Abstract
Cellular senescence is a complex biological process that leads to irreversible cell-cycle arrest. Various extrinsic and intrinsic insults are associated with the onset of cellular senescence and frequently accompany genomic or epigenomic alterations. Cellular senescence is believed to contribute to tumor suppression, immune response, and tissue repair as well as aging and age-related diseases. Long noncoding RNAs (lncRNAs) are >200 nucleotides long, poorly conserved, and transcribed in a manner similar to that of mRNAs. They are tightly regulated during various cellular and physiological processes. Although many lncRNAs and their functional roles are still undescribed, the importance of lncRNAs in a variety of biological processes is widely recognized. RNA-binding proteins (RBPs) have a pivotal role in posttranscriptional regulation as well as in mRNA transport, storage, turnover, and translation. RBPs interact with mRNAs, other RBPs, and noncoding RNAs (ncRNAs) including lncRNAs, and they are involved in the regulation of a broad spectrum of cellular processes. Like other cell fate regulators, lncRNAs and RBPs, separately or cooperatively, are implicated in initiation and maintenance of cellular senescence, aging, and age-related diseases. Here, we review the current understanding of both lncRNAs and RBPs and their association with oxidative stress, senescence, and age-related diseases.
1. Introduction
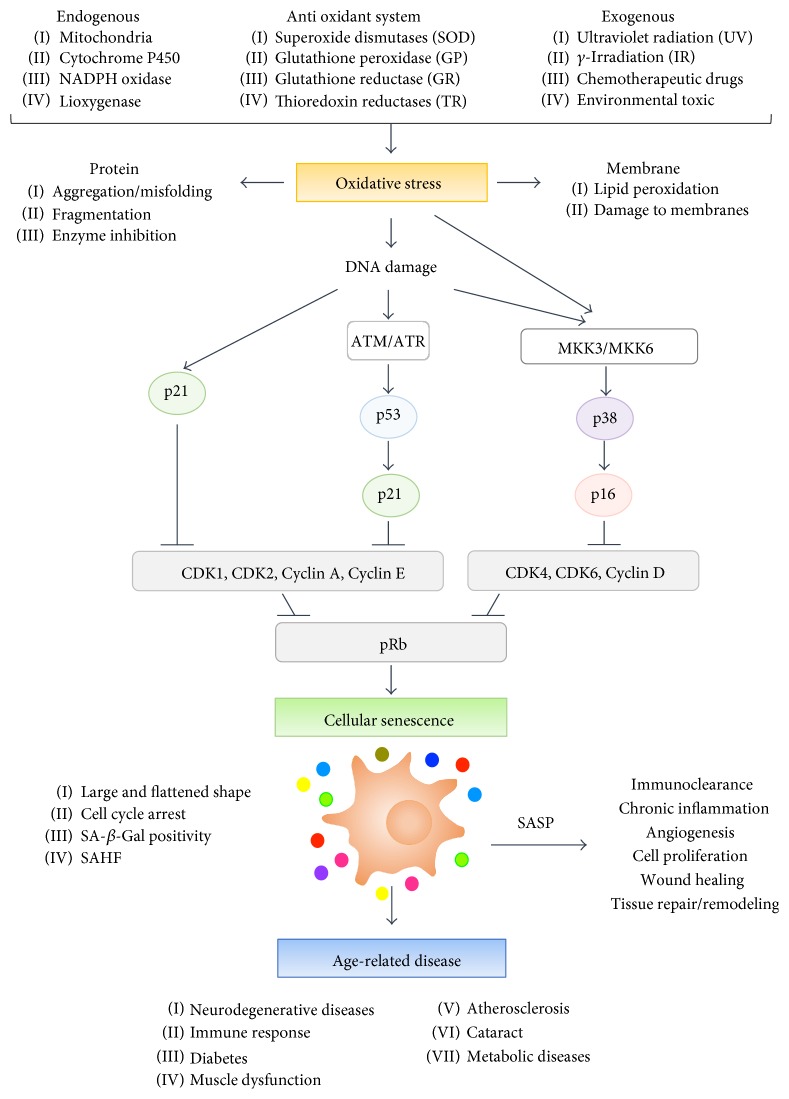
Cellular senescence is a biological process in which cells cease growth permanently. Hayflick and Moorehead firstly described replicative senescence (RS), which is an exhaustion of replicative potential in human diploid fibroblasts after continuous cultivation [1, 2]. More than a half-century from that first description that early concept of cellular senescence has been remarkably extended in recent days. RS can be considered a defense mechanism that limits proliferation potential of older cells containing irreparable and dangerous mutations. In contrast to RS, which is driven by telomere shortening, cells can prematurely undergo senescence in response to diverse forms of cellular stresses. Stress-induced cellular senescence (SIPS) can be triggered by DNA damage, oncogenic mutations, strong mitotic signals, genomic instability, lack of nutrients, improper cell contacts, and many other factors [3]. An excess of reactive oxygen species (ROS) specifically participates in induction and maintenance of cellular senescence. ROS including superoxide anion, hydrogen peroxide, and hydroxyl radicals are inevitably generated as byproducts of aerobic metabolism and are also derived from radiation, chemotherapeutic agents, carcinogens, and other intrinsic and extrinsic factors. Physiological ROS level regulates signal transduction, gene expression, and proliferation. However, ROS shifts from physiological to pathophysiological level are referred to as oxidative stress. Oxidative stress results in damage to lipids, proteins, and nuclear and mitochondrial DNA and is involved in various changes, such as epigenetic modification and signaling pathways, finally resulting in cellular senescence [4]. Cellular senescence programs are induced by persistent activation of the p53/p21 stress response pathway and/or the RB/p16 tumor suppressor pathway. Senescent cells are characterized by a variety of phenotypes including enlarged and flattened morphology, senescence-associated β-galactosidase (SA-β-Gal) activity, formation of senescence-associated heterochromatin foci (SAHF), and altered gene expression and protein processing [5]. In the last decade, many research groups have demonstrated that anticancer drugs and ionizing radiation can effectively induce SIPS in cancer cells in vitro and in vivo [6]. Currently, in accord with the role of senescence-associated secretory phenotypes (SASP) in tumor prevention, therapy-induced senescence is considered a powerful strategy for cancer treatment [7]. Although senescent cells irreversibly lose their dividing capability, they are metabolically active and secrete a myriad of SASP-related factors including cytokines, chemokines, growth factors, and proteases [1]. SASP can influence multiple facets of tissue microenvironments and contribute to the inflammatory response and many other aging phenotypes. Since cellular senescence can potentially contribute to various physiological and pathological aging processes, cellular senescence is an important hallmark of human aging and an attractive target for therapeutic exploitation [6–10]. Aging is the gradual deterioration of the physical, mental, and biological state of an organism with time, eventually resulting in increased vulnerability to death. Moreover, aging is the irreversible loss of physiological integrity and the major risk factor in various age-related diseases such as neurodegenerative diseases, immune response, metabolic diseases, muscle dysfunction, atherosclerosis, and cataract. Various factors and processes have been implicated in the initiation, regulation, and progression of the aging process. An “oxidative stress theory of aging” was proposed long ago, and oxidative stress is primarily associated with cellular senescence and aging. Several studies have shown that transcriptional events are implicated in the regulation of gene expression during oxidative stress responses, cellular senescence, and the pathogenesis of age-related diseases [11–14]. Activation of transcriptional factors such as p53, NF-κB, HIF-1α, CEBP, STAT, and E2F1 governs mRNA expression via promoter activation, microRNA induction, and epigenetic regulation in senescence, aging, and age-related disease [11]. Schematic relationships among DNA damage and oxidative stress, cellular senescence, and age-related diseases are shown in Figure 1. Herein, we revisit current knowledge of the mechanistic, functional, and pathological roles of long noncoding RNAs (lncRNAs) and RNA-binding proteins (RBPs) that are primarily related to DNA damage, oxidative stress, cellular senescence, aging, and age-related diseases. In this review, we will not describe lncRNAs and RBPs associated with telomeres and cancer because those topics have already been extensively introduced in other reviews [15–18].
Figure 1.
Schematic relationships among DNA damage and oxidative stress, cellular senescence, and age-related diseases.
2. Long Noncoding RNAs
The lncRNAs are transcripts more than 200 nucleotide long that have no protein-coding potential. Moreover, they are poorly conserved, transcribed from the intergenic and intronic regions of genome primarily by polymerase II, 5′ methyl-capped, and polyadenylated in manner similar to that of mRNAs [16]. The lncRNAs modulate gene expression at all regulation levels: transcriptional, posttranscriptional, translational, and posttranslation. They can regulate gene expression via interaction with chromatin modifiers, RBPs, DNA, and RNA [15]. To date, many lncRNAs have been characterized. Most of those are nuclear localized and act as enhancer RNAs (eRNAs), chromatin modifiers via recruitment of various DNA methyltransferases, and histone modifiers via Polycomb repressive complexes or histone methyltransferases [16]. Some lncRNAs are transported to the cytoplasm and regulate translation or mRNA stability. Moreover, lncRNAs affect key cellular processes such as proliferation, differentiation, quiescence, senescence, stress and immune response, and many other cellular functions related to the biology of aging [19].
2.1. DNA Damage Response and Oxidative Stress
2.1.1. LincRNA-p21
LincRNA-p21 is 3.1 kb long and is transcribed from the opposite strand to p21 (CDKN1A) in a p53-dependent manner [20]. LincRNA-p21, which is also induced by hypoxia and/or hypoxia inducible factor-1α (HIF-1α), is able to bind HIF-1α and VHL, and it disrupts the VHL–HIF-1α interaction. This disassociation attenuates VHL-mediated HIF-1α ubiquitination and causes HIF-1α accumulation. These results indicate a positive feedback loop between HIF-1α and lincRNA-p21 under hypoxia [21]. In addition, LincRNA-p21 is highly inducible by UVB through a p53-dependent pathway and plays key role in the UVB-induced apoptotic pathway [22].
2.1.2. LincRNA-RoR
LincRNA-RoR, a 2.6 kb long transcript, was first described as having potentially important functions in embryonic stem cells and induced pluripotent stem cells (iPSCs) [19]. LincRNA-RoR regulates genes involved in the p53 response, such as responses to oxidative stress and DNA damage [23]. Depletion of p53 can partially rescue the apoptotic phenotype by ablation of lincRNA-RoR. LincRNA-RoR dramatically represses DNA damage-induced p53 compared to that in unstressed cells. Depletion of lincRNA-RoR did not regulate p53 mRNA levels, suggesting posttranscriptional regulation of p53. Mechanistically, lincRNA-RoR has a 28-base heterogeneous nuclear ribonucleoprotein I- (hnRNP I-) binding motif and directly interacts with phosphorylated hnRNP I in the cytoplasm. The interaction between lincRNA-RoR and phosphorylated hnRNP I directly represses p53 translation and results in the modulation of cell-cycle progression and apoptosis. Thus, lincRNA-RoR and p53 act within an autoregulatory feedback loop in response to cellular stress [24]. A recent study revealed that lincRNA-RoR can epigenetically regulate the expression of TESC by recruiting G9A methyltransferase in the TESC promoter [25].
2.1.3. Pint
Pint (p53-induced noncoding transcript), previously named lincRNA-Mkln1, has highly conserved canonical p53-binding motifs in the promoter and is a transcriptional target of p53 [20]. Pint is a nuclear lincRNA and is transcribed from an intergenic region on mouse chromosome 6. Pint has three p53 response elements and is directly regulated by p53 upon DNA damage [26]. Depletion of Pint significantly decreases cell proliferation, and overexpression of Pint conversely increases cell growth. Pint directly interacts with Polycomb repressive complex 2 (PRC2) and represses expression of PRC2 targeting genes via H3K27 trimethylation. PINT, the pint human ortholog, is also regulated by p53. However, overexpressed PINT diminishes tumor cell proliferation, indicating both analogy and difference between murine Pint and human ortholog PINT [26].
2.1.4. PANDA
To detect functional noncoding RNAs (ncRNAs) in the regulatory region of human cell-cycle genes, ultrahigh-density array fabrication was performed and the lncRNA PANDA (P21-associated ncRNA DNA damage-activated) was identified at the CDKN1A locus [27]. PANDA is specifically induced by DNA damage in a p53-dependent manner. PANDA is a 5′-capped, polyadenylated lncRNA located approximately 4.5 kb upstream of the CDKN1A transcriptional start site. In human fibroblasts treated with doxorubicin, PANDA prevents NF-YA activation, through its association with NF-YA, finally suppressing transcription of proapoptotic genes. Thus, PANDA induced by DNA damage impedes apoptosis through recruitment of NF-YA [27]. Another study reported that PANDA can stabilize p53 proteins in response to DNA damage [28]. In addition, it was revealed that silencing of PANDA causes G1 arrest via an increase in the mRNA level of cyclin-dependent kinase inhibitor p18 [29].
2.1.5. LncRNA-JADE
LncRNA-JADE is induced by ATM-NF-кB signaling and is mainly localized in the nucleus in the DNA damage response (DDR) [30]. In response to DDR, increased lncRNA-JADE interacts with breast cancer type 1 susceptibility protein (Brca1) and induces expression of Jade1, a major component of human acetylase binding to ORC 1 (HBO1) histone acetylation complex. Consequently, depletion of lncRNA-JADE renders sensitivity to DNA damaging drugs through the functional link between DDR and histone H4 acetylation in the DDR. [30].
2.1.6. H19
H19 was first described as an imprinted ncRNA transcript at the Igf2 locus. A number of studies have reported that H19 is upregulated in both primary and metastatic tumors and is closely involved in migration, angiogenesis, and inflammatory diseases [31]. HIF-1α and p53 are involved in the upregulation of H19 in hypoxic cancer cells [32]. Recently, it was demonstrated that H19 expression is elevated under hypoxic conditions in mesenchymal stem cells [33]. In addition, overexpression of H19 in diabetic rats can attenuate oxidative stress, inflammation, and apoptosis [34].
2.1.7. ANRIL
ANRIL (antisense noncoding RNA in the INK locus) is transcribed in the antisense direction to the INK4B-ARF-INK4A locus and is transcriptionally upregulated by the transcription factor E2F1 in an ATM-dependent manner after DNA damage. Such elevated levels of ANRIL suppress the expression of INK4A-ARF-INK4B in the late-DDR stage, allowing negative feedback to the DDR. Thus, ANRIL helps the cell to return to a normal status at the completion of DNA repair [35].
