and physical examinations, complete blood count (CBC), routine organ function tests, computed tomography (CT), magnetic resonance imaging (MRI), and histological differentiation of tumor graded according to the Fuhrman’s nuclear grading system.
Treatment
Sorafenib and sunitinib were used as first-line treatment in patients with mRCC. Sorafenib 400 mg was administered twice daily orally in a 4-week cycle, and sunitinib 50 mg was prescribed daily orally for the first 4 weeks in a 6-week cycle (4-week on/2-week off) until disease progression, intolerable AEs, or death was reported. Dose titrations were done considering the patient’s tolerance.
Follow-up and outcome measures
All the patients were suggested to have monthly disease assessment after the treatment to estimate the treatment response and AEs. At baseline and 3 months after the treatment, the SF-36 was completed during outpatient visits. The National Cancer Institute Common Terminology Criteria for Adverse Events v3.0 [17] was used for diagnosis and grading of AEs, based on which dose titrations were carried out. The treatment was terminated in patients who experienced serious AEs, disease progression, or unacceptable toxicity (≥4-week delay in recovery to a permissible level of toxicity despite 2 dose reductions), as defined by the response evaluation criteria in solid tumors (RECIST) [18]. After treatment, patients were followed up every month until they experienced discomfort or death. PFS and OS were assessed as endpoints. PFS was defined as the duration from the onset of targeted therapy to disease progression or death as assessed by the treating physicians or the last visiting day recorded if the disease did not progress. OS was defined as the duration from the onset of targeted therapy to death or the last visiting day. The prognostic outcomes were assessed according to the Memorial Sloan-Kettering Cancer Center (MSKCC) grading model [19] and the International Metastatic Renal-Cell Carcinoma Database Consortium (IMDC) prognostic model [20].
Statistical analyses
All analyses were made using the SAS version 9.4 (SAS Corporation, Cary, NC, USA). Categorical variables are presented as counts and percentages and were compared between the sorafenib and sunitinib groups with the Pearson Chi squared test or the Fisher exact test as appropriate. The Kaplan–Meier method was used to estimate survival curves, and the log-rank test to compare PFS and OS between the sorafenib and sunitinib groups. Patients alive at the end of the study were censored at the last follow-up. SF-36 scores are presented as mean ± standard deviation (SD) and were compared using the unpaired t test. The means of individual scores were defined as cutoff points. Forward stepwise logistic regression analysis was used to determine associations between clinical characteristics and SF-36 scores. All statistical tests were 2-sided, and P < 0.05 was considered significant.
Results
Clinicopathologic characteristics
A total of 184 patients, with a median age of 60 years (range 24–83 years), were selected, and 141 of them were men. Of them, 110 received sorafenib and 74 received sunitinib as first-line treatment. No significant differences in baseline clinical characteristics were found between the two groups (Table 1). Fifteen (13.6%) patients in the sorafenib group and 10 (13.5%) in the sunitinib group received second-line targeted therapy due to disease progression.
Table 1.
Baseline clinicopathologic and prognostic characteristics of 184 patients with metastatic renal cell carcinoma (mRCC)
| Variable | Total [cases (%)] | Sorafenib group [cases (%)] | Sunitinib group [cases (%)] | P value |
|---|---|---|---|---|
| Total | 184 | 110 | 74 | |
| Sex | 0.336 | |||
| Man | 141 (76.6) | 87 (84.3) | 54 (73.0) | |
| Woman | 43 (23.4) | 23 (25.7) | 20 (27.0) | |
| Age (years) | 0.152 | |||
| <65 | 139 (75.5) | 79 (71.8) | 60 (81.1) | |
| ≥65 | 45 (24.5) | 31 (28.2) | 14 (18.9) | |
| Histology | 0.872 | |||
| Clear cell | 176 (95.7) | 105 (95.5) | 71 (96.0) | |
| Others | 8 (4.3) | 5 (4.5) | 3 (4.0) | |
| Prior nephrectomy | 0.516 | |||
| Yes | 150 (81.5) | 88 (80.0) | 62 (83.8) | |
| No | 34 (18.5) | 22 (20.0) | 12 (16.2) | |
| Prior cytokine therapy | 0.118 | |||
| Yes | 60 (32.6) | 31 (28.2) | 29 (39.2) | |
| No | 124 (67.4) | 79 (71.8) | 45 (60.8) | |
| Fuhrman grade | 0.636 | |||
| 1–2 | 106 (57.6) | 64 (58.2) | 42 (56.8) | |
| 3–4 | 64 (34.8) | 35 (31.8) | 29 (39.2) | |
| Unknown | 14 (7.6) | 11 (10.0) | 3 (4.0) | |
| Number of metastatic sites | 0.084 | |||
| 1 | 134 (72.8) | 75 (68.2) | 59 (79.7) | |
| ≥2 | 50 (27.2) | 35 (31.8) | 15 (20.3) | |
| Metastatic sites | ||||
| Lung | 139 (75.5) | 81 (73.6) | 58 (78.4) | 0.463 |
| Lymph nodes | 44 (23.9) | 29 (26.4) | 15 (20.3) | 0.342 |
| Bone | 19 (10.3) | 12 (10.9) | 7 (9.5) | 0.751 |
| Liver | 15 (8.2) | 12 (10.9) | 3 (4.1) | 0.096 |
| Others | 13 (7.1) | 7 (6.4) | 6 (8.1) | 0.651 |
| MSKCC grade | 0.598 | |||
| Favorable | 86 (46.7) | 49 (44.6) | 37 (50.0) | |
| Intermediate | 73 (39.7) | 46 (41.8) | 27 (36.5) | |
| Poor | 25 (13.6) | 15 (13.6) | 10 (13.5) | |
| IMDC risk | 0.199 | |||
| Good | 100 (54.3) | 57 (51.8) | 43 (58.1) | |
| Intermediate | 73 (39.7) | 44 (40.0) | 29 (39.2) | |
| Poor | 11 (6.0) | 9 (8.2) | 2 (2.7) | |
MSKCC Memorial Sloan-Kettering Cancer Center, IMDC International Metastatic Renal Cell Carcinoma Database Consortium
OS and PFS
Kaplan–Meier curves of PFS and OS are illustrated in Fig. 1. With a median follow-up of 23 months (95% confidence interval [CI] 18–29 months), the median PFS and OS of all the 184 patients were 11 months (95% CI 8–12 months) and 23 months (95% CI 19–27 months), respectively. The median PFS and OS were 10 months (95% CI 7–13 months) and 24 months (95% CI 15–31 months), respectively, in the sorafenib group, which did not significantly differ from the median PFS (11.5 months; 95% CI 9–12 months; P = 0.366) and OS (23 months; 95% CI 18–25 months; P = 0.552) in the sunitinib group.
Fig. 1.
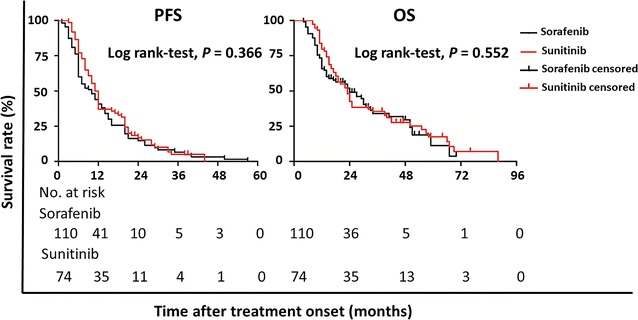
Kaplan–Meier curves of progression-free survival (PFS) and overall survival (OS) of patients with metastatic renal cell carcinoma treated with sorafenib and sunitinib. No significant differences in both PFS and OS were observed between the two groups
Adverse events
The comparison of AEs between the sorafenib and sunitinib groups is summarized in Table 2. The five most common AEs after treatment onset were hand-foot syndrome (67.3%), diarrhea (62.7%), fatigue (38.2%), nausea (37.3%), and hypertension (20.9%) in the sorafenib group and were hand–foot syndrome (59.5%), fatigue (44.6%), thrombocytopenia (40.5%), nausea (39.2%), and leukocytopenia (36.5%) in the sunitinib group. The treatments were well tolerated with few grade 1–2 AEs. The 3 most common grade 3–4 AEs were hand–foot syndrome (10.8%), diarrhea (1.8%), and anemia (1.8%) in the sorafenib group and were diarrhea (4.1%), leukocytopenia (4.1%), and hypertension (3.6%) in the sunitinib group. In the sorafenib group, 11 (10.0%) patients required dose reduction. In the sunitinib group, 9 (12.2%) patients required dose reduction.
Table 2.