2.1.8. LncRNA-LET
LncRNA-LET (lncRNA low expression in tumor) transcripts are underexpressed in tumor tissues compared to their expression in paired nontumor tissues [36]. Moreover, LncRNA-LET is downregulated by hypoxia-induced histone deacetylase 3 (HDAC3) under hypoxic conditions. LncRNA-LET is associated with degradation of nuclear factor of activated T cells 90 kDa (NF90) protein via the regulation of ubiquitin-proteasome pathway. Since NF90 stabilizes HIF-1α mRNA without altering HIF-1α transcriptional activity, lncRNA-LET finally decreases HIF-1α stability due to its association with NF90. These findings illustrate that lncRNA-LET could be a key regulator of hypoxia signaling [36].
2.1.9. LINK-A
LINK-A (long intergenic noncoding RNA for kinase activation) is 1.5 kb long and mainly localized in the cytoplasm [37]. LINK-A facilitates breast tumor kinase (BRK) activation through the recruitment of BRK to the EGFR : GPNMB heterodimeric complex upon HB-EGF stimulation. Consequently, activated BRK induces phosphorylation of HIF-1α at Tyr565 and inhibits hydroxylation of HIF-1α, finally resulting in HIF-1α stabilization. In addition, LINK-A interacts with leucine-rich repeat kinase 2 (LRRK2) and enhances phosphorylation of HIF-1α at Ser797. Phosphorylation of Ser797 increases transcriptional activation of HIF-1α via HIF-1α–p300 interaction. These events illustrate the magnitude and diversity of cytoplasmic lncRNA LINK-A in signal transduction related to HIF-1α under normoxic conditions [37].
2.2. Cellular Senescence and Aging
2.2.1. 7SL
7SL is a 300 bp long transcript and an RNA component of signal recognition proteins (SRP) [38]. 7SL is widely upregulated in cancer tissues and involved in cell proliferation. 7SL decreases p53 translation and accumulation by interacting with the 3′-untranslated region (3′-UTR) of TP53 mRNA, which encodes tumor suppressor p53. Depletion of 7SL increases the occupancy of HuR to TP53 mRNA and p53 production. 7SL-depleted cells undergo cellular senescence and autophagy, indicating that 7SL promotes cell growth via p53 suppression [39].
2.2.2. HOTAIR
HOTAIR (HOX antisense intergenic RNA) was first identified as HOX lncRNA located in the HOXC locus through transcriptomic analyses of HOX loci [40]. This antisense lncRNA increases the occupancy of Suz12 on the HOXD locus and silences HOXD locus genes by changing the chromatin structure [40]. HOTAIR enhances cancer progression and malignancy by leading to altered H3K27 methylation due to retargeting of PRC2 [41]. Depletion of HOTAIR induces cell-cycle arrest in various cancer types. In addition, HOTAIR can contribute to cellular senescence via a positive feedback loop cascade of an NF-кB–HOTAIR axis [42].
2.2.3. UCA1
UCA1 (urothelial carcinoma-associated 1), an lncRNA with a length of 1.4 kb, was first identified in bladder cell carcinoma [43]. UCA1, a direct target of coactivator of AP1 and estrogen receptor α (CAPERα)/T-box3 (TBX3) repression, sequesters hnRNP I, which suppresses transcription of CDKN2A and destabilizes CDKN2A mRNA [44]. Oncogenic stress dissociates the CAPERα/TBX3 corepressor and activates UCA1. CAPERα/TBX3 and UCA1 coordinately induce oncogene-induced senescence (OIS). In addition, UCA1 can bind with hnRNP I and competitively inhibit hnRNP I binding to p27 mRNA [45]. hnRNP I enhances translation of p27 mRNA, and there is a negative correlation between p27 expression and UCA1 level.
2.2.4. LincRNA-p21
Overexpressed lincRNA-p21 increases p21 expression at both mRNA and protein levels, and it impedes cell-cycle progression [46]. LincRNA-p21 is necessary for the recruitment of hnRNP K to the p53 response element and for increasing the binding efficiency of p53 on the p21 promoter region. Moreover, lincRNA-p21 affects the G1/S checkpoint and p21 levels through deregulated expression and altered chromatin state of some Polycomb target genes. Thus, lincRNA-p21 is required for the positive regulation of p21 expression and finally is involved in cellular senescence [46].
2.2.5. ANRIL
ANRIL is a 3.8 kb long lncRNA transcribed in an antisense orientation from the INK4B/ARF/INK4A gene cluster, and it overlaps with the promoter of p14/ARF and the two exons of p15/CDKN2B [47]. ANRIL is required for the recruitment of chromobox (CBX7), a component of PRC1, to the INK4B/ARF/p16 gene locus. This complex exhibits high-affinity binding to methylated histone H3 at lysine 27 (H3K27me) and represses the transcription of INK4b/ARF/INK4a [48]. Moreover, depletion of ANRIL disrupts the binding of suppressor of zeste 12 protein homolog (Suz12), a component of PRC2, to INK4B locus, and increases the expression of p15 [49, 50]. Recent studies have reported that ANRIL promotes silencing of KLF2 and P21 transcription via epigenetic silencing [51]. Such epigenetic transcriptional repression of INK4B/ARF/INK4A by ANRIL, which is associated with senescence, was reviewed by Aguilo et al. [52].
2.2.6. ANRASSF1
ANRASSF1 (antisense intronic noncoding RASSF1) is an intronic lncRNA transcribed from the antisense to RAS-association domain family member 1A (RASSF1A) gene [53]. RASSF1A, a tumor suppressor gene, is associated with cell-cycle arrest and senescence via p53-independent regulation of p21 [54]. Highly expressed ANRASSF1 recruits PRC2 to the RASSF1A promoter and increases the H3K27me3 level, resulting in decreased RASSF1A expression. Therefore, ANRASSF1 mediates cellular senescence through the epigenetic inactivation of the RASSF1A gene [53].
2.2.7. PANDA
PANDA (P21 associated ncRNA DNA damage-activated) is capable of interacting with scaffold-attachment-factor A (SAFA) [55]. SAFA is a nuclear protein that is able to bind DNA and RNA, including ncRNA, and is involved in transcriptional and posttranscriptional regulation by acting as an adaptor molecule for DNA-RNA-protein interactions [56]. In proliferating cells, the SAFA and PANDA interaction recruits Polycomb repressive complex 1 (PRC1) and PRC2 complexes to senescence target genes including CDKN1A in order to silence their expression [55]. Thus, PANDA depletion leads to senescence phenotypes by derepression of p21 due to disruption of SAFA-PANDA-PRC interactions. However, in senescent cells, PANDA sequesters transcription factor NF-YA and limits the expression of NF-YA-E2F-coregulated proliferation-promoting genes. Therefore, PANDA levels modulate cell fates to enter or exit from senescence [55].
2.2.8. FAL1
FAL1 (focally amplified lncRNA on chromosome 1) was identified from a genome-wide analysis of somatic copy number alterations [57]. FAL1 interacts with epigenetic repressor BMI1 protein, a subcomponent of PRC1, and increases BMI1 stability. Thus, FAL1 can negatively regulate a large number of genes such as CDKN1A, FAS, and BTG2. In addition, FAL1 promotes tumor proliferation and represses senescence primarily by decreasing CDKN1A transcription [58].
2.2.9. MIR31HG
Whereas MIR31HG lncRNA is upregulated during OIS, its depletion promotes p16-dependent senescence phenotypes [59]. MIR31HG interacts with the INK4A and MIR31HG genomic loci and mediates repression of the INK4A locus with Polycomb group (PcG) proteins. MIR31HG plays a role during OIS as a transcriptional regulator of p16 via direct interaction with PcG proteins [59].
2.2.10. SALNR
SALNR (senescence-associated long noncoding RNA) expression is downregulated in senescent human fibroblasts. SALNR interacts with NF90, a RNA-binding protein involved in microRNA (miRNA) biogenesis, and regulates its nuclear localization. SALNR and the NF90 complexes impede premature senescence through the regulation of senescence-associated miRNAs, specifically miR-181a and miR-22 [60].
2.2.11. VAD
VAD is a vlincRNA (very long intergenic ncRNA) that is differentially expressed in RAF-induced senescence and is localized in the chromatin. VAD is involved in the maintenance of senescence features. Mechanistically, VAD modulates chromatin structure in cis and increases the expression of INK4 genes in trans. VAD decreases the occupancy of the repressive histone variant H2A.Z at INK4 promoters during senescence induction [61].
2.3. Age-Related Diseases
2.3.1. Neurodegenerative Diseases
(1) Alzheimer's Disease. Alzheimer's disease (AD) is the most common neurodegenerative disease and accounts for the majority of dementia cases. Amyloid β (Aβ) plaques and neurofibrillary tangles are the two primary pathological hallmarks of AD. The amyloid cascade hypothesis suggests that deposition of Aβ might be cause of neuronal dysfunction and death of brain tissue in AD. Recently, the cleavage patterns of amyloid precursor protein (APP) to Aβ peptides (Aβ1–40 and Aβ1–42) by secretases, small oligomers of Aβ (2~12 peptides), Aβ concentration, and Aβ stability have been proposed as important factors in AD [62].
BC200 is a 200 bp long RNA pol III-transcribed lncRNA that is predominantly expressed in the brain [63]. BC200 is downregulated in normal aged brains, but BC200 is significantly upregulated in AD brains. Specifically, BC200 is highly expressed in AD-related regions (e.g., Broadmann's area 9) compared to its expression in nonrelated regions (e.g., area 17) [64].
BACE1-AS (BACE1-antisense transcript) is a 2 kb long transcript from the antisense strand of β-secretase-1 (BACE1) and is a crucial enzyme in AD pathology. BACE1-AS regulates BACE1 mRNA and protein expression in vitro and in vivo. In response to cell stress, elevated BACE1-AS increases BACE1 mRNA and protein levels due to RNA duplex formation, generating additional Aβ 1–42 peptides [65]. Moreover, modulation of BACE1 and the BACE1-AS transcript can participate in the alteration of oligomeric Aβ aggregation pattern and Aβ-related hippocampal neurogenesis [66].
NDM29 (neuroblastoma differentiation marker 29) is a cytoplasmic lncRNA transcribed by polymerase (pol) III. NDM29 is highly expressed in neuroblastoma cells and is involved in neuroblastoma maturation [67]. In addition, elevated NDM29 expression is detected in the brain of AD patients. NDM29-dependent cell maturation induces APP synthesis and results in an increase of Aβ secretion. Moreover, an increase in the production of copies of NDM29 transcripts can be driven by inflammatory stimuli [68].
17A is a 159 bp long lncRNA synthesized by RNA pol III that induces an increase of GABA B2 receptor splice variant B, which affects GABA-B function. Thus, 17A impairs GABA-B signaling and might enhance Aβ secretion. In addition, 17A is upregulated in AD compared to its level in control tissues [69]. Other lncRNAs such as 51A, NAT-Rad18, and GNDFOS might also be involved in AD [70, 71].
(2) Parkinson's Disease. Parkinson's disease (PD) is a common and complex neurodegenerative disease characterized by defects in the body's motor functions (slow or lack of movements, and tremor). The classical features of PD are associated with Lewy bodies and loss of dopaminergic neurons in the substantia nigra pars compacta in the midbrain. The resultant deficiency of dopamine ultimately induces movement disorder, which is a characteristic of PD [72].
AS Uchl1 (antisense to mouse ubiquitin carboxy-terminal hydrolase L1) is a 1.2 kb lncRNA transcribed from the opposite strand of the ubiquitin carboxy-terminal hydrolase L1 (Uchl1) gene and induces UChl1 translation. AS Uchl1 is expressed in mesencephalic regions, which are degenerated in PD. AS Uchl1 is regulated by Nur11, a major transcription factor functioning in the differentiation and maintenance of dopaminergic neurons. Expressions of AS Uchl1 and UCHL1 have been decreased in PD models in vitro and in vivo [73].
NaPINK1 is transcribed from the antisense direction of the PINK1 gene, which is implicated in PD through an association with unbalanced mitochondrial homeostasis. As naPINK1 is able to stabilize PINK1 splice variant (svPINK1) expression in neurons via a dsRNA-mediated mechanism, naPINK1 might be involved in PD through regulation of the PINK1 locus [71, 74].
(3) Huntington's Disease. Huntington's disease (HD) is a dominantly inherited disease characterized by chorea, psychiatric problems, and dementia. HD is caused by mutation in the huntingtin (HTT) gene. Expansion of the CAG-triplet repeat sequence within the first exon of HTT results in abnormal protein production, which gradually leads to death of brain cells [75].
TUG1 (taurine upregulated gene 1) was first identified in a screen for genes upregulated by taurine in developing retinal cells [76] and, subsequently, in a genome analysis to examine lncRNAs physically associated with chromatin-modifying complexes [77]. Depletion of TUG1 increases the phenotypes of apoptosis [76], and TUG1 expression is elevated in HD [78]. Mechanistically, TUG1, a direct transcriptional target of p53, combines with enhancer of zeste homolog 2 (EZH2), a component of the PRC2 complex, and epigenetically regulates gene expression. TUG1 and EZH2 bind to the promoter of homeobox B2 (HOXB7) and represses HOXB7 expression [79].
MEG3 is highly expressed in adult human and mouse brains and is differentially expressed in HD patients. MEG3 can associate with the PRC2 complex and is found in the chromatin region in the nucleus, suggesting that MEG3 might be involved in epigenetic regulation in HD [16, 78]. Other lncRNAs such as HAR1 (human accelerated region 1), NEAT1 (nuclear paraspeckle assembly transcript 1), and DGCR5 (DiGeorge syndrome critical region gene 5) also exhibit altered expression in HD patients, as shown by microarray studies [16, 78].
HTTAS (huntingtin antisense) is a natural antisense transcript at the HD CAG repeat. HTTAS is mainly spliced into HTTAS-V1 (exons 1 and 3) and HTTAS-V2 (exons 2 and 3). HTTAS-V1 expression is reduced in human HD frontal cortex, and its overexpression negatively regulates HTT transcription [80].
2.3.2. Immune Response
The immune response is a wide variety of physiological and pathological processes originating from immune system activation. It is triggered by pathogens, antigens, tissues injury, and other noxious stimulations. The innate immune response provides immediate defense against infection and is evolutionarily conserved. The adaptive immune response is highly specific to particular pathogens and provides long-lasting protection. Adaptive immune responses are mainly mediated antibody and cell-mediated immune responses. The inflammatory response is considered an innate immune response. Inflammation functions to eliminate the initial cause of the original insult and to initiate tissue repair, which are regulated by immune mediators including cytokines, chemokines, and soluble inflammatory proteins [81].