Comparison of adverse events in the sorafenib and sunitinib groups
| Adverse event | Sorafenib group [cases (%)] | Sunitinib group [cases (%)] | P value* | ||||
|---|---|---|---|---|---|---|---|
| All grade | Grade 1–2 | Grade 3–4 | All grade | Grade 1–2 | Grade 3–4 | ||
| Hypertension | 23 (20.9) | 22 (20.0) | 1 (0.9) | 23 (36.0) | 20 (32.4) | 3 (3.6) | 0.050 |
| Hand–foot syndrome | 74 (67.3) | 66 (60.0) | 8 (7.3) | 44 (59.5) | 44 (59.5) | 0 (0.0) | 0.279 |
| Diarrhea | 69 (62.7) | 67 (60.9) | 2 (1.8) | 26 (35.2) | 23 (31.1) | 3 (4.1) | <0.001 |
| Nausea | 41 (37.3) | 41 (37.3) | 0 (0.0) | 29 (39.2) | 29 (39.2) | 0 (0.0) | 0.793 |
| Fatigue | 42 (38.2) | 42 (38.2) | 0 (0.0) | 33 (44.6) | 33 (44.6) | 0 (0.0) | 0.385 |
| Alopecia | 9 (8.2) | 9 (8.2) | 0 (0.0) | 7 (9.5) | 7 (9.5) | 0 (0.0) | 0.763 |
| Leukocytopenia | 12 (10.9) | 12 (10.9) | 0 (0.0) | 27 (36.5) | 24 (32.4) | 3 (4.1) | <0.001 |
| Anemia | 20 (18.2) | 18 (16.4) | 2 (1.8) | 9 (12.2) | 9 (12.2) | 0 (0.0) | 0.272 |
| Thrombocytopenia | 12 (10.9) | 12 (10.9) | 0 (0.0) | 30 (40.5) | 28 (37.8) | 2 (2.7) | <0.001 |
| Hypothyroidism | 4 (3.6) | 4 (3.6) | 0 (0.0) | 13 (17.6) | 13 (17.6) | 0 (0.0) | 0.001 |
| Elevation of ALT | 11 (10.0) | 10 (9.1) | 1 (0.9) | 10 (13.5) | 10 (13.5) | 0 (0.0) | 0.462 |
ALT alanine aminotransferase
* Grade 1–2 and grade 3–4 toxicities were combined for the comparison
Diarrhea was more common in the sorafenib group than in the sunitinib group (66.4% vs. 31.1%, P < 0.001), whereas higher rates of hematologic toxicities such as leukocytopenia (32.4% vs. 10.9%, P < 0.001), thrombocytopenia (37.8% vs. 10.9%, P < 0.001), and hypothyroidism (17.6% vs. 3.6%, P = 0.001) were observed in the sunitinib group than in the sorafenib group.
QoL
The baseline and post-treatment SF-36 scores did not significantly differ between the two groups (Table 3). There was no significant difference in mean SF-36 scores at all the 8 dimensions between the sorafenib and sunitinib groups at both baseline and 3 months after treatment.
Table 3.
Quality of life of mRCC patients at baseline and 3 months after treatment with sorafenib and sunitinib
| SF-36 dimension | At baseline (score) | P value | Three months after treatment (score) | P value | ||
|---|---|---|---|---|---|---|
| Sorafenib group (n = 110) | Sunitinib group (n = 74) | Sorafenib group (n = 110) | Sunitinib group (n = 74) | |||
| PF | 68.9 ± 20.9 | 67.8 ± 19.5 | 0.548 | 66.7 ± 24.0 | 67.1 ± 20.3 | 0.914 |
| RP | 38.2 ± 36.6 | 45.6 ± 34.2 | 0.167 | 36.5 ± 37.5 | 42.7 ± 40.1 | 0.527 |
| BP | 77.2 ± 16.0 | 78.7 ± 20.2 | 0.587 | 76.2 ± 16.7 | 77.4 ± 19.7 | 0.646 |
| GH | 56.0 ± 16.3 | 54.4 ± 20.2 | 0.531 | 54.7 ± 15.1 | 50.8 ± 19.4 | 0.152 |
| VT | 70.1 ± 20.6 | 71.0 ± 16.1 | 0.759 | 68.7 ± 21.7 | 69.3 ± 15.8 | 0.858 |
| SF | 80.5 ± 18.6 | 82.1 ± 15.7 | 0.531 | 78.5 ± 19.2 | 80.2 ± 17.3 | 0.571 |
| RE | 59.1 ± 6.3 | 63.1 ± 33.4 | 0.452 | 55.5 ± 39.7 | 58.6 ± 37.4 | 0.594 |
| MH | 71.1 ± 12.5 | 75.2 ± 9.8 | 0.050 | 69.7 ± 15.7 | 75.8 ± 14.4 | 0.055 |
PF physical functioning, RP role-physical, BP bodily pain, GH general health, VT vitality, SF social functioning, RE role-emotional, MH mental health
Univariate analysis indicated that the occurrence of grade 3–4 AEs were associated with decreased role-physical score (P = 0.013) and bodily pain score (P = 0.003) (Table 4). The associations remained significant in multivariate analyses (P = 0.017 and 0.005) (Table 5).