THRIL (TNFα- and hnRNP L-related immunoregulatory lincRNA) is an approximately 2 kb lncRNA that changes expression upon activation of innate immune signaling in macrophages. THRIL recruits heterogeneous nuclear ribonucleoprotein L (hnRNP L) to the TNFα promoter and increases the secretion of TNFα, an inflammatory cytokine. An increase in TNFα downregulates THRIL expression via a negative feedback mechanism. Moreover, THRIL is associated with maintaining expression of many innate immunity-associated genes [82].
Lnc-DC is exclusively expressed in human conventional dendritic cells and is involved in dendritic cell (DC) differentiation and DC capacity to stimulate T cell activation. Lnc-DC prevents signal transducer and activator of transcription 3 (STAT3) dephosphorylation by SHP1 through a direct association with STAT3. Lnc-DC is known as a specific regulator of DC differentiation and function [83].
Lnc-IL7R, which overlaps with the 3′-UTR of interleukin-7 receptor α (IL7R) gene, shows altered expression in response to LPS stimulation. Lnc-IL7R functionally diminishes the LPS-induced inflammatory response and is mechanistically involved in trimethylation of H3K27 at the E-selectin and VCAM-1 promoters. Lnc-IL7R is a regulator of proinflammatory genes via epigenetic modification [84].
LincRNA-EPS is downregulated in response to inflammatory triggers. Gain-of-function and rescue studies have revealed that lincRNA-EPS represses transcriptions of immune response genes by interacting with hnRNP L. LincRNA-EPS has a critical role in restraining lethal inflammatory responses [85].
2.3.3. Diabetes
Diabetes is a metabolic disease associated with high blood sugar levels. Diabetes can be caused by the pancreas not producing insulin (type 1 diabetes (T1DM)) or by insulin resistance (type 2 diabetes (T2DM)). The majority of type 1 diabetes cases are attributed to a T cell-mediated autoimmune attack, which leads to loss of the insulin-producing beta cells of the islets of Langerhans in the pancreas. It is traditionally termed juvenile diabetes and is partially inherited. T2DM is the most common type of diabetes. Insulin resistance in T2DM might be combined with reduced insulin secretion and defective responsiveness of insulin receptors [86].
RNCR3 is involved in diabetes-induced retinal neurodegeneration [87]. Knockdown of RNRC3 reduces the release of cytokines and results in fewer apoptotic retinal cells and improved visual function. RNCR3 increases in response to high glucose stress in vitro and in vivo and regulates retinal endothelial cell function through the RNCR3/KLF2/miR-185-5p network [88].
MEG3 is reduced in the retinas of STZ-induced diabetic mice and in endothelial cells under high glucose and oxidative stress. MEG3 knockdown aggravates diabetes-related retinal vessel dysfunction, which is mainly mediated by activation of PI3K/AKT signaling [89]. MEG3 expression is upregulated in hepatocytes through histone acetylation in high-fat diet and ob/ob mice. In addition, MEG3 is involved in hepatic insulin resistance via an increase in FoxO1 expression [90].
HI-LNC25 was first identified in a transcriptome mapping study of human pancreatic islets and β cells [87]. HI-LNC25 is a β cell-specific lncRNA and an integral component of β cell differentiation and maturation [87]. Depletion of HI-LNC25 decreases expression of GLIS3 mRNA, which is associated with pancreatic β cell function and mass maintenance [91]. KCNQ1OT1 and HI-LNC45, which were previously genetically associated with T2DM [92], are significantly dysregulated in diabetes islets [91].
2.3.4. Muscle Dysfunction
Muscle development is a multistep process that includes myogenesis, muscle differentiation, and regeneration. Myogenesis is a tightly regulated developmental program to direct myoblasts to form muscle fibers. Myogenic pathways are primarily governed by transcription factors, MyoD, Myf5, myogenin, and MRF4 at the molecular level. Impairment of these processes might be a cause of muscle dysfunction and is an age-related pathological phenomenon [93].
SRA (steroid receptor RNA activator) was initially characterized as an lncRNA functioning to enhance steroid receptor-dependent gene expression [94]. SRA has an unusual property that functions as both SRA RNA and SRAP protein through alternative splicing. The ratio between SRA RNA and SRAP increases during myogenic differentiation, but there is no increase in myotonic dystrophy patients. SRA RNA is an enhancer of myogenic differentiation and myogenic conversion through regulation of MyoD activity [95].
MUNC (MyoD upstream noncoding RNA) is transcribed from the upstream of myogenic differentiation (MyoD), a master transcriptional regulatory factor in muscle differentiation and specifically expressed in skeletal muscle. MUNC depletion reduces myoblast differentiation and impairs muscle regeneration in vivo. MUNC is involved in gene expressions of MyoD, Myogenin, and Myh3 (myosin heavy chain) by acting in trans. MUNC also stimulates the transcription of other genes that are not recognized as MyoD-inducible genes. MUNC is an evolutionarily conserved promyogenic lncRNA that acts directly or indirectly on multiple promoters to increase myogenic gene expression [96].
Linc-RAM (linc RNA activator of myogenesis) is a skeletal muscle-specific lncRNA that localizes in both cytoplasm and nucleus of myoblast. Depletion of linc-RAM impairs myoblast differentiation and muscle regeneration. Mechanistically, linc-RAM promotes assembly of the MyoD-Baf60c-Brg1 complex and facilitates the recruitment of the SWI/SNF core on target myogenic genes, resulting in transcription of myogenic differentiation genes [97].
Other muscle-specific lncRNAs such as linc-MD1 and lncRNA Dum are also involved in the control of muscle gene expression and muscle regeneration [98, 99].
2.3.5. Atherosclerosis
Atherosclerosis is the primary cause of heart disease and stroke. It is a chronic disease of the large arteries and is characterized by narrowing or closing of an artery with lipids and fibrous elements. Pathological studies have provided evidence of the critical role of endothelium in mediating inflammation and accumulation of oxidized low-density lipoproteins (LDL) in the intima to recruit monocytes and form macrophage-derived foam cells [100].
SENCR (smooth muscle and endothelial cell-enriched migration/differentiation-associated long noncoding RNA) is an antisense transcript from the first intron of friend leukemia virus integration 1 (FLI1) and is localized in the cytoplasm. SENCR is highly expressed in both smooth muscle and endothelial cells [101]. SENCR impedes migration and proliferation of smooth muscle cells through the regulation of FoxO1 and TRPC6 expression [102]. In addition, SENCR is associated with the regulation of endothelial cell differentiation and angiogenic capacity of human umbilical endothelial cells (HUVECs) [103].
Recent studies have reported several lncRNAs that are involved in atherosclerosis-related smooth muscle cell, endothelial cell, macrophage, and lipid metabolism regulation, suggesting a potential function of such lncRNAs in atherosclerosis development [104].
2.3.6. Cataract
Cataract is characterized by the clouding of an eye's lens. Cataract accounts for half the cases of blindness. Lens proteins denature and degrade over time, and this process is accelerated by age and diseases such as diabetes and hypertension. ROS may be mechanistically involved in cataractogenesis. The only treatment for cataract is surgery in the current state of technology [105].
LncRNA-MIAT (lncRNA myocardial infarction associated transcript) is highly expressed in patients with cataracts and is involved in the maintenance of human lens epithelial cells (HLECs) whose dysfunction results in cataract formation. MIAT regulates viability, proliferation, and migration of human HLECs in response to oxidative stress. Mechanistically, MIAT acts as a competing endogenous RNA (ceRNA) and can regulate HLEC function through a feedback loop with AKT and miR-150-5p [106].
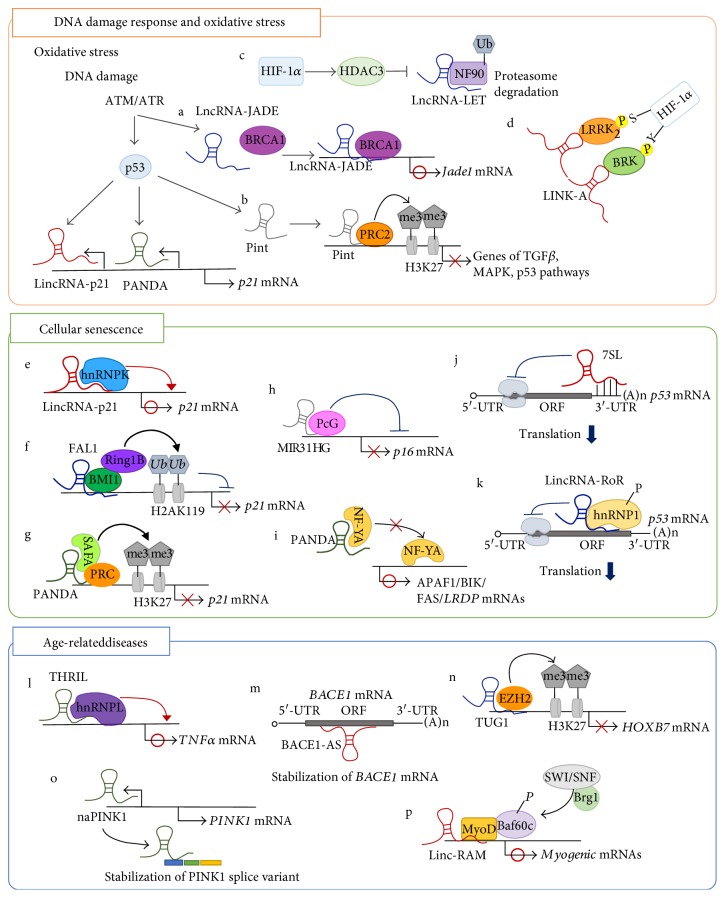
A mechanistic diagram of representative lncRNAs involved in DNA damage and oxidative stress, cellular senescence, and age-related diseases is shown in Figure 2.
Figure 2.
A mechanistic diagram of representative IncRNAs involved in DNA damage and oxidative stress, cellular senescence, and related diseases.
3. RNA-Binding Proteins
The RBPs have a pivotal role in mediating posttranscriptional regulation of gene expression by affecting pre-mRNA splicing and maturation as well as mRNA transport, storage, turnover, and translation [107]. RBPs can regulate a broad spectrum of cellular process including cell proliferation, death, differentiation, and development, and differential expression or altered activity of certain RBPs is involved in the pathogenesis of several human diseases [107–109]. RBPs interact with mRNAs via a limited set of modular RNA-binding domains (RBDs), such as the heterogeneous nuclear RNA K-homology (KH) domain, RNA recognition motif (RRM), and the zinc-finger (Znf) domain [110]. In addition, RBPs interact with other RBPs and/or ncRNAs such as miRNAs and lncRNAs via cooperative or competitive interaction [111, 112].
3.1. DNA Damage and Oxidative Stress
3.1.1. HuR
HuR is a member of human antigen (Hu) family and governs turnover and translation of target mRNAs involved in the regulation of cell proliferation, growth, survival, and differentiation in response to various stresses [113]. HuR has been reported to have protective roles during DDR and oxidative stress by governing RNA stability and translation of various target mRNAs including VEGF, HIF-1α, p53, c-myc, SIRT1, and prothymosin α. The roles of HuR in the regulation of stress response have been extensively reviewed by others [114, 115]. A recent report has shown that HuR targets and upregulates heme oxygenase 1 (HO1) during oxidative stress [116]. HuR is also implicated in DDR by directly regulating RNA metabolism of p53, WEE1, and non-POU domain-containing octamer-binding protein (NONO, also known as p54NRB) [117, 118]. Esophageal cancer-related gene 2 (ECRG2), a DNA damage-inducible tumor suppressor, can regulate XIAP-mediated cell death by downregulating HuR expression [119].
3.1.2. Heterogeneous Nuclear Ribonucleoproteins
Heterogeneous nuclear ribonucleoproteins (hnRNPs) are nuclear proteins regulating a broad spectrum of RNA metabolism including alternative splicing, translocation, and translation [120]. hnRNP A0 is phosphorylated by MAPK-activated protein kinase 2 (MK2) and stabilizes GADD45α mRNA during DDR [121]. In response to UV radiation or hypoxic stress, hnRNP A18 is induced and has protective roles by increasing the expression of UV- or stress-response genes such as replication protein A (RPA2), thioredoxin (TRX), or HIF-1α [122, 123]. hnRNP A1 is reported to regulate alternative splicing of hdm2 and UVE-triggered translation of Apaf-1 in response to UV exposure [124]. After ionizing radiation, hnRNP C has been found in DNA-damage sites and regulates BRCA gene expression and homologous recombination. Depletion of hnRNP C reduces the abundance of key HR proteins including BRCA1/2, RAD51, and BRIP1 by affecting alternative splicing [125]. hnRNP H/F is reported to increase after DNA damage and to enhance p53 expression by interfering 3′-end processing of p53 mRNA, thereby regulating apoptosis [126]. hnRNP I (also known as PTB) has been known to increase HIF-1α-mediated gene expression by enhancing translation of HIF-1α in hypoxia [127].
3.1.3. FUS
FUS (also known as hnRNP P2) binds RNA and single- and double-stranded DNA, and it affects multiple steps of DNA/RNA metabolism. FUS has been observed in sites with laser-induced DNA double-strand breaks (DSBs) and regulates DSB repair [128]. FUS also has an important role in the DDR in neurons by directly interacting with histone deacetylase 1 (HDAC1), and recruitment of FUS and HDAC1 is essential for DDR signaling [129].
3.1.4. T Cell-Restricted Intracellular Antigens
T cell-restricted intracellular antigen-1 (TIA-1) is a member of RNA-binding protein involved in alternative pre-mRNA splicing and mRNA translation. TIA-1 is a component of stress granules (SGs) triggered by hypoxia, ischemia, and anoxia, and it has essential roles in regulating mRNAs involved in oxidative stress and DDR through its associations with other SG components [130]. A recent study has shown that TIA-1 oxidation, mediated by reactive oxygen species (ROS), suppresses SG formation and increases cell death after oxidative stress [131]. TIA-related protein (TIAR) has been reported to increase and regulate neuronal cell death after cerebral ischemic injury [132]. After UVC-induced DNA damage, TIAR is dissociated from C-rich motif-containing mRNAs, including Apaf-1 mRNA, and enhances their translation [133].
3.1.5. Wig1
Wig1 (also known as ZMAT3) is a transcriptional target gene of p53 and has a zinc-finger domain that binds to double-strand RNA (dsRNA) [134]. Wig1 has been known to stabilize p53 mRNA by protecting it from deadenylation, thereby enhancing the p53-mediated stress response [135]. Depletion of Wig1 is responsible for increases in cell death and cell-cycle arrest upon DNA damage. Wig1 functions as a survival factor during stress response by regulating FAS and 14-3-3σ [136].