Table 4.
Univariate analysis of associations between clinical characteristics and SF-36 scores of mRCC patients
| Variable | PF score | RP score | BP score | GH score | VT score | SF score | RE score | MH score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex (man vs. woman) | 1.690 (0.832–3.434) | 0.147 | 1.384 (0.698–2.744) | 0.353 | 0.750 (0.354–1.591) | 0.453 | 0.879 (0.425–1.814) | 0.987 | 0.793 (0.400–1.572) | 0.507 | 0.976 (0.493–1.932) | 0.944 | 1.709 (0.823–3.552) | 0.151 | 0.857 (0.433–1.699) | 0.659 |
| Age (<65 vs. ≥65 years) | 0.838 (0.431–1.631) | 0.604 | 0.962 (0.493–1.879) | 0.910 | 0.764 (0.364–1.607) | 0.479 | 1.020 (0.503–2.067) | 0.438 | 1.830 (0.926–3.618) | 0.082 | 0.982 (0.505–1.910) | 0.957 | 1.151 (0.585–2.266) | 0.684 | 1.412 (0.724–2.751) | 0.311 |
| MSKCC grade (favorable vs. others) | 1.432 (0.609–3.364) | 0.410 | 1.646 (0.707–3.833) | 0.248 | 0.850 (0.335–2.155) | 0.732 | 1.717 (1.132–4.028) | 0.034 | 0.881 (0.381–2.040) | 0.768 | 1.646 (0.701–3.868) | 0.253 | 1.475 (0.576–3.284) | 0.474 | 1.062 (0.458–2.459) | 0.899 |
| IMDC risk (good vs. others) | 0.442 (0.465–5.794) | 0.442 | 0.436 (0.112–1.695) | 0.231 | 0.478 (0.100–2.283) | 0.355 | 1.171 (0.330–4.153) | 0.807 | 0.536 (0.152–1.892) | 0.322 | 0.851 (0.251–2.887) | 0.796 | 1.321 (0.374–4.667) | 0.666 | 1.395 (0.411–4.733) | 0.593 |
| AE grade (1–2 vs. 3–4) | 0.802 (0.312–2.074) | 0.804 | 3.845 (1.327–11.139) | 0.013 | 4.513 (1.689–12.127) | 0.003 | 1.544 (0.589–4.047) | 0.377 | 0.860 (0.334–2.219) | 0.756 | 1.876 (0.706–4.985) | 0.207 | 2.234 (0.772–6.469) | 0.138 | 2.715 (0.987–7.466) | 0.053 |
| Treatment (sorafenib vs. sunitinib) | 1.199 (0.662–2.173) | 0.550 | 1.096 (0.607–1.977) | 0.761 | 1.340 (0.718–2.504) | 0.358 | 0.617 (0.327–1.164) | 0.136 | 0.733 (0.406–1.325) | 0.304 | 1.074 (0.596–1.938) | 0.811 | 1.377 (0.749–2.531) | 0.303 | 1.243 (0.689–2.243) | 0.470 |
PF physical functioning, RP role-physical, BP bodily pain, GH general health, VT vitality, SF social functioning, RE role-emotional, MH mental health, IMDC International Metastatic Renal Cell Carcinoma Database Consortium
Table 5.
Multivariate analyses of associations between clinical characteristics and SF-36 scores of mRCC patients
| Variable | PF score | RP score | BP score | GH score | VT score | SF score | RE score | MH score | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Sex (man vs. woman) | 1.611 (0.786–3.302) | 0.193 | 1.419 (0.692–2.909) | 0.339 | 0.737 (0.337–1.613) | 0.446 | 0.884 (0.421–1.885) | 0.744 | 0.861 (0.428–1.730) | 0.674 | 0.955 (0.476–1.917) | 0.898 | 1.673 (0.796–3.513) | 0.174 | 0.850 (0.423–1.710) | 0.649 |
| Age (<65 vs. ≥65 years) | 0.689 (0.347–1.402) | 0.312 | 0.821 (0.401–1.680) | 0.589 | 0.721 (0.329–1.580) | 0.414 | 0.794 (0.378–1.665) | 0.541 | 1.636 (0.802–3.337) | 0.176 | 0.814 (0.406–1.632) | 0.562 | 1.033 (0.506–2.109) | 0.929 | 1.310 (0.61–2.636) | 0.449 |
| MSKCC grade (favorable vs. others) | 1.184 (0.441–3.179 | 0.738 | 2.557 (0.905–7.339) | 0.076 | 1.089 (0.371–3.195) | 0.877 | 1.927 (0.721–5.153) | 0.191 | 0.887 (0.334–2.357) | 0.811 | 1.939 (0.714–5.265) | 0.194 | 1.128 (0.413–3.085) | 0.814 | 0.804 (0.305–2.122) | 0.660 |
| IMDC risk (good vs. others) | 0.689 (0.347–1.402) | 0.713 | 0.202 (0.041–1.003) | 0.050 | 0.465 (0.371–3.195) | 0.399 | 0.665 (0.157–2.809) | 0.579 | 0.454 (0.109–1.887) | 0.277 | 0.527 (0.129–2.157) | 0.373 | 1.077 (0.258–4.499) | 0.919 | 1.529 (0.383–6.104) | 0.548 |
| AE grade (1–2 vs. 3–4) | 0.699 (0.268–1.828) | 0.465 | 3.711 (1.263–10.910) | 0.017 | 4.259 (1.561–11.622) | 0.005 | 1.411 (0.532–3.747) | 0.489 | 0.758 (0.289–1.989) | 0.573 | 1.718 (0.641–4.603) | 0.282 | 2.035 (0.695–5.960) | 0.329 | 2.465 (0.889–6.835) | 0.083 |
| Treatment (sorafenib vs. sunitinib) | 1.141 (0.620–2.099) | 0.671 | 1.001 (0.539–1.859) | 0.998 | 1.335 (0.694–2.568) | 0.387 | 0.595 (0.311–1.139 | 0.117 | 0.735 (0.401–1.348) | 0.320 | 1.033 (0.564–1.890) | 0.917 | 1.364 (0.731–2.545) | 0.721 | 1.340 (0.730–3.462) | 0.345 |
PF physical functioning, RP role-physical, BP bodily pain, GH general health, VT vitality, SF social functioning, RE role-emotional, MH mental health, IMDC International Metastatic Renal Cell Carcinoma Database Consortium
Discussion
In the present study, we found that sorafenib has comparable efficacy and lower toxicity than sunitinib as first-line therapy for mRCC. AEs were associated with QoL impairments at the role-physical and bodily pain dimensions.
In the present study, the median PFS were 10 months in the sorafenib group and 11.5 months in the sunitinib group (P = 0.366). Our findings were similar to those from a study conducted in Korea by Park et al. [14]. In our study, the median OS were 24 months in the sorafenib group and 23 months in the sunitinib group (P = 0.552). Our data of OS were shorter than those from the Sheng et al. [15] study, but similar to those from the Park et al. [14] study, and proved that both sorafenib and sunitinib are equally effective as first-line treatment of mRCC in Chinese patients.
In addition to efficacy, toxicities of TKIs are significant factors to be considered while prescribing sorafenib and sunitinib. Randomized clinical trials reported that severe or grade 3–4 toxicities occurred in one-third of the patients treated with sorafenib [7] and two-thirds of the patients treated with sunitinib [21]. Hematologic toxicities are more common with sunitinib than sorafenib in our study. However, the severity of hematologic toxicities could be reduced with discontinuation of sunitinib [21, 22]. Increased risks of bleeding and poor healing are also associated with sunitinib [23], which may severely affect the QoL of patients. In our study, the rates of hematologic toxicities such as leukocytopenia (P < 0.001) and thrombocytopenia (P < 0.001) were significantly higher in the sunitinib group than in the sorafenib group, which were consistent with the results of previous studies [14, 15]. Hypothyroidism was also more common in the sunitinib group (P = 0.001), whereas diarrhea was more common in the sorafenib group (P < 0.001). The rates of TKI-related AEs vary among different ethnicities. Ye et al. [6] indicated that Chinese patients were more likely to experience hand-foot syndrome with sorafenib treatment than Western patients, and the occurrence rate in our center was 67.3%. A study in Japan showed that the most frequent AE was elevated lipase followed by hand-foot syndrome and that 10.7% patients had serious AEs [9]. The TARGET study conducted in a Western population showed that diarrhea, rash, fatigue, and hand-foot syndrome were common AEs and hypertension and cardiac ischemia were serious AEs in patients receiving sorafenib treatment [7]. Toxicities of TKIs are also associated with the patient nutritional status. For example, Antoun et al. [24] reported that a low body mass index could be a predictor for a high rate of AEs in patients with mRCC treated with sorafenib. Thus, diverse patient clinical characteristics may lead to diverse rates of AEs in various studies.