3.2. Cellular Senescence and Aging
3.2.1. HuR
HuR is implicated in cellular senescence and the aging process based on its involvement in regulating stability and translation of various target mRNAs including p21, p16, cyclin A, cyclin B1, c-fos, and SIRT1 [137]. Recent reports have shown that loss of HuR is related to a shorter life span in Drosophila as well as to several senescence-associated phenotypes in mouse embryonic fibroblasts (MEF) [138, 139]. The HuR level is downregulated in RS and aging, and its expression is controlled by positive feedback mechanisms [140]. Coactivator-associated arginine methyltransferase 1 (CARM1) has been known as a regulator of HuR by inducing methylation on R217 residue of HuR, and loss of CARM1 downregulates HuR activity in RS [141].
3.2.2. AU-Rich Element RNA-Binding Protein 1
AU-rich element RNA-binding protein 1 (AUF1; also known as hnRNP D) includes four alternative spliced isoforms (p37, p40, p42, and p45) containing two RRMs and regulates mRNA stability and turnover. In addition, AUF1 has been shown to affect proliferation, stress response, immune response, and cellular senescence. AUF1 is differentially regulated during aging and cellular senescence [140]. AUF regulates the mRNA stability of p21 and p16 in a competitive or cooperative manner with HuR and influences cellular senescence [142, 143]. Pont et al. reported that AUF1-deficient mice exhibit decreased telomerase level and activity, increased DNA damage at telomere ends, enhanced cellular senescence, and rapid premature aging [144].
3.2.3. TIA-1/TIAR
TIA-1 and TIAR regulate alternative splicing, SG formation, and translation of various target genes including TNFα, COX-2, c-myc, calmodulin 2, small nuclear ribonucleoprotein polypeptide F (SNRPF), and caspase-8 in response to various cellular stresses [145]. It has been shown that TIA-1 is downregulated during RS and aging [140]. TIA-1/TIAR depletion promotes cellular senescence of MEF cells [146]. However, the detailed mechanisms underlying TIA-1/TIAR-mediated regulation of cellular senescence or aging need to be elucidated.
3.2.4. CUGBP1
CUG triplet repeat, RNA-binding protein 1 (CUGBP1) is a member of the CELF/BRUNOL protein family containing two N-terminal RRMs and regulates pre-mRNA alternative splicing, mRNA editing, and translation [147]. CUGBP1 has a role in enhancing p21 expression and regulates cellular senescence [148]. CUGBP1 binds to the 5′-UTR of p21 and increases translation of p21 by competing with calreticulin. In senescent cells, increased phosphorylation of CUGBP1 promotes binding to p21 mRNA.
CUGBP1 has been known to increase with aging in fat tissue and to regulate CCAAT/enhancer-binding protein β (C/EBPβ) expression [149]. CUGBP1 binds to C/EBPβ mRNA and enhances its translation, thereby accumulating C/EBPβ-liver-enriched inhibitory protein (C/EBPβ-LIP), a dominant inhibitor of differentiation, in fat cells. Augmented expression of CUGBP1 is responsible for the impairment of adipogenesis in aged-fat tissues. In old liver, CUGBP1 phosphorylation at S302 residue by GSK3β facilitates the association of CUGBP1 with eukaryotic initiation factor 2 (eIF2) and increases translation of HDAC1 and C/EBPβ, which are responsible for epigenetic regulation of gene expression [150, 151].
3.2.5. Tristetraprolin
Tristetraprolin (TTP) is an ARE-binding protein involved in destabilizing target mRNAs, and its expression is upregulated during cellular senescence and aging [152]. TTP is elevated in B lymphocytes from aged mice compared to the level in cells from young mice, and it destabilizes transcription factor E47 mRNA [153]. Sanduja et al. have reported that TTP promotes cellular senescence by destabilizing E6-AP ubiquitin ligase mRNA [154]. E6-AP downregulation mediated by TTP results in p53 and hTERT accumulation in cells.
3.2.6. Wig1
Wig1 is also implicated in the regulation of cellular senescence. Kim et al. reported that Wig1 prevents cellular senescence by regulating p21 expression [155]. Wig1 binds to the stem-loop structure near the miRNA-binding site of p21 mRNA and recruits the RNA-induced silencing complex (RISC) by interacting with Ago2, thereby destabilizing p21 mRNA. Depletion of Wig1 results in a decrease of miR-mediated p21 mRNA decay and promotes cellular senescence via p21 upregulation in various cell types.
3.3. Age-Related Diseases
3.3.1. Neurodegenerative Diseases
Neuronal cells have their own systems for regulating RNA expression in response to various stimuli via RBPs that are uniquely expressed in neuronal cells. Accumulating evidence indicates that abnormalities in RNA metabolism are a common feature of neurodegeneration [156, 157]. Therefore, mutations or dysregulation of RBPs is widely involved in the pathogenesis of neurodegenerative diseases, including amyotrophic lateral sclerosis, AD, HD, and PD, by governing RNA metabolism [158].
(1) TDP-43. TDP-43 was identified as the major component of ubiquitin-positive neuronal inclusion bodies observed in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration (FTLD) patients [159]. TDP-43 functions as a translational repressor and known to have essential roles in transcriptional regulation and miRNA maturation [160]. Also, TDP-43 regulates axonal transport of RNA granules by interacting with hnRNP A2/B1 [161, 162]. Mutations of TDP-43 genes found in ALS patients are related to delocalization and aggregation of TDP-43. Formation of insoluble aggregates of TDP-43 in the cytoplasm alters interactions between TDP-43 and its target mRNAs having important functions in the brain, thereby indicating the involvement of TDP-43 in ALS/FTLD pathogenesis [163].
(2) FUS. Mutations in the gene coding FUS are found in 5% of familial ALS patients and in rare sporadic cases. Like TDP-43, mutations on FUS gene facilitate delocalization and abnormal aggregation of FUS to cytoplasm and affect the alternative splicing of its target genes [164, 165]. Also, FUS mutations are responsible for an increase in DNA damage in ALS patients [129].
(3) HuD. HuD (also known as nELAVL or ELAVL4) is expressed in the brain and has been implicated in various aspects of RNA metabolism [166]. HuD functions as a pivotal regulator of neurogenesis, axonal growth, and neuronal function, and dysregulation of HuD results in neuronal defects [167]. HuD has been reported to increase in the brain of AD patients and to stabilize APP mRNA, β-site APP-cleaving enzyme 1 (BACE1) mRNA, and BACE1 antisense (BACE1-AS) lncRNAs, thereby facilitating the accumulation of the toxic APP cleavage product Aβ [168].
(4) FMRP. Fragile X mental retardation protein (FMRP) is a gene product encoded by fragile X mental retardation 1 (FMR1) and plays essential roles in normal cognitive development and female reproductive function [169]. Mutations on the FXR1 gene lead to fragile X syndrome (FXS), autism, AD, and PD by dysregulating translation of its target genes [170]. FMRP inhibits APP mRNA translation by recruiting APP mRNA into P-bodies [171]. FMRP has been shown to decrease in the brain of sporadic AD patients [172].
(5) hnRNPs. hnRNP A1 has essential roles in the regulation of pre-mRNA processing, transport, and translation of mRNAs [173]. Loss of hnRNP A1 expression or presences of mutations (D262) are observed in ALS patients [162]. In addition, hnRNP A1 shows a decrease in the AD brain and has been known to regulate alternative splicing of RAGE and APP mRNAs [174, 175].
hnRNP A2/B1 affects alternative splicing of ALS-associated D-amino acid oxidase, and ALS mutant (hnRNP A2B1 D290V) dysregulates cellular stress responses [162, 176]. hnRNP A2/B1 and hnRNP B1 are also differentially expressed in the AD brain [177].
hnRNP C has been known to stabilize APP mRNA or enhance its translation by competing with FMRP, therefore positively regulates APP expression [171]. Borreca et al. reported augmented expression of hnRNP C in the brain of sporadic AD patients [172].
3.3.2. Metabolic Diseases
Metabolic disease is associated with the risk of developing T2DM, obesity, cardiovascular disease (CVD), and coronary heart disease (CHD) [178, 179]. Increasing evidence indicates that dysregulation of RNA metabolism in metabolically active and insulin-sensitive organs, such as the pancreas, liver, muscle, and adipose tissues, is actively implicated in the pathogenesis of metabolic diseases [180].
(1) HuD. HuD is also found in the islets of the pancreas and mediates RNA quality control of pancreatic β cells [181]. In the pancreatic islets of a T2DM mouse model, HuD expression is downregulated [163]. HuD regulates insulin biosynthesis by associating with 5′-UTR of insulin2 mRNA and repressing its translation [181]. In addition, HuD regulates autophagosome formation and lipid synthesis via translation regulation of ATG5 and INSIG1, respectively [182, 183]. Moreover, HuD regulates apoptosis of pancreatic β cells [184].
(2) CUGBP1. CUGBP1 has been reported to regulate insulin resistance and alternative splicing of the insulin [185]. In addition, CUGBP1 negatively regulates insulin secretion by stabilizing phosphodiesterase subtype 3B (PDE3B) [186]. CUGBP1 expression is higher in diabetic heart and pancreas, and single nucleotide polymorphisms (SNPs) on the CUGBP1 locus are associated with obesity [186, 187].
(3) RBFOX2. RBFOX2 (also known as RBM9) is a RNA-binding protein and a homolog of C. elegans Fox-1 and regulates alternative splicing by directly binding to the consensus (U)GCAUG motif in the target pre-mRNAs [188]. A recent study by Nutter et al. showed that 73% of transcripts misspliced in diabetic hearts have RBFOX2-binding sites, and a dominant negative form of RBFOX2 (DN-RBFOX2) was found in diabetic hearts [189]. DN-RBFOX2 precedes diabetic cardiac complications, as well as delays intracellular calcium transients in cardiomyocytes by blocking RBFOX2-mediated alternative splicing.
(4) IGF2BP2/IMP2. Insulin-like growth factor 2 mRNA-binding protein 2 (IGF2BP2/IMP2) belongs to IGF2 mRNA-binding protein (IMP) family and is known to regulate IGF2 translation by interacting with the 5′-UTR of IGF2 mRNA [190]. Genome-wide association studies have shown that the human IGF2BP/IMP2 gene contains SNPs associated with T2DM [191, 192]. Dai et al. demonstrated that mice lacking IGF2BP2/IMP2 resist diet-induced obesity and have improved glucose tolerance, insulin sensitivity, and longer lifespan through the increased translation of UCP1 or mitochondrial components [193].
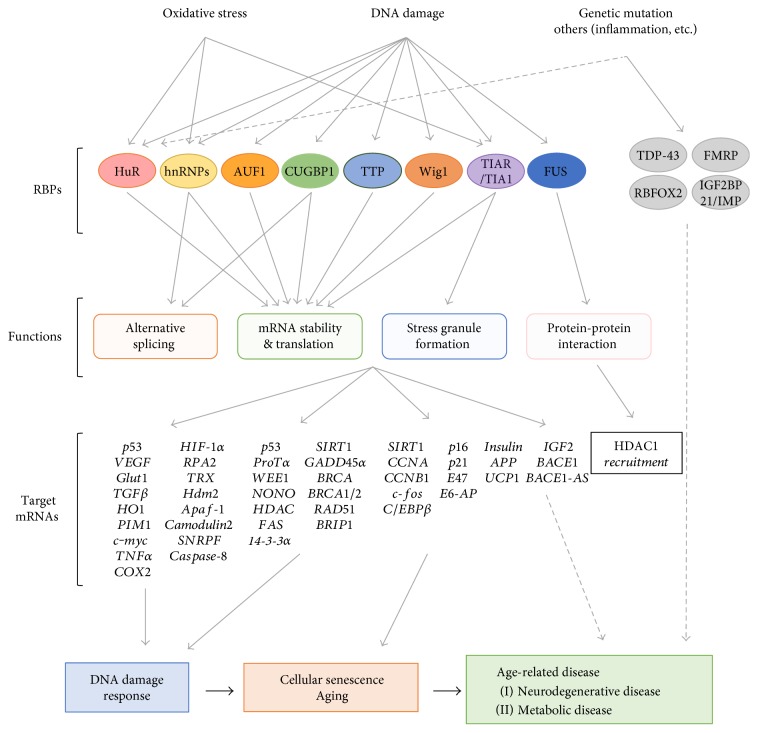
Representative RBPs involved in DNA damage and oxidative stress, cellular senescence, and age-related diseases are shown in Figure 3.
Figure 3.
Representative RBPs involved in DNA damage and oxidative stress, cellular senescence, and age-related diseases.
4. Conclusion
Increasing evidence indicates that ncRNAs and RBPs are essential regulators of various cellular processes, and dysregulation of these RNA regulators is implicated in the pathogenesis of several diseases including neurodegenerative diseases, metabolic diseases, and cancer. In this review, we tried to discuss the regulatory lncRNAs and RBPs that are involved in stress response, cellular senescence, and the pathogenesis of age-related diseases including neurodegenerative diseases, metabolic diseases, immune response, and muscle dysfunction (Tables 1 and 2). We have limited our discussion to lncRNAs and RBPs because miRNAs have been intensively reviewed by others [194–196]. Although the list of reviewed lncRNAs and RBPs is extensive, additional RNA regulators are certainly going to be uncovered in future studies of stress-related responses and age-related diseases.
Table 1.