Assessment of the changes in QoL after receiving sorafenib or sunitinib treatment is important. Herrmann et al. [25] reported that pretreatment QoL could be a predictor of overall response and OS. Several studies have reported that QoL was not decreased significantly after sorafenib [26] or sunitinib treatment [27]. Furthermore, a phase II randomized controlled trial reported that patients receiving sorafenib treatment had better QoL assessed using the functional assessment of cancer therapy-kidney symptom index (FKSI) and greater treatment satisfaction assessed using the Treatment Satisfaction Questionnaire for Medication (TSQM) compared with patients receiving interferon-alpha treatment [28]. In our study, we observed no significant differences in mean SF-36 scores at all the 8 dimensions between the sorafenib and sunitinib groups at 3 months after the treatment. In a Japanese population, SF-36 scores before and at 3 months after TKI treatment also showed no significant differences [11]. We further analyzed associations between clinicopathologic characteristics and SF-36 scores. There were no significant associations between SF-36 scores and age, sex, MSKCC grade, IMDC risk, or treatment. However, the grade of AEs (grade 1–2 vs. grade 3–4) was independently associated with QoL at the physical functioning (P = 0.017) and bodily pain dimensions (P = 0.005). This result suggested that timely management of AEs of targeted therapy may eliminate their impact on QoL. Furthermore, a Japanese study observed that the 2-day on/1-day off dosing schedule of sunitinib significantly improved QoL compared with the 4-week on/2-week off dosing schedule [29]. Hence, comparison of the efficacy of TKIs with different dosing schedules is warranted for further validation.
This study has several limitations. First, this was a retrospective study at a single center with limited number of patients, which may cause potential bias of the inferences. The study population was limited to Chinese patients. In addition, to be eligible for the analysis, patients needed to have survived beyond 3 months so as to complete the SF-36 questionnaire at both baseline and 3 months after treatment. Second, a few patients who were intolerant to the drugs had dose titrations, and some switched to second-line targeted therapy, which might have influenced their QoL and survival. Third, the median follow-up was relatively short.
Conclusions
We demonstrated that sorafenib and sunitinib, as first-line treatment agents, had comparable efficacy on mRCC. Grade 3–4 AEs of TKIs may impair QoL at role-physical and body pain dimensions. Apart from this, neither of the agents had a negative impact on the overall QoL. Multi-center studies with long-term follow-up are warranted to further validate the findings of the present study.
Authors’ contributions
WC, WK, and BD conceptualised and designed the study. JZ, YC, and WX were involved in data collection and analysis. YH, LZ, and JH were involved in interpretation of results and manuscript writing. All authors read and approved the final manuscript.
Acknowledgements
The authors acknowledge Dr. Amit Bhat (Indegene Pvt Ltd, Bangalore, India) for providing medical writing support for this manuscript.
Competing interests
The authors declare that they have no competing interests.
Funding
This study was supported by the National Natural Science Foundation of China (Nos. 81402084, 81472378), the Shanghai Municipal Commission of Health and Family Planning (No. 2013SY027), and the Incubating Program for Clinical Research and Innovation of Renji Hospital (No. PYXJS16-008).
Abbreviations
- AEs
adverse events
- CI
confidence interval
- BP
bodily pain
- CBC
complete blood count
- CT
computed tomography
- GH
general health
- KPS
Karnofsky performance status
- MH
mental health
- mRCC
metastatic renal cell carcinoma
- MRI
magnetic resonance imaging
- MSKCC
Memorial Sloan-Kettering Cancer Centre
- OS
overall survival
- PF
physical functioning
- PFS
progression-free survival
- QoL
quality of life
- RE
role-emotional
- RECIST
response evaluation criteria in solid tumors
- RP
role-physical
- SD
standard deviation
- SF
social functioning
- SF-36
36-Item Short Form Health Survey Questionnaire
- TKIs
tyrosine kinase inhibitors
- VT
vitality
- IMDC
International Metastatic Renal Cell Carcinoma Database Consortium
Contributor Information
Wen Cai, Email: caiwen@hotmail.com.