A list of lncRNAs involved in DNA damage response, oxidative stress, cellular senescence, and age-related diseases.
| LncRNAs | Functions | References |
|---|---|---|
| DNA damage response | ||
| LincRNA-p21 | Represses gene expression with hnRNP K | [20–22] |
| LincRNA-RoR | Suppresses p53 translation with hnRNP I and inhibits p53-mediated cell-cycle arrest and apoptosis | [23–25] |
| Pint | Connects p53 activation with epigenetic silencing by PRC2 | [20, 26] |
| PANDA | Regulates proapoptotic genes with NF-YA | [27–29] |
| LncRNA-JADE | Connects the DNA damage response to histone H4 acetylation | [30] |
| Oxidative stress | ||
| H19 | Upregulated by oxidative stress | [31–34] |
| ANRIL | Represses the expression of INK4A-ARF-INK4B | [35] |
| LncRNA-LET | Degrades NF90 via ubiquitin-proteasome pathway | [36] |
| LINK-A | Regulates the stabilization of HIF-1α | [37] |
| Cellular senescence | ||
| 7SL | Promotes cell growth via suppression of p53 | [38, 39] |
| HOTAIR | Represses transcription of HOXD with PRC2 | [40–42] |
| UCA1 | Negative correlation between p27 and UCA in breast cancer tissue | [43–45] |
| LincRNA-p21 | Influences the p53 tumor suppressor pathway by regulating p53-mediated p21 expression | [46] |
| ANRIL | Regulates CDKN2A/B by epigenetic mechanisms | [47–52] |
| ANRASSF1 | Represses the expression of RASSF1A | [53, 54] |
| PANDA | Interacts with PRC1, PRC2, and NF-YA and represses the transcription of senescence-promoting genes | [55, 56] |
| FAL1 | Oncogenic activity of FAL1 is repression of p21 | [57, 58] |
| MIR31HG | Interacts with both INK3A and PcG proteins and represses INK4A | [59] |
| SALNR | Regulates NF90 activity | [60] |
| VAD | Regulates chromatin structure and increases the expression of INK4 | [61] |
| Neurodegenerative diseases | ||
| BC200 | Upregulation of BC200 related to the severity of AD | [63, 64] |
| BACE1-AS | Regulates BACE1 mRNA and generates Aβ 1–42 | [65, 66] |
| NDM29 | Induces APP and increases Aβ secretion | [67, 68] |
| 17A | Enhances Aβ secretion by impairing GABA-B signaling | [69, 71] |
| AS Uchl1 | Induces Uchl1 expression by increasing its translation | [73] |
| naPINK1 | Regulates the stabilization of svPINK1 expression | [71, 74] |
| TUG1 | Downstream target of p53 and regulates cell-cycle genes | [76–79] |
| MEG3 | Epigenetically regulates chromatin in HD | [16, 78] |
| HTTAS-V1 | Overexpression of HTTAS-V1 reduces HTT transcripts | [80] |
| Immune response | ||
| THRIL | Regulates TNFα expression and is associated with childhood acute inflammatory diseases | [82] |
| Lnc-DC | Exclusively expressed in dendritic cells and regulates DC differentiation | [83] |
| Lnc-IL7R | Diminishes LPS-induced inflammatory response | [84] |
| LincRNA-EPS | Regulated in macrophages to control the expression of immune response genes | [85] |
| Diabetes | ||
| RNCR3 | Regulates retinal endothelial cell function via RNCR3/KLF2/miR-185-5p | [87, 88] |
| MEG3 | Downregulates MEG3 in the retinas of STZ-induced diabetic mice | [89, 90] |
| HI-LNC25 | Regulates β cell differentiation and maturation | [91, 92] |
| Muscle dysfunction | ||
| SRA | Enhances the activity of nuclear receptors and regulates differentiation of MyoD | [94, 95] |
| MUNC | Facilitates the function of MyoD in skeletal myogenesis | [96] |
| Linc-RAM | Promotes assembly of MyoD-Baf60-Brg1 complex and increases the transcription of myogenic differentiation genes | [97] |
| Atherosclerosis | ||
| SENCR | Impedes migration and proliferation of smooth muscle cells by regulating FOXO1 and TRPC6 expression | [101–103] |
| Cataracts | ||
| LncRNA-MIAT | Upregulated in patients with cataracts and involved in the maintenance of LECs | [106] |
Table 2.
A list of RBPs involved in DNA damage response, oxidative stress, cellular senescence, and age-related diseases.
| RBPs | Functions | References |
|---|---|---|
| DNA damage response and oxidative stress | ||
| HuR | Protection roles in oxidative stress and DNA damage by regulating RNA metabolism (reviewed in 114, 115) Regulates HO1, WEE1, and NONO expression during stress response |
[114–119] |
| hnRNP A0 | Phosphorylation of hnRNP A0 by MK2 promotes GADD45α mRNA stabilization | [121] |
| hnRNP A18 | Increases gene expression involved in stress-response | [122, 123] |
| hnRNP A1 | Involved in alternative splicing of hdm2 and Apaf-1 translation | [124] |
| hnRNP C | Regulates BRCA gene expression and homologous recombination after ionizing irradiation | [125] |
| hnRNP H/F | Increased in DNA damage response and upregulates p53 expression | [126] |
| hnRNP I | Enhances translation of HIF-1α in hypoxia | [127] |
| FUS | Interacts with HDAC1 and regulates DNA damage response | [129] |
| TIA-1/TIAR | TIA-1/TIAR are involved in SG formation after stress response and decrease HIF-1α translation TIA-1 oxidation by ROS suppresses SG formation and increases cell death TIAR increases Apaf-1 translation after UVC-induced DNA damage |
[130, 131, 133] |
| Wig1 | Stabilizes p53 mRNA and enhancing p53-mediated stress response | [136] |
| Cellular senescence and aging | ||
| HuR | HuR loss is related to shorter life span and enhanced senescence-associated phenotypes (reviewed in 137) CARM1 downregulates HuR activity in replicative senescence |
[137–139, 141] |
| AUF1 | Involved in cellular senescence by regulating mRNA stability of p21 and p16, and AUF1 KO mice show enhanced cellular senescence and rapid premature aging | [142–144] |
| TIA-1/TIAR | Down-regulated in cellular senescence and TIA-1/TIAR depletion promotes cellular senescence of MEF cells | [140, 146] |
| CUGBP1 | CUGBP1 phosphorylation promotes the binding to p21 mRNA in senescent cells Regulates C/EBPβ and HDAC1 in the liver and fat of old mice |
[148, 150, 151] |
| TTP | Upregulated in senescent cells and contributes to p53 accumulation by destabilizing E6-AP mRNA | [140, 154] |
| Wig1 | Prevents premature senescence by destabilizing p21 mRNA | [155] |
| Neurodegenerative diseases | ||
| TDP-43 | Functions as a translational repressor Regulates axonal transport of RNA granules by interacting with hnRNP A2/B1 Mutants form of TDP-43 found in ALS patients are prone to aggregation |
[160–162] |
| FUS | Interacts with DNA/RNA and regulates DNA/RNA metabolism Mutation found in ALS patients are related to abnormal aggregation of FUS in cytoplasm and dysregulation of alternative splicing |
[164] |
| HuD | Has pivotal roles in neurogenesis, axonal growth, and neuronal functions Upregulated in the brain of AD patients and promotes Aβ accumulation |
[166, 168] |
| FMRP | Mutations on FXP1 gene are linked to FXS, AD, and PD by dysregulation translation of target genes Downregulated in the brain of sporadic AD patients and regulates APP translation |
[170–172] |
| hnRNP A1 | Loss of hnRNP A1 or mutations on D262 residue is found in the ALS patients Downregulated in the brain of AD patients and affects to splicing of RAGE and APP mRNAs |
[174, 175] |
| hnRNP A2/B1 | Mutation on D290 residue dysregulates cellular stress response in ALS Differentially expressed in the brain of AD and affects alternative splicing |
[176, 177] |
| hnRNP C | Upregulated in the brain of AD patients Stabilizes APP mRNA and enhances translation of APP |
[171, 172] |
| Metabolic diseases | ||
| HuD | Downregulated in the pancreas of T2DM Regulates insulin biosynthesis, autophagosome formation, lipid synthesis, and apoptosis in pancreatic β cells |
[181–184] |
| CUGBP1 | Upregulated in the diabetic hearts and the pancreas and regulates insulin secretion and insulin resistance Obesity-related SNPs on CUGBP1 influence alternative splicing, translation, and turnover of target mRNAs |
[185–187] |
| RBFOX2 | Plays essential roles in alternative splicing In diabetic hearts, majority of misspliced transcripts have RBFOX2-binding sites |
[188, 189] |
| IGF2BP2/IMP | SNPs on IGF2BP2/IMP2 genes are associated to T2DM IMP2 KO mice show better glucose tolerance, insulin sensitivity, and longer lifespan |
[191–193] |
The results of studies undertaken to uncover the roles of lncRNAs and RBPs during stress response, cellular senescence, and the pathogenesis of age-related diseases are prompting several questions for immediate consideration. For example, what are the molecular targets of lncRNAs and RBPs? What signaling pathways control the expression and function of lncRNAs and RBPs during the stress response or in the pathogenesis of age-related diseases? How do they contribute to the stress response and cellular senescence? Do lncRNAs and RBPs interplay in order to fine tune RNA metabolism? How are RNA regulators including lncRNAs, miRNAs, and RBPs differentially expressed in age-related diseases? As we begin to consider these questions, the importance of functional networking between RBPs and ncRNAs is coming to the forefront [197–199].
A deeper and more comprehensive knowledge of the fine mechanisms involving lncRNAs and RBPs in the regulation of RNA metabolism is warranted because regulatory lncRNAs and RBPs are promising novel targets for intervention in physiopathologies with underlying deficiencies in stress response, cellular senescence, and the aging process.
Acknowledgments
This work was supported by grants awarded to Jae-Seon Lee from the Basic Science Research Program (no. 2014R1A2A1A11051988), Nuclear Research and Development Program (no. 2012-M2B2B1-2012055637), and Medical Research Center (MRC) (no. 201409392). Eun Kyung Lee and Chongtae Kim are supported by the National Research Foundation of Korea (NRF) (2014R1A2A1A11053431 and 2016R1A6A3A11931343).
Conflicts of Interest
The authors declare that they have no competing interests.
Authors' Contributions
Chongtae Kim and Donghee Kang equally contributed to this work.
References
- 1.Campisi J. Aging, cellular senescence, and cancer. Annual Review of Physiology. 2013;75:685–705. doi: 10.1146/annurev-physiol-030212-183653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hayflick L., Moorehead P. S. The serial cultivation of human diploid cell strains. Experimental Cell Research. 1961;25(3):585–621. doi: 10.1016/0014-4827(61)90192-6. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Porath I., Weinberg R. A. The signals and pathways activating cellular senescence. The International Journal of Biochemistry and Cell Biology. 2005;37(5):961–976. doi: 10.1016/j.biocel.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Davalli P., Mitic T., Caporali A., Lauriola A., D’Arca D. ROS, cell senescence, and novel molecular mechanisms in aging and age-related diseases. Oxidative Medicine and Cellular Longevity. 2016;2016:18. doi: 10.1155/2016/3565127.3565127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coppé J.-P., Patil C. K., Rodier F., et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biology. 2008;6(12, article e301) doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee M., Lee J.-S. Exploiting tumor cell senescence in anticancer therapy. Biochemistry and Molecular Biology Reports. 2014;47(2):51–59. doi: 10.5483/BMBRep.2014.47.2.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shay J. W., Roninson I. B. Hallmarks of senescence in carcinogenesis and cancer therapy. Oncogene. 2004;23(16):2919–2933. doi: 10.1038/sj.onc.1207518. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz-Espín D., Serrano M. Cellular senescence: from physiology and pathology. Nature Review Molecular Cell Biology. 2014;15(7):482–496. doi: 10.1038/nrm3823. [DOI] [PubMed] [Google Scholar]
- 9.López-Otín C., Blasco M. A., Partridge L., Serrano M., Kroemer G. The hallmarks of aging. Cell. 2013;153(6):1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childs B. G., Durik M., Baker D. J., van Deursen J. M. Cellular senescence in aging and age-related diseases: from mechanisms to therapy. Nature Medicine. 2015;21(12):1424–1435. doi: 10.1038/nm.4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christmann M. I., Kaina B. Transcriptional regulation of human DNA repair genes following genotoxic stress: trigger mechanisms, inducible responses and genotoxic adaptation. Nucleic Acids Research. 2013;41(18):8403–8420. doi: 10.1093/nar/gkt635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lanigan F., Geraghty J. G., Bracken A. P. Transcriptional regulation of cellular senescence. Oncogene. 2011;30:2901–2911. doi: 10.1038/onc.2011.34. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Zhang Y., Xu H., Bu G. Transcriptional regulation and its misregulation in Alzheimer’s disease. Molecular Brain. 2013;6:p. 44. doi: 10.1186/1756-6606-6-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Desvergne B., Michalik L., Wahli W. Transcriptional regulation of metabolism. Physiological Reviews. 2006;88(2):465–514. doi: 10.1152/physrev.00025.2005. [DOI] [PubMed] [Google Scholar]
- 15.Grammatikakis I., Panda A. C., Abdelmohsen K., Gorospe M. Long noncoding RNAs (lncRNAs) and the molecular hallmarks of aging. Aging (Albany NY) 2014;6(12):992–1009. doi: 10.18632/aging.100710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kour S., Rath P. C. Long noncoding RNAs in aging and age-related diseases. Ageing Research Reviews. 2016;26:1–21. doi: 10.1016/j.arr.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 17.Schmitt A. M., Chang H. Y. Long noncoding RNAs in cancer pathways. Cancer Cell. 2016;29(4):452–463. doi: 10.1016/j.ccell.2016.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prensner J. R., Chinnaiyan A. M. The emergence of lncRNAs in cancer biology. Cancer Discovery. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Montes M., Lund A. H. Emerging roles of lncRNAs in senescence. The FEBS Journal. 2016;283(13):2414–2426. doi: 10.1111/febs.13679. [DOI] [PubMed] [Google Scholar]
- 20.Huarte M., Guttman M., Feldser D., et al. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142(3):409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang F., Zhang H., Mei Y., Wu M. Reciprocal regulation of HIF-1α and lincRNA-p21 modulates the Warburg effect. Molecular Cell. 2014;53(1):88–100. doi: 10.1016/j.molcel.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 22.Hall J. R., Messenger Z. J., Tam H. W., Phillips S. L., Recio L., Smart R. C. Long noncoding RNA lincRNA-p21 is the major mediator of UVB-induced and p53-dependent apoptosis in keratinocytes. Cell Death and Diseases. 2015;6, article e1700 doi: 10.1038/cddis.2015.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Loewer S., Cabili M. N., Guttman M., et al. Long intergenic non-coding RNA-RoR modulates reprogramming of human diploid pluripotent stem cells. Nature Genetics. 2010;42(12):1113–1117. doi: 10.1038/ng.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang A., Zhou N., Huang J., et al. The human long non-coding RNA-RoR is a p53 repressor in response to DNA damage. Cell Research. 2013;23(3):340–350. doi: 10.1038/cr.2012.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan J., Xing Y., Wen X., et al. Long non-coding RNA ROR decoys gene-specific histone methylation to promote tumorigenesis. Genome Biology. 2015;16:p. 139. doi: 10.1186/s13059-015-0705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marín-Béjar O., Marchese F. P., Athie A., et al. Pint lincRNA connects the p53 pathway with epigenetic silencing by the Polycomb repressive complex 2. Genome Biology. 2013;14(9, article R104) doi: 10.1186/gb-2013-14-9-r104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hung T., Wang Y., Lin M. F., et al. Extensive and coordinated transcription of noncoding RNAs within cell-cycle promoters. Nature Genetics. 2011;43(7):621–629. doi: 10.1038/ng.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kotake Y., Kitagawa K., Ohhata T., et al. Long non-coding RNA, PANDA, contributes to the stabilization of p53 tumor suppressor protein. Anticancer Research. 2016;36(4):1605–1611. [PubMed] [Google Scholar]
- 29.Kotake Y., Goto T., Naemura M., Inoue Y., Okamoto H., Tahara K. Long noncoding RNA PANDA positively regulates proliferation of osteosarcoma cells. Anticancer Research. 2017;37(1):81–85. doi: 10.21873/anticanres.11292. [DOI] [PubMed] [Google Scholar]
- 30.Wan G., Hu X., Liu Y., et al. A novel non-coding RNA lncRNA-JADE connects DNA damage signaling to histone H4 acetylation. The EMBO Journal. 2013;32(21):2833–2847. doi: 10.1038/emboj.2013.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matouk I., Raveh E., Ohana P., et al. The increasing complexity of the oncofetal h19 gene locus: functional dissection and therapeutic intervention. International Journal of Molecular Science. 2013;14(2):4298–4316. doi: 10.3390/ijms14024298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matouk I. J., Mezan S., Mizrahi A., et al. The oncofetal H19 RNA connection: hypoxia, p53 and cancer. Biochimica et Biophysica Acta. 2010;1803(4):443–451. doi: 10.1016/j.bbamcr.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 33.Ravid O., Shoshani O., Sela M., et al. Relative genomic stability of adipose tissue derived mesenchymal stem cells: analysis of ploidy, H19 long non-coding RNA and p53 activity. Stem Cell Research and Therapy. 2014;5(6):p. 139. doi: 10.1186/scrt529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li X., Wang H., Yao B., Xu W., Chen J., Zhou X. lncRNA H19/miR-675 axis regulates cardiomyocyte apoptosis by targeting VDAC1 in diabetic cardiomyopathy. Scientific Reports. 2016;6, article 36340 doi: 10.1038/srep36340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wan G., Mathur R., Hu X., et al. Long non-coding RNA ANRIL(CDKN2B-AS) is induced by the ATM-E2F1 signaling pathway. Cellular Signalling. 2013;25(5):1086–1095. doi: 10.1016/j.cellsig.2013.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang F., Huo X. S., Yuan S. X., et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Molecular Cell. 2013;49(6):1083–1096. doi: 10.1016/j.molcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 37.Lin A., Li C., Xing Z., et al. The LINK-A lncRNA activates normoxic HIF1α signaling in triple-negative breast cancer. Nature Cell Biology. 2016;18(2):213–224. doi: 10.1038/ncb3295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walter P., Blobel G. Signal recognition particle contains a 7S RNA essential for protein translocation across the endoplasmic reticulum. Nature. 1982;299(5885):691–698. doi: 10.1038/299691a0. [DOI] [PubMed] [Google Scholar]
- 39.Abdelmohsen K., Panda A. C., Kang M. J., et al. 7SL RNA represses p53 translation by competing with HuR. Nucleic Acids Research. 2014;42(15):10099–10111. doi: 10.1093/nar/gku686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rinn J. L., Kertesz M., Wang J. K., et al. Functional demarcation of active and silent chromatin domains in human HOX loci by noncoding RNAs. Cell. 2007;129(7):1311–1323. doi: 10.1016/j.cell.2007.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta R. A., Shah N., Wang K. C., et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464(7291):1071–1076. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Özeş A. R., Miller D. F., Özeş O. N., et al. NF-κB-HOTAIR axis links DNA damage response, chemoresistance and cellular senescence in ovarian cancer. Oncogene. 2016;35(41):5350–5361. doi: 10.1038/onc.2016.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang F., Li X., Xie X., Zhao L., Chen W. UCA1, a non-protein-coding RNA up-regulated in bladder carcinoma and embryo, influencing cell growth and promoting invasion. The FEBS Journal. 2008;582(13):1919–1927. doi: 10.1016/j.febslet.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 44.Kumar P. P., Emechebe U., Smith R., et al. Coordinated control of senescence by lncRNA and a novel T-box3 co-repressor complex. eLife. 2014;3 doi: 10.7554/eLife.02805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang J., Zhou N., Watabe K., et al. Long non-coding RNA UCA1 promotes breast tumor growth by suppression of p27 (Kip1) Cell Death and Disease. 2014;5, article e1008 doi: 10.1038/cddis.2013.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dimitrova N., Zamudio J. R., Jong R. M., et al. LincRNA-p21 activates p21 in cis to promote Polycomb target gene expression and to enforce the G1/S checkpoint. Molecular Cell. 2014;54(5):777–790. doi: 10.1016/j.molcel.2014.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pasmant E., Laurendeau I., Héron D., Vidaud M., Vidaud D., Bièche I. Characterization of a germ-line deletion, including the entire INK4/ARF locus, in a melanoma-neural system tumor family: identification of ANRIL, an antisense noncoding RNA whose expression coclusters with ARF. Cancer Research. 2007;67(8):3963–3969. doi: 10.1158/0008-5472.CAN-06-2004. [DOI] [PubMed] [Google Scholar]
- 48.Yap K. L., Li S., Muñoz-Cabello A. M., et al. Molecular interplay of the noncoding RNA ANRIL and methylated histone H3 lysine 27 by polycomb CBX7 in transcriptional silencing of INK4a. Molecular Cell. 2010;38(5):662–674. doi: 10.1016/j.molcel.2010.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kotake Y., Nakagawa T., Kitagawa K., et al. Long non-coding RNA ANRIL is required for the PRC2 recruitment to and silencing of p15(INK4B) tumor suppressor gene. Oncogene. 2011;30(16):1956–1962. doi: 10.1038/onc.2010.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Qiu J. J., Wang Y., Liu Y. L., Zhang Y., Ding J. X., Hua K. Q. The long non-coding RNA ANRIL promotes proliferation and cell cycle progression and inhibits apoptosis and senescence in epithelial ovarian cancer. Oncotarget. 2016;7(22):32478–32492. doi: 10.18632/oncotarget.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nie F. Q., Sun M., Yang J. S., et al. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Molecular Cancer Therapeutics. 2015;14(1):268–277. doi: 10.1158/1535-7163.MCT-14-0492. [DOI] [PubMed] [Google Scholar]
- 52.Aguilo F., Zhou M. M., Walsh M. J. Long noncoding RNA, polycomb, and the ghosts haunting INK4b-ARF-INK4a expression. Cancer Research. 2011;71(16):5365–5369. doi: 10.1158/0008-5472.CAN-10-4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckedorff F. C., Ayupe A. C., Crocci-Souza R., et al. The intronic long noncoding RNA ANRASSF1 recruits PRC2 to the RASSF1A promoter, reducing the expression of RASSF1A and increasing cell proliferation. PLoS Genetics. 2013;9(8, article e1003705) doi: 10.1371/journal.pgen.1003705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Thaler S., Hähnel P. S., Schad A., Dammann R., Schuler M. RASSF1A mediates p21Cip1/Waf1-dependent cell cycle arrest and senescence through modulation of the Raf-MEK-ERK pathway and inhibition of Akt. Cancer Research. 2009;69(5):1748–1757. doi: 10.1158/0008-5472.CAN-08-1377. [DOI] [PubMed] [Google Scholar]
- 55.Puvvula P. K., Desetty R. D., Pineau P., et al. Long noncoding RNA PANDA and scaffold-attachment-factor SAFA control senescence entry and exit. Nature Communications. 2014;5:p. 5323. doi: 10.1038/ncomms6323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helbig R., Fackelmayer F. O. Scaffold attachment factor A (SAF-A) is concentrated in inactive X chromosome territories through its RGG domain. Chromosoma. 2003;112(4):173–182. doi: 10.1007/s00412-003-0258-0. [DOI] [PubMed] [Google Scholar]
- 57.Zhong X., Hu X., Zhang L. Oncogenic long noncoding RNA FAL1 in human cancer. Molecular and Cellular Oncology. 2015;2(2, article e977154) doi: 10.4161/23723556.2014.977154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hu X., Feng Y., Zhang D., et al. A functional genomic approach identifies FAL1 as an oncogenic long noncoding RNA that associates with BMI1 and represses p21 expression in cancer. Cancer Cell. 2014;26(3):344–357. doi: 10.1016/j.ccr.2014.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montes M., Nielsen M. M., Maglieri G., et al. The lncRNA MIR31HG regulates p16(INK4A) expression to modulate senescence. Nature Communications. 2015;6:p. 6967. doi: 10.1038/ncomms7967. [DOI] [PubMed] [Google Scholar]
- 60.Wu C. L., Wang Y., Jin B., Chen H., Xie B. S., Mao Z. B. Senescence-associated long non-coding RNA (SALNR) delays oncogene-induced senescence through NF90 regulation. The Journal of Biological Chemistry. 2015;290(50):30175–30192. doi: 10.1074/jbc.M115.661785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazorthes S., Vallot B., Briois S., et al. A vlincRNA participates in senescence maintenance by relieving H2AZ-mediated repression at the INK4 locus. Nature Communications. 2015;6:p. 5971. doi: 10.1038/ncomms6971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. Alzheimer’s disease. Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 63.Tiedge H., Chen W., Brosius J. Primary structure, neural-specific expression, and dendritic location of human BC200 RNA. Journal of Neuroscience. 1993;13(6):2382–2390. doi: 10.1523/JNEUROSCI.13-06-02382.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mus E., Hof P. R., Tiedge H. Dendritic BC200 RNA in aging and in Alzheimer’s disease. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(25):10679–10684. doi: 10.1073/pnas.0701532104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Faghihi M. A., Modarresi F., Khalil A. M., et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of β-secretase expression. Nature Medicine. 2008;14(7):723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Modarresi F., Faghihi M. A., Patel N. S., Sahagan B. G., Wahlestedt C., Lopez-Toledano M. A. Knockdown of BACE1-AS nonprotein-coding transcript modulates beta-amyloid-related hippocampal neurogenesis. International Journal of Alzheimer’s Disease. 2011;2011:11. doi: 10.4061/2011/929042.929042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gavazzo P., Vella S., Marchetti C., Nizzari M., Cancedda R., Pagano A. Acquisition of neuron-like electrophysiological properties in neuroblastoma cells by controlled expression of NDM29 ncRNA. Journal of Neurochemistry. 2011;119(5):989–1001. doi: 10.1111/j.1471-4159.2011.07492.x. [DOI] [PubMed] [Google Scholar]
- 68.Massone S., Ciarlo E., Vella S., et al. NDM29, a RNA polymerase III-dependent non coding RNA, promotes amyloidogenic processing of APP and amyloid β secretion. Biochimica et Biophysica Acta. 2012;1823(7):1170–1177. doi: 10.1016/j.bbamcr.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 69.Massone S., Vassallo I., Fiorino G., et al. 17A, a novel non-coding RNA, regulates GABA B alternative splicing and signaling in response to inflammatory stimuli and in Alzheimer disease. Neurobiology of Disease. 2011;41(2):308–317. doi: 10.1016/j.nbd.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 70.Luo Q., Chen Y. Long noncoding RNAs and Alzheimer’s disease. Journal of Clinical Interventions in Aging. 2016;11:867–872. doi: 10.2147/CIA.S107037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wu P., Zuo X., Deng H., Liu X., Liu L., Ji A. Roles of long noncoding RNAs in brain development, functional diversification and neurodegenerative diseases. Brain Research Bulletin. 2013;97:69–80. doi: 10.1016/j.brainresbull.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 72.Kalia L. V., Lang A. E. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 73.Carrieri C., Forrest A. R., Santoro C., et al. Expression analysis of the long non-coding RNA antisense to Uchl1 (AS Uchl1) during dopaminergic cells' differentiation in vitro and in neurochemical models of Parkinson’s disease. Frontiers in Cellular Neuroscience. 2015;9:p. 114. doi: 10.3389/fncel.2015.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Scheele C., Petrovic N., Faghihi M. A., et al. The human PINK1 locus is regulated in vivo by a non-coding natural antisense RNA during modulation of mitochondrial function. BMC Genomics. 2007;8:p. 74. doi: 10.1186/1471-2164-8-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Labbadia J., Morimoto R. I. Huntington’s disease: underlying molecular mechanisms and emerging concepts. Trends in Biochemical Sciences. 2013;38(8):378–385. doi: 10.1016/j.tibs.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Young T. L., Matsuda T., Cepko C. L. The noncoding RNA taurine upregulated gene 1 is required for differentiation of the murine retina. Current Biology. 2005;15(6):501–512. doi: 10.1016/j.cub.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 77.Khalil A. M., Guttman M., Huarte M., et al. Many human large intergenic noncoding RNAs associate with chromatin-modifying complexes and affect gene expression. Proceedings of the National Academy of Sciences. 2009;106(28):11667–11672. doi: 10.1073/pnas.0904715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Johnson R. Long non-coding RNAs in Huntington’s disease neurodegeneration. Neurobiology of Disease. 2012;46(2):245–254. doi: 10.1016/j.nbd.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 79.Zhang E. B., Yin D. D., Sun M., et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death and Disease. 2014;5, article e1243 doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Chung D. W., Rudnicki D. D., Yu L., Margolis R. L. A natural antisense transcript at the Huntington’s disease repeat locus regulates HTT expression. Human Molecular Genetics. 2011;20(17):3767–3477. doi: 10.1093/hmg/ddr263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454(7203):428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 82.Li Z., Chao T. C., Chang K. Y., et al. The long noncoding RNA THRIL regulates TNFα expression through its interaction with hnRNPL. Proceedings of the National Academy of Sciences. 2014;111(3):1002–1007. doi: 10.1073/pnas.1313768111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang P., Xue Y., Han Y., et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344(6181):310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 84.Cui H., Xie N., Tan Z., et al. The human long noncoding RNA lnc-IL7R regulates the inflammatory response. European Journal of Immunology. 2014;44(7):2085–2095. doi: 10.1002/eji.201344126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Atianand M. K., Hu W., Satpathy A. T., et al. A long noncoding RNA lincRNA-EPS acts as a transcriptional brake to restrain inflammation. Cell. 2016;165(7):1672–1685. doi: 10.1016/j.cell.2016.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bell G. I., Polonsky K. S. Diabetes mellitus and genetically programmed defects in beta-cell function. Nature. 2001;414(6865):788–791. doi: 10.1038/414788a. [DOI] [PubMed] [Google Scholar]