Wen Kong, Email: dr_kongwen@sina.cn.
Baijun Dong, Email: dongbaijun@hotmail.com.
Jin Zhang, Email: med-zhangjin@vip.sina.com.
Yonghui Chen, Email: cyh1488@163.com.
Wei Xue, Email: xuewei@renji.com.
Yiran Huang, Email: yrhuangrenji@163.com.
Lixin Zhou, Phone: +86-13701961919, Email: zhou_li_xin@hotmail.com.
Jiwei Huang, Phone: +86-13651682825, Email: jiweihuang@outlook.com.
References
- 1.Gore ME, Szczylik C, Porta C. Safety and efficacy of sunitinib for metastatic renal-cell carcinoma: an expanded-access trial. Lancet Oncol. 2009;10:757–763. doi: 10.1016/S1470-2045(09)70162-7. [DOI] [PubMed] [Google Scholar]
- 2.Patard JJ, Pignot G, Escudier B, Eisen T, Bex A, Sternberg C, et al. ICUD-EAU International Consultation on Kidney Cancer 2010: treatment of metastatic disease. Eur Urol. 2011;60:684–690. doi: 10.1016/j.eururo.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 3.Harshman LC, Xie W, Bjarnason GA, Knox JJ, MacKenzie M, Wood L, et al. Conditional survival of patients with metastatic renal-cell carcinoma treated with VEGF-targeted therapy: a population-based study. Lancet Oncol. 2012;13:927–935. doi: 10.1016/S1470-2045(12)70285-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heng DY, Xie W, Regan MM, Warren MA, Golshayan AR, Sahi C, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27:5794–5799. doi: 10.1200/JCO.2008.21.4809. [DOI] [PubMed] [Google Scholar]
- 5.Choueiri TK, Duh MS, Clement J, Brick AJ, Rogers MJ, Kwabi C, et al. Angiogenesis inhibitor therapies for metastatic renal cell carcinoma: effectiveness, safety and treatment patterns in clinical practice-based on medical chart review. BJU Int. 2010;105:1247–1254. doi: 10.1111/j.1464-410X.2009.08972.x. [DOI] [PubMed] [Google Scholar]
- 6.Ye DW, Zhang HL. Critical appraisal of sorafenib in the treatment of Chinese patients with renal cell carcinoma. OncoTargets Ther. 2014;7:925–935. doi: 10.2147/OTT.S41828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akaza H, Tsukamoto T, Murai M, Nakajima K, Naito S. Phase II study to investigate the efficacy, safety, and pharmacokinetics of sorafenib in Japanese patients with advanced renal cell carcinoma. Jpn J Clin Oncol. 2007;37:755–762. doi: 10.1093/jjco/hym095. [DOI] [PubMed] [Google Scholar]
- 8.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ. Targeted therapies for metastatic renal cell carcinoma: an overview of toxicity and dosing strategies. Oncologist. 2008;13:1084–1096. doi: 10.1634/theoncologist.2008-0120. [DOI] [PubMed] [Google Scholar]
- 9.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 10.Li L, Wang HM, Shen Y. Chinese SF-36 Health Survey: translation, cultural adaptation, validation, and normalisation. J Epidemiol Community Health. 2003;57:259–263. doi: 10.1136/jech.57.4.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miyake H, Harada K, Inoue TA, Fujisawa M. Assessment of health-related quality of life in Japanese patients with metastatic renal cell carcinoma during treatment with tyrosine kinase inhibitors. Med Oncol. 2014;31:190. doi: 10.1007/s12032-014-0190-6. [DOI] [PubMed] [Google Scholar]
- 12.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–731. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 13.Hutson TE, Lesovoy V, Al-Shukri S, Stus VP, Lipatov ON, Bair AH, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14:1287–1294. doi: 10.1016/S1470-2045(13)70465-0. [DOI] [PubMed] [Google Scholar]
- 14.Park SJ, Lee JL, Park I, Park K, Ahn Y, Ahn JH, et al. Comparative efficacy of sunitinib versus sorafenib as first-line treatment for patients with metastatic renal cell carcinoma. Chemotherapy. 2012;58:468–474. doi: 10.1159/000346484. [DOI] [PubMed] [Google Scholar]
- 15.Sheng X, Chi Z, Cui C, Si L, Li S, Tang B, et al. Efficacy and safety of sorafenib versus sunitinib as first-line treatment in patients with metastatic renal cell carcinoma: largest single-center retrospective analysis. Oncotarget. 2016;7:27044–27054. doi: 10.18632/oncotarget.7395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo J, Ma J, Sun Y, Qin S, Ye D, Zhou F, He Z, et al. Chinese guidelines on the management of renal cell carcinoma (2015 edition) Chin Clin Oncol. 2016;5(1):12. doi: 10.3978/j.issn.2304-3865.2015.11.01. [DOI] [PubMed] [Google Scholar]
- 17.Common Terminology Criteria for Adverse Events v4.0 (CTCAE). Washington, DC: US Department of Health and Human Services. 2010. http://www.hrc.govt.nz/sites/default/files/CTCAE%20manual%20-%20DMCC.pdf. Accessed 15 Aug 2015.