- 87.Liu C., Li C. P., Wang J. J., Shan K., Liu X., Yan B. RNCR3 knockdown inhibits diabetes mellitus-induced retinal reactive gliosis. Biochemical and Biophysical Research Communications. 2016;479(2):198–203. doi: 10.1016/j.bbrc.2016.09.032. [DOI] [PubMed] [Google Scholar]
- 88.Shan K., Li C. P., Liu C., Liu X., Yan B. RNCR3: a regulator of diabetes mellitus-related retinal microvascular dysfunction. Biochemical and Biophysical Research Communications. 2017;482(4):777–783. doi: 10.1016/j.bbrc.2016.11.110. [DOI] [PubMed] [Google Scholar]
- 89.Qiu G. Z., Tian W., Fu H. T., Li C. P., Liu B. Long noncoding RNA-MEG3 is involved in diabetes mellitus-related microvascular dysfunction. Biochemical and Biophysical Research Communications. 2016;471(1):135–141. doi: 10.1016/j.bbrc.2016.01.164. [DOI] [PubMed] [Google Scholar]
- 90.Zhu X., Wu Y. B., Zhou J., Kang D. M. Upregulation of lncRNA MEG3 promotes hepatic insulin resistance via increasing FoxO1 expression. Biochemical and Biophysical Research Communications. 2016;469(2):319–325. doi: 10.1016/j.bbrc.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 91.Morán I., Akerman I., van de Bunt M., et al. Human β cell transcriptome analysis uncovers lncRNAs that are tissue-specific, dynamically regulated, and abnormally expressed in type 2 diabetes. Cell Metabolism. 2012;16(4):435–448. doi: 10.1016/j.cmet.2012.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Voight B. F., Scott L. J., Steinthorsdottir V., et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nature Genetics. 2010;42(7):579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Amack J. D., Mahadevan M. S. Myogenic defects in myotonic dystrophy. Developmental Biology. 2004;265(2):294–301. doi: 10.1016/j.ydbio.2003.07.021. [DOI] [PubMed] [Google Scholar]
- 94.Lanz R. B., McKenna N. J., Onate S. A., et al. A steroid receptor coactivator, SRA, functions as an RNA and is present in an SRC-1 complex. Cell. 1999;97(1):17–27. doi: 10.1016/S0092-8674(00)80711-4. [DOI] [PubMed] [Google Scholar]
- 95.Hubé F., Velasco G., Rollin J., Furling D., Francastel C. Steroid receptor RNA activator protein binds to and counteracts SRA RNA-mediated activation of MyoD and muscle differentiation. Nucleic Acids Research. 2011;39(2):513–525. doi: 10.1093/nar/gkq833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mueller A. C., Cichewicz M. A., Dey B. K., et al. MUNC, a long noncoding RNA that facilitates the function of MyoD in skeletal myogenesis. Molecular and Cellular Biology. 2015;35(3):498–513. doi: 10.1128/MCB.01079-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu X., Zhang Y., Li T., et al. Long non-coding RNA Linc-RAM enhances myogenic differentiation by interacting with MyoD. Nature Communications. 2017;8, article 14016 doi: 10.1038/ncomms14016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cesana M., Cacchiarelli D., Legnini I., et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang L., Zhao Y., Bao X., et al. LncRNA Dum interacts with Dnmts to regulate Dppa2 expression during myogenic differentiation and muscle regeneration. Cell Research. 2015;25(3):335–350. doi: 10.1038/cr.2015.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lusis A. J. Atherosclerosis. Nature. 2000;407(6801):233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bell R. D., Long X., Lin M., et al. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34(6):1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zou Z. Q., Xu J., Li L., Han Y. S. Down-regulation of SENCR promotes smooth muscle cells proliferation and migration in db/db mice through up-regulation of FoxO1 and TRPC6. Biomedicine and Pharmacotherapy. 2015;74:35–41. doi: 10.1016/j.biopha.2015.06.009. [DOI] [PubMed] [Google Scholar]
- 103.Boulberdaa M., Scott E., Ballantyne M., et al. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Molecular Therapy Family of Journals. 2016;24(5):978–990. doi: 10.1038/mt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Liu Y., Zheng L., Wang Q., Hu Y. W. Emerging roles and mechanisms of long noncoding RNAs in atherosclerosis. International Journal of Cardiology. 2017;228:570–582. doi: 10.1016/j.ijcard.2016.11.182. [DOI] [PubMed] [Google Scholar]
- 105.Lam D., Rao S. K., Ratra V., et al. Cataract. Nature Reviews Disease Primer. 2015;1, article 15014 doi: 10.1038/nrdp.2015.14. [DOI] [PubMed] [Google Scholar]
- 106.Shen Y., Dong L. F., Zhou R. M., et al. Role of long non-coding RNA MIAT in proliferation, apoptosis and migration of lens epithelial cells: a clinical and in vitro study. Journal of Cellular and Molecular Medicine. 2016;20(3):537–548. doi: 10.1111/jcmm.12755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lukong K. E., Chang K. W., Khandjian E. W., Richard S. RNA-binding proteins in human genetic disease. Trends in Genetics. 2008;24(8):416–425. doi: 10.1016/j.tig.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Moore M. J. From birth to death: the complex lives of eukaryotic mRNAs. Science. 2005;309(5740):1514–1518. doi: 10.1126/science.1111443. [DOI] [PubMed] [Google Scholar]
- 109.Keene J. D. RNA regulons: coordination of post-transcriptional events. Nature Review Genetics. 2007;8(7):533–543. doi: 10.1038/nrg2111. [DOI] [PubMed] [Google Scholar]
- 110.Lunde B. M., Moore C., Varani G. RNA-binding proteins: modular design for efficient function. Nature Review Molecular and Cellular Biology. 2007;8(6):479–490. doi: 10.1038/nrm2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schmitz U., Lai X., Winter F., Wolkenhauer O., Vera J., Gupta S. K. Cooperative gene regulation by microRNA pairs and their identification using a computational workflow. Nucleic Acids Research. 2014;42(12):7539–7552. doi: 10.1093/nar/gku465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mavrakis K. J., Van Der Meulen J., Wolfe A. L., et al. A cooperative microRNA-tumor suppressor gene network in acute T-cell lymphoblastic leukemia (T-ALL) Nature Genetics. 2011;43(7):673–678. doi: 10.1038/ng.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hinman M. N., Lou H. Diverse molecular functions of Hu proteins. Cellular and Molecular Life Science. 2008;65(20):3168–3181. doi: 10.1007/s00018-008-8252-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Masuda K., Abdelmohsen K., Gorospe M. RNA-binding proteins implicated in the hypoxic response. Journal of Cellular and Molecular Medicine. 2009;13(9a):2759–2769. doi: 10.1111/j.1582-4934.2009.00842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kai M. Roles of RNA-binding proteins in DNA damage response. International Journal of Molecular Science. 2016;17(3):p. 310. doi: 10.3390/ijms17030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Amadio M., Scapagnini G., Davinelli S., Calabrese V., Govoni S., Pascale A. Involvement of ELAV RNA-binding proteins in the post-transcriptional regulation of HO-1. Frontiers in Cellular Neuroscience. 2014;8:p. 459. doi: 10.3389/fncel.2014.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lal S., Burkhart R. A., Beeharry N., et al. HuR posttranscriptionally regulates WEE1: implications for the DNA damage response in pancreatic cancer cells. Cancer Research. 2014;74(4):1128–1140. doi: 10.1158/0008-5472.CAN-13-1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Alfano L., Costa C., Caporaso A., Antonini D., Giordano A., Pentimalli F. HUR protects NONO from degradation by mir320, which is induced by p53 upon UV irradiation. Oncotarget. 2016;7(47):78127–78139. doi: 10.18632/oncotarget.13002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lucchesi C., Sheikh M. S., Huang Y. Negative regulation of RNA-binding protein HuR by tumor-suppressor ECRG2. Oncogene. 2016;35(20):2565–2573. doi: 10.1038/onc.2015.339. [DOI] [PubMed] [Google Scholar]
- 120.Han S. P., Tang Y. H., Smith R. Functional diversity of the hnRNPs: past, present and perspectives. Biochemical Journal. 2010;430(3):379–392. doi: 10.1042/BJ20100396. [DOI] [PubMed] [Google Scholar]
- 121.Reinhardt H. C., Hasskamp P., Schmedding I., et al. DNA damage activates a spatially distinct late cytoplasmic cell-cycle checkpoint network controlled by MK2-mediated RNA stabilization. Molecular Cell. 2010;40(1):34–49. doi: 10.1016/j.molcel.2010.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Yang R., Weber D. J., Carrier F. Post-transcriptional regulation of thioredoxin by the stress inducible heterogenous ribonucleoprotein A18. Nucleic Acids Research. 2006;34(4):1224–1236. doi: 10.1093/nar/gkj519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chang E. T., Parekh P. R., Yang Q., Nguyen D. M., Carrier F. Heterogenous ribonucleoprotein A18 (hnRNP A18) promotes tumor growth by increasing protein translation of selected transcripts in cancer cells. Oncotarget. 2016;7(9):10578–10593. doi: 10.18632/oncotarget.7020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Cammas A., Pileur F., Bonnal S., et al. Cytoplasmic relocalization of heterogeneous nuclear ribonucleoprotein A1 controls translation initiation of specific mRNAs. Molecular Biology of the Cell. 2007;18(12):5048–5059. doi: 10.1091/mbc.E07-06-0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Anantha R. W., Alcivar A. L., Ma J., et al. Requirement of heterogeneous nuclear ribonucleoprotein C for BRCA gene expression and homologous recombination. PLoS One. 2013;8(4, article e61368) doi: 10.1371/journal.pone.0061368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Decorsière A., Cayrel A., Vagner S., Millevoi S. Essential role for the interaction between hnRNP H/F and a G quadruplex in maintaining p53 pre-mRNA 3′-end processing and function during DNA damage. Genes and Development. 2011;25(3):220–225. doi: 10.1101/gad.607011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Galban S., Kuwano Y., Pullmann R., Jr., et al. RNA-binding proteins HuR and PTB promote the translation of hypoxia-inducible factor 1alpha. Molecular and Cellular Biology. 2008;28(1):93–107. doi: 10.1128/MCB.00973-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mastrocola A. S., Kim S. H., Trinh A. T., Rodenkirch L. A., Tibbetts R. S. The RNA-binding protein fused in sarcoma (FUS) functions downstream of poly(ADP-ribose) polymerase (PARP) in response to DNA damage. Journal of Biological Chemistry. 2013;288(34):24731–24741. doi: 10.1074/jbc.M113.497974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang W. Y., Pan L., Su S. C., et al. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nature Neuroscience. 2013;16(10):1383–1391. doi: 10.1038/nn.3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Anderson P., Kedersha N. Stress granules: the Tao of RNA triage. Trends in Biochemical Sciences. 2008;33(3):141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 131.Arimoto-Matsuzaki K., Saito H., Takekawa M. TIA1 oxidation inhibits stress granule assembly and sensitizes cells to stress-induced apoptosis. Nature Communications. 2016;7, article 10252 doi: 10.1038/ncomms10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jin K., Li W., Nagayama T., et al. Expression of the RNA-binding protein TIAR is increased in neurons after ischemic cerebral injury. Journal of Neuroscience Research. 2000;59(6):767–774. doi: 10.1002/(SICI)1097-4547(20000315)59:6<767::AID-JNR9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 133.Kim H. S., Kuwano Y., Zhan M., et al. Elucidation of a C-rich signature motif in target mRNAs of RNA-binding protein TIAR. Molecular and Cellular Biology. 2007;27(19):6806–6817. doi: 10.1128/MCB.01036-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hellborg F., Qian W., Mendez-Vidal C., et al. Human wig-1, a p53 target gene that encodes a growth inhibitory zinc finger protein. Oncogene. 2001;20(39):5466–5474. doi: 10.1038/sj.onc.1204722. [DOI] [PubMed] [Google Scholar]
- 135.Vilborg A., Glahder J. A., Wilhelm M. T., et al. The p53 target Wig-1 regulates p53 mRNA stability through an AU-rich element. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(37):15756–15761. doi: 10.1073/pnas.0900862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bersani C., Xu L. D., Vilborg A., Lui W. O., Wiman K. G. Wig-1 regulates cell cycle arrest and cell death through the p53 targets FAS and 14-3-3sigma. Oncogene. 2014;33(35):4407–4417. doi: 10.1038/onc.2013.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Masuda K., Kuwano Y., Nishida K., Rokutan K. General RBP expression in human tissues as a function of age. Ageing Research Reviews. 2012;11(4):423–431. doi: 10.1016/j.arr.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 138.Toba G., Yamamoto D., White K. Life-span phenotypes of elav and Rbp9 in Drosophila suggest functional cooperation of the two ELAV-family protein genes. Archives of Insect Biochemistry and Physiology. 2010;74(4):261–265. doi: 10.1002/arch.20377. [DOI] [PubMed] [Google Scholar]
- 139.Hashimoto M., Tsugawa T., Kawagishi H., Asai A., Sugimoto M. Loss of HuR leads to senescence-like cytokine induction in rodent fibroblasts by activating NF-kappaB. Biochimica et Biophysica Acta. 2014;1840(10):3079–3087. doi: 10.1016/j.bbagen.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 140.Masuda K., Marasa B., Martindale J. L., Halushka M. K., Gorospe M. Tissue- and age-dependent expression of RNA-binding proteins that influence mRNA turnover and translation. Aging (Albany NY) 2009;1(8):681–698. doi: 10.18632/aging.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pang L., Tian H., Chang N., et al. Loss of CARM1 is linked to reduced HuR function in replicative senescence. BMC Molecular Biology. 2013;14:p. 15. doi: 10.1186/1471-2199-14-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Chang N., Yi J., Guo G., et al. HuR uses AUF1 as a cofactor to promote p16INK4 mRNA decay. Molecular and Cellular Biology. 2010;30(15):3875–3886. doi: 10.1128/MCB.00169-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Guo G. E., Ma L. W., Jiang B., Yi J., Tong T. J., Wang W. G. Hydrogen peroxide induces p16(INK4a) through an AUF1-dependent manner. Journal of Cellular Biochemistry. 2010;109(5):1000–1005. doi: 10.1002/jcb.22474. [DOI] [PubMed] [Google Scholar]