- 18.Duffaud F, Therasse P. New guidelines to evaluate the response to treatment in solid tumors. Bull Cancer. 2001;87:881–886. [PubMed] [Google Scholar]
- 19.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22(3):454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 20.Heng DY, Xie W, Regan MM, Harshman LC, Bjarnason GA, Vaishampayan UN, et al. External validation and comparison with other models of the International Metastatic Renal-Cell Carcinoma Database Consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi: 10.1016/S1470-2045(12)70559-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon-alfa in metastatic renal cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 22.Porta C, Szczylik C, Bracarda S, Hawkins R, Bjarnason GA, Oudard S, et al. Short and long-term safety with sunitinib in an expanded access trial in matastatic renal cell carcinoma (mRCC) J Clin Oncol. 2008;26(15 Suppl):5114. doi: 10.1200/jco.2008.26.15_suppl.5114. [DOI] [Google Scholar]
- 23.Van der Veldt AA, Boven E, Helgason HH, van Wouwe M, Berkhof J, de Gast G, et al. Predictive factors for severe toxicity of sunitinib in unselected patients with advanced renal cell cancer. Br J Cancer. 2008;99:259–265. doi: 10.1038/sj.bjc.6604456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antoun S, Lanoy E, Iacovelli R, Albiges-Sauvin L, Loriot Y, Merad-Taoufik M, et al. Skeletal muscle density predicts prognosis in patients with metastatic renal cell carcinoma treated with targeted therapies. Cancer. 2013;119:3377–3384. doi: 10.1002/cncr.28218. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann E, Gerss J, Bierer S, Köpke T, Bolenz C, Hertle L, et al. Pre-treatment global quality of health predicts progression free survival in metastatic kidney cancer patients treated with sorafenib or sunitinib. J Cancer Res Clin Oncol. 2009;135:61–67. doi: 10.1007/s00432-008-0438-7. [DOI] [PubMed] [Google Scholar]
- 26.Cella D, Escudier B, Rini B, Chen C, Bhattacharyya H, Tarazi J, et al. Patient-reported outcomes for axitinib vs sorafenib in metastatic renal cell carcinoma: phase III (AXIS) trial. Br J Cancer. 2013;108:1571–1578. doi: 10.1038/bjc.2013.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cella D, Michaelson MD, Bushmakin AG, Cappelleri JC, Charbonneau C, Kim ST, et al. Health-related quality of life in patients with metastatic renal cell carcinoma treated with sunitinib vs interferon-alpha in a phase III trial: final results and geographical analysis. Br J Cancer. 2010;102:658–664. doi: 10.1038/sj.bjc.6605552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Szczylik C, Cella D, Eisen T, Shah S, Laferriere N, Scheuring U, et al. Comparison of kidney cancer symptoms and quality of life (QoL) in renal cell cancer (RCC) patients receiving sorafenib vs interferon-alpha. J Clin Oncol. 2008;26:9603. doi: 10.1200/jco.2008.26.15_suppl.9603. [DOI] [Google Scholar]
- 29.Miyake H, Harada K, Miyazaki A, Fujisawa M. Improved health-related quality of life of patients with metastatic renal cell carcinoma treated with a 2 weeks on and 1 week off schedule of sunitinib. Med Oncol. 2015;32:78. doi: 10.1007/s12032-015-0528-8. [DOI] [PubMed] [Google Scholar]