- 144.Pont A. R., Sadri N., Hsiao S. J., Smith S., Schneider R. J. mRNA decay factor AUF1 maintains normal aging, telomere maintenance, and suppression of senescence by activation of telomerase transcription. Molecular Cell. 2012;47(1):5–15. doi: 10.1016/j.molcel.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Waris S., Wilce M. C., Wilce J. A. RNA recognition and stress granule formation by TIA proteins. International Journal of Molecular Science. 2014;15(12):23377–23388. doi: 10.3390/ijms151223377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sanchez-Jimenez C., Izquierdo J. M. T-cell intracellular antigen (TIA)-proteins deficiency in murine embryonic fibroblasts alters cell cycle progression and induces autophagy. PLoS One. 2013;8(9, article e75127) doi: 10.1371/journal.pone.0075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Vlasova I. A., Tahoe N. M., Fan D., et al. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Molecular Cell. 2008;29(2):263–270. doi: 10.1016/j.molcel.2007.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Iakova P., Wang G. L., Timchenko L., et al. Competition of CUGBP1 and calreticulin for the regulation of p21 translation determines cell fate. The EMBO Journal. 2004;23(2):406–417. doi: 10.1038/sj.emboj.7600052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Timchenko N. A., Wang G. L., Timchenko L. T. RNA CUG-binding protein 1 increases translation of 20-kDa isoform of CCAAT/enhancer-binding protein beta by interacting with the alpha and beta subunits of eukaryotic initiation translation factor 2. Journal of Biological Chemistry. 2005;280(21):20549–20557. doi: 10.1074/jbc.M409563200. [DOI] [PubMed] [Google Scholar]
- 150.Timchenko L. T., Salisbury E., Wang G. L., et al. Age-specific CUGBP1-eIF2 complex increases translation of CCAAT/enhancer-binding protein beta in old liver. Journal of Biological Chemistry. 2006;281(43):32806–32819. doi: 10.1074/jbc.M605701200. [DOI] [PubMed] [Google Scholar]
- 151.Jin J., Wang G. L., Salisbury E., Timchenko L., Timchenko N. A. GSK3beta-cyclin D3-CUGBP1-eIF2 pathway in aging and in myotonic dystrophy. Cell Cycle. 2009;8(15):2356–2359. doi: 10.4161/cc.8.15.9248. [DOI] [PubMed] [Google Scholar]
- 152.Ross C. R., Brennan-Laun S. E., Wilson G. M. Tristetraprolin: roles in cancer and senescence. Ageing Research Reviews. 2012;11(4):473–484. doi: 10.1016/j.arr.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Frasca D., Landin A. M., Riley R. L., Blomberg B. B. Mechanisms for decreased function of B cells in aged mice and humans. Journal of Immunology. 2008;180(5):2741–2746. doi: 10.4049/jimmunol.180.5.2741. [DOI] [PubMed] [Google Scholar]
- 154.Sanduja S., Kaza V., Dixon D. A. The mRNA decay factor tristetraprolin (TTP) induces senescence in human papillomavirus-transformed cervical cancer cells by targeting E6-AP ubiquitin ligase. Aging (Albany NY) 2009;1(9):803–817. doi: 10.18632/aging.100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim B. C., Lee H. C., Lee J. J., et al. Wig1 prevents cellular senescence by regulating p21 mRNA decay through control of RISC recruitment. The EMBO Journal. 2012;31(22):4289–4303. doi: 10.1038/emboj.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Darnell R. B. RNA protein interaction in neurons. Annual Review Neuroscience. 2013;36:243–270. doi: 10.1146/annurev-neuro-062912-114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lenzken S. C., Achsel T., Carrì M. T., Barabino S. M. L. Neuronal RNA -binding proteins in health and disease. Wiley Interdisciplinary Reviews: RNA. 2014;5:565–576. doi: 10.1002/wrna.1231. [DOI] [PubMed] [Google Scholar]
- 158.Holt C. E., Bullock S. L. Subcellular mRNA localization in animal cells and why it matters. Science. 2009;326(5957):1212–1216. doi: 10.1126/science.1176488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sreedharan J., Blair I. P., Tripathi V. B., et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science. 2008;319(5870):1668–1672. doi: 10.1126/science.1154584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ling S. C., Polymenidou M., Cleveland D. W. Converging mechanisms in ALS and FTD: disrupted RNA and protein homeostasis. Neuron. 2013;79(3):416–438. doi: 10.1016/j.neuron.2013.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sofola O. A., Jin P., Qin Y., et al. RNA-binding proteins hnRNP A2/B1 and CUGBP1 suppress fragile X CGG premutation repeat-induced neurodegeneration in a Drosophila model of FXTAS. Neuron. 2007;55(4):565–571. doi: 10.1016/j.neuron.2007.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kim H. J., Kim N. C., Wang Y. D., et al. Mutations in prion-like domains in hnRNPA2B1 and hnRNPA1 cause multisystem proteinopathy and ALS. Nature. 2013;495(7442):467–473. doi: 10.1038/nature11922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee E. B., Lee V. M., Trojanowski J. Q. Gains or losses: molecular mechanisms of TDP43-mediated neurodegeneration. Nature Reviews Neuroscience. 2011;13(1):38–50. doi: 10.1038/nrn3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vance C., Rogelj B., Hortobagyi T., et al. Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6. Science. 2009;323(5918):1208–1211. doi: 10.1126/science.1165942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kwiatkowski T. J., Jr., Bosco D. A., Leclerc A. L., et al. Mutations in the FUS/TLS gene on chromosome 16 cause familial amyotrophic lateral sclerosis. Science. 2009;323(5918):1205–1208. doi: 10.1126/science.1166066. [DOI] [PubMed] [Google Scholar]
- 166.Perrone-Bizzozero N., Bird C. W. Role of HuD in nervous system function and pathology. Frontiers in Bioscience (Schol Ed) 2013;5:554–563. doi: 10.2741/s389. [DOI] [PubMed] [Google Scholar]
- 167.Hayashi S., Yano M., Igarashi M., Okano H. J., Okano H. Alternative role of HuD splicing variants in neuronal differentiation. Journal of Neuroscience Research. 2015;93(3):399–409. doi: 10.1002/jnr.23496. [DOI] [PubMed] [Google Scholar]
- 168.Kang M. J., Abdelmohsen K., Hutchison E. R., et al. HuD regulates coding and noncoding RNA to induce APP→Aβ processing. Cell Reports. 2014;7(5):1401–1409. doi: 10.1016/j.celrep.2014.04.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Richter J. D., Bassell G. J., Klann E. Dysregulation and restoration of translational homeostasis in fragile X syndrome. Nature Reviews Neuroscience. 2015;16(10):595–605. doi: 10.1038/nrn4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Li Z., Zhang Y., Ku L., Wilkinson K. D., Warren S. T., Feng Y. The fragile X mental retardation protein inhibits translation via interacting with mRNA. Nucleic Acids Research. 2001;29(11):2276–2283. doi: 10.1093/nar/29.11.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Lee E. K., Kim H. H., Kuwano Y., et al. hnRNP C promotes APP translation by competing with FMRP for APP mRNA recruitment to P bodies. Nature Structure and Molecular Biology. 2010;17(6):732–739. doi: 10.1038/nsmb.1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Borreca A., Gironi K., Amadoro G., Ammassari-Teule M. Opposite dysregulation of fragile-X mental retardation protein and heteronuclear ribonucleoprotein C protein associates with enhanced APP translation in Alzheimer disease. Molecular Neurobiology. 2016;53(5):3227–3234. doi: 10.1007/s12035-015-9229-8. [DOI] [PubMed] [Google Scholar]
- 173.He F., Krans A., Freibaum B. D., Taylor J. P., Todd P. K. TDP-43 suppresses CGG repeat-induced neurotoxicity through interactions with HnRNP A2/B1. Human Molecular Genetics. 2014;23(19):5036–5051. doi: 10.1093/hmg/ddu216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Liu X. Y., Li H. L., Su J. B., et al. Regulation of RAGE splicing by hnRNP A1 and Tra2beta-1 and its potential role in AD pathogenesis. Journal of Neurochemistry. 2015;133(2):187–198. doi: 10.1111/jnc.13069. [DOI] [PubMed] [Google Scholar]
- 175.Berson A., Barbash S., Shaltiel G., et al. Cholinergic-associated loss of hnRNP-A/B in Alzheimer’s disease impairs cortical splicing and cognitive function in mice. EMBO Molecular Medicine. 2012;4(8):730–742. doi: 10.1002/emmm.201100995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Martinez F. J., Pratt G. A., Van Nostrand E. L., et al. Protein-RNA networks regulated by normal and ALS-associated mutant HNRNPA2B1 in the nervous system. Neuron. 2016;92(4):780–795. doi: 10.1016/j.neuron.2016.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mizukami K., Ishikawa M., Iwakiri M., et al. Immunohistochemical study of the hnRNP A2 and B1 in the hippocampal formations of brains with Alzheimer’s disease. Neuroscience Letters. 2005;386(2):111–115. doi: 10.1016/j.neulet.2005.05.070. [DOI] [PubMed] [Google Scholar]
- 178.Hotamisligil G. S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 179.Wilson P. W., D’Agostino R. B., Parise H., Sullivan L., Meigs J. B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation. 2005;112(20):3066–3072. doi: 10.1161/CIRCULATIONAHA.105.539528. [DOI] [PubMed] [Google Scholar]
- 180.Kim W., Lee E. K. Post-transcriptional regulation in metabolic diseases. RNA Biology. 2012;9(6):1–9. doi: 10.4161/rna.20091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lee E. K., Kim W., Tominaga K., et al. RNA-binding protein HuD controls insulin translation. Molecular Cell. 2012;45(6):826–835. doi: 10.1016/j.molcel.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kim C., Lee H., Kang H., et al. RNA-binding protein HuD reduces triglyceride production in pancreatic beta cells by enhancing the expression of insulin-induced gene 1. Biochimica et Biophysica Acta. 2016;1859(4):675–685. doi: 10.1016/j.bbagrm.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Kim C., Kim W., Lee H., et al. The RNA-binding protein HuD regulates autophagosome formation in pancreatic beta cells by promoting autophagy-related gene 5 expression. Journal of Biological Chemistry. 2014;289(1):112–121. doi: 10.1074/jbc.M113.474700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Juan-Mateu J. A., Rech T. H., Villate O., et al. Neuron-enriched RNA-binding proteins regulate pancreatic beta cell function and survival. Journal of Biological Chemistry. 2017;292(8):3466–3480. doi: 10.1074/jbc.M116.748335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sen S., Talukdar I., Webster N. J. SRp20 and CUG-BP1 modulate insulin receptor exon 11 alternative splicing. Molecular and Cellular Biology. 2009;29(3):871–880. doi: 10.1128/MCB.01709-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Zhai K., Gu L., Yang Z., et al. RNA-binding protein CUGBP1 regulates insulin secretion via activation of phosphodiesterase 3B in mice. Diabetologia. 2016;59(9):1959–1967. doi: 10.1007/s00125-016-4005-5. [DOI] [PubMed] [Google Scholar]
- 187.Verma S. K., Deshmukh V., Liu P., et al. Reactivation of fetal splicing programs in diabetic hearts is mediated by protein kinase C signaling. Journal of Biological Chemistry. 2013;288(49):35372–35386. doi: 10.1074/jbc.M113.507426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Yeo G. W., Coufal N. G., Liang T. Y., Peng G. E., Fu X. D., Gage F. H. An RNA code for the FOX2 splicing regulator revealed by mapping RNA-protein interactions in stem cells. Nature Structure and Molecular Biology. 2009;16(2):130–137. doi: 10.1038/nsmb.1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nutter C. A., Jaworski E. A., Verma S. K., et al. Dysregulation of RBFOX2 is an early event in cardiac pathogenesis of diabetes. Cell Reports. 2016;15(10):2200–2213. doi: 10.1016/j.celrep.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Dai N., Rapley J., Angel M., Yanik M. F., Blower M. D., Avruch J. mTOR phosphorylates IMP2 to promote IGF2 mRNA translation by internal ribosomal entry. Genes and Development. 2011;25(11):1159–1172. doi: 10.1101/gad.2042311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Saxena R., Voight B. F., Lyssenko V., et al. Genome-wide association analysis identifies loci for type 2 diabetes and triglyceride levels. Science. 2007;316(5829):1331–1336. doi: 10.1126/science.1142358. [DOI] [PubMed] [Google Scholar]
- 192.Scott L. J., Mohlke K. L., Bonnycastle L. L., et al. A genome-wide association study of type 2 diabetes in Finns detects multiple susceptibility variants. Science. 2007;316(5829):1341–1345. doi: 10.1126/science.1142382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Dai N., Zhao L., Wrighting D., et al. IGF2BP2/IMP2-deficient mice resist obesity through enhanced translation of Ucp1 mRNA and other mRNAs encoding mitochondrial proteins. Cell Metabolism. 2015;21(4):609–621. doi: 10.1016/j.cmet.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Martinez-Sanchez A., Rutter G. A., Latreille M. MiRNAs in beta-cell development, identity, and disease. Frontiers in Genetics. 2016;7:p. 226. doi: 10.3389/fgene.2016.00226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Olivieri F., Albertini M. C., Orciani M., et al. DNA damage response (DDR) and senescence: shuttled inflamma-miRNAs on the stage of inflamm-aging. Oncotarget. 2015;6(34):35509–35521. doi: 10.18632/oncotarget.5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Y., Yu B., He J., Chen D. From nutrient to MicroRNA: a novel insight into cell signaling involved in skeletal muscle development and disease. International Journal of Biological Sciences. 2016;12(10):1247–1261. doi: 10.7150/ijbs.16463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Castello A., Fischer B., Eichelbaum K., et al. Insights into RNA biology from an atlas of mammalian mRNA-binding proteins. Cell. 2012;149(6):1393–1406. doi: 10.1016/j.cell.2012.04.031. [DOI] [PubMed] [Google Scholar]
- 198.Neelamraju Y., Hashemikhabir S., Janga S. C. The human RBPome: from genes and proteins to human disease. Journal of Proteomics. 2015;127(Part A):61–70. doi: 10.1016/j.jprot.2015.04.031. [DOI] [PubMed] [Google Scholar]
- 199.Li J. H., Liu S., Zheng L. L., et al. Discovery of protein-lncRNA interactions by integrating large-scale CLIP-Seq and RNA-Seq datasets. Frontiers in Bioengineering and Biotechnology. 2014;2:p. 88. doi: 10.3389/fbioe.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]